Abstract
Methamphetamine administration causes long-term deficits to dopaminergic systems that, in humans, are thought to be associated with motor slowing and memory impairment. Methamphetamine interacts with the dopamine transporter (DAT) and increases extracellular concentrations of dopamine that, in turn, binds to a number of dopamine receptor subtypes. Although the relative contribution of each receptor subtype to the effects of methamphetamine is not fully known, non-selective dopamine D2/D3 receptor antagonists can attenuate methamphetamine-induced changes to dopamine systems. The present study extended these findings by testing the role of the dopamine D3 receptor subtype in mediating the long-term dopaminergic, and for comparison serotonergic, deficits caused by methamphetamine. Results indicate that the dopamine D3 receptor selective antagonist, PG01037, attenuated methamphetamine-induced decreases in striatal DAT, but not hippocampal serotonin (5HT) transporter (SERT), function, as assessed 7 days after treatment. However, PG01037 also attenuated methamphetamine-induced hyperthermia. When methamphetamine-induced hyperthermia was maintained by treating rats in a warm ambient environment, PG01037 failed to attenuate the effects of methamphetamine on DAT uptake. Furthermore, PG01037 did not attenuate methamphetamine-induced decreases in dopamine and 5HT content. Taken together, the present study demonstrates that dopamine D3 receptors mediate, in part, the long-term deficits in DAT function caused by methamphetamine, and that this effect likely involves an attenuation of methamphetamine-induced hyperthermia.
Keywords: Methamphetamine, dopamine D3 receptors, D3 antagonist, PG01037, dopamine transporter, serotonin transporter
1. Introduction
Methamphetamine use and dependence is a serious public health concern with a significant societal impact, including a burden on psychiatric and medical resources. Although several neurotransmitter systems are presumably involved in the reinforcing (Achat-Mendes et al., 2012; Munzar et al., 1999) and neurochemical (Eisch et al., 1996; Stephans and Yamamoto, 1994) effects of methamphetamine, there has been a large focus on dopamine systems (Koob and Volkow, 2010). Methamphetamine use can produce both acute and long-term changes in dopaminergic neurons, as demonstrated in rodents (Hadlock et al., 2010; Metzger et al., 2000), non-human primates (Melega et al., 1997), and humans (McCann et al., 1998; Wilson et al., 1996). In humans, these effects of methamphetamine are associated with motor slowing and memory impairment (Volkow et al., 2001) and may be related to psychiatric symptoms (Sekine et al., 2001).
Methamphetamine interacts with the dopamine transporter (DAT) and facilitates the release of dopamine (Kahlig et al., 2005; Raiteri et al., 1979). In turn, this enhances extracellular concentrations of dopamine (Kuczenski et al., 1995; O’Dell et al., 1991) that binds to a number of dopamine receptor subtypes. Preclinical studies indicate that high-dose methamphetamine administration causes long-term changes to dopaminergic systems, including long-term decreases in DAT activity, tyrosine hydroxylase activity, and striatal dopamine content (Kogan et al., 1976; Nakayama et al., 1993; Seiden et al., 1976; Wagner et al., 1980). Although the mechanisms underlying these long-term deficits are not completely understood, several dopamine receptor subtypes have been implicated in mediating the effects of methamphetamine. For example, nonselective dopamine D2/D3 receptor antagonists can attenuate methamphetamine-induced dopamine overflow (O’Dell et al., 1993), decreases in DAT activity (Gross et al., 2011; Hadlock et al., 2010), and decreases in dopamine content (Broening et al., 2005; Sonsalla et al., 1986). However, the ability of these antagonists to attenuate the effects of methamphetamine has primarily been attributed, at least in part, to antagonism of methamphetamine-induced hyperthermia (Albers and Sonsalla, 1995). A large body of literature suggests that dopamine D2 receptors mediate changes in body temperature induced by direct-acting dopamine receptor agonists (Chaperon et al., 2003; Collins et al., 2007); thus, and to the extent that methamphetamine-induced hyperthermia is mediated by dopamine D2, and not D3, receptors, selective dopamine D3 receptor ligands might be useful to investigate the association between methamphetamine-induced hyperthermia and subsequent long-term deficits.
