Abstract
Ischemia-reperfusion (IR) injury is significantly worse in aged hearts, but the underlying mechanisms are poorly understood. Age-related damage to mitochondria may be a critical feature, which manifests in an exacerbation of IR injury. Silent information regulator of transcription 3 (SIRT3), the major mitochondrial NAD+-dependent lysine deacetylase, regulates a variety of functions, and its inhibition may disrupt mitochondrial function to impact recovery from IR injury. In this study, the role of SIRT3 in mediating the response to cardiac IR injury was examined using an in vitro model of SIRT3 knockdown (SIRT3kd) in H9c2 cardiac-derived cells and in Langendorff preparations from adult (7 mo old) wild-type (WT) and SIRT3+/− hearts and aged (18 mo old) WT hearts. SIRT3kd cells were more vulnerable to simulated IR injury and exhibited a 46% decrease in mitochondrial complex I (Cx I) activity with low O2 consumption rates compared with controls. In the Langendorff model, SIRT3+/− adult hearts showed less functional recovery and greater infarct vs. WT, which recapitulates the in vitro results. In WT aged hearts, recovery from IR injury was similar to SIRT3+/− adult hearts. Mitochondrial protein acetylation was increased in both SIRT3+/− adult and WT aged hearts (relative to WT adult), suggesting similar activities of SIRT3. Also, enzymatic activities of two SIRT3 targets, Cx I and MnSOD, were similarly and significantly inhibited in SIRT3+/− adult and WT aged cardiac mitochondria. In conclusion, decreased SIRT3 may increase the susceptibility of cardiac-derived cells and adult hearts to IR injury and may contribute to a greater level of IR injury in the aged heart.
Keywords: SIRT3, heart, aging, ischemia, reperfusion, mitochondria, acetylation
myocardial infarction (MI) remains a major clinical problem in the Western world. Much of the morbidity and mortality associated with MI is due to the lack of adequate protection of the myocardium when ischemic areas are reperfused after surgical or catheter-based interventions (23). The heart is an energetically demanding organ, and its function relies on mitochondrial ATP generation. Irreversible damage to mitochondria during MI results in a shortage of ATP supply, oxidative stress, and eventually, cardiomyocyte death. This pathologic process is termed ischemia-reperfusion (IR) injury.
Aging is a major risk factor for MI, and recovery from IR injury is greatly compromised in aged hearts (6, 25). A number of observations draw a link among the loss of mitochondrial function, aging, and increased susceptibility of aged hearts to IR injury (6, 11, 25, 28, 58). However, the underlying mechanisms of this link remain ambiguous.
Within the aging field, a great deal of attention has been directed toward the silent information regulator of transcription (SIRT) family of proteins (4, 44). SIRTs are class III NAD+-dependent histone deacetylases involved in gene silencing (12a). Importantly, SIRT-mediated protein deacetylation has emerged as an important post-translational modification involved in signaling in several cell compartments (30). Although a direct role for nuclear/cytosolic SIRT1 in cardioprotection against IR injury has been reported (22, 39, 40), the role of other SIRTs in modulating IR injury is less clear.
Since mitochondria and metabolism are sensitive targets for damage during IR injury (36), the role of mitochondrial SIRTs in cardioprotection is of particular interest. SIRT3 is the major mitochondrial deacetylase (31), and several proteins implicated in regulating the response to IR have been identified as SIRT3 targets (47). They include cyclophilin D (18, 49), isocitrate dehydrogenase 2 (ICDH2) (59), Ku70 (52), MnSOD (46), and complex I (Cx I) (3). Whereas the number of SIRT3 targets that may be involved in cardiac IR injury continues to grow, the interplay between those targets and SIRT3 in the context of IR is not known.
SIRT3 protein expression is lower in old, sedentary adults (27). In addition, SIRT3 activity may be compromised by low levels of NAD+, as seen in aged hearts (7, 50). Notably, SIRT3 can be inhibited by oxidants (15), which may be generated at higher rates in aged hearts (5). Therefore, if the protective effects of SIRT3 rely on its enzymatic activity, this might be impaired in aged individuals. Indeed, adult SIRT3−/− mice share a number of cardiac phenotypes with aged wild-type (WT) mice, such as cardiac hypertrophy and fibrosis (18, 51). Nevertheless, direct inhibition of SIRT3 activity in aged hearts has not been reported, and the relationship between downregulation of SIRT3 and poor recovery of aged hearts from IR injury is unclear. In this study, we examined the connection between SIRT3 and IR injury in the context of aging. Our data support a link between SIRT3 downregulation and vulnerability of both adult and aged hearts to IR injury.
METHODS
Animals and materials.
Male WT [7 mo (“adult”) and 18 mo (“aged”)] and SIRT3+/− [7 mo (adult)] mice, on a C57BL/6 background, were used in this study. All mice were maintained in a pathogen-free vivarium under recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with a 12:12-h, light-dark cycle and food and water available ad libitum. All experimental protocols were approved by the Association for Assessment and Accreditation of Laboratory Animal Care-accredited University of Rochester Committee on Animal Resources. All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise mentioned.
Small interfering RNA transfection in H9c2 cells and in vitro-simulated IR injury.
