Abstract
The induction of an anti-viral state by type I interferons (IFN) was evaluated in primary trigeminal ganglion cell cultures using herpes simplex virus type 1 (HSV-1). Cells treated with mouse IFN-β consistently showed the greatest resistance to HSV-1 infection in comparison to cells treated with IFN-α1, IFN-α4, IFN-α5, IFN-α6, or IFN-α9. The anti-viral efficacy was dose-dependent and correlated with the induction of the IFN-inducible, anti-viral genes, 2′–5′ oligoadenylate synthetase (OAS) and double-stranded RNA-dependent protein kinase. In trigeminal ganglion cells deficient in the downstream effector molecule of the OAS pathway, RNAse L, the anti-viral state induced by IFN-β was lost.
Keywords: IFN-α, IFN-β, HSV-1, PKR, OAS, neuroimmunology
1. Introduction
Herpes simplex virus type 1 (HSV-1) is a highly successful human pathogen establishing a latent infection in sensory ganglion neurons following axoplasmic transport from the initial site of entry (Hay et al., 1998). The prevalence of the virus in the human population is due, in part, to the immune evading mechanisms developed by the virus through co-evolution with the host (Friedman et al., 1984; Jennings et al., 1985; Hill et al., 1995; van Strijp et al., 1988; Jerome et al., 1998; Orange et al., 2002). During latency, evidence suggests the virus continually undergoes spontaneous, incomplete reactivation (Feldman et al., 2002) which may explain the chronic expression of cytokines within the latently-infected ganglion (Shimeld et al., 1995; Liu et al., 1996; Halford et al., 1996a, 1997). Presently, it is not entirely clear what prevents viral reactivation although candidates include neutralizing antibodies (Mikloska et al., 1999) as well as infiltrating leukocytes, interferon (IFN)-γ and other soluble mediators (Noisakran and Carr, 1999; Liu et al., 2000, 2001). However, the virus can ultimately overcome immune surveillance experimentally shown using hyperthermic stress (Sawtell and Thompson, 1992), an event that eliminates infiltrated T lymphocytes from the trigeminal ganglion (TG) cell cultures (Carr et al., 1998).
Type I IFNs (including IFN-α and IFN-β) are potent cytokines generated in response to viral infections that control HSV-1 replication and spread following ocular infection (Hendricks et al., 1991). While IFNs are effective in blocking HSV-1 replication at the transcriptional level (De Stasio and Taylor, 1990), HSV-1 encodes for a number of proteins including γ134.5 (Chou et al., 1995; Cheng et al., 2001), ICP0 (Mossman et al., 2000; Härle et al., 2002a; Eidson et al., 2002), and US11 (Peters et al., 2002) that counter the activation or activity of IFN-inducible proteins.
In addition, HSV-1 has also been found to antagonize JAK1/STAT1 signaling following IFN receptor stimulation preventing the formation of the IFN-stimulated gene factor 3 (ISGF3) complex and induction of IFN-stimulatory genes (Yokota et al., 2001). The intricate relationship between HSV-1 and one IFN-stimulatory pathway double-stranded RNA-dependent protein kinase (PKR) is exemplified by the restoration of a wild type phenotype using an ICP34.5 deletion mutant in PKR-deficient mice (Leib et al., 2000).
The present study was undertaken to further explore the relationship between type I IFNs and HSV-1 infection focusing on the TG. Specifically, primary TG cell cultures were established and evaluated for resistance to HSV-1 infection in the presence or absence of type I IFNs. Previously, we reported L929 cells transiently transfected with type I IFN transgenes showed various degrees of resistance to HSV-1 infection through a PKR-dependent pathway independent of the OAS pathway (Härle et al., 2002b). Consistent with the previous study, the present investigation found IFN-β elicited the greatest anti-viral efficacy in TG cells compared to IFN-α species. However, in the absence of RNase L, the downstream effector molecule of 2′–5′ oligoadenylate (OAS), the resistance to viral infection mediated by IFN-β was lost suggesting tissue specificity of the IFN activated, anti-viral pathway OAS.
2.0 Materials and methods
2.1 Virus and cell line
Vero cells and L929 cells originally obtained from the American Type Culture Collection (ATCC, Manassas, VA.) were cultured in RPMI-1640 (Invitrogen, Carlsbad, CA) or DMEM (for L929 cells, ATCC) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and antibiotic/antimycotic solution (AAS, Invitrogen) at 37° C, 5% CO2, and 95% humidity. HSV-1 stock (McKrae strain) was prepared as previously described (Halford et al., 1996a). Vesicular stomatitis virus (VSV) was a gift from Dr. Robert Fleishmann (Dept. Microbiology, UTMB).
