Abstract
Early-life ethanol feeding (ELAF) alters the metabolic function of proopiomelanocortin (POMC)-producing neurons and the circadian expression of clock regulatory genes in the hypothalamus. We investigated whether the circadian mechanisms control the action of ELAF on metabolic signaling genes in POMC neurons. Gene expression measurements of Pomc and a selected group of metabolic signaling genes, Stat3, Sirt1, Pgc1-α, and Asb4 in laser-captured microdissected POMC neurons in the hypothalamus of POMC-enhanced green fluorescent protein mice showed circadian oscillations under light/dark and constant darkness conditions. Ethanol programmed these neurons such that the adult expression of Pomc, Stat3, Sirt, and Asb4 gene transcripts became arrhythmic. In addition, ELAF dampened the circadian peak of gene expression of Bmal1, Per1, and Per2 in POMC neurons. We crossed Per2 mutant mice with transgenic POMC-enhanced green fluorescent protein mice to determine the role of circadian mechanism in ELAF-altered metabolic signaling in POMC neurons. We found that ELAF failed to alter arrhythmic expression of most circadian genes, with the exception of the Bmal1 gene and metabolic signaling regulating genes in Per2 mutant mice. Comparison of the ELAF effects on the circadian blood glucose in wild-type and Per2 mutant mice revealed that ELAF dampened the circadian peak of glucose, whereas the Per2 mutation shifted the circadian cycle and prevented the ELAF dampening of the glucose peak. These data suggest the possibility that the Per2 gene mutation may regulate the ethanol actions on Pomc and the metabolic signaling genes in POMC neurons in the hypothalamus by blocking circadian mechanisms.
Chronic alcohol drinking is a lifestyle factor that may influence the risk of type 2 diabetes mellitus. In the United States, 1 in 13 pregnant women drink alcohol and many of them even binge drink (1). Studies with laboratory rats and mice have shown that ethanol exposure prenatally (equivalent to the first two trimesters of human pregnancy) and/or postnatally (equivalent to the third trimester of human pregnancy) increases the gluconeogenic enzyme fructose-1,6-bisphosphatase (2), reduces insulin production and secretion (3), increases insulin resistance in the liver (4) and muscle (5), and impairs pancreatic β-cell dysfunction (5, 6).
In addition, a high-fat diet worsens glucose intolerance in early-life ethanol feeding (ELAF) rats during the adult period (7) as well as increases adiposity and disrupts pancreatic morphology in ELAF-exposed adult guinea pig offspring (3). ELAF-induced insulin resistance may partly be caused by cell-signaling defects in proopiomelanocortin (POMC) neurons in the hypothalamus. The POMC neurons are known to be targets of leptin and insulin (8, 9), and abnormal signaling of these metabolic hormones in POMC neurons are associated with obesity, insulin resistance, and type 2 diabetes (10, 11). A hypothalamic POMC-derived peptide, α-MSH, has been shown to be lower in subjects with type 2 diabetes (12). These data, together with the evidence that ELAF suppresses the expression of the Pomc gene and secretion of the POMC-derived peptide β-endorphin (13, 14), suggest that developmental alcohol exposure affects metabolic sensing of POMC neurons to alter leptin and insulin responses, but the mechanisms of ELAF action on POMC neurons are unknown.
One possibility is that ELAF alters the metabolic sensing of POMC neurons by affecting the circadian mechanism. Animal studies have revealed that ELAF alters the circadian function of the master circadian pacemaker and circadian expression of the Pomc gene in the hypothalamus (15–17). We therefore tested whether ELAF alters the clock machinery to disrupt circadian expression of metabolic signaling genes in POMC neurons. We provide evidence here for the first time implicating a role for the circadian mechanism in ELAF-induced alteration of metabolic signals in POMC neurons.
Materials and Methods
Animals
Experiments were conducted using male mice, which were individually housed in 12-hour light, 12-hour dark cycles [lights on at 7:00 am, defined as Zeitgeber time (ZT) = 0]. Animal care and treatment were performed in accordance with institutional guidelines, and protocols were approved by the Rutgers Institutional Animal Care and Facilities Committee and complied with National Institutes of Health policy. Adult C57BL/6J mice and Per2 mutants (mPer2Brdml) of the same genetic background (C57BL/6J) were obtained from the Jackson Laboratory. Transgenic mice expressing the fluorescent protein enhanced green fluorescent protein (EGFP) in POMC neurons (POMC-EGFP) were obtained from Dr Malcolm Low (Oregon Health & Science University, Portland, Oregon). His laboratory generated these mice by introducing the EGFP cassette (fluorescent protein) into the 5′ untranslated region of exon 2 of the mouse Pomc genomic clone containing 13 kb of 5′ and 2 kb of 3′ flanking sequences (18). The transgene was microinjected into pronuclei of C57BL/6J mouse embryos at the one-cell stage (Jackson Laboratories). These mice are fertile and have normal growth and development. The founder line had the expected distribution of positive cells based on the known pattern of POMC-expressing cells in the arcuate nucleus, the nucleus of the solitary tract, pituitary melanotrophs, and corticotrophs. Per2 mutants (m Per2Brdml) were crossed with POMC-EGFP transgenic mice to produce Per2 mutant POMC-EGFP (POMC-EGFP-Per2mut) mice. This mouse has EGFP expression in POMC cells and mutant Per2 in the entire mouse. After the sixth generation, a pair of transgenic homozygous POMC-EGFP-Per2mut mice was generated.
To determine the POMC-EGFP allele, the following primers were us: forward, TATATCATGGCCGACAAGCA, and reverse, GAACTCCAGCAGGACCATGT. The PCR cycling reaction conditions were as follows: 94°C for 3 minutes, 35 cycles of 94°C for 30 seconds, 60°C for 1 minute, 72°C for 1 minute, 72°C for 10 minutes, and 4°C for 1 minute. The expected product size was 220 bp. To determine the Per2 mutant allele, four primers were used: wild-type primer, forward, CTTGGGTGGAGAGGCTATTC, reverse, AGGTGAGATGACAGGAGATC; and mutant primer, forward, CATTGGGAGGCACAAGTCAG, reverse, GAGCTGCGAACACATCCTCA. The PCR cycling reaction conditions were the following: 94°C for 3 minutes, 12 cycles of 94°C for 20 seconds, 64°C for 30 seconds, 72°C for 35 seconds, 25 cycles of 94°C for 20 seconds, 58°C for 30 seconds, 72°C for 35 seconds, 72°C for 2 minutes, and 10°C for 1 minute. Two types of different products were observed: the wild-type allele (350 bp) or the mutant allele (280 bp) (Supplemental Figure 1). The transgenic POMC-EGFP-Per2mut mice contained a POMC-EGFP allele and a Per2 mutant allele (280 bp). These animals were fertile and had normal growth and development.
