Significance
Genetic linkage to major histocompatibility complex class II proteins has been observed in many immunological disorders; yet, little is known about the underlying mechanisms of these associations. For chronic beryllium disease (CBD), the linkage to HLA-DPB1 alleles encoding a glutamic acid at position 69 of the β-chain is well established. We tested whether the presence of HLA-DP2 is sufficient for the development of a disease-specific model of CBD. HLA-DP2 transgenic mice developed a beryllium-specific adaptive immune response composed of CD4+ T cells that secrete Th1-type cytokines and are HLA-DP2-restricted, thus replicating the major features of the human disease. In addition, regulatory T cells modulate granuloma formation in the lungs of beryllium oxide-exposed HLA-DP2 transgenic mice.
Keywords: metal hypersensitivity, MHC presentation, genetic susceptibility
Abstract
Susceptibility to chronic beryllium disease (CBD) is linked to certain HLA-DP molecules, including HLA-DP2. To elucidate the molecular basis of this association, we exposed mice transgenic (Tg) for HLA-DP2 to beryllium oxide (BeO) via oropharyngeal aspiration. As opposed to WT mice, BeO-exposed HLA-DP2 Tg mice developed mononuclear infiltrates in a peribronchovascular distribution that were composed of CD4+ T cells and included regulatory T (Treg) cells. Beryllium-responsive, HLA-DP2–restricted CD4+ T cells expressing IFN-γ and IL-2 were present in BeO-exposed HLA-DP2 Tg mice and not in WT mice. Using Be-loaded HLA-DP2–peptide tetramers, we identified Be-specific CD4+ T cells in the mouse lung that recognize identical ligands as CD4+ T cells derived from the human lung. Importantly, a subset of HLA-DP2 tetramer-binding CD4+ T cells expressed forkhead box P3, consistent with the expansion of antigen-specific Treg cells. Depletion of Treg cells in BeO-exposed HLA-DP2 Tg mice exacerbated lung inflammation and enhanced granuloma formation. These findings document, for the first time to our knowledge, the development of a Be-specific adaptive immune response in mice expressing HLA-DP2 and the ability of Treg cells to modulate the beryllium-induced granulomatous immune response.
Chronic beryllium disease (CBD) is a granulomatous lung disorder that occurs in susceptible individuals exposed to beryllium (Be) in the workplace (1). Genetic susceptibility to CBD has been linked to major histocompatibility complex class II (MHCII) alleles, particularly HLA-DP. HLA-DPB1 alleles with a glutamic acid at position 69 of the β-chain (βGlu69), which includes DPB1*02:01, are strongly associated with disease susceptibility (2). Functional studies have shown that the HLA-DP molecules that mediate Be presentation to T cells match those implicated in disease susceptibility (3, 4). An HLA-DP2 crystal structure revealed a unique solvent-exposed acidic pocket located between the peptide backbone and the HLA-DP2 β-chain α-helix, formed by three glutamic acid residues from the β-chain, including βGlu69 (5). Mutagenesis of any of these residues resulted in loss of the ability of HLA-DP2 to present Be to T cells (5), establishing that the HLA contribution to the development of CBD is due to the ability of βGlu69-containing HLA-DP molecules to bind and present Be to pathogenic CD4+ T cells.
Regulatory T (Treg) cells play a key role in maintaining peripheral T-cell tolerance, in particular, by suppressing the activation and expansion of effector T cells (6, 7). We have previously shown that there is variation in the frequency of Treg cells in the bronchoalveolar lavage (BAL) of CBD patients and that the frequency of forkhead box P3 (FoxP3)-expressing Treg cells in the lung is inversely associated with disease severity (8). However, the lack of a viable animal model has precluded study of whether this T-cell subset participates in the regulation of disease severity as well as a determination of the factors that contribute to their presence in the lung.
Here, we exposed mice transgenic (Tg) for HLA-DP2 (the most prevalent βGlu69-containing molecule) to Be oxide (BeO), a form of the metal that is used in the workplace. BeO-exposed HLA-DP2–expressing mice developed mononuclear infiltrates in the lung, a Be-specific adaptive immune response in lung and spleen, and an expansion of Treg cells in the lung. Depletion of Treg cells exacerbated the Be-specific CD4+ T-cell alveolitis and converted the peribronchovascular mononuclear cell infiltrates into well-formed granulomas. Our findings strongly suggest that expression of the correct MHCII molecule in mice accompanied by an environmental exposure drives disease development, and identify an important role for Treg cells in modulating the Be-induced granulomatous response.
Results
HLA-DP2 Tg Mice Develop Peribronchovascular Mononuclear Infiltrates After BeO Exposure.
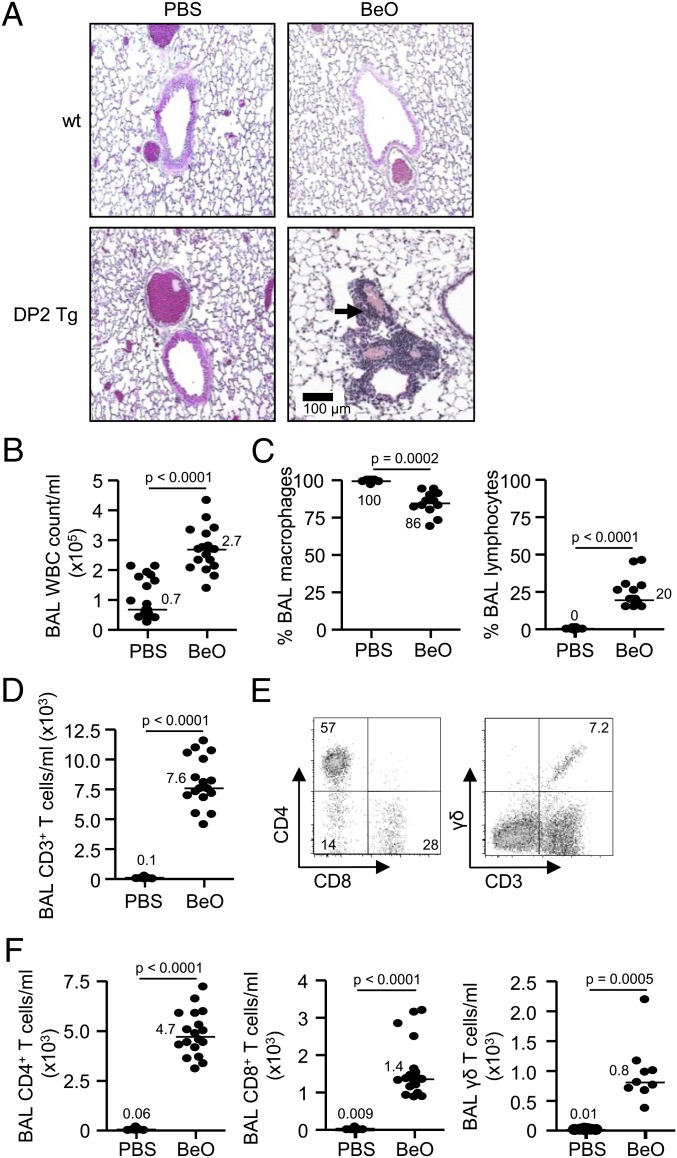
HLA-DP2 Tg mice were previously generated on an FVB/N background (9). HLA-DP2 Tg mice and wild-type (WT) FVB/N mice were sensitized by oropharyngeal aspiration of 100 µg BeO on days 0, 1, and 2 and challenged with BeO (100 µg) on days 14, 15, 18, and 19 before being killed on day 21. As seen in Fig. 1A, HLA-DP2 Tg mice exposed to BeO develop mononuclear infiltrates in a peribronchovascular distribution. Conversely, no cellular infiltrates were seen in the lungs of BeO-treated WT mice or in any PBS-treated mice.
