Abstract
Adiponectin (APN), the most abundant adipocyte-secreted adipokine, regulates energy homeostasis and exerts well-characterized insulin-sensitizing properties. The peripheral or central effects of APN regulating bone metabolism are beginning to be explored but are still not clearly understood. In the present study, we found that APN-knockout (APN-KO) mice fed a normal diet exhibited decreased trabecular structure and mineralization and increased bone marrow adiposity compared with wild-type (WT) mice. APN intracerebroventricular infusions decreased uncoupling protein 1 (UCP1) expression in brown adipose tissue, epinephrine and norepinephrine serum levels, and osteoclast numbers, whereas osteoblast osteogenic marker expression and trabecular bone mass increased in APN-KO and WT mice. In addition, centrally administered APN increased hypothalamic tryptophan hydroxylase 2 (TPH2), cocaine- and amphetamine-regulated transcript (CART), and 5-hydroxytryptamine (serotonin) receptor 2C (Htr2C) expressions but decreased hypothalamic cannabinoid receptor-1 expression. Treatment of immortalized mouse neurons with APN demonstrated that APN-mediated effects on TPH2, CART, and Htr2C expression levels were abolished by downregulating adaptor protein containing pleckstrin homology domain, phosphotyrosine domain, and leucine zipper motif (APPL)-1 expression. Pharmacological increase in sympathetic activity stimulated adipogenic differentiation of bone marrow stromal cells (BMSC) and reversed APN-induced expression of the lysine-specific demethylases involved in regulating their commitment to the osteoblastic lineage. In conclusion, we found that APN regulates bone metabolism via central and peripheral mechanisms to decrease sympathetic tone, inhibit osteoclastic differentiation, and promote osteoblastic commitment of BMSC.
Keywords: adiponectin; hypothalamus; adaptor protein containing pleckstrin homology domain, phosphotyrosine domain, and leucine zipper motif 1; bone marrow stromal cells; tryptophan hydroxylase 2
adiponectin (APN), the most abundant adipocyte-secreted adipokine (50), exerts well-characterized insulin-sensitizing, anti-inflammatory, antiatherosclerotic, and antidiabetic properties (25, 42).
Different forms of APN have been detected in plasma, including trimers, hexamers, and high-molecular-weight multimers of full-length APN (fAPN) (26, 39). The trimeric form of globular APN (gAPN), which results from proteolytic cleavage of fAPN, has also been found in plasma (43). APN concentration in cerebrospinal fluid (CSF) is about 0.01–0.04% to that of serum in mice (14, 27), although higher concentrations of APN in CSF and serum were reported for female than for male mice (27) and for fasting vs. fed mice (14).
The signaling mechanisms responsible for the peripheral and central actions of APN are not completely understood. Two different APN receptors (AdipoR) have been identified, AdipoR1 and AdipoR2 (49). AdipoR1 shows high affinity for gAPN, and AdipoR2 has an intermediate affinity for both fAPN and gAPN (49). The expression profile of AdipoR1 is quite ubiquitous and is most abundant in skeletal muscle (49), whereas AdipoR2 is most abundant in liver (4). AdipoR1 and AdipoR2 are also expressed widely in the brain, including in regions of the mouse hypothalamus, brainstem, cortical neurons, and endothelial cells (4, 9, 14, 37). The adaptor protein containing pleckstrin homology domain, phosphotyrosine domain, and leucine zipper motif (APPL1) has been shown to bind to AdipoR1 and AdipoR2 and act as a link between the receptors and their downstream signaling molecules (20).
APN-knockout (APN-KO) mice were shown to be insulin resistant and prone to develop atherosclerosis, but their body weight was normal (13, 19). In addition, their bone phenotype was reported to be normal, except for a slight increase in bone mass in aged mice (46). When the effects of mechanical loading or long-term adaptation and compensation on bone metabolism were eliminated by performing bone explantation in APN-KO mice, APN inhibited osteoclastogenesis and bone resorption (40). Similarly, mice treated with an APN adenovirus for 2 wk showed an increase in trabecular bone, which was associated with a decrease in the number of osteoclasts compared with control mice (24). Furthermore, sustained release of APN was shown to improve peri-implant osteogenesis in ovariectomized rabbits by suppressing osteoclastic activity both in vivo and in vitro (17). A number of in vitro studies have also confirmed that APN can signal directly in osteoclast precursor cells to inhibit osteoclastogenesis and bone resorption (24, 40, 48) and in osteoblasts to increase their proliferation and differentiation (16, 24) and their receptor activator of nuclear factor-κB ligand (RANKL) production (18). In addition to the direct effects of APN on bone cells affecting osteoclastogenesis and osteoblast differentiation, APN signals in neurons of the locus coeruleus (10) and decreases the sympathetic tone in osteoblasts, which is supposed to increase bone mass accrual (6, 7, 34).
The involvement of APN in regulating energy metabolism has also been investigated, but conflicting results have been reported. Thus, APN has been shown to reduce (31), increase (14), or have no effect on food intake (27). Similarly, APN was reported to either increase (27) or reduce energy expenditure (EE) (14).
In this study, we investigated the ability of APN to regulate bone metabolism by central mechanisms by applying intracerebroventricular (icv) infusions of recombinant APN into APN-KO and wild-type (WT) mice. We then questioned whether central APN could decrease sympathetic tone and increase trabecular bone mass by inducing expression of TPH2, the rate-limiting enzyme in the synthesis of brain-derived serotonin (44), and further evaluated whether APN could also inhibit osteoclast differentiation and bone marrow stromal cells (BMSC) adipogenic commitment. In our study, we identified a new central APN mechanism via APPL1/tryptophan hydroxylase 2 (TPH2) that decreases sympathetic tone and increases trabecular bone mass. We also found that, in addition to inhibiting osteoclastic differentiation, APN inhibits the adipogenic commitment of BMSC via epigenetic mechanisms to favor their osteogenic differentiation.
MATERIALS AND METHODS
Recombinant gAPN and fAPN.
pEt15b bacterial expression vector encoding the COOH-terminal part of human APN (amino acids 106–244) was used to express gAPN as a His-tagged protein in BL21(DE3) bacterial cells. His-tagged gAPN was affinity purified using Ni-Agarose beads, washed extensively, and eluted from the Ni-Agarose resin, as described previously (20, 40). Purified recombinant mouse fAPN was purchased from BioVendor (Candler, NC).
APPL1 shRNA lentivirus preparation.
Lentiviral-shRNA constructs (Sigma-Aldrich) for specific knockdown of APPL1 expression and the scramble control nonspecific plasmid (Sigma-Aldrich) were donated by Dr. Yuchang Fu from the University of Alabama at Birmingham, as described previously (38). To generate the lentivirus, 7.5 μg of each lentiviral-shRNA plasmid plus 5 μg of vesicular Stomatitis Virus-G protein and 7.5 μg of pGag/Pol packaging plasmids were cotransfected in human embryonic kidney-293T cells in 10-cm plates with the calcium precipitation method.
Animal experimentation and surgical procedures.
Male APN-KO (Jax no. 008195) and WT (C57BL/6J, Jax no. 000664) mice weighing 20–25 g were purchased from the Jackson Laboratory (Bar Harbor, ME). Under general anesthesia, 4- to 6-wk-old mice were fixed on a stereotaxic instrument (Stoelting, Wood Dale, IL). The calvaria was exposed, a 0.7-mm hole was drilled in the exposed calvaria, and a 28-gauge cannula (Brain infusion kit II; Durect, Cupertino, CA) was implanted into the third ventricle according to the following coordinates: midline, −1 mm AP, 3 mm ventral (0 point bregma). The cannula was secured to the skull with cyanoacrylate and attached with Tygon tubing to an osmotic pump (Durect) placed in the dorsal subcutaneous tissue of the mice. Recombinant gAPN (20 ng/h), fAPN (BioVendor), or artificial CSF (119 mM NaCl, 26.2 mM NaHCO3, 2.5 mM KCl, 1 mM NaH2PO4, 1.3 mM MgCl2, and 10 mM glucose) was delivered at a rate of 0.22 μl/h for 28 days. The mice were housed individually after the surgery.
In vivo experiments with APPL1-shRNA lentiviral or scramble control were performed as follows. After 1 wk of acclimation to the facility, 5-wk-old male mice were implanted with a 22-gauge cannula in the third ventricle. Ten days postsurgery cannulation, animals were randomized separated into two groups. Approximately 1 × 1011 particles of APPL1-shRNA lentiviral or scramble control were injected through icv cannulas into the third ventricle of the mice. The mice were euthanized 3 mo after the lentivirus central administration, and the samples were collected. The surgery cannulation was confirmed by histology analysis. All mice were maintained under standardized conditions with 12:12-h light-dark cycle. All protocols using mice were carried out under the Association for Assessment and Accreditation of Laboratory Animal Care guidelines and with approval from the Tufts University Institutional Animal Care and Use Committee.
