Abstract
The generation of insulin-secreting cells from nonendocrine pancreatic epithelial cells (NEPEC) has been demonstrated for potential clinical use in the treatment of diabetes. However, previous methods either had limited efficacy or required viral vectors, which hinder clinical application. In this study, we aimed to establish an efficient method of insulin-secreting cell generation from NEPEC without viral vectors. We used nonislet fractions from both research-grade human pancreata from brain-dead donors and clinical pancreata after total pancreatectomy with autologous islet transplantation to treat chronic pancreatitis. It is of note that a few islets could be mingled in the nonislet fractions, but their influence could be limited. The NeuroD1 gene was induced into NEPEC using an effective triple lipofection method without viral vectors to generate insulin-secreting cells. The differentiation was promoted by adding a growth factor cocktail into the culture medium. Using the research-grade human pancreata, the effective method showed high efficacy in the differentiation of NEPEC into insulin-positive cells that secreted insulin in response to a glucose challenge and improved diabetes after being transplanted into diabetic athymic mice. Using the clinical pancreata, similar efficacy was obtained, even though those pancreata suffered chronic pancreatitis. In conclusion, our effective differentiation protocol with triple lipofection method enabled us to achieve very efficient insulin-secreting cell generation from human NEPEC without viral vectors. This method offers the potential for supplemental insulin-secreting cell transplantation for both allogeneic and autologous islet transplantation.
Introduction
Cell therapy as a treatment for diabetes requires a source of human insulin-secreting cells that can respond to glucose in a physiologic manner. Allogeneic islet cell transplantation has been performed for the treatment of type 1 diabetes with promising results (Shapiro et al., 2000, 2006; Matsumoto et al., 2005, 2011; Matsumoto, 2010); however, it often needs multiple transplantations to achieve insulin independence. Therefore, increasing the functional transplanted beta cell mass should improve the clinical outcomes. On the other hand, autologous islet transplantation after total pancreatectomy has been performed for the treatment of chronic pancreatitis with severe abdominal pain (Sutherland et al., 2008). All isolated islets were transplanted into the patient; however, less than half of the patients could maintain insulin independence (Sutherland et al., 2008; Matsumoto, 2011; Takita et al., 2011). To improve the efficacy of autologous islet transplantation, it is necessary to increase the transplanted beta cell mass. Thus, the generation and transplantation of insulin-secreting cells from nonendocrine pancreatic epithelial cells (NEPEC) derived from the remaining pancreatic tissues after islet isolation has the potential to improve the efficacy of both allogeneic and autologous islet transplantation. It has been demonstrated that insulin-secreting cells can be generated from nonendocrine pancreatic cells under certain conditions (Bonner-Weir et al., 2000; Ramiya et al., 2000; Seaberg et al., 2004; Suzuki et al., 2004; Zhao et al., 2005; Hao et al., 2006; Noguchi et al., 2006; Yamamoto et al., 2006; Inada et al., 2008). However, previous methods had limited efficacy or required viral vectors, which hinder clinical application. In addition, so far there is no report to generate them from pancreata with chronic pancreatitis. In this study, we introduced the human NeuroD1 gene into human NEPEC from both cadaveric donors and removed pancreata with chronic pancreatitis using an effective nonviral gene transfection protocol, since a previous study suggested that NeuroD1 could facilitate the differentiation of pancreatic nonendocrine cells in vitro (Noguchi et al., 2006). This method promoted their differentiation into glucose-responsive insulin-secreting cells that were able to ameliorate diabetes in vivo after transplantation.
Materials and Methods
Plasmid constructs
The plasmid encoding human NeuroD1 under human cytokeratin19 promoter (pCK19-hND) was produced as shown previously (Kagaya et al., 2001; Shimoda et al., 2010a). After transfection of this plasmid into human islets, the NeuroD1 expression did not increase (data not shown). We produced and tested the plasmids encoding some reporter genes such as GFP and DsRed under the human CK19 promoter and transfected them to some cell lines expressing or not expressing CK19 and confirmed that it functioned in only CK19-expressing cells. Briefly, we used Panc-1 cell line for CK19+ cells and HFL-1 cell line for CK19− cells. The efficacy of the transfection of pCK19-GFP, pCK19-DsRed, and pCK19-hND into Panc-1 by single lipofection was about 50–70%. In contrast, no transfected gene expression was detected in HFL-1 cells.
Disease-free human pancreata from brain-dead donors
Fifteen donor pancreata were procured from deceased multiorgan donors after obtaining consent for research through local organ procurement organizations (Southwest Transplant Alliance, Dallas, TX, and LifeGift, Fort Worth, TX) (Matsumoto et al., 2010, 2011). This study was approved by the institutional review board of Baylor Research Institute (Dallas, TX). Islets were isolated using the semiautomated method described previously with our modifications (Matsumoto et al., 2006; Ikemoto et al., 2009; Noguchi et al., 2009). The islets used in this study were handpicked for further purification. The donor and isolation variables were as follows: donor age 46.0±3.6 years; 8 men and 7 women; body mass index (BMI) 30.3±1.3 kg/m2; cold ischemic time 183±10 min.
Culturing and development of NEPEC
The pancreatic nonislet fraction obtained from the remnant of islet purification was used. We preliminarily examined the remnant cells with quantitative RT-PCR and found that amylase expression was high and the expression levels of NeuroD1, insulin, C-Peptide, Sox9, and Pdx1 were very low as expected by pancreatic cell proportion. CK19 expression was also low compared with normal pancreas. The cells were cultured to generate NEPEC as shown previously (Hao et al., 2006; Shimoda et al., 2010a). Briefly, it was cultured in suspension in RPMI 1640 medium (Cellgro, Manassas, VA) with 5.5 mM glucose and 10% (v/v) fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) and antibiotics for 2 days. G418 (40 μg/ml; Invitrogen, Carlsbad, CA) was added in the culture medium for 4 days to deplete fibroblasts. Without G418, the fibroblastic cells rapidly increased and became dominant (Hao et al., 2006; Shimoda et al., 2010a). We counted and calculated the ratio of fibroblastic cell numbers to the total cells at day 7 after plating in the groups with G418 treatment (NEPEC) and without the treatment and found that more than 80% (84.3±4.3%) of cells were fibroblastic in the non-G418 group. On the other hand, less than 1% (0.7±0.2%) of cells were fibroblastic in the G418 treatment group. Samples of the suspension culture cells with G418 at days 0 and 4 were stained with dithizone to check for contamination of islets. The ratio of the area of dithizone-positive cells was calculated using more than 20 randomly captured photomicrographs at a magnification of 100×. Data are presented as mean±standard error of three independent experiments. Afterward, NEPECs were plated on dishes with the same medium without G418 and spread into a cell monolayer. After 7 days, each preparation from a single pancreas was divided into four groups as described in the following section. We also performed a preliminary experiment to test the transfection efficiency to NEPECs by lipofection using a plasmid encoding GFP reporter gene under CMV promoter (pCMV-EGFP). As a result, the transfection efficacy was about 15%, and there was no remarkable change of cellular appearance.
