Abstract
BACKGROUND
The authors conducted a phase 1/2 study of tipifarnib in combination with idarubicin and cytarabine (IA) in 95 patients with previously untreated acute myeloid leukemia (AML) or high-risk myelodysplastic syndrome.
METHODS
Induction consisted of idarubicin 12 mg/m2 a day on days 1-3, cytarabine 1.5 g/m2 intravenously continuously daily on days 1-4 (days 1-3 if age ≥60 years), and tipifarnib, with the first cohort (n = 6) receiving 200 mg orally twice a day and all others receiving 300 mg twice a day for 21 days every 28 days. Consolidation consisted of 5 courses of idarubicin 8 mg/m2 a day on days 1-2, cytarabine 0.75 g/m2 a day on days 1-3, and tipifarnib 300 mg twice a day for 14 days every 4-6 weeks. Maintenance with tipifarnib 300 mg twice a day for 21 days every 4-6 weeks was continued for 6 months.
RESULTS
With a median follow-up of 33 months, 61 patients achieved complete remission (CR) (64%), and 9 achieved complete remission with incomplete platelet recovery (CRp) (9%). The median duration of CR was not reached. Median overall survival was 17 months. The most common grade 3 adverse events were gastrointestinal toxicities, liver dysfunction, and skin rash. Compared with historical IA, IA and tipifarnib showed a better CR duration (P = .04) and a trend toward a higher CR rate in patients with chromosome 5/7 abnormalities.
CONCLUSIONS
The combination of IA and tipifarnib is safe and active. Further studies exploring different dosages and schedules are warranted, particularly in patients with poor-risk AML.
Keywords: acute myeloid leukemia, tipifarnib, combination, myelodysplastic syndrome, farnesyl transferase inhibitor
The backbone of acute myeloid leukemia (AML) therapy remains centered on cytarabine in combination with an anthracycline such as idarubicin or daunorubicin. Approximately 50% to 70% of patients achieve complete remission (CR), but most will relapse and succumb to their disease or associated complications.1-3 Patients with high-risk myelodysplastic syndrome (MDS; defined by marrow blasts >10%) are also frequently treated with AML regimens, hypomethylating agents, and/or allogeneic stem cell transplantation. However, they have a poor outcome, with a median survival of 12 months or less.4-9 Therefore, new therapies and more effective treatment regimens are needed.
Farnesyltransferase inhibitors are a novel class of anticancer agents that competitively inhibit farnesyltransferase (FTase), an enzyme that catalyzes the transfer of a farnesyl moiety to the C-terminal cysteine residue of substrate proteins.10 A host of intracellular proteins, substrates for prenylation via FTase, play a role in the growth and proliferation of AML cells.11 Interruption of prenylation may prevent substrates from being functional, which, in turn, may result in the inhibition of cellular events that depend on the function of those substrates. Tipifarnib is an orally available, nonpeptidomimetic farnesyltransferase inhibitor (FTI) with significant in vitro antitumor activity across a wide range of tumors.12 Tipifarnib has shown clinical activity in myeloid malignancies including in those in elderly patients with AML, high-risk MDS, and myeloproliferative disorders.13-18 In a phase 1 study in patients with refractory leukemias, tipifarnib induced a response rate of 29%, including 2 CRs.15 The treatment was generally well tolerated, with the major toxicities consisting of mild to moderate myelosuppression, nausea, vomiting, and diarrhea at the maximum tolerated dose (MTD) of 600 mg twice daily. In a phase 2 study using 600 mg twice a day for 21 days every 4 weeks in previously untreated elderly patients and those with refractory or relapsed AML, a CR rate of 14% was reported.17 In patients with MDS treated at 600 mg twice a day for 28 days every 6 weeks, a response rate of 30% was reported.16
The favorable toxicity profile, antileukemic activity, and novel mechanism of action of tipifarnib make it attractive for use in combination therapy. Several studies had suggested a synergy between tipifarnib and chemotherapeutic agents, and FTIs have been suggested to inhibit multidrug resistance, a common mechanism of resistance to chemotherapy in AML, particularly among the elderly.19,20 In vitro studies showed that the antiproliferative effects in human AML cells are additive when tipifarnib is combined with cladribine or fludarabine21 and synergistic when tipifarnib is combined with bortezomib22 or daunorubicin,20 possibly reflecting, in the latter case, competitive inhibition of P-glycoprotein.
