Abstract
Aims
Myeloperoxidase (MPO), a cardiovascular risk factor in humans, is an in vivo catalyst for lipoprotein modification via intermediate formation of reactive chlorinating species. Among the different lipoprotein classes, anti-atherogenic high-density lipoprotein (HDL) represents a major target for modification by hypochlorous acid (HOCl), generated from H2O2 by MPO in the presence of physiological chloride concentrations. As MPO was identified as an HDL-associated protein that could facilitate selective oxidative modification of its physiological carrier, the aim of the present study was to investigate whether and to what extent modification of HDL by HOCl affects the binding affinity of MPO in vitro.
Methods and results
We show that binding affinity of 125I-labelled MPO to HDL markedly increases as a function of increasing extent of HOCl modification of HDL. In contrast to native HDL, HOCl–HDL potently inhibits MPO binding/uptake by endothelial cells and effectively attenuates metabolism of MPO by macrophages. Reduction of HDL-associated chloramines with methionine strongly impaired binding affinity of MPO towards HOCl–HDL. This indicates that N-chloramines generated by HOCl are regulators of the high-affinity interaction between HOCl–HDL and positively charged MPO. Most importantly, the presence of HOCl–HDL is almost without effect on the halogenating activity of MPO.
Conclusion
We propose that MPO-dependent modification of HDL and concomitant increase in the binding affinity for MPO could generate a vicious cycle of MPO transport to and MPO-dependent modification at sites of chronic inflammation.
Keywords: Hypochlorous acid, ApoA-I, Inflammation, Neutrophils, MPO–hydrogen peroxide–halide system
1. Introduction
Classically, the ‘oxidation hypothesis’ of atherosclerosis is predominantly focused on the role of oxidation in the modification of low-density lipoprotein (LDL) and phospholipids, generating pro-atherogenic species capable of promoting cholesterol deposition, foam cell formation, and smooth muscle cell proliferation.1 Several lines of evidence indicate that oxidants generated by myeloperoxidase (MPO) are critically involved in these processes. The MPO–H2O2 system generates tyrosyl and nitrosyl radicals, and in the presence of physiological chloride concentrations hypochlorous acid (HOCl) is formed. Besides its function as antimicrobial agent,2 HOCl is able to initiate modification reactions targeting a wide range of biological substrates, including anti-oxidants, amines, amino acids, sulphides, nucleotides, DNA, lipids, and (lipo)proteins.3–8 MPO is present in lesion material, and in situ experiments revealed co-localization of HOCl-modified epitopes with lipid-rich areas, macrophages, and MPO in lesion material.8–13 Density gradient centrifugation of human plaque homogenate and subsequent immunoblot analysis of the LDL-buoyant fraction further revealed that the apolipoprotein moiety of LDL is modified by the MPO–H2O2–chloride system.14
However, over the past few years, there is growing evidence that anti-atherogenic high-density lipoprotein (HDL) becomes ‘dysfunctional’ through oxidative modification mediated by MPO.8,15 Chlorination of HDL or its major apolipoprotein moiety, apoA-I, impairs the removal of cholesterol from cultured cells by ATP-binding cassette transporter A116–19 but promotes cellular lipid uptake and foam cell formation in vitro.20 Different lines of evidence, e.g. (i) increased content of MPO-generated chlorinated tyrosine in circulating and lesion-derived apoA-I, (ii) a specific interaction site between apoA-I and MPO, and (iii) co-purification of MPO with HDL-like particles recovered from human atherosclerotic lesions, support a specific mechanism for oxidative modification of HDL by MPO in vivo.21 In situ experiments revealed co-localization of specific epitopes derived from HOCl with apoA-I and MPO in human atheroma.8,13,22
Among the prime targets of MPO-dependent modification of HDL are amino acid side chains of cysteine, methionine, tyrosine, and lysine.20,22 Conversion of positively charged ε-amino groups of lysine residues by HOCl results in the formation of N-chloramines that might affect the interaction with MPO. Therefore, the present study aimed at investigating the interrelationship between a triade of factors that likely affect the pathology in the diseased vessel wall, namely the relationship between the extent of HOCl modification of HDL, the ability of HOCl-modified HDL to interact with positively charged MPO, and the effects of these modified particles on the halogenating activity of lipoprotein-bound MPO.
2. Methods
2.1 Materials
NaOCl/HOCl, organic solvents, and potassium bromide were obtained from Sigma. Radiochemicals were purchased from PerkinElmer Life Sciences. Dulbecco’s modified Eagle’s medium (DMEM) was from Invitrogen, and foetal calf serum (FCS) was obtained from Roche Applied Science (Vienna). Plasticware used for tissue culture was obtained from Costar (Vienna). MPO was from Planta Naturstoffe (Vienna). All other chemicals were obtained from Merck except where indicated.
2.2 Isolation of plasma lipoproteins
HDL (HDL subclass 3, d = 1.125–1.21 g/mL) was prepared by discontinuous density ultracentrifugation of plasma obtained from normolipidemic blood donors.23 HDL was recovered from the tubes and dialysed against 10 mM phosphate-buffered saline (PBS; pH 7.4, 0.15 M NaCl). The protein concentration of the resulting solution was determined by the Lowry procedure using bovine serum albumin (BSA) as a standard. Before use, HDL was desalted, and the preservatives were removed by dialysis or size exclusion chromatography on Econopac 10-DG columns (Bio-Rad).
2.3 Modification of high-density lipoprotein
Modification of HDL was performed as described.24 Briefly, one milligram of HDL per millilitre of PBS (pH 7.4) was incubated in the absence of free amino acids/carbohydrate with NaOCl solution (added as single addition and gentle vortexing) at molar ratios of HOCl:HDL ranging from 50:1 to 200:1 for 60 min (0°C, under argon) or, when indicated, up to 12 h at 37°C. To some HDL preparations, methionine (a five-fold molar excess over HOCl) was added at the end of the modification procedure.