2.2 Plasmid DNA constructs
All murine IFN transgenes were cloned into the eukaryotic expression vector pkCMVintPolylinker (5,087 bp, Vical Inc., San Diego, CA.) containing a simian virus 40 polyadenylation signal and a kanamycin resistance gene (Cull et al., 2002. The IFN genes (575 to 626 bp) are expressed under the control of a human cytomegalovirus immediate-early enhancer/promoter. The plasmid constructs were transformed into Escherichia coli strain INVαF′ (Invitrogen) and grown in Terrific broth containing 50 μg kanamycin/ml followed by purification using a plasmid maxiprep kit (Bio-Rad). After the plasmid isolation, restriction enzyme digestion assays were conducted and the products analyzed by agarose gel electrophoresis.
2.3 Establishment of TG cell cultures
TG cell cultures were prepared as described (Halford et al., 1996b) using female ICR outbred mice (25–34 g, Harlan Spague Dawley, Indianapolis, IN.), C57BL/6J wild type mice (The Jackson Laboratory, Bar Harbor, ME.), or mice with a homozygous disruption in the gene encoding RNase L (Zhou et al., 1997) as the source of the TG cells. The cells were cultured in 1.0 ml DMEM containing 0.375% HCO3 supplemented with 10% FBS and AAS (referred to as complete DMEM). The cultures were maintained at 37° C, 5% CO2, and 95% humidity. After 7–12 days in culture, the cells were either transfected with plasmid vector alone or plasmids containing type I IFN transgenes or exposed to various concentrations of type I IFN.
2.4 Transfection of TG or L929 cells
Seven to twelve days post initiation of culture, TG cells (1.0 +/− 0.3 × 105 cells/well) in 6-well microtiter plates were transfected with 2 μg of plasmid DNA and 10 μl of Superfect (Qiagen, Valencia, CA.) in 700 μl of complete DMEM for 3 hr. The cells were then washed once with prewarmed (37° C) phosphate buffered saline (PBS, pH 7.4) and 2.0 ml of fresh, prewarmed complete DMEM was added to each well. Twenty-four hr post transfection, supernatants were collected and cells were infected with HSV-1 at a multiplicity of infection (MOI) of 0.5. The viral inoculum was removed 1 hr post infection (p.i.) and replaced with fresh complete DMEM. After 24 hr, the cells were freeze-thawed and the clarified supernatants (10,000 × g, 1 min) were assayed for HSV-1 titer by plaque assay using Vero cells. In a separate experiment, known concentrations of biologically active IFN (3.2 – 32.0 units) were added to the TG cell cultures 24 hr prior to infection with HSV-1 (MOI = 0.5).
L929 cells were transfected as previously described (Härle et al., 2002b) to generate IFN-α1, IFN-α4, IFN-α5, IFN-α6, IFN-α9, and IFN-β enriched supernatants. The supernatants were evaluated for the quantity of biologically active IFN by bioassay.
2.5 IFN bioassay
To determine the concentration of biologically active IFN secreted at the end of the 24 hr post transfection period, supernatants from the transfected cells were serially diluted and incubated with L929 cells for 24 hr in 96 well plates. Following the incubation period, the supernatant was removed and the cells were infected with VSV (MOI = 0.05). When the cytopathic effect (CPE) in the control wells was maximal (32 hr p.i.), the cells were stained with crystal violet. In each assay, a standard curve using mouse recombinant IFN-β (Petska Biomedical Laboratories, New Brunswick, NJ) was used. Fifty percent inhibition of CPE was equivalent to 1.0 unit/ml IFN.
2.6 Reverse transcription and real-time PCR
Total TG cell RNA was isolated 12 hr p.i. or 24 hr post IFN-treatment (for OAS and PKR gene expression) in Ultraspect RNA isolation reagent (Biotecx Inc., Houston, TX.) according to the manufacturer’s protocol. First strand cDNA was synthesized using avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI) and an oligo (dT) primer (Promega). Real time PCR analysis of targeted gene expression was carried out using the iCycler (Bio-Rad) as previously described (Al-khatib et al., 2002).
2.7 Immunohistochemistry
Dissociated TG cells obtained from ICR mice were grown in 24 well microtiter plates coated with laminin and collagen. Seven days following the initiation of the culture, the cells were fixed for 30 min in 3% paraformaldehyde in PBS containing 0.1% Triton X-100 (pH 7.5) and then rinsed three times in 1.0 ml of PBS containing 0.1% Triton X-100 (pH 7.5). Non-specific binding sites were blocked with 10% normal goat serum for 30 min at room temperature. Cells were incubated overnight with rabbit anti-glial fibrillary acidic protein (GFAP) antibody (1:200 dilution, DAKO, Carpintera, CA.), anti-microtubule associated protein (MAP) antibody (1:50 dilution, Accurate Chemical Corp., Westbury, NY) or anti-neuron-specific enolase antibody (1:100 dilution, Chemicon, Temecular, CA) in PBS (pH 7.5) containing 1% normal goat serum at 4° C. Following the overnight incubation, the cells were then rinsed three times with 1.0 ml PBS containing 0.1% Triton X-100 and incubated with Texas red or peroxidase-conjugated goat anti-rabbit IgG (1:200 dilution, Vector Laboratories, Burlingame, CA.) in PBS containing 10% normal goat serum for 1 hr. The cells were then rinsed three times for 5 min each with PBS and substrate (3,3′-diaminobenzidine) was added to peroxidase-labeled cells. Peroxidase- and fluorescently-labeled cells were viewed using a Nikon E800 fluorescent microscope. Cells treated with secondary antibody only served as controls.