Dopamine D3 receptors have been suggested as targets for medication development to treat substance use disorders and for its purported role in mediating abuse-related effects of drugs, including methamphetamine (Heidbreder et al., 2005; Higley et al., 2011; Newman et al., 2005); however, it is unclear whether these receptors are involved in other effects of methamphetamine, such as long-term deficits. Compounds that selectively target dopamine D3 receptors have been developed, including PG01037 (functions as a selective dopamine D3 receptor antagonist in vivo; Baladi et al., 2010; Collins et al., 2005). The present study utilized PG01037 to investigate the role of dopamine D3 receptors in mediating the long-term dopaminergic, and for comparison, serotonergic deficits caused by methamphetamine.
2. Material and Methods
2.1. Animals
Male Sprague-Dawley rats (250–300 g upon arrival; Charles River Laboratories) were housed in an environmentally controlled room under a 14/10 light/dark cycle, with food and water provided ad libitum. On the day of the experiment, rats were housed in plastic cages (n = 4 rats/cage) and were maintained in an ambient temperature of ~24°C. Rats received PG01037 (4 × 32 mg/kg/injection, s.c.) or vehicle (4 × 1 ml/kg/injection, s.c.) 30 min before each administration of methamphetamine (4 × 7.5 mg/kg/injection, s.c.; 2-h intervals) or vehicle (4 × 1 ml/kg/injection, s.c.; 2-h intervals). Core (rectal) body temperatures were recorded using a digital rectal thermometer (Physitemp Instruments, Clifton, NJ). Rectal temperatures were recorded immediately before the first injection (i.e. PG01037 or vehicle) and then 30 min before and after every injection of either methamphetamine or vehicle, depending on the group. For experiments where body temperature was manipulated, some cages in which rats received methamphetamine were placed in a warm ambient environment to enhance drug-induced hyperthermia. All experiments were approved by the University of Utah Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Body Temperature
In studies designed to investigate the impact of PG01037 pretreatment on methamphetamine-induced alterations in core (rectal) body temperature, temperatures were measured by inserting a thermal probe into the rectum. Animals were adapted to the experimental procedure by measuring body temperature before studies with drug commenced. Cumulative dose-response curves were generated for methamphetamine alone (1–32 mg/kg s.c.) with increasing doses administered every 15 min and in combination with the D3 receptor-selective antagonist PG01037 (32 and 56 mg/kg s.c.). Beginning 10 min after each injection, body temperature was recorded. The antagonist was given 30 min before administration of the first dose of methamphetamine. Experimental sessions were separated by at least 7 days.
2.3. Synaptosomal [3H]dopamine and [3H]5HT Uptake
Synaptosomal uptake of [3H]dopamine through DAT and [3H]5HT through the serotonin transporter (SERT) was determined 7 days after the last methamphetamine administration and according to methods reported previously (Fleckenstein et al., 1997; Hadlock et al., 2011). In brief, freshly dissected striatal (for DAT uptake) or hippocampal (for SERT) tissue was homogenized in ice-cold 0.32 M sucrose and centrifuged (800g for 12 min; 4°C). The supernatant (S1) was then centrifuged (22,000g for 15 min; 4°C), and the resulting pellet (P2) was resuspended in ice-cold 0.32 M sucrose. Assays were conducted in modified Krebs’ buffer (126 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 16 mM sodium phosphate, 1.4 mM MgSO4, 11 mM dextrose, 1 mM ascorbic acid; pH 7.4). Each assay tube contained synaptosomal tissue (i.e., resuspended P2 obtained from 1.5 mg of original wet weight striatal tissue) and 1 mM pargyline. Nonspecific values were determined in the presence of 1 mM cocaine (for DAT) or 10 mM fluoxetine (for SERT). After preincubation of assay tubes for 10 min at 37°C, assays were initiated by the addition of [3H]dopamine or [3H]5HT (0.5 nM final concentration). Samples were incubated at 37°C for 3 min, then filtered through Whatman GF/B filters soaked previously in 0.05% polyethylenimine. Filters were washed rapidly 3 times with 3 ml of ice-cold 0.32 M sucrose using a Brandel filtering manifold. Radioactivity trapped in filters was counted using a liquid scintillation counter. Remaining resuspended P2 samples were assayed for protein concentrations according to the previous methods (Lowry et al., 1951).
