Abstract
The prodigious rate at which malaria parasites proliferate during asexual blood-stage replication, midgut sporozoite production, and intrahepatic development creates a substantial requirement for essential nutrients, including fatty acids that likely are necessary for parasite membrane formation. Plasmodium parasites obtain fatty acids either by scavenging from the vertebrate host and mosquito vector or by producing fatty acids de novo via the type two fatty acid biosynthesis pathway (FAS-II). Here, we study the FAS-II pathway in Plasmodium falciparum, the species responsible for the most lethal form of human malaria. Using antibodies, we find that the FAS-II enzyme FabI is expressed in mosquito midgut oocysts and sporozoites as well as liver-stage parasites but not during the blood stages. As expected, FabI colocalizes with the apicoplast-targeted acyl carrier protein, indicating that FabI functions in the apicoplast. We further analyze the FAS-II pathway in Plasmodium falciparum by assessing the functional consequences of deleting fabI and fabB/F. Targeted deletion or disruption of these genes in P. falciparum did not affect asexual blood-stage replication or the generation of midgut oocysts; however, subsequent sporozoite development was abolished. We conclude that the P. falciparum FAS-II pathway is essential for sporozoite development within the midgut oocyst. These findings reveal an important distinction from the rodent Plasmodium parasites P. berghei and P. yoelii, where the FAS-II pathway is known to be required for normal parasite progression through the liver stage but is not required for oocyst development in the Anopheles mosquito midgut.
INTRODUCTION
Plasmodium falciparum undergoes multiple developmental changes as it cycles between its human host and its Anopheles mosquito vector. Infection of the mosquito begins with the ingestion of male and female gametocytes during a blood meal from a parasitized human host. Fertilization within the mosquito midgut yields invasive ookinetes that traverse the midgut epithelium and then transform into oocysts (1). This triggers sporogony, a process that produces thousands of midgut sporozoites within ∼10 to 14 days. Mature sporozoites released from the mature oocyst migrate to the salivary glands, rendering the mosquito infectious to humans (1). During a blood meal, these sporozoites are injected into the human host and migrate to the liver, where they traverse the endothelial lining to invade a human hepatocyte. Liver-stage parasites rapidly replicate to form tens of thousands of exoerythrocytic merozoites, which are released as merosomes into the bloodstream after 5 to 7 days (2–4). This event initiates asexual blood-stage development, which is responsible for all clinical manifestations. The rapid expansion of the parasite biomass during these replicative stages of the life cycle requires many nutrients, among which are fatty acids, the precursors of phospholipids necessary for parasite membrane formation. Plasmodium parasites, like other apicomplexan parasites, obtain fatty acids either by scavenging them from the host and vector (5) or by producing them de novo via the type two fatty acid synthesis (FAS-II) pathway (6). This pathway, also present in bacteria, Mycobacterium spp., plants, and other parasite pathogens, including Toxoplasma gondii, catalyzes rounds of fatty acid elongation through the action of four key enzymes, FabB/F, FabG, FabZ, and FabI (Fig. 1) (7). This process differs from the mammalian type I pathway that incorporates all enzymatic functionalities on a single large polypeptide. In Plasmodium, the FAS-II pathway is localized to the parasite apicoplast, a secondary plastid of algal origin (8–10).
FIG 1.
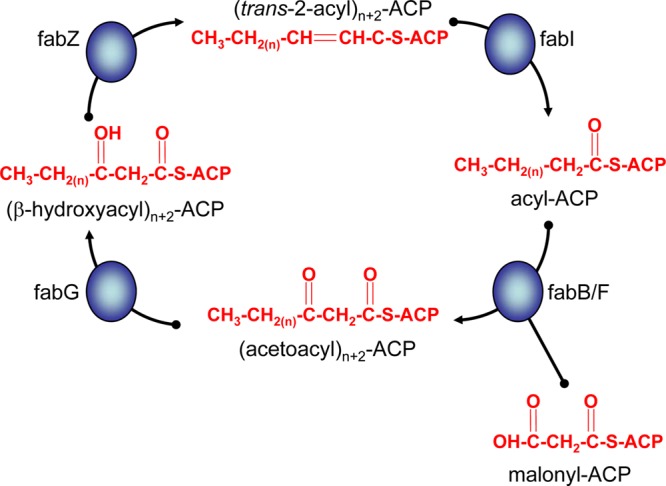
Fatty acid elongation in the Plasmodium apicoplast. Initiation of fatty acid synthesis leads to the formation of the four-carbon butyryl-acyl carrier protein (ACP). The chain length of this acyl-ACP is extended by a further two carbons through the condensation of the acyl-ACP with malonyl-ACP. The reaction is catalyzed by β-ketoacyl-ACP synthase II (fabB/F) and leads to the formation of acetoacyl-ACP (n + 2 denotes the addition of a further two carbons to the chain length of the elongating fatty acid). Acetoacyl-ACP is reduced to β-hydroxyacyl-ACP by β-ketoacyl-ACP reductase (fabG), dehydrated to trans-2-acyl-ACP by the action of β-hydroxyacyl-ACP dehydratase (fabZ), and finally reduced to acyl-ACP by enoyl-ACP reductase (fabI). Successive cycles of this elongation process lead to the formation of C14-ACP.
Research into the requirement of FAS-II for parasite survival using reverse genetics in two rodent models of malaria (P. yoelii and P. berghei), as well as in P. falciparum, have demonstrated that FAS-II is not required for asexual blood-stage replication (11–14). These discoveries reversed the long-held dogma that FAS-II was required for blood stages and, by extension, was an excellent drug target for malaria control due to the absence of FAS-II in the human host (15). Nevertheless, pathways of Plasmodium lipid metabolism remain an attractive target for malaria treatment (16, 17). Studies on rodent models of malaria demonstrated that FAS-II was only required for liver-stage development. In the case of P. yoelii infection in BALB/c mice, the requirement was absolute. Liver-stage parasites lacking FabB/F did not complete their development and did not release merozoites into the bloodstream (13). On the other hand, P. berghei liver-stage parasites lacking FabI in C57BL/6 mice or FabB/F in either BALB/c or C57BL/6 mice were severely but incompletely attenuated, resulting in breakthrough infection that developed into normal blood-stage growth (11, 14). Furthermore, deletion of P. yoelii genes coding for the apicoplast-targeted pyruvate dehydrogenase complex (PDH) (18), which is essential for the creation of acetyl-coenzyme A (CoA), a precursor of FAS-II, as well as deletion of the gene coding for P. berghei apicoplast-targeted octanoyl transferase (19), which is necessary for the lipoylation of the PDH E2 subunit, led to late liver-stage arrest (19, 20). Thus, studies on models of rodent malaria have shown that the deletion of genes involved in all aspects of FAS-II cause growth arrest during liver-stage development.
FAS-II genes are highly syntenic between rodent and human Plasmodium spp., allowing the identification of all FAS-II genes in P. falciparum and the prediction that the FAS-II enzymes share a function in the parasite life cycle. Genetically attenuated P. falciparum parasites that fail to complete liver-stage development would be attractive whole-parasite vaccines (21, 22). Because P. falciparum parasites lacking FabI showed no phenotypic change during blood-stage replication (13, 14), we expected to observe attenuation at the liver stage, as had been observed in the rodent malaria models. To test this hypothesis, we engineered P. falciparum parasites lacking functional FabI (PfΔfabI) or FabB/F (PfΔfabB/F). The results of this analysis are provided below.
MATERIALS AND METHODS
Parasite culture.