H9c2 cardiac-derived cells (American Type Culture Collection, Manassas, VA) were transfected with SIRT3 small interfering RNA (siRNA) and subjected to in vitro IR injury; the major steps of this procedure have been described previously (41). Briefly, H9c2 cells were plated on 22-mm, 12-well plates (Greiner Bio-One, Monroe, NC) and transfected with 50 nM SIRT3 siRNA [SIRT3 knockdown (SIRT3kd); Qiagen, Valencia, CA] or scrambled control siRNA (Ctrl). Successful knockdown was verified after 72 h using immunoblotting.
For in vitro-simulated IR injury, cells were incubated in normoxic buffer (DMEM, 10 mM glucose, 10 mM HEPES, pH 7.4, at 37°C). Simulated ischemia (4 h) started when cells were transferred into a hypoxic chamber with 0% O2 (Coy, Grass Lake, MI), and media were replaced with hypoxic buffer (glucose-free DMEM, 10 mM HEPES, pH 6.5, at 37°C). Upon reperfusion, the media were replaced with normoxic buffer, and cells were incubated at 37°C (room air + 5% CO2) for 2 h. Cell death was then assayed by measuring lactate dehydrogenase (LDH) release using a Cytotoxicity Detection Kit (Roche Diagnostics, Indianapolis, IN), according to the manufacturer's protocol.
Cx I activity and O2 consumption rate measurements.
Cx I activity was measured by monitoring rotenone-sensitive NADH oxidation at 340 nm (8) in freeze/thawed Ctrl and SIRT3kd H9c2 cells and in mitochondria isolated from WT adult, SIRT3+/− adult, and WT aged hearts, before and after IR injury. For O2 consumption rate (OCR) measurements, H9c2 cells were plated on V7-PS 24-well plates (Seahorse Bioscience, Billerica, MA). One-half of the wells was transfected with 50 nM SIRT3 siRNA (Qiagen) and the other one-half with Ctrl siRNA. Seventy-two hours later, OCR was measured using a Seahorse Bioscience XF24 Flux Analyzer, as described previously (17), with a standard baseline, oligomycin (1 μg/ml), FCCP (500 nM), antimycin A (5 μM; “BOFA” protocol). Reserve capacity was calculated as a difference in OCR between baseline and FCCP-uncoupled respiration rate.
Mouse Langendorff-perfused heart.
Following avertin anesthesia, hearts were extirpated and perfused in constant flow mode (4 ml·min−1·100 mg−1) with Krebs-Henseleit (KH) buffer, comprised of 118 mM NaCl, 4.7 mM KCl, 25 mM NaHCO3, 10 mM glucose, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 2.5 mM CaCl2 (buffer was equilibrated with 95% O2 + 5% CO2, pH 7.4, at 37°C). Global ischemia (25 min) was induced by stopping flow and immersing the heart in deoxygenated KH buffer. Reperfusion (1 h) was begun by raising flow back to the pre-ischemic rate over a period of 1 min. Functional parameters measured include heart rate [(HR) = beats/min], left ventricular-developed pressure [(LVDP) = left ventricular pressure (LVP)systolic − LVPdiastolic], rate pressure product [(RPP) = LVDP·HR], and ischemic hypercontracture (differences between pre-ischemic LVPdiastolic and maximum LVP during ischemia). For infarct size measurements (using the same hearts as for the functional measurements), the heart was cut transversely into five sections, which were incubated in 1.0% 2,3,5-triphenyltetrazolium chloride (TTC) at 37°C for 20 min, followed by fixation in 10% neutral-buffered formalin for 24 h. The area at risk was the whole heart, and infarct was indicated by the areas unstained with TTC. Slices were photographed, and infarct/whole heart ratios were determined with Photoshop software (39). For IR experiments, the following groups were used: adult WT (n = 9), adult SIRT3+/− (n = 7), and aged WT (n = 12).
Isolated mitochondrial preparation.
Following euthanasia, hearts were removed and immersed in 2 ml ice-cold isolation medium (IM), comprised of 300 mM sucrose, 20 mM Tris-HCl, 2 mM EGTA, pH 7.35, at 4°C. Tissue was chopped, then washed, and homogenized in 2 ml IM. The homogenate was centrifuged at 1,000 g for 5 min. The pellet was discarded and the supernatant centrifuged at 7,000 g for 10 min. The pellet was resuspended in 1.5 ml IM and then centrifuged at 10,000 g for 5 min. The final pellet was resuspended in 50 μl IM. Protein was determined by the Lowry method against a standard curve constructed using BSA.
MnSOD activity.
Isolated heart mitochondria were suspended at 50 μg/ml in phosphate buffer (pH 7.35, 25°C) containing xanthine oxidase (2 U/ml), xanthine (10 mM), cytochrome c (2 mM), and KCN (1 mM). Xanthine/xanthine oxidase produces superoxide, which reduces cytochrome c at a linear rate (measured spectrophotometrically at 550 nm). SOD intersects this reduction, and the SOD content of mitochondrial samples was determined from a standard curve constructed using known amounts of SOD. The presence of KCN both inhibits Cu/ZnSOD, rendering the assay specific for MnSOD, and inhibits mitochondrial cytochrome c oxidase, preventing reoxidation of reduced cytochrome c.
Western blotting.