The goal of the current study was to investigate the combination of tipifarnib with an established induction therapy regimen of idarubicin and cytarabine in patients with AML and high-risk MDS.
MATERIALS AND METHODS
Study Group
Previously untreated adults with AML (defined by ≥20% blasts) or high-risk MDS (>10% blasts) ages 15 to 70 years were eligible after informed consent was obtained according to institutional guidelines. Previous therapy with hydroxyurea (but no other chemotherapy) and hematopoietic growth factors was allowed. Additional eligibility criteria were: 1) Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; 2) adequate liver function (serum bilirubin ≤2 mg/dL, alanine aminotransferase [ALT] or aspartate aminotransferase up to 2.5 × the upper limit of normal) and renal function (serum creatinine ≤2 mg/dL); 3) ability to take oral medication; 4) absence of the chromosomal translocation t(15;17); 5) absence of any active and uncontrolled infection or any other severe concurrent disease; and 6) no known allergy to imidazole drugs. Nursing and pregnant women were excluded. Approval for the study was granted by the institutional review board of The University of Texas M. D. Anderson Cancer Center. The study was conducted in accordance with the basic principles of the Declaration of Helsinki.
Treatment Schedule
The treatment plan consisted of induction therapy with cytarabine 1.5 g/m2 over 24 hours daily on days 1-4 (age <60 years) or days 1-3 (age ≥60 years) and idarubicin 12 mg/m2 intravenously over 1 hour daily on days 1-3. Patients achieving a response received consolidation with 5 additional courses every 4-6 weeks with cytarabine 0.75 g/m2 over 24 hours daily for 3 days and idarubicin 8 mg/m2 intravenously over 1 hour daily for 2 days. Tipifarnib was given twice a day for 21 days during induction therapy, for 14 days with each course of consolidation, and as a single agent for maintenance therapy for 3 weeks every 4-6 weeks for 6 courses.
Tipifarnib was given at a dose of 200 mg orally twice a day for the first 6 patients to determine tolerability and at the target dose of 300 mg orally twice a day during induction, consolidation, and maintenance to all other patients after no unexpected toxicity was identified in patients treated at the lower dose. Toxicity was graded according to the NCI Common Terminology Criteria version 3.0. During induction therapy, patients who experienced grades 3-4 extramedullary toxicities, prolonged myelosuppression, or life-threatening myelosuppression-related complications and who were not in CR after 1 course received a second induction course at −2 dose level. Patients who experienced grade 2 extramedullary toxicities received the second course at −1 dose level (Table 1). Patients who developed toxicity requiring dose reduction at the lowest dose level of tipifarnib were taken off the study. Patients who showed no significant antitumor effect after 2 courses were taken off the study. During consolidation, the dose of chemotherapy treatment in subsequent courses was reduced by 25% for grades 3-4 extramedullary toxicities, or for severe life-threatening infections. The dose of tipifarnib was similarly reduced to the next lower dose, that is, 200 or 100 mg twice a day.
Table 1.
Dose Modifications During Induction Therapy
| Dose Level |
Idarubicin (mg/m2/d × 3) |
Cytarabine (g/m2/24 h × 3-4) days |
Tipifarnib (mg PO BID × 21 d every course) |
|---|---|---|---|
| 0 | 12 | 1.5 | 300 |
| −1 | 8 | 1.0 | 200 |
| −2 | 6 | 0.75 | 100 |
The pretreatment evaluation included history, physical examination, complete blood count (CBC) with differential and platelet count, a complete chemistry survey, and marrow aspiration with cytogenetic analysis. Follow-up studies included CBC, differential, and platelet count as well as chemistry profile every 4-7 days during remission induction and every 1-4 weeks during maintenance therapy. Marrow aspiration and cytogenetics (if abnormal at start) were performed on days 21-28 and then an aspiration every 1-2 weeks as required until CR, then every 3-6 months in year 1. Supportive measures for optimal medical care were provided throughout the study and included admission to a laminar air flow room for the duration of the induction for patients ≥50 years old, use of prophylactic antibiotics, and irradiated blood-product support as indicated.