NEPEC differentiation protocol (triple lipofection method)
Seven days after plating, the pCK19-hND plasmid was transfected with lipofectamine2000 (Invitrogen) on days 0, 2, and 4, according to the manufacturer's protocol. At the same time, a set of five growth factors (named F5) was added in the culture medium for 7 days (days 0–7). The supplemented growth factors were 10 mM nicotinamide, 1% (v/v) insulin–transferrin–selenium, 10 ng/ml basic fibroblast growth factor, 50 ng/ml exendin-4 (Sigma, St. Louis, MO), and 10 ng/ml bone morphogenetic protein 4 (Pepro Tech, Rocky Hill, NJ). In this study, NEPEC were divided into four groups: (1) nontreated NEPEC (NEPEC group); (2) NEPEC with five growth factors added in culture medium (F5 group); (3) NEPEC with transfection of pCK19-hND plasmid (ND group); (4) NEPEC with both pCK19-hND and the growth factors (ND+F5 group). The cells were evaluated at day 7 with the following assays.
Quantitative real-time PCR
For the four groups and human islets, the whole cells in each culture plate were collected, and the total RNA was prepared from TRIzol (Invitrogen) according to the manufacturer's instructions and was reverse-transcribed using the SuperScript III First-Strand Synthesis System (Invitrogen). Then, 1 μl of cDNA was used as a template and analyzed by RT2 qPCR Primer Assays (SABiosciences, Frederick, MD) on Mx 3000P (Stratagene, La Jolla, CA). The number of amplification cycles was normalized to the endogenous control GAPDH and displayed as fold change. Then, the relative quantification value to a reference group (NEPEC group or human islets) was calculated.
Immunohistochemistry
The samples were fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100, and blocked in 20% Aquablock (East Coast Biologics, North Berwick, ME). The following antibodies were used for immunohistochemistry: rabbit anti-NeuroD1 (#AB15580), mouse anti-proinsulin C-peptide (#C-PEP-01), rabbit anti-somatostatin (#AB5494), rabbit anti-neurogenin-3 (#AB5684) (Millipore, Billerica, MA), guinea pig anti-insulin (#ab7842), rabbit anti-Ki67 (#ab15580) (Abcam, Cambridge, MA), mouse anti-cytokeratin 19 (#RCK108; Dako, Glostrup, Denmark), mouse anti-glucagon (#K79bB10; Sigma), goat anti-PDX1 (#A-17), goat anti-Nkx6.1 (#C-14), mouse anti-amylase (#G-10), and rabbit anti-Sox9 (#H-90) (Santa Cruz, Santa Cruz, CA). The antigens were visualized using appropriate secondary antibodies conjugated with fluorescein isothiocyanate, Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA), Alexa-fluor-488, and Alexa-fluor-568 (Invitrogen). Then, 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) was added to the sections for nuclear staining. Images were captured on an epifluorescent microscope and analyzed using MetaMorph software (Molecular Devices, Sunnyvale, CA).
Insulin/C-peptide release assay and cellular C-peptide content
In this study, secreted and cellular C-peptide was measured as well as insulin to eliminate the possibility that cells cultured with insulin did not truly differentiate and could slowly release absorbed insulin (Hansson et al., 2004). The human insulin and C-peptide levels in culture supernatants were measured by enzyme-linked immunosorbent assay kits (Alpco, Salem, NH). The cells were incubated in the fresh RPMI 1640 with 10% FBS. The supernatants were collected 24 hr after incubation and analyzed. The cellular C-peptide content was examined by acid–ethanol extraction. The values were normalized by the cell number.
Glucose stimulation test
After two washes and preincubation in the low glucose solution (RPMI 1640 containing 2.8 mM glucose without serum) for 1 hr, the differentiated cells were incubated with RPMI 1640 containing 2.8 mM glucose at 37°C for 1 hr followed by incubation with the same medium containing 25 mM glucose at 37°C for 1 hr. The conditioned supernatant was collected and analyzed. Human islets were used as a control. The values were normalized by the cell number.
In vivo transplantation assay
Diabetes was induced in 10–13-week-old male nude mice (Athymic Nude-Foxn1nu; Harlan, Houston, TX) using a single tail vein injection of streptozotocin (STZ; 150 mg/kg; Sigma). Animals were considered to be diabetic after two consecutive blood glucose measurements ≥350 mg/dl. NEPEC, F5, ND, and ND+F5 cells at day 7 were detached from the culture dishes using Accutase (Innovative Cell Technologies, San Diego, CA), and grafts containing 10,000,000 cells of each group were transplanted under the left kidney capsule in the diabetic nude mice. Each mouse received the cells derived from a single pancreas without mixing cells from different pancreata. The nonfasting blood glucose levels were measured three times a week for 30 days. Before STZ administration, before transplantation, and at day 3, 10, 20, and 30 after transplantation, nonfasting blood was taken for measurement of serum mouse and human C-peptide levels. The intraperitoneal glucose tolerance test was performed at day 31. Nontransplanted diabetic nude mice were used as a control (STZ group). ND+F5 cells were transplanted into normal nude mice and examined as well. The graft-bearing kidney was removed at day 32 to establish that the graft was functional, as determined by the elevation of blood glucose postnephrectomy. To investigate the cell proliferation of the graft in the early period after transplantation, the graft-bearing kidney was taken at day 3 as well. The grafts were fixed in 4% paraformaldehyde at 4°C overnight and equilibrated in 30% sucrose at 4°C overnight and cryopreserved with Tissue Tek optimal cutting temperature compound (Sakura Finetek USA, Torrance, CA) at −80°C. Sections were cut at 8 μm increments and analyzed by hematoxylin–eosin staining and immunohistochemistry as described previously (Shimoda et al., 2010b) and above. For assessment of cell proliferation, the grafts were stained with insulin and Ki67. The ratio of PDX1-positive, NeuroD1-positive, Nkx6.1-positive, and Ki67-positive cells in the insulin-positive cells were measured, respectively, by counting the positive cell and the total cell numbers using more than 20 photomicrographs at a magnification of 200×.
The native pancreas was taken from nontreated mice (wild, n=5), STZ-induced diabetic mice (STZ, n=5), and the diabetic mice after transplantation of the ND+F5 cells at day 35 (ND+F5, n=5), and analyzed by immunohistochemistry. The ratio of insulin-positive cells to the total cell number was measured by counting the cells using 20 photomicrographs at a magnification of 40× for each pancreas.
NEPEC derived from pancreas with chronic pancreatitis
The pancreatic nonendocrine tissues were obtained from the five patients who had been clinically diagnosed with chronic pancreatitis and received total pancreatectomy with autologous islet transplantation from August 2010 to August 2011 at Baylor University Medical Center. This study was approved by the institutional review board at Baylor Research Institute (Dallas, TX). The development, differentiation, and assessment of NEPEC were performed in the same manner as with the brain-dead donors.
Statistical analysis
Data were expressed as mean±standard error. Statistically significant differences between two groups were determined by Student's t-test. For the comparison of more than two experimental groups, analysis of variance (ANOVA) was used and then t-test with Bonferroni adjustments was used. The repeated-measures ANOVA (RM-ANOVA) test was used to examine differences in blood glucose values.
Results
Nonendocrine pancreatic cells from disease-free human pancreata
Dithizone-positive contaminated islets in the nonislet fraction were very few (Fig. 1A and B), and they comprised 0.5±0.2% of the sample after isolation and further decreased to 0.08±0.03% after culturing with G418.
FIG. 1.