For immunoblotting, mitochondria and H9c2 cell pellets were lysed in SDS-containing sample loading buffer. Samples were resolved on 12% or 16% SDS-PAGE gel, transferred to nitrocellulose, stained with Ponceau S, and probed with antibodies [anti-SIRT3 and anti-acetylation lysine (Cell Signaling Technology, Danvers, MA); anti-NADH dehydrogenase (ubiquinone) 1 α subcomplex subunit 9 (NDUFA9) and anti-adenine nucleotide translocase 1 (Abcam-MitoSciences, Cambridge, MA); and anti-β-actin and anti-voltage-dependent anion channels (Calbiochem-Millipore, Darmstadt, Germany)] at 1:1,000 dilutions. In some cases, membranes were stripped and reprobed. A horseradish peroxidase-linked secondary antibody (GE Biosciences, Pittsburgh, PA) was used at 1:2,500 dilution, with enhanced chemiluminescence detection (Pierce, Rockford, IL, or GE Biosciences).
Statistics.
Significance between the two groups was determined using Student's t-test. Multiple comparisons were performed assuming a normal distribution using ANOVA with Tukey's multiple comparison test (Prism 6.0 for Windows; GraphPad, La Jolla, CA).
RESULTS
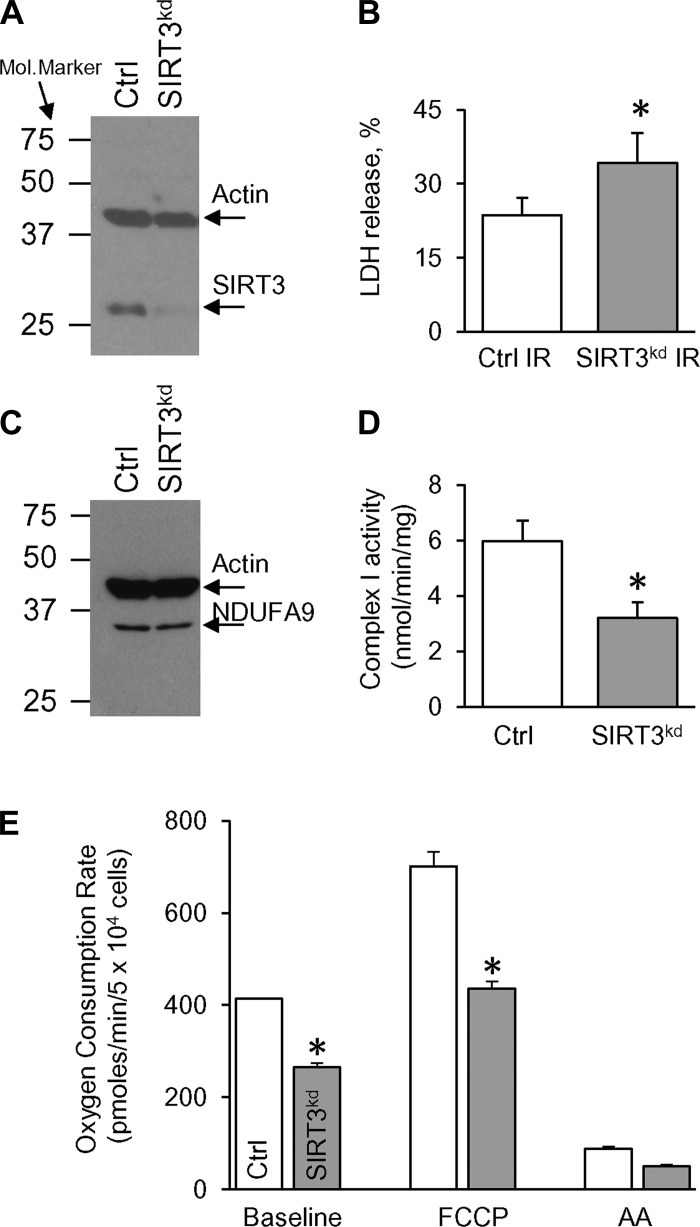
SIRT3-deficient H9c2 cells (SIRT3kd) were used to test a role for SIRT3 in simulated IR injury. Figure 1A demonstrates that 50 nM of siRNA decreased the SIRT3 protein level down to 10% vs. nonsilencing siRNA (Ctrl), with no changes in the β-actin level. To check whether downregulation of SIRT3 modulates simulated IR injury, both Ctrl and SIRT3kd cells were subjected to 4 h of simulated ischemia, followed by 2 h of reperfusion. Figure 1B demonstrates that LDH release, measured at the end of the simulated IR protocol, was significantly higher in SIRT3kd cells (vs. Ctrl IR), indicating higher cell death in the SIRT3kd group.
Fig. 1.
Silent information regulator of transcription 3 knockdown (SIRT3kd) exacerbates simulated ischemia-reperfusion (IR) injury and changes cellular bioenergetics. SIRT3 protein was knocked down in H9c2 cardiac-derived cells. Seventy-two hours post-transfection, cells were harvested for Western blot analysis and functional assays. A: SIRT3 and β-actin expressions in control (Ctrl) vs. SIRT3kd cells. A blot image representative of 5 independent experiments is shown. B: lactate dehydrogenase (LDH) release from Ctrl and SIRT3kd cells during simulated IR injury. LDH release is expressed as a percentage of total LDH. C: NADH dehydrogenase (ubiquinone) 1 α subcomplex subunit 9 (NDUFA9) and β-actin expressions in Ctrl vs. SIRT3kd cells. D: complex I (Cx I) activity in Ctrl vs. SIRT3kd cells. E: changes in O2 consumption rate (OCR) upon injections of FCCP (500 nM) and antimycin A (AA; 5 μM). OCR was measured in Ctrl vs. SIRT3kd cells in the XF24 Flux Analyzer (Seahorse Bioscience, Billerica, MA). All data are shown as means ± SE, n = 4–5 runs; *P < 0.05 vs. Ctrl using t-test.