Fig. 1.
Characterization of mononuclear cells in the BAL of BeO or PBS-treated HLA-DP2 transgenic mice. (A) Representative H&E staining of lungs from wild-type (WT) FVB/N and HLA-DP2 Tg mice after exposure to PBS or 100 µg BeO is shown (10× magnification). A total of nine mice from each treatment group were examined by H&E from three separate experiments. Arrows denote areas of peribronchovascular mononuclear cell infiltration. (B) Total WBC counts from BAL fluid of HLA-DP2 Tg mice after exposure to BeO or PBS are shown. (C) Percentage of BAL macrophages and lymphocytes in HLA-DP2 Tg mice after exposure to BeO or PBS is shown. (D) Number of CD3+ T cells in the BAL of HLA-DP2 Tg mice after exposure to BeO or PBS is shown. (E) Representative density plots showing the expression of CD4 and CD8 on gated CD3+ T cells or the expression of the γδ TCR on CD3+ T cells in the BAL of BeO-exposed HLA-DP2 Tg mice. (F) The number of CD4+, CD8+, and γδ T cells in the BAL of HLA-DP2 Tg mice after exposure to BeO or PBS is shown. The solid bars represent the median value for each treatment group, and data represent up to 18 mice from three separate experiments.
To determine the cellular composition of the mononuclear infiltrates in the HLA-DP2 Tg mice, BAL was performed on PBS- and BeO-exposed HLA-DP2 Tg mice. A significantly increased number of WBCs was seen in the BAL of BeO-exposed HLA-DP2 Tg mice (P < 0.0001) (Fig. 1B). The increased number of WBCs in the BAL was accompanied by a significant decrease in the percentage of macrophages and a significant increase in the percentage of lymphocytes (Fig. 1C). There was greater than a 100-fold increase in the number of CD3+ T cells in the BAL of BeO- compared with PBS-exposed HLA-DP2 Tg mice (Fig. 1D). In the representative density plots shown in Fig. 1E, 57% and 28% of the CD3+ T cells expressed CD4 and CD8 T-cell phenotypes, respectively, with a small percentage of the CD3+ T cells expressing a γδ T-cell receptor (TCR). Overall, a significantly increased number of CD4+, CD8+, and γδ T cells was seen in the lungs of HLA-DP2 Tg mice exposed to BeO compared with control animals at 21 d (Fig. 1F). Similar to the human disease, these findings confirm the presence of lymphocytic alveolitis in BeO-exposed HLA-DP2 Tg mice with a predominance of CD4+ T cells.
HLA-DP2 Transgenic Mice Develop a Be-Specific Adaptive Immune Response That Is CD4+ T-Cell Dependent and HLA-DP2 Restricted.
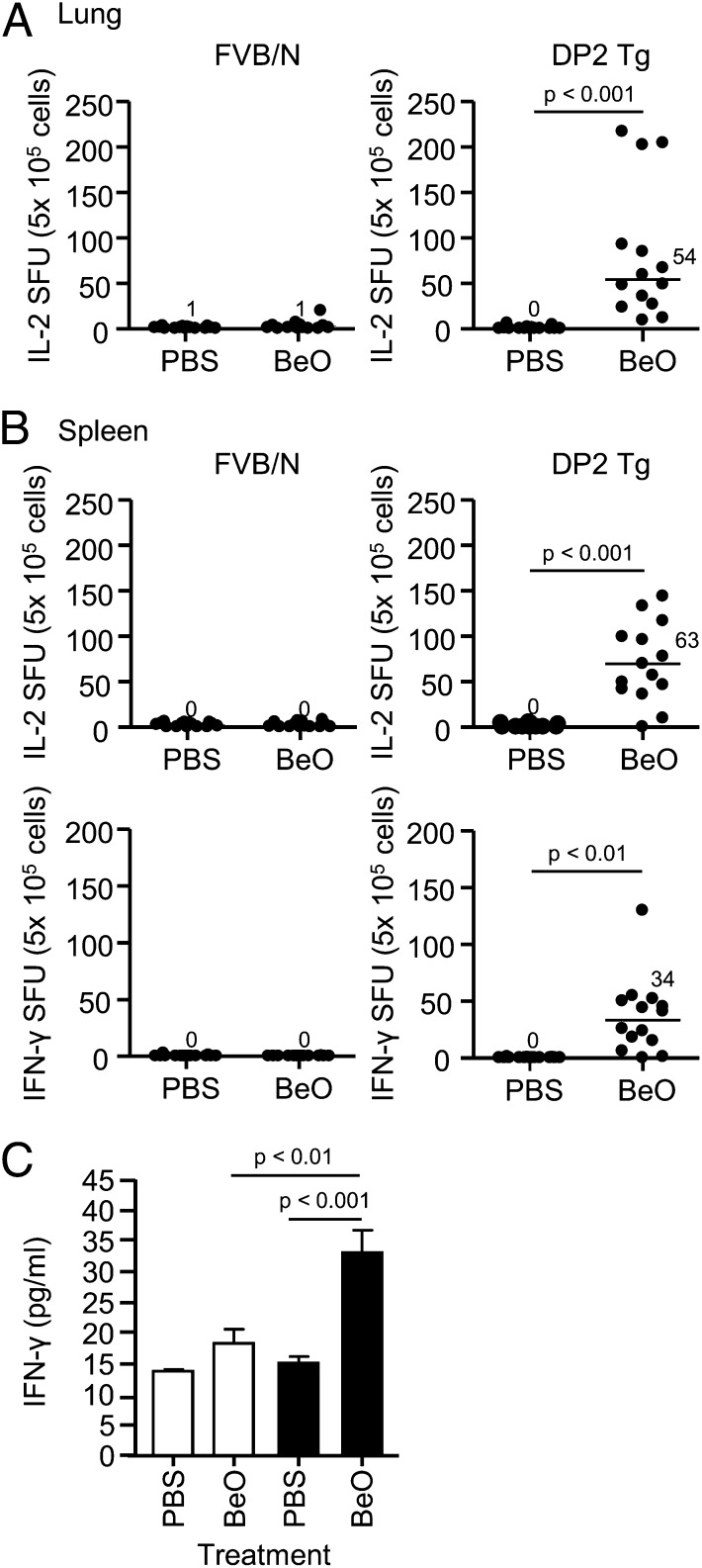
To document an adaptive immune response to Be, we used IFN-γ and IL-2 enzyme-linked immunosorbent spot (ELISPOT) assays as a measure of T-cell response (10, 11). Be-responsive, IL-2–secreting T cells [median, 54 spot forming units (SFUs); range, 9.3–217] were only present in the lung of HLA-DP2 Tg mice exposed to BeO (Fig. 2A). IL-2–secreting T cells in spleen were also only identified in BeO-treated HLA-DP2 Tg mice (63 SFUs; range, 0–144) (Fig. 2B). Although we were unable to perform IFN-γ ELISPOTs using lung cells due to the high background of IFN-γ–secreting cells in the PBS-treated lungs of both WT and HLA-DP2 Tg