Pancreatic nonendocrine cells after isolation and NEPEC after differentiation protocol. (A and B) Representative figures of the human pancreatic nonislet fraction (A) immediately after islet isolation and (B) 4 days after suspension culture with G418. The arrow indicates a contaminated islet detected by dithizone staining. Scale bars: 100 μm. (C) A ratio of cell proliferation of the four groups. The cell number at day 1 after starting cell attachment culture was used as the starting point (=1.0). At day 7, the differentiation procedure started. There was no significant difference of proliferation among the four groups. (D) Morphology of NEPECs of the four groups at day 7 after differentiation. Scale bars: 100 μm. (E) Effective induction of human NeuroD1 into NEPEC, shown through immunohistochemistry of the four groups 7 days after the start of differentiation. Most CK19-positive cells produced NeuroD1 in the ND and ND+F5 groups. Green, CK19; red, human NeuroD1; blue, DAPI. Scale bars: 50 μm. (F and G) Gene expression profile of the differentiated NEPEC. The human islets and the four groups of cells were harvested 7 days after the initiation of differentiation. A panel of genes was assessed by quantitative real-time PCR. For statistical analysis, ANOVA and t-test with Bonferroni adjustments were used. (F) Relative quantification represents the fold change of expression between each group and the NEPEC group (NEPEC=1.0). (G) Relative quantification represents the fold change of expression between each group and human islets (islets=1.0). For CK19, the relative values of the 4 groups were presented (NEPEC=1.0). Data are presented as mean±standard error of five independent experiments. *p<0.01 in ND+F5 group vs. all other groups. **p<0.01 in ND+F5 group vs. NEPEC and F5 groups. ANOVA, analysis of variance; GCG, glucagon; GK, glucokinase; NEPEC, nonendocrine pancreatic epithelial cells; Ngn3, neurogenin-3; PPY, pancreatic polypeptide; SST, somatostatin.
Differentiation by human NeuroD1 gene induction and growth factors
The NEPECs proliferated well during adhesive culture. The proliferation rates of the cells in all four groups were similar (Fig. 1C), which indicated that there was low toxicity associated with the transfection. The morphology of all four groups of cells was indistinguishable 7 days after induction (Fig. 1D). Almost all NEPEC expressed human CK19, and most ND and ND+F5 cells also stained for human NeuroD1, whereas NEPEC and F5 cells did not (Fig. 1E). None of the cells expressed amylase (data not shown). At the same time, NeuroD1 gene expression was quantified (Fig. 1F). The ND and ND+F5 group had an approximate 10,000-fold greater expression level of the NeuroD1 gene than the NEPEC group. The insulin gene expression in the ND+F5 group was significantly higher than in other groups and was approximately 3% that of the human islets (Fig. 1G). Glucokinase gene expression in the ND+F5 group was significantly higher than in the NEPEC and F5 groups. CK19 was still expressed in the ND+F5 group, because about 90% of cells expressed CK19 as strongly as NEPEC (Fig. 1E). The expression of glucagon, somatostatin, and pancreatic polypeptide did not differ among the four groups. Amylase expression was not detectable within 40 cycles in all groups (data not shown).
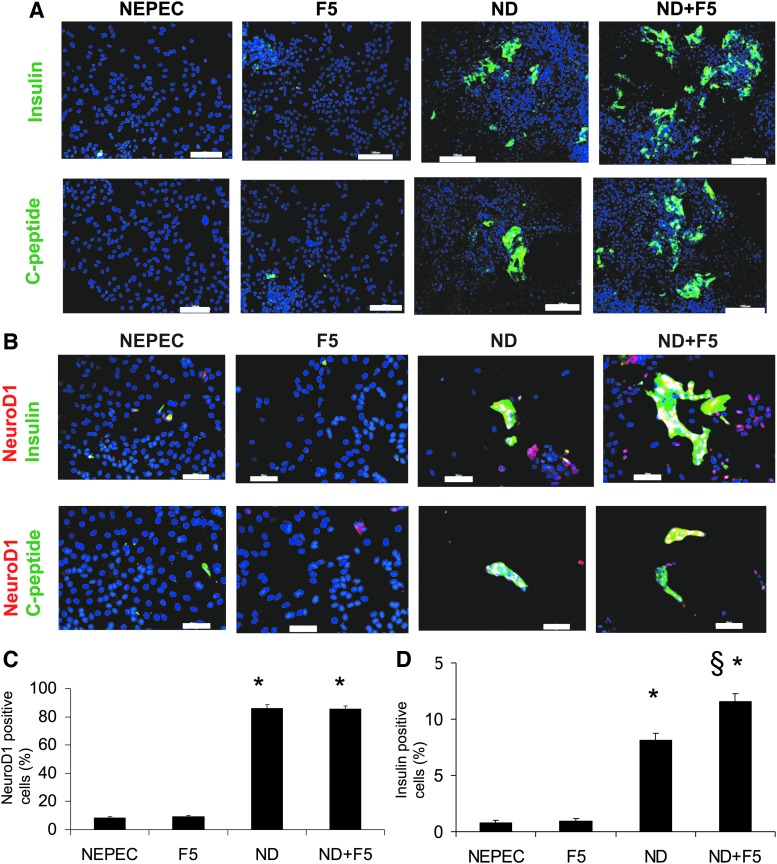
Number of NeuroD1- and insulin-positive cells
In the ND+F5 group, the number of insulin-positive cells clearly increased (Fig. 2A–D). Many of those cells were NeuroD1 positive as well. The ND group had fewer insulin-positive cells than the ND+F5 group. On the other hand, the NEPEC and F5 groups had very few insulin-positive cells. C-peptide staining showed similar results with respect to insulin. The ratio of NeuroD1-positive cells in the ND and ND+F5 groups was significantly higher than in the NEPEC and F5 groups (NEPEC, 8.1±0.7%; F5, 9.1±0.8%; ND, 87.2±2.3%; ND+F5, 85.3±2.0%; p<0.00001 in ND and ND+F5 vs. NEPEC and F5; Fig. 2C). The ratio of insulin-positive cells in the ND+F5 group was significantly higher than in the other three groups (NEPEC, 0.8±0.2%; F5, 0.9±0.2%; ND, 8.3±0.6%; ND+F5, 11.9±0.7%; p<0.00001 in ND and ND+F5 vs. NEPEC and F5; p<0.002 in ND+F5 vs. other groups; Fig. 2D). In the ND+F5 group, most of insulin-positive cells coexpressed NeuroD1 (92.3±2.4%). The ratio of insulin-positive cells in the NEPEC group was higher than in the suspension culture cells after treatment with G418 (0.08%; Fig. 1B), probably because the culture condition might change the characteristic of the cells even without growth factors. Data were obtained from five different pancreata.
FIG. 2.
NeuroD1 promotes NEPEC differentiation. (A) Immunostaining for human insulin (upper panels) and C-peptide (lower panels) of the four groups at day 7 after differentiation. Green, insulin or C-peptide; blue, DAPI. Scale bar: 100 μm. Magnification: 100×. (B) Immunostaining for human NeuroD1, insulin (upper panels), and C-peptide (lower panels) of the four groups at day 7 after differentiation. Green, insulin or C-peptide; red, human NeuroD1; blue, DAPI. Scale bar: 50 μm. Magnification: 200×. Percentage of the cells expressing NeuroD1 (C) and insulin (D) as determined by immunohistochemistry at day 7 after differentiation. The NeuroD1 or insulin-positive cells were counted using more than 20 photomicrographs at a magnification of 200×. *p<0.00001 between the ND group vs. NEPEC and F5 and between ND+F5 vs. NEPEC and F5 (C and D). §p<0.002 between ND+F5 vs. all other groups (D). Values are presented as mean±standard error of five independent experiments. For statistical analysis, ANOVA and t-test with Bonferroni adjustments were used.