P. falciparum line NF54 (wild type [WT]) and mutant PfΔfabB/F and PfΔfabI cloned parasites (see below) were used for analysis. PfΔfabB/F blood stages were cultured in a semiautomated culture system using standard in vitro culture conditions for P. falciparum, and induction of gametocyte production in these cultures was performed as previously described (23, 24). PfΔfabI B1 and PfΔfabI C10 gametocytes were induced as described previously (25). In vitro PfΔfabI E6 and reference NF54 blood-stage cultures were maintained in RPMI 1640 (25 mM HEPES, 2 mM l-glutamine) supplemented with 50 μM hypoxanthine and 10% A+ human serum in an atmosphere of 5% CO2, 5% O2, and 90% N2. Cells were subcultured into O+ erythrocytes. Gametocyte cultures were initiated at 5% hematocrit and 0.8 to 1% parasitemia (mixed stages) and maintained for up to 17 days with daily medium changes. Fresh human red blood cells were obtained from the Dutch National Blood Bank (Sanquin, Nijmegen, the Netherlands; permission was granted by donors for the use of blood products for malaria research) or from the Interstate Blood Bank (Memphis, TN).
Generation of PfΔfabB/F parasites.
We deleted the P. falciparum fabB/F gene (PFF 1275c; PF3D7_0626300) from chromosome six of NF54 parasites (see Fig. S1 in the supplemental material) using a modified construct based on plasmid pHHT-FRT-green fluorescent protein (GFP)-Pf52 (26). Targeting regions were generated by PCR using primers P9 and P10 for the 5′ target region and primers P11 and P12 for the 3′ target region (see Table S1). The 5′ and 3′ target regions were cloned into pHHT-FRT-GFP-Pf52 digested with BssHII plus BsiWI and XmaI plus NheI, respectively, resulting in the plasmid pHHT-FRT-GFP-Pf-fabB/F. All DNA fragments were amplified by PCR amplification (Phusion; Finnzymes) from P. falciparum NF54 genomic DNA (27), and all PCR fragments were sequenced after TOPO TA (Invitrogen) subcloning. Transfection and selection of mutant parasites were performed as described previously (26), resulting in the selection of two parasite lines, PfΔfabB/F 1 and PfΔfabB/F 2. Cloning of these transgenic parasites was performed as described previously (28). The parasite line PfΔfabB/F 1 was subsequently transfected with pMV-FLPe to remove the drug resistance selectable marker cassette using FLPe recombinase as described previously (26), resulting in the cloned parasite PfΔfabB/F 1 *FLPe. Genotype analysis of the PfΔfabB/F clones was performed by an Expand long-range dNTPack (Roche) diagnostic PCR (LR-PCR) and Southern blot analysis (29). Genomic DNA of blood stages from WT or PfΔfabB/F parasites was isolated and analyzed by LR-PCR using primer pair P7 and P8 (see Table S1) for correct integration of constructs in the P. falciparum fabB/F locus by double-crossover integration. The LR-PCR program used an elongation step of 62°C (30) for 10 min and an annealing step of 48°C for 30 s. For Southern blot analysis (29), genomic DNA was digested with EcoRI and the DNA hybridized to a radioactive probe consisting of the 3′ target region for the P. falciparum fabB/F gene deletion (described above).
Generation of PfΔfabI parasites.
To create single-crossover mutant parasites in the endogenous P. falciparum fabI locus (PFF 0730c; PF3D7_0615100), we created the pcam-bsd-PfΔfabI plasmid, comprising the blasticidin S-deaminase (bsd) selectable marker and a 0.6-kb P. falciparum fabI fragment. This fragment, which corresponds to amino acids 98 to 295 of the encoded P. falciparum fabI sequence, was PCR amplified using primers p5 and p6 (see Table S1 in the supplemental material) and the sequence verified. This insert was subcloned into the pcam-bsd plasmid (31) using NotI and BamHI sites (see Fig. S2A in the supplemental material). Transfected NF54 parasites were selected using 2.5 μg/ml blasticidin hydrochloride as described previously (32). Integration of the plasmid by homologous recombination and single-site crossover was detected by PCR on day 119 posttransfection (see Fig. S2B) and was confirmed by Southern blotting hybridization (see Fig. S2C). The recombinant clones PfΔfabI B1 and PfΔfabI C10 were obtained by limiting dilution (33). The generation of the PfΔfabI parasite clone E6 by double-crossover homologous recombination has been described previously (13). Briefly, the PfΔfabI E6 parasite lacks the complete fabI gene. The resulting fabI genomic location harbored the drug-selectable human dhfr marker, resulting in resistance to the Plasmodium-specific dihydrofolate reductase (DHFR) inhibitor WR99210.
Analysis of gametocyte production.
Gametocyte cultures were quantified on days 8 and 12 to 14 postinduction by counting the number of stage II and mature (stage IV and V) gametocytes, respectively, in Giemsa-stained thin blood films (24). Exflagellation of male gametocytes was determined in small samples from the cultures by stimulating the gametocytes in fetal calf serum (pH 8.0) at room temperature for 10 to 20 min. Exflagellation centers were counted under the light microscope in 10 to 25 homogeneous fields of red blood cells at ×400 magnification as described previously (34).
P. falciparum sporozoite production and analysis.
Membrane feeding assays were performed as described previously (34). Briefly, 14-day cultures from NF54 or PfΔfabB/F (1 and 2) or PfΔfabI (B1 and C10) gametocytes were fed to female Anopheles stephensi mosquitoes. From day 7 onwards, the mosquitoes were dissected and examined for midgut oocysts as described previously (34, 35). The statistical analysis of oocyst production was performed with the nonparametric Wilcoxon rank-sum test. On days 14 to 19 postfeeding, the mosquitoes were dissected and analyzed for the presence of salivary gland sporozoites as described previously (36). On days 9 to 30 postfeeding, the mosquitoes were dissected and mounted in phosphate-buffered saline (PBS) using cover slides. The midguts were analyzed on a fluorescence microscope (Leica DMR with a DC300F camera) at ×630 magnification. For generating sporozoites from PfΔfabI E6, A. stephensi mosquitoes (originating from the Walter Reed Army Institute of Research) were maintained at 27°C and 75% humidity on a 12-h light/dark cycle. Larval stages were reared by following standard protocols as described in the MR4 manual and maintained on finely ground Tetramin fish food. Adult mosquitoes were maintained on 8% dextrose in water containing 0.05% para-aminobenzoic acid (PABA). Non-blood-fed adult female mosquitoes were fed on gametocyte cultures 3 to 7 days postemergence. Gametocyte cultures were quickly spun down and the pelleted infected erythrocytes diluted to a 40% hematocrit with fresh A+ human serum and O+ erythrocytes. Mosquitoes were allowed to feed through parafilm for up to 20 min. Following blood feeding, mosquitoes were maintained for up to 19 days at 27°C at 75% humidity and provided with 8% dextrose solution in PABA water. Infection prevalence was checked at days 7 to 10 by examining dissected midguts under light microscopy for the presence of oocysts with salivary gland dissections performed at days 14 to 19.
Immunofluorescence assay of P. falciparum FabI in midgut oocysts and sporozoites.
Mosquito midguts were isolated and fixed with 4% (vol/vol) paraformaldehyde (PFA) in 1× PBS for 1 h at room temperature in a microcentrifuge tube. Midguts were washed with 1× PBS and then permeabilized and blocked with 1× PBS containing 3% bovine serum albumin (BSA) and 0.25% Triton X-100 for 2 h at room temperature. Midguts were washed with 1× PBS, and primary antibodies were diluted in 1× PBS plus 3% BSA and incubated with the midguts at 4°C overnight with end-over-end rotation. Following three washes with 1× PBS, fluorescent secondary antibodies were diluted with 1× PBS plus 3% BSA and incubated with midguts for 2 h with end-over-end rotation at room temperature. Midguts were washed three times with 1× PBS, stained with 4′,6-diamidino-2-phenylindole (DAPI) in 1× PBS for 5 to 10 min at room temperature, and washed once more with 1× PBS. Midguts were mounted on a polylysine-coated microscope slide and overlaid with ProLong antifade reagent (Molecular Probes, CA) and a coverslip.
