Abstract
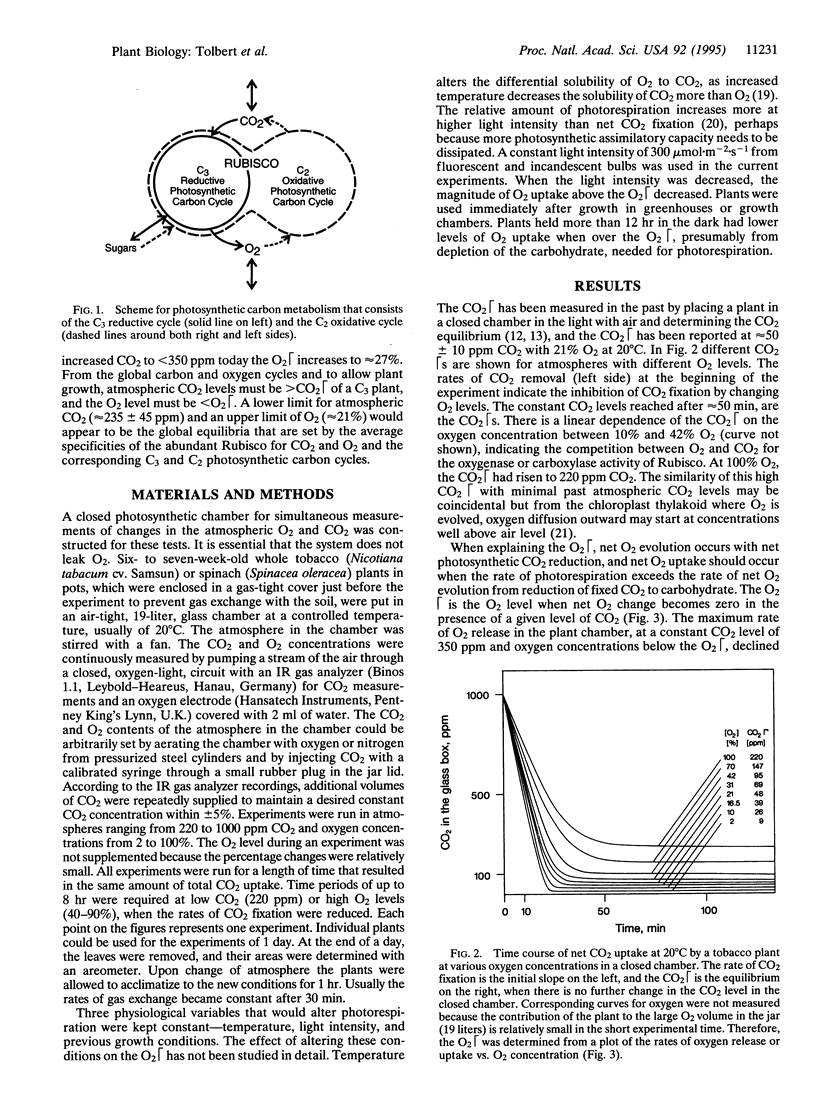
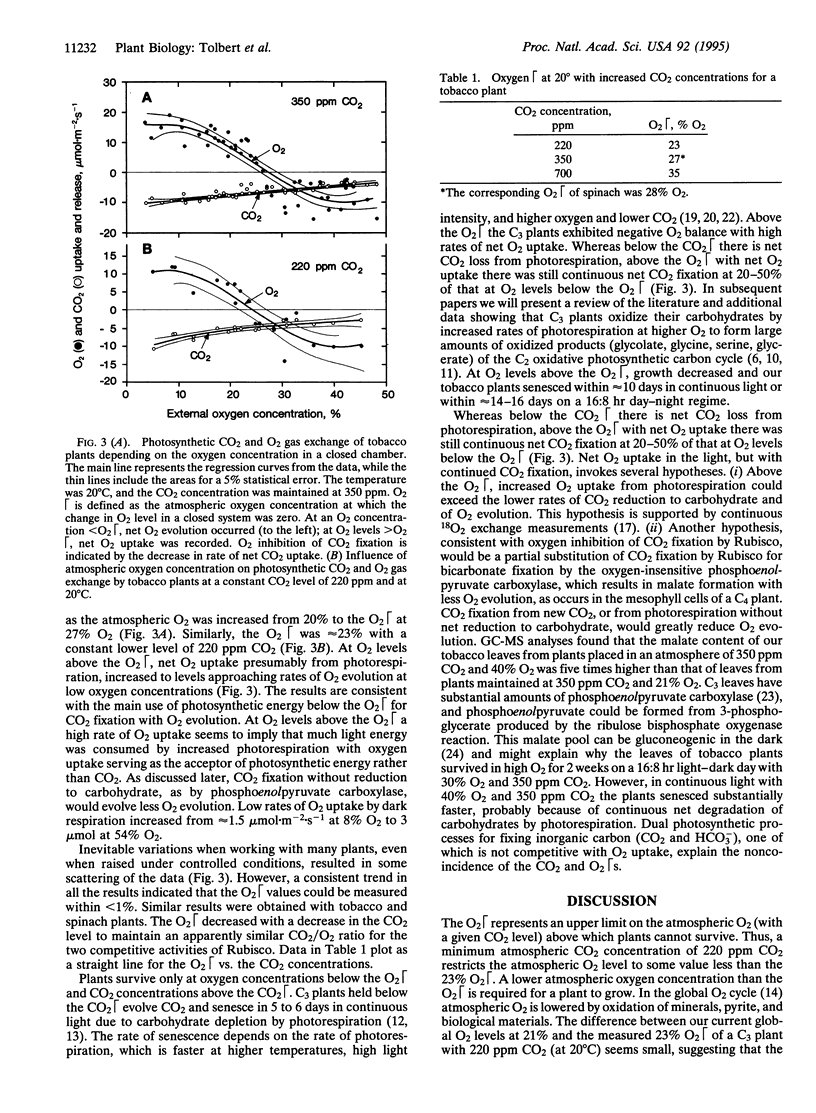
The O2 and CO2 compensation points (O2 and CO2) of plants in a closed system depend on the ratio of CO2 and O2 concentrations in air and in the chloroplast and the specificities of ribulose bisphosphate carboxylase/oxygenase (Rubisco). The photosynthetic O2 is defined as the atmospheric O2 level, with a given CO2 level and temperature, at which net O2 exchange is zero. In experiments with C3 plants, the O2 with 220 ppm CO2 is 23% O2; O2 increases to 27% with 350 ppm CO2 and to 35% O2 with 700 ppm CO2. At O2 levels below the O2, CO2 uptake and reduction are accompanied by net O2 evolution. At O2 levels above the O2, net O2 uptake occurs with a reduced rate of CO2 fixation, more carbohydrates are oxidized by photorespiration to products of the C2 oxidative photosynthetic carbon cycle, and plants senesce prematurely. The CO2 increases from 50 ppm CO2 with 21% O2 to 220 ppm with 100% O2. At a low CO2/high O2 ratio that inhibits the carboxylase activity of Rubisco, much malate accumulates, which suggests that the oxygen-insensitive phosphoenolpyruvate carboxylase becomes a significant component of the lower CO2 fixation rate. Because of low global levels of CO2 and a Rubisco specificity that favors the carboxylase activity, relatively rapid changes in the atmospheric CO2 level should control the permissive O2 that could lead to slow changes in the immense O2 pool.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict C. R., Beevers H. Formation of Sucrose From Malate in Germinating Castor Beans . II. Reaction Sequence From Phosphoenol-Pyruvate to Sucrose. Plant Physiol. 1962 Mar;37(2):176–178. doi: 10.1104/pp.37.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner R. A., Canfield D. E. A new model for atmospheric oxygen over Phanerozoic time. Am J Sci. 1989 Apr;289(4):333–361. doi: 10.2475/ajs.289.4.333. [DOI] [PubMed] [Google Scholar]