mice, significantly increased IFN-γ levels were seen in lung homogenates from BeO-exposed HLA-DP2 Tg mice compared with either PBS-exposed Tg mice or BeO-exposed WT mice (Fig. 2C). Conversely, no background IFN-γ secretion in spleen was seen in PBS-treated mice (Fig. 2B), and IFN-γ–secreting splenocytes were only detected in BeO-treated HLA-DP2 Tg mice (Fig. 2B). A positive correlation was noted between the number of Be-responsive, IL-2–secreting cells in spleen and lung of BeO-exposed Tg mice (r = 0.75, P = 0.002) (Fig. S1). Be-induced cytokine secretion was inhibited by in vitro (Fig. S2A) and in vivo (Fig. S2B) depletion of CD4+ T cells as well as after treatment with an anti–HLA-DP2 mAb (Fig. S2C). Collectively, these findings confirm the presence of a Be-specific adaptive immune response that is CD4 T-cell dependent and HLA-DP2 restricted.
Fig. 2.
Identification of a Be-specific adaptive immune response in the lung and spleen of HLA-DP2 transgenic (Tg) mice. Frequency of Be-specific T cells in lung (A) and spleen (B) of wild-type (WT) FVB/N (Left) and HLA-DP2 Tg (Right) mice using IFN-γ and IL-2 ELISPOT is shown. Data are expressed as the mean spot-forming units (SFUs) per 5 × 105 cells, and median values are indicated with solid lines. Data shown represent 15 mice per group from five separate experiments. (C) IFN-γ levels in supernatants of lung homogenates were analyzed by Luminex from WT FVB/N (empty bars) and HLA-DP2 transgenic (Tg) (filled bars) mice exposed to PBS or BeO for 3 wk. Data represent a total of 11 mice from three separate experiments.
Be-Specific CD4+ T Cells in HLA-DP2 Tg Mice Recognize the Same Ligands as T Cells Derived from Patients with CBD.
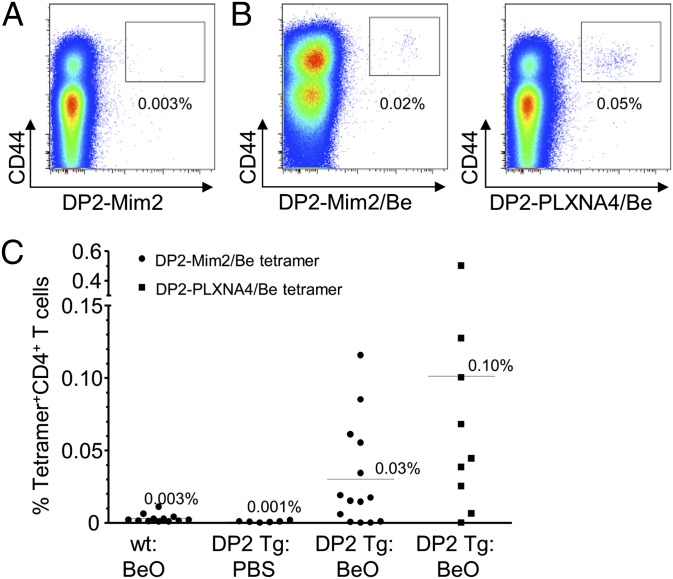
Using Be-loaded HLA-DP2–mimotope-2 and –plexin A4 tetramers that bind to a subset of Be-responsive CD4+ T cells derived from the lung of HLA-DP2–expressing patients with CBD (12), we queried whether HLA-DP2–expressing mice exposed to BeO would possess activated CD4+ T cells that recognize the same αβTCR ligands as their counterparts in humans. Total lung cells were analyzed by flow cytometry by excluding B220+, F4/80+, CD11c+, and CD8+ cells and gating on CD3+CD4+ T cells. In the absence of Be loading, no HLA-DP2–mimotope-2 tetramer staining of CD4+ T cells was detected in the lung of BeO-exposed HLA-DP2 Tg mice (Fig. 3A). However, distinct populations of CD4+CD44+ T cells binding to both Be-loaded HLA-DP2–peptide tetramers were detected in the lung of BeO-exposed HLA-DP2 Tg mice (0.02% for DP2–mimotope-2 and 0.05% for DP2–plexin A4 in the representative density plots shown in Fig. 3B, respectively). In addition, no DP2 tetramer staining was detected in the spleen of PBS or BeO-exposed mice. Overall, 10 of 14 (71%) and 8 of 9 (89%) BeO-exposed HLA-DP2 Tg mice had detectable populations of CD4+CD44+ T cells in the lung that bound Be-loaded HLA-DP2 tetramers expressing either mimotope-2 or plexin A4 (Fig. 3C). These data show that T cells derived from the lung of Be-exposed HLA-DP2 Tg mice share the same specificity as those in the lung of human subjects with CBD.