Characteristics of insulin-positive cells
Immunohistochemical analysis showed that insulin-positive cells in the ND+F5 group expressed CK19 very weakly, whereas the cells that were strongly CK19 positive did not express insulin (Fig. 3A). The insulin-positive cells strongly coexpressed C-peptide and PDX1 (Fig. 3B and C). Some insulin-positive cells coexpressed neurogenin-3 (Fig. 3D). A few glucagon-positive cells were found near the insulin-positive cells, but they did not coexpress insulin (Fig. 3E). On the other hand, somatostatin, amylase, and Sox9 were not expressed (Fig. 3F–H).
FIG. 3.
Characteristics of insulin-positive cells in ND+F5 at day 7 in vitro. (A) CK19 expression was very low in the insulin-positive cells. The cells strongly coexpressed C-peptide (B), PDX1 (C), and neurogenin-3 (D), whereas glucagon (E), somatostatin (F), amylase (G), and Sox9 (H) were negative. Original magnification: 400×. Scale bar: 25 μm. In micrographs, DAPI was used for nuclear staining (blue). (I–N) Insulin secretion potency and glucose responsiveness. (I) Assessment of cellular C-peptide content in the four groups at day 7 after differentiation (n=5). Values were normalized by cell numbers (per 1×106 cells). (J) The ratio of the C-peptide content of the four groups to human islets. *p<0.001 between the ND group vs. NEPEC and F5, and between ND+F5 vs. NEPEC and F5. §p<0.01 between ND+F5 vs. ND group. (K and L) Human insulin (K) and C-peptide (L) levels in the culture media of the four groups at day 7 after differentiation (n=5). Values were normalized by cell numbers. *p<0.001 between ND+F5 vs. NEPEC, F5, ND, and islets. (M and N) C-peptide secretion in response to glucose. (M) The culture medium was replaced with a fresh medium with 2.8 mM (white bar) or 25 mM glucose (black bar) for 1 hr. C-peptide levels were measured and normalized by cell number (n=5). Human islets were used as a control. *p<0.001 between 2.8 mM vs. 25 mM. (N) SI of the four groups (N=5). SI was calculated by dividing the C-peptide concentration of 25 mM glucose by that of 2.8 mM glucose. *p<0.001 between ND+F5 vs. NEPEC, F5, ND, and islets. All values are presented as mean±standard error. For statistical analysis, ANOVA and t-test with Bonferroni adjustments were used. SI, stimulation index.
Insulin secretion potency of the differentiated NEPEC
The ND+F5 group had significantly higher cellular C-peptide content than the other groups (NEPEC, 0.8±0.2; F5, 1.6±0.2; ND, 12.1±0.4; ND+F5, 22.8±2.6 pmol/1×106 cells; p<0.001 in ND and ND+F5 vs. NEPEC and F5 groups; p<0.01 in ND+F5 vs. ND; Fig. 3I), and about 1.2% of the content of human islets (Fig. 3J). The ND+F5 group had the highest insulin level in the culture medium among the four groups but a lower level than the islets (NEPEC, 2.5±0.6; F5, 3.6±0.6; ND, 15.7±2.8; ND+F5, 26.6±6.0; islet, 333.3±20.2 pmol/1×106 cells; p<0.003 in ND+F5 vs. other groups; Fig. 3K). In addition, C-peptide levels in ND+F5 were also the highest among the 4 groups (NEPEC, 3.3±0.5; F5, 5.4±1.0; ND, 14.1±1.5; ND+F5, 27.4±5.0; islet, 317.9±10.3 pmol/1×106 cells; p<0.003 in ND+F5 vs. other groups; Fig. 3L). The glucose-stimulated C-peptide secretion test showed that there was a significant difference between the 2.8 and 25 mM glucose conditions in the ND+F5 group and islets (Fig. 3M), and ND+F5 cells had the strongest potency (stimulation index: NEPEC, 0.9±0.1; F5, 1.0±0.0; ND, 1.2±0.1; ND+F5, 2.2±0.7; islet, 7.5±1.0; p<0.001 in ND+F5 vs. other groups; Fig. 3M and N).
Function of engrafted differentiated NEPEC
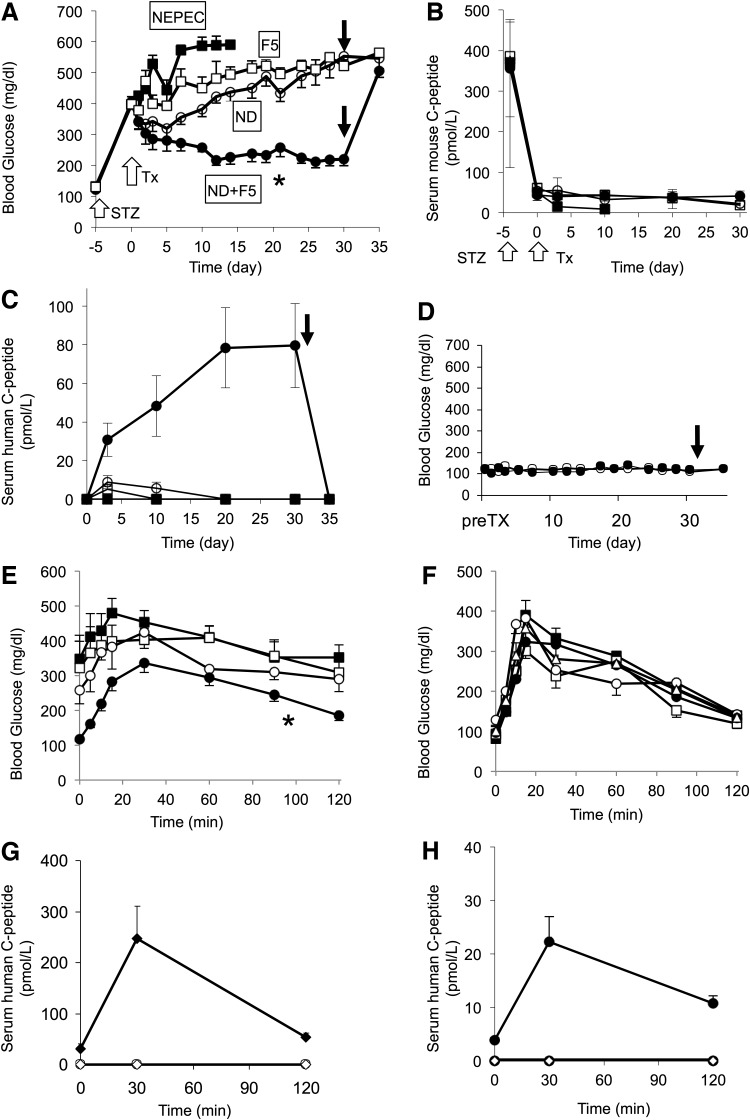
The ND+F5 cells at day 7 after differentiation were transplanted under the kidney capsule of diabetic nude mice (n=16). For this experiment, five independent pancreatic preparations were used separately. NEPEC (n=5), F5 (n=5), and ND cells (n=8) were used as a control. After transplantation, nonfasting blood glucose levels decreased to around 200 mg/dl for the ND+F5 group, whereas none of the NEPEC- and F5-transplanted mice showed a decrease in blood glucose, and all of NEPEC mice died by day 15 with diabetes (Fig. 4A). The ND group showed a small effect, but it deteriorated after 30 days. Serum mouse C-peptide was detected at a low level before transplant, and it did not change up to day 30 (Fig. 4B). In contrast, serum human C-peptide was not detectable in nonfasting condition before transplant, whereas it was detected and gradually increased after transplantation (Fig. 4C). After removal of the kidney containing the ND+F5 cells from the mice, blood glucose levels returned to pretransplant diabetic levels (∼500 mg/dl), confirming that the transplant was responsible for reducing the blood glucose. In addition, the human C-peptide levels returned to be undetectable after removal of the graft (Fig. 4C).