Response Criteria
A CR required bone marrow blasts ≤5% and recovery of normal hematopoiesis with an absolute neutrophil count (ANC) of 1 × 109/L or more and platelet count of 100 × 109/L or more in addition to disappearance of all clinical or radiologic evidence of disease. CRp had similar criteria to CR but with platelet counts from 20 to <100 × 109/L. Partial response (PR) required blood recovery as for CR but with both a decrease in marrow blasts of ≥50% and ≤25% abnormal cells in the marrow.
Statistical Analysis
The trial objectives were to assess the safety and tolerability of tipifarnib when used in combination with idarubicin and cytarabine as initial therapy for patients with AML. To further assess the possible impact of adding tipifarnib, results were compared with a historical cohort of patients treated with the same chemotherapy but without tipifarnib. The impact of clinical features on outcome was an exploratory endpoint.
Because this combination had not been used before, an initial cohort of 6 patients was first treated at a reduced dose of tipifarnib. Once the lower-dose tipifarnib combination was determined to be safe, patients were treated with the target dose (ie, 300 mg twice a day) to a maximum of 90 patients. The target dose would start accrual if grades 3-4 toxicity attributable to tipifarnib was observed in fewer than 2 of the 6 patients treated at the 200-mg twice-daily dose. Patients who were eligible for the trial and received any dose of idarubicin, cytarabine, or tipifarnib were included in estimating CR rates and were counted as treatment failures if response could not be assessed for any reason. Duration of CR and overall survival were estimated according to the Kaplan-Meier method. CR duration was dated from the start of CR to first evidence of recurrence. The efficacy and toxicity profile of this combination were compared with the historical M. D. Anderson experience with 108 consecutive patients having the same inclusion criteria and treated with the same chemotherapy without tipifarnib between 2001 and 2005.
For the phase 2 portion of the study, the design was adapted to account for patient characteristics. The trial would be terminated after the first 45 patients were evaluated if 20 or fewer CRs were observed. With the trial continuing to maximum accrual of 90 patients, the combination regimen would be recommended for further study in this group of patients if at least 58 CRs were observed. The probability that the combination would be recommended under the assumption of a CR rate of 74% was .91. Outcome by other disease characteristics was assessed in a descriptive manner.
RESULTS
Study Group
A total of 95 patients were enrolled in the study: 6 at the lower dose and 89 at the target tipifarnib dose of 300 mg twice a day. The patient characteristics are summarized in Table 2. The median age was 50 years (range, 17-61 years). Sixty-three patients (66%) had an ECOG performance status of 1. There were 82 patients with AML as defined by the World Health Organization classification (≥20% blasts as the cutoff between AML and high-risk MDS). Forty patients (42%) had antecedent hematologic disorders prior to their diagnosis, for a median of 2 months (range, 1-108 months). More than half the patients had abnormal karyotypes, which included abnormalities of chromosomes 5 and/or 7 in 19 patients (20%), translocation t(8:21) in 2 (2%), inversion 16 in 3 (3%), and other abnormalities in 29 (31%). Of 83 patients whose marrow samples were tested for FLT3 abnormalities, 13 (16%) showed internal tandem duplications (ITDs), and 6 (7%) showed mutations in aspartic acid residue 835 (D835) of the tyrosine kinase loop (2 patients had both mutations).
Table 2.
Patient Characteristics
| Median (range)/N (%) | ||||
|---|---|---|---|---|
| Parameter | IA+Tipifarnib, N=95 |
IA (Historical Control), N=108 |
P | |
| Age (y) | 50 (17-61) | 52 (17-61) | .517 | |
| PS (1) | 63 (66) | 59 (55) | .089 | |
| WBCs (×109/L) | 5.3 (0.5-161.5) | 10.0 (0.5-390.0) | .047 | |
| Platelets (×109/L) | 62 (9-676) | 58 (5-635) | .159 | |
| AHD (mo) | 2 (1-108) | 0 (0-144) | .807 | |
| 40 (42) | 38 (35) | .312 | ||
| Disease type | AML | 82 (86) | 100 (93) | .144 |
| MDS | 13 (14) | 8 (7) | ||
| CG | Diploid | 41 (43) | 56 (52) | .617 |
| t(8;21)/inversion 16 | 5 (5) | 3 (3) | ||
| Chromosome 5 or 7 abnormalities |
19 (20) | 16 (15) | ||
| Other | 29 (31) | 31 (29) | ||
| Not done | 1 (1) | 2 (2) | ||
| FLT3 (+)a | ITD | 13/83 (16) | 18/57 (32) | .080 |
| Point mutation | 6/83 (7) | 4/57 (7) | ||
IA, idarubicin and cytarabine; ECOG PS, performance status; WBCs, white blood cells; AHD, antecedent of hematologic disorder; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CG, cytogenetic; inv, inversion; ITD, internal tandem duplication.