Sporozoites were isolated and fixed with 4% (vol/vol) PFA in 1× PBS for 40 min at room temperature. Fixed sporozoites were applied to a polylysine-treated microscope slide and washed twice with 1× PBS and then permeabilized and blocked with 3% (wt/vol) BSA plus 0.2% (vol/vol) Triton X-100 in 1× PBS for 1 h at room temperature. Immunofluorescence assays (IFAs) were performed essentially as described for mosquito stages; however, the microscope slide was housed in a wet chamber at 4°C overnight for the primary antibody incubation, and washes were applied directly to the slide. Fluorescence analysis used an inverted microscope (Eclipse TE2000-E; Nikon), and images were acquired using an Olympus IX70 Delta Vision deconvolution microscope.
Primary antibodies used were a mouse monoclonal antibody to the P. yoelii acyl carrier protein (ACP; PYYM_0306500) and a rabbit polyclonal antibody to P. falciparum FabI. Recombinant P. yoelii ACP lacking the bipartite leader sequence and containing a poly-His N-terminal epitope tag was expressed in the E. coli strain BL21(DE3) transfected with a pET plasmid containing codon-optimized cDNA sequence for P. yoelii ACP (resulting in the expression of amino acids 61 to 142). Recombinant protein was purified and used for monoclonal antibody production after mouse immunization. Mouse monoclonal antibody was diluted to 2 μg/ml for IFAs. Recombinant P. falciparum FabI lacking the bipartite leader sequence and containing a poly-His N-terminal epitope tag was expressed in the E. coli strain BL21(DE3) transfected with a pET plasmid containing codon-optimized cDNA sequence for P. falciparum FabI (resulting in the expression of amino acids 77 to 432). Recombinant protein was purified and used for polyclonal antibody production in rabbits. Rabbit serum was diluted 1:500 for IFAs.
Immunofluorescence analysis of FabI in P. falciparum liver stages in FRG huHep mice.
Animal studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (37). Seattle Biomedical Research Institute has an OLAW Animal Welfare Assurance (A3640-01), and animal studies were carried out in compliance with the guidelines of their Institutional Animal Care and Use Committee. FRG huHep mice (38) were injected intravenously with approximately 3 × 106 sporozoites, and livers were harvested from euthanized mice at 3, 5, and 7 days postinfection. Livers were perfused with 1× PBS and fixed in 4% (vol/vol) PFA in 1× PBS, and lobes were cut into 50-μm sections using a Vibratome apparatus (Ted Pella Inc., Redding, CA). For IFAs, sections were permeabilized in 1× Tris-buffered saline (TBS) containing 3% (vol/vol) H2O2 and 0.25% (vol/vol) Triton X-100 for 30 min at room temperature. Sections were then blocked in 1× TBS containing 5% (vol/vol) dried milk (TBS-M) for at least 1 h and incubated with primary antibody in TBS-M at 4°C overnight. After washing in 1× TBS, fluorescent secondary antibodies were added in TBS-M for 2 h at room temperature in a manner similar to that described above. After further washing, the section was incubated in 0.06% (wt/vol) KMnO4 for 2 min to quench background fluorescence. The section was then washed with 1× TBS and stained with 1 μg/ml DAPI in 1× TBS for 5 to 10 min at room temperature to visualize DNA and mounted with ProLong antifade reagent (Molecular Probes, CA). Primary antibodies were diluted in TBS-M as described above.
RESULTS
P. falciparum FAS-II enzymes are expressed in the midgut oocyst.
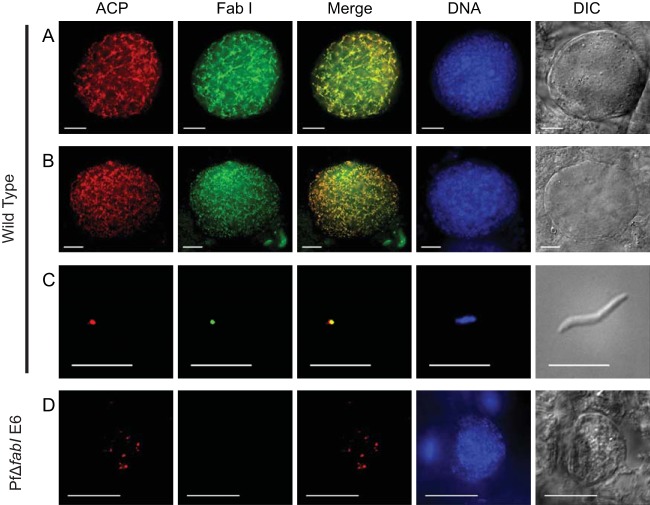
In P. yoelii, FAS-II enzyme transcription was found to be upregulated in liver stages compared to blood stages (13), and FAS-II proteins were also identified in the proteome of P. yoelii liver stages (39). In P. falciparum a detailed analysis of the expression of FAS-II enzymes has been lacking. Thus, we examined FAS-II expression in P. falciparum transcriptome and proteome data sets. During asexual blood-stage development, fabB/F, fabG, and fabZ transcription increased in mature trophozoites and schizonts, while transcription of fabI was low throughout asexual development (40). During the sexual stages, RNA sequence (RNA-Seq) data revealed that fabI, fabZ, and fabG transcription was increased in the gametocyte stage and was present in ookinetes, whereas fabB/F was present in gametocytes but not in ookinetes (41). Only FabI and FabG were identified in the gametocyte proteome (42), and only FabG was identified in oocyst-derived sporozoites and salivary gland-derived sporozoites (36). To test whether FabI was expressed during oocyst development in the apicoplast, we performed IFAs on developing oocysts 9 days after an infectious blood meal, on mature oocysts on day twelve, and on midgut sporozoites. Figure 2A shows that apicoplast-targeted FabI was strongly expressed in the day 9 oocyst and was colocalized with the known apicoplast marker, ACP. The extremely branched structure of the apicoplast at this time point is in agreement with apicoplast-targeted fluorophore expression in the rodent malaria P. berghei oocyst (43). The colocalized expression of FabI and ACP was punctate in the mature day 12 oocyst (Fig. 2B), at a time when individual sporozoites are being formed and the organelles are segregating into each individual sporozoite. Expression of both FabI and ACP was also apparent in the fully mature midgut oocyst sporozoite (Fig. 2C). FabI and ACP expression were not detected in the salivary gland sporozoite by IFAs, suggesting that the FAS-II pathway operates at a much lower level and may not be necessary for parasite survival at this stage.
FIG 2.
Apicoplast-targeted expression of P. falciparum FabI during oocyst development. Expression of FabI in the developing oocyst was analyzed by parasite IFA. (A) At 9 days after a wild-type infectious blood meal, FabI was clearly expressed and was colocalized with the known apicoplast-targeted lumen protein acyl carrier protein (ACP). The apicoplast is highly branched at this stage of development, as evident from the expression pattern. (B) Midgut oocyst maturation at 12 days was accompanied by the colocalized expression of FabI with ACP, and individual apicoplast entities were apparent in the mature sporoblast. (C) Isolated midgut oocyst sporozoites showed FabI and ACP colocalization that abutted the nucleus, which is typical of the apicoplast in this life cycle stage. DNA was stained with DAPI. (D) PfΔfabI oocysts at day 12 after the blood feed confirmed the absence of FabI expression. Apicoplast development, based on acyl carrier protein (ACP) expression, did not show the highly branched organellar structure seen in wild-type parasite oocysts at the same time point, and the oocysts were also smaller (compare to panel B). Scale bar, 10 μm. DIC, differential interference contrast.
Deletion of the P. falciparum fabB/F gene abolishes sporozoite production in mosquitoes.