FIG. 4.
Improvement of diabetes after transplantation of the cells. (A) Nonfasting blood glucose levels in diabetic nude mice with subcapsular kidney transplantation for NEPEC (black squares, n=5), F5 (white squares, n=5), ND (white circles, n=8), and ND+F5 (black circles, n=16). At day 32, nephrectomy was performed (black arrow). The blood glucose levels in the ND+F5 group were significantly decreased (*p<0.001 vs. F5 and ND by RM-ANOVA). White arrows indicate the STZ administration and the transplantation of the cells. (B and C) Serum mouse and human C-peptide levels in mice transplanted with NEPEC (black squares, n=5), F5 (white squares, n=5), ND (white circles, n=8), and ND+F5 (black circles, n=16). Mouse C-peptide (B) and human C-peptide (C) before STZ injection, before transplant and at days 3, 10, 20, and 30 after transplant. White arrows indicate the STZ administration and the transplantation of the cells. At day 32, nephrectomy was performed (black arrow, C). (D) Nonfasting blood glucose levels in normal nude mice with ND+F5 transplanted (black circles, n=5). Untreated nude mice were used as a control (white circle, n=5). The arrow indicates nephrectomy at day 32. The ND+F5 group showed no remarkable change. (E–H) Glucose tolerance test at day 31. After the mice had fasted for 12 hr, glucose (2 g/kg) was intraperitoneally injected. The blood glucose levels were measured for 120 min after injection. At 0, 30, and 120 min, blood was taken for measurement of serum human C-peptide levels. (E) Blood glucose levels after a glucose tolerance test in diabetic nude mice for STZ control (black squares, n=5) and F5 (white squares, n=5), ND (white circles, n=8), and ND+F5 (black circles, n=16). The transplant of ND+F5 cells lowered glucose levels during the test (*p<0.02 vs. STZ by RM-ANOVA). (F) Blood glucose levels in normal nude mice for untreated control (white triangles, n=5), NEPEC (black squares, n=5), F5 (white squares, n=5), ND (white circles, n=5), and ND+F5 (black circles, n=5). There was no significant difference between the five groups. (G and H) Serum human C-peptide levels during the glucose tolerance test. All values are presented as mean±standard error. (G) F5 (white circle, n=5) or ND cells (white diamond, n=8) or ND+F5 cells (black circles, n=16) were transplanted into diabetic nude mice. Human C-peptide was not detected in the NEPEC group or the ND group. (H) ND cells (white diamond, n=5) or ND+F5 cells (black circles, n=5) were transplanted into normal nude mice. Untreated normal nude mice were used for control (white circle, n=5). Human C-peptide was not detected in control and ND group mice. RM-ANOVA, repeated-measures ANOVA; STZ, streptozotocin; Tx, Transplantation.
To determine whether ND+F5 cells might cause oversecretion of insulin after transplant, the cells were transplanted into normal nude mice (n=5). Their blood glucose stayed in a normal range for 30 days, and no effect was found after removal of the graft (Fig. 4D). This suggests that the endogenous beta cells maintain the blood glucose within normal range and the insulin secretion by the transplanted cells was not automatic but respondent to the blood glucose level. The glucose tolerance tests performed at day 31 posttransplantation showed that the blood glucose levels in the ND+F5 group were significantly lower than those in the STZ group (Fig. 4E). The F5 and ND groups showed a pattern similar to that of the STZ group. We also performed the test for normal nude mice with or without transplantation (Fig. 4F). There was no difference between the groups. During the glucose challenge, serum human C-peptide was detected in mice transplanted with ND+F5 cells (Fig. 4G and H). When ND+F5 cells were transplanted into normal nude mice, the serum C-peptide levels were lower than those in diabetic mice. The mice in the F5 and ND groups did not have a detectable level of human C-peptide (Fig. 4G and H).
Histological and immunohistochemical analysis of the graft
We examined the grafts of the ND+F5 group at day 32 after transplantation by histology and immunofluorescence. Typical grafts contained epithelial cell clusters that included insulin-positive cells bordered by areas of connective tissue and the surface of renal cells (Fig. 5A and B). Some ductal structures were seen mainly in the thick-stratified part of the graft (Fig. 5C). Within the grafts, insulin-positive cells were detected (Fig. 5D), and they mainly existed as thin-layer cells without CK19 expression. The cells forming ducts were CK19 positive, and there were very few insulin-positive cells in the thick part of the graft (Fig. 5E). These insulin-positive cells were also C-peptide positive (Fig. 5F). The beta cell transcription factors PDX1, Nkx6.1, and NeuroD1 were expressed in the most of insulin-positive cells (Fig. 5G–J). Glucagon-, somatostatin-, and amylase-positive cells were not detected (Fig. 5K–M). Some insulin-positive cells coexpressed Ki67 (Fig. 5N and O). The rate of Ki67-positive cells in the insulin-positive cells at day 3 after transplant was 12.4%, whereas it was 0.47% at day 32 (Fig. 5P), indicating that the transplanted cells could proliferate in the early period after transplant and the proliferating cells decreased afterward.
FIG. 5.
ND+F5 cells differentiated toward beta cells in vivo. Histological and immunohistochemical characteristics of the kidney subcapsular region of ND+F5 transplantation at day 32. (A–C) Histological sections of the transplant site. (A and B) The transplanted cells were detected as a thin layer on the kidney surface. (C) Some cells formed ductal structures. Magnification: (A) 40×; (B and C) 200×. Scale bars: (A) 250 μm; (B and C) 50 μm. Arrows indicate the graft. (D–L) Immunofluorescence analysis of grafts. (D–E) Staining for CK19 (green) and insulin (red). (F) Staining for insulin (green) and C-peptide (red). (G–I) Staining for insulin (green), PDX1 (red), NeuroD1 (red), and Nkx6.1 (red). (J) Ratio of PDX1-, NeuroD1-, Nkx6.1-positive cells in the insulin-positive cells at day 32 after transplant. For statistical analysis, ANOVA and t-test with Bonferroni adjustments were used. (K–M) Staining for insulin (red), glucagon (green), somatostatin (green), and amylase (green). (N and O) Cell proliferation of the insulin-positive cells in the graft of the ND+F5 cells at day 3 (N) and day 32 (O) after transplant. Staining for insulin (green), Ki67 (red), and DAPI (blue). (P) Ratio of Ki67-positive cells in the insulin-positive cells at day 3 and day 32 after transplant. In all micrographs, DAPI was used for nuclear staining (blue). Scale bars: (D and E) 100 μm; others, 25 μm. Original magnification: (D and E) 100×; others, 400×. White line, border of graft; K, kidney; G, graft; Ins, insulin; GCG, glucagon. The schema of the engrafted cells and kidney surface is illustrated (B, D, and E). K, kidney; G, graft cells; C, connective tissue.