In addition to being vulnerable to simulated IR injury, SIRT3kd cells exhibited remarkable metabolic disturbances. Indeed, despite no changes in the levels of NDUFA9 (Fig. 1C), SIRT3kd cells exhibited significantly less Cx I activity (compared with Ctrl; Fig. 1D). A link between Cx I and cardioprotection is suggested by the observation that fully functional Cx I is necessary for endogenous cardioprotection (38). Further supporting a role for SIRT3 in mitochondrial metabolism, SIRT3kd cells exhibited a lower OCR compared with Ctrl (Fig. 1E). A similar bioenergetic profile has been shown with SIRT3kd in C2C12 skeletal myoblasts (24, 54). A significant reduction in the reserve capacity in SIRT3kd cells (171 ± 20 pMoles/min vs. 287 ± 18 pMoles/min in Ctrl, P < 0.05; Fig. 1E) may predict poor tolerance to stress, and this may further exacerbate recovery from IR injury.
Next, with the use of a more physiologic approach of the Langendorff-perfused heart, we investigated if SIRT3+/− adult hearts were more susceptible to IR injury compared with the WT adult. We also performed experiments using aged (18 mo) WT hearts with the objective of determining whether the biochemical and physiological changes seen in SIRT3+/− hearts (see below) were phenocopied by aging.
Pre-ischemic values of LVDP and HR are shown in Table 1. No significant differences were seen, although both LVDP and HR were slightly increased in SIRT3+/− hearts. During ischemia, the magnitude of hypercontracture was similar across all three groups (Table 1).
Table 1.
Cardiac function (LVDP and HR) at baseline (before ischemia) and IHC in WT adult, SIRT3+/− adult, and WT aged hearts
| WT Adult | SIRT3+/− Adult | WT Aged | |
|---|---|---|---|
| LVDP, mmHg | 123.7 ± 8.8 | 136.6 ± 6.9 | 126.6 ± 7.4 |
| HR, min−1 | 360 ± 20 | 390 ± 15 | 369 ± 21 |
| IHC, mmHg | 65.9 ± 5.6 | 71.8 ± 7.4 | 71.4 ± 5.4 |
Left ventricular-developed pressure (LVDP) was calculated as a difference between systolic and diastolic ventricular pressure. Heart rate (HR) was calculated as a number of strokes/min. Ischemic hypercontracture (IHC) was measured as a difference between left ventricular (LV) diastolic pressure and maximum LV pressure during ischemia. Values are means ± SE, n = 7–12; P < 0.05 vs. wild-type (WT) adult (ANOVA). SIRT3, silent information regulator of transcription 3.
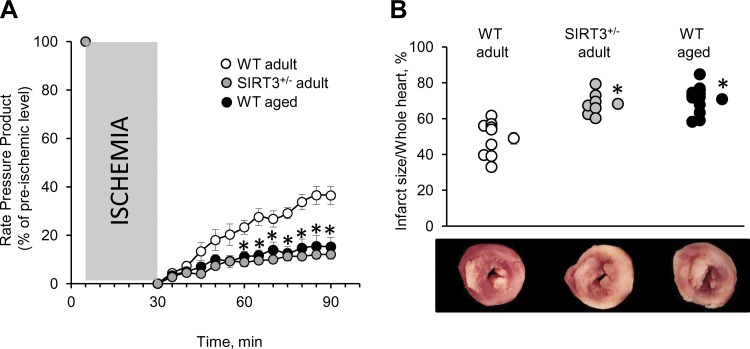
Upon reperfusion, we observed significantly worse recovery of the RPP in all three groups (vs. pre-ischemic level; Fig. 2A). Importantly, both adult SIRT3+/− and aged WT hearts were more susceptible to IR injury (worse RPP recovery) compared with adult WT hearts (Fig. 2A). This corresponded to an increase in the size of infarcted area in SIRT3+/− adult and WT aged hearts compared with adult WT (Fig. 2B). These results indicate that IR injury caused marked functional disturbances in SIRT3+/− adult and WT aged hearts.
Fig. 2.
IR injury in wild-type (WT) adult vs. SIRT3+/− adult vs. WT aged hearts. Three groups of animals were tested: WT adult (7 mo), SIRT3+/− adult (7 mo), and WT aged (18 mo). All hearts were subjected to IR, comprising 25 min ischemia and 1 h reperfusion (see methods for details). A: changes in the rate pressure products during IR (presented as percentages of pre-ischemic levels). B: myocardial infarction after IR injury. Top: individual data points for each condition are shown on the left, with average quantified infarct/whole heart ratios on the right. Bottom: typical cross-sections of hearts stained for infarct (white) and viable tissue (red). All data are shown as means ± SE, n = 9 for adult WT, n = 7 for adult SIRT3+/−, and n = 12 for aged WT; *P < 0.05 vs. adult WT (B); *significance for both SIRT3+/− adult and WT aged groups vs. WT adults (A).