Metabolic analysis.
Preweighed mice (8 wk old, 23.5–24.7 g) were housed individually for 3 days prior to being transferred into calorimeter cages (Oxymax Monitoring System, Standard Syringe Pump, Harvard Apparatus) and then acclimatized to the cages for 24 h before the recordings commenced. Afterward, mice were randomized into groups of matching mean body weights. Mice were subsequently monitored in the system for 24 h. To reduce the effect of exposure to the new environment, the metabolic chamber was soiled with the home cage bedding of each subject. Oxygen uptake (V̇o2) and carbon dioxide production (V̇co2) were measured, starting at 1700, in individual mice at 15-min intervals for a total of 24 h under a consistent environmental temperature (24 ± 1°C) and given free access to chow and water. This interval of time provided a sufficient number of samples for the light and the dark phases to ensure a consistent evaluation of the EE that was not altered by the increase in activity levels in the light phase of the cycle determined by the transfer of the animals into the calorimeter. V̇o2 and V̇co2 were expressed as the volume of O2 and CO2 consumed per kilogram 0.75 body weight per hour with an air flow of 0.75 l/min. EE was calculated using a rearrangement of the Weir equation, following the manufacturer's recommendations (Oxymax Monitoring System, Standard Syringe Pump, Harvard Apparatus).
Tissue preparation and biochemical analysis.
Blood samples were collected by cardiac puncture and serum samples used for ELISA and high-performance liquid chromatography (HPLC) analyses. The hypothalamus was dissected for RNA and protein extraction. The femurs were removed and fixed in 10% formalin. Brown adipose tissue (BAT) was collected to study sympathetic tone. The APN serum level was measured by ELISA (Novex; Life Technologies, Carlsbad, CA). Serum epinephrine and norepinephrine levels were analyzed by HPLC in the Dr. Raymond F. Johnson Neurochemistry Core Laboratory.
Microcomputed tomography analysis.
Trabecular bone architecture was assessed using a microcomputed tomography (μCT) system (Viva CT 40; SCANCO Medical). Femur bone specimens were stabilized with gauze in a 2-ml centrifuge tube filled with 70% ethanol and fastened in the specimen holder of the μCT scanner. One-hundred and thirty μCT slices, corresponding to a 1.365-mm region distal from the growth plate, were acquired at an isotropic spatial resolution of 10.5 μm. A global threshold technique was applied to binarize three-dimensional grayscale μCT images, where the minimum between the bone and bone marrow peaks in the voxel gray value histogram is the threshold value. The trabecular bone compartment was segmented by a semiautomatic contouring method and subjected to a model-independent morphological analysis by the standard software provided by the manufacturer of the μCT scanner. Three-dimensional morphological parameters, including bone volume/trabecular volume (BV/TV), trabecular number (Tb.N), trabecular thickness, trabecular separation (Tb.Sp), and tissue bone mineral density (T-BMD), were evaluated. The T-BMD differs from bone mineral density and can also be used to estimate tissue mineral density (3).
Bone histological analysis and immunohistochemistry.
For histological analysis, femurs were demineralized in 15% EDTA, dehydrated in graded alcohols and xylene, embedded in paraffin, and cut serially into 5-μm sagittal sections. The sections were stained with hematoxylin and eosin (H & E). For immunohistochemistry, sections were deparaffinized and incubated with different antibodies to detect expression of special AT-rich sequence-binding protein 2 (SATB2; 1:1,000; Abcam, Cambridge, MA), osterix (OSX; 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), osteocalcin (OCN) (1:1,000; Abcam), c-fos (1:1,000l Santa Cruz Biotechnology), or Tyr-hydroxylase (1:1,000; Santa Cruz Biotechnology), followed by staining with histostain-plus kit (AEC, Mouse). For tartrate-resistant acid phosphatase (TRAP) stainings, we used a TRAP staining kit, following the manufacturer's recommendations (Sigma-Aldrich, St. Louis, MO). Differentiated osteoclasts were then identified as red-stained cells with three or more nuclei and counted as described previously (40).
Embryonic mouse hypothalamus N1 cell culture and experimental conditions.
Immortalized mouse N1 hypothalamic neurons (CELLutions Biosystems) were maintained in DMEM culture medium containing 10% FBS at 37°C in 5% CO2-95% air. N1 cells were serum-starved overnight and left untreated or treated with 10−10 M gAPN or fAPN. Cells were transduced with the APPL1 lentivirus at a multiplicity of infection of 50 with 8 μg/ml polybrene (Sigma-Aldrich). Viral particles were harvested from the supernatant 24, 36, 48, and 60 h after transfection. Stable cell lines expressing lentiviral shRNA were selected via puromycin for 2 wk.
BMSC isolation and culture conditions.
Mouse BMSC were isolated from femur and tibia bone marrow of male mice (4–6 wk old). Isolated cells were cultured in DMEM containing 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified atmosphere with 5% CO2. Third-passage cells were used for experimentation. Adipogenic differentiation of BMSC was assessed in an adipogenic induction medium consisting of 0.1 μM dexamethasone, 10 μg/ml insulin, and 0.5 mM 3-isobutyl-1-methylxanthine. To mimic the effect of increasing sympathetic activity on BMSC adipogenesis, we used the nonselective β-adrenergic agonist isoproterenol (ISO) at concentrations of 0.1 μM and APN (gAPN 0.5 mg/ml and fAPN 0.1 mg/ml). On day 14 posttreatment, Oil Red O staining was performed to detect lipid droplets formation.
Quantitative real-time polymerase chain reaction.
Total RNA was extracted from samples using TRIzol (Invitrogen, Carlsbad, CA). The first strand of cDNA was generated using SuperScript III reverse transcriptase (Life Technologies) and oligo(dT)20 primer (Life Technologies). A quantitative real-time reverse transcription-PCR assay was performed using iQTM SYBR Green Supermix (Bio-Rad, Hercules, CA) on a Bio-Rad iQ5 thermal cycler. The evaluation of relative differences in the PCR product amounts was carried out by the comparative cycle threshold method, using β-actin as a control. The sequence of primers was as follows: β-actin sense 5′-GGACCTGACGGACTACCTCATG-3′, antisense 5′-TCTTTGATGTCACGCACGATTT-3′; CB1 sense 5′-CCTTGCAGATACAACCTT-3′, antisense 5′-TGCCATGTCTCCTTTGATA-3′; cocaine- and amphetamine-regulated transcript (CART) sense 5′-CTACTCTGCCGTGGATGATGC-3′, antisense 5′-GGACTTCTTGCAACGCTTCG-3′; 5-hydroxytryptamine (serotonin) receptor 2C (Htr2C) sense 5′-AGCAGTGCGTAGTCCTGTTG-3′, antisense 5′-CTTTCGTCCCTCAGTCCAAT-3′; TPH2 sense 5′-GCAAGACAGCGGTAGTGTTCT-3′, antisense 5′-AGG GCC CCC TTC ATG AGG TC-3′; uncoupling protein 1 (UCP1) sense 5′-GTG AAG GTC AGA ATG CAA GC-3′, antisense 5′-AGG GCC CCC TTCATG AGGTC-3′; peroxisome proliferator-activated receptor (PPAR)γ sense 5′-CCCAAGGGAGGAATAGCTTCT-3′, antisense 5′-CTCTGCGATGCGGTTCCAA-3′, lysine (K)-specific demethylase 4B (KDM4B) sense 5′-AGGGACTTCAACAGATATGTGGC-3′, antisense 5′-GATGTCATCATACGTCTGCCG-3′; lysine (K)-specific demethylase 6B (KDM6B) sense 5′-TGAAGAACGTCAAGTCCATTGTG-3′, antisense 5′-TCCCGCTGTACCTGACAGT-3′.
Western blot analysis.
The whole protein lysates were prepared by using RIPA lysis buffer (Santa Cruz Biotechnology). SDS-PAGE and Western blot analyses were performed using NuPAGE 4–12% Bis-Tris gradient gels and 0.45-μm Invitrogen polyvinylidene fluoride membranes (Life Technologies). The antibodies were obtained for APPL1 (1:2,000; Cell Signaling Technology), TPH2 (1:1,000; Sigma Aldrich), and β-actin (1:1,000; Santa Cruz Biotechnology). The secondary antibodies were horseradish peroxidase-linked goat anti-rabbit IgG (Santa Cruz Biotechnology). Blots were visualized using Pierce enhanced chemiluminescence reagents (Thermo Fisher Scientific).
Statistical analysis.