Alternatively, modification of HDL coated to microtitre wells by the MPO–H2O2–chloride system was performed in PBS (100 μL, 50 mM, pH 7.4) by adding H2O2 (3.3–100 μM). MPO (10 nM, Planta Naturstoffe) was added at the start. The reaction mixture was incubated for 1 h at 37°C.
All modified HDL preparations were passed over a PD10 column to remove unreacted NaOCl or methionine and were used immediately for experiments. The relative electrophoretic mobility (REM) of HOCl–HDL was assessed by agarose gel electrophoresis.25 For amino acid analysis, HDL and HOCl–HDL (450 μg of protein) were lyophilized in 5 mL ampoules and purged with nitrogen before hydrolysis in constant boiling 6 N HCl (24 h, 120°C).20 Amino acid analyses were performed on a Biochrom analyser (Pharmacia Biotech) using norleucin as internal standard.
2.4 Circular dichroism spectrometry
Circular dichroism (CD) spectra were performed on a PiStar-180 spectrometer equipped with a thermostatic cell holder from Applied Photophysics (Leatherhead, UK). For recording far-UV spectra (250–190 nm), the quartz cuvette had a path length of 1 mm. Spectral bandwidth: 5 nm; step size: 1 nm; scan time: 624 s; protein concentration: 0.1 mg/mL MPO and 0.25 mg/mL HDL or HOCl–HDL. In the near-UV and visible region (250–500 nm), conditions were as follows: pathlength: 10 mm; spectral bandwidth: 5 nm; step size: 1 nm; scan time: 1612 s; protein concentration: 0.5 mg/mL MPO and 1 mg/mL HDL or HOCl–HDL. All CD measurements were performed in 10 mM PBS (pH 7.4) at 25°C.26 Each spectrum was automatically corrected with the baseline to remove birefringence of the cell. The instrument was flushed with nitrogen with a flow rate of 5 L/min.
2.5 Polarographic measurements of myeloperoxidase activity
The halogenation activity of MPO incubated with HDL or HOCl–HDL for 30 min was measured by continuously monitoring H2O2 consumption polarographically with a platinum electrode covered with a hydrophilic membrane and fitted to the Amperometric Biosensor Detector 3001 (Universal Sensors, Inc., USA). The applied electrode potential at pH 7.4 was 650 mV and the H2O2 electrode filling solution was prepared freshly half-daily.27 The electrode was calibrated against known concentrations of H2O2. In detail, the consumption of H2O2 (100 μM) in the presence of 100 μM bromide in 100 mM PBS (pH 7.4) was followed (25°C). Reactions were started by addition of MPO (10 μg/mL) either free or incubated for 30 min with HDL or HOCl–HDL (1 mg/mL). One unit is defined as consumption of 1 μmol H2O2 per minute at 25°C.
2.6 Protein labelling procedures
Labelling of MPO with 125INa was performed as described28 using N-bromosuccinimide as the coupling agent. Routinely, 100 μCi of 125INa was used to label 1 mg of protein. This procedure resulted in specific activities between 200 and 400 cpm/ng of protein.
2.7 Myeloperoxidase-binding assays
Microtitre wells (Nunc Maxisorp, VWR) were coated with 100 μL of a solution containing either BSA or apoA-I or HDL (each 100 μg/mL, 15 mM sodium carbonate, 35 mM sodium bicarbonate, pH 9.6) overnight at 4°C as described.28 Subsequently, the wells were washed with washing buffer (ice-cold PBS containing 0.05% Tween-20). In some experiments, adsorbed (lipo)proteins were oxidized onto the wells by adding 100 μL of HOCl solutions added as reagent NaOCl (3.3, 11, 33, and 100 mM in PBS, pH 7.4) or generated by the MPO–H2O2–chloride-system (1 μg/mL MPO) for 1 h at 4°C. For controls, adsorbed (lipo)proteins were treated with H2O2 (3.3, 11, 33, and 100 μM in PBS, pH 7.4) only. After washing and blocking [2 h at 37°C with PBS containing non-immune IgG (10 mg/mL)], the wells were incubated with 125I-labelled MPO (100 μL at indicated concentrations; either alone or in the presence of an excess of unlabelled competitors) in DMEM (containing 1 mg BSA/mL) for 2 h at 37°C. Then the supernatant was removed, and the wells were washed four times with washing buffer. Bound 125I-MPO was quantified by counting radioactivity in the individual wells.
2.8 Coupling of lipoproteins to Sepharose and myeloperoxidase binding
One milligram of HDL or HOCl–HDL (oxidant:lipoprotein molar ratio of 200:1) was coupled to CN–Br-activated Sepharose (GE Health-care) according to the manufacturer’s instructions. The amount of lipoproteins coupled to Sepharose was estimated by quantitation of unbound lipoproteins in the supernatant. After washing and blocking (1 M ethanolamine, 0.1 M NaHCO3, 0.5 M NaCl, pH 9.0), 10 μg 125I-MPO was added to HDL- or HOCl–HDL coupled to Sepharose-beads or to Sepharose-beads alone (control) in the presence of 1 mg/mL BSA. After gentle rotation (30 min, room temperature), the beads were spun down and the radioactivity was measured in the supernatant. Subsequently, the beads were washed and spun down again to measure bead-associated radioactivity.
2.9 Cells
Human umbilical venous endothelial cells (kindly supplied by Dr R. Heller, University of Jena, Germany) were isolated and cultured in M199 medium, containing 15% (v/v) FCS, 5% (v/v) human serum, and 7.5 μg/mL endothelial cell supplement.29 RAW264.7 macrophages (ATCC) were cultured in DMEM containing 10% (v/v) FCS, 2 mM glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin.