2.4. Dopamine and 5HT Content Determination
Seven days after drug treatment, animals were decapitated, and striatal tissue was immediately removed and frozen on aluminum foil placed over dry ice. Tissue was obtained from the striatum contralateral to that used for synaptosomal [3H]dopamine uptake. Samples were stored at −80°C until assayed. Monoamine levels were determined in tissue homogenates using HPLC, with electrochemical detection (Chapin et al., 1986). Briefly, on the day of the assay, tissue samples (approximately 10 mg of striatal tissue) were thawed in 500 ml of ice-cold tissue buffer [0.1 M phosphate-citrate buffer (pH 2.5) containing 15% methanol], sonicated for 3 to 5 s, and then centrifuged (22,000g for 15 min at 4°C). Tissue pellets were retained and dissolved in 1 N NaOH, and protein content was determined according to the method of Lowry et al. (1951). The supernatant (S1) was then centrifuged (22,000g for 10 min at 4°C), and the resulting supernatant (S2) was injected onto an HPLC system equipped with a Partisphere C18 reverse-phase analytical column (5-mm spheres; 110 3 4.6 mm) and a reverse-phase guard column (Whatman Inc., Clifton, NJ). The mobile phase consisted of 0.05 M sodium phosphate, 0.03 M citrate buffer (pH 2.86) containing 0.1 M EDTA, 0.035% sodium octyl sulfate, and 25% methanol. Monoamines were detected with an electrochemical detector with the working electrode potential set at +0.70 V relative to an Ag+/AgCl reference electrode.
2.5. Drugs
(±)-Methamphetamine hydrochloride (Research Triangle Institute, Research Triangle Park, NC) was dissolved in 0.9% sterile saline, with the dose described as the free base form. PG01037 (N-{4 [4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide HCl) was synthesized by Jianjing Cao (Medicinal Chemistry Section, National Institute on Drug Abuse, Baltimore, MD) using methods reported previously (Grundt et al., 2005) and dissolved in 10% β-cyclodextrin). The doses and pretreatment times for methamphetamine (Hadlock et al., 2010; McGuire et al., 2011) and PG01037 (Baladi et al., 2010; Collins et al., 2005) were selected based on previous publications. All drugs were administered s.c. in a volume of 1 ml/kg.
2.6. Data Analysis
Synaptosomal [3H]dopamine and [3H]5HT uptake data are expressed as average (± S.E.M.) fmol of radioligand per microgram (μg) protein and plotted as a function of treatment group. Values obtained were consistent with those reported previously (Howard et al., 2011; Metzger et al., 2000; Tata and Yamamoto, 2008). Comparisons were made within an experiment (versus across separate experiments) such that animals within a given experiment were treated concurrently, tissues were processed simultaneously and assay conditions to which samples were exposed were identical. Statistical analyses for uptake and monoamine content assays were conducted with an ANOVA with post hoc Bonferroni’s for multiple comparisons.
Body temperature data are expressed as the average (± S.E.M.) temperature in degrees Celsius and plotted as a function of time or the average (± S.E.M.) temperature across experiment time for each individual rat (after the first methamphetamine injection) and plotted as a function of treatment group. Statistical analyses were conducted with an ANOVA with post hoc Bonferroni’s for multiple comparisons. For the body temperature experiment using cumulative doses of methamphetamine, data are expressed as a change in degrees Celsius from baseline (i.e., body temperature determined after vehicle administration) averaged among six rats (± S.E.M.) and plotted as a function of dose. Differences between methamphetamine dose-response curves in the presence and absence of antagonist were analyzed by simultaneously fitting straight lines to the linear portion of the dose-response curves by means of GraphPad Prism (GraphPad Software Inc., San Diego, CA). The linear portion included doses that spanned the 50% level of effect and included not more than one dose with greater than 75% effect and not more than one dose with less than 25% effect. Differences between slopes and intercepts of the curves were analyzed with the F ratio test (GraphPad Prism), as detailed elsewhere (Koek et al., 2006). To calculate ED50 values for methamphetamine-induced hyperthermia in the absence and presence of antagonist, a common maximum effect was selected for individual rats. The 95% CLs were calculated from ED50 values averaged among rats. To evaluate changes in potency as a result of antagonist treatment, a dose ratio was calculated for each rat by dividing the ED50 obtained in the presence of antagonist by the ED50 obtained in the absence of antagonist. When the 95% CLs of the dose ratio did not include 1, the antagonist was considered to significantly change the potency of the drug relative to its potency in the absence of antagonist. For all tests, significance was set at P<0.05.