Fig. 3.
Be-responsive CD4+ T cells in the lung of HLA-DP2 transgenic (Tg) mice recognize the same αβTCR ligand as their counterparts in human lung. (A) Representative density plots depicting ex vivo lung CD4+CD44hi T cells from a BeO-exposed HLA-DP2 Tg mouse stained with an HLA-DP2–mimotope-2 tetramer without loaded Be. (B) Representative density plots of ex vivo lung cells from a BeO-exposed HLA-DP2 Tg mouse stained with either a Be-loaded HLA-DP2–mimotope-2 (DP2-Mim2/Be; Left) or HLA-DP2–plexin A4 (DP2-PLXN4/Be; Right) tetramer. (C) Cumulative percentage of ex vivo CD4+ T cells from the lungs of PBS- or BeO-treated WT FVB/N and HLA-DP2 Tg mice positively staining with HLA-DP2/mimotope-2/Be and HLA-DP2–PLXNA4/Be tetramers is shown.
BeO Exposure Leads to an Expansion of FoxP3-Expressing Treg Cells in the BAL of Be-Exposed HLA-DP2 Tg Mice.
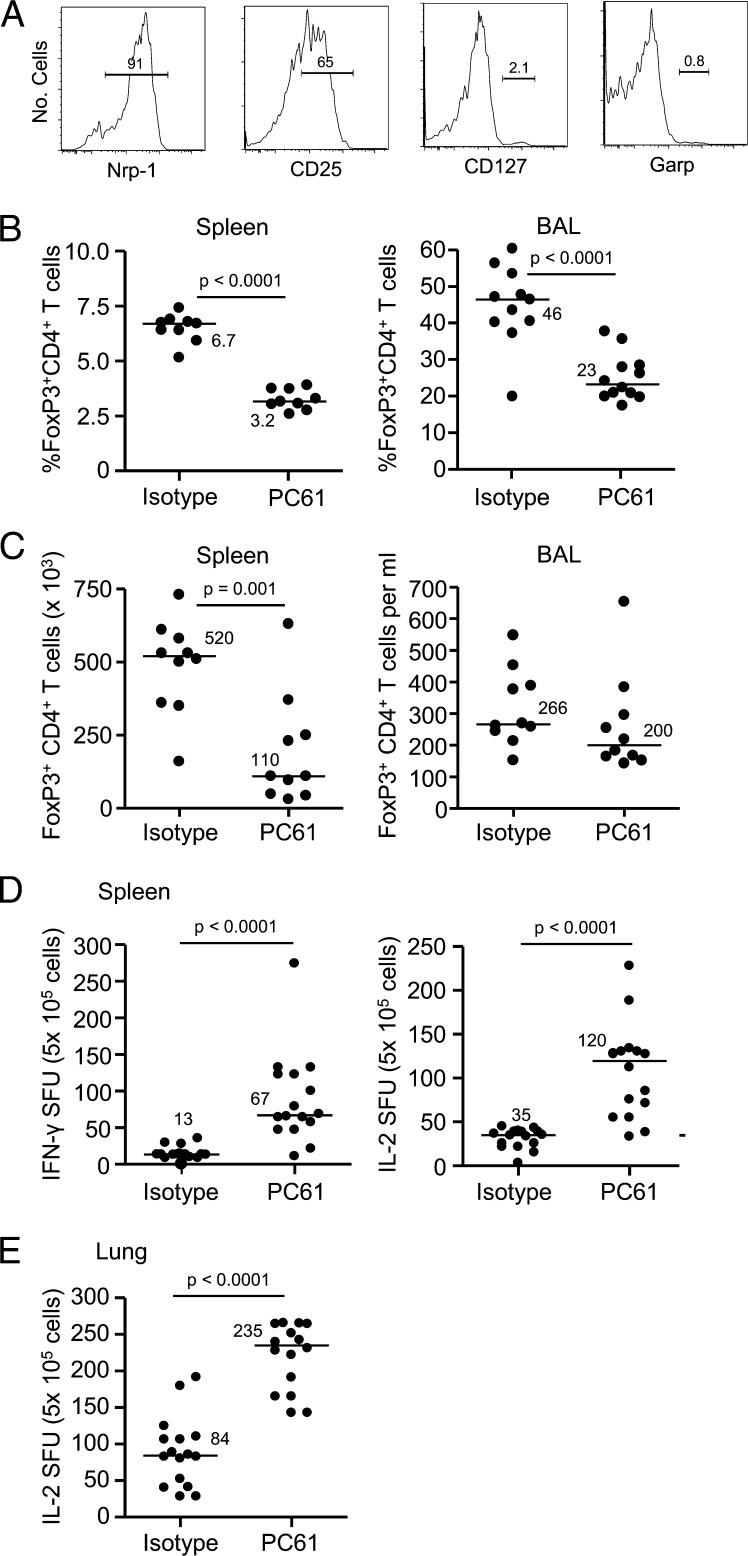
Despite the induction of a Be-specific adaptive immune response in BeO-exposed HLA-DP2 Tg mice, we noted a significant expansion of FoxP3+ CD4+ T cells in the BAL (35 ± 2.7%) of BeO-exposed mice compared with PBS-treated mice (10 ± 2.2%; P < 0.0001). The expanded FoxP3-expressing Treg cells are characterized by the expression of neuropilin-1 (Nrp-1) and CD25 and the lack of CD127 and glycoprotein A repetitions predominant (GARP) expression (Fig. 4A). Overall, 90 ± 0.2% of the FoxP3+ CD4+ T cells in the BAL of BeO-exposed HLA-DP2 Tg mice expressed Nrp-1, whereas only 1.0 ± 0.3% expressed Garp (Fig. 4A).