Two patients had both mutations on IA+tipifarnib; 1 patient had both mutations on IA.
Response and Outcome
Sixty-one patients (64%) achieved CR and 9 (9%) CRp, for an overall response rate of 74%. Twenty-one patients (22%) had resistant disease, and 4 (4%) died during induction, on days 8, 11, 27, and 55, for a failure rate of 26% (Table 3). Of 41 patients with diploid cytogenetics, 30 (73%) had CR and 4 (10%) CRp (OR, 83%). The CR rate was 58% (11 of 19) in patients with abnormalities of chromosomes 5 and/or 7 and 55% (16 of 29) in those harboring other cytogenetic abnormalities. The CR rates were 72% in 43 patients younger than 50 years, 67% in 18 patients older than 50 years with diploid cytogenetic analysis, and 53% in 34 patients older than 50 years with unfavorable cytogenetic analysis. Seventeen patients had abnormalities of FLT3 in the form of ITDs and/or D835. CR rates were 67% (44 of 66) in FLT3 wild-type patients and 76% (13 of 17) in those with FLT3 mutations.
Table 3.
Responses to Idarubicin, Cytarabine, and Tipifarnib by Disease Characteristics
| Parameter | No. | CR (%) |
|---|---|---|
| Overall | 95 | 61 (64) |
| Karyotype | ||
| Diploid | 41 | 30 (73) |
| Chromosome 5 or 7 abnormalities | 19 | 11 (58) |
| t(8;21)/inversion 16 | 5 | 4 (80) |
| Other abnormalities | 29 | 16 (55) |
| Not done | 1 | 0 (0) |
| FLT3 status | ||
| Mutated | 17 | 13 (76) |
| Wild type | 66 | 44 (67) |
| Not done | 12 | 4 (33) |
| Group | ||
| <50 y | 43 | 31 (72) |
| ≥50 y, diploid | 18 | 12 (67) |
| ≥50 y, other | 34 | 18 (53) |
CR indicates complete response.
Three patients (1 diploid and 2 abnormal cytogenetics) who failed to achieve CR following the first induction course received a second induction with identical doses and schedules of tipifarnib and IA; none of them achieved CR. Median time to CR was 30 days (range, 21-71 days) for all patients, 29 days (range, 21 to 60 days) in the diploid group, and 34 days (range, 23-71 days) in the abnormal cytogenetic group. Median time to CRp was 31 days (range, 18-67 days).
Four patients (4%) died during the first induction course. One patient (age 43 years) had an acute myocardial infarction, followed by congestive heart failure, and died on day 8 of the induction course. A 28-year-old patient had sepsis, with disseminated intravascular coagulopathy, and died on day 11. One 52-year-old patient developed a diffuse alveolar hemorrhage and probable fungal pneumonia and died on day 27. One patient (age 44 years) had concomitant bacterial and fungal septicemia that led to multiple organ failure; he died on day 55 before recovering his counts.
