Abstract
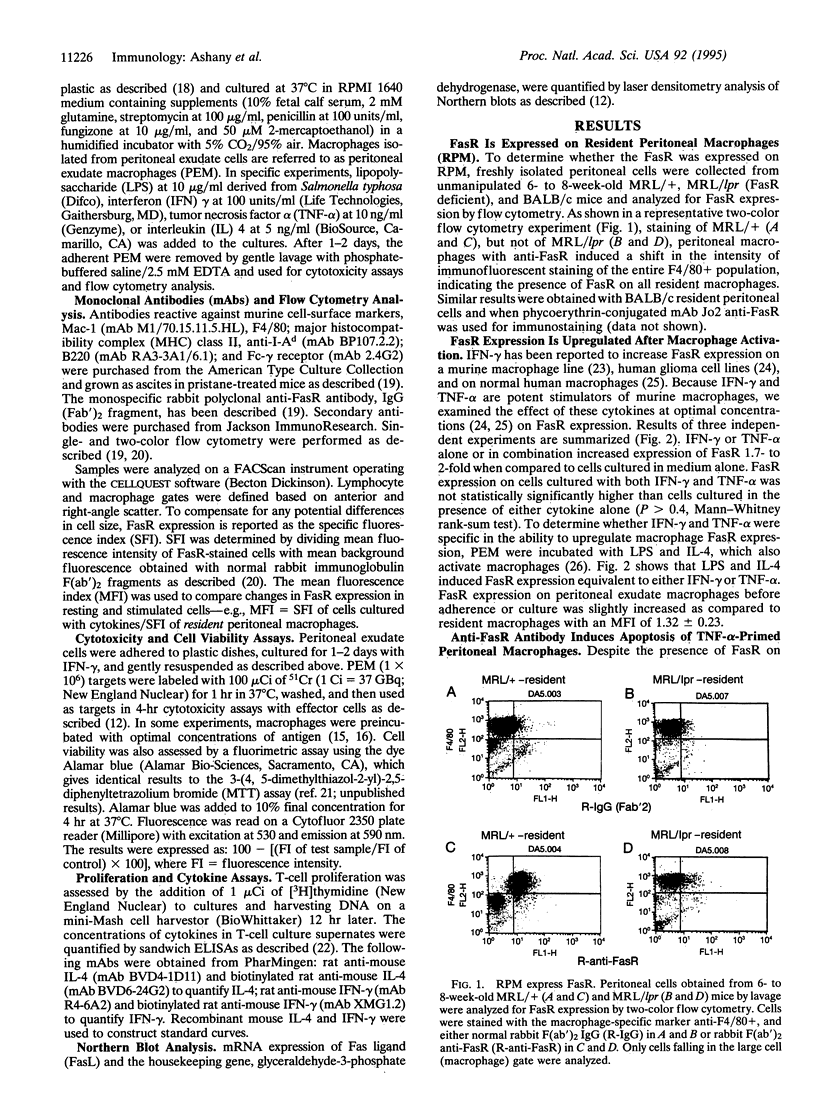
The Fas/APO-1 cytotoxic pathway plays an important role in the regulation of peripheral immunity. Recent evidence indicates that this regulatory function operates through deletion of activated T and B lymphocytes by CD4+ T cells expressing the Fas ligand. Because macrophages play a key role in peripheral immunity, we asked whether Fas was involved in T-cell-macrophage interactions. Two-color flow cytometry revealed that Fas receptor (FasR) was expressed on resting murine peritoneal macrophages. FasR expression was upregulated after activation of macrophages with cytokines or lipopolysaccharide, although only tumor necrosis factor-alpha rendered macrophages sensitive to anti-FasR antibody-mediated death. To determine the consequence of antigen presentation by macrophages to CD4+ T cells, macrophages were pulsed with antigen and then incubated with either Th1 or Th2 cell lines or clones. Th1, but not Th2, T cells induced lysis of 60-80% of normal macrophages, whereas macrophages obtained from mice with mutations in the FasR were totally resistant to Th1-mediated cytotoxicity. Macrophage cytotoxicity depended upon specific antigen recognition by T cells and was major histocompatibility complex restricted. These findings indicate that, in addition to deletion of activated lymphocytes, Fas plays an important role in deletion of activated macrophages after antigen presentation to Th1 CD4+ T cells. Failure to delete macrophages that constitutively present self-antigens may contribute to the expression of autoimmunity in mice deficient in FasR (lpr) or Fas ligand (gld).