Fig. 4.
Treg cells are expanded in the lung of BeO-exposed HLA-DP2 transgenic (Tg) mice and modulate Be-specific CD4+ T cells. (A) Representative histograms of neuropilin-1 (Nrp-1), CD25, CD127, and Garp expression on FoxP3+ CD4+ T cells in the BAL of BeO-exposed HLA-DP2 Tg mice are shown. (B) Percentage of FoxP3+ CD4+ T cells in the spleen (Left) and BAL (Right) at day 21 of BeO exposure in HLA-DP2 Tg mice after exposure to isotype control or PC61 is shown. (C) Number of FoxP3+ CD4+ T cells in the spleen (Left) and BAL (Right) at day 21 of BeO exposure in HLA-DP2 Tg mice after exposure to isotype control or PC61 is shown. Frequency of Be-specific T cells in spleen (D) and lung (E) of BeO-exposed HLA-DP2 Tg mice using IFN-γ and IL-2 ELISPOT 21 d after exposure to isotype control or PC61 is shown. Data are expressed as the mean spot-forming units (SFUs) per 5 × 105 cells. For B–E, median values are indicated with solid lines, and data shown represent at least nine mice per group from two separate experiments.
Antigen-Specific Treg Cells Expand in the Lung of BeO-Exposed HLA-DP2 Tg Mice.
To determine the role of Treg cells in the development of Be-induced lung inflammation, we depleted CD25+ T cells using an anti-CD25 mAb (clone PC61) 3 d before BeO exposure. As shown in Fig. S3A, PC61-treated HLA-DP2 Tg mice had a significantly lower frequency of FoxP3+ CD4+ T cells in blood at day 8 (P < 0.0001), consistent with effective depletion of Treg cells. Compared with treatment of BeO-exposed HLA-DP2 Tg mice with an isotype control mAb, PC61 treatment resulted in a significant increase in the percentage of lymphocytes (P = 0.008) and a decreased percentage of macrophages (P = 0.003) in the BAL at day 21 (Fig. S3B). Importantly, the percentage of FoxP3-expressing CD4+ T cells in the spleen and lung (P < 0.0001) at day 21 was significantly decreased in HLA-DP2 Tg mice that received PC61 (Fig. 4B). Whereas the number of FoxP3-expressing CD4+ T cells in spleen was significantly decreased in PC61-treated HLA-DP2 Tg mice, no differences were seen in the BAL (Fig. 4C). However, the increased influx of lymphocytes into the lung of PC61-treated mice (Fig. S3B) likely alters the effector/regulatory T-cell ratio. The depletion of Treg cells was also associated with a significant increase in the number of Be-responsive IFN-γ– and IL-2–secreting cells in the spleen (P < 0.0001; Fig. 4D). In addition, a 2.8-fold increase in the number of Be-responsive IL-2–secreting T cells in the lung of PC61-treated HLA-DP2 Tg mice was seen (P < 0.0001; Fig. 4E). Overall, these findings are consistent with an exaggerated Be-induced adaptive immune response in the spleen and lung, suggesting that the expanded Treg cells seen in the lung of BeO-exposed HLA-DP2 Tg mice play a key role in dampening the expansion of Be-specific CD4+ T cells in the target organ.
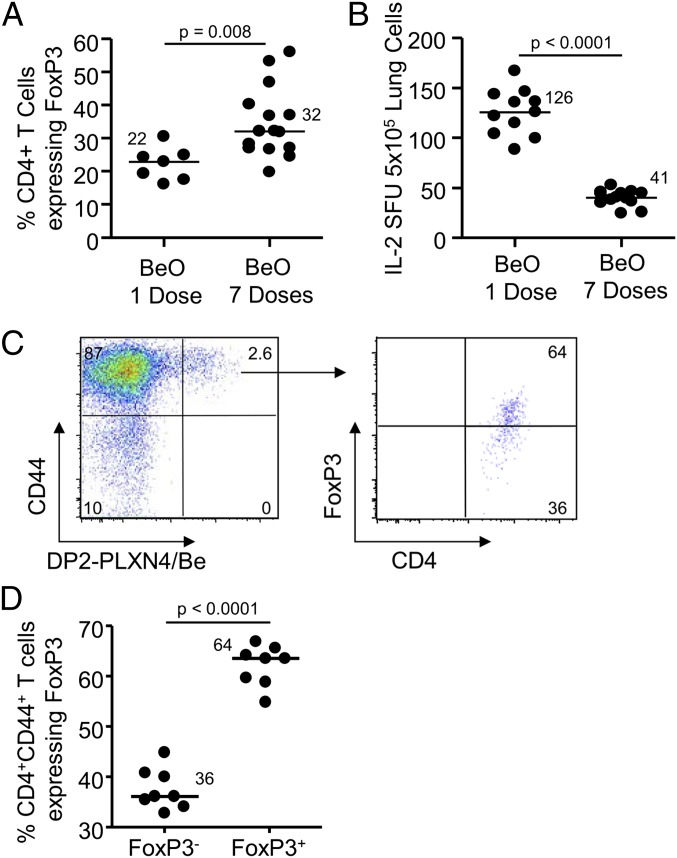
Based on the expansion of Treg cells in the BAL of HLA-DP2 Tg mice subjected to multiple exposures of BeO, we queried whether a single dose of BeO would suffice in the induction of Be-specific CD4+ effector T cells in the lung and result in a decreased number of FoxP3-expressing Treg cells. As shown in Fig. 5A, our multiple exposure regimen induced a significantly greater percentage of FoxP3-expressing CD4+ T cells in BAL and a significantly decreased number of Be-responsive IL-2–secreting T cells in lung (Fig. 5B). Thus, multiple exposures to intratracheal BeO dampen the Be-induced adaptive immune response through proliferation of Treg cells.
Fig. 5.