2.8 Statistics
One-way analysis of variance (ANOVA) and Tukey’s t-test were used to determine significant (p<.05) differences between the vector and IFN-α/β transgene- or protein-treated groups.
3.0 Results
3.1 Phenotypic characterization of TG cell culture
Dissociated TG cells cultured for 7 days were assessed for the percentage of neurons and Schwann cells using immunohistochemical means. Using either anti-neuron-specific enolase or MAP antibodies, 1.5 +/− 0.5% of the cells were defined as neurons whereas 3.1 +/− 1.0% of the cells expressed high levels of GFAP (Schwann cells) (Fig. 1). A significant proportion of cells (> 80%) expressed low levels of GFAP and may represent quiescent Schwann cells.
Figure 1. Phenotypic profile of TG cell cultures.
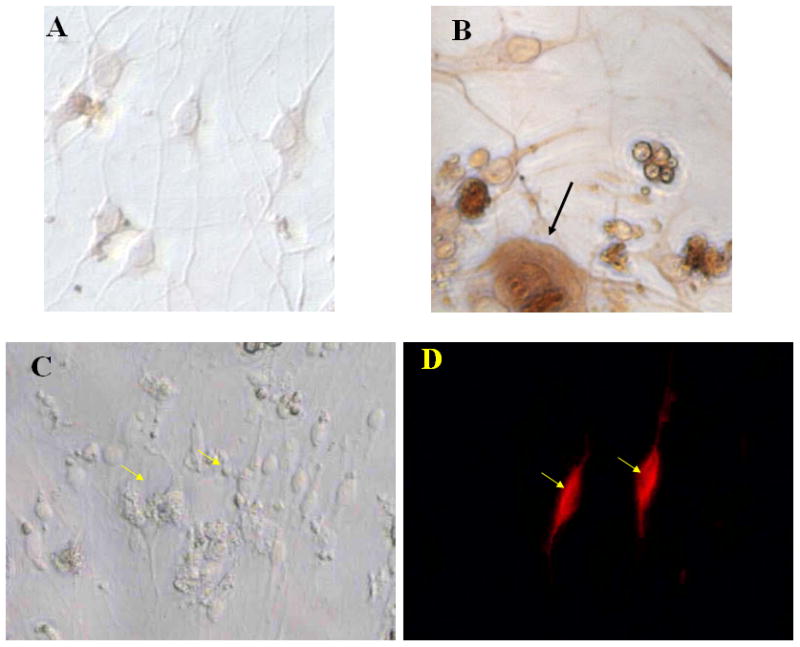
Primary trigeminal ganglion cells (1×105 cells) were cultured for 7 days, fixed, and then assessed for the percentage of neurons and Schwann cells by immunohistochemical means. (A) and (B) show a typical staining pattern for microtubule associated protein staining of neurons (B) and negative control (A). (C) and (D) show a typical staining pattern for GFAP-labeled cells under fluorescent light (D) and light field (C) microscopy. Arrows indicate neuron (black) and Schwann cells (yellow) magnified 200X.
3.2 Resistance to HSV-1 infection by transfected TG cells
TG cell cultures were evaluated for resistance to HSV-1 infection following transfection with plasmid DNA containing different type I IFN transgenes. TG cell cultures were highly susceptible to HSV-1 infection (MOI = 0.5) yielding 698,441 +/− 86,631 plaque forming units (pfu)/culture within 24 hr. There is no biologically active IFN detected in these cultures following HSV-1 infection (limit of detection is 1.0 unit). Cells transfected with plasmid vector or plasmid vector containing type I IFN transgenes were less susceptible to infection with cells transfected with the IFN-β showing the greatest degree of resistance to HSV-1 infection (Fig. 2). The degree of anti-viral efficacy comparing the type I IFN transgenes was not due to differences in the amount of biologically active IFN secreted by the cells as all transfected cultures (except vector-transfected cells) secreted similar amounts of IFN (Fig. 2). Since the vector-transfected cells were less susceptible to infection compared to non-transfected cells but did not consistently produce measurable levels of IFN (only 3/7 cultures tested produced at least 1.0 unit of IFN), the process of transfection itself or the plasmid vector alone induced an IFN-independent, anti-viral state in the targeted cells. Consequently, a different approach was necessary in order to compare the anti-viral efficacies of type I IFNs against HSV-1 using primary TG cells.
Figure 2. Transfection of primary TG cells with IFN-β transgene suppresses HSV-1 replication.