Overall, 70 patients received a total of 152 courses of consolidation therapy. Seventeen patients received only 1 cycle of consolidation, 9 received 2, and 26 received 3 or more, with 19 completing all 5 planned cycles. The main reasons for not completing consolidation chemotherapy as planned were allogeneic stem cell transplantation (n = 18), treatment toxicities (n = 14), and disease relapse (n = 13). Seventeen patients received a total of 82 courses of maintenance therapy with tipifarnib at a median dose of 100 mg twice a day (range, 100-200 mg twice a day), for a median of 5 months (range,1-8 months). Of the 17 patients, 8 remained in remission for 50+ months (range, 45 to 64+ months), whereas 9 patients relapsed after a median of 18 months (range, 12-34 months), 5 of whom later died after a median of 22 months (range, 14-34 months). Eighteen patients (median age, 52 years; range, 34-60 years) received consolidation therapy with allogeneic stem cell transplantation: 16 in CR and 2 in CRp. Five of them (28%) had chromosome 5 or 7 abnormalities compared with 14 of 77 (18%) who did not receive transplantation (P = .16). Of the 18 transplanted patients, 6 (33%; 1 with chromosome 7 abnormality) were alive 22+ months (range, 11 to 33+ months) after transplantation, 5 (28%) relapsed and died, and 7 (39%) died in CR or CRp of transplant-related complications.
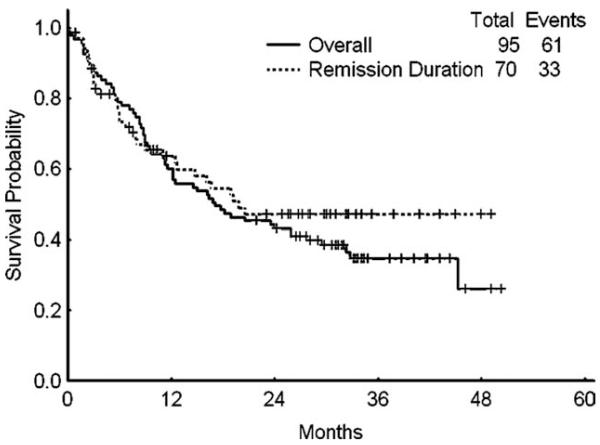
With a median follow-up of 33 months (range, 23-50 months), 27 of 61 patients who achieved CR (44%) were still in remission (6 after transplant). The median remission duration for all patients who achieved CR was not reached (range, 1 to >49 months). In contrast, patients who achieved CRp had a median remission duration of only 3 months (range, 1-35 months). Median overall survival for the intent-to-treat population was 17 months (range, 0 to >50 months). Median overall survival was 32 months (range, 2 to >50 months) for those patients who achieved CR, 12 months (range, 2 to >43 months) for those who achieved CRp, and 8 months (range, 0 to >34 months) for nonresponders (Fig. 1).
Figure 1.
Survival and remission duration are shown.
Side Effects
No grades 3-4 tipifarnib-related toxicities were observed among the first 6 patients enrolled in the phase 1 portion of the study and who received tipifarnib 200 mg twice a day; therefore, the dose of tipifarnib was increased to 300 mg twice a day and was given to 89 patients enrolled in part 2 of the study. Table 4 summarizes the toxicities observed with the combination therapy during induction. The most frequently observed side effects were diarrhea, nausea and vomiting, skin rash, mucositis, and hyperbilirubinemia. Tipifarnib was held for a median of 5 days (range, 0-18 days) during induction therapy. Fifty-three patients (56%) had tipifarnib dose reductions during induction, 21 (40%) during consolidation, and 3 (18%) during maintenance. The most common causes for treatment interruption and dose reduction were gastrointestinal toxicities, prolonged myelosuppression, and sepsis. During consolidation, the median time to neutrophil recovery (ANC ≥ 1 × 109/L) was 26 days (range, 0-64 days) and for platelet recovery (≥100 × 109/L) was 37 days (range, 0-65 days). Neutropenia and thrombocytopenia grades 3-4 during maintenance therapy were uncommon. Median time to next course was 43 days (range, 15-161 days) during consolidation and 32 days (range, 24-154 days) during maintenance. Tipifarnib was given at a dose of 200 mg twice a day in 96 of the 152 cycles of consolidation (63%) and in 44 of the 83 cycles of maintenance (53%) and at 100 mg twice a day in 31 cycles of consolidation (20%) and in 23 cycles of maintenance (28%).
Table 4.