Entrained under light/dark (LD) or constant darkness (DD) conditions
In most studies, animals were maintained under a standard light-dark cycle (12 h, 12 h) with lights on beginning at 7:00 am for at least 2 weeks. Mice were killed at 4-hour intervals across the LD cycle. Brains were collected and immediately frozen for RNA extraction and mRNA measurement or used for laser-capture microdissection (LCM) collection of POMC neurons followed by RNA extraction and mRNA measurement. To determine whether these genes were expressed in a rhythmic manner under constant conditions, POMC and metabolic signaling genes were studied in POMC-EGFP mice housed under DD for a period of 2 weeks. Mice were killed at 4-hour intervals over a 48-hour period such that all time points were repeated twice. Brains were collected and processed for LCM capturing of enriched POMC neurons and for gene expression assays by quantitative PCR.
Postnatal ethanol treatment
Both the transgenic POMC-EGFP (wild type) and POMC-EGFP-Per2mut mice were bred, and their offspring were fed by oral gavage on an ethanol-containing milk formula or a control diet between postnatal day (PD) 2 and PD 7. On the day of treatment, pups from each litter were divided into three groups and were given milk formula containing 11.34% ethanol (vol/vol), yielding a total daily ethanol dose of 2.5 g/kg (AF) or isocaloric maltose (PF), or they were left in the litter with the mother (AD) as described previously (19, 20). The feeding was conducted at 10:00 am and 12:00 pm daily for 5 days. This dose of ethanol gives rise to blood alcohol concentrations of approximately 0.2 mg/dL (19, 20). After each feeding, the pups were immediately returned to the litter. The pups were kept with their dams until weaning at PD 21. These animals were separated by sex and group housed in12-hour light and 12-hour dark cycles. At PD 90, the male offspring were killed at 4-hour intervals over a 24-hour period, and their brains were collected and stored at −80°C for gene expression studies.
Laser-capture microdissection
Brains were sectioned at 20 μm and processed for the dehydration steps. The slides were dehydrated in 75% ethanol for 30 seconds, 95% ethanol for 30 seconds, 100% ethanol for 1 minute (twice), and xylene for 5 minutes. After these steps, the POMC cells containing EGFP fluorescence were viewed using a 488-nm filter and captured by using the PixCell LCM system (Arcturus). The laser spot size was 7 μm. The power amplitude and pulse duration of the PixCell laser were adjusted for each slide (65–75 mW, 750–850 msec). The thermoplastic film-coated caps containing the captured cells were incubated in lysis buffer from the Picopure RNA isolation kit for 35 minutes (Molecular Devices) and stored at −80°C for gene expression studies.
Total RNA extraction and RT-PCR
The LCM-isolated POMC cells were processed for RNA isolation using Picopure RNA isolation kit and converted to cDNA by a high-capacity cDNA reverse transcriptase kit (Applied Biosystems). The cDNA yield was subjected to RT-PCR using the specific 5′ nuclease assay on an ABI Prism 7500 sequence detector (Applied Biosystems). The primers for Pomc (Mm00435874_m1), Stat3 (Mm01219775_m1), Asb4 (Mm00480830_m1), Sirt1 (Mm01168521_m1), Pgc1α (Mm00731216_s1), Per1 (Mm00501813_m1), Per2 (Mm00478113_m1), Per3 (Mm00478120_m1), Bmal1 (Mm00500226_m1), Clock (Mm00455950_m1), and Gapdh (4352932E) primers were acquired from Applied Biosystems. The RT-PCR was completed at 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Relative quantity of mRNA was calculated by relating the PCR threshold cycle obtained from the tested sample to relative standard curves generated from a serial dilution of cDNA prepared from the total RNA. The glyceraldehyde-3-phosphate dehydrogenase mRNA in each sample was measured as an internal control. Relative mRNA levels at each time point were indicated as a percentage of the maximum value over the 24-hour period of the control group for in vivo studies.
Glucose measurement
Adult male C57BL/6 (wild type) and Per2Brdml (Per2 mutant) mice offspring of mothers fed alcohol (AF) or control diets (PF or AD) during PD 2 and PD 7 were used in this study. At 90 days, these mice were fasted for 18 hours and then used for blood collection (2.0 μL) by tail vein punctures. Blood glucose levels were measured at 4-hour intervals during a circadian cycle using a Contour blood glucose monitoring system.
Statistical analysis
Data were analyzed using a one-way ANOVA with a Dunnett post hoc test to assess the differences between the time points of the same group. In the Dunnett post hoc test, the lowest value was chosen as the control. Two-way ANOVA with the Bonferroni posttest was used to assess the differences between the AD, PF, and AF groups over time. Culture experiment data were analyzed using an ANOVA with a Newman-Keuls post hoc test. A value of P < .05 was considered statistically significant. The F values and degrees of freedom data are shown in Supplemental Tables 1–5.
Results
POMC mRNA levels show circadian oscillation in the POMC neuronal population within the arcuate nucleus of the hypothalamus