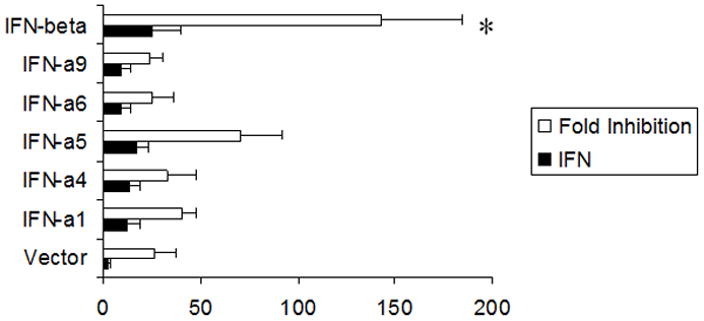
Primary trigeminal ganglion cells (1×105 cells) in culture were transfected with 2 μg of pkCMV plasmid DNA alone (Vector) or plasmid vector DNA containing the indicated type I IFN transgene. Twenty-four hr post-transfection, the supernatant was removed and assayed for IFN content by bioassay (Units/ml) and the cells were subsequently infected with HSV-1 (MOI=0.5). Twenty-four hr post infection, the cultures were freeze-thawed and viral titers determined. The amount of biologically active IFN is depicted in solid bars whereas the fold inhibition is reflected in the open bars. This figure is a summary of six experiments. *p<.05 comparing the vector- to IFN-β-transfected groups as determined by ANOVA and Tukey’s t-test. Bars represent mean +/− SEM.
3.3 IFN-β antagonizes HSV-1 replication at the transcriptional level
Type I IFN-enriched supernatants obtained from transiently transfected L929 cells were added to TG cell cultures 24 hr prior to HSV-1 infection. In a dose-dependent fashion, IFN-β was found to suppress HSV-1 replication compared to cells treated with supernatant from vector-transfected L929 cells as measured by viral titers recovered in the infected TG cell cultures (Fig. 3). Only the highest concentration of IFN-α4 tested (32 units/ml) was found to antagonize HSV-1 replication in comparison to the other IFN-α species tested (Fig. 3). In a similar fashion, TG cell cultures treated with IFN-β were found to show the greatest inhibition of steady state HSV-1 lytic gene expression as measured by real time PCR twelve hr post infection (Fig. 4). In comparison to TG cell cultures incubated with supernatant from plasmid vector-transfected L929 cells, IFN-β-enriched supernatant (32 units/ml) suppressed immediate early (infection cell protein 27, ICP27), early (thymidine kinase, TK), and late (virion protein 16, VP16) lytic gene expression. By comparison, the same concentration of the IFN-α species only suppressed the immediate early and late gene but not the early lytic gene TK.
Figure 3. IFN-β antagonizes HSV-1 replication in a dose-dependent fashion in primary TG cell cultures.
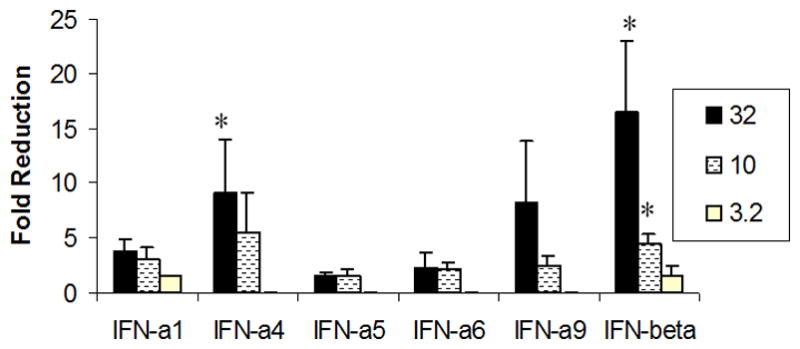
TG cell cultures were treated with the indicated concentration of type I IFN and subsequently infected with HSV-1 (MOI=0.5) 24 hr later. Following a 24 hr incubation period, the cultures were freeze-thawed and viral titers were determined by plaque assay. This figure is a summary of four experiments with each treatment conducted in duplicate. *p<.05 comparing the type I IFN-treated group to the vector supernatant-treated control as determined by ANOVA and Tukey’s t-test. Bars represent mean +/− SEM.
Figure 4. IFN-β suppresses HSV-1 lytic gene expression.
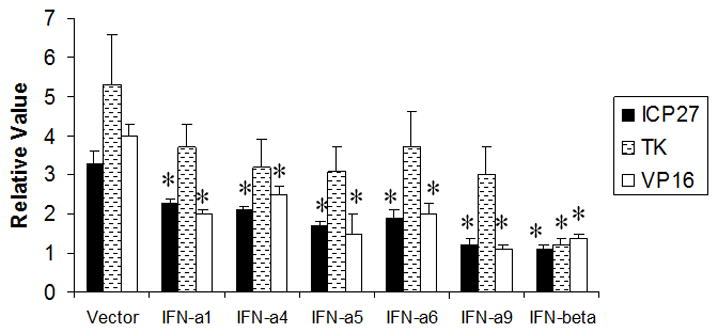
IFN-treated (32 units/culture) primary TG cells were infected 24 hr post-treatment with HSV-1 (MOI=0.5). Twelve hr post-infection, RNA was isolated from each culture and used to generate cDNA. Using oligonucleotide primers specific for the immediate early (ICP27), early (TK), and late (VP16) lytic HSV-1 genes, the relative amounts of each targeted gene was determined by real- time PCR. The relative level for each gene expressed in the TG cells was normalized to the relative value of GAPDH for each group. Undiluted supernatant from vector-transfected cells (Vector) served as the control. This figure is a representative of two experiments each conducted in triplicate. *p<.05 comparing the IFN-treated to vector supernatant-treated group as determined by ANOVA and Tukey’s t-test. Bars represent mean +/− SEM.