Adverse Events in ≥5% of All Patients During Induction (Regardless of Causality)
| Adverse Events During Induction |
Any Grade |
Grade ≥3 |
|---|---|---|
| No. | No. | |
| Diarrhea | 68 | 33 |
| Nausea/vomiting | 56 | 9 |
| Rash/pruritis | 51 | 18 |
| Hepatic (bilirubin/SGPT) | 31 | 13 |
| Mucositis/stomatitis/colitis/gastritis | 29 | 10 |
| Pain | 28 | 5 |
| Fatigue | 20 | 7 |
| Neurologic (anxiety, mood alteration, seizure, vision) |
15 | 3 |
| Cardiac | 15 | 1 |
| Hypokalemia | 14 | 13 |
| Edema | 12 | 1 |
| Respiratory | 11 | 2 |
| Constipation | 11 | 0 |
| Hypoalbuminemia | 10 | 3 |
| Hyperglycemia | 10 | 2 |
| Hypophosphatemia | 8 | 6 |
| Anorexia/weight loss | 8 | 2 |
| Renal | 8 | 1 |
| Hypocalcemia | 6 | 2 |
| Hemorrhage/bleeding | 5 | 3 |
Comparison With Historical Patients Receiving Idarubicin and Cytarabine
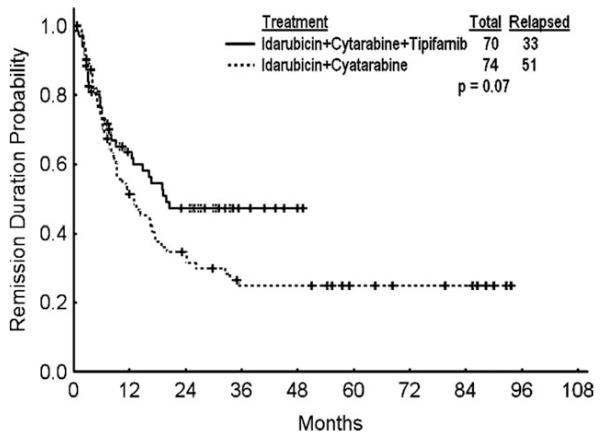
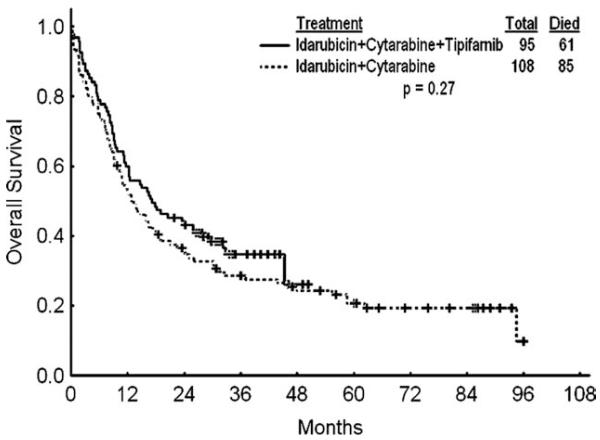
We compared the efficacy and safety of tipifarnib-based combination with historical experience at M. D. Anderson using the same chemotherapy without tipifarnib in matched patients (by age and year of treatment) receiving idarubicin and cytarabine therapy (Table 5). The objective response rates were similar. There was a trend for higher CR rates in favor of patients with chromosome 5 and 7 abnormalities treated with the tipifarnib-based combination (58% vs 19%; P = .054), but there was no effect on survival (P = .12). The median number of consolidation courses for patients who achieved CR treated with chemotherapy was 3 (range, 1-5), compared with 4 (range, 0-4) for those in the historical control group. There was a better median complete remission duration (CRD) with the tipifarnib-based combinations: the median CRD was not reached for patients treated with tipifarnib-based combinations and was 13 months with chemotherapy alone (P = .04); the 18-month CRD rates were 54% and 38%, respectively (Fig. 2). Median overall survival rates were 17 versus 13 months; the 18-month survival rates were 48% and 41% (P = .27; Fig. 3). Patients receiving tipifarnib-based combinations had a higher rate of grades 3-4 diarrhea (35% versus 8%; P < .001). The 6- and 12-week mortality rates were 3% and 12%, respectively, with the tipifarnib-based therapy versus 8% and 16%, respectively, with chemotherapy alone (P = .27).
Table 5.