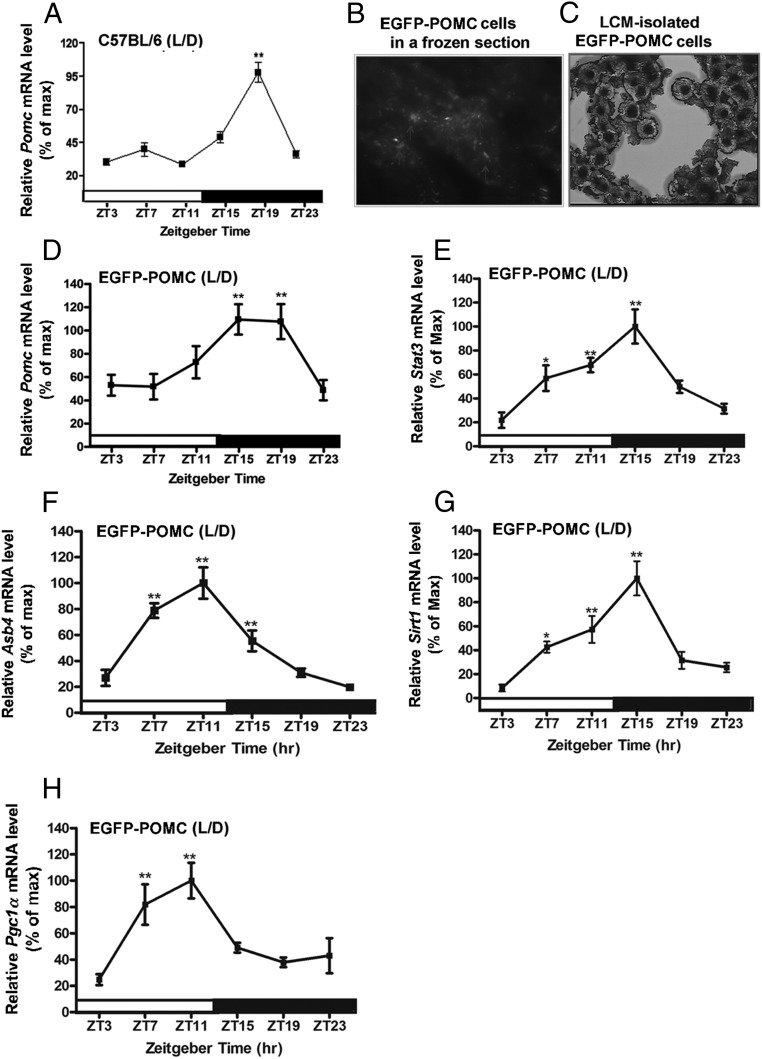
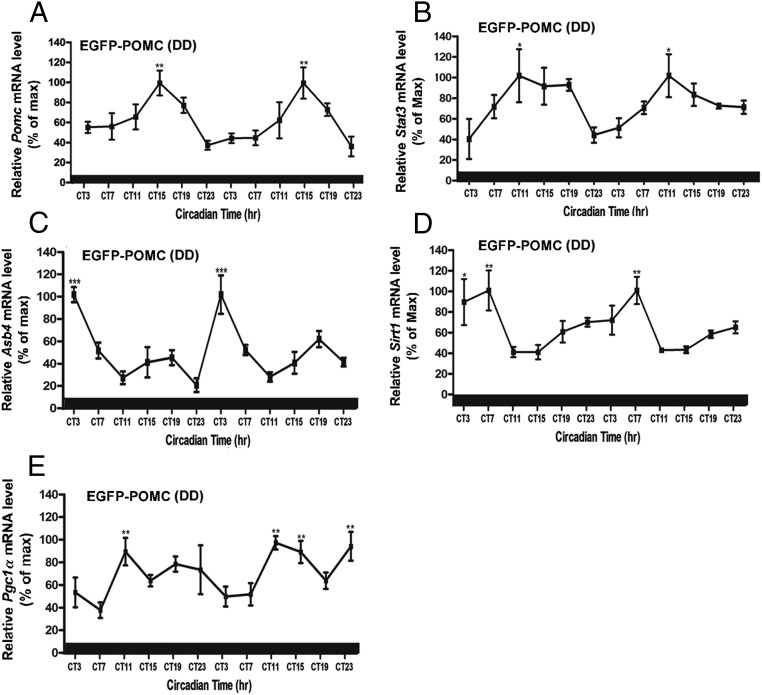
In rats, the daily rhythm of Pomc mRNA in the arcuate nucleus (16) followed the rhythmic pattern of c-fos expression in POMC neurons (21, 22). The data from the present study show a rhythmic expression of Pomc mRNA in the arcuate nucleus tissue of C57BL/6J mice maintained in the LD cycle (Figure 1A). The Pomc mRNA levels in this strain of mice were lower in daytime and started to increase at the onset of the dark phase, with the peak between ZT15 and ZT19, and then showed a gradual decrease until a nadir between ZT23 and ZT3. To measure gene transcript changes specifically within the POMC neurons, we used the LCM methods and POMC-EGFP transgenic mice as an animal model. The POMC-EGPF neurons are visible under a fluorescence microscope (Figure 1B) and can be isolated by the LCM methods (Fig. 1C). Like in C57BL/6J mice, the Pomc mRNA expression in the LCM-isolated neurons of POMC-EGFP transgenic mice housed in the LD cycle displayed the circadian variations over time with the identical rhythmic pattern (Figure 1, A and D). The daily Pomc rhythm pattern in LCM-isolated POMC neurons did not change when the mice were housed in the DD condition (Figure 2A), suggesting that the Pomc gene expresses in a circadian manner.
Figure 1.
Circadian expression of genes related to metabolic signaling in POMC neurons in the hypothalamus of wild-type and EGFP-POMC C57BL/6 mice under LD conditions. A, Daily rhythms in mRNA levels of the Pomc gene in the hypothalamic tissue of wild-type mice. B, Identification of POMC neurons (white arrowheads) by EGFP fluorescence. C, POMC neurons isolated in a LCM cap under a light microscope. D, Daily rhythms in mRNA levels of the Pomc gene in POMC neurons isolated by LCM from the hypothalamus of EGFP-POMC mice. E–H, Daily rhythms in the mRNA levels of metabolic-related genes (Stat3, E; Asb4, F; Sirt1, G; Pgc1a, H) in the POMC neurons of EGFP-POMC mice. The isolated Pomc cells correspond to brains collected at 10:00 am, 2:00 pm, 6:00 pm, 10:00 pm, 2:00 am, and 6:00 am. These time points correspond with ZT3, ZT7, ZT11, ZT15, ZT19, and ZT23, respectively. The mRNA level of Pomc or other metabolic gene at each time point was normalized with the Gapdh mRNA value, and the ratio was used to calculate the percentage of the maximum value over a 24-hour period. *, **, ***, Significantly different (P < .05, P < .01, P < .001, respectively) from the lowest value, as per a one-way ANOVA with the Dunnett posttest. Black bar represents the dark period. Data are mean ± SEM of five to six animals per time point.
Figure 2.
Circadian expression of genes related to metabolic signaling in POMC neurons in the hypothalamus of EGFP-POMC mice under DD. A, Daily rhythms in the mRNA levels of the Pomc gene in POMC neurons isolated by LCM from the hypothalamus. B–E, Daily rhythms in the mRNA levels of metabolic related genes (Stat3, B; Asb4, C; Sirt1, D; Pgc1a, E) in POMC neurons. The mRNA level of Pomc or other metabolic gene at each time point was normalized with the Gapdh mRNA value, and the ratio was used to calculate the percentage of the maximum value over a 24-hour or a 48-hour period. *, **, ***, Significantly different (P < .05, P < .01, P < .001, respectively) from the lowest value, as per a one-way ANOVA with the Dunnett posttest. Black bar represents the dark period. Data are mean ± SEM of five to six animals per time point.
Stat3, Sirt1, Pgc1-α, and Asb4 mRNA levels show circadian oscillations in POMC neurons in the arcuate nucleus of the hypothalamus
Energy and reward homeostasis is essential for maintaining energy balance, and its disruption may lead to metabolic disorders, including obesity and diabetes. If circadian functions of POMC neurons control body metabolism, it would be expected to see circadian oscillations in the expression of insulin- and leptin-signaling molecules in these neurons. To investigate this possibility, we measured the mRNA levels of a selective group of insulin- and leptin-signaling genes (Stat3, Sirt1, Pgc1-α, Asb4) by real-time PCR in LCM-isolated POMC neurons obtained at different time points from POMC-EGFP mice housed under LD and DD environmental conditions. Circadian oscillations of transcripts levels were found in all the signaling factors studied (Figure 1, E and H). It was observed that under LD conditions, rhythm peaks of Sta3 and Sirt1 occurred at a similar Zeitgeber time (ZT15). This time is during the early night, which corresponds to the active state and initiation of feeding time in mice, reflecting their role in the metabolic function. Peaks of Asb4 and Pgc1α mRNA rhythms occurred at an earlier time period (ZT11), which was during the elevated phase cycle of Stat3 and Sirt1. Under constant darkness, the mRNA level of Stat3 mRNA shows its peak at circadian time (CT) 11, and mRNA levels of Pgc1α, Sirt1, and Asb4 peaked at an advanced time (CT11, CT7, and CT3, respectively) (Figure 2, B–E). Because all the metabolic signaling genes showed rhythmic profiles under DD conditions, these signaling molecules are also expressed in POMC neurons in a circadian manner.