Results are presented as means ± SD. Statistical significance was evaluated by one-way analysis of variance (ANOVA; Figs. 1 and 2) or an independent-samples t-test (Figs. 3–9). Two-way ANOVA with Tukey's honestly significantly different post hoc test was used to determine significant genotype × treatment interaction for the various end point parameters (Figs. 1C and 2B). All statistical analyses were performed using SPSS statistic 17.0. Values of P < 0.05 were considered statistically significant.
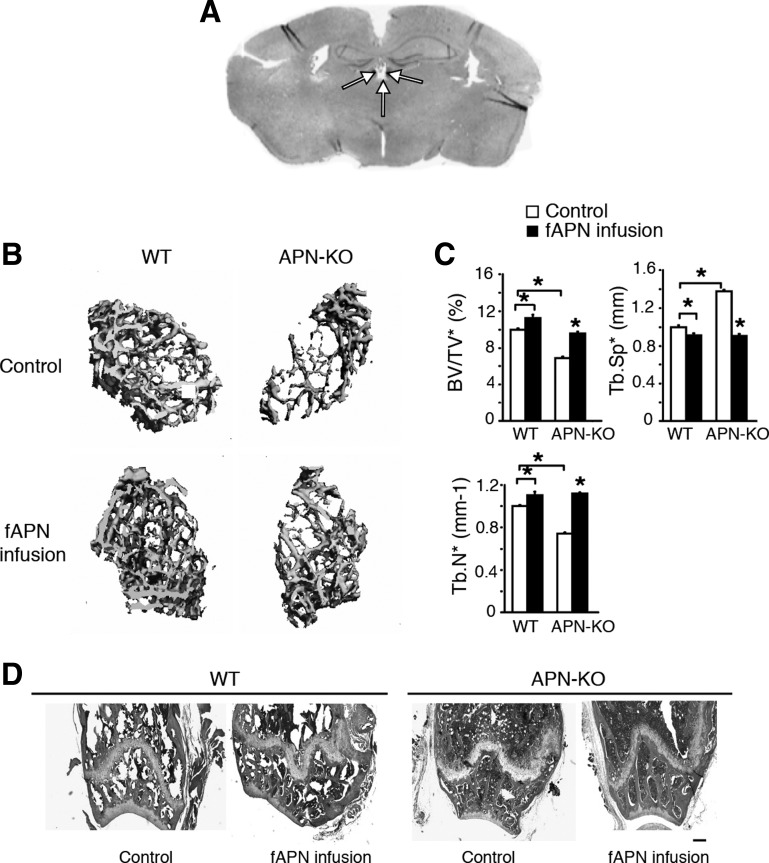
Fig. 1.
Intracerebroventricular (icv) full-length adiponectin (fAPN) infusion increases bone trabecular bone mass in APN-knockout (APN-KO) and wild-type (WT) mice after 28 days of infusion. A: representative microphotograph of the coronal section shows the placements (indicated by arrows) of a cannula in the 3rd ventricle. B: 3-dimensional (3D) microcomputed tomography (μCT) images of the distal femur metaphyseal region in APN-KO and WT mice with or without 28 days of fAPN infusion (n = 5). Scale bars, 100 μm. C: trabecular bone volume normalized by tissue volume (BV/TV), trabecular no. (Tb.N), and trabecular separation (Tb.Sp). All data are means ± SD; n = 5 mice. *P < 0.05 as determined by 1-way ANOVA. Two-way ANOVA revealed significant changes due to APN treatment or mouse genotype for all of the bone parameters investigated (P < 0.05). Two-way ANOVA and Tukey honestly significantly different (HSD) post hoc test revealed a significant genotype × treatment interaction for Tb.Sp (P < 0.05). D: hematoxylin and eosin (H & E)-stained sections of the distal femur metaphyseal region. Scale bars, 200 μm.
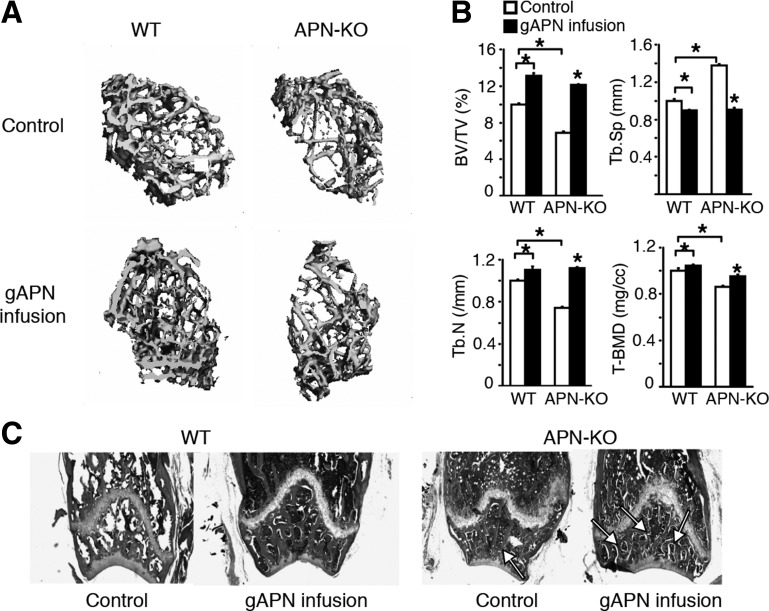
Fig. 2.
Intracerebroventricular (icv) globular adiponectin (gAPN) infusion increases trabecular bone mass and decreases bone marrow adiposity in APN-KO and WT mice. A: 3D μCT images of the distal femur metaphyseal region in APN-KO and WT mice with or without 28 days of gAPN infusion (n = 5). Scale bar, 100 μm. B: BV/TV, Tb.N, Tb.Sp, and tissue bone mineral density (T-BMD). Values are means ± SD for n = 5 mice/group. *P < 0.05 as determined by 1-way ANOVA. Two-way ANOVA revealed significant changes due to APN treatment or mouse genotype for all of the bone parameters investigated (P < 0.05). Two-way ANOVA and Tukey HSD post hoc test revealed a significant genotype × treatment interaction for Tb.Sp (P < 0.05). C: H & E-stained sections of the distal femur metaphyseal region. Scale bar, 200 μm.
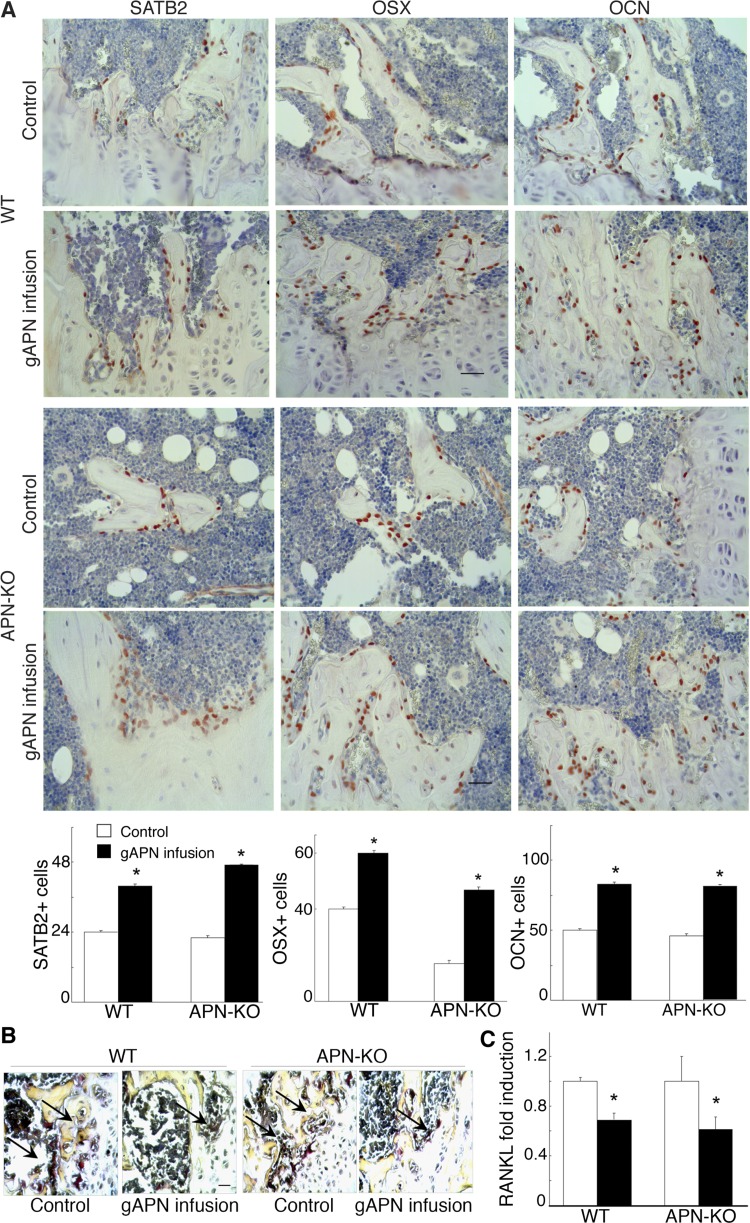
Fig. 3.