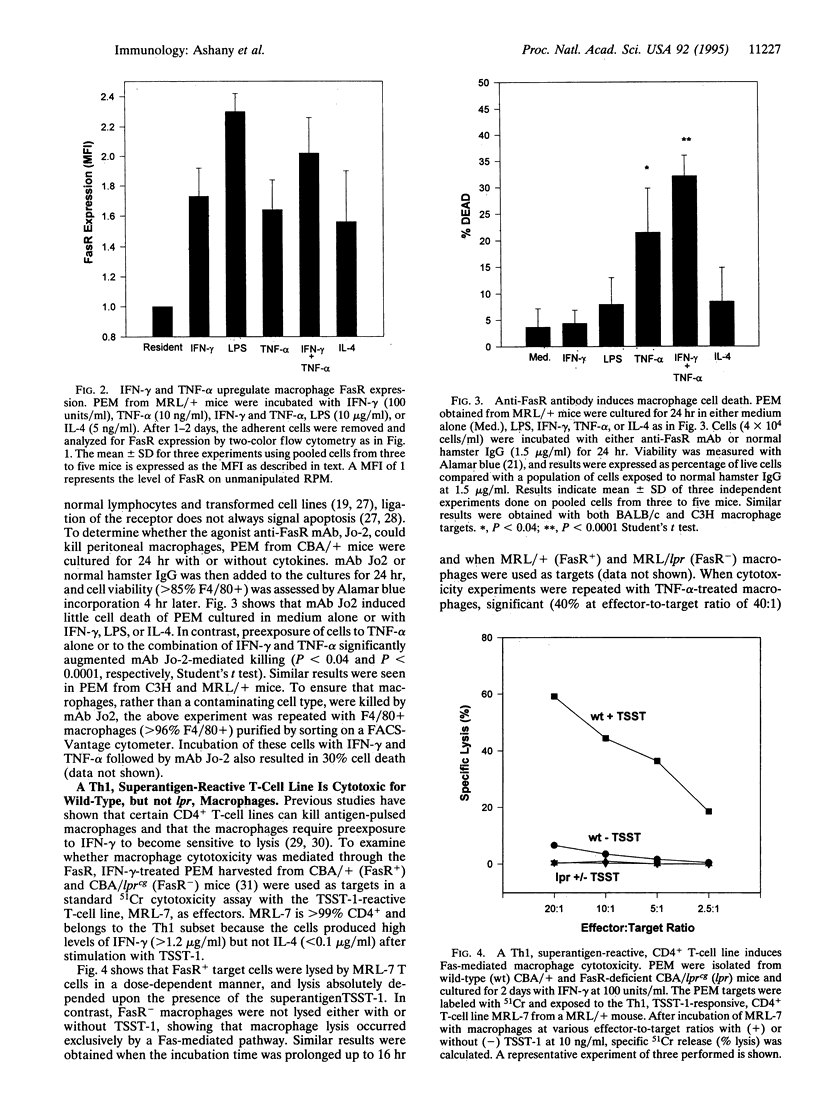
Full text
PDF

Selected References
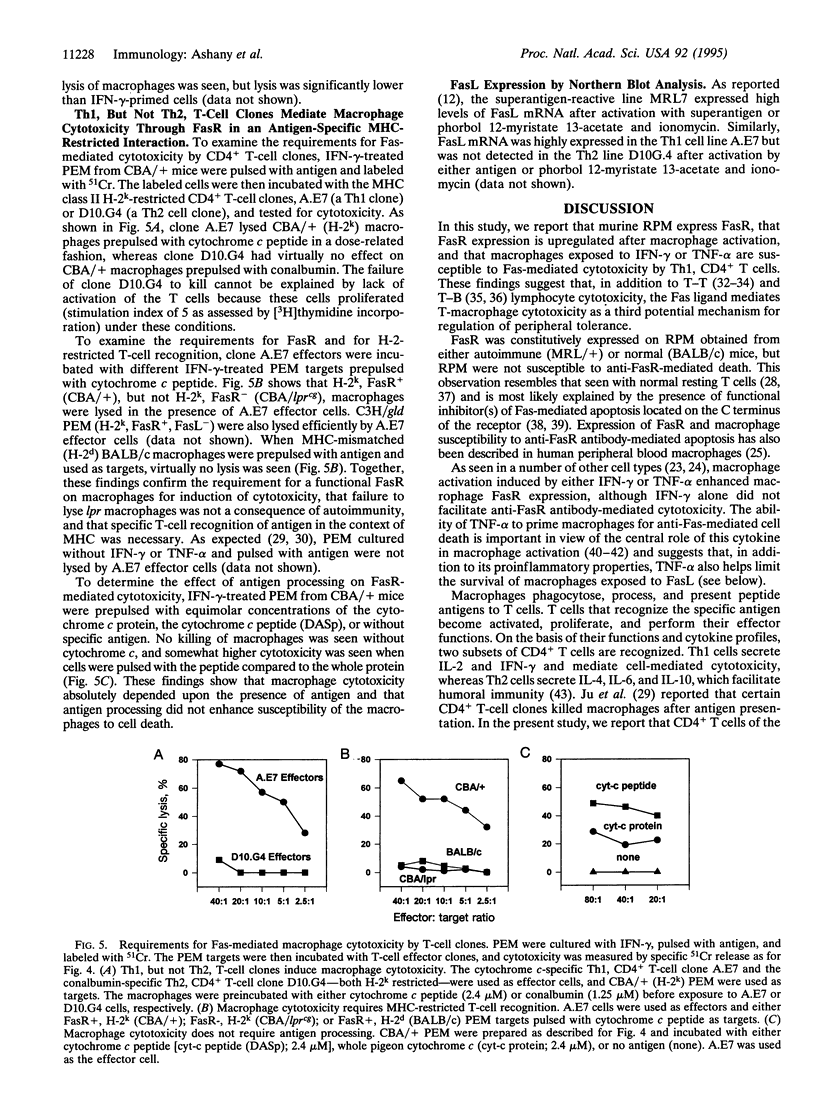
These references are in PubMed. This may not be the complete list of references from this article.