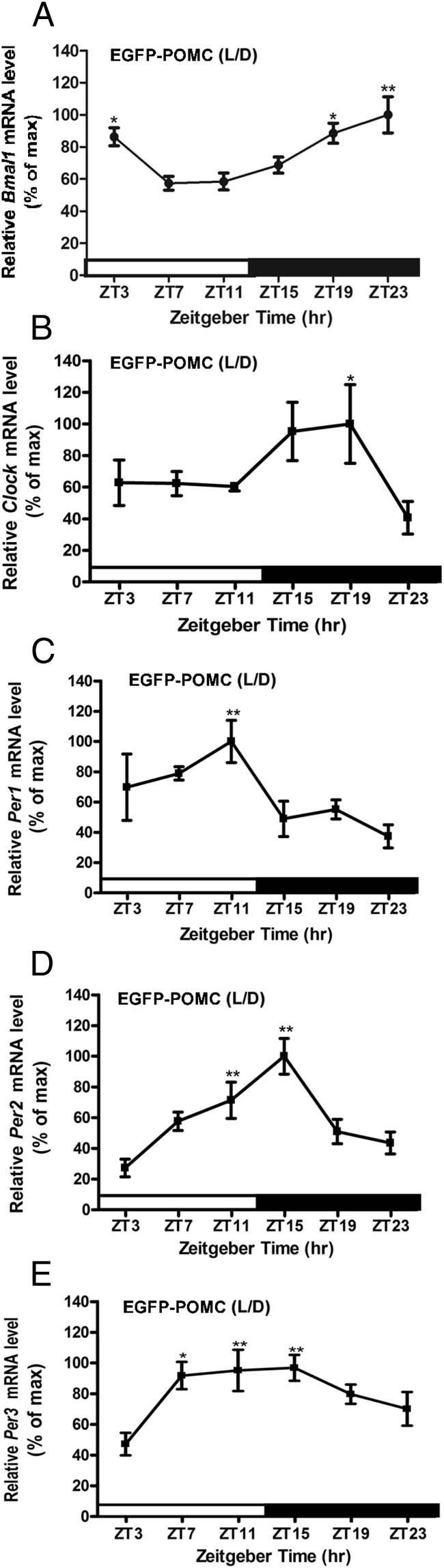
Circadian oscillation of canonical clock genes in POMC neuron
Because Pomc and metabolic signaling genes show circadian oscillation in transcripts expression, we examined whether POMC neurons present clock gene oscillations. For that purpose, we measured mRNA levels of five representative clock genes (Bmal1, Clock, Per1, Per2, and Per3), which are known to help maintain circadian rhythm, in POMC neurons isolated by LCM at different time points in the hypothalamus of POMC-EGFP mice under the LD condition. The mRNA oscillations of the positive clock genes Bmal1 and Clock peaked between ZT19 and ZT23 and showed the lowest levels from ZT7 to ZT11 (Figure 3, A and B). These time points coincided with the rising mRNA levels of Per1 and Per2, their negative counterparts in the molecular clock. Per1 mRNA levels peaked at ZT11 and were lowest from ZT15 to ZT23 (Figure 3C). Per2 mRNA levels were found to be lowest between ZT23 and ZT3 and were highest at ZT11 (Figure 3D). Per3 mRNA levels peaked between ZT7 and ZT15 and were found to be lower levels between ZT23 and ZT3 (Figure 3E). These data are consistent with previous reports describing clock gene expression in other mouse peripheral tissues (23) and suggest that, like many other cells, POMC cells also have a rhythmic expression of canonical clock genes.
Figure 3.
Daily rhythms in mRNA levels of clock genes in POMC neurons in the hypothalamus of EGFP-POMC mice maintained under L/D. A, Bmal1; B, Clock; C, Per1; D, Per2; E, Per3. Relative mRNA levels of various genes were calculated as in Figure 1. *, **, Significantly different (P < .05, .01) from the lowest value, as per one-way ANOVA with the Dunnett post hoc test. Black bar represents the dark period. Data are mean ± SEM of N = 5–6 animals per time point.
Early-life exposure to ethanol disrupts circadian oscillations of POMC and metabolic genes in POMC neurons
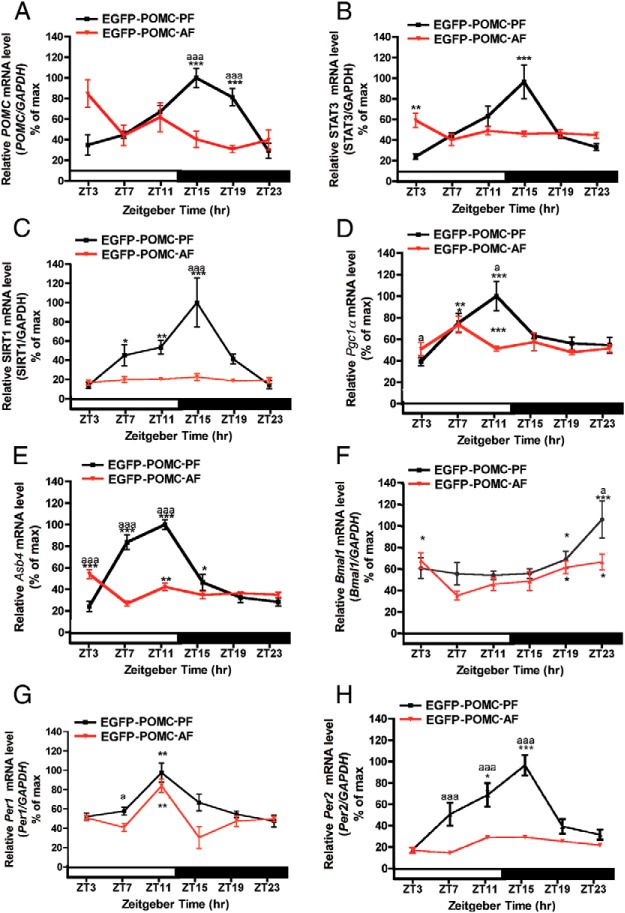
We determined whether ELAF alters mRNA expression of POMC and metabolic signaling genes in POMC neurons. Our results demonstrate that daily expression levels of Pomc, Stat3, Sirt1, Pgc1α, and Asb4 mRNA show identical rhythmic profiles in both AD (Figure 1, D–H) and PF animals (Figure 4, A–E), suggesting that the milk formula feeding method did not significantly alter the circadian expression of signaling genes in POMC neurons. However, postnatal ethanol feeding using milk formula programmed these neurons such that the adult expression of Pomc, Stat3, Sirt1, and Asb4 mRNA levels became arrhythmic in these cells (AF groups). In addition, the mRNA levels of these genes in the AF mice in general remained at the trough levels of the oscillatory phases of these genes observed in the AD and PF mice. One exception is that the Pgc1α mRNA levels in the AF mice maintained oscillatory profiles (Figure 4D). However, the peak of the Pgc1α mRNA oscillation in the ethanol-treated group showed an earlier peak at ZT7 compared with the AD and PF control groups, which peaked at ZT11. These data demonstrate that postnatal ethanol exposure significantly alters the circadian expression of POMC and various metabolic signaling genes in POMC neurons.