2.10 Cell culture studies
Association studies of 125I-MPO to cells in the absence or presence of native or HOCl-modified HDL was performed at 37°C as described.22 After the incubation period, the medium was aspirated, and cells were rinsed two times with Tris-buffered saline [containing 5% (w/v) BSA] followed by two washes with Tris-buffered saline only. To determine cell-bound 125I-MPO, DMEM containing heparin (100 U/mL DMEM) was added, and the cells were incubated for further 30 min to release cell-surface-associated 125I-MPO. The cells were separated, the supernatants were collected, and the radioactivity was measured to estimate heparin-releasable (bound) MPO. Subsequently, the cells were lysed with 0.3 N NaOH to measure radioactivity (internalized and cell-associated, nonheparin-releasable) and protein content. Degradation of 125I-MPO was estimated by measuring the non-trichloroacetic acid-precipitable radioactivity in the medium as described.30
3. Results
3.1 Interaction of HOCl-modified lipoproteins with myeloperoxidase
During the first set of experiments, we tested whether modification of HDL by HOCl alters binding characteristics of MPO to the modified HDL particle. For this purpose, a micro-plate-based assay was performed. Microtitre wells were first coated with (lipo)proteins and excess of binding sites was blocked. Subsequently, wells were incubated with 125I-MPO. Figure 1A shows dose-dependent binding of 125I-MPO to coated wells, which were not saturated at the concentrations used. Binding of 125I-MPO to wells coated with either HDL or apoA-I at same protein concentrations is similar but almost five-fold higher compared with wells coated with BSA (Figure 1A), a protein recently reported to act as an in vivo carrier for MPO.31 Figure 1A further shows that 125I-MPO binding to slightly modified HDL (oxidant:lipoprotein molar ratio of 50:1) is similar to HDL while a higher extent of modification (oxidant:lipoprotein molar ratio of 200:1) is paralleled by increased 125I-MPO binding.
Figure 1. Myeloperoxidase binding to HOCl-modified lipoproteins.
(A) Albumin (Alb), apolipoprotein A-I (apoA-I), native high-density lipoprotein (HDL), and HOCl–HDL (oxidant:lipoprotein molar ratio of 50:1 and 200:1) were coated to microtitre plates. After washing and blocking of unspecific binding sites, 125I-MPO (0.6, 3, and 15 μg/mL) was added (37°C, 1 h). Subsequently, the wells were washed and the radioactivity was counted. (B) High-density lipoprotein was immobilized onto wells of plastic dishes and modified by indicated concentrations of HOCl added as reagent or generated by the myeloperoxidase–H2O2–chloride system in vitro. H2O2, in the absence of myeloperoxidase and chloride, was used as a control. After washing and blocking of unspecific binding sites, 125I-MPO (1 μg/mL) was added to coated wells at 37°C for 1 h. Subsequently, the wells were washed and the radioactivity was counted. (C) Dose–response curves of 125I-MPO (1 μg/mL) binding competition to high-density lipoprotein-coated microtitre wells by high-density lipoprotein or HOCl–high-density lipoprotein (oxidant:lipoprotein molar ratio of 50:1, 100:1, and 200:1) at indicated concentrations. (D) One milligram high-density lipoprotein or HOCl–high-density lipoprotein (oxidant:lipoprotein molar ratio of 200:1) coupled to Sepharose-beads or Sepharose-beads alone (control) was incubated with 125I-MPO (10 μg/mL) at 25°C. After 30 min, beads were spun down and radioactivity of free (supernatant) or bound (Sepharose-bound or Sepharose–lipoprotein-bound) 125I-MPO was measured. Results represent mean ± SD (n = 3) of one experiment out of three.
In a further experiment, HDL was coated to microtitre wells and subsequently modified with increasing concentrations of HOCl added as reagent or generated by the MPO–H2O2–chloride system (Figure 1B). 125I-MPO binding to HDL increased with increasing extent of HOCl modification reaching a plateau at ~30 μM HOCl. Treatment of HDL with H2O2 in the absence of MPO did not affect 125I-MPO binding to HDL (Figure 1B).
Next, we tested whether HOCl–HDL is able to compete for 125I-MPO binding to HDL. This was determined on the basis of the ability of native and modified lipoproteins to inhibit 125I-MPO binding to HDL that has been coated to microtitre wells. Dose–response curves show that HOCl–HDL preparations effectively blocked 125I-MPO binding to HDL (Figure 1C). The ability of the different HOCl–HDL preparations to inhibit 125I-MPO binding to HDL was directly related to the extent of HOCl modification. The calculated IC50 values are listed in Table 1.
Table 1. Binding constants for HOCl–high-density lipoprotein:myeloperoxidase interaction.
| HDL | 5.8 ± 2.2 |
| HOCl–HDL (50:1) | 1.3 ± 0.4 |
| HOCl–HDL (100:1) | 0.104 ± 0.083 |
| HOCl–HDL (200:1) | 0.0107 ± 0.012 |
IC50 (mg/mL) values for the inhibition of 125I-MPO binding to HDL-coated microtitre wells by HDL and HOCl–HDL (at indicated oxidant: lipoprotein molar ratio) are given. Calculations were performed by non-linear regression analysis (Graph Pad Prism).
To further confirm an increased binding affinity of MPO to HOCl–HDL, native and modified HDL was coupled to Sepharose (described in the Methods section) and incubated with 125I-MPO. MPO binding was quantitated by measuring Sepharose-bound and unbound (soluble) radioactivity. About 90% of added 125I-MPO was associated with HOCl–HDL–Sepharose, whereas only 25% was associated with HDL–Sepharose (Figure 1D). About 10% of added 125I-MPO is bound to Sepharose alone. From these experiments (Figure 1), we conclude that MPO is a preferential ligand for HOCl–HDL.