3. Results
Methamphetamine (4 × 7.5 mg/kg/injection, s.c., 2-h intervals) decreased striatal DAT uptake as assessed 7 days after the final injection (Fig. 1A; P<0.05; F(3, 30)=7.44, P<.0009). PG01037 (32 mg/kg/injection, s.c.), administered 30 min before each methamphetamine or vehicle injection, attenuated the effects of methamphetamine on DAT uptake. In contrast, PG01037 pretreatment did not attenuate the methamphetamine-induced deficits in hippocampal SERT function at this time point (0.51 ± 0.03, 0.25 ± 0.05*, 0.6 ± 0.07 and 0.36 ± 0.07* fmol/μg protein for vehicle/vehicle, vehicle/methamphetamine, PG01037/saline, PG01037/methamphetamine, respectively; *P<0.05; F(3, 31)=7.01, P<0.001). PG01037 pretreatment likewise did not attenuate methamphetamine-induced decreases in striatal dopamine or hippocampal serotonin content (Table 1; F(3, 29)=16.15, P<0.0001, F(3, 28)=7.940, P<0.001, respectively) as assessed 7 days later. Administration of the antagonist alone did not impact DAT or SERT uptake or striatal monoamine content.
Figure 1.
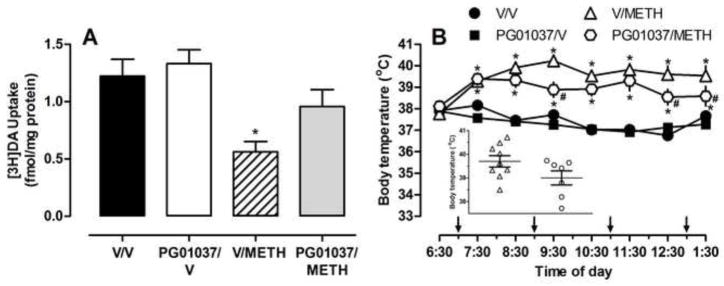
The effects of PG01037 on methamphetamine-induced decreases in striatal DAT (A) and hyperthermia (B). Rats (n = 6–9/group) received PG010137 (4 × 32 mg/kg/injection, s.c.) or vehicle (4 × 1 ml/kg/injection, s.c.) 30 min before each administration of methamphetamine (4 × 7.5 mg/kg/injection, s.c., 2-h intervals) or vehicle (4 × 1 ml/kg/injection, s.c., 2-h intervals) and were sacrificed 7 days later. Ordinates: mean (± S.E.M.) fmol of [3H]dopamine per μg protein or body temperature. Abscissa: treatment group; V = vehicle and METH = methamphetamine or time across experiment; arrows indicate methamphetamine administration. Inset figure: mean (± S.E.M.) body temperature across time after first methamphetamine injection for each individual rat. * Methamphetamine values significantly different from respective vehicle control group (P<0.05). # PG01037/methamphetamine values significantly different from other methamphetamine group (P<0.05).
Table 1.
Effect of PG01037 on methamphetamine-induced decreases in dopamine and 5HT content
| Group | Dopamine | 5HT | Dopamine | 5HT |
|---|---|---|---|---|
| Warm ambient environment | ||||
| Vehicle/Vehicle | 90.7 (±6.5)a | 4.9 (±0.4) | 115.5 (±17.9) | 2.8 (±0.4) |
| PG01037/Vehicle | 81.6 (±9.6) | 4.2 (±0.6) | 126.6 (±29.1) | 3.5 (±0.6) |
| Vehicle/methamphetamine | 27.1b (±5.1) | 2.4b (±0.3) | 40.4b (±12.1) | 1.3b (±0.2) |
| PG010137/methamphetamine | 38.1b (±9.2) | 2.5b (±0.4) | 30.2b (±7.3) | 1.6b (±0.3) |
Average monoamine content (± S.E.M.) of 6–9 rats per group
Methamphetamine values significantly different from vehicle control values (P<0.05)
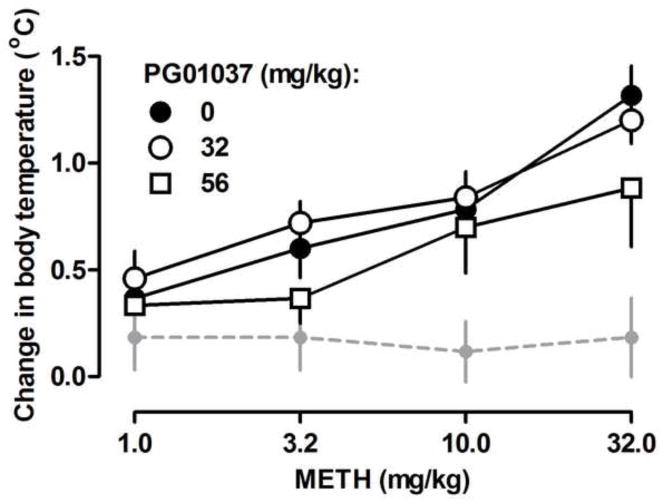