Figure 4.
Changes in the circadian expression of metabolic and clock genes in POMC neurons in the hypothalamus of adult EGFP-POMC mice given a diet of milk formula containing ethanol (AF) or pair-fed isocaloric diet (PF) or left in the litter with the mother (AD) during the postnatal period. A–H, Daily rhythms in the mRNA levels of Pomc (A), metabolic-related genes (Stat3, B; Sirt, C; Pgc1a, D; Asb4, E), and clock genes (Bmal1, F; Per1, G; Per2, H). Relative mRNA levels of various genes were calculated as in Figure 1. *,**,***, Significantly different (P < .05, P < .01, P < .001, respectively) between means at different time points among the PF and AF groups from the lowest value, as per a one-way ANOVA with the Dunnett posttest. a, aa, aaa, Significant difference (P < .05, P < .01, P < .001, respectively) between AF and PF as per a two-way ANOVA with the Bonferroni post hoc test. Black bar represents the dark period. Data are mean ± SEM of five to six animals per time point.
Early-life exposure to ethanol disrupts circadian oscillation of canonical clock genes in POMC neurons
Next, the possibility that alcohol targets the cellular clock mechanism to alter POMC neuronal functions was tested. The mRNA levels of Bmal1, Per1, and Per2 in POMC neurons in control (AD and PF) and alcohol-fed (AF) POMC-EGFP mice under LD conditions were compared. The circadian profile of Bmal1, Per1, and Per2 in AD (Figure 3, A, C, and D) and PF groups (Figure 4, F–H) showed identical rhythm patterns, suggesting that the milk formula feeding had no significant effect on the circadian profile of these genes. The circadian profiles of Bmal1 and Per1 mRNA in the AF group displayed significantly lower levels during the peak circadian phase (P < .05) than in the AD and PF groups. However, the circadian profile for the Per2 gene demonstrated a much more robust difference in the rhythmic profile. The Per2 mRNA levels in the AF group were lower at all time points than the levels in the AD and PF groups. Additionally, the Per2 mRNA levels did not show any significant rhythm in POMC neurons of AF mice. These data demonstrate that early-life exposure to alcohol alters the circadian profile of Per2 and other key regulatory genes that maintain the cellular circadian clock machinery. Early-life exposure to ethanol failed to alter the daily expression of Pomc and metabolic genes in POMC neurons in Per2 mutant mice.
Because postnatal ethanol markedly affects Per2 gene expression, the possibility is raised that this gene may be involved in the regulation of ethanol's actions on the circadian function of POMC neurons. To test this possibility, we created a mouse line POMC-EGFP-Per2mut by crossing Per2 mutant mice (mPer2Brdml) with transgenic POMC-EGFP mice. The Per2mut mice (mPer2Brdml) carry a mutant mPer2 gene with a deletion in the PAS Per, period circadian protein; Arnt, aryl hydrocarbon receptor nuclear trranslocator protein; and Sim, single-minded protein) dimerization domain, which is critical for the interaction with other clock proteins, thus rendering a loss-of-function mutation of the PER2 protein (24). We crossed Per2mut mice with transgenic POMC-EGFP mice to generate a POMC-EGFP mouse line with Per2mut in all tissues (POMC-EGFP-Per2mut). Genotype analysis identified both the POMC-EGFP and Per2 mutant allele in POMC-EGFP-Per2mut mice (Supplemental Figure 1).
To investigate the effects of the Per2 mutation on the molecular clock components in the POMC neurons, we analyzed by RT-PCR the daily mRNA rhythms of Per1, Per2, and Bmal1 in POMC neurons. Expression profiles of circadian clock genes in the POMC neurons of Per2 mutant mice were different from those of wild-type mice: Per1, Per2, and Bmal1 mRNA levels in the POMC neurons of Per2 mutant mice failed to display a significant daily rhythm (compare data of Figures 3, A, C, and D, and 5, A–C). These results suggest that the Per2 mutation causes significant changes in the circadian expression of some key clock genes in a POMC neuronal cell population. This can be envisioned as an overall defect in the molecular circadian clock physiology in the POMC neuronal population of Per2 mutant mice.
Figure 5.
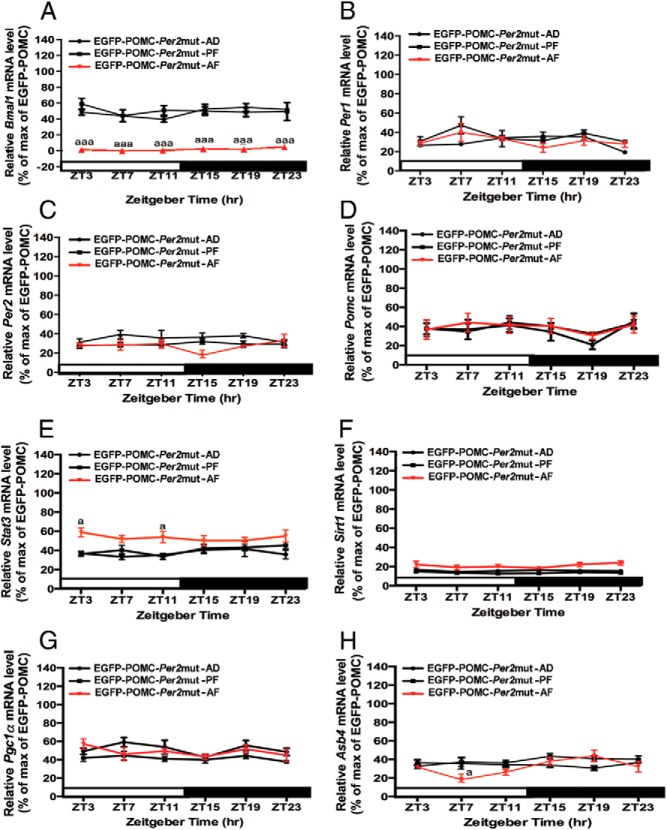
Changes in circadian expression of metabolic and clock genes in POMC neurons in the hypothalamus of adult Per2 mutant (EGFP-POMC-Per2mut) mice given a diet of milk formula containing ethanol (AF) or pair-fed isocaloric diet (PF) or left in the litter with the mother (AD) during the postnatal period. A–H, Daily rhythms in the mRNA levels of clock genes (Bmal1, A; Per1, B; Per2, C), Pomc (D), and metabolic related genes (Stat3, E; Sirt, F; Pgc1a, G; Asb4, H). Relative mRNA levels of various genes were calculated as in Figure 1. a, aaa, Significant difference (P < .05, P < .001) between AF and AD or PF as per a two-way ANOVA with the Bonferroni post hoc test. Black bar represents the dark period. Data are mean ± SEM of five to six animals per time point.