To underscore that binding of MPO is dependent on the extent of HOCl modification of HDL, binding data from Figure 1A were plotted against parameters indicative for the extent of HOCl modification of HDL/apoA-I; the REM of modified HDL particles was calculated and the concentration of two amino acids highly susceptible to HOCl modification was estimated. Figure 2 shows that binding of MPO to modified HDL particles is directly related to an increasing REM value (Figure 2A) and to the extent of modified lysine and tyrosine residues in the apolipoprotein moiety of HDL (Figure 2B and C).
Figure 2. Characterization of HOCl–high-density lipoprotein.
Data from Figure 1A (myeloperoxidase binding to native and modified high-density lipoprotein preparations at the highest myeloperoxidase concentrations) were plotted against relative electrophoretic mobility (REM) values (A) and the extent of lysine (B) and tyrosine modification (C) of native high-density lipo-protein and modified high-density lipoprotein (indicated oxidant:lipoprotein molar ratio). Relative electrophoretic mobility values and amino acid analyses were estimated as described in the Methods section.
3.2 High-density lipoprotein-associated chloramines affect binding affinity of myeloperoxidase to high-density lipoprotein
As HOCl modification of (lipo)proteins is paralleled by formation of N-chloramines, we tested whether these chloramines are responsible for the increased affinity of HOCl–HDL towards MPO. For this purpose, HDL was modified with HOCl under two different conditions. First, HDL was modified at 0°C for 1 h; where indicated, methionine was added to reverse N-chloramine formation. Second, HDL was modified at 37°C for 12 h, a condition known to lead to breakdown of chloramines18; again, methionine was added at the end of the incubation period as indicated. MPO-binding affinity towards these HOCl–HDL preparations was determined on the basis of their ability to inhibit 125I-MPO binding to native HDL that has been coated to microtitre wells. HDL, modified with reagent HOCl at 0 and 37°C effectively inhibited 125I-MPO binding to native HDL (Figure 3A and B). Depending on the temperature used for modification of HDL, the addition of methionine strongly altered the affinity of modified HDL towards 125I-MPO. As expected, addition of methionine to HOCl–HDL (modified at 37°C) did not affect the binding affinity to 125I-MPO; however, addition of methionine at 0°C impaired binding affinity (Figure 3A and B). From these experiments, we conclude that chloramines (formed at 0 and 37°C) or unstable secondary products of chloramines18 may contribute to the increased binding affinity of MPO to HOCl–HDL.
Figure 3. Myeloperoxidase binding and competition experiments.
(A and B) Competition experiments of 125I-MPO (1 μg/mL) binding to high-density lipoprotein-coated microtitre wells by high-density lipoprotein or HOCl–high-density lipoprotein (oxidant:lipoprotein molar ratio of 200:1) at indicated concentrations. Modification of high-density lipoprotein was performed at 0°C (1 h) or 37°C (12 h) in the absence or presence of methionine (Met) added at a five-fold molar excess over HOCl at the end of the incubation period. Results represent mean ± SD (n = 3) of one experiment out of three.
3.3 Activity and structural features of myeloperoxidase bound to lipoproteins
As MPO binds with high affinity to HOCl–HDL (Figures 1–3), we tested whether lipoprotein-bound MPO is still enzymatically active. In principle, N-chloramines present in HOCl–HDL might directly inactivate MPO or alternatively modify the structure of the enzyme in a way that influences H2O2 and halide binding and their redox transformation in further consequence. To test this issue, MPO was incubated with HDL or HOCl–HDL and enzymatic activity was estimated by measuring consumption of H2O2. MPO binding to unmodified HDL does not affect its enzymatic activity (Figure 4). Importantly, even when incubated with HOCl–HDL, MPO retained ~75–80% of its activity (Figure 4). This indicates that HOCl–HDL acts as a carrier for MPO with only modest loss of enzymatic function. Addition of methionine to HDL modified by HOCl at 0°C tended to slightly reverse the observed decrease in MPO activity.
Figure 4. Measurement of lipoprotein-associated myeloperoxidase activity.
High-density lipoprotein or HOCl–high-density lipoprotein [prepared at indicated oxidant:lipoprotein molar ratio at 0 or 37°C, without or with methionine (Met) added at the end of incubation time] were co-incubated with myeloperoxidase. After 30 min, 100 μM H2O2 and 100 μM bromide were added and myeloperoxidase activity monitored continuously by measuring H2O2 consumption polarographically (see the Methods section). Values are given as percentage of activity of free myeloperoxidase (100% corresponds to 3.8 units). Results represent mean ± SD (n = 3) of one experiment out of three.