P. falciparum FabB/F is a key enzyme in the FAS-II cycle (Fig. 1) and is critical in determining the length of the elongating fatty acid. To examine the function of this FAS-II enzyme, we generated PfΔfabB/F gene deletion mutants using a double-crossover gene deletion method that allowed subsequent removal of the resistance markers fused to GFP following a second round of FLP-mediated sequence excision (26). The schematics of the gene deletion are depicted in Fig. S1 in the supplemental material. Two independent mutants were generated (PfΔfabB/F 1 and PfΔfabB/F 2) which contain the human DHFR selectable marker fused to GFP in the place of the fabB/F gene. This fusion was expressed under the control of the calmodulin promoter in order to identify recombinant parasites through GFP fluorescence, and the DHFR-GFP expression cassette was flanked by FLP recombination target (FRT) sites. In a subsequent round of transfection, the complete resistance marker cassette from the mutant PfΔfabB/F 1 clone was removed using the FRT/FLPe recombinase system (26) that selected for recombination between the FRT sites. This event excised the DHFR-GFP cassette and controlled for the influence of GFP expression on the knockout (KO) clone. This procedure resulted in FabB/F-deficient parasites (see Fig. S1A). Correct integration of the targeting construct pHHT-FRT-GFP-fabB/F (p-fabB/F), as well as removal of the resistance marker cassette using FLPe, was confirmed using long-range PCR (see Fig. S1B) and Southern blot analysis (see Fig. S1C). Asexual blood-stage development of all PfΔfabB/F mutants was comparable to that of WT parasites (data not shown).
We observed no difference in development of either stage II or stage IV to V gametocytes in PfΔfabB/F 1 and PfΔfabB/F 2 mutant parasites compared to the WT parent line (Table 1 and data not shown). Furthermore, parasites from day 14 gametocyte cultures of PfΔfabB/F 1 and PfΔfabB/F 2 were able to exflagellate, and these were fed to A. stephensi mosquitoes. Both clones produced high numbers of oocysts with no significant difference between PfΔfabB/F clones and WT parasites. Additionally, all of the fed mosquitoes were infected in these experiments (Table 1). We next determined whether sporozoites developed inside PfΔfabB/F oocysts sampled 14 to 17 days after mosquito infection. Despite the high oocyst burdens, no PfΔfabB/F midgut sporozoites were observed in a total of over 92 oocysts. In contrast, the WT strain produced 3,000 ± 1,000 (means ± standard deviations [SD]) midgut sporozoites per oocyst analyzed (data not shown). We also observed no salivary gland sporozoites from any of the ΔfabB/F clones (ascertained from a total of ∼150 mosquitoes), in contrast to 106,000 ± 13,000 (means ± SD) salivary gland sporozoites obtained per mosquito infected with WT parasites (Table 1). Similar results, i.e., an absence of salivary gland sporozoites, were obtained with the PfΔfabB/F 1*FLPe clone (Table 1) where the hdhfr-gfp cassette had been deleted, indicating that this loss of sporozoite production was not caused by expression of the DHFR resistance marker-GFP fusion protein.
TABLE 1.
P. falciparum ΔfabB/F and ΔfabI parasites produce infectious gametocytes and oocysts but fail to produce sporozoitese
| Parasite line | No. (range) of stage II gametocytes | No. (range) of stage II gametocytes per 1,000 RBC | Exflagellationa | Oocyst productionb (means ± SD) | No. infected/no. dissected mosquitoes | % Infected mosquitoes | No. of salivary gland sporozoites per mosquitoc (means ± SD) |
|---|---|---|---|---|---|---|---|
| Wild type | 6 (2–9) | 37 (28–44) | +++ | 30 ± 16.2 | 20/20 | 100 | 106,000 ± 13,000 |
| PfΔfabB/F 1 (DXO) | 8 (6–11) | 45 (36–66) | +++ | 28 ± 9.2 | 20/20 | 100 | 0 |
| PfΔfabB/F 1 *FLPe | 2 | 23 | ++ | 23 ± 19.3 | 10/10 | 100 | 0 |
| PfΔfabB/F 2 (DXO) | 7 (5–9) | 40 (31–58) | +++ | 41 ± 19.6 | 20/20 | 100 | 0 |
| Wild type | ND | ND | +++ | 43.7 ± 35.9 | 24/24 | 100 | 60,348 |
| PfΔfabI C10 (SXO) | ND | ND | + | ND | ND | ND | 547d |
| PfΔfabI B1 (SXO) | ND | ND | + | ND | ND | ND | 841d |
| Wild type | ND | ND | +++ | 35 ± 6 | 18/20 | 90 | 81,000 ± 12,400 |
| PfΔfabI E6 (DXO) | ND | ND | +++ | 29 ± 3 | 18/20 | 90 | 0 |
Exflagellation of male gametocytes was determined in samples by counting exflagellation centers in 10 to 25 homogeneous fields at ×400 magnification. A mean of <2 per field was scored as +, 2 to 10 as ++, and >10 as +++.
These values show the mean ± SD oocyst numbers per midgut on days 7 to 10 after blood feeding. No significant difference was seen between the mutant and wild-type parasites (P > 0.05 in Wilcoxon matched pairs tests).
Salivary gland sporozoite production was analyzed on days 14 to 19 after blood feed. No sporozoites were ever observed in PfΔfabB/F or PfΔfabI E6 KO parasites that had employed double-crossover events to delete the target gene.
The very low numbers of sporozoites observed in the PfΔfabI C10 and B1 gene disruption clones (∼1% of the wild type) is likely a result of rare intralocus recombination events that can restore the original wild-type locus in parasites engineered using single-crossover strategies.
RBC, red blood cells; ND, not determined; SXO, single crossover recombination; DXO, double crossover recombination.
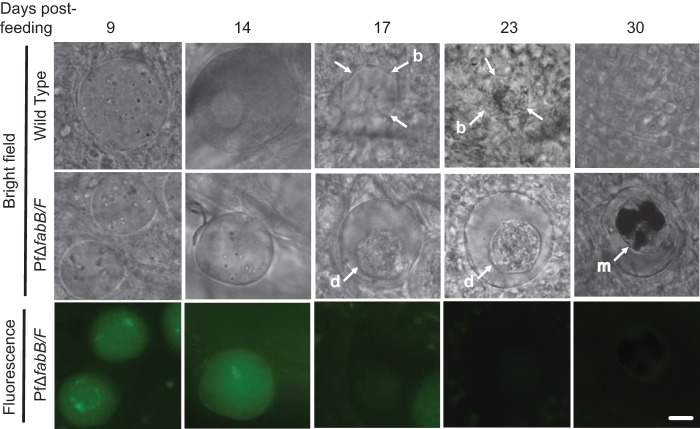
We next performed light and fluorescence microscopy to analyze PfΔfabB/F 1 oocysts at different stages after mosquito infection (Fig. 3). At day nine postfeeding, PfΔfabB/F oocysts were observed to be morphologically similar to WT oocysts, and GFP was expressed. At day 14 postfeeding, the formation of oocyst sporozoites during sporogony was seen in WT oocysts, as evidenced by large numbers of long, slender forms visible at high magnification within the oocyst (Fig. 3). With PfΔfabB/F mosquito infections, oocysts appeared smaller than their WT counterparts at day 14, and sporozoites were not observed at any of the time points analyzed postfeeding. Moreover, from day 17 to day 23 postfeeding, the PfΔfabB/F oocysts appeared to degenerate (Fig. 3, d) within the oocyst wall, and GFP expression was barely observable. At these same time points, WT oocysts had ruptured, and blisters (Fig. 3, b) remained visible on the epithelium of the mosquito midgut. These blisters were not observed in mosquitoes infected with PfΔfabB/F parasites. By day 30 postfeeding, no evidence for WT oocyst existence could be found, but the PfΔfabB/F oocysts had continued to degenerate and became melanized (Fig. 3, m) forms that were essentially GFP negative. These analyses showed that PfΔfabB/F oocysts had formed but were incapable of producing midgut sporozoites, resulting in subsequent oocyst degeneration and melanization by the mosquito immune system.