Effect of transplant of ND+F5 on endogenous pancreas
The β-cell number in the pancreas of STZ-induced diabetic mice substantially decreased. After transplant of the ND+F5 cells, the β-cell number did not significantly change (Supplementary Fig. S1A–D; Supplementary Data are available online at www.liebertpub.com/hgtb). These were compatible with the fact that serum mouse C-peptide level was not different between before and after transplant (Fig. 4B).
NEPEC derived from pancreas with chronic pancreatitis
We developed NEPEC using the pancreatic nonislet fraction after islet isolation for total pancreatectomy with autologous islet transplantation (n=5). The donor and isolation variables were as follows: donor age 42.2±4.1 years; 3 men and 2 women; BMI 24.3±1.7 kg/m2; duration of symptoms 4.8±1.0 years; cold ischemic time 36±5 min. The cells were differentiated into ND+F5 cells in vitro using the same protocol as that for deceased donors and evaluated at day 7 after in vitro differentiation. The characteristics of ND+F5 cells from both groups (normal pancreas vs. chronic pancreatitis) were quite similar (Supplementary Table S1). The efficacy of transfection of the NeuroD1 gene, percentage of insulin-positive cells, cellular insulin content, and insulin secretion potency were comparable in both groups. ND+F5 cells at day 7 after differentiation were transplanted under the kidney capsule of diabetic nude mice (n=12). Nonfasting blood glucose was measured for 30 days (Fig. 6A) and the levels in most of the mice decreased to around 200 mg/dl. After removal of the kidney containing the ND+F5 cells from the mice, the levels returned to pretransplant diabetic levels (∼450 mg/dl). Human C-peptide was detected in the mice (Fig. 6B). Glucose tolerance tests were performed at day 31 posttransplantation. The blood glucose levels showed a pattern similar to that of the ND+F5 groups from normal pancreata (Fig. 6C), and serum human C-peptide was detected in mice during the tests (Fig. 6D). These data indicate that ND+F5 cells from chronic pancreatitis functioned to lower blood glucose after transplantation, as well as those from normal pancreata.
FIG. 6.
Effects of transplantation of the ND+F5 cells derived from pancreata with chronic pancreatitis. (A) Nonfasting blood glucose levels in diabetic nude mice with subcapsular kidney transplantation for ND+F5 (n=12). At day 32, nephrectomy was performed (arrow). The blood glucose increased to pretransplant levels. White arrows indicate the STZ administration and the transplantation of the cells. (B) Human C-peptide before transplant and at days 3, 10, 20, and 30 after transplant. At day 32, nephrectomy was performed (black arrow). (C) Blood glucose levels after a glucose tolerance test in diabetic nude mice (day 31) for ND+F5 (n=12). (D) Serum human C-peptide levels during the glucose tolerance test at day 31 (n=12). All values are presented as mean±standard error. (E–J) Histological and immunohistochemical characteristics of the kidney subcapsular region of ND+F5 transplantation at day 32. (E) A histological section of the transplant site. The transplanted cells were detected as a thin layer on the kidney surface. Magnification: 200×. Scale bar: 50 μm. (F–J) Immunofluorescence analysis of grafts. (F and G) Staining for CK19 (green) and insulin (red). (H–J) Staining for insulin (green), PDX1 (red), NeuroD1 (red), and Nkx6.1 (red). In all micrographs, DAPI was used for nuclear staining (blue). Scale bars: (D and E) 50 μm; (H–J) 25 μm. Original magnification: (F and G) 200×; (H–J) 400×. White line, border of graft; K, kidney; G, graft; Ins, insulin. The schema of the engrafted cells and kidney surface is illustrated (E). K, kidney; G, graft cells; C, connective tissue.
The graft cells were between the connective tissue and the surface of renal cells (Fig. 6E). The insulin-positive cells were seen in the thin layer of the graft, without CK19 expression (Fig. 6F). CK19-positive ductal structures were seen in the thick-stratified part of the graft with a few insulin-positive cells (Fig. 6G). The beta cell transcription factors PDX1, NeuroD1, and Nkx6.1 were expressed in most of insulin-positive cells (Fig. 6H–J). These findings show that the grafts derived from patient pancreata with chronic pancreatitis were comparable to those from the cadaveric donor pancreata.
Discussion
We report here for the first time that insulin-secreting cells can be generated from the pancreatic nonendocrine fraction obtained from human pancreata with chronic pancreatitis, as compared with those from normal pancreata. In our protocol, an effective triple lipofection method without viral vectors was used to induce insulin production. The triple lipofection method in this study produced high transfection efficacy for NEPECs. Although the mechanism is not clear, to repeat lipofection at a suitable interval might be important. At a rough estimate, 107 NEPECs were obtained from 0.25 ml of nonislet tissue after islet isolation. The ND+F5 cells included about 12% insulin-positive cells. In general, 20–40 ml of nonislet tissue was obtained per pancreas. Therefore, approximately 1–2×108 insulin-positive cells could be obtained per pancreas. Although the current differentiation protocol requires further improvement, it can be said that this method is moving closer to clinical application. Supplemental transplantation of the generated insulin-secreting cells from the autologous pancreas might be an excellent candidate for clinical application to improve the effect of autologous islet transplantation and achieve a high insulin-free rate. In contrast to allotransplantation, the recipients of autologous islet transplants do not require immunosuppressive drugs and are not at risk for autoimmune destruction or alloimmune rejection. A possible problem is that pancreatic tissues with chronic pancreatitis may be damaged and unsuitable as a source of the generated cells. However, it was reported that partial pancreatic ductal obstruction could enhance the islet neogenesis-associated proteins that stimulate beta cell regeneration (Rosenberg, 1988). In addition, chronic inflammation could promote regeneration of islet progenitor cells in nonislet tissues (Phillips et al., 2007). Interestingly, our data indicated that ND+F5 cells derived from pancreata with chronic pancreatitis had a similar potency to those from normal pancreata.