- Andjelić S., Drappa J., Lacy E., Elkon K. B., Nikolić-Zugić J. The onset of Fas expression parallels the acquisition of CD8 and CD4 in fetal and adult alpha beta thymocytes. Int Immunol. 1994 Jan;6(1):73–79. doi: 10.1093/intimm/6.1.73. [DOI] [PubMed] [Google Scholar]
- Beverly B., Kang S. M., Lenardo M. J., Schwartz R. H. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992 Jun;4(6):661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- Brunner T., Mogil R. J., LaFace D., Yoo N. J., Mahboubi A., Echeverri F., Martin S. J., Force W. R., Lynch D. H., Ware C. F. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995 Feb 2;373(6513):441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Lane W. S., Gorga J. C., Stern L. J., Vignali D. A., Strominger J. L. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992 Aug 27;358(6389):764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- Chu J. L., Ramos P., Rosendorff A., Nikolić-Zugić J., Lacy E., Matsuzawa A., Elkon K. B. Massive upregulation of the Fas ligand in lpr and gld mice: implications for Fas regulation and the graft-versus-host disease-like wasting syndrome. J Exp Med. 1995 Jan 1;181(1):393–398. doi: 10.1084/jem.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Dang-Vu A. P., Pisetsky D. S., Weinberg J. B. Functional alterations of macrophages in autoimmune MRL-lpr/lpr mice. J Immunol. 1987 Mar 15;138(6):1757–1761. [PubMed] [Google Scholar]
- Davidson W. F., Calkins C., Hügins A., Giese T., Holmes K. L. Cytokine secretion by C3H-lpr and -gld T cells. Hypersecretion of IFN-gamma and tumor necrosis factor-alpha by stimulated CD4+ T cells. J Immunol. 1991 Jun 15;146(12):4138–4148. [PubMed] [Google Scholar]
- Dhein J., Walczak H., Bäumler C., Debatin K. M., Krammer P. H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995 Feb 2;373(6513):438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Drappa J., Brot N., Elkon K. B. The Fas protein is expressed at high levels on CD4+CD8+ thymocytes and activated mature lymphocytes in normal mice but not in the lupus-prone strain, MRL lpr/lpr. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10340–10344. doi: 10.1073/pnas.90.21.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G. J., Rhynhart K., Nadler L. M. Selective induction of B7/BB-1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991 Oct 15;137(2):429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- Gordon S., Clarke S., Greaves D., Doyle A. Molecular immunobiology of macrophages: recent progress. Curr Opin Immunol. 1995 Feb;7(1):24–33. doi: 10.1016/0952-7915(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Hansburg D., Fairwell T., Schwartz R. H., Appella E. The T lymphocyte response to cytochrome c. IV. Distinguishable sites on a peptide antigen which affect antigenic strength and memory. J Immunol. 1983 Jul;131(1):319–324. [PubMed] [Google Scholar]
- Hecht T. T., Longo D. L., Matis L. A. The relationship between immune interferon production and proliferation in antigen-specific, MHC-restricted T cell lines and clones. J Immunol. 1983 Sep;131(3):1049–1055. [PubMed] [Google Scholar]
- Heidenreich S., Weyers M., Gong J. H., Sprenger H., Nain M., Gemsa D. Potentiation of lymphokine-induced macrophage activation by tumor necrosis factor-alpha. J Immunol. 1988 Mar 1;140(5):1511–1518. [PubMed] [Google Scholar]
- Itoh N., Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993 May 25;268(15):10932–10937. [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Ju S. T., Cui H., Panka D. J., Ettinger R., Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4185–4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S. T., DeKruyff R. H., Dorf M. E. Inducer T-cell-mediated killing of antigen-presenting cells. Cell Immunol. 1986 Sep;101(2):613–624. doi: 10.1016/0008-8749(86)90171-1. [DOI] [PubMed] [Google Scholar]