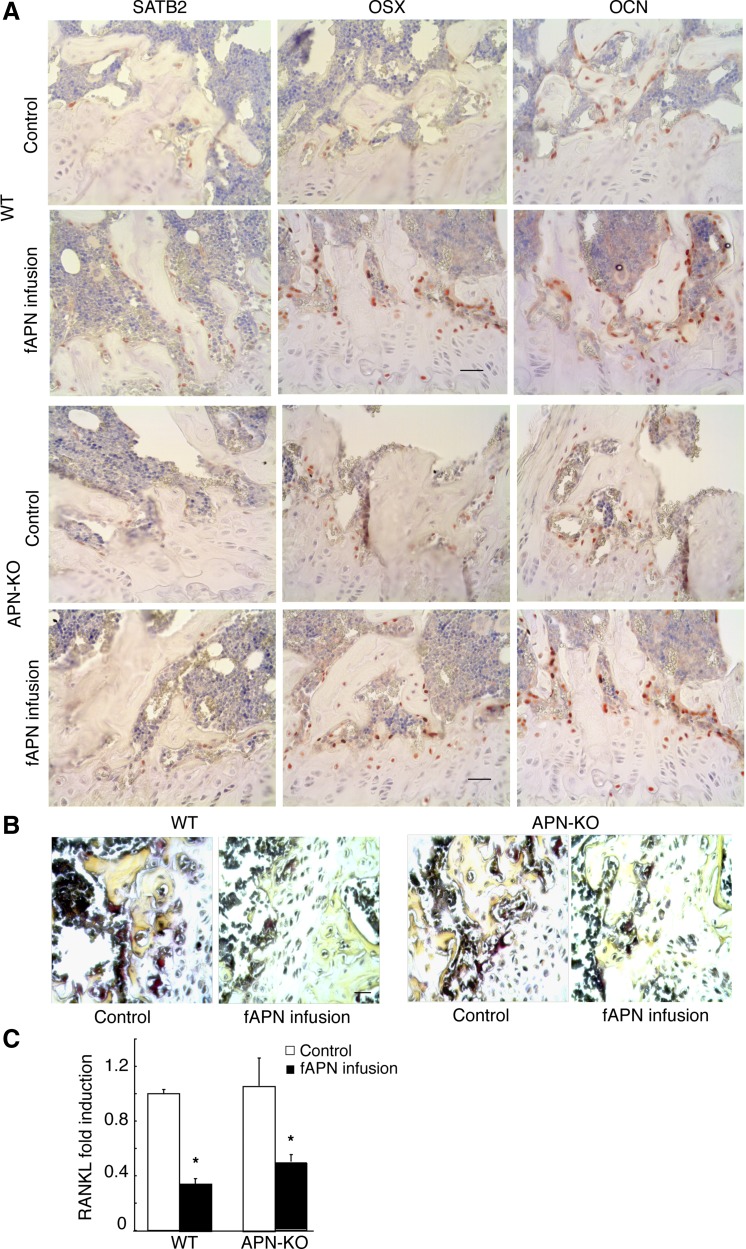
Changes in osteogenic and osteoclastic markers after gAPN or vehicle icv infusion in APN-KO and WT mice for 28 days. A: immunohistochemical analysis of special adipose tissue-rich sequence-binding protein 2 (SATB2), osterix (OSX), and osteocalcin (OCN; stained red) in distal femur trabecular bone sections. Scale bars, 20 μm. B: light micrographs of tartrate-resistant acid phosphatase (TRAP) staining (dark purple) from tibia bone sections. Scale bars, 20 μm. C: quantitative RT-PCR analysis of receptor activator of nuclear factor-κB ligand (RANKL) expression in bone marrow stromal cells (BMSC) normalized to β-actin mRNA expression. Scale bars, 200 μm. All data are means ± SD; n = 5 mice. *P < 0.05 as determined by independent-samples t-test.
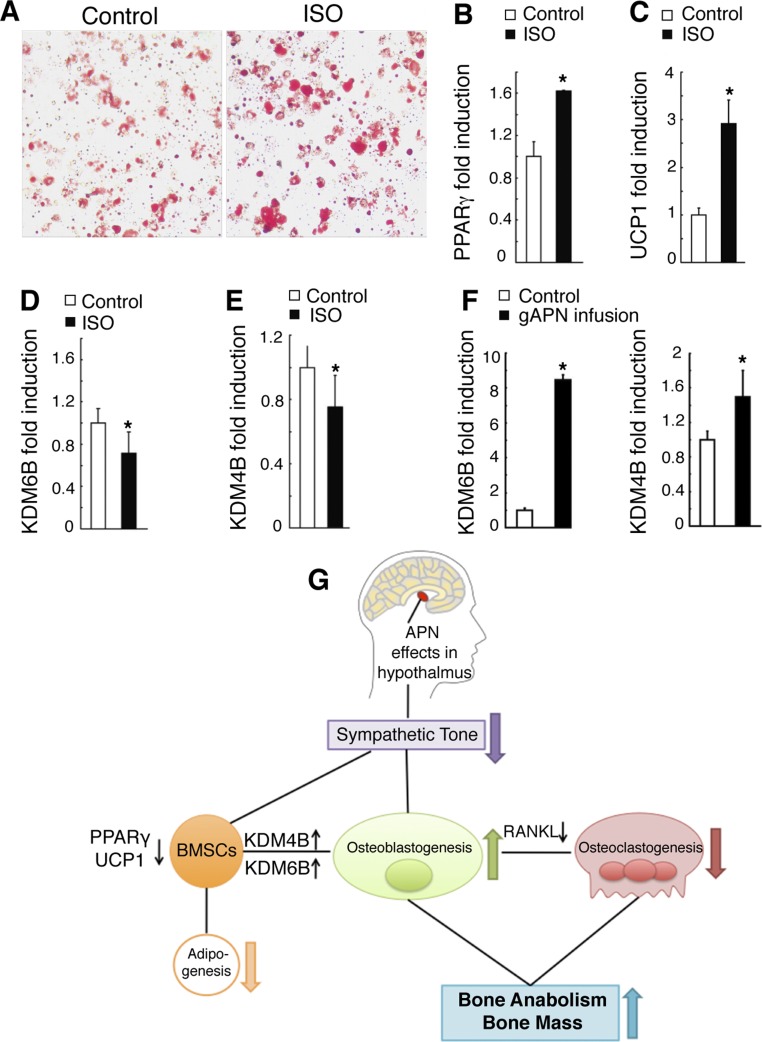
Fig. 9.
APN inhibits the adipogenic commitment of BMSC to favor osteoblastic differentiation. A: BMSC primary cultures were treated with vehicle and isoproterenol (ISO; to mimic an increase in sympathetic activity) in adipogenesis induction medium for 7 days, followed by Oil Red O staining. B–E: gene expression changes from cells in A as analyzed by quantitative RT-PCR analysis of peroxisome proliferator-activated receptor-γ (PPARγ; B), UCP1 (C), lysine (K)-specific demethylase 6B (KDM6B; D), and lysine (K)-specific demethylase 4B (KDM4B; E). F: increased KDM6B and KDM4B mRNA expression in BMSC isolated from APN-infused APN-KO mice. G: schematic diagram. All data are means ± SD; n = 5 mice. *P < 0.05 as determined by independent-samples t-test.
RESULTS
Central infusion of APN rescues the deficient bone trabecular phenotype of APN-KO mice.
To investigate APN regulation of bone metabolism by central mechanisms, we delivered gAPN or fAPN via icv infusion for 28 days into the third ventricle of APN-KO and WT mice (Fig. 1A). We then used μCT (Figs. 1, B and C, and 2, A and B) and histological analysis (Figs. 2C and 1D) to analyze changes in femur bone structure and mineral density following 28 days of gAPN or fAPN icv infusion. gAPN or fAPN icv infusions significantly increased trabecular bone volume, T-BMD, and Tb.N and Tb.Sp distance in both APN-KO and WT mice (Figs. 1C and 2B). Control APN-KO mice displayed decreased bone trabecular density and mineralization and increased bone marrow adiposity compared with control WT mice (Figs. 1, B and C, and 2, A and B). However, gAPN (Fig. 2, A and B) and fAPN infusion (Fig. 1, B and C) partially rescued the deficient bone phenotype in APN-KO mice. Histological analysis of trabecular bone further confirmed the ability of gAPN (Fig. 2C) and fAPN (Fig. 1D) icv infusions to rescue the comparatively lower trabecular bone mass and higher marrow adipogenesis of APN-KO mice (Figs. 1D and 2C).