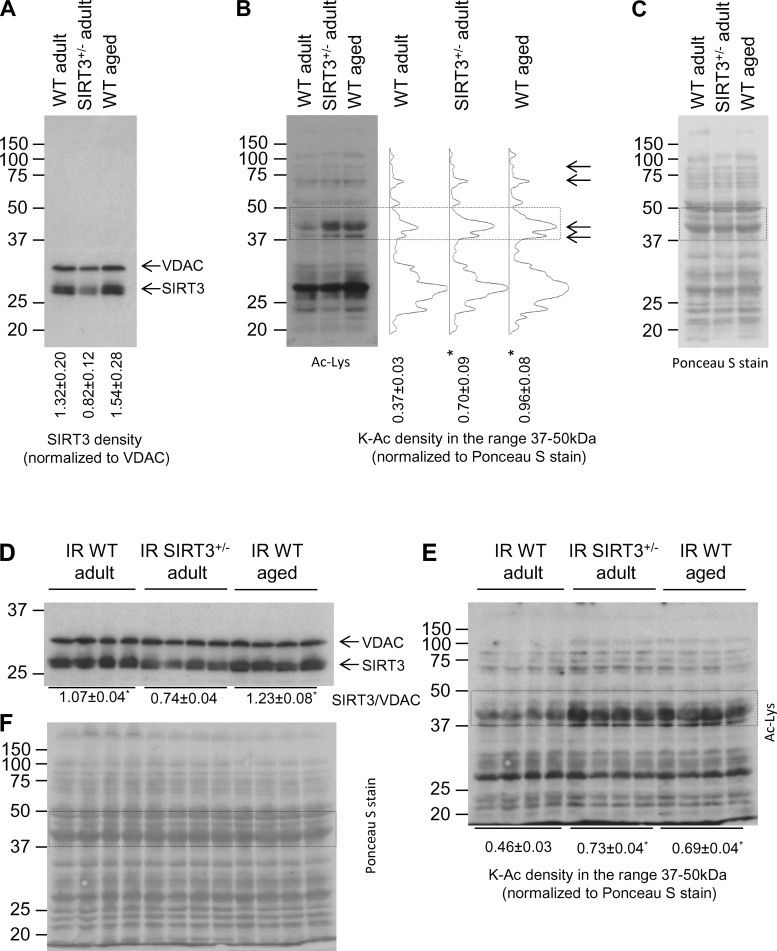
Having established that adult SIRT3+/− and WT aged hearts possessed increased sensitivity to IR injury relative to young WT controls, we proceeded to investigate whether SIRT3 was downregulated in WT aged hearts. First, we examined the level of SIRT3 protein using immunoblot analysis of mitochondrial extract from WT adult, SIRT3+/− adult, and WT aged hearts. As expected, SIRT3 protein expression was decreased in SIRT3+/− adult hearts; however, no changes between WT adult and aged hearts were found (Fig. 3A). These results, however, do not eliminate the possibility that SIRT3 enzymatic activity might be impaired in WT aged hearts. Although the reliability of enzymatic SIRT activity assays has been questioned recently (42), mitochondrial protein acetylation has been used previously as a surrogate marker for SIRT3 activity in the heart (26, 55); thus we analyzed protein acetylation in mitochondrial samples isolated from WT adult, SIRT3+/− adult, and WT aged hearts. Figure 3B demonstrates that mitochondrial proteins were more acetylated in both SIRT3+/− adult and WT aged hearts compared with WT adult, with the most marked changes in protein acetylation observed in the 37- to 50-kDa range. The densitometry profiles revealed that acetylated patterns in the SIRT3+/− adult group matched those found in the WT aged group (Fig. 3B), indicating a link between downregulation of SIRT3 activity and protein acetylation in WT aged hearts. Figure 3C shows equal protein loading across the groups, indicating that increased signals observed in SIRT3+/− adult and WT aged heart mitochondria (Fig. 3B) were exclusively due to protein post-translational modifications in the form of acetylation.
Fig. 3.
Mitochondrial protein expression and acetylation in WT adult vs. SIRT3+/− adult vs. WT aged hearts. Mitochondria were isolated and lysed for Western blotting, as detailed in methods. The same blot was probed with antibodies indicated in the pictures. A: mitochondrial SIRT3 and voltage-dependent anion channel (VDAC) expressions before ischemia. SIRT3/VDAC ratio is shown below the blot. B: global lysine acetylation (K-Ac) of mitochondrial samples before ischemia. Right: corresponding densitometry profiles of the acetylation lysine (Ac-Lys) signal intensity in the 20- to 150-kDa range. Bottom: Ac-Lys densitometry in the 37- to 50-kDa range, normalized to protein across the same molecular mass range (delineated with the dotted rectangle) from Ponceau S-stained membranes (see C). C: corresponding Ponceau S-stained membrane (loading control for A and B). D: mitochondrial SIRT3 and VDAC expressions after IR injury. E: global K-Ac of mitochondrial samples after IR injury. F: corresponding Ponceau S-stained membrane (loading control for D and E). Data are shown as means ± SE, n = 3 for pre-ischemic immunoblots, and n = 4 for IR injuries; *P < 0.05 vs. WT adult (ANOVA).
Next, we tested the effect of IR injury on mitochondrial protein expression and lysine acetylation. After IR injury, SIRT3 protein level was similar in adult and aged WT hearts (Fig. 3D). Mitochondrial acetylation, normalized to corresponding loading controls, was significantly higher in adult SIRT3+/− and aged WT hearts relative to adult WT (Fig. 3, E and F). These data indicate that IR injury did not change the pre-ischemic profiles of SIRT3 protein expression and mitochondrial acetylation.