BeO induces the expansion of antigen-specific Treg cells that alter the functional capacity of Be-specific effector T cells. (A) Percentage of FoxP3-expressing CD4+ T cells in BAL of HLA-DP2 Tg mice exposed to one dose vs. seven doses of BeO is shown. (B) Frequency of Be-specific T cells in lung of HLA-DP2 Tg mice exposed to either one or seven doses of BeO using IL-2 ELISPOT is shown. Data are expressed as the mean spot-forming units (SFUs) per 5 × 105 cells, and median values are indicated with solid lines. (C) Representative density plot of Be-loaded HLA-DP2–plexin A4 (DP2-PLXN4/Be) tetramer staining of BAL CD4+CD44+ T cells from a BeO-exposed HLA-DP2 Tg mice is shown. Gating on tetramer-binding CD4+CD44+ T cells, a substantial portion of the tetramer-binding T cells coexpress FoxP3. The gate for FoxP3 was set based on FoxP3 expression in CD4+CD44+ tetramer-negative T cells and applied to CD4+CD44+ tetramer-positive T cells. (D) Frequency of antigen-specific CD4+ T cells in BAL of HLA-DP2 Tg mice exposed to BeO with and without FoxP3 expression is shown. Median values are indicated with solid lines. Data represent a total of eight mice per group from two separate experiments.
To determine whether the HLA-DP2–plexin A4/Be tetramer-binding T-cell population contained antigen-specific Treg cells, we performed BAL on BeO-exposed HLA-DP2 Tg mice and determined the frequency of tetramer-binding CD4+CD44+ T cells that coexpressed FoxP3. As shown in the representative density plot and cumulative data (Fig. 5 C and D), a significantly greater percentage of HLA-DP2–plexin A4/Be tetramer-binding CD4+ T cells expressed FoxP3. Collectively, our data show that both effector CD4+ T cells and FoxP3-expressing Treg cells are specific for the same HLA-DP2–plexin A4/Be complex.
Treg Cells Modulate Be-Induced Granulomatous Inflammation.
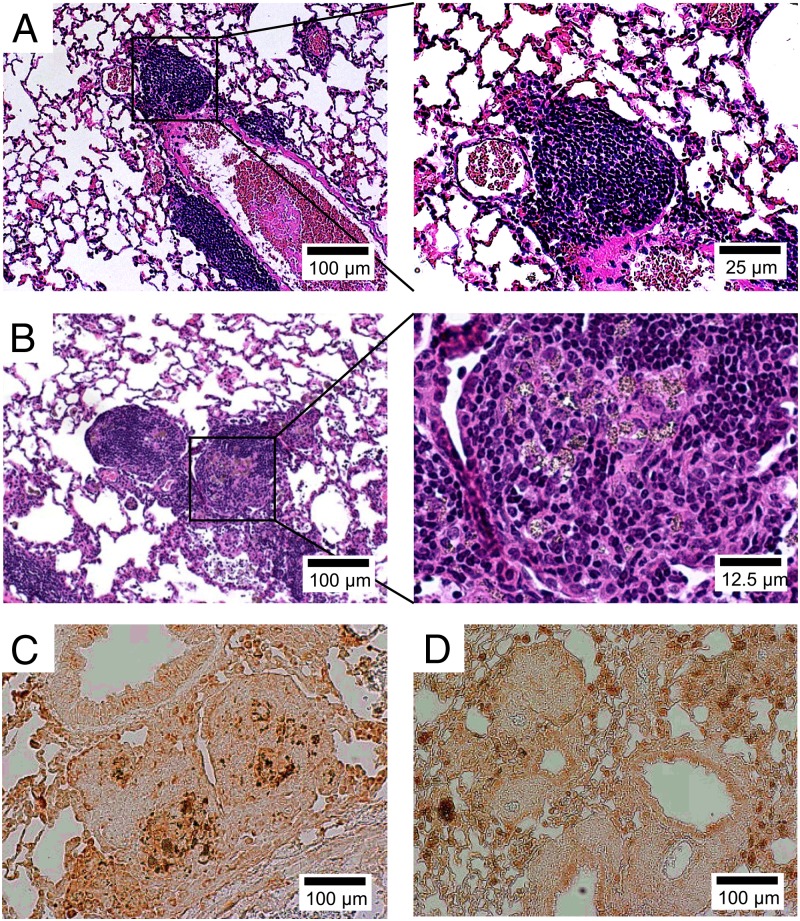
As shown in Figs. 1A and 6A, intratracheal BeO exposure of HLA-DP2 Tg mice resulted in the development of mononuclear infiltrates in a peribronchovascular distribution without obvious granuloma formation. Conversely, PC61 treatment was associated with the development of lymphocytic infiltrates surrounding particulate-laden macrophages (Fig. 6B), consistent with granulomatous inflammation. Immunohistochemistry for Mac-1, a macrophage marker, confirmed the accumulation of macrophages within the lymphoid aggregates of PC61-treated, BeO-exposed HLA-DP2 Tg mice (Fig. 6C), whereas mice exposed only to BeO showed diffuse macrophage staining without localization within the mononuclear infiltrates (Fig. 6D). Collectively, our findings show the expansion of Treg cells within the lungs of BeO-exposed HLA-DP2 Tg mice and the ability of these cells to modulate Be-specific CD4+ T-cell expansion and granulomatous inflammation in a murine model of CBD.
Fig. 6.
Treg cells modulate Be-induced granulomatous inflammation. Representative H&E staining of lung from BeO-exposed HLA-DP2 transgenic (Tg) mice that were treated with an isotype control (A) or an anti-CD25 (B) mAb is shown. Magnification (10×) is shown (A and B, Left) and an enlarged view (40× magnification) of the area enclosed within the black box (A and B, Right) is shown. Representative immunohistochemical staining for the macrophage marker, Mac-1, is shown in lung tissue obtained from BeO-exposed HLA-DP2 Tg mice with (C) and without (D) treatment with an anti-CD25 depleting mAb, PC61, at 21 d. Histology images are representative of a total of at least nine mice from each treatment group from three separate experiments.
Discussion
The current study describes a murine model of CBD using HLA-DP2 Tg mice exposed to BeO. This humanized model displays multiple features of the human disease including (i) peribronchovascular mononuclear infiltrates in the lung; (ii) Be-specific, Th1-polarized CD4+ T cells in lung and spleen; (iii) HLA-DP2-restricted adaptive immune response; (iv) Be-responsive T cells that recognize identical ligands as those T cells derived from patients with CBD; and (v) an inverse relationship between the frequency of Treg cells in the lung and disease severity. These findings provide evidence of a gene by environment interaction where the presence of a single MHCII molecule and an environmental exposure drives the development of a Be-induced adaptive immune response in mice and humans. Importantly, we also show that Treg cells modulate Be-induced granuloma formation.