In line, binding of MPO to both HDL and HOCl–HDL did not alter the overall secondary structure of the haem protein. This is evident by inspection of far-UV CD spectra (Supplementary material online, Figure S1A and B) that clearly shows that the spectrum of MPO incubated with HDL or HOCl–HDL results in a superimposition of the CD spectra of free MPO and the corresponding lipoprotein particles. In addition, the UV–Vis spectrum of MPO incubated with HDL or HOCl–HDL (not shown) was identical to that of free MPO with main absorption maxima at 430, 570, 622, and 690 nm, which are characteristic of a six-coordinated high-spin aquo ferric haem.32 This indicates intact haeme coordination and reactivity, which are underlined by the polarographic measurements of H2O2 consumption (Figure 4). Nevertheless, binding of MPO to HDL or HOCl–HDL is reflected by some loss of haem ellipticity and changes in the absorption region of aromatic amino acids in the corresponding CD spectra (Supplementary material online, Figure S1C and D). The effect was more pronounced in the MPO/HOCl–HDL system. Its near-UV CD spectrum differs significantly from that of MPO/HDL system reflecting also modifications of aromatic amino acids in HDL by HOCl. Indeed, the specific binding site for MPO on apoA-I is located in helix 8, where, in addition to a lysine residue, tyrosine 192 has been identified as the most potent amino acid for nitration and chlorination, respectively.17–19,21
3.4 Cell association of myeloperoxidase is impaired by HOCl–high-density lipoprotein
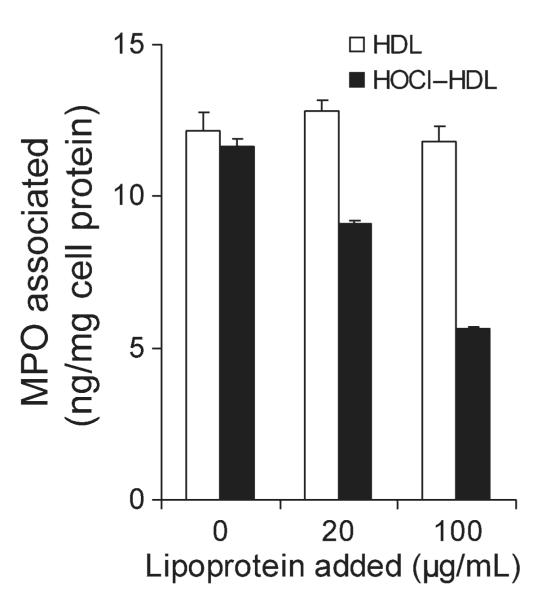
After release from activated phagocytes, MPO adheres to the endothelial layer.10,33 Immunofluorescence double-labelling experiments revealed co-localization of HOClmodified epitopes with MPO and apoA-I on endothelial cells lining the blood vessel.8 We therefore tested whether HOCl–HDL interferes with cell association (the sum of binding and internalization) of MPO. Even in the presence of 50% FCS, HOCl–HDL inhibited cell association of 125I-MPO to human endothelial cells in a concentration-dependent manner (Figure 5). Native HDL was without significant effects on 125I-MPO binding.
Figure 5. Cell association of 125I-MPO by endothelial cells.
Human endothelial cells were incubated (1 h, 37°C) in DMEM (containing 50% foetal calf serum) with 125I-MPO (200 ng/mL) in the absence or presence of indicated concentrations (20 and 100 μg/mL) of high-density lipoprotein or HOCl–high-density lipoprotein (oxidant:lipoprotein molar ratio of 200:1). Subsequently, the cells were washed and lysed, total cell-associated radioactivity was counted, and cell protein content was measured. Results represent mean ± SD (n = 3) of one experiment out of three.
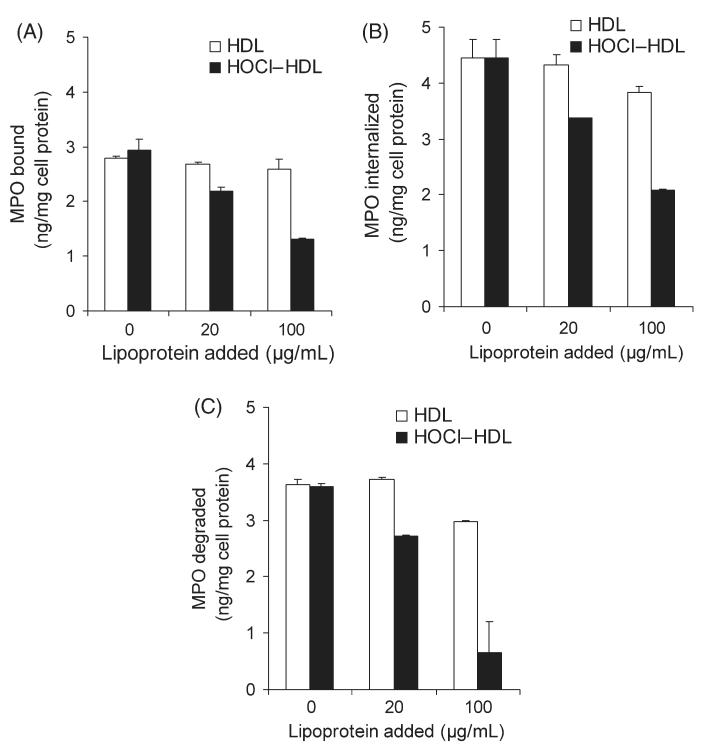
In human atherosclerotic lesions, MPO is also detected in association with macrophages.9–11 Therefore, the next series of experiments were performed with macrophages; cell association (binding and internalization) and degradation of 125I-MPO were measured. HOCl–HDL effectively inhibited cell association, i.e. binding (Figure 6A) and internalization (Figure 6B) of 125I-MPO in a concentration-dependent manner even in the presence of 50% FCS. In line with these findings (Figure 6A and B), lysosomal degradation of 125I-MPO was also inhibited by HOCl–HDL in a concentration-dependent manner up to 80% (Figure 6C). Native HDL showed weak inhibitory activity (up to 15%) on cell association and degradation of 125I-MPO (Figure 6A–C).
Figure 6. Binding, internalization, and degradation of 125I-MPO by macrophages.
RAW macrophages were incubated (2 h, 37°C) in DMEM (containing 50% foetal calf serum) with 125I-MPO (200 ng/mL) in the absence or presence of indicated concentrations (20 and 100 mg/mL) of high-density lipoprotein or HOCl–high-density lipoprotein (oxidant:lipoprotein molar ratio of 200:1) to determine binding (A), internalization (B), and degradation of 125I-MPO (C). After the incubation period, the cells were rinsed, and incubated with Dulbecco’s modified Eagle’s medium containing heparin (100 U/mL) for further 20 min to release cell surface-bound myeloperoxidase (heparin-releasable) (A). Then the cells were lysed and radioactivity counted to measure internalized myeloperoxidase (heparin-resistant) (B) and to measure protein content of the cells. Degradation of 125I-MPO (C) was determined as described in the Methods section. Results represent mean ± SD (n = 3) of one experiment out of three.