Methamphetamine dose-dependently increased body temperature and a single injection of PG01037 (32 and 56 mg/kg) did not attenuate the hyperthermic effects of methamphetamine (i.e., 95% CLs of dose ratios did include 1; Fig. 2). Similarly, results presented in Fig. 1B demonstrate that repeated methamphetamine injections increased core body temperature by approximately 2–3°C (Fig. 1B, F(3, 31)=167.50, P<0.0001). However, and in contrast to Figure 2, multiple administrations of PG01037 attenuated methamphetamine-induced increases in body temperature at certain time points throughout the course of treatment [F(7, 31)=4.60, P<0.0001]. For example, in methamphetamine-treated rats at the fourth temperature recording (i.e. 9:30 a.m.), the body temperature of rats pretreated with PG01037 was significantly lower than the body temperature of rats pretreated with vehicle (Fig. 1B; P<0.0001). Administration of the antagonist alone did not alter body temperature (Fig. 1B). When body temperature was plotted as the average temperature throughout the course of treatment for each individual rat, the group means were not different from each other; however, there was a trend for some rats that received PG01037 to have an overall lower temperature (Fig. 1B inset).
Figure 2.
Methamphetamine-induced hyperthermia when methamphetamine was administered alone and with different doses of PG01037 (n = 6). Abscissa, dose in milligrams per kilogram of body weight; dashed gray line indicate body temperature across time after saline administration. Ordinate, mean (± S.E.M.) change in body temperature.
To examine whether the ability of PG01037 to attenuate the effects of multiple methamphetamine injections on DAT uptake was related to attenuation of methamphetamine-induced hyperthermia, in the second experiment, rats were treated in a warm ambient environment (a common practice used to promote methamphetamine-induced hyperthermia; see, for example Bowyer et al., 1993; Hadlock et al., 2010; McFadden et al., 2011; Myles et al., 2008). Results indicate that PG01037 failed to attenuate the methamphetamine-induced decreases in DAT (Fig. 3A, F(3, 32)=31.24, P<0.0001). Further, PG01037 did not attenuate the long-term deficits in SERT function (0.58 ± 0.10, 0.62 ± 0.11, 0.21 ± 0.06,* 0.22 ± 0.03* fmol/μg protein for vehicle/vehicle, vehicle/methamphetamine, PG01037/saline, PG01037/methamphetamine, respectively; *P <0.05; F(3, 30)=8.70, P<0.001, F(3, 30)=6.16, P<0.01, respectively), nor the decreases in dopamine and 5HT content (Table 1), as assessed 7 days after drug administration. Methamphetamine increased core body temperature (Fig. 3B, F(3, 31)=238.8, P<0.0001) and body temperatures were not different between methamphetamine-treated groups pretreated with PG01037 or vehicle (Fig. 3B). Administration of the antagonist alone did not impact DAT or SERT uptake, striatal monoamine content, or body temperature.
Figure 3.
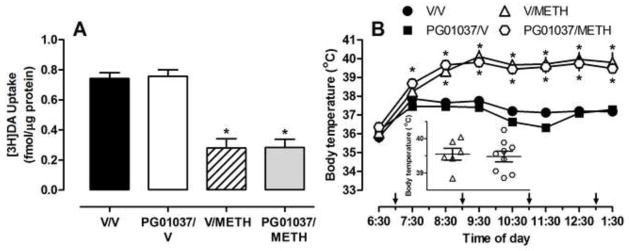
The effects of PG01037 on methamphetamine-induced decreases in striatal DAT (A) and hyperthermia (B) when rats were treated in a warm ambient environment. Rats (n = 6–9/group) received PG010137 (4 × 32 mg/kg/injection, s.c.) or vehicle (4 × 1 ml/kg/injection, s.c.) 30 min before each administration of methamphetamine (4 × 7.5 mg/kg/injection, s.c., 2-h intervals) or vehicle (4 × 1 ml/kg/injection, s.c., 2-h intervals) and were sacrificed 7 days later. See figure 2 for other details.