FIG 3.
Oocysts from P. falciparum ΔfabB/F parasites do not show sporozoite formation, do degenerate, and are melanized. Light and fluorescence microscopy images of oocysts from WT and PfΔfabB/F 1 parasites at increasing time points from day 9 to day 30 after feeding of A. stephensi mosquitoes. The mosquito midguts were dissected and mounted for light microscopy (bright field) and fluorescence microscopy to analyze GFP expression (fluorescence), as the PfΔfabB/F 1 parasite contains the hdhfr-gfp resistance marker. b, blister formation after bursting of oocysts; d, degenerating oocyst within the oocyst wall; m, melanization. Scale bar, 10 μm.
Gene disruption of P. falciparum fabI severely attenuates salivary gland sporozoite production.
In a separate experimental approach, we examined the function of P. falciparum fabI by generating gene disruption mutants using a single-crossover recombination approach (see Fig. S2A in the supplemental material) in NF54 parasites (44). Two clones were isolated, and correct integration of the targeting construct in these clones was confirmed using PCR (see Fig. S2B) and Southern blot analysis (see Fig. S2C). Asexual blood-stage development of both P. falciparum ΔfabI mutant clones was comparable to that of WT parasites (data not shown). When we initiated gametocyte cultures, we observed that the PfΔfabI single-crossover recombination clones C10 and B1 generated fewer stage V gametocytes (0.36% and 0.15%, respectively) than WT parasites, which produced 1.5% gametocytes on average. The morphology of the PfΔfabI gametocytes was identical to that of WT gametocytes and male gametocytes were able to exflagellate, suggesting that PfΔfabI single-crossover recombination did not prevent gamete formation. Based on our findings with the fabB/F-deleted parasites, we surmise that this partial loss of gametocyte production was a consequence of long-term culturing, as is known to occur quite frequently in P. falciparum cultured blood-stage parasites (45). To assess the transmissibility of our PfΔfabI clones, we fed day 18 gametocyte cultures to A. stephensi mosquitoes and assessed whether our FAS-II gene disruption mutants could produce salivary gland sporozoites. We found an ∼100-fold reduction in the amount of salivary gland sporozoites produced by PfΔfabI mutants compared to the WT (Table 1). Following salivary gland dissection of groups of 25 mosquitoes per parasite, we observed 547 and 841 salivary gland sporozoites with our PfΔfabI C10 and B1 clones, respectively, compared to 60,348 produced by the WT parent. This low level of sporozoite production likely reflects internal recombination events that are known to occur at a low frequency in parasites that have undergone single-crossover integration events as a result of the internal gene fragment duplication. These recombination events can lead to marker excision and reconstitution of the original functional locus (46). We were, however, unable to confirm this because of the low number of sporozoites per mosquito. We can rule out the alternative interpretation that the truncated FabI protein was partially functional despite not being targeted to the apicoplast, because the forward primer (P5; see Table S1 in the supplemental material) used to amplify the fabI homologous fragment contained a stop codon followed by two unrelated base pair insertions to prematurely terminate and change the reading frame of the protein. This design ensured that even if partial translation occurred, it could only produce a nonfunctional peptide.
Gene deletion of P. falciparum fabI abolishes sporozoite production in mosquitoes.
To independently test the requirement of the FAS-II pathway for sporozoite production, we analyzed the PfΔfabI E6 parasite clone (13) that was generated previously by a double-crossover approach that deleted fabI (47). That parasite had not been previously examined for its developmental progression in mosquitoes. This double-crossover-mediated gene deletion mutant (E6) revealed no defect either in gametocyte production or infectivity to mosquitoes. Upon feeding PfΔfabI E6 day 14 gametocyte cultures to A. stephensi mosquitoes, we observed normal levels of infectivity, with oocyst numbers that were comparable to those of WT parasites assayed in parallel (Table 1). At days 14 to 19 postfeeding, we were readily able to isolate sporozoites from the salivary glands of WT-infected mosquitoes. In contrast, mosquitoes fed with PfΔfabI E6 mutant parasites contained no detectable salivary gland sporozoites (Table 1), which is in agreement with the absence of sporozoites in salivary glands of mosquitoes infected with PfΔfabB/F gene deletion parasites. The combined data show that PfΔfabI mutants were infectious to mosquitoes, as determined by the production of oocysts, but could not successfully complete sporogony and form infectious salivary gland sporozoites. We also confirmed that FabI was not expressed in PfΔfabI E6 oocysts, in contrast to its expression in WT parasites (Fig. 2D). Additionally, apicoplast development based on ACP expression in the PfΔfabI E6 KO line did not show the highly branched organellar structures seen in WT parasite oocysts at day 12 of development (Fig. 2B) and were also smaller in size, in agreement with results for the PfΔfabB/F parasite (Fig. 3).
P. falciparum FabI is expressed during parasite liver-stage development.
To date, no expression data have been reported for FAS-II enzymes during P. falciparum liver-stage development. Rodent malaria liver stages require FAS-II for normal development (13, 14), and we hypothesized that P. falciparum liver-stage development also relies on parasite FAS-II. Although we were unable to generate PfΔfabI salivary gland sporozoites to formally test this hypothesis, we examined the expression of both FabI and ACP during the development of WT P. falciparum liver stages in the humanized livers of chimeric FRG huHep mice (38). Interestingly, and in agreement with rodent malaria liver-stage FAS-II expression data, FabI was expressed throughout the liver stage of development and was colocalized with the apicoplast resident protein ACP (Fig. 4). The growth of the liver-stage parasite from day 3 (Fig. 4A) to day 5 (Fig. 4B) was mirrored by an increasingly complex apicoplast structure, as has been shown previously for liver-stage apicoplast development in P. berghei (43). Liver-stage maturation results in the release of thousands of exoerythrocytic merozoites, and Fig. 4C shows a mature liver-stage parasite that contains the nuclei of the forming merozoites, each with its own apicoplast, as apparent by the colocalized expression of FabI and ACP.
FIG 4.
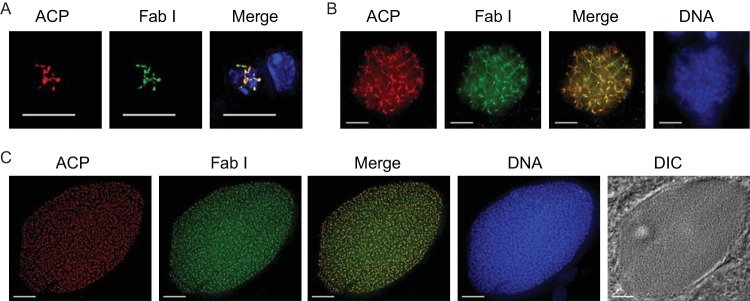
P. falciparum FabI is expressed throughout liver-stage development. Expression of FabI was analyzed in the livers of FRG huHep mice infected with P. falciparum sporozoites by IFA. (A) At 3 days of infection, the branched structure of the apicoplast was apparent and FabI expression colocalized with the apicoplast-targeted lumen protein acyl carrier protein (ACP). (B) At 5 days, the liver-stage parasite had grown in size and complexity, as seen by increased DNA replication and apicoplast structure, and FabI and ACP were expressed. (C) Maturation of the liver-stage parasite at 7 days led to the production of thousands of exoerythrocytic merozoites, each with its own nucleus and apicoplast. Individual nuclei and apicoplasts expressing FabI and ACP were apparent. DNA was stained with DAPI. Scale bar, 10 μm.