3.4 IFN-β hinders HSV-1 replication through the OAS/RNase L pathway
Exposure to type I IFNs results in the activation of two prominent anti-viral pathways PKR and OAS. Indeed, when TG cell cultures were incubated with type I IFN-enriched supernatant the expression of both PKR and OAS1a mRNA were significantly (p<.05) elevated compared to cultures incubated with supernatant from vector-transfected cells (Fig. 5). Those cultures exposed to the IFN-β containing supernatant showed the greatest increase in both OAS and PKR expression by real time PCR. The induction of the OAS pathway results in the activation of latent endoribonucleases RNase L which cleaves mRNA and rRNA ultimately inhibiting protein synthesis (Clemens and Williams, 1978; Floyd-Smith et al., 1981). To further explore the relationship between the activation of the OAS pathway by type I IFNs and the establishment of an anti-viral state, TG cell cultures were prepared from RNase L null mice. TG cells from wild type or RNase L null mice infected with HSV-1 (MOI=0.5) yielded similar levels of HSV-1 24 hr post infection (Fig. 6a). However, in the absence of RNase L, IFN-β exposed TG cells exhibited no resistance to HSV-1 infection in comparison to wild type TG cells (Fig. 6b). We interpret these results to suggest that the OAS pathway is involved in antagonizing HSV-1 but only in IFN-pretreated TG cells.
Figure 5. Type I IFN augments the expression of the IFN-stimulatory genes, OAS and PKR.
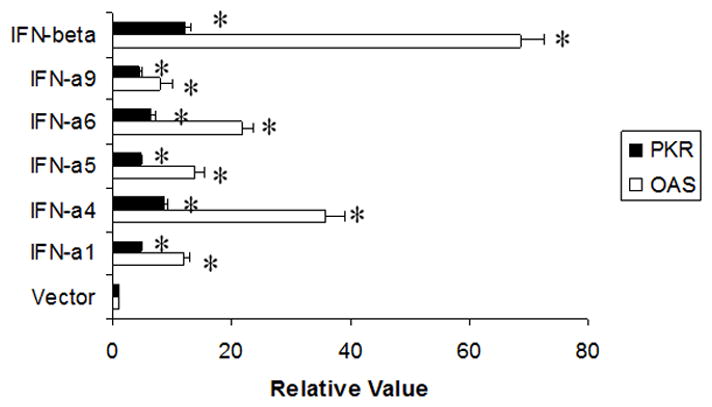
TG cells (1×105 cells) were stimulated with 32 units of the indicated type I IFN or (control) vector supernatant. Twenty-four hr post treatment, the RNA was isolated from the treated cells and used to generate cDNA. Oligonucleotide primers specific for OAS(1a, 1c) or PKR were used to amplify the targeted gene by real-time PCR. The relative level for each gene expressed in the TG cells was normalized to the relative value of GAPDH. This figure is a representative of two experiments each conducted in triplicate. *All IFN-treated groups significantly (p<.05) elevated OAS and PKR gene expression relative to the control (vector)-treated group as determined by ANOVA and Tukey’s t-test. Bars represent mean +/− SEM.
Figure 6. The anti-viral efficacy mediated by IFN-β is dependent on OAS/RNase L.
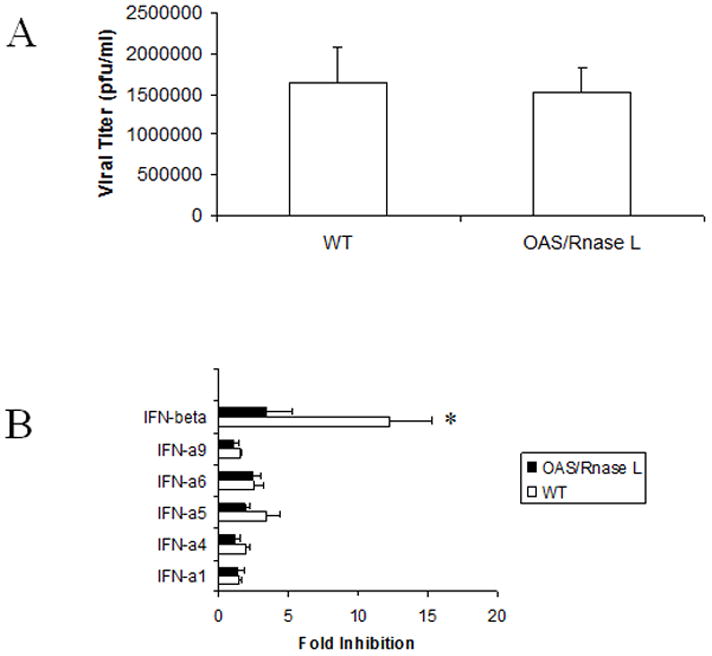
TG cells from wild type (WT) C57BL/6 or RNase L knockout mice (OAS/RNase L) were cultured and either infected with HSV-1 (MOI=0.5) prior to (A) or (B) after treatment with 32 units of the indicated IFN or control (vector). Twenty-four hr post infection, the cell cultures were freeze-thawed and viral titers determined. This figure is a summary of four experiments with each experiment conducted in duplicate. *p<.05 comparing the IFN-β treated, WT TG cells compared to the vector-treated WT controls or the OAS/RNase L TG cells treated with IFN-β as determined by ANOVA and Tukey’s t-test. Bars represent mean +/− SEM.