4. Discussion
The current study examined the contribution of dopamine D3 receptors to the long-term effects of methamphetamine on dopamine and 5HT systems. Results indicate that a selective dopamine D3 receptor antagonist, PG01037, attenuates the long-term methamphetamine-induced decreases in DAT uptake. This effect of PG01037 was most likely due to D3 receptor-mediated antagonism of methamphetamine-induced hyperthermia, particularly during the latter time course of the repeated methamphetamine regimen. This is suggested by findings that PG01037 did not acutely impact methamphetamine-induced hyperthermia, whether during the first 2 h of the repeated methamphetamine regimen (Fig. 1) or during the 90-min course of the cumulative-dose regimen (Fig. 2). Rather, the attenuation of methamphetamine-induced hyperthermia was observed at several time points beginning 2.5 h after the initiation of methamphetamine treatment (Fig. 1).
It is well established that hyperthermia contributes to the neuronal damage induced by various amphetamine-like compounds, including methamphetamine (Albers and Sonsalla, 1995; Bowyer et al., 1993; Bowyer et al., 1992; Farfel and Seiden, 1995; Metzger et al., 2000; Schmidt et al., 1990). Moreover, when methamphetamine-induced hyperthermia is prevented by manipulating ambient temperature or by pharmacological antagonism, long-term methamphetamine-induced deficits are attenuated (Albers and Sonsalla, 1995; Bowyer et al., 1993). Despite the importance of methamphetamine-induced hyperthermia to the neurotoxic effects of methamphetamine, the receptor mechanism(s) by which methamphetamine increases body temperature is unclear, and might involve dopamine (Bowyer et al., 1993), 5HT (Metzger et al., 2000), and NMDA systems (Bowyer et al., 2001). Furthermore, the receptor mechanism(s) mediating the effects of methamphetamine on body temperature might differ depending on not only the time period after initial drug exposure (as noted above), but also ambient temperature (see, for review Sabol et al., 2013). In support of this notion, in the current study, PG01037 attenuates methamphetamine-induced hyperthermia during the repeated methamphetamine treatment in a normal, but not a warmer, ambient environment. The complex interaction of these central, as well as peripheral mechanisms contributing to methamphetamine-induced hyperthermia (i.e., locomotor activity, metabolism) is an important future area of study (for review see Kiyatkin, 2013).
Findings that PG01037 attenuates the long-term methamphetamine-induced DAT deficits are similar to previous reports wherein nonselective dopamine D1 and D2/D3 receptor antagonists attenuate long-term methamphetamine-induced dopaminergic deficits by reducing the hyperthermic effects of methamphetamine (Gross et al., 2011; Broening et al., 2005; Sonsalla et al., 1986). However, and in contrast to the effect of nonselective dopamine D2/D3 receptor antagonists (Broening et al., 2005; O’Dell et al., 1993), PG01037 failed to attenuate methamphetamine-induced decreases in striatal dopamine content. It is conceivable that a larger dose of PG01037 is necessary to attenuate methamphetamine-induced decreases in striatal dopamine content, although selectivity for D3 receptors over other DA receptor subtypes might be lost. Alternatively, it might be possible that compensatory mechanisms occur that prevent methamphetamine-induced decreases in DAT function but not dopamine content or that the dopamine receptor subtype(s) that mediate the effects of methamphetamine on DAT function and DA content differ. Related to the latter point, evidence that dopamine receptor antagonists can differentially impact the effects of methamphetamine on markers of dopaminergic neuronal integrity is found throughout the literature. For example, pretreatment with the dopamine D1 receptor antagonist SCH23390 differentially antagonizes long-term methamphetamine-induced decreases in tyrosine hydroxylase activity and dopamine content (Sonsalla et al., 1986). Furthermore, in dopamine D2 receptor knockout mice, methamphetamine causes long-term deficits in tyrosine hydroxylase, but not DAT levels (Granado et al., 2011). Taken together with the current data, these findings suggest that multiple dopamine (and perhaps non-dopamine) receptor subtypes may mediate the multiple aspects of methamphetamine-induced deficits.
PG01037 failed to attenuate the effects of methamphetamine on SERT uptake and 5HT content. This is consistent with other reports demonstrating that nonselective dopamine D2/D3 receptor antagonists fail to attenuate methamphetamine-induced decreases in SERT uptake and most likely reflects the paucity of dopaminergic neurons in the hippocampus (Haughey et al., 2000). In addition, and similar to other studies using nonselective dopamine D2/D3 receptor antagonists, PG01037 did not attenuate the effects of methamphetamine on striatal 5HT content (Broening et al., 2005; Sonsalla et al., 1986).