DISCUSSION
In this study, we have established that P. falciparum malaria parasites have an absolute requirement for FAS-II for midgut oocyst sporozoite production during the mosquito stages of the parasite life cycle. It has long been known that Plasmodium possesses its own FAS-II pathway, which is of cyanobacterial origin and localizes to the apicoplast (9). Due to the prokaryotic origin of FAS-II and earlier evidence suggesting a deleterious effect of FAS-II inhibition on parasite blood-stage replication, FAS-II was once thought to be a prime target for blood-stage antimalarial therapy (15). However, recent research utilizing gene knockout strategies in P. falciparum, P. yoelii, and P. berghei have clearly demonstrated that FAS-II is not essential for blood-stage replication (13, 14). Nevertheless, the studies on P. falciparum asexual blood-stage replication were carried out in vitro, and there is the distinct possibility that in vivo, the blood-stage parasite requires FAS-II, as has been suggested previously (48). The study presented here shows that unlike the rodent malaria parasites P. berghei and P. yoelii, where FAS-II is only essential for liver-stage development (13, 14), the human malaria parasite P. falciparum requires FAS-II for mosquito-stage oocyst sporozoite production. This finding challenges the assumption that rodent and human malaria parasites utilize similar nutrient acquisition strategies, although it is also possible that our choice of malaria parasite and mosquito vector combination is uncovering phenotypic differences that may not be seen in natural settings.
Although the oocysts increased in size normally in P. falciparum parasites harboring knockouts in both fabI and fabB/F until day 9 of infection, sporogony did not proceed normally. These data imply that P. falciparum is dependent on de novo synthesis of its own fatty acids for complete midgut sporozoite maturation. We saw expression of FabI in the P. falciparum midgut sporozoite but not the salivary gland sporozoite, which contrasts with the expression pattern of P. yoelii FabI, where expression is seen in the salivary gland sporozoite but not the midgut oocyst sporozoite (13). We believe this difference is due to the fact that in P. falciparum, FAS-II is essential for oocyst sporozoite production but not for sporozoite viability and that our IFAs detect remnant expression of the protein. For P. yoelii, however, FAS-II is not necessary for midgut sporozoite production; thus, it is not expressed by the oocyst sporozoite but is necessary for liver-stage development and is present in the salivary gland sporozoite, in preparation for the transition to liver-stage replication. It is also possible that our IFAs were unable to detect a lower level of FabI expression in the P. falciparum salivary gland sporozoite.
Oocyst sporozoite production in a laboratory setting within the A. stephensi mosquito takes place at a higher rate for P. yoelii than for both P. berghei and P. falciparum; however P. yoelii does not rely on FAS-II for oocyst sporozoite production while P. falciparum does. One aspect of midgut oocyst development that differs between rodent malarias and human malarias is the number of sporozoites per mature midgut oocyst. Enumeration of this value is rarely carried out, but using A. dirus as the vector mosquito, it has been shown that the average number of P. falciparum and P. vivax sporozoites per oocyst is estimated at approximately 3,400 and 3,700, respectively (49). In contrast, the rodent malaria oocyst within the midgut of A. stephensi mosquitoes contains approximately 800 P. berghei sporozoites (50) and 1,000 P. yoelii sporozoites (51). Thus, the human malaria oocysts studied contain approximately four times as many sporozoites as their rodent counterparts. An increased number of sporozoites produced in approximately the same length of time would obviously require a significantly greater amount of membrane production. Thus, we propose that P. falciparum relies on FAS-II to produce the large amounts of fatty acids necessary for the phospholipid component of the complex membrane structures found within this life cycle stage. It is interesting that in the case of the PfΔfabB/F oocysts, sporozoite production from the sporoblast was clearly impaired, even though the oocyst appeared to increase in size in the same way as the WT oocyst. Thus, fatty acid synthesis is likely essential for sporoblast development. Conversely, the developing rodent malaria oocyst can complete its sporozoite development without requiring the FAS-II pathway.
An alternative hypothesis is that the P. falciparum sporozoite requires a unique fatty acid that only the parasite is able to create using its own enzymatic machinery. The fatty acid itself could then be incorporated into a unique phospholipid moiety necessary for membrane integrity or as part of the glycosylphosphatidylinositol (GPI) anchor necessary for the membrane attachment of proteins to the parasite plasma membrane. Indeed, the most abundant protein on the sporozoite surface, circumsporozoite protein (CSP), was shown to utilize an essential GPI anchor for its attachment to the parasite plasma membrane (52). This raises the possibility that FAS-II is required simply to produce enough fatty acid for the GPI anchor of CSP and potentially other sporozoite surface proteins.
The FAS-II pathway takes place within the Plasmodium parasite's apicoplast, and to date no studies have demonstrated the fate of the fatty acids produced de novo by FAS-II and if they are even retained within the apicoplast. A further hypothesis is that the fatty acids produced by FAS-II are incorporated into phospholipids required for the expansion of the developing apicoplast within the replicating parasite. This scenario predicts that the sole purpose for FAS-II is to ensure the integrity of the organelle within which the pathway resides. Indeed, fatty acid synthesis has also been studied in the related apicomplexan Toxoplasma gondii, and in this parasite, a conditional null mutant of the FAS-II component acp severely compromised parasite growth in culture and caused defects in apicoplast biogenesis and a consequent loss of the organelle (53). Further studies on T. gondii fatty acid biosynthesis have since shown that both the apicoplast and the endoplasmic reticulum cooperate in the synthesis of very long fatty acids (54). Interestingly, it was recently shown that P. falciparum blood stages could be cultured in vitro without an apicoplast, as long as the growth medium was supplemented with isopentenyl pyrophosphate (IPP) (55). IPP is the product of the apicoplast-targeted nonmevalonate isoprenoid precursor biosynthetic pathway, and targeting of this pathway by drugs is lethal to asexual blood-stage parasites (56). The research by Yeh and DeRisi concluded that isoprenoid precursor biosynthesis is the only essential function of the apicoplast during asexual blood-stage growth (55). It is tempting to hypothesize that if one could supplement fatty acids and IPP in the mosquito feed, then one could create apicoplast-null P. falciparum oocysts.
Our original assumption was that P. falciparum parasites, like their rodent malaria counterparts, would not require FAS-II until late in liver-stage development. Thus, we assumed that P. falciparum parasites lacking FAS-II genes would behave as genetically attenuated parasites (GAPs) that could be tested for their ability to act as experimental vaccines (22), with the caveat that P. berghei FAS-II GAPs are not fully attenuated (14). Because our P. falciparum FAS-II knockout parasites failed to complete sporogony, we were unable to formally test the hypothesis that FAS-II is essential for liver-stage development. An alternative experimental design to test this hypothesis would be to employ a promoter swap strategy, such that the FAS-II gene is expressed in sporozoites but not in late liver stages, for example, using the CSP or TRAP promoter that is upregulated during mosquito stages (1). A variation of this strategy would be to express rapamycin-inducible Di-Cre recipient clones of P. falciparum under early-liver-stage-specific promoters to excise FAS-II genes only during liver-stage development (57). An alternative strategy could utilize regulatable tags, which have successfully been used to demonstrate the essentiality of blood-stage expressed proteins (58, 59) but, to date, not for pre-erythrocytic stages.
Our finding of major phenotypic differences between knockout P. falciparum and rodent Plasmodium parasites adds a degree of caution to the often-stated assumption that rodent malaria parasites behave similarly to human malaria parasites. P. falciparum liver-stage parasites nevertheless still remain an ideal target for both drug and vaccine design, since preventing the transition from liver-stage maturation to blood-stage parasitemia prevents clinical disease. This point was further reiterated from a recent clinical trial involving 40 human volunteers that demonstrated that intravenous injection of irradiated P. falciparum sporozoites conferred protection against malaria (60). Further studies are clearly warranted to identify pathways required exclusively for P. falciparum liver-stage development that would serve to produce robust protection as a prophylactic genetically attenuated vaccine.
Supplementary Material
ACKNOWLEDGMENTS
We thank the following people from RUNMC (Nijmegen) for technical support: Marga Vegte-Bolmer, Rianne Siebelink-Stoter, Wouter Graumans, Jolanda Klaassen, Laura Pelser-Posthumus, Astrid Pouwelsen, and Jacqueline Kuhnen.