These results demonstrated that either the globular or the full-length forms of APN were effective in increasing trabecular bone density and mineralization after 28 days of icv administration.
Central APN infusion increases osteoblast differentiation and decreases osteoclast differentiation and RANKL expression.
Next, we evaluated changes in osteogenic gene expression by immunostaining femur sections of APN-infused groups and controls. APN-infused mice showed significantly increased (P < 0.05) numbers of osteoblasts expressing SATB2, OSX, or OCN compared with control mice (Figs. 3A and 4A). However, TRAP+ staining of femur sections revealed a significantly lower number of TRAP+ mature osteoclasts in APN-infused groups than in control mice (Figs. 3B and 4B). In agreement with central APN inhibiting osteoclastogenesis, expression of RANKL, a key osteoclast-differentiating factor (15), was also significantly decreased (P < 0.05) in gAPN- (Fig. 3C) and fAPN-infused mice (Fig. 4C) compared with control mice.
Fig. 4.
Changes in osteogenic and osteoclastic markers after fAPN or vehicle icv infusion in APN-KO and WT mice for 28 days. A: immunohistochemical analysis of distal femur trabecular bone sections for SATB2, OSX, and OCN (stained red). Scale bars, 20 μm. B: light micrographs of TRAP staining (dark purple) from tibia bone sections. Scale bars, 20 μm. C: RANKL expression in BMSC from APN-KO and WT mice normalized to β-actin mRNA expression. All data are means ± SD; n = 5 mice. *P < 0.05 as determined by independent-samples t-test.
Central APN infusion decreased UCP1 expression in BAT and increased EE.
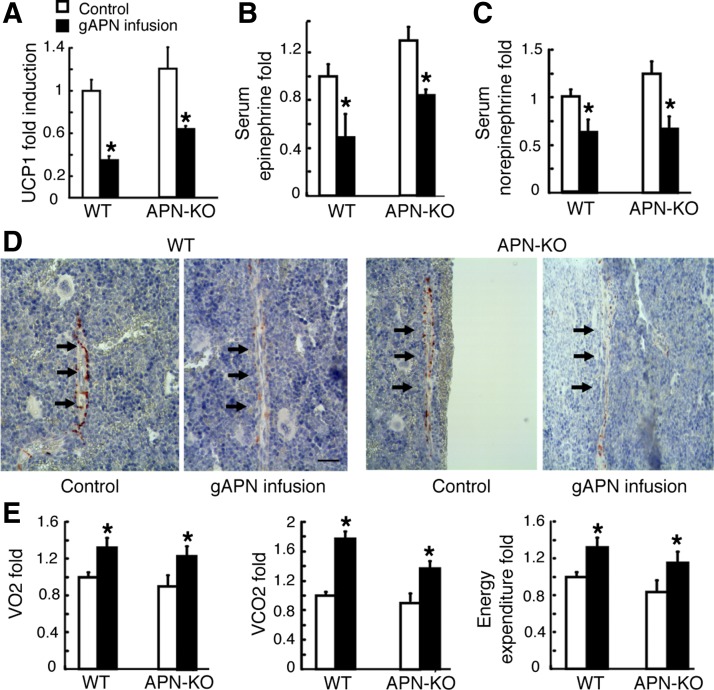
We then measured UCP1 expression to evaluate sympathetic tone in BAT (8, 29). We found that UCP1 expression was decreased in both gAPN- (Fig. 5A) and fAPN-infused mice (Fig. 6A). Moreover, serum levels of epinephrine (Fig. 5B) and norepinephrine (Fig. 5C) were significantly reduced in gAPN infusion groups compared with the control (P < 0.05). Immunohistochemical analysis of femurs showed that axons from the gAPN-infused mice (Fig. 5D) were less immunoreactive to the Tyr hydroxylase, a marker of sympathetic fibers (36), compared with control mice.
Fig. 5.
Changes in sympathetic activity markers and energy expenditure (EE) after gAPN-infused APN-KO and WT mice with respect to control mice. A: uncoupling protein 1 (UCP1) quantitative real-time polymerase chain reaction (qRT-PCR) analysis in brown adipose tissue (BAT). B and C: serum epinephrine and norepinephrine levels detected by HPLC. D: immunohistochemical analysis of Tyr hydroxylase in femora. Arrows indicate positive Tyr hydroxylase staining of the nerve fiber. Scale bars, 20 μm. E: oxygen uptake (V̇o2), carbon dioxide production (V̇co2), and EE were measured by indirect calorimetry. All data are means ± SD; n = 5 mice; *P < 0.05 as determined by independent-samples t-test. In all figures, the control group is set to a scale of 1.0 and the result expressed in relative fold.
Fig. 6.
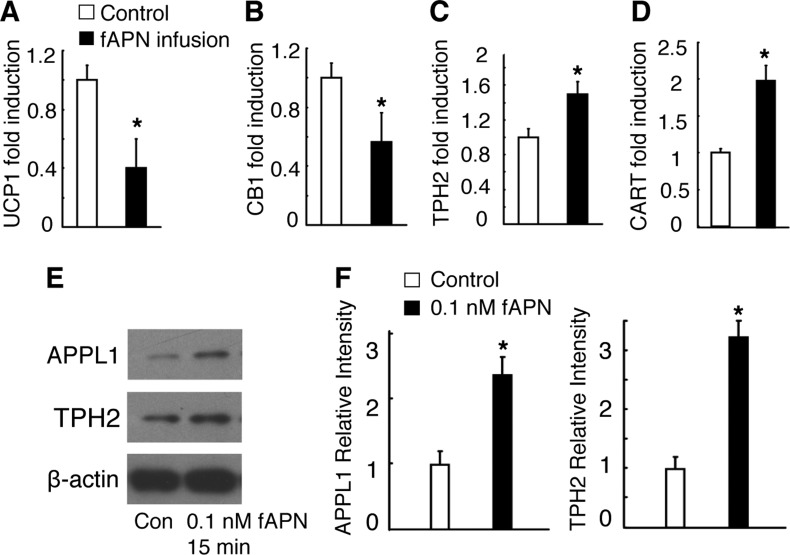
Sympathetic activity and gene expressions change after fAPN icv infusion. A: UCP1 expression in BAT normalized to β-actin mRNA expression. All data are means ± SD; n = 5 mice. B–D: gene expression in the hypothalamus after fAPN icv or vehicle infusion in WT mice. Cannabinoid receptor 1 (CB1; B), tryptophan hydroxylase 2 (TPH2; C), and cocaine- and amphetamine-regulated transcript (CART; D), all normalized to β-actin mRNA expression. All data are means ± SD; n = 5 mice. E and F: adaptor protein containing pleckstrin homology domain, phosphotyrosine domain, and leucine zipper motif (APPL1) and TPH2 expression in embryonic mouse hypothalamus N1 (mHypoE-N1) cells after 0.1 nM fAPN or vehicle treatment. The experiments were performed 3 times with similar results. *P < 0.05 as determined by independent-samples t-test.
Furthermore, V̇o2, V̇co2, and EE (P < 0.05; Fig. 5E) were found to increase significantly within the first 24 h of gAPN infusion in APN-KO and WT mice compared with the controls.
APN alters expression of hypothalamic regulators of sympathetic tone or bone metabolism.
We next explored targets of APN in the mouse hypothalamus using c-Fos immunohistochemistry (Fig. 7A). c-Fos protein was used as a marker for the activation of neurons in the hypothalamus since electrical or chemical stimulation leads to enhanced expression of c-fos mRNA and protein expression (22). gAPN icv infusion increased nuclear Fos-positive staining in the arcuate nucleus (ARC), which was shown previously to express AdipoR1 and AdipoR2 in rodents (4, 9, 14).
Fig. 7.