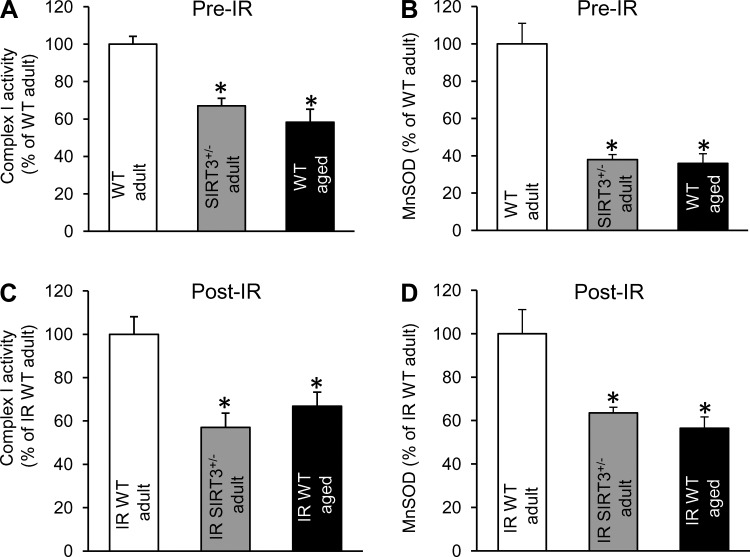
To provide additional support for the possible downregulation of SIRT3 activity in aged WT hearts, we demonstrated (Fig. 4, A and B) that pre-ischemic activities of SIRT3 targets, Cx I and MnSOD, were significantly depressed in both SIRT3+/− adult and WT aged cardiac mitochondria compared with the WT adult. Figure 4, C and D, demonstrates that mitochondria isolated from both SIRT3+/− adult and WT aged hearts after IR injury also showed reduction in the activities of Cx I and MnSOD relative to adult WT.
Fig. 4.
Cx I and MnSOD enzymatic activities in mitochondria from WT adult vs. SIRT3+/− adult vs. WT aged hearts. Mitochondria were isolated from nonischemic heart (Pre-IR) and at the end of the reperfusion protocol (Post-IR). For enzymatic assays, frozen mitochondria were used; data expressed as percent of adult WT. A: Cx I activity Pre-IR. B: MnSOD activity Pr-IR. C: Cx I activity Post-IR. D: MnSOD activity Post-IR. Data are shown as means ± SE, n = 3 for Pre-IR, and n = 4 for Post-IR; *P < 0.05 vs. WT adult (ANOVA).
DISCUSSION
The main findings of this study were as follows: 1) SIRT3-deficient (SIRT3kd), cardiac-derived cells were more susceptible to simulated IR injury and exhibited lower Cx I activity and functional reserve capacity than control cells; 2) under IR injury conditions, SIRT3+/− adult and WT aged hearts showed a similar phenotype of injury, resulting in significant depression of RPP and a larger MI compared with WT adult hearts; 3) mitochondria isolated from WT aged hearts possessed a higher level of protein acetylation (vs. WT adult), and those acetylated bands matched with those detected in mitochondria isolated from SIRT3+/− hearts; and 4) activities of SIRT3 targets Cx I and MnSOD were diminished significantly in mitochondria isolated from both adult SIRT3+/− and aged WT hearts.
SIRT3 is important in stress responses, but its role may vary depending on the stressor. For example, SIRT3 plays a vital role in the accommodation of cellular metabolism to nonfatal stress conditions, such as dietary restriction (20). In contrast, SIRT3−/− mice were able to tolerate dobutamine-induced stress related to robust inotropic stimulation in a manner similar to their WT littermates (54). Since SIRT3 activity has been linked to regulation of mitochondrial function (21, 33), such discrepancies in stress tolerance might be related to the degree of mitochondrial involvement in each particular type of stress. Importantly, SIRT3 is required to maintain an endogenous cellular resistance to more severe cardiac stress, resulting in hypertrophy (18, 51). In this study, SIRT3 was shown to play an important role in a clinically relevant model of severe cardiac stress associated with IR injury (Figs. 1, A and B, and 2).
The downstream mechanisms by which SIRT3 appears to afford protection against IR injury remain unclear and might be related to one or more of the SIRT3-regulated, stress-response pathways. One SIRT3 target, which may impact IR injury, is the permeability transition pore, a nonselective mitochondrial channel that opens early during cardiac reperfusion and initiates mitochondrial swelling via membrane disruption (19). SIRT3 deacetylates cyclophilin D, a component of the permeability transition pore and prevents its opening (18, 49). SIRT3 may also enhance antioxidant defense via deacetylation and activation of MnSOD (46). Furthermore, deacetylation and activation of mitochondrial ICDH2 lead to an increase of NADPH pools that enhance antioxidant capacity (59). In addition, SIRT3 deacetylates Ku70, which promotes its interaction with BAX protein, preventing translocation to mitochondria (52). Previously, an important role of the electron transport chain, in particular for Cx I and II, in IR injury was demonstrated (9, 37, 57). SIRT3 interacts with and deacetylates several subunits of Cx I and II to regulate their activity (3, 10). Even these limited observations suggest that SIRT3, via regulation of mitochondrial enzymes, can play an important role in recovery from IR injury.