The NIH (R56 AI080685 to S.H.I.K. and A.M.V. and R01 AI085584 to D.A.F.), the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (grant no. 1481; to S.H.I.K.), the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and Top Institute Pharma (Netherlands) project T4-102 all provided partial funding for this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 2 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00264-13.
REFERENCES
- 1.Aly AS, Vaughan AM, Kappe SH. 2009. Malaria parasite development in the mosquito and infection of the mammalian host. Annu. Rev. Microbiol. 63:195–221. 10.1146/annurev.micro.091208.073403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graewe S, Rankin KE, Lehmann C, Deschermeier C, Hecht L, Froehlke U, Stanway RR, Heussler V. 2011. Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathog. 7:e1002224. 10.1371/journal.ppat.1002224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindner SE, Miller JL, Kappe SH. 2012. Malaria parasite pre-erythrocytic infection: preparation meets opportunity. Cell. Microbiol. 14:316–324. 10.1111/j.1462-5822.2011.01734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prudencio M, Rodriguez A, Mota MM. 2006. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat. Rev. Microbiol. 4:849–856. 10.1038/nrmicro1529 [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishnan S, Serricchio M, Striepen B, Butikofer P. 2013. Lipid synthesis in protozoan parasites: a comparison between kinetoplastids and apicomplexans. Prog. Lipid Res. 52:488–512. 10.1016/j.plipres.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarun AS, Vaughan AM, Kappe SH. 2009. Redefining the role of de novo fatty acid synthesis in Plasmodium parasites. Trends Parasitol. 25:545–550. 10.1016/j.pt.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 7.Marrakchi H, Zhang YM, Rock CO. 2002. Mechanistic diversity and regulation of type II fatty acid synthesis. Biochem. Soc. Trans. 30:1050–1055 [DOI] [PubMed] [Google Scholar]
- 8.Ralph SA, van Dooren GG, Waller RF, Crawford MJ, Fraunholz MJ, Foth BJ, Tonkin CJ, Roos DS, McFadden GI. 2004. Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Microbiol. 2:203–216. 10.1038/nrmicro843 [DOI] [PubMed] [Google Scholar]
- 9.Waller RF, Keeling PJ, Donald RG, Striepen B, Handman E, Lang-Unnasch N, Cowman AF, Besra GS, Roos DS, McFadden GI. 1998. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 95:12352–12357. 10.1073/pnas.95.21.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dooren GG, Striepen B. 2013. The algal past and parasite present of the apicoplast. Annu. Rev. Microbiol. 67:271–289. 10.1146/annurev-micro-092412-155741 [DOI] [PubMed] [Google Scholar]
- 11.Annoura T, Ploemen IH, van Schaijk BC, Sajid M, Vos MW, van Gemert GJ, Chevalley-Maurel S, Franke-Fayard BM, Hermsen CC, Gego A, Franetich JF, Mazier D, Hoffman SL, Janse CJ, Sauerwein RW, Khan SM. 2012. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine 30:2662–2670. 10.1016/j.vaccine.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 12.Baschong W, Wittlin S, Inglis KA, Fairlamb AH, Croft SL, Kumar TR, Fidock DA, Brun R. 2011. Triclosan is minimally effective in rodent malaria models. Nat. Med. 17:33–34. 10.1038/nm0111-33 [DOI] [PubMed] [Google Scholar]
- 13.Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, Cowman AF, Kappe SH. 2009. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell. Microbiol. 11:506–520. 10.1111/j.1462-5822.2008.01270.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu M, Kumar TR, Nkrumah LJ, Coppi A, Retzlaff S, Li CD, Kelly BJ, Moura PA, Lakshmanan V, Freundlich JS, Valderramos JC, Vilcheze C, Siedner M, Tsai JH, Falkard B, Sidhu AB, Purcell LA, Gratraud P, Kremer L, Waters AP, Schiehser G, Jacobus DP, Janse CJ, Ager A, Jacobs WR, Jr, Sacchettini JC, Heussler V, Sinnis P, Fidock DA. 2008. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe 4:567–578. 10.1016/j.chom.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waller RF, Ralph SA, Reed MB, Su V, Douglas JD, Minnikin DE, Cowman AF, Besra GS, McFadden GI. 2003. A type II pathway for fatty acid biosynthesis presents drug targets in Plasmodium falciparum. Antimicrob. Agents Chemother. 47:297–301. 10.1128/AAC.47.1.297-301.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben Mamoun C, Prigge ST, Vial H. 2010. Targeting the lipid metabolic pathways for the treatment of malaria. Drug Dev. Res. 71:44–55. 10.1002/ddr.20347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrader FC, Glinca S, Sattler JM, Dahse HM, Afanador GA, Prigge ST, Lanzer M, Mueller AK, Klebe G, Schlitzer M. 2013. Novel type II fatty acid biosynthesis (FAS II) inhibitors as multistage antimalarial agents. Chem. Med. Chem. 8:442–461. 10.1002/cmdc.201200407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foth BJ, Stimmler LM, Handman E, Crabb BS, Hodder AN, McFadden GI. 2005. The malaria parasite Plasmodium falciparum has only one pyruvate dehydrogenase complex, which is located in the apicoplast. Mol. Microbiol. 55:39–53. 10.1111/j.1365-2958.2004.04407.x [DOI] [PubMed] [Google Scholar]
- 19.Falkard B, Kumar TR, Hecht LS, Matthews KA, Henrich PP, Gulati S, Lewis RE, Manary MJ, Winzeler EA, Sinnis P, Prigge ST, Heussler V, Deschermeier C, Fidock D. 2013. A key role for lipoic acid synthesis during Plasmodium liver stage development. Cell. Microbiol. 15:1585–1604. 10.1111/cmi.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pei Y, Tarun AS, Vaughan AM, Herman RW, Soliman JM, Erickson-Wayman A, Kappe SH. 2010. Plasmodium pyruvate dehydrogenase activity is only essential for the parasite's progression from liver infection to blood infection. Mol. Microbiol. 75:957–971. 10.1111/j.1365-2958.2009.07034.x [DOI] [PubMed] [Google Scholar]
- 21.Butler NS, Vaughan AM, Harty JT, Kappe SH. 2012. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 33:247–254. 10.1016/j.it.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 22.Khan SM, Janse CJ, Kappe SH, Mikolajczak SA. 2012. Genetic engineering of attenuated malaria parasites for vaccination. Curr. Opin. Biotechnol. 23:908–916. 10.1016/j.copbio.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 23.Ifediba T, Vanderberg JP. 1981. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 294:364–366. 10.1038/294364a0 [DOI] [PubMed] [Google Scholar]
- 24.Ponnudurai T, Lensen AH, Meis JF, Meuwissen JH. 1986. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitology 93:263–274. 10.1017/S003118200005143X [DOI] [PubMed] [Google Scholar]
- 25.Fivelman QL, McRobert L, Sharp S, Taylor CJ, Saeed M, Swales CA, Sutherland CJ, Baker DA. 2007. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol. Biochem. Parasitol. 154:119–123. 10.1016/j.molbiopara.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 26.van Schaijk BC, Vos MW, Janse CJ, Sauerwein RW, Khan SM. 2010. Removal of heterologous sequences from Plasmodium falciparum mutants using FLPe-Recombinase. PLoS One 5:e15121. 10.1371/journal.pone.0015121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponnudurai T, Leeuwenberg AD, Meuwissen JH. 1981. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop. Geograph. Med. 33:50–54 [PubMed] [Google Scholar]