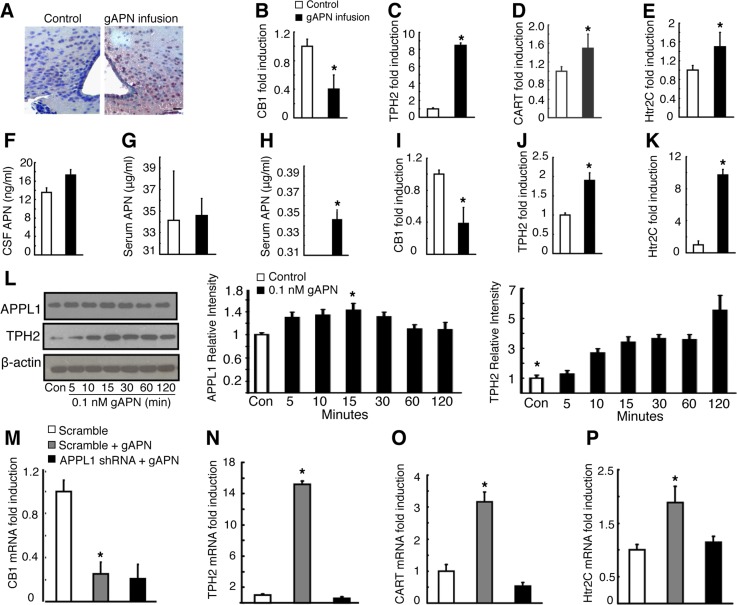
Intracerebroventricular gAPN acts in the hypothalamus. A: immunohistochemistry showing induction of Fos-immunoreactive cells (dark purple) in coronal sections of arcuate nucleus. Scale bar, 20 μm. B–E: CB1 (B), TPH2 (C), CART (D), and 5-hydroxytryptamine (serotonin) receptor 2C (Htr2C; E) hypothalamic gene expressions following infusion in WT mice. F and G: changes in cerebrospinal fluid (CSF; F) and serum APN concentration (G) following infusion in WT mice. H: changes in serum APN concentration following infusion in APN-KO mice. H–K: CB1 (I), TPH2 (J), and Htr2C (K) quantitative RT-PCR analysis following gAPN treatment in mHypoE-N1 cells. L: APPL1 and TPH2 protein expression in gAPN-induced mHypoE-N1 cells. M–O: CB1 (M), TPH2 (N), CART (O), and Htr2C (P) quantitative RT-PCR analysis following gAPN treatment with or without pretreatment with APPL1 shRNA lentivirus in mHypoE-N1 cells. All quantitative RT-PCR was normalized to β-actin mRNA expression. All data are means ± SD; n = 5 mice. *P < 0.05 as determined by independent-samples t-test.
Because cannabinoid receptor 1 (CB1) (5, 35), TPH2 (44), Htr2C (47), and CART (7) are well known for mediating central actions on bone metabolism (5), we analyzed their hypothalamic expression following icv infusions of gAPN (Fig. 7, B–E) or fAPN (Fig. 6, B–D). Hypothalamic CB1 expression (Figs. 6B and 7B) decreased concurrently with increased TPH2 (Figs. 6C and 7C) and CART expression (Figs. 6D and 7D). HTr2C expression was also decreased upon gAPN icv infusion (Fig. 7E).
To further confirm that infused APN acted centrally and not peripherally, we measured APN levels in CSF (Fig. 7F) and in serum (Fig. 7G) in gAPN-infused and control WT mice. Serum APN levels in both gAPN-treated and control WT groups were not significantly different (P > 0.05). However, negligible levels of serum APN were detected in gAPN-infused APN-KO mice but not in control mice (Fig. 7H).
APPL1 requirement by APN to induce expression changes in CB1, TPH2, CART, and Htr2C in vitro.
To further explore the molecular and signaling mechanisms of central APN, we treated embryonic mouse hypothalamus N1 (mHypoE-N1) cells with gAPN. We found CB1 (Fig. 7I) gene expression to be decreased, whereas TPH2 (Fig. 7J) and Htr2C (Fig. 7K) gene expressions increased compared with control cells. Whereas TPH2 protein expression was increased by gAPN (Fig. 7L) or fAPN (Fig. 6, E and F) in a time-dependent manner, APPL1 protein expression increased significantly only at the 15-min time point (Fig. 7L).
To evaluate whether recombinant APN mediated the effects by activating hypothalamic AdipoR1/R2 signaling via APPL1, we pretreated the mHypoE-N1 cells with specific APPL1 shRNA lentivirus. We found that downregulation of APPL1 expression did not alter APN-induced CB1 expression (Fig. 7M); however, APN changes on TPH2 (Fig. 7N), CART (Fig. 7O), and Htr2C (Fig. 7P) expression were significantly reversed by the APPL1 shRNA lentivirus pretreatment.
Hypothalamic APPL1 downregulation in vivo decreased hypothalamic TPH2 expression and increased BAT UCP expression.
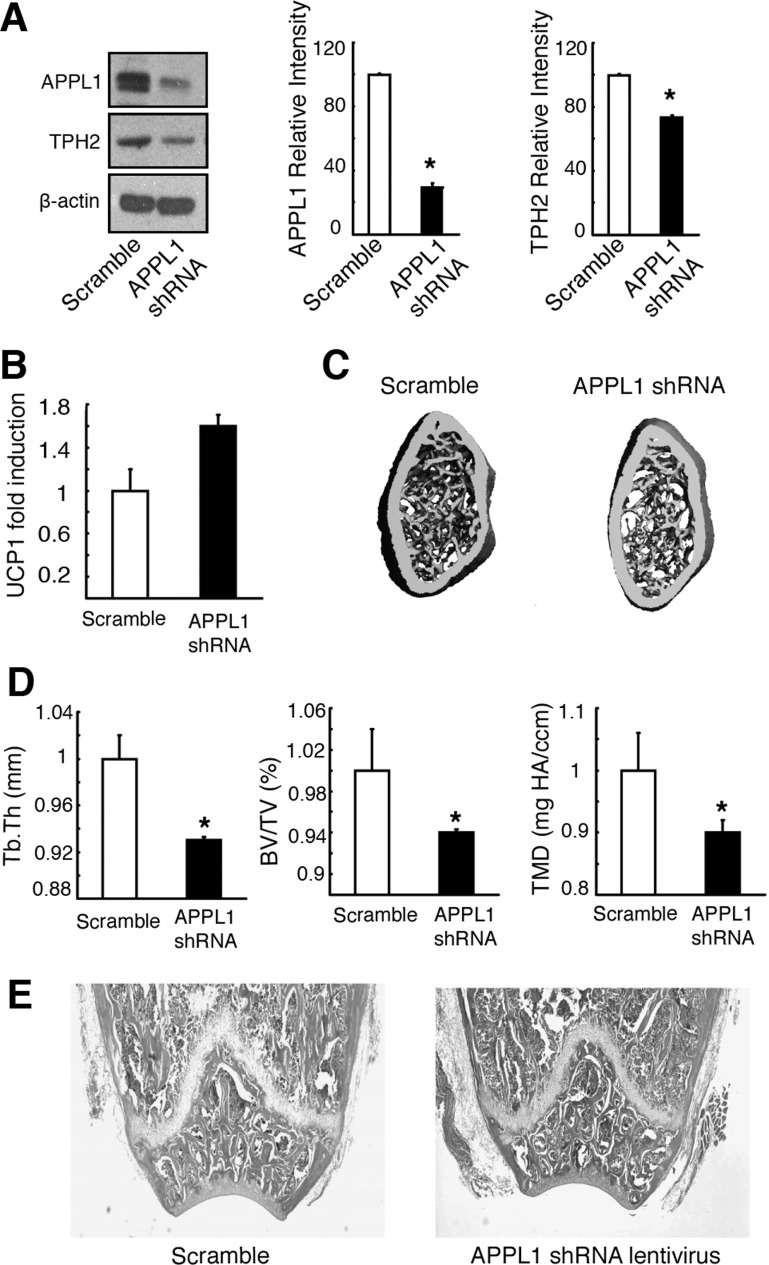
To verify whether central APN mediated the effects observed by activating hypothalamic AdipoR1/R2 signaling in vivo via APPL1, APPL1 shRNA lentivirus was administered by icv infusion in WT mice. We found that decreased protein expression of hypothalamic APPL1 in the APPL1 shRNA lentivirus-treated group (Fig. 8A) correlated with decreased TPH2 expression (Fig. 8A), increased BAT UCP1 expression (Fig. 8B), and decreased trabecular bone structure parameters (Fig. 8, C–E) compared with the scramble control group. This suggested that central APN affected sympathetic tone and bone metabolism partly by inducing the hypothalamic APPL1-TPH2 signaling pathway.
Fig. 8.
APPL1 is required by central APN to regulate bone metabolism and sympathetic tone. A: APPL1 shRNA lentivirus pretreatment reversed APN's induction effects in mHypoE-N1 cells. B: BAT UCP1 expression increased following APPL1 shRNA lentivirus treatment in vivo. C–E: bone trabecular mass decreased in the APPL1 shRNA lentivirus-treated group. C: 3D μCT images of the distal femora metaphyseal regions. Scale bar, 100 μm. D: changes in bone parameters, including trabecular thickness (Tb.Th), trabecular bone volume normalized by tissue volume (BV/TV), and tissue mineral density (TMD). All data are means ± SD; n = 5 mice. *P < 0.05 as determined by independent-samples t-test. E: H & E-stained sections of the distal femora metaphyseal regions. Scale bar, 200 μm.