4.0 Discussion
The present study found that in comparison to 5 murine IFN-α species, IFN-β showed substantially greater efficacy against HSV-1 infection in primary TG cell cultures as measured by viral titer and lytic gene expression, and this protective effect was mediated through the OAS/RNase L pathway. However, the absence of a functional OAS pathway did not have a significant impact on HSV-1 replication in untreated TG cells. These results imply that naive TG cells infected with HSV-1 do not utilize the OAS/RNase L as a means to antagonize viral replication. Candidate alternative pathways that may function in these cells include PKR (Khabar et al., 2000; Härle et al., 2002b) or pathways independent of OAS or PKR (Zhou et al., 1997, 1999). One intriguing aspect of the present findings is that all type I IFNs tested elevated OAS expression in the TG cells but only IFN-β and marginally, IFN-α4 antagonized HSV-1 replication. We interpret these results to suggest a threshold of OAS must be achieved in order to significantly impact on viral replication (IFN-α4 and IFN-β induced the greatest increase in OAS message). It is likely that the relationship between OAS activation and viral replication is dependent on the infectious dose of HSV-1 as well. Specifically, we have recently found that increasing the viral inoculum used to infect cells overrides any effect that the activation of the OAS pathway might have on viral replication (Al-khatib et al., 2003). Consistent with this observation, HSV-1 encodes for inhibitors of RNase L activation thus eroding the anti-viral state induced by OAS activation (Cayley et al., 1984). Consequently, a reciprocal relationship exists between OAS activation and viral titer. Specifically, as viral protein levels increase, the antiviral effect associated with OAS activation is less effective due to direct hindrance in the formation of active RNase L. One probable outcome of this interaction is the generation of compensatory anti-viral pathways independent of OAS activation that are capable of antagonizing HSV-1 (Zhou et al., 1999).
The reduction in viral yield in the IFN-β-treated cultures coincided with suppression of representative genes of all three classes of HSV-1 lytic genes. Paradoxically, all IFN-α species tested also significantly reduced immediate early and late gene expression measuring ICP27 and VP16 respectively. Therefore, the only difference was IFN-β suppression of the early lytic gene, thymidine kinase (TK) expression. The three classes of HSV-1 lytic genes are expressed in a coordinated, cascade fashion with the early lytic gene family (which includes TK) responsible for viral DNA replication (Turner and Jenkins, 1997). Consequently, the issue of why IFN-β suppresses TK gene expression but does not substantially impact further on late gene expression (i.e., VP16) in comparison with IFN-α (which have no apparent effect on TK mRNA levels) remains unresolved. In no way was this evaluation comprehensive in that only a single gene from each class of HSV-1 lytic genes was measured. Therefore, it is difficult to discern if this pattern of viral gene expression by IFN-β is global or specific for the candidate genes evaluated in this study. However, it is intriguing to note that TK is required for reactivation (Coen et al., 1989) making IFN-β a candidate molecule to further study in relationship to this event (HSV-1 reactivation).
Collectively, the present study reinforces earlier work showing IFN-β confers a greater anti-viral effect against HSV-1 in comparison to multiple IFN-α species (Härle et al., 2002b). The central role of IFN-β as an important anti-viral cytokine is substantiated using mice lacking IFN-β (Deonarain et al., 2000). These animals are highly susceptible to vaccinia virus infection and cells derived from these mice show attenuation in virus-induced IFN-α and OAS expression. However, IFN-α species appear to afford greater anti-cytomegalovirus efficacy compared to IFN-β depending on the viral pathogen under study (Cull et al., 2002) with further synergies in efficacy displayed with combinations of IFN-α and -β (Bartlett et al., 2002). Consequently, the future challenge will be to identify those pathways associated with the efficacy of the type I IFN species against the specific viral pathogen and the signaling cascades linked to the activation of the cellular events surrounding the establishment of this anti-viral environment. In so doing, it may be possible to target the induction of selective transcriptional regulatory elements controlling the expression of specific anti-viral genes and thus, circumvent the unwarranted side-effects associated with type I IFN exposure.