Recent studies have reported the methods to generate insulin-positive cells derived from healthy human pancreatic nonislet cells under specific conditions in vitro (Bonner-Weir et al., 2000; Bogdani et al., 2003; Todorov et al., 2006; Gao et al., 2007; Yatoh et al., 2007). However, those cells had a low level of insulin compared with mature beta cells and did not reverse diabetes after implantation. Zhao et al. (2005) reported that insulin-producing cells were derived from human pancreatic nonendocrine cells using PDX1 gene induction and the cells reversed hyperglycemia in mice. Compared with their method, our method has some advantages: high transfection efficacy (85% vs. 30%), shorter time period required to differentiate the insulin-producing cells, no need to transplant the cells inside the renal parenchyma, and the unique CK19 promoter that could function in only CK19-positive cells to eliminate the possibility that the reversal of diabetes was caused by proliferation of residual beta cells. It is difficult to exclude all contaminating islets from the initial pancreatic tissue. However, Russ et al. (2009, 2011) studied using genetic cell-lineage tracing and reported that the expanding human islet cells in tissue culture could be induced to redifferentiate into insulin-producing cells and there was no evidence that the insulin-expressing cells were derived from a non-beta-cell origin in their culture condition, which is consistent with our data showing that the NEPEC and F5 group cells did not differentiate into the insulin-producing cells. It indicates that NEPECs are not derived from the endocrine cells. We previously reported that NeuroD1 gene induction stimulated the differentiation of NEPEC into insulin-producing cells, comprising approximately 8% of NEPEC (Shimoda et al., 2010a). NeuroD1 is a basic helix–loop–helix transcription factor that is found in the pancreas, intestine, and central nervous system (Naya et al., 1995). In NeuroD1 gene knockout experiments, the mutant mice developed severe diabetes with ketoacidosis and died after birth (Naya et al., 1997). Previous reports have shown that adenoviral-mediated introduction of NeuroD1 induced beta cell neogenesis in the liver and reversed diabetes in mice with betacellulin gene therapy (Kojima et al., 2003) or PDX-1/VP16 gene therapy (Kaneto et al., 2005). Another study suggested that NeuroD1 is not essential for early differentiation but has an important role in later-stage differentiation and maintenance of beta cells (Chao et al., 2007). Recently, we demonstrated that NeuroD1 gene delivery into pancreas reversed STZ-induced diabetes in rats (Chen et al., 2010). These studies indicate that NeuroD1 is an important gene for determining the lineage to pancreatic endocrine cells. Furthermore, NeuroD1 can promote the differentiation of pancreatic progenitor cells into beta cells in vitro (Noguchi et al., 2006). Taken together, the overexpression of NeuroD1 can be a highly effective method to differentiate NEPEC into beta cells. However, the induction of the NeuroD1 gene without the five growth factors, which were reported to maturate human embryonic stem cells and induce pluripotent stem cell-derived pancreatic progenitor cells into insulin-producing cells (Zhang et al., 2009), was not sufficient to reverse diabetes, as shown in this study (the ND group). Our current protocol allowed us to increase the ratio of insulin-positive cells in vitro (the ND+F5 group).
The mechanism to generate fully mature beta cells is still unknown. In our method, the insulin secretion gradually decreased 3 weeks after induction of differentiation, and the induced NeuroD1 expression became lower 2 weeks after transfection in vitro (data not shown). It indicates that the differentiation protocol could not differentiate NEPEC completely and could not maintain it. In vivo condition is required for further differentiation and maintenance. Indeed, our results indicate that the transplanted cells proliferated and further differentiated in the early period resulting in improving blood glucose control with a relatively limited number of insulin-positive cells (11.9% of 10,000,000 cells), but the mechanism is unclear. In fact, it was reported that another type of engineered human insulin-producing cells required more cells (Ravassard et al., 2011). In addition, ND+F5 cells were not fully matured beta cells even under in vivo conditions. Their phenotype became more similar to that of mature beta cells (CK19−, PDX1+, Nkx6.1+, NeuroD1+, glucagon−, amylase−); however, their morphology was still unlike that of islets, clearly indicating that the cells were immature. Their characteristics should be investigated more by other beta cell markers, including MafA and Nkx2.2, and by microstructure, but they were not performed because of technical difficulties so that further examinations are required. Interestingly, the insulin-positive cells mainly appeared as thin-layer cells in the graft. Although the exact cause has yet to be determined, one possibility is that their survival might require sufficient oxygen and nutrition and thick grafts might lack these factors. It is a dilemma that many functional cells are required to establish normoglycemic control, but the number of transplantable cells is limited. In the rodent model, cotransplantation of other cells might stimulate graft vascularization by producing some factors such as vascular endothelial growth factor, resulting in improved beta cell graft survival (Baeyens et al., 2009). Promoting vascularization might improve our protocol. However, even though the cell survival would be improved, they would not have three-dimensional structure like islets. In addition, although most of the cells overexpressed NeuroD1 in ND+F5, only 11.9% of cells became insulin positive in vitro. The reason is unknown, but there may be two possibilities. First, NeuroD1 is an essential but not sufficient to induce full maturation. Other unknown factors might be required. Second, there may be a few “special” cells in the CK19-positive cell population, such as stem or progenitor cells, although they are indistinguishable by morphology, and only these cells could be differentiated into beta cells. There may be other undiscovered mechanisms, and further studies are required.
Another point of uncertainty is the exact origin of the new insulin secretory cells. In this study, they were probably generated from epithelial cells because almost all fibroblasts were eliminated before monolayer culture, and the pCK19-hND plasmid worked in only CK19-positive cells (CK19 is a marker of epithelial cells). There are two major types of pancreatic epithelial cells: ductal cells and acinar cells. It has been reported that acinar cells can be transdifferentiated to have duct cell-like properties (Minami et al., 2005; Zhao et al., 2005). In contrast, others found that the ductal cells are the main source (Bonner-Weir et al., 2000; Leng and Lu, 2005; Yatoh et al., 2007). In addition, it is theoretically possible that a few contaminated beta cells dedifferentiated and somehow expressed CK19, and then redifferentiated to insulin-producing cells by pCK19-hND transfection. Therefore, a part of insulin-positive cells might be derived from original islet cells because it is almost impossible to completely exclude all islets from the pancreatic nonislet tissues before culture. This is a limitation of this study. However, their contributions would be slight because contaminated islets were too few to be dominant during culture. Thus, this question remains to be solved.
Conclusively, our new highly effective method for beta cell generation without viral vectors might help this field move toward clinical application for the cure of diabetes.
Supplementary Material
Acknowledgments
We thank Y. Tamura, A.M. Rahman, and G. Olsen of Annette C. and Harold C. Simmons Transplant Institute for their technical support, and Cynthia Orticio of Baylor Research Institute for editorial assistance. This work was supported, in part, by National Institutes of Health R01 Grant HL072430-01 (Grayburn), a research grant from the Japan IDDM Network and the Baylor All Saints Health Foundation.
Author Disclosure Statement
The authors declare no conflict of interest.