Since the importance of these pathways might be tissue specific (13), we examined two of them and found both to be relevant for SIRT3+/− and aged cardiomyocytes. First, MnSOD activity was depressed significantly in mitochondria isolated from both adult SIRT3+/− and aged WT compared with adult WT (Fig. 4, B and D), and this is likely to increase the superoxide level. Second, Cx I activity was decreased at both the cellular (SIRT3kd vs. Ctrl; Fig. 1D) and organ (adult SIRT3+/− and aged WT hearts vs. adult WT; Fig. 4, A and C) levels. In this regard, a number of studies demonstrated that the SIRT3 downregulation and high mitochondrial protein acetylation coincided with cardiac mitochondrial disturbances associated with electron transport chain defects (26, 55). Specifically, increased mitochondrial protein acetylation has been shown in Cx I-deficient mice with cardiac-specific deletion of the NADH dehydrogenase (ubiquinone) Fe-S protein 4 (NDUSF4) (26) and in a mouse model of Freidreich's ataxia, which has multiple defects in the electron transport chain (55). Furthermore, mice harboring defects in the electron transport chain and SIRT3−/− mice both developed heart failure faster than control mice (18, 26, 55) and recapitulated the cardiac phenotype of WT aged hearts (12). With the further establishment of a connection between increased susceptibility of aged hearts to cardiac stress and the role of downregulated SIRT3 in this process, we demonstrated that IR injury resulted in less functional recovery upon reperfusion and larger infarctions in both SIRT3+/− adult and WT aged hearts compared with WT adult hearts (Fig. 2).
Although SIRT3 protein levels are the same in WT adult vs. aged hearts (Fig. 3A), it is possible that SIRT3 enzymatic activity can be attenuated by the age-related increase in 4-hydroxynonenal adduction to the cysteine residue (15, 35) or other nonidentified modifications. Such an impairment of SIRT3 activity is suggested by the high level of mitochondrial protein acetylation detected in WT aged hearts (Fig. 3B). Also, since IR injury did not alter the patterns or levels of increased protein acetylation in adult SIRT3+/− and aged WT mitochondria relative to adult WT (Fig. 3E), acute changes in protein acetylation may not be a primary modulator of IR injury.
Hyperacetylation of mitochondrial proteins has been widely used as a marker of SIRT3 activity in noncardiac tissues (13, 14, 21, 24, 31, 56). Hence, we demonstrated increased mitochondrial acetylation in SIRT3+/− adult hearts and used that as a surrogate marker for SIRT3 activity (Fig. 3B). Importantly, acetylated profiles were similar in both SIRT3+/− adult and WT aged cardiac mitochondria (Fig. 3B). Interestingly, hyperacetylation of mitochondrial proteins has been shown previously in cardiac mitochondria isolated from spontaneously hypertensive heart failure-prone rats with a decreased SIRT3 protein level (16). In addition, mitochondrial acetylation, as a consequence of SIRT3 depression, was increased during postinfarction heart failure in diabetic rats (45). Overall, hyperacetylation of cardiac mitochondria may be an early warning sign of SIRT3 downregulation and can be associated with an inability of the heart to respond adequately to cardiac stress.
Since SIRT3 orchestrates multiple signaling pathways relevant to IR injury, it is hard to distinguish which are critical for cardioprotection. Although we demonstrated potential roles for Cx I and MnSOD, further studies are required to clarify protective downstream targets. Thus the manipulation of individual SIRT3 targets might not be sufficient to afford cardioprotection against IR injury. In this regard, our results suggest that the development of therapeutic approaches that target SIRT3 activity may be a promising pathway to protect the aged heart from IR injury. It has been reported that SIRT3 is upregulated in response to caloric restriction (43) and mediates resistance to oxidative stress (46). Notably, caloric restriction increases a healthy lifespan (2, 34, 53), may protect the aged heart against IR injury (48), and restores resistance (endogenous protection) of the aged heart to IR injury (1, 32). Thus a caloric restriction mimetic or specific SIRT3 activators may serve as effective tools to protect the aged heart against IR injury.
In conclusion, SIRT3 deficiency exacerbates cardiac IR injury at the cellular and organ levels and may contribute to age-related loss of resistance to IR injury.
GRANTS
Funding for this work was provided by a grant from the American Heart Foundation Founder's Affiliate (12GRNT12060233) to G. A. Porter and by a grant from the National Heart, Lung, and Blood Institute (RO1 HL-071158) to P. S. Brookes.
DISCLOSURES
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: G.A.P., P.S.B., and S.M.N. conception and design of research; W.R.U. and S.M.N. performed experiments; G.A.P., W.R.U., and S.M.N. analyzed data; G.A.P., P.S.B., and S.M.N. interpreted results of experiments; W.R.U. and S.M.N. prepared figures; S.M.N. drafted manuscript; G.A.P., W.R.U., P.S.B., and S.M.N. edited and revised manuscript; G.A.P., W.R.U., P.S.B., and S.M.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. David Sinclair for the SIRT3+/− mice. We thank Dr. Robert Dirksen, Dr. Keith Nehrke, Dr. Mary Wines-Samuelson, Jonathan Malecki, and Alina Ainbinder (University of Rochester) for C57BL/6 WT aged mice and Drs. Andrew Wojtovich and Tatsiana Stefanos (University of Rochester) for critical evaluation of the manuscript.