Acknowledgments
The authors would like to thank Benitta John-Philip for her expert technical expertise. This work was supported by USPHS grant AI53108 (D.J.J.C.), CA44059 (R.H.S.), The Presbyterian Health Foundation of Oklahoma, NEI core grant EY12190, an unrestricted grant from Research to Prevent Blindness (RPB) Inc., and an RPB Stein Research Professorship award (D.J.J.C.).
References
- Al-khatib K, Williams BRG, Silverman RH, Halford WP, Carr DJJ. Absence of PKR attenuates the anti-HSV-1 activity of an adenoviral vector expressing murine IFN-β. J Interferon Cytokine Res. 2002;22:861–871. doi: 10.1089/107999002760274872. [DOI] [PubMed] [Google Scholar]
- Al-khatib K, Williams BRG, Silverman RH, Halford W, Carr DJJ. The murine double-stranded RNA-dependent protein kinase PKR and the murine 2′,5′ oligoadenylate synthetase-dependent RNase L are required for IFN-β mediated resistance against herpes simplex virus type 1 in primary trigeminal ganglion cultures. Virology. 2003 doi: 10.1016/s0042-6822(03)00298-8. in press. [DOI] [PubMed] [Google Scholar]
- Bartlett EJ, Cull VS, Brekalo NL, Lenzo JC, James CM. Syergy of type I interferon-A6 and interferon-B naked DNA immunotherapy for cytomegalovirus infection. Immunol Cell Biol. 2002;80:425–435. doi: 10.1046/j.1440-1711.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Carr DJJ, Noisakran S, Halford WP, Lukacs N, Asensio V, Campbell IL. Cytokine and chemokine production by HSV-1 latently infected trigeminal ganglion cell cultures: Effects of hyperthermic stress. J Neuroimmunol. 1998;85:111–121. doi: 10.1016/s0165-5728(97)00206-3. [DOI] [PubMed] [Google Scholar]
- Cayley PJ, Davies JA, McCullagh KG, Kerr IM. Activation of the ppp(A2′p)nA system in interferon-treated, herpes simplex virus-infected cells and evidence for novel inhibitors of the ppp(A2′p)nA-dependent RNase. Eur J Biochem. 1984;143:165–174. doi: 10.1111/j.1432-1033.1984.tb08355.x. [DOI] [PubMed] [Google Scholar]
- Cheng G, Brett ME, He B. Val193 and Phe195 of the γ134.5 protein of herpes simplex virus type 1 are required for viral resistance to interferon-α/β. Virology. 2001;290:115–120. doi: 10.1006/viro.2001.1148. [DOI] [PubMed] [Google Scholar]
- Chou J, Chen JJ, Gross M, Roizman B. Association of a Mr 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2α and premature shutoff of protein synthesis after infection with γ134.5− mutants of herpes simplex virus 1. Proc Natl Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens MJ, Williams BR. Inhibition of cell-free protein synthesis by pppA2′p5′A2′p5′A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978;12:565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Coen DM, Kosz-Vnenchak M, Jacobson JG, Leib DA, Bogard CL, Schaffer PA, Tyler KL, Knipe DM. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull VS, Bartlett EJ, James CM. Type I interferon gene therapy protects against cytomegalovirus-induced myocarditis. Immunology. 2002;106:428–437. doi: 10.1046/j.1365-2567.2002.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonarain R, Alcami A, Alexiou M, Dallman MJ, Gewert DR, Porter CG. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J Virol. 2000;74:3404–3409. doi: 10.1128/jvi.74.7.3404-3409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stasio PR, Taylor MW. Specific effect of interferon on the herpes simplex virus type 1 transactivation event. J Virol. 1990;64:2588–2593. doi: 10.1128/jvi.64.6.2588-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson KM, Hobbs WE, Manning BJ, Carlson P, DeLuca NA. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J Virol. 2002;76:2180–2191. doi: 10.1128/jvi.76.5.2180-2191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci USA. 2002;99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd-Smith G, Slattery E, Lengyel P. Interferon action: RNA cleavage pattern of a (2′–5′) oligoadenylate-dependent endonuclease. Science. 1981;29:1030–1032. doi: 10.1126/science.6165080. [DOI] [PubMed] [Google Scholar]
- Friedman HM, Cohen GR, Eisenberg RJ, Seidel CA, Cines DB. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component in infected cells. Nature. 1984;309:633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996a;157:3542–3549. [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJJ. Mechanisms of herpes simplex virus type 1 reactiavation. J Virol. 1996b;70:5051–5060. doi: 10.1128/jvi.70.8.5051-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJJ. Acyclovir blocks cytokine gene expression in trigeminal ganglia latently infected with herpes simplex virus type 1. Virology. 1997;238:53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- Härle P, Sainz B, Jr, Carr DJJ, Halford WP. The immediate-early protein, ICPO, is essential for the resistance of herpes simplex virus to interferon-α/β. Virology. 2002a;293:295–304. doi: 10.1006/viro.2001.1280. [DOI] [PubMed] [Google Scholar]