APN promotes osteogenic differentiation of BMSC by inhibiting adipogenesis.
BMSC have the potential to differentiate into different cell lineages, including osteoblasts and adipocytes (11, 21, 32, 33). Since administration of APN decreased sympathetic activity in our study and in a previous report (10), and APN-KO exhibited higher marrow adiposity compared with WT mice (Figs. 1D and 2C), we explored the putative role of sympathetic activity in regulating adipogenic commitment of BMSC. To that end, BMSC were treated with the β-adrenergic agonist ISO, and then adipogenesis was evaluated by Oil Red O staining. Increased positive Oil Red staining was found in the ISO-treated cells compared with control cells (Fig. 9A). Additionally, mRNA levels of PPARγ and UCP1 were significantly increased in ISO-treated cells compared with the controls (Fig. 9, B and C).
To gain insight into the mechanisms underlying ISO induction of adipogenic differentiation of BMSC, we evaluated expression levels of the lysine-specific histone demethylases KDM4B and KDM6B. Because KDM4B and KDM6B remove methylated histone lysine residues and enable dynamic and reversible regulation of transcription (1, 12, 30, 51), they considerably increase osteogenic and reduce adipogenic differentiation (51). We found that expression levels of KDM6B (Fig. 9D) and KDM4B (Fig. 9E) were greatly suppressed after ISO treatment compared with the untreated control. In addition, BMSC isolated from APN-infused APN-KO mice exhibited increased KDM4B and KDM6B expression than BMSC from control mice (Fig. 9F).
DISCUSSION
The present study found that icv infusion of either gAPN or fAPN into APN-KO and WT mice decreased sympathetic tone compared with control groups. Thus, APN-infused mice exhibited decreased UCP1 expression in BAT, lower serum levels of epinephrine and norepinephrine, and decreased immunoreactivity to anti-Tyr hydroxylase. Recently, the ability of APN to inhibit the activity of the sympathetic nervous system was also suggested by reporting an age-dependent increase in sympathetic tone markers in APN-KO mice compared with WT mice (10). Those same authors subcutaneously infused fAPN into APN-KO mice and found that it bound to neurons of the locus coeruleus, where the sympathetic nervous system originates from (28), and decreased UCP1 expression in BAT (10). Thus, central APN in either the globular or full-length form can consistently decrease sympathetic activity in mice.
In addition to decreasing sympathetic tone, gAPN- or fAPN-infused mice exhibited increased bone trabecular mass and volume, as evidenced by increased BV/TV, T-BMD, and Tb.N and decreased Tb.Sp. These results correlated with increased mRNA expression of osteoblast marker genes such as OCN, OSX, and SATB2 and decreased osteoclast numbers in APN-infused groups compared with controls. The ability of central APN to decrease sympathetic tone and increase bone trabecular mass (Fig. 9G) was in agreement with previous reports demonstrating that the sympathetic nervous system inhibits bone formation and favors bone resorption to regulate bone mass accrual (6, 7, 34). It is important to mention that in our study negligible levels of serum APN were detected in gAPN-infused APN-KO mice that were well below physiological APN levels, reported in the range of 2 to 20 μg/ml by other investigators (27, 37). Furthermore, differences in serum APN levels between control and gAPN-infused WT mice were not statistically significant. Therefore, the negligible leakage of centrally infused gAPN into the circulation of APN-KO mice may be a sign that the integrity of their blood-brain barrier is slightly compromised. In agreement with this possibility, APN-KO mice exhibited larger disruption of the blood-brain barrier and leakage than WT mice under sepsis-induced conditions (41). Therefore, our study strongly supports that gAPN or fAPN icv infusion decreased sympathetic tone and regulated bone metabolism by central APN effects derived from the binding of APN to AdipoR1/AdipoR2 in the hypothalamus or other central compensatory mechanisms but not by peripheral mechanisms.
In agreement with central APN mediating its effects via binding to AdipoR1/R2 receptors in the ARC and/or in other brain regions that would release neurotransmitters acting in the ARC region, c-Fos immunostaining substantially increased in the ARC after gAPN icv infusion. Several lines of evidence shown in this study and the bibliography support the notion that central APN decreased sympathetic tone and regulated bone remodeling partly by promoting brain-derived serotonin synthesis. First, gAPN or fAPN icv infusion increased expression of hypothalamic TPH2, the enzyme that catalyzes the rate-limiting step in the biosynthesis of brain-derived serotonin (44), and that of its Htr2c receptors in the hypothalamus. Second, specific APPL1 shRNA lentivirus pretreatment in immortalized mouse hypothalamic neurons reversed gAPN-induced effects on the expression of TPH2 and Htr2c. Finally, specific APPL1 shRNA lentivirus treatment in vivo decreased expression of hypothalamic TPH1, decreased bone trabecular mass, and increased UCP1 expression. Brain-derived serotonin was previously shown to be released by brainstem (47), which expresses AdipoR1/R2 receptors (37), and favor bone mass accrual through binding to Htr2c receptors on the ventromedial hypothalamic nuclei (47). Taken together, the ability of central APN to increase the expression of TPH2, Htr2C, and trabecular mass while decreasing sympathetic tone agrees with previous evidence showing that serotonin decreases sympathetic tone and increases bone mass (47). This hypothesis does not exclude the possibility that gAPN icv infusion triggers the release of other neurotransmitters that may cooperate with or oppose serotonin in regulating sympathetic tone and regulating bone mass. In agreement with this scenario, gAPN icv infusion caused CB1 expression downregulation, which was not reversed by pretreatment with specific APPL1 shRNA lentivirus in mouse hypothalamic neurons. Previous reports have shown that CB1 signaling inhibits norepinephrine release by sympathetic neurons, thus balancing the tonic sympathetic restrain of bone formation (6). Since CB1 expression decreased in an APPL1-independent manner in our study, the gAPN-mediated effects on CB1 expression are likely to be a compensatory mechanism that is not triggered by direct binding of APN to its receptors in the hypothalamus.
In addition to decreasing sympathetic tone, gAPN also increased hypothalamic expression of the neurotransmitter CART (5, 7). Furthermore, CART upregulation by gAPN was reversed by pretreatment with specific APPL1 shRNA lentivirus in immortalized mouse hypothalamic neurons. Since CART was previously shown to decrease RANKL expression and inhibit osteoclastogenesis (5, 7), the effects of central APN on decreasing RANKL expression in our study (Fig. 9G) may result from an increase in hypothalamic CART expression. Taking together these results and previously published reports (10, 40), we propose that APN may be able to inhibit bone resorption by peripheral and central mechanisms. Whereas previous reports have shown that APN induces opposing central and peripheral effects on osteoblasts (10), we propose that APN may inhibit osteoclast differentiation and bone resorption by direct binding of circulating APN to AdipoR1/AdipoR2 on osteoclasts/precursor cells to inhibit RANKL-induced osteoclast differentiation (40) and by decreasing RANKL expression upon CART upregulation. In our study, we also found that gAPN icv led to an increase in V̇o2, V̇co2, and EE, which was unexpected based on the inhibitory effect on sympathetic tone markers. Since hypothalamic CART may reduce appetite and increase EE under certain circumstances (23), further investigation on the effects of central APN on CART expression may help explain the conflicting results reported in the literature about the effects of APN on food intake (14, 27, 31) and EE (10, 14, 27). An alternative explanation for the conflictive effects of APN on EE may be that different APN concentrations and the form of APN may involve differential use of AdipoR1 or AdipoR2 signaling in the hypothalamus. In fact, mice deficient in AdipoR1 and AdipoR2 exert opposite effects on EE and physical activity (2). Thus, AdipoR1 deficiency resulted in decreased EE and spontaneous locomotor activity, whereas AdipoR2 deficiency had the opposite effect. In addition, AdipoR1 shows high affinity for gAPN, whereas AdipoR2 has an intermediate affinity for both fAPN and gAPN (49). Taken together, the central effects of APN on energy metabolism cannot be reconciled at this point and will require further investigation to determine whether preferential activation of AdipoR1 in the hypothalamus by gAPN may result in increased EE, whereas the activation of AdipoR1/R2 or AdipoR2 by fAPN may result in decreased EE.