Until the present study, no viable murine model of Be-induced disease existed. The likely explanation is the absence of equivalent glutamic acid residues at the required positions of murine MHCII β-chains that confer genetic susceptibility (Table S1). Multiple genetic studies have linked βGlu69-containing HLA-DP alleles to the development of CBD (2). In addition, our recent HLA-DP2 crystal structure identified a cluster of glutamic acid residues at positions 26, 68, and 69 of the β-chain (5), and functional analysis confirmed the importance of these amino acids as the putative Be binding site within the TCR binding footprint (5, 13). The dramatic change in the ability of FVB/N mice to mount a Be-specific, adaptive immune response with expression of the HLA-DP2 transgene confirms our hypothesis.
The Be-specific adaptive immune response in humans is characterized by a T-cell alveolitis composed of Th1-polarized CD4+ T cells that recognize Be in an HLA-DP–restricted manner (3, 4). In BeO-exposed HLA-DP2 Tg mice, Be-responsive T cells also express CD4 and secrete IFN-γ and IL-2 upon Be reexposure in vitro, mirroring the human disease. Remarkably, a subset of these lung CD4+ T cells recognizes the same αβTCR ligands as their counterparts in the human lung. We have previously identified a set of Be-dependent mimotopes and endogenous peptides, including those derived from plexin A, that stimulate T-cell hybridomas expressing two related HLA-DP2–restricted Vβ5.1+ T-cell receptors (12). Be-saturated HLA-DP2 tetramers presenting both of these ligands stained CD4+ T cells from ex vivo BAL cells of all HLA-DP2–expressing patients with CBD evaluated. The plexin A epitopes in mice are identical to those in humans, and detection of mimotope-2– and plexin A4-specific CD4+ T cells derived from the lung of HLA-DP2 Tg mice strongly validates our murine model of Be-induced disease.
Although our mouse model replicates many features of the human disease, we did not identify granulomatous inflammation in the lungs of HLA-DP2 Tg mice. However, following the depletion of Treg cells, characteristic granulomatous inflammation with Be-laden macrophages surrounded by lymphocytes and an exaggerated Be-specific CD4+ T-cell response was seen. The large expansion of Treg cells in the lung of BeO-exposed HLA-DP2 Tg mice is also consistent with other murine studies showing that Treg cells may constitute up to 50% of the CD4+ T-cell pool in a target organ during inflammation (14). In a murine model of tuberculosis, the expansion of Mycobacterium tuberculosis-specific Treg cells delayed priming of effector T cells in the lymph node and the development of a protective immune response (15). These findings are consistent with the current study, suggesting a role of Treg cells in the maintenance of a tolerant lung microenvironment. In the case of tuberculosis, the expanded Treg cell subset is thought to “hijack” the immune response, allowing the expansion of the microorganism (16). On the other hand, expansion of Treg cells in response to exposure to noninfectious particulates, such as Be, represents the appropriate immunologic response in an attempt to maintain tolerance in the lung.
Although Nrp-1 was initially proposed as a naturally occurring Treg-cell–specific marker (14, 17), additional studies have shown that peripherally induced Treg cells are capable of up-regulating Nrp-1, especially in the lung (18, 19). Consequently, it remains uncertain whether the expanded Treg cells in the lungs of BeO-exposed HLA-DP2 Tg mice are naturally occurring or peripherally induced. Our data suggest that these Treg cells are functional and capable of suppressing Be-specific, effector CD4+ T cells in vivo. Importantly, the expanded Treg cell subset in the lung contains a population of cells specific for the HLA-DP2–plexin A4/Be complex, similar to Be-specific effector CD4+ T cells.
We have previously observed a relative decrease in the number of FoxP3+ CD4+ T cells in the BAL of patients with CBD compared with healthy control subjects that was due to the dramatic increase in the number of Be-specific effector CD4+ T cells in the lung (8). Furthermore, the frequency of Treg cells in the BAL inversely correlated with disease severity as measured by loss of lung diffusing capacity, suggesting the importance of Treg cells in the immunopathogenesis of Be-induced disease (8). The relative deficiency of Treg cells in the BAL of patients with CBD diverge from the expanded Treg cell subset present in BeO-exposed HLA-DP2 Tg mice. Multiple potential differences exist between Be-exposed humans and mice, including genetic and environmental variations in the hosts, the presence or absence of therapeutic interventions, and the nature of the exposure. For example, Be workers are exposed over prolonged periods of time, which is in contrast to the acute exposure regimen used in the current study. As mentioned above, our repeated exposure protocol may induce tolerance through the expansion of FoxP3-expressing Treg cells. In this regard, a single BeO exposure of HLA-DP2 Tg mice results in significantly fewer FoxP3+ CD4+ T cells in the BAL compared with the exposure protocol used here and generates a Be-specific CD4+ T-cell response in between that seen with the standard BeO exposure and BeO-exposed mice treated with PC61. Artificially reducing the numbers of Treg cells in Be-exposed mice has allowed us to provide mechanistic insight into our previous clinical findings of an inverse relationship between the presence of Treg cells and disease severity. Altogether, these data show that Treg cells suppress Be-specific effector CD4+ T-cell responses, alveolitis, and granuloma formation in Be-exposed humans and mice.
In summary, this is the first report to our knowledge of a humanized HLA-DP2 model of Be-induced disease that replicates the majority of findings seen in the human disease and identifies an important role of Treg cells in Be-induced lung inflammation. Collectively, our findings suggest that lung inflammation in this murine model of CBD represents a continuum from mononuclear infiltrates to granulomatous inflammation with Treg cells modulating granuloma formation and organization. In a rare disease where human studies are limited, this model will allow a dissection of the mechanisms underlying the ability of Be to initiate an adaptive immune response and the role of Treg cells in this process, ultimately leading to a better understanding of the immunopathogenesis of CBD.
Materials and Methods
Detailed materials and methods are provided in SI Materials and Methods.
Mice.
FVB/N WT mice were purchased from The Jackson Laboratories and HLA-DP2 Tg (9) mice were obtained from Terry Gordon (New York University, NY, NY). The HLA-DP2 Tg mice were generated as previously described (20). Characterization of HLA-DP expression in HLA-DP2 Tg mice is shown in Fig. S4. Importantly, peritoneal macrophages derived from HLA-DP2 Tg mice can present peptide antigens and induce IL-2 secretion from T-cell hybridomas expressing HLA-DP2–restricted TCRs specific for either a dengue-virus–derived peptide (designated DV-13) or a hepatitis-B-virus–derived peptide (designated HepB1) (Fig. S5). All experiments were approved by the Institutional Animal Care and Use Committee of the University of Colorado, Denver.