In conclusion, the present study has demonstrated that dopamine D3 receptors mediate, in part, the long-term deficits in DAT function caused by methamphetamine, and that this effect likely involves an attenuation of methamphetamine-induced hyperthermia. However, other non-hyperthermic mechanisms remain to be elucidated. Investigating the receptor-mediated mechanisms contributing to methamphetamine-related changes to dopamine systems might enhance understanding of neurodegenerative disorders involving alterations in dopamine systems, such as Parkinson’s disease.
Acknowledgments
This work was supported by grants from the National Institute of Health DA00869, DA13367, DA019447, DA11389, and DA00378.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Achat-Mendes C, Platt DM, Spealman RD. Antagonism of metabotropic glutamate 1 receptors attenuates behavioral effects of cocaine and methamphetamine in squirrel monkeys. The Journal of pharmacology and experimental therapeutics. 2012 doi: 10.1124/jpet.112.196295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. The Journal of pharmacology and experimental therapeutics. 1995;275:1104–1114. [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Dopamine D3 receptors mediate the discriminative stimulus effects of quinpirole in free-feeding rats. The Journal of pharmacology and experimental therapeutics. 2010;332:308–315. doi: 10.1124/jpet.109.158394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. The Journal of pharmacology and experimental therapeutics. 1992;260:817–824. [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. The Journal of pharmacology and experimental therapeutics. 1993;268:1571–1580. [PubMed] [Google Scholar]
- Bowyer JF, Holson RR, Miller DB, O’Callaghan JP. Phenobarbital and dizocilpine can block methamphetamine-induced neurotoxicity in mice by mechanisms that are independent of thermoregulation. Brain research. 2001;919:179–183. doi: 10.1016/s0006-8993(01)03051-7. [DOI] [PubMed] [Google Scholar]
- Broening HW, Morford LL, Vorhees CV. Interactions of dopamine D1 and D2 receptor antagonists with D-methamphetamine-induced hyperthermia and striatal dopamine and serotonin reductions. Synapse. 2005;56:84–93. doi: 10.1002/syn.20130. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Tricklebank MD, Unger L, Neijt HC. Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology. 2003;44:1047–1053. doi: 10.1016/s0028-3908(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Chapin DS, Lookingland KJ, Moore KE. Effects of LC mobile phase composition on retention times for biogenic amines, and their precursors and metabolites. Curr Sep. 1986;7:68–71. [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. The Journal of pharmacology and experimental therapeutics. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, O’Dell SJ, Marshall JF. Striatal and cortical NMDA receptors are altered by a neurotoxic regimen of methamphetamine. Synapse. 1996;22:217–225. doi: 10.1002/(SICI)1098-2396(199603)22:3<217::AID-SYN3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Farfel GM, Seiden LS. Role of hypothermia in the mechanism of protection against serotonergic toxicity. II. Experiments with methamphetamine, p-chloroamphetamine, fenfluramine, dizocilpine and dextromethorphan. The Journal of pharmacology and experimental therapeutics. 1995;272:868–875. [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. The Journal of pharmacology and experimental therapeutics. 1997;282:834–838. [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Oliva I, O’Shea E, Martin ED, Colado MI, Moratalla R. Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol Dis. 2011;42:391–403. doi: 10.1016/j.nbd.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Gross NB, Duncker PC, Marshall JF. Striatal dopamine D1 and D2 receptors: widespread influences on methamphetamine-induced dopamine and serotonin neurotoxicity. Synapse. 2011;65:1144–1155. doi: 10.1002/syn.20952. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. Journal of medicinal chemistry. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Chu PW, Walters ET, Hanson GR, Fleckenstein AE. Methamphetamine-induced dopamine transporter complex formation and dopaminergic deficits: the role of D2 receptor activation. The Journal of pharmacology and experimental therapeutics. 2010;335:207–212. doi: 10.1124/jpet.110.166660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. The Journal of pharmacology and experimental therapeutics. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Metzger RR, Hanson GR. The effects of methamphetamine on serotonin transporter activity: role of dopamine and hyperthermia. Journal of neurochemistry. 2000;75:1608–1617. doi: 10.1046/j.1471-4159.2000.0751608.x. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain research Brain research reviews. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. Journal of psychopharmacology. 2011;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CD, Keefe KA, Garris PA, Daberkow DP. Methamphetamine neurotoxicity decreases phasic, but not tonic, dopaminergic signaling in the rat striatum. J Neurochem. 