- Härle P, Cull V, Agbaga MP, Silverman RH, Williams BRG, James C, Carr DJJ. Differential effect of murine alpha/beta interferon transgenes on antagonization of herpes simplex virus type 1 replication. J Virol. 2002b;76:6558–6567. doi: 10.1128/JVI.76.13.6558-6567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay KA, Edris WA, Gaydos A, Tenser RB. Herpes simplex virus latency after direct ganglion virus inoculation. J Neurovirol. 1998;4:531–538. doi: 10.3109/13550289809113497. [DOI] [PubMed] [Google Scholar]
- Hendricks RL, Weber PC, Taylor JL, Koumbis A, Tumpey TM, Glorioso JC. Endogenously produced interferon α protects mice from herpes simplex virus type 1 corneal disease. J Gen Virol. 1991;72:1601–1610. doi: 10.1099/0022-1317-72-7-1601. [DOI] [PubMed] [Google Scholar]
- Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- Jennings SR, Rice PL, Kloszewski ED, Anderson RW, Thompson DL, Tevethis SS. Effect of herpes simplex virus type 1 and 2 on surface expression of class I major histocompatibility complex antigens on infected cells. J Virol. 1985;56:757–766. doi: 10.1128/jvi.56.3.757-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome KR, Tait JF, Koelle DM, Corey L. Herpes simplex virus type 1 renders infected cells resistant to cytotoxic T-lymphocyte-induced apoptosis. J Virol. 1998;72:436–441. doi: 10.1128/jvi.72.1.436-441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabar KSA, Dhalla M, Siddiqui Y, Zhou A, Al-Ahdal N, Der SD, Silverman RH, Williams BRG. Effect of deficiency of the double-stranded RNA-dependent protein kinase, PKR, on antiviral resistance in the presence or absence of ribonuclease L: HSV-1 replication is particularly sensitive to deficiency of the major IFN-mediated enzymes. J Interferon Cytokine Res. 2000;20:653–659. doi: 10.1089/107999000414835. [DOI] [PubMed] [Google Scholar]
- Leib DA, Machalek MA, Williams BRG, Silverman RH, Virgin HW. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc Natl Acad Sci USA. 2000;97:6097–6101. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Khanna KM, Chen XP, Fink DJ, Hendricks RL. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Khanna KM, Carriere BN, Hendricks RL. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikloska Z, Sanna PP, Cunningham AL. Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro. J Virol. 1999;73:5934–5944. doi: 10.1128/jvi.73.7.5934-5944.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman KL, Saffran HA, Smiley JR. Herpes simplex virus ICPO mutants are hypersensitive to interferon. J Virol. 2000;74:2052–2056. doi: 10.1128/jvi.74.4.2052-2056.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noisakran S, Carr DJJ. Lymphocytes delay kinetics of HSV-1 reactivation from in vitro explants of latent infected trigeminal ganglia. J Neuroimmunol. 1999;95:126–135. doi: 10.1016/s0165-5728(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Orange JS, Fassett MS, Koopman LA, Boyson JE, Strominger JL. Viral evasion of natural killer cells. Nature Immunol. 2002;3:1006–1012. doi: 10.1038/ni1102-1006. [DOI] [PubMed] [Google Scholar]
- Peters GA, Khoo D, Mohr I, Sen GC. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J Virol. 2002;76:11054–11064. doi: 10.1128/JVI.76.21.11054-11064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NM, Thompson RL. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld C, Whiteland JL, Nicholls SM, Grinfeld E, Easty DL, Gao H, Hill TJ. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- Turner SL, Jenkins FJ. The roles of herpes simplex virus in neuroscience. J Neurovirol. 1997;3:110–125. doi: 10.3109/13550289709015801. [DOI] [PubMed] [Google Scholar]
- Van Strijp JA, van Kessel KP, Miltenburg LA, Fluit AC, Verhoef J. Attachment of human polymorphonuclear leukocytes to herpes simplex virus-infected fibroblasts mediated by antibody-independent complement activation. J Virol. 1988;62:847–850. doi: 10.1128/jvi.62.3.847-850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota SI, Yokosawa N, Kubota T, Suzutani T, Yoshida I, Miura S, Jimbow K, Fujii N. Herpes simplex virus type 1 suppressess the interferon signaling pathway by inhibiting phosphorylation of STATs and janus kinases during an early infection stage. Virology. 2001;286:119–124. doi: 10.1006/viro.2001.0941. [DOI] [PubMed] [Google Scholar]
- Zhou A, Paranjape J, Brown TL, Nie H, Naik S, Dong B, Chang A, Trapp B, Fairchild R, Colmenares C, Silverman RH. Interferon action and apoptosis are detective in mice devoid of 2′–5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16:3297–3304. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Paranjape JM, Der SD, Williams BRG, Silverman RH. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258:435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]