Comparison of the levels of Bmal1 mRNA in POMC neurons between the AF-, PF-, and AD-treated groups shows that ELAF reduced the levels of the gene transcript at all time points (Figures 3A, 4F, and 5A). However, ELAF failed to affect the Per1 or Per2 gene expression profiles in Per2 mutants (Figures 3C, 3D, 4G, 4H, 5B, and 5C). It should be noted that ELAF markedly affected Per2 gene expression at all circadian time points in wild-type POMC-EGFP mice (Figure 4H).
We also analyzed the effects of the Per2 mutation on ELAF modulation of Pomc and metabolic gene expression during the adult period. Our analyses demonstrated that one of the major effects of the Per2 mutation on circadian expression of Pomc and metabolic genes was that Per2 mutation prevented rhythmic expression of all these genes in AD, PF, and AF mice (Figure 5, D–H). Another major effect was that the Per2 mutation prevented or suppressed ELAF inhibitory effects on Pomc and all metabolic genes studied (compare data shown in Figures 4 and 5). One subtle effects was that, unlike what was seen in wild-type mice (Figures 1E and 4B), the mean level of Stat3 mRNA was moderately elevated at ZT11 in the AF group as compared with the AD and PF groups in Per2 mutant mice (Figure 5E). Because the actions of postnatal ethanol and Per2 mutation were in general similar on Pomc and metabolic signaling genes and because Per2 mutation in general prevented or suppressed ethanol to alter Pomc and metabolic signaling gene expression, these data suggest the possibility that the Per2 gene may by blocking the circadian mechanism mediate ethanol's action on Pomc and metabolic signaling genes in POMC neurons in the hypothalamus.
Early-life exposure to ethanol disrupts the circadian rhythm of blood glucose in wild-type mice but not in Per2 mutant mice
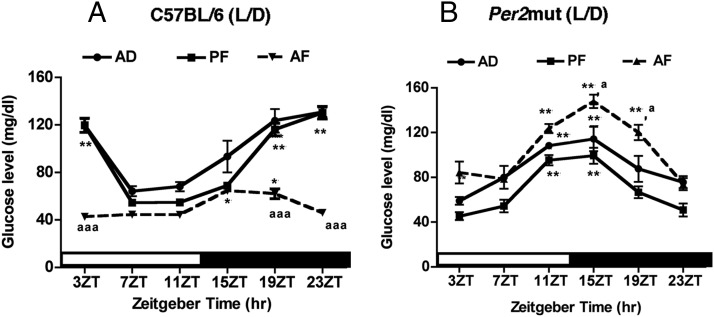
Previous experiments identified several metabolic genes that respond to ELAF treatments. These genes are known to play a significant role in controlling glucose homeostasis (9). Hence, we evaluated the changes in the glucose rhythm after the ELAF treatment in C57BL/6 mice. Furthermore, to determine the role of the Per2 gene in controlling the ELAF effect, we compared glucose rhythms in AF-, PF-, and AD-treated C57BL/6 and Per2 mutant mice during the adult period. The data indicate that the glucose levels in the control groups (AD and PF) were rhythmic in C57BL/6 mice. Peak glucose levels in these mice were observed between 15ZT and 23ZT, and the nadir points were between 7ZT and 11ZT. In comparison, control (AD and PF treated) Per2 mutant mice exhibited a glucose rhythm shift that resulted in an earlier peak at 11 ZT and 15ZT (Figure 6, A and B), suggesting that PER2 may regulate glucose rhythm in part by serving as a timekeeper to maintain the resetting cue between the nadir and peak points. The postnatal ethanol decreased glucose levels and abolished the glucose rhythm in C57BL/6 mice. ELAF treatment did not alter the rhythmic profiles of blood glucose in Per2 mice. ELAF treatment resulted in a moderate elevation of blood glucose levels during the peak periods in Per2 mutant mice (Figure 6B). These data suggest that intact circadian machinery is a prerequisite for the ELAF action on the glucose rhythm.
Figure 6.
Changes in diurnal expression of blood glucose in adult C57BL/6 or Per2 mutant male mice given milk formula containing ethanol diet (AF) or pair-fed isocaloric diet (PF) or left in the litter with the mother (AD) during the postnatal period. *,**,***, Significantly different (P < .05, P < .01, P < .001, respectively) between means at different time points among the PF and AF groups from the lowest value, as per a one-way ANOVA with the Dunnett posttest. a, aa, aaa, Significantly different (P < .05, P < .01, P < .001, respectively) between AF, PF, and AD as per a two-way ANOVA with the Bonferroni post hoc test. Black bar represents the dark period. Data are mean ± SEM of five to six animals per time point.
Discussion
The present data suggest that postnatal alcohol exposure in mice, similar to human fetal alcohol exposure during the third trimester of pregnancy, can be considered as a risk factor for developing metabolic disorders in adulthood. Many studies suggest that circadian mechanisms are critically involved in glucose homeostasis and other metabolic functions (25–27). In addition, altered sleep patterning has been implicated in abnormal insulin and leptin signaling (28–30), suggesting a role of the circadian clock mechanism in mediating metabolic signaling pathways in the central nervous system. Supporting this view is the evidence that the disruption of clock components leads to hypoinsulinemia and diabetes (31) and that the Per2 mutation affects key aspects of diabetes, including retinal vascular damage, neuronal loss in the bone marrow, and endothelial dysfunction (32). It should be noted that patients with fetal alcohol spectrum disorders are known to have altered sleep patterning (33, 34) and metabolic problems (34, 35). In view of this evidence, one can predict the involvement of the circadian clock in mediating metabolic sensing in the hypothalamus. In this study, we demonstrated for the first time that ELAF induces changes in the daily expression of several metabolic sensing genes (Stat3, Sirt1, Pgc1α, and Asb4) in the POMC neurons and alters the circadian rhythm of blood glucose levels during the adult period that are regulated possibly by the circadian mechanism.