The sympathetic nervous system has been shown to induce osteoclastogenesis by enhancing RANKL expression via β2-adrenergic receptors on osteoblasts (7). In this study, we showed that ISO, a sympathetic agonist, increased BMSC adipogenesis and downregulated the expression of histone demethylases KDM4B and KDM6B. Furthermore, in APN-KO mice, marrow adiposity decreased after central APN infusion compared with the APN-KO mice infused with vehicle control. The latter was observed concurrently with increased KDM4B and KDM6B expression in BMSC of APN-infused mice. Methylation marks normally removed by KDM4B and KDM6B and adipogenesis were found to increase in BMSC from osteoporotic ovariectomized mice or aging mice (51). Furthermore, mRNA expression of KDM4B and KDM6B and trabecular bone mass were decreased significantly in 18-mo-old mice compared with 2-mo-old mice (51). Our results cannot resolve the conflictive effects of APN in bone metabolism reported by different laboratories that used older mice than in our study or ovariectomiced APN-KO mice. Thus, it was reported that 8-wk-old ovariectomiced female APN-KO mice exhibited higher bone trabecular mass 10 wk after surgery than WT mice subjected to the same procedures (45). In addition, trabecular bone volume and trabecular number were higher in 14-wk-old APN-KO male mice than in WT littermates (46). Furthermore, APN-KO mice were also described to exhibit high bone mass at 12 wk and low bone mass after 24 wk (10).
Taken together, our results and those of previous investigators point out the notion that APN controls bone metabolism by central and peripheral effects, which may oppose each other in osteoblasts (10) but not in other bone cells such as osteoclast/precursor cells or BMSC. Although these results are very exciting, further research will be needed to compare the therapeutic potential of central and peripheral APN to increase bone mass in animal models of bone loss-associated diseases.
GRANTS
This work was supported by National Institute of Dental Research Grants R01-DE-16710 and R01-DE-21464 (to J. Chen), the International Association for Dental Research and Academy of Osseointegration (Innovation in Implant Sciences Award to J. Chen), and the Tufts Center for Neuroscience Research (P30 NS047243).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.W., Q.T., Z.T., and J. Chen conception and design of research; Y.W., Q.T., J.Z., D.M., J. Cheng, and H.J. performed experiments; Y.W., Q.T., P.V., L.Q.D., H.J., M.R., E.M., and J. Chen analyzed data; Y.W., Q.T., P.V., L.Q.D., M.R., E.M., and J. Chen interpreted results of experiments; Y.W., Q.T., P.V., D.M., J. Cheng, and J. Chen prepared figures; Y.W., Q.T., P.V., and J. Chen drafted manuscript; Y.W., Q.T., P.V., J.Z., D.M., L.Q.D., J. Cheng, M.R., E.M., Z.T., and J. Chen edited and revised manuscript; Y.W., Q.T., P.V., J.Z., D.M., L.Q.D., J. Cheng, H.J., M.R., E.M., Z.T., and J. Chen approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Y. Fu and L. Tian at the University of Alabama at Birmingham for providing APPL1 lentiviral-shRNA plasmids, Jin Tang for sample collection, tissue processing, and H & E staining, Gabriel McDonald at Boston University for technical assistance with μCT scanning and analysis, Jennifer Newman at Tufts University for assistance with metabolic cages and indirect calorimetry, and X. Han, X. Yu, and J. Lin at the Forsyth Institute for imaging collection.
REFERENCES
- 1.Agger K, Christensen J, Cloos PA, Helin K. The emerging functions of histone demethylases. Curr Opin Genet Dev 18: 159–168, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bjursell M, Ahnmark A, Bohlooly-Y M, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, Berg AL, Oscarsson J, Lindén D. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 56: 583–593, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25: 1468–1486, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Coope A, Milanski M, Araujo EP, Tambascia M, Saad MJ, Geloneze B, Velloso LA. AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett 582: 1471–1476, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Driessler F, Baldock PA. Hypothalamic regulation of bone. J Mol Endocrinol 45: 175–181, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Elefteriou F. Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys 473: 231–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434: 514–520, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Festuccia WT, Blanchard PG, Richard D, Deshaies Y. Basal adrenergic tone is required for maximal stimulation of rat brown adipose tissue UCP1 expression by chronic PPAR-γ activation. Am J Physiol Regul Integr Comp Physiol 299: R159–R167, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Guillod-Maximin E, Roy AF, Vacher CM, Aubourg A, Bailleux V, Lorsignol A, Penicaud L, Parquet M, Taouis M. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol 200: 93–105, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, Clarke CJ, Hannun YA, DePinho RA, Guo EX, Mann JJ, Karsenty G. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab 17: 901–915, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai M, Rosen CJ. PPARγ: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol 6: 629–636, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet 7: 715–727, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277: 25863–25866, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6: 55–68, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Lee HW, Kim SY, Kim AY, Lee EJ, Choi JY, Kim JB. Adiponectin stimulates osteoblast differentiation through induction of COX2 in mesenchymal progenitor cells. Stem Cells 27: 2254–2262, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Luo E, Hu J, Bao C, Li Y, Tu Q, Murray D, Chen J. Sustained release of adiponectin improves osteogenesis around hydroxyapatite implants by suppressing osteoclast activity in ovariectomized rabbits. Acta Biomater 8: 734–743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res 21: 1648–1656, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8: 731–737, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8: 516–523, 2006 [DOI] [PubMed] [Google Scholar]
- 21.McCauley LK. c-Maf and you won't see fat. J Clin Invest 120: 3440–3442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci 14: 421–451, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Murphy KG. Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief Funct Genomic Proteomic 4: 95–111, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 331: 520–526, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Padmalayam I, Suto M. Role of adiponectin in the metabolic syndrome: current perspectives on its modulation as a treatment strategy. Curr Pharm Des 19: 5755–5763, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem 278: 9073–9085, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med 10: 524–529, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Rush RA, Geffen LB. Dopamine beta-hydroxylase in health and disease. Crit Rev Clin Lab Sci 12: 241–277, 1980 [DOI] [PubMed] [Google Scholar]
- 29.Scarpace PJ, Matheny M. Leptin induction of UCP1 gene expression is dependent on sympathetic innervation. Am J Physiol Endocrinol Metab 275: E259–E264, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell 25: 1–14, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, Zolotukhin S. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci USA 100: 14217–14222, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol 5: 442–447, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol 9: 1273–1285, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell 111: 305–317, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Tam J, Trembovler V, Di Marzo V, Petrosino S, Leo G, Alexandrovich A, Regev E, Casap N, Shteyer A, Ledent C, Karsak M, Zimmer A, Mechoulam R, Yirmiya R, Shohami E, Bab I. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J 22: 285–294, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Thoenen H, Angeletti PU, Levi-Montalcini R, Kettler R. Selective induction by nerve growth factor of tyrosine hydroxylase and dopamine- -hydroxylase in the rat superior cervical ganglia. Proc Natl Acad Sci USA 68: 1598–1602, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol 165: 313–327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian L, Luo N, Zhu X, Chung BH, Garvey WT, Fu Y. Adiponectin-AdipoR1/2-APPL1 signaling axis suppresses human foam cell formation: differential ability of AdipoR1 and AdipoR2 to regulate inflammatory cytokine responses. Atherosclerosis 221: 66–75, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol 440: 213–221, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Tu Q, Zhang J, Dong LQ, Saunders E, Luo E, Tang J, Chen J. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. J Biol Chem 286: 12542–12553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vachharajani V, Cunningham C, Yoza B, Carson J, Jr, Vachharajani TJ, McCall C. Adiponectin-deficiency exaggerates sepsis-induced microvascular dysfunction in the mouse brain. Obesity (Silver Spring) 20: 498–504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villarreal-Molina T, Posadas-Romero C, Romero-Hidalgo S, Antúnez-Argüelles E, Bautista-Grande A, Vargas-Alarcón G, Kimura-Hayama E, Canizales-Quinteros S, Juárez-Rojas JG, Posadas-Sánchez R, Cardoso-Saldaña G, Medina-Urrutia A, González-Salazar Mdel C, Martínez-Alvarado R, Jorge-Galarza E, Carnevale A. The ABCA1 gene R230C variant is associated with decreased risk of premature coronary artery disease: the genetics of atherosclerotic disease (GEA) study. PLoS One 7: e49285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 278: 40352–40363, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol 66: 1673–1680, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Wang F, Wang PX, Wu XL, Dang SY, Chen Y, Ni YY, Gao LH, Lu SY, Kuang Y, Huang L, Fei J, Wang ZG, Pang XF. Deficiency of adiponectin protects against ovariectomy-induced osteoporosis in mice. PLoS One 8: e68497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB, Costa JL, Orpe A, Broom N, Naot D, Reid IR, Cornish J. In vitro and in vivo effects of adiponectin on bone. Endocrinology 150: 3603–3610, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138: 976–989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi N, Kukita T, Li YJ, Martinez Argueta JG, Saito T, Hanazawa S, Yamashita Y. Adiponectin inhibits osteoclast formation stimulated by lipopolysaccharide from Actinobacillus actinomycetemcomitans. FEMS Immunol Med Microbiol 49: 28–34, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K, Zhou X, Park NH, Wang CY. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell 11: 50–61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]