Exposure of Mice.
Six- to eight-week-old WT and HLA-DP2 Tg FVB/N mice were exposed to 50 μL of either sterile PBS or 100 μg BeO (National Institute of Standards and Technology, standard reference material 1877) (21, 22) via oropharyngeal aspiration (23) on days 0, 1, and 2. Mice were lightly anesthetized with isoflurane before inhalation. A limulus amebocyte assay (Sigma-Aldrich) confirmed that all preparations contained <5.0 endotoxin units/mL of endotoxin. After sensitization, mice were boosted with either 100 μg BeO or PBS via oropharyngeal aspiration on days 14, 15, 18, and 19 and killed on day 21. In select experiments, HLA-DP2 Tg mice was intraperitoneally injected with either PBS or the depleting CD4 mAb GK1.5 (100 μg in 50 μL PBS) 2 d before BeO exposure and three times per week until being killed at day 21. Enumeration of CD4+ T cells in the spleen on the day of killing showed a 96 ± 1.4% reduction in CD4+ T cells compared with PBS-treated mice.
Statistical Analysis.
A one-way ANOVA and unpaired t test were used to determine significance of differences between groups (Prism 4, GraphPad Software). P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors thank Lisa Maier for helpful discussions and Ainsley Weston, Sally Tinkle, and Edward Rubin for the generation of the HLA-DP2 Tg mice. This work is supported by the following National Institutes of Health Grants: HL62410, HL111760, and ES11810 (to A.P.F.); and ES011473, 212-2010-M-35006, and DE-FG02-08ER64670 (to T.G.). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of their respective organizations.
Footnotes
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408048111/-/DCSupplemental.
The authors declare no conflict of interest.
References
- 1.Fontenot AP, Maier LA. Genetic susceptibility and immune-mediated destruction in beryllium-induced disease. Trends Immunol. 2005;26(10):543–549. doi: 10.1016/j.it.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.McCanlies EC, Kreiss K, Andrew M, Weston A. HLA-DPB1 and chronic beryllium disease: A HuGE review. Am J Epidemiol. 2003;157(5):388–398. doi: 10.1093/aje/kwg001. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot AP, Torres M, Marshall WH, Newman LS, Kotzin BL. Beryllium presentation to CD4+ T cells underlies disease-susceptibility HLA-DP alleles in chronic beryllium disease. Proc Natl Acad Sci USA. 2000;97(23):12717–12722. doi: 10.1073/pnas.220430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lombardi G, et al. HLA-DP allele-specific T cell responses to beryllium account for DP-associated susceptibility to chronic beryllium disease. J Immunol. 2001;166(5):3549–3555. doi: 10.4049/jimmunol.166.5.3549. [DOI] [PubMed] [Google Scholar]
- 5.Dai S, et al. Crystal structure of HLA-DP2 and implications for chronic beryllium disease. Proc Natl Acad Sci USA. 2010;107(16):7425–7430. doi: 10.1073/pnas.1001772107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 7.Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 8.Mack DG, et al. Deficient and dysfunctional regulatory T cells in the lungs of chronic beryllium disease subjects. Am J Respir Crit Care Med. 2010;181(11):1241–1249. doi: 10.1164/rccm.201001-0025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarantino-Hutchison LM, et al. Genetic determinants of sensitivity to beryllium in mice. J Immunotoxicol. 2009;6(2):130–135. doi: 10.1080/15476910902977399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin AK, et al. Beryllium-specific CD4+ T cells in blood as a biomarker of disease progression. J Allergy Clin Immunol. 2011;128:1100–1106 e1101-1105. doi: 10.1016/j.jaci.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pott GB, et al. Frequency of beryllium-specific, TH1-type cytokine-expressing CD4+ T cells in patients with beryllium-induced disease. J Allergy Clin Immunol. 2005;115(5):1036–1042. doi: 10.1016/j.jaci.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Falta MT, et al. Identification of beryllium-dependent peptides recognized by CD4+ T cells in chronic beryllium disease. J Exp Med. 2013;210(7):1403–1418. doi: 10.1084/jem.20122426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bill JR, et al. Beryllium presentation to CD4+ T cells is dependent on a single amino acid residue of the MHC class II β-chain. J Immunol. 2005;175(10):7029–7037. doi: 10.4049/jimmunol.175.10.7029. [DOI] [PubMed] [Google Scholar]
- 14.Yadav M, Stephan S, Bluestone JA. Peripherally induced Tregs - role in immune homeostasis and autoimmunity. Front Immunol. 2013;4:232. doi: 10.3389/fimmu.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207(7):1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson RP, Shafiani S, Urdahl KB. Foxp3+ regulatory T cells in tuberculosis. Adv Exp Med Biol. 2013;783:165–180. doi: 10.1007/978-1-4614-6111-1_9. [DOI] [PubMed] [Google Scholar]
- 17.Yadav M, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722, S1711-1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss JM, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–1742, S1721. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhamne C, Chung Y, Alousi AM, Cooper LJ, Tran DQ. Peripheral and thymic foxp3+ regulatory T cells in search of origin, distinction, and function. Front Immunol. 2013;4:253. doi: 10.3389/fimmu.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan B, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1986. [Google Scholar]
- 21.Stefaniak AB, et al. Surface area of respirable beryllium metal, oxide, and copper alloy aerosols and implications for assessment of exposure risk of chronic beryllium disease. AIHA J (Fairfax, Va) 2003;64(3):297–305. doi: 10.1080/15428110308984820. [DOI] [PubMed] [Google Scholar]
- 22.Winchester MR, et al. Certification of beryllium mass fraction in SRM 1877 Beryllium Oxide Powder using high-performance inductively coupled plasma optical emission spectrometry with exact matching. Anal Chem. 2009;81(6):2208–2217. doi: 10.1021/ac802251n. [DOI] [PubMed] [Google Scholar]
- 23.Rao GV, et al. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J Toxicol Environ Health A. 2003;66(15):1441–1452. doi: 10.1080/15287390306417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.