Previous studies have demonstrated that the hypothalamic POMC neuron is regulated by nutrient-sensing and metabolic signals and is a key component in metabolic homeostasis (36, 37). Mice with POMC neuronal abnormalities fed ad libitum exhibit normal to moderate hyperinsulinemia, and when subjected to a restricted protocol, they develop hyperglycemia, glucose intolerance, and dyslipidemia (38). Leptin, a hormone derived from adipose tissue, conveys critical information about peripheral energy storage and availability to the POMC neurons. The anorexigenic actions of leptin are mediated by the long form of the leptin receptor, which activates both Janus kinase 2-dependent and -independent pathways, including the signal transducer and activator of transcription-3 pathway. In addition, leptin and insulin integrate with the central melanocortin system to coordinate alterations in energy and glucose balance. Furthermore, various signaling molecules (eg, Asb4, Pgc1a, and Sirt1) participate in the integration of leptin and insulin sensing to control POMC neuronal function. All these metabolic genes are suggested to have a symbiotic relationship with POMC in metabolic signaling in the hypothalamus.
The data presented here suggest that developmental alcohol exposure prevents the clock mechanism to operate in POMC neurons, primarily by suppressing the expression of the Per2 gene. These results raise the possibility that the Per2 gene may be blocking these effects of ethanol on hypothalamic POMC neurons that are mediated by circadian mechanisms. This view is supported by several previous indirect evidences. For example, Agapito et al (14) demonstrated that the Per2 mutation prevents β-endorphin stimulatory and inhibitory responses to acute and chronic ethanol challenges in a cell culture system. Also, prenatal ethanol decreases Per2 mRNA levels in the arcuate nucleus, in which many POMC neuronal cells are localized (39). Additionally, a population of POMC neurons produces and releases glutamate (40), which is also a target of Per2 mutation (41). However, further study is needed to establish the mediatory role of Per2 in fetal alcohol effects on POMC neurons.
How the Per2 gene mutation alters ethanol's action on POMC-producing neurons is not well understood at present. One possibility is that the Per2 gene mutation leads to insufficient production of PER2 proteins, which leads to abnormalities in the clock mechanism governing POMC neuronal function. In this regard, it should be mentioned that our present study indicates a phase advancement of blood glucose rhythm in Per2 mutant mice, suggesting a defect in the molecular circadian clock physiology in the absence of this clock gene.
A similar phase advance and shorter circadian period in the rhythm of the Per2 mutant transcript have been described in the suprachiasmatic nucleus (42). Albrecht et al (43) showed that mice carrying mutant mPer2 genes, when tested for responses to a light pulse at ZT 14 and ZT 22, did not advance or delay the clock, which suggests that Per genes not only are light-responsive components of the circadian oscillator but also are involved in the resetting of the circadian clock. However, mice carrying mutant mPer2 genes showed a rhythmic expression profile of arylalkylamine N-acetyltransferase, a rate-limiting melatonin synthesis enzyme, and robust photoperiodic responses of the Tshb, Dio2, and Dio3 genes, suggesting that the Per2 clock gene is not necessary for the photoperiodic response in mice (24). Data from our study identify a complete loss of rhythm in POMC neurons in Per2 mutant mice in the LD condition. Similarly, the loss of the hypothalamic α-MSH and plasma glucorticoid rhythms were observed in Per2 mutant mice (45). Per2mut mice have a disrupted clock mechanism in the spleen, even in the LD condition (23). These data suggest that a significant uncoupling takes place between the suprachiasmatic nucleus and the cellular clock in the POMC and other cells in Per2 mice. The other possibility is that PER2 is directly binding to the Pomc gene to alter ethanol's response. This concept seems somewhat heretical, given the current paradigm that clock proteins inhibit expression by posttranslational modifications of the positive elements such as Clock and Bmal1 (46). However, it is clear, at least in Drosophila, that PER2 is associated with DNA (47). Moreover, recent studies in rat pituitary GH3 cells have shown PER2 proteins acting directly on the promoter of pituitary prolactin (48). Therefore, one can assume that a similar process exists in other genes, perhaps including POMC. More studies are necessary to determine exactly how the Per2 gene controls POMC neuronal functions and mediates the action of ethanol.
In conclusion, the data presented here show that ELAF suppresses Per2 gene expression and alters the function of the circadian clock system to affect metabolic sensing and POMC production in POMC neurons in the hypothalamus. In this context, it is interesting to note that the specific Per2 polymorphism 10870, which reduces the function of this gene, is evident in chronic alcoholic patients (40, 49). Interestingly, the Per2 polymorphism contributes to changes in glucose metabolism because the Per2 single-nucleotide polymorphism is associated with the metabolic syndrome (44). Furthermore, a perturbation of circadian clock components has shown to be associated with islet pathophysiology in human diabetes mellitus type 2 (50). Therefore, disturbances in the Per2 gene-regulated metabolic signaling mechanism in the POMC neurons may play a significant role in the development of metabolic disorders in patients with fetal alcohol spectrum disorders.
Acknowledgments
We thank Ms Jackie C. Barreira and Dr Peter Kuhn for technical assistances and Dr Kathy Manger for editorial assistance.
This work was supported by National Institutes of Health Grants R01 AA015718 and R37 AA08757.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AD
- left in the litter with the mother
- AF
- diet of milk formula containing ethanol
- CT
- circadian time
- DD
- constant darkness
- EGFP
- enhanced green fluorescent protein
- ELAF
- early-life ethanol feeding
- LCM
- laser-capture microdissection
- LD
- light/dark
- PD
- postnatal day
- PF
- pair-fed isocaloric diet
- POMC
- proopiomelanocortin
- ZT
- Zeitgeber time.