4. Discussion
Both abundant staining for MPO8–11 and the high extent of 3-chlorotyrosine or nitrotyrosine on apoA-I21 support halogenation/nitration activity of the MPO-H2O2 system in human lesion material where apoA-I has been found to co-localize with HOCl-modified epitopes.13,22 The massive accumulation of 2-chlorohexadecanal,34 a reactive aldehyde, generated from HOCl-mediated attack of lipoproteinand cell-associated ether phospholipids, provides further evidence for MPO-mediated activity in human lesion material.
Panzenboeck et al.20 first demonstrated that HOCl, added as reagent or generated by the MPO–H2O2–chloride system, transforms HDL into a modified lipoprotein particle with characteristics similar to lipoproteins commonly thought to initiate foam cell formation in vivo. Compared with HDL, HOCl–HDL is preferentially taken up and degraded by macrophages.20 This uptake is not due to aggregation, i.e. inter-particle cross-linking of apoA-I in modified HDL, as confirmed by static and dynamic light scattering experiments.20 In search for a candidate receptor that mediates uptake of HOCl–HDL, we could previously identify SR-BI,22 a class B scavenger receptor highly upregulated in human atherosclerotic lesions.35 In addition to an enhanced cellular lipid uptake, a decreased lipid acceptor activity of modified HDL13,17,19,24 supported the concept that modification of HDL by HOCl, added as reagent or generated by the MPO–H2O2–chloride system, generates a dysfunctional lipoprotein particle.16,20 Subsequently, a number of studies have underlined an important role for MPO in the oxidative modification of HDL in vitro and in vivo.18,19,21,36,37
HDL isolated from human atheroma contains MPO, and the protein moiety of HDL represents a specific target for nitration and chlorination within human atheroma.21 Most importantly, ~15% of the HDL-like particles recovered from human aortic lesions contain at least one oxidative modification; this amount may increase up to 50% in certain patients.21 As apoA-I is the major HDL-associated apolipoprotein, attempts have been made to identify specific regions and/or segments in the primary structure prone to be modified by the MPO–H2O2 system in vivo and in vitro.17,19,21,38
Here, we show that modification of HDL by the MPO product HOCl markedly increases binding affinity of HDL for MPO (Figure 1B). Using different experimental approaches, we show that binding of MPO to modified HDL increases as a function of the extent of HOCl modification (Figure 1A). Notably, we observed that binding affinity of MPO to native HDL is much weaker compared with HOCl–HDL. The observed increase in binding affinity is due to a reduction of the positive charge of the protein via formation of N-chloramines (and/or breakdown products of corresponding N-chloramines), leading to increased interaction with positively charged MPO. N-chloramines can decompose into the carbonyl aminoadipic acid semialdehyde, which can be further oxidized to 2-aminoadipic acid accounting up to 44% of total lysine loss.18 Most importantly, an oxidant:lipoprotein molar ratio of 50:1 and 200:1, as used in the present study, results in the loss of 15 or 45% lysine residues (Figure 2B) and 40 and 85% of tyrosine residues (Figure 2C) but increased REM of 23 and 79% (Figure 2A)—data in good agreement with previous findings.39,40 It has to be noted that upon binding of MPO to the corresponding HDL particle, changes of the overall secondary structure of MPO could not be detected, whereas observed deviations in the near-UV and visible CD region suggest re-arrangements of its three-dimensional structure. In any case, these re-arrangements only slightly affected the enzymatic capacity of MPO to follow the halogenation cycle and to oxidize a halide in the presence of H2O2. The maximum observed decrease in the activity of 10 μg MPO/mL incubated with 1 mg/mL HOCl–HDL for 30 min was ~25% compared with free MPO.
We could show that HOCl–HDL inhibits degradation of MPO by macrophages thereby possibly sustaining active MPO levels. Furthermore, we show that HOCl–HDL inhibits cell association of MPO with endothelial cells (Figure 5). This must be seen in the context that MPO released from activated neutrophils rapidly associates with endothelial cells. Assuming local activity of the MPO–H2O2–chloride system, low concentrations of HOCl at sites of inflammation cause very rapid shortening of actin microfilaments, cell retraction and increased endothelial permeability,41 and even endothelial cell lysis.42,43 Moreover, cell-bound MPO undergoes transcytosis across the intact endothelium, where the enzyme may modify extracellular matrix proteins.33
In summary, we propose that modification of HDL by MPO-derived HOCl results in the formation of a potent carrier for enzymatically active MPO. Most importantly, association of MPO with HOCl–HDL protects this peroxidase from cellular uptake and degradation. Furthermore, monocytes/macrophages, when exposed to enzymatically active MPO, may exhibit an increased capacity for phagocytosis and intracellular killing of micro-organisms.44 However, in parallel, the cellular uptake of MPO is accompanied by modulation of the activation state of monocytes/macrophages,45 leading to the release of cytokines and formation of MPO-generated radicals.46 On the other hand, when MPO is trapped by HOCl–HDL extracellularly, the enzymatically highly active MPO may then act as a catalyst for oxidative modifications of lipids and/or proteins that in turn may contribute to inflammatory diseases, e.g. atherosclerosis, glomerulosclerosis, arthritis, cystic fibrosis, and asthma.47–51
Supplementary Material
Acknowledgements
The authors are grateful to Dr M. Vadon (Medical University of Graz, Graz, Austria) for providing human plasma, and G. Radspieler for performing amino acid analyses. The expert technical assistance of M. Sundl and S. Zirkl is appreciated.
Funding: This work was supported by grants from the Austrian Science Fund (FWF, P17013-B05, P19074-B05, F3007-B05, P20664-B11) to P.G.F., W.S., and E.M.
Footnotes
Conflict of interest: none declared.