Comparison with Age and Treatment-Era Matched Patients Who Received Idarubicin and Cytarabine
| Idarubicin and Cytarabine (CI) |
Idarubicin, Cytarabine, and Tipifarnib (CI) |
P | |
|---|---|---|---|
| Overall, no. | 108 | 95 | |
| CR rate, % | 60 (50-69) | 64 (54-74) | .59 |
| OR rate, % | 70 (59-77) | 74 (54-74) | |
| Median CRD, mo | 13 (12.9-13.1) | NR | .04 |
| Median, OS, mo | 13 (12.7-13.1) | 17 (16.9-17.2) | .27 |
| Diploid, no. | 56 | 41 | |
| CR rate, % | 75 (62-86) | 73 (57-86) | .23 |
| OR rate, % | 77 (64-87) | 83 (68-93) | |
| Chromosome 5 or 7 abnormalities, no. | 16 | 19 | |
| CR rate, % | 19 (4-46) | 58 (34-80) | .054 |
| OR rate, % | 32 (11-59) | 69 (43-87) |
CI indicates confidence interval; T, tipifarnib; CR, complete response; OR, objective response; CRD, complete response duration; OS, overall survival; NR, not reached.
Figure 2.
Remission duration with idarubicin and cytarabine versus with idarubicin, cytarabine, and tipifarnib (including patients in CR and CRp) is shown.
Figure 3.
Overall survival idarubicin and cytarabine versus with idarubicin, cytarabine, and tipifarnib is shown.
DISCUSSION
This study evaluated the efficacy and safety of a combination therapy of tipifarnib, idarubicin, and cytarabine in adults with AML. Prior investigations using single-agent tipifarnib at a dose of 600 mg orally twice a day for 21 days every 28-day cycle, reported CR rates of 4% in patients with refractory disease and 14% in elderly patients with previously untreated AML.17-23 In this study, using tipifarnib 300 mg orally twice a day in combination with idarubicin and cytarabine, a CR rate of 64% was achieved with an acceptable safety profile. Median survival was 17 months, and median CR duration was 19 months.
Overall, this study did not demonstrate any significantly increased antileukemia activity with the addition of tipifarnib. Except for a possible trend toward more durable responses with the tipifarnib-based combination compared with historical controls, the CR and survival rates were not different from what would be expected with chemotherapy alone. In our study, the dose of tipifarnib was 300 mg twice a day, lower than the MTD identified in the phase 1 trial (600 mg twice a day given for 21 days every 28 days), and in more than 50% of the consolidation courses, tipifarnib was reduced to 100-200 mg twice a day. A recent report found no apparent increase in response rate with higher tipifarnib doses when used as a single agent; therefore, the benefit of a higher dose intensity is unclear.24 Despite the possible trend for more durable responses, the role of tipifarnib and the contribution of the extended therapy during consolidation and maintenance can only be assessed in a randomized trial.
Other studies have failed also to demonstrate a considerable benefit of tipifarnib used alone or in combination with chemotherapy. Brandwein et al failed to show a significant benefit by adding tipifarnib to chemotherapy.25 In their study, the combination of tipifarnib, given at a dose of 600 mg twice a day for 10 days per cycle, and conventional “3 + 7” induced an objective response rate of 54% in previously untreated patients with AML ages 60 years and older; this rate was not significantly different from what was obtained with chemotherapy alone. A phase 3 randomized trial in patients with AML aged 70 years and older randomized to tipifarnib versus best supportive care did not show a survival advantage for tipifarnib. The CR rate in that study was only 8%.26
Although the response rates to tipifarnib as a single agent may be independent of the cytogenetic risk group,17 the CR rate in the present study for patients with adverse risk cytogenetics (58%) was higher than the response rate of 19% in patients treated with identical chemotherapy in the historical cohort. This is in line with a previous report from Brandwein et al, who reported a response rate of 57% in patients receiving tipifarnib-based combination versus 30% in patients treated with standard induction regimen.25 Karp et al also reported that tipifarnib maintenance may benefit patients with secondary AML and adverse cytogenetics; the CRD was longer in patients who received tipifarnib maintenance therapy compared with that in the historical control (HR, 0.11; P = .02).27 Based on these observations, patients with adverse cytogenetics might be a subset in whom further exploration of tipifarnib-based chemotherapy combinations may be warranted. Furthermore, Raponi and colleagues have identified and validated a 2-gene expression ratio, RASGRP1/APTX, that has predictive utility in both newly diagnosed and relapsed or refractory AML treated with tipifarnib.28 The stratification with this classifier significantly predicts for improved overall survival that is independent of other prognostic factors including a previously described genomic signature. Similarly, in 11 patients with relapsed/refractory mantle cell lymphoma treated with tipifarnib, the RASGRP1/APTX gene expression ratio was higher in the responder, whereas AKAP13 expression was higher in the nonresponders.29 This gene analysis was not available for the present study, but it is possible that patients could be selected this way in future studies to identify those most likely to benefit.