References
- 1. Centers for Disease Control and Prevention (CDC). Alcohol use and binge drinking among women of childbearing age—United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2012;61:534–538 [PubMed] [Google Scholar]
- 2. Fofana B, Yao XH, Rampitsch C, Cloutier S, Wilkins JA, Nyomba BL. Prenatal alcohol exposure alters phosphorylation and glycosylation of proteins in rat offspring liver. Proteomics. 2010;10:417–434 [DOI] [PubMed] [Google Scholar]
- 3. Dobson CC, Mongillo DL, Brien DC, et al. Chronic prenatal ethanol exposure increases adiposity and disrupts pancreatic morphology in adult guinea pig offspring. Nutr Diabetes. 2012;2:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yao XH, Nyomba BL. Hepatic insulin resistance induced by prenatal alcohol exposure is associated with reduced PTEN and TRB3 acetylation in adult rat offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1797–R1806 [DOI] [PubMed] [Google Scholar]
- 5. Chen L, Yao XH, Nyomba BL. In vivo insulin signaling through PI3-kinase is impaired in skeletal muscle of adult rat offspring exposed to ethanol in utero. J Appl Physiol. 2005;99:528–534 [DOI] [PubMed] [Google Scholar]
- 6. Ting JW, Lautt WW. The effect of acute, chronic, and prenatal ethanol exposure on insulin sensitivity. Pharmacol Ther. 2006;111:346–373 [DOI] [PubMed] [Google Scholar]
- 7. Chen L, Nyomba BL. Glucose intolerance and resistin expression in rat offspring exposed to ethanol in utero: modulation by postnatal high-fat diet. Endocrinology. 2003;144:500–508 [DOI] [PubMed] [Google Scholar]
- 8. Coppari R, Bjørbæk C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11:692–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coll AP, Loraine Tung YC. Pro-opiomelanocortin (POMC)-derived peptides and the regulation of energy homeostasis. Mol Cell Endocrinol. 2009;300:147–151 [DOI] [PubMed] [Google Scholar]
- 11. Singh RB, Gupta S, Dherange P, et al. Metabolic syndrome: a brain disease. Can J Physiol Pharmacol. 2012;90:1171–1183 [DOI] [PubMed] [Google Scholar]
- 12. Alkemade A, Yi CX, Pei L, et al. AgRP and NPY expression in the human hypothalamic infundibular nucleus correlate with body mass index, whereas changes in αMSH are related to type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E925–E933 [DOI] [PubMed] [Google Scholar]
- 13. Agapito MA, Barreira JC, Logan RW, Sarkar DK. Evidence for possible period 2 gene mediation of the effects of alcohol exposure during the postnatal period on genes associated with maintaining metabolic signaling in the mouse hypothalamus. Alcohol Clin Exp Res. 2013;37:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agapito M, Mian N, Boyadjieva NI, Sarkar DK. Period 2 gene deletion abolishes β-endorphin neuronal response to ethanol. Alcohol Clin Exp Res. 2012;34:1613–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allen GC, Farnell YZ, Maeng JU, West JR, Chen WJ, Earnest DJ. Long-term effects of neonatal alcohol exposure on photic reentrainment and phase-shifting responses of the activity rhythm in adult rats. Alcohol. 2005;37:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–1554 [DOI] [PubMed] [Google Scholar]
- 17. Sakata-Haga H, Dominguez HD, Sei H, Fukui Y, Riley EP, Thomas JD. Alterations in circadian rhythm phase shifting ability in rats following ethanol exposure during the third trimester brain growth spurt. Alcohol Clin Exp Res. 2006;30:899–907 [DOI] [PubMed] [Google Scholar]
- 18. Overstreet LS, Hentges ST, Bumaschny VF, et al. A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci. 2004;24:3251–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodlett CR, Pearlman AD, Lundahl KR. Binge neonatal alcohol intubations induce dose-dependent loss of Purkinje cells. Neurotoxicol Teratol. 1998;20:285–292 [DOI] [PubMed] [Google Scholar]
- 20. Sarkar DK, Kuhn P, Marano J, Chen C, Boyadjieva N. Alcohol exposure during the developmental period induces β-endorphin neuronal death and causes alteration in the opioid control of stress axis function. Endocrinology. 2007;148:2828–2834 [DOI] [PubMed] [Google Scholar]
- 21. Jamali AK, Tramu G. Daily cycle of fos expression within hypothalamic POMC neurons of the male rat. Brain Res. 1997;771:45–54 [DOI] [PubMed] [Google Scholar]
- 22. Jamali KA, Tramu G. Control of rat hypothalamic pro-opiomelanocortin neurons by a circadian clock that is entrained by the daily light-off signal. Neuroscience. 1999;93:1051–1061 [DOI] [PubMed] [Google Scholar]
- 23. Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFN-γ. J Interferon Cytokine Res. 2006;26:645–649 [DOI] [PubMed] [Google Scholar]
- 24. Zheng B, Larkin DW, Albrecht U, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173 [DOI] [PubMed] [Google Scholar]
- 25. Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic β-cell loss and dysfunction. J Biol Rhythms. 2011;26:423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boer-Martins L, Figueiredo VN, Demacq C, et al. Relationship of autonomic imbalance and circadian disruption with obesity and type 2 diabetes in resistant hypertensive patients. Cardiovasc Diabetol. 2011;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zirlik S, Hauck T, Fuchs FS, Neurath MF, Konturek PC, Harsch IA. Leptin, obestatin and apelin levels in patients with obstructive sleep apnoea syndrome. Med Sci Monit. 2011;17:CR159–CR164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–R903 [DOI] [PubMed] [Google Scholar]
- 31. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhatwadekar AD, Yan Y, Qi X, et al. Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow. Diabetes. 2013;62:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jan JE, Asante KO, Conry JL, et al. Sleep health issues for children with FASD: clinical considerations. Int J Pediatr. 2010;2010:639048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burd L, Selfridge R, Klug M, Bakko S. Fetal alcohol syndrome in the United States corrections system. Addict Biol. 2004;9:169–176 [DOI] [PubMed] [Google Scholar]
- 35. de la Monte SM, Wands JR. Role of central nervous system insulin resistance in fetal alcohol spectrum disorders. J Popul Ther Clin Pharmacol. 2010;17:e390–e404 [PMC free article] [PubMed] [Google Scholar]
- 36. Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070 [DOI] [PubMed] [Google Scholar]
- 37. Balthasar N, Dalgaard LT, Lee CE, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505 [DOI] [PubMed] [Google Scholar]
- 38. Smart JL, Tolle V, Low MJ. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest. 2006;116:495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen CP, Kuhn P, Advis JP, Sarkar DK. Prenatal ethanol exposure alters the expression of period genes governing the circadian function of β-endorphin neurons in the hypothalamus. J Neurochem. 2006;97:1026–1033 [DOI] [PubMed] [Google Scholar]
- 40. Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29:13684–13690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spanagel R, Pendyala G, Abarca C, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42 [DOI] [PubMed] [Google Scholar]
- 42. Ikegami K, Iigo M, Yoshimura T. Circadian clock gene Per2 is not necessary for the photoperiodic response in mice. PLoS One. 2013;8:e58482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16:100–104 [DOI] [PubMed] [Google Scholar]
- 44. Englund A, Kovanen L, Saarikoski ST, et al. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang S, Liu A, Weidenhammer A, et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150:2153–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kondratov RV, Shamanna RK, Kondratova AA, Gorbacheva VY, Antoch MP. Dual role of the CLOCK/BMAL1 circadian complex in transcriptional regulation. FASEB J. 2006;20:530–532 [DOI] [PubMed] [Google Scholar]
- 47. Yoshitane H, Takao T, Satomi Y, Du NH, Okano T, Fukada Y. Roles of CLOCK phosphorylation in suppression of E-box-dependent transcription. Mol Cell Biol. 2009;29:3675–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bose S, Boockfor FR. Episodes of prolactin gene expression in GH3 cells are dependent on selective promoter binding of multiple circadian elements. Endocrinology. 2010;151:2287–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Comasco E, Nordquist N, Göktürk C, et al. The clock gene PER2 and sleep problems: association with alcohol consumption among Swedish adolescents. Ups J Med Sci. 2010;115:41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stamenkovic JA, Olsson AH, Nagorny CL, et al. Regulation of core clock genes in human islets. Metabolism. 2012;61:978–985 [DOI] [PubMed] [Google Scholar]