- 28.van Schaijk BC, Janse CJ, van Gemert GJ, van Dijk MR, Gego A, Franetich JF, van de Vegte-Bolmer M, Yalaoui S, Silvie O, Hoffman SL, Waters AP, Mazier D, Sauerwein RW, Khan SM. 2008. Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS One 3:e3549. 10.1371/journal.pone.0003549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Russel WD. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 30.Su XZ, Wu Y, Sifri CD, Wellems TE. 1996. Reduced extension temperatures required for PCR amplification of extremely A+T-rich DNA. Nucleic Acids Res. 24:1574–1575. 10.1093/nar/24.8.1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidhu AB, Valderramos SG, Fidock DA. 2005. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 57:913–926. 10.1111/j.1365-2958.2005.04729.x [DOI] [PubMed] [Google Scholar]
- 32.Sidhu AB, Verdier-Pinard D, Fidock DA. 2002. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298:210–213. 10.1126/science.1074045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodyer ID, Taraschi TF. 1997. Plasmodium falciparum: a simple, rapid method for detecting parasite clones in microtiter plates. Exp. Parasitol. 86:158–160. 10.1006/expr.1997.4156 [DOI] [PubMed] [Google Scholar]
- 34.Ponnudurai T, Lensen AH, Van Gemert GJ, Bensink MP, Bolmer M, Meuwissen JH. 1989. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98:165–173. 10.1017/S0031182000062065 [DOI] [PubMed] [Google Scholar]
- 35.Ponnudurai T, van Gemert GJ, Bensink T, Lensen AH, Meuwissen JH. 1987. Transmission blockade of Plasmodium falciparum: its variability with gametocyte numbers and concentration of antibody. Trans. R. Soc. Trop. Med. Hyg. 81:491–493. 10.1016/0035-9203(87)90172-6 [DOI] [PubMed] [Google Scholar]
- 36.Lasonder E, Janse CJ, van Gemert GJ, Mair GR, Vermunt AM, Douradinha BG, van Noort V, Huynen MA, Luty AJ, Kroeze H, Khan SM, Sauerwein RW, Waters AP, Mann M, Stunnenberg HG. 2008. Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog. 4:e1000195. 10.1371/journal.ppat.1000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. The National Academies Press, Washington, DC [Google Scholar]
- 38.Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, Camargo N, Bial J, Ploss A, Kappe SH. 2012. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J. Clin. Investig. 122:3618–3628. 10.1172/JCI62684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, Daly TM, Bergman LW, Kappe SH. 2008. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc. Natl. Acad. Sci. U. S. A. 105:305–310. 10.1073/pnas.0710780104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartfai R, Hoeijmakers WA, Salcedo-Amaya AM, Smits AH, Janssen-Megens E, Kaan A, Treeck M, Gilberger TW, Francoijs KJ, Stunnenberg HG. 2010. H2A.Z demarcates intergenic regions of the Plasmodium falciparum epigenome that are dynamically marked by H3K9ac and H3K4me3. PLoS Pathog. 6:e1001223. 10.1371/journal.ppat.1001223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Barragan MJ, Lemieux J, Quinones M, Williamson KC, Molina-Cruz A, Cui K, Barillas-Mury C, Zhao K, Su XZ. 2011. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genomics 12:587. 10.1186/1471-2164-12-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, Younis Younis S, Sauerwein R, Alano P. 2010. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol. Cell. Proteomics 9:1437–1448. 10.1074/mcp.M900479-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanway RR, Witt T, Zobiak B, Aepfelbacher M, Heussler VT. 2009. GFP-targeting allows visualization of the apicoplast throughout the life cycle of live malaria parasites. Biol. Cell 101:415–430. 10.1042/BC20080202 [DOI] [PubMed] [Google Scholar]
- 44.Wu Y, Kirkman LA, Wellems TE. 1996. Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc. Natl. Acad. Sci. U. S. A. 93:1130–1134. 10.1073/pnas.93.3.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graves PM, Carter R, McNeill KM. 1984. Gametocyte production in cloned lines of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 33:1045–1050 [DOI] [PubMed] [Google Scholar]
- 46.Tsai YL, Hayward RE, Langer RC, Fidock DA, Vinetz JM. 2001. Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect. Immun. 69:4048–4054. 10.1128/IAI.69.6.4048-4054.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maier AG, Braks JA, Waters AP, Cowman AF. 2006. Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol. Biochem. Parasitol. 150:118–121. 10.1016/j.molbiopara.2006.06.014 [DOI] [PubMed] [Google Scholar]
- 48.Daily JP, Scanfeld D, Pochet N, Le Roch K, Plouffe D, Kamal M, Sarr O, Mboup S, Ndir O, Wypij D, Levasseur K, Thomas E, Tamayo P, Dong C, Zhou Y, Lander ES, Ndiaye D, Wirth D, Winzeler EA, Mesirov JP, Regev A. 2007. Distinct physiological states of Plasmodium falciparum in malaria-infected patients. Nature 450:1091–1095. 10.1038/nature06311 [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg R, Rungsiwongse J. 1991. The number of sporozoites produced by individual malaria oocysts. Am. J. Trop. Med. Hyg. 45:574–577 http://www.ncbi.nlm.nih.gov/pubmed/1951866 [DOI] [PubMed] [Google Scholar]
- 50.Shimizu S, Osada Y, Kanazawa T, Tanaka Y, Arai M. 2010. Suppressive effect of azithromycin on Plasmodium berghei mosquito stage development and apicoplast replication. Malar. J. 9:73. 10.1186/1475-2875-9-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindner SE, Mikolajczak SA, Vaughan AM, Moon W, Joyce BR, Sullivan WJ, Jr, Kappe SH. 2013. Perturbations of Plasmodium Puf2 expression and RNA-seq of Puf2-deficient sporozoites reveal a critical role in maintaining RNA homeostasis and parasite transmissibility. Cell. Microbiol. 15:1266–1283. 10.1111/cmi.12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Q, Fujioka H, Nussenzweig V. 2005. Mutational analysis of the GPI-anchor addition sequence from the circumsporozoite protein of Plasmodium. Cell. Microbiol. 7:1616–1626. 10.1111/j.1462-5822.2005.00579.x [DOI] [PubMed] [Google Scholar]
- 53.Mazumdar J, Wilson HE, Masek K, Hunter AC, Striepen B. 2006. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc. Natl. Acad. Sci. U. S. A. 103:13192–13197. 10.1073/pnas.0603391103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramakrishnan S, Docampo MD, Macrae JI, Pujol FM, Brooks CF, van Dooren GG, Hiltunen JK, Kastaniotis AJ, McConville MJ, Striepen B. 2012. Apicoplast and endoplasmic reticulum cooperate in fatty acid biosynthesis in apicomplexan parasite Toxoplasma gondii. J. Biol. Chem. 287:4957–4971. 10.1074/jbc.M111.310144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeh E, Derisi JL. 2011. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 9:e1001138. 10.1371/journal.pbio.1001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573–1576. 10.1126/science.285.5433.1573 [DOI] [PubMed] [Google Scholar]
- 57.Collins CR, Das S, Wong EH, Andenmatten N, Stallmach R, Hackett F, Herman JP, Muller S, Meissner M, Blackman MJ. 2013. Robust inducible Cre recombinase activity in the human malaria parasite Plasmodium falciparum enables efficient gene deletion within a single asexual erythrocytic growth cycle. Mol. Microbiol. 88:687–701. 10.1111/mmi.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muralidharan V, Oksman A, Iwamoto M, Wandless TJ, Goldberg DE. 2011. Asparagine repeat function in a Plasmodium falciparum protein assessed via a regulatable fluorescent affinity tag. Proc. Natl. Acad. Sci. U. S. A. 108:4411–4416. 10.1073/pnas.1018449108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russo I, Oksman A, Goldberg DE. 2009. Fatty acid acylation regulates trafficking of the unusual Plasmodium falciparum calpain to the nucleolus. Mol. Microbiol. 72:229–245. 10.1111/j.1365-2958.2009.06639.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL, VRC 312 Study Team 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341:1359–1365. 10.1126/science.1241800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.