2011;118:668–676. doi: 10.1111/j.1471-4159.2011.07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. The hidden side of drug action: brain temperature changes induced by neuroactive drugs. Psychopharmacology. 2013;225:765–780. doi: 10.1007/s00213-012-2957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Carter LP, Wu H, Coop A, France CP. Discriminative stimulus effects of flumazenil: perceptual masking by baclofen, and lack of substitution with γ-hydroxybutyrate and its precursors 1,4-butanediol and γ-butyrolactone. Behav Pharmacol. 2006;17:239–247. doi: 10.1097/00008877-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. European journal of pharmacology. 1976;36:363–371. doi: 10.1016/0014-2999(76)90090-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. The Journal of neuroscience. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of biological chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Andrenyak DM, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methampheatmine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacol Exp Ther. 2011;340:295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Baladi MG, France CP. Eating high-fat chow enhances sensitization to the effects of methamphetamine on locomotion in rats. Eur J Pharmacol. 2011;658:156–159. doi: 10.1016/j.ejphar.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Raleigh MJ, Stout DB, Lacan G, Huang SC, Phelps ME. Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain research. 1997;766:113–120. doi: 10.1016/s0006-8993(97)00548-9. [DOI] [PubMed] [Google Scholar]
- Metzger RR, Haughey HM, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid decrease in dopamine transporter function: role of dopamine and hyperthermia. The Journal of pharmacology and experimental therapeutics. 2000;295:1077–1085. [PubMed] [Google Scholar]
- Munzar P, Baumann MH, Shoaib M, Goldberg SR. Effects of dopamine and serotonin-releasing agents on methamphetamine discrimination and self-administration in rats. Psychopharmacology. 1999;141:287–296. doi: 10.1007/s002130050836. [DOI] [PubMed] [Google Scholar]
- Myles BJ, Jarrett LA, Broom SL, Anton Speaker H, Sabol KE. The effects of methamphetamine on core body temperature in the rat- Part I: chronic treatment and ambient temperature. Psychopharmacology. 2008;198:301–311. doi: 10.1007/s00213-007-1061-z. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Koyama T, Yamashita I. Long-lasting decrease in dopamine uptake sites following repeated administration of methamphetamine in the rat striatum. Brain research. 1993;601:209–212. doi: 10.1016/0006-8993(93)91712-2. [DOI] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. Journal of medicinal chemistry. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Weihmuller FB, Marshall JF. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain research. 1991;564:256–260. doi: 10.1016/0006-8993(91)91461-9. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Weihmuller FB, Marshall JF. Methamphetamine-induced dopamine overflow and injury to striatal dopamine terminals: attenuation by dopamine D1 or D2 antagonists. Journal of neurochemistry. 1993;60:1792–1799. doi: 10.1111/j.1471-4159.1993.tb13405.x. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Cerrito F, Cervoni AM, Levi G. Dopamine can be released by two mechanisms differentially affected by the dopamine transport inhibitor nomifensine. The Journal of pharmacology and experimental therapeutics. 1979;208:195–202. [PubMed] [Google Scholar]
- Sabol KE, Yancey DM, Anton Speaker H, Mitchell SL. Methampheatmine and core temperature in the rat: Ambient temperature, dose, and the effect of a D2 receptor blocker. Psychopharmcology. 2013;228:551–561. doi: 10.1007/s00213-013-3059-z. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Black CK, Abbate GM, Taylor VL. Methylenedioxymethamphetamine-induced hyperthermia and neurotoxicity are independently mediated by 5-HT2 receptors. Brain research. 1990;529:85–90. doi: 10.1016/0006-8993(90)90813-q. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug and alcohol dependence. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. The American journal of psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Gibb JW, Hanson GR. Roles of D1 and D2 dopamine receptor subtypes in mediating the methamphetamine-induced changes in monoamine systems. The Journal of pharmacology and experimental therapeutics. 1986;238:932–937. [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Tata DA, Yamamoto BK. Chronic stress enhances methamphetamine-induced extraceullar glutamate and excitotoxicity in the rat striatum. Synapse. 2008;62:325–336. doi: 10.1002/syn.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. The American journal of psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain research. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature medicine. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]