References
- Baeyens L., Bonné S., Bos T., et al. (2009). Notch signaling as gatekeeper of rat acinar-to-beta-cell conversion in vitro. Gastroenterology 136, 1750–1760 [DOI] [PubMed] [Google Scholar]
- Bogdani M., Lefebvre V., Buelens N., et al. (2003). Formation of insulin-positive cells in implants of human pancreatic duct cell preparations from young donors. Diabetologia 46, 830–838 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Taneja M., Weir G.C., et al. (2000). In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. USA 97, 7999–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C.S., Loomis Z.L., Lee J.E., et al. (2007). Genetic identification of a novel NeuroD1 function in the early differentiation of islet alpha, PP and epsilon cells. Dev. Biol. 312, 523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Shimoda M., Wang M., et al. (2010). Regeneration of pancreatic islets in vivo by ultrasound-targeted gene therapy. Gene Ther. 17, 1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Ustinov J., Korsgren O., et al. (2007). Maturation of in vitro-generated human islets after transplantation in nude mice. Mol. Cell Endocrinol. 264, 28–34 [DOI] [PubMed] [Google Scholar]
- Hansson M., Tonning A., Frandsen U., et al. (2004). Artifactual insulin release from differentiated embryonic stem cells. Diabetes 53, 2603–2609 [DOI] [PubMed] [Google Scholar]
- Hao E., Tyrberg B., Itkin-Ansari P., et al. (2006). Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat. Med. 12, 310–316 [DOI] [PubMed] [Google Scholar]
- Ikemoto T., Noguchi H., Shimoda M., et al. (2009). Islet cell transplantation for the treatment of type 1 diabetes in the USA. J. Hepatobiliary Pancreat. Surg. 16, 118–123 [DOI] [PubMed] [Google Scholar]
- Inada A., Nienaber C., Katsuta H., et al. (2008). Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. USA 105, 19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya M., Kaneko S., Ohno H., et al. (2001). Cloning and characterization of the 5′-flanking region of human cytokeratin 19 gene in human cholangiocarcinoma cell line. J. Hepatol. 35, 504–511 [DOI] [PubMed] [Google Scholar]
- Kaneto H., Nakatani Y., Miyatsuka T., et al. (2005). PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes 54, 1009–1022 [DOI] [PubMed] [Google Scholar]
- Kojima H., Fujimiya M., Matsumura K., et al. (2003). NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat. Med. 9, 596–603 [DOI] [PubMed] [Google Scholar]
- Leng S.H., and Lu F.E. (2005). Induction of pancreatic duct cells of neonatal rats into insulin-producing cells with fetal bovine serum: a natural protocol and its use for patch clamp experiments. World J. Gastroenterol. 11, 6968–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S. (2010). Islet cell transplantation for type 1 diabetes. J. Diabetes 2, 16–22 [DOI] [PubMed] [Google Scholar]
- Matsumoto S. (2011). Autologous islet cell transplantation to prevent surgical diabetes. J. Diabetes 3, 376–380 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Okitsu T., Iwanaga Y., et al. (2005). Insulin independence after living-donor distal pancreatectomy and islet allotransplantation. Lancet 365, 1642–1644 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Okitsu T., Iwanaga Y., et al. (2006). Successful islet transplantation from nonheartbeating donor pancreata using modified Ricordi islet isolation method. Transplantation 82, 460–465 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Noguchi H., Shimoda M., et al. (2010). Seven consecutive successful clinical islet isolations with pancreatic ductal injection. Cell Transplant. 19, 291–297 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Takita M., Chaussabel D., et al. (2011). Improving efficacy of clinical islet transplantation with iodixanol based islet purification, thymoglobulin induction and blockage of IL-1 beta and TNF alpha. Cell Transplant. 20, 1641–1647 [DOI] [PubMed] [Google Scholar]
- Minami K., Okuno M., Miyawaki K., et al. (2005). Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc. Natl. Acad. Sci. USA 102, 15116–15121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya F.J., Stellrecht C.M., and Tsai M.J. (1995). Tissue-specific regulation of the insulin gene by a novel basic helix-loop-helix transcription factor. Genes Dev. 9, 1009–1019 [DOI] [PubMed] [Google Scholar]
- Naya F.J., Huang H.P., Qiu Y., et al. (1997). Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 11, 2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Xu G., Matsumoto S., et al. (2006). Induction of pancreatic stem/progenitor cells into insulin-producing cells by adenoviral-mediated gene transfer technology. Cell Transplant. 15, 929–938 [DOI] [PubMed] [Google Scholar]
- Noguchi H., Ikemoto T., Naziruddin B., et al. (2009). Iodixanol-controlled density gradient during islet purification improves recovery rate in human islet isolation. Transplantation 87, 1629–1635 [DOI] [PubMed] [Google Scholar]
- Phillips J.M., O'Reilly L., Bland C., et al. (2007). Patients with chronic pancreatitis have islet progenitor cells in their ducts, but reversal of overt diabetes in NOD mice by anti-CD3 shows no evidence for islet regeneration. Diabetes 56, 634–640 [DOI] [PubMed] [Google Scholar]
- Ramiya V.K., Maraist M., Arfors K.E., et al. (2000). Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat. Med. 6, 278–282 [DOI] [PubMed] [Google Scholar]
- Ravassard P., Hazhouz Y., Pechberty S., et al. (2011). A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J. Clin. Invest. 121, 3589–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. (1988). Induction of islet cell neogenesis in the adult pancreas: the partial duct obstruction model. Microsc. Res. Tech. 43, 337–346 [DOI] [PubMed] [Google Scholar]
- Russ H.A., Ravassard P., Kerr-Conte J., et al. (2009). Epithelial-mesenchymal transition in cells expanded in vitro from lineage-traced adult human pancreatic beta cells. PLoS ONE 4, e6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ H.A., Sintov E., Anker-Kitai L., et al. (2011). Insulin-producing cells generated from dedifferentiated human pancreatic beta cells expanded in vitro. PLoS ONE 6, e25566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg R.M., Smukler S.R., Kieffer T.J., et al. (2004). Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 22, 1115–1124 [DOI] [PubMed] [Google Scholar]
- Shapiro A.M., Lakey J.R., Ryan E.A., et al. (2000). Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238 [DOI] [PubMed] [Google Scholar]
- Shapiro A.M., Ricordi C., Hering B.J., et al. (2006). International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 355, 1318–1330 [DOI] [PubMed] [Google Scholar]
- Shimoda M., Chen S., Noguchi H., et al. (2010a). Neurogenic differentiation 1 directs differentiation of cytokeratin 19-positive human pancreatic non-endocrine cells into insulin producing cells. Transplant. Proc. 42, 2071–2074 [DOI] [PubMed] [Google Scholar]
- Shimoda M., Chen S., Noguchi H., et al. (2010b). In vivo non-viral gene delivery of human vascular endothelial growth factor improves revascularisation and restoration of euglycaemia after human islet transplantation into mouse liver. Diabetologia 53, 1669–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D.E., Gruessner A.C., Carlson A.M., et al. (2008). Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation 86, 1799–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Nakauchi H., and Taniguchi H. (2004). Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes 53, 2143–2152 [DOI] [PubMed] [Google Scholar]
- Takita M., Naziruddin B., Matsumoto S., et al. (2011). Body mass index reflects islet isolation outcome in islet autotransplantation for patients with chronic pancreatitis. Cell Transplant. 20, 313–322 [DOI] [PubMed] [Google Scholar]
- Todorov I., Omori K., Pascual M., et al. (2006). Generation of human islets through expansion and differentiation of non-islet pancreatic cells discarded (pancreatic discard) after islet isolation. Pancreas 32, 130–138 [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yamato E., Taniguchi H., et al. (2006). Stimulation of cAMP signaling allows isolation of clonal pancreatic precursor cells from adult mouse pancreas. Diabetologia 49, 2359–2367 [DOI] [PubMed] [Google Scholar]
- Yatoh S., Dodge R., Akashi T., et al. (2007). Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes 56, 1802–1809 [DOI] [PubMed] [Google Scholar]
- Zhang D., Jiang W., Liu M., et al. (2009). Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 19, 429–438 [DOI] [PubMed] [Google Scholar]
- Zhao M., Amiel S.A., Christie M.R., et al. (2005). Insulin-producing cells derived from human pancreatic non-endocrine cell cultures reverse streptozotocin-induced hyperglycaemia in mice. Diabetologia 48, 2051–2061 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.