- Ju S. T., Panka D. J., Cui H., Ettinger R., el-Khatib M., Sherr D. H., Stanger B. Z., Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995 Feb 2;373(6513):444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Kaye J., Janeway C. A., Jr The Fab fragment of a directly activating monoclonal antibody that precipitates a disulfide-linked heterodimer from a helper T cell clone blocks activation by either allogeneic Ia or antigen and self-Ia. J Exp Med. 1984 May 1;159(5):1397–1412. doi: 10.1084/jem.159.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klas C., Debatin K. M., Jonker R. R., Krammer P. H. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993 Jun;5(6):625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- Kägi D., Vignaux F., Ledermann B., Bürki K., Depraetere V., Nagata S., Hengartner H., Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994 Jul 22;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., O'Fallon S., Somoza C., Phillips J. H., Linsley P. S., Okumura K., Ito D., Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995 Jan 1;154(1):97–105. [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990 Dec 15;145(12):4306–4310. [PubMed] [Google Scholar]
- Lowin B., Hahne M., Mattmann C., Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994 Aug 25;370(6491):650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A., Moriyama T., Kaneko T., Tanaka M., Kimura M., Ikeda H., Katagiri T. A new allele of the lpr locus, lprcg, that complements the gld gene in induction of lymphadenopathy in the mouse. J Exp Med. 1990 Feb 1;171(2):519–531. doi: 10.1084/jem.171.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nagata S., Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995 Jan;16(1):39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Ogasawara J., Suda T., Nagata S. Selective apoptosis of CD4+CD8+ thymocytes by the anti-Fas antibody. J Exp Med. 1995 Feb 1;181(2):485–491. doi: 10.1084/jem.181.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Yonehara S., Crump W. L., 3rd, Grimm E. A. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992 Mar;140(1):197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- Richardson B. C., Buckmaster T., Keren D. F., Johnson K. J. Evidence that macrophages are programmed to die after activating autologous, cloned, antigen-specific, CD4+ T cells. Eur J Immunol. 1993 Jul;23(7):1450–1455. doi: 10.1002/eji.1830230708. [DOI] [PubMed] [Google Scholar]
- Richardson B. C., Lalwani N. D., Johnson K. J., Marks R. M. Fas ligation triggers apoptosis in macrophages but not endothelial cells. Eur J Immunol. 1994 Nov;24(11):2640–2645. doi: 10.1002/eji.1830241111. [DOI] [PubMed] [Google Scholar]
- Rothstein T. L., Wang J. K., Panka D. J., Foote L. C., Wang Z., Stanger B., Cui H., Ju S. T., Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995 Mar 9;374(6518):163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- Sander B., Höidén I., Andersson U., Möller E., Abrams J. S. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. Cytokine detection by immunoassay and intracellular immunostaining. J Immunol Methods. 1993 Dec 3;166(2):201–214. doi: 10.1016/0022-1759(93)90361-a. [DOI] [PubMed] [Google Scholar]
- Sato T., Irie S., Kitada S., Reed J. C. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995 Apr 21;268(5209):411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- Schon-Hegrad M. A., Holt P. G. Improved method for the isolation of purified mouse peritoneal macrophages. J Immunol Methods. 1981;43(2):169–173. doi: 10.1016/0022-1759(81)90020-x. [DOI] [PubMed] [Google Scholar]
- Stalder T., Hahn S., Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J Immunol. 1994 Feb 1;152(3):1127–1133. [PubMed] [Google Scholar]
- Suda T., Okazaki T., Naito Y., Yokota T., Arai N., Ozaki S., Nakao K., Nagata S. Expression of the Fas ligand in cells of T cell lineage. J Immunol. 1995 Apr 15;154(8):3806–3813. [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Itoh N., Yonehara S., Copeland N. G., Jenkins N. A., Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992 Feb 15;148(4):1274–1279. [PubMed] [Google Scholar]
- Weller M., Frei K., Groscurth P., Krammer P. H., Yonekawa Y., Fontana A. Anti-Fas/APO-1 antibody-mediated apoptosis of cultured human glioma cells. Induction and modulation of sensitivity by cytokines. J Clin Invest. 1994 Sep;94(3):954–964. doi: 10.1172/JCI117462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer T., Jones P. P. Combined effects of tumor necrosis factor-alpha, prostaglandin E2, and corticosterone on induced Ia expression on murine macrophages. J Immunol. 1990 Aug 15;145(4):1167–1175. [PubMed] [Google Scholar]