References
- 1.Stocker R, Keaney JF., Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 2.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 3.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase–hydrogen peroxide–chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985;840:204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg JJ, Winterbourn CC, Kuypers FA. Hypochlorous acid-mediated modification of cholesterol and phospholipid: analysis of reaction products by gas chromatography–mass spectrometry. J Lipid Res. 1993;34:2005–2012. [PubMed] [Google Scholar]
- 5.Prütz WA. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch Biochem Biophys. 1996;332:110–120. doi: 10.1006/abbi.1996.0322. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 7.Malle E, Marsche G, Arnhold J, Davies MR. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim Biophys Acta. 2006;1761:392–415. doi: 10.1016/j.bbalip.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Malle E, Marsche G, Panzenboeck U, Sattler W. Myeloperoxidase-mediated oxidation of high-density lipoproteins: fingerprints of newly recognized potential proatherogenic lipoproteins. Arch Biochem Biophys. 2006;445:245–255. doi: 10.1016/j.abb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malle E, Waeg G, Schreiber R, Gröne E, Sattler W, Gröne HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur J Biochem. 2000;267:4495–4503. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malle E, Wäg G, Thiery J, Sattler W, Gröne HJ. Hypochlorite-modified (lipo)proteins are present in rabbit lesions in response to dietary cholesterol. Biochem Biophys Res Commun. 2001;289:894–900. doi: 10.1006/bbrc.2001.6074. [DOI] [PubMed] [Google Scholar]
- 13.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97:1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholls SJ, Zheng L, Hazen SL. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc Med. 2005;15:212–219. doi: 10.1016/j.tcm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Bergt C, Reicher H, Malle E, Sattler W. Hypochlorite modification of high density lipoprotein: effects on cholesterol efflux from J774 macrophages. FEBS Lett. 1999;452:295–300. doi: 10.1016/s0014-5793(99)00677-8. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L, Settle M, Brubaker G, Schmitt D, Hazen SL, Smith JD, et al. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. J Biol Chem. 2005;280:38–47. doi: 10.1074/jbc.M407019200. [DOI] [PubMed] [Google Scholar]
- 18.Peng DQ, Wu Z, Brubaker G, Zheng L, Settle M, Gross E, et al. Tyrosine modification is not required for myeloperoxidase-induced loss of apolipo-protein A-I functional activities. J Biol Chem. 2005;280:33775–33784. doi: 10.1074/jbc.M504092200. [DOI] [PubMed] [Google Scholar]
- 19.Shao B, Bergt C, Fu X, Green P, Voss JC, Oda MN, et al. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J Biol Chem. 2005;280:5983–5993. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 20.Panzenboeck U, Raitmayer S, Reicher H, Lindner H, Glatter O, Malle E, et al. Effects of reagent and enzymatically generated hypochlorite on physicochemical and metabolic properties of high density lipoproteins. J Biol Chem. 1997;272:29711–29720. doi: 10.1074/jbc.272.47.29711. [DOI] [PubMed] [Google Scholar]
- 21.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsche G, Hammer A, Oskolkova O, Kozarsky KF, Sattler W, Malle E. Hypochlorite-modified high density lipoprotein, a high affinity ligand to scavenger receptor class B, type I, impairs high density lipoprotein-dependent selective lipid uptake and reverse cholesterol transport. J Biol Chem. 2002;277:32172–32179. doi: 10.1074/jbc.M200503200. [DOI] [PubMed] [Google Scholar]
- 23.Malle E, Ibovnik A, Steinmetz A, Kostner GM, Sattler W. Identification of glycoprotein IIb as the lipoprotein(a)-binding protein on platelets. Lipoprotein(a) binding is independent of an arginyl-glycyl-aspartate tripeptide located in apolipoprotein(a) Arterioscler Thromb. 1994;14:345–352. doi: 10.1161/01.atv.14.3.345. [DOI] [PubMed] [Google Scholar]
- 24.Marsche G, Levak-Frank S, Quehenberger O, Heller R, Sattler W, Malle E. Identification of the human analog of SR-BI and LOX-1 as receptors for hypochlorite-modified high density lipoprotein on human umbilical venous endothelial cells. FASEB J. 2001;15:1095–1097. doi: 10.1096/fj.00-0532fje. [DOI] [PubMed] [Google Scholar]
- 25.Malle E, Ibovnik A, Leis HJ, Kostner GM, Verhallen PF, Sattler W. Lysine modification of LDL or lipoprotein(a) by 4-hydroxynonenal or malondialdehyde decreases platelet serotonin secretion without affecting platelet aggregability and eicosanoid formation. Arterioscler Thromb Vasc Biol. 1995;15:377–384. doi: 10.1161/01.atv.15.3.377. [DOI] [PubMed] [Google Scholar]
- 26.Furtmüller PG, Zederbauer M, Jantschko W, Helm J, Bogner M, Jakopitsch C, et al. Active site structure and catalytic mechanisms of human peroxidases. Arch Biochem Biophys. 2006;445:199–213. doi: 10.1016/j.abb.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Furtmüller PG, Jantschko W, Regelsberger G, Jakopitsch C, Arnhold J, Obinger C. Reaction of lactoperoxidase compound I with halides and thiocyanate. Biochemistry. 2002;41:11895–11900. doi: 10.1021/bi026326x. [DOI] [PubMed] [Google Scholar]