REFERENCES
- 1.Abete P, Testa G, Ferrara N, de Santis SD, Capaccio P, Viati L, Calabrese C, Cacciatore F, Longobardi G, Condorelli M, Napoli C, Rengo F. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am J Physiol Heart Circ Physiol 282: H1978–H1987, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Ahmet I, Tae HJ, de Cabo CR, Lakatta EG, Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol 51: 263–271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105: 14447–14452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo CR. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov 11: 443–461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bejma J, Ramires P, Ji LL. Free radical generation and oxidative stress with ageing and exercise: differential effects in the myocardium and liver. Acta Physiol Scand 169: 343–351, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res 83: 247–261, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in Wistar rats. PLoS One 6: e19194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394: 627–634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19: 753–759, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 49: 304–311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 110: 1109–1124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119: 2789–2797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med 39: 335–345, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Marcos PJ, Jeninga EH, Canto C, Harach T, de Boer VC, Andreux P, Moullan N, Pirinen E, Yamamoto H, Houten SM, Schoonjans K, Auwerx J. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci Rep 2: 425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz KS, Galligan JJ, Hirschey MD, Verdin E, Petersen DR. Mitochondrial acetylome analysis in a mouse model of alcohol-induced liver injury utilizing SIRT3 knockout mice. J Proteome Res 11: 1633–1643, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fritz KS, Galligan JJ, Smathers RL, Roede JR, Shearn CT, Reigan P, Petersen DR. 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification. Chem Res Toxicol 24: 651–662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grillon JM, Johnson KR, Kotlo K, Danziger RS. Non-histone lysine acetylated proteins in heart failure. Biochim Biophys Acta 1822: 607–614, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo S, Olm-Shipman A, Walters A, Urciuoli WR, Devito S, Nadtochiy SM, Wojtovich AP, Brookes PS. A cell-based phenotypic assay to identify cardioprotective agents. Circ Res 110: 948–957, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2: 914–923, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans 38: 841–860, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49: 186–199, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122: 2170–2182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito H. No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nat Clin Pract Cardiovasc Med 3: 499–506, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci USA 108: 14608–14613, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res 66: 233–244, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18: 239–250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes 57: 2933–2942, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys 420: 287–297, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Libert S, Guarente L. Metabolic and neuropsychiatric effects of calorie restriction and sirtuins. Annu Rev Physiol 75: 669–684, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27: 8807–8814, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long P, Nguyen Q, Thurow C, Broderick TL. Caloric restriction restores the cardioprotective effect of preconditioning in the rat heart. Mech Ageing Dev 123: 1411–1413, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Lu Z, Scott I, Webster BR, Sack MN. The emerging characterization of lysine residue deacetylation on the modulation of mitochondrial function and cardiovascular biology. Circ Res 105: 830–841, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minor RK, Allard JS, Younts CM, Ward TM, de Cabo CR. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci 65: 695–703, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreau R, Heath SH, Doneanu CE, Lindsay JG, Hagen TM. Age-related increase in 4-hydroxynonenal adduction to rat heart alpha-ketoglutarate dehydrogenase does not cause loss of its catalytic activity. Antioxid Redox Signal 5: 517–527, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol 42: 812–825, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, Brookes PS. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol 46: 960–968, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res 89: 643–649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadtochiy SM, Yao H, McBurney MW, Gu W, Guarente L, Rahman I, Brookes PS. SIRT1-mediated acute cardioprotection. Am J Physiol Heart Circ Physiol 301: H1506–H1512, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nadtochiy SM, Zhu Q, Urciuoli W, Rafikov R, Black SM, Brookes PS. Nitroalkenes confer acute cardioprotection via adenine nucleotide translocase 1. J Biol Chem 287: 3573–3580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285: 8340–8351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, III, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 1: 771–783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park S, Mori R, Shimokawa I. Do sirtuins promote mammalian longevity? A critical review on its relevance to the longevity effect induced by calorie restriction. Mol Cells 35: 474–480, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parodi-Rullan R, Barreto-Torres G, Ruiz L, Casasnovas J, Javadov S. Direct renin inhibition exerts an anti-hypertrophic effect associated with improved mitochondrial function in post-infarction heart failure in diabetic rats. Cell Physiol Biochem 29: 841–850, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Sack MN. Emerging characterization of the role of SIRT3-mediated mitochondrial protein deacetylation in the heart. Am J Physiol Heart Circ Physiol 301: H2191–H2197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol 295: H2348–H2355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci 123: 894–902, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab 23: 420–428, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119: 2758–2771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 28: 6384–6401, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo CR. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102: 519–528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vassilopoulos A, Pennington DJ, Andresson T, Rees D, Fearnley I, Ham A, Yan Y, Flynn CR, Jones K, Kim HS, Deng C, Walker J, Gius D. SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient and exercise-induced stress. Antioxid Redox Signal. 2014. March 6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner GR, Pride PM, Babbey CM, Payne RM. Friedreich's ataxia reveals a mechanism for coordinate regulation of oxidative metabolism via feedback inhibition of the SIRT3 deacetylase. Hum Mol Genet 21: 2688–2697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winnik S, Gaul DS, Preitner F, Lohmann C, Weber J, Miranda MX, Liu Y, van Tits LJ, Mateos JM, Brokopp CE, Auwerx J, Thorens B, Luscher TF, Matter CM. Deletion of Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: implications for cardiovascular risk factor development. Basic Res Cardiol 109: 399, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wojtovich AP, Brookes PS. The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels. Basic Res Cardiol 104: 121–129, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wojtovich AP, Nadtochiy SM, Brookes PS, Nehrke K. Ischemic preconditioning: the role of mitochondria and aging. Exp Gerontol 47: 1–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem 287: 14078–14086, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]