- Canvin D. T., Berry J. A., Badger M. R., Fock H., Osmond C. B. Oxygen exchange in leaves in the light. Plant Physiol. 1980 Aug;66(2):302–307. doi: 10.1104/pp.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., André M. Effect of CO(2), O(2), and Light on Photosynthesis and Photorespiration in Wheat. Plant Physiol. 1980 Dec;66(6):1032–1036. doi: 10.1104/pp.66.6.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M. Photorespiration, Warburg effect and glycolate. Ann N Y Acad Sci. 1969 Dec 19;168(2):356–368. doi: 10.1111/j.1749-6632.1969.tb43123.x. [DOI] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. N., Krenzer E. G., Jr, Brun W. A. Carbon dioxide compensation points in related plant species. Science. 1969 Apr 11;164(3876):187–188. doi: 10.1126/science.164.3876.187. [DOI] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger H. M., Beck E. Oxygen Concentration in Isolated Chloroplasts during Photosynthesis. Plant Physiol. 1977 Dec;60(6):903–906. doi: 10.1104/pp.60.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER J. S., BRITTAIN E. G. Oxygen as a factor in photosynthesis. Biol Rev Camb Philos Soc. 1962 Feb;37:130–170. doi: 10.1111/j.1469-185x.1962.tb01607.x. [DOI] [PubMed] [Google Scholar]
- Widholm J. M., Ogren W. L. Photorespiratory-induced senescence of plants under conditions of low carbon dioxide. Proc Natl Acad Sci U S A. 1969 Jul;63(3):668–675. doi: 10.1073/pnas.63.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]