- 28.Marsche G, Semlitsch M, Hammer A, Frank S, Weigle B, Demling N, et al. Hypochlorite-modified albumin colocalizes with RAGE in the artery wall and promotes MCP-1 expression via the RAGE-Erk1/2 MAP-kinase pathway. FASEB J. 2007;21:1145–1152. doi: 10.1096/fj.06-7439com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuszkowski A, Gräbner R, Marsche G, Unbehaun A, Malle E, Heller R. Hypochlorite-modified low density lipoprotein inhibits nitric oxide synthesis in endothelial cells via an intracellular dislocalization of endothelial nitric-oxide synthase. J Biol Chem. 2001;276:14212–14221. doi: 10.1074/jbc.M007659200. [DOI] [PubMed] [Google Scholar]
- 30.Artl A, Marsche G, Lestavel S, Sattler W, Malle E. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler Thromb Vasc Biol. 2000;20:763–772. doi: 10.1161/01.atv.20.3.763. [DOI] [PubMed] [Google Scholar]
- 31.Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc Natl Acad Sci USA. 2004;101:7699–7704. doi: 10.1073/pnas.0401712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malle E, Furtmüller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thukkani AK, McHowat J, Hsu FF, Brennan ML, Hazen SL, Ford DA. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 2003;108:3128–3133. doi: 10.1161/01.CIR.0000104564.01539.6A. [DOI] [PubMed] [Google Scholar]
- 35.Hirano K, Yamashita S, Nakagawa Y, Ohya T, Matsuura F, Tsukamoto K, et al. Expression of human scavenger receptor class B type I in cultured human monocyte-derived macrophages and atherosclerotic lesions. Circ Res. 1999;85:108–116. doi: 10.1161/01.res.85.1.108. [DOI] [PubMed] [Google Scholar]
- 36.Pussinen PJ, Metso J, Keva R, Hirschmugl B, Sattler W, Jauhiainen M, et al. Plasma phospholipid transfer protein-mediated reactions are impaired by hypochlorite modification of high density lipoprotein. Int J Biochem Cell Biol. 2003;35:192–202. doi: 10.1016/s1357-2725(02)00130-9. [DOI] [PubMed] [Google Scholar]
- 37.Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, Smith JD, et al. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol. 2007;14:861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 38.Bergt C, Oettl K, Keller W, Andreae F, Leis HJ, Malle E, et al. Reagent or myeloperoxidase-generated hypochlorite affects discrete regions in lipid-free and lipid-associated human apolipoprotein A-I. Biochem J. 2000;346:345–354. [PMC free article] [PubMed] [Google Scholar]
- 39.Opper C, Schüssler G, Sattler W, Malle E. Effects of hypochlorite-modified low density lipoproteins and high density lipoproteins on platelet function. Platelets. 1998;9:339–341. doi: 10.1080/09537109876582. [DOI] [PubMed] [Google Scholar]
- 40.Zabe M, Feltzer RE, Malle E, Sattler W, Dean WL. Effects of hypochloritemodified low-density and high-density lipoproteins on intracellular Ca2+ and plasma membrane Ca2+-ATPase activity of human platelets. Cell Calcium. 1999;26:281–287. doi: 10.1054/ceca.1999.0081. [DOI] [PubMed] [Google Scholar]
- 41.Tatsumi T, Fliss H. Hypochlorous acid and chloramines increase endothelial permeability: possible involvement of cellular zinc. Am J Physiol. 1994;267:H1597–H1607. doi: 10.1152/ajpheart.1994.267.4.H1597. [DOI] [PubMed] [Google Scholar]
- 42.Mathy-Hartert M, Deby-Dupont G, Deby C, Jadoul L, Vandenberghe A, Lamy M. Cytotoxicity towards human endothelial cells, induced by neutrophil myeloperoxidase: protection by ceftazidime. Mediators Inflamm. 1995;4:437–443. doi: 10.1155/S0962935195000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C, Patel R, Eiserich JP, Zhou F, Kelpke S, Ma W, et al. Endothelial dysfunction is induced by proinflammatory oxidant hypochlorous acid. Am J Physiol Heart Circ Physiol. 2001;281:H1469–H1475. doi: 10.1152/ajpheart.2001.281.4.H1469. [DOI] [PubMed] [Google Scholar]
- 44.Lefkowitz SS, Gelderman MP, Lefkowitz DL, Moguilevsky N, Bollen A. Phagocytosis and intracellular killing of Candida albicans by macrophages exposed to myeloperoxidase. J Infect Dis. 1996;173:1202–1207. doi: 10.1093/infdis/173.5.1202. [DOI] [PubMed] [Google Scholar]
- 45.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci USA. 2005;102:431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lefkowitz DL, Mills K, Morgan D, Lefkowitz SS. Macrophage activation and immunomodulation by myeloperoxidase. Proc Soc Exp Biol Med. 1992;199:204–210. doi: 10.3181/00379727-199-43348. [DOI] [PubMed] [Google Scholar]
- 47.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 48.Gröne HJ, Gröne EF, Malle E. Immunohistochemical detection of hypochlorite-modified proteins in glomeruli of human membranous glomerulonephritis. Lab Invest. 2002;82:5–14. doi: 10.1038/labinvest.3780390. [DOI] [PubMed] [Google Scholar]
- 49.Davies JM, Horwitz DA, Davies KJ. Potential roles of hypochlorous acid and N-chloroamines in collagen breakdown by phagocytic cells in synovitis. Free Radic Biol Med. 1993;15:637–643. doi: 10.1016/0891-5849(93)90167-s. [DOI] [PubMed] [Google Scholar]
- 50.Van Der Vliet A, Nguyen MN, Shigenaga MK, Eiserich JP, Marelich GP, Cross CE. Myeloperoxidase and protein oxidation in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2000;279:L537–L546. doi: 10.1152/ajplung.2000.279.3.L537. [DOI] [PubMed] [Google Scholar]
- 51.Aldridge RE, Chan T, van Dalen CJ, Senthilmohan R, Winn M, Venge P, et al. Eosinophil peroxidase produces hypobromous acid in the airways of stable asthmatics. Free Radic Biol Med. 2002;33:847–856. doi: 10.1016/s0891-5849(02)00976-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.