The combination regimen was well tolerated. The major toxicities were gastrointestinal, including transient liver test abnormalities, nausea and vomiting, diarrhea, and mucositis. Except for diarrhea, the toxicities were not substantially more than those observed with standard chemotherapy alone. Gastrointestinal toxicities were frequently seen with tipifarnib single-agent therapy, although this was observed at higher doses.15 In a recent phase 1 study combining tipifarnib and standard induction therapy in elderly patients, more severe gastrointestinal toxicities were reported at a dose of 600 mg twice a day.25 Although myelosuppression is a common toxicity of tipifarnib, there was no significant prolonged myelosuppression, and the median time to neutrophil and platelet recovery was comparable to that observed with standard chemotherapy alone. There was no increase in the 6- and 12-week mortality rates.
In summary, the results of this study suggest that the combination of idarubicin, cytarabine, and tipifarnib in patients with AML and high-risk MDS can be safely administered. A possible benefit of this combination among patients with high-risk cytogenetic abnormalities deserves additional investigation.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Estey E. Treatment of relapsed and refractory acute myeloid leukemia. Leukemia. 2000;14:476–479. doi: 10.1038/sj.leu.2401568. [DOI] [PubMed] [Google Scholar]
- 2.Leopold LH, Willemze R. The treatment of acute myeloid leukemia in first relapse: a comprehensive review of the literature. Leuk Lymphoma. 2002;43:1715–1727. doi: 10.1080/1042819021000006529. [DOI] [PubMed] [Google Scholar]
- 3.Ravandi F, Kantarjian H, Giles F, Cortes J. New agents in acute myeloid leukemia and other myeloid disorders. Cancer. 2004;100:441–454. doi: 10.1002/cncr.11935. [DOI] [PubMed] [Google Scholar]
- 4.Fenaux P, Mufti G, Santini V, et al. Azacitidine (AZA) treatment prolongs overall survival (OS) in higher-risk MDS patients compared with conventional cure regimens (CCR): results of the AZA-001 phase III study. Blood. 2007;110:250a. Abstract 817. [Google Scholar]
- 5.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, O’Brien S, Huang X, et al. Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience. Cancer. 2007;109:1133–1137. doi: 10.1002/cncr.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 10.End DW. Farnesyl protein transferase inhibitors and other therapies targeting the Ras signal transduction pathway. Invest New Drugs. 1999;17:241–258. doi: 10.1023/a:1006380320290. [DOI] [PubMed] [Google Scholar]
- 11.Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–3652. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 12.End DW, Smets G, Todd AV, et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61:131–137. [PubMed] [Google Scholar]
- 13.Mesa RA, Camoriano JK, Geyer SM, et al. A phase II trial of tipifarnib in myelofibrosis: primary, post-polycythemia vera and post-essential thrombocythemia. Leukemia. 2007;21:1964–1970. doi: 10.1038/sj.leu.2404816. [DOI] [PubMed] [Google Scholar]
- 14.Cortes JE, Albitar M, Thomas D, et al. Efficacy of the farnesyl transferase inhibitor, ZARNESTRAT (R115777), in chronic myeloid leukemia and other hematological malignancies. Blood. 2003;101:1692–1697. doi: 10.1182/blood-2002-07-1973. [DOI] [PubMed] [Google Scholar]
- 15.Karp JE, Lancet JE, Kaufmann SH, et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase I clinical-correlative trial. Blood. 2001;97:3361–3369. doi: 10.1182/blood.v97.11.3361. [DOI] [PubMed] [Google Scholar]
- 16.Kurzrock R, Kantarjian HM, Cortes JE, et al. Farnesyltransferase inhibitor R115777 in myelodysplastic syndrome: clinical and biologic activities in the phase 1 setting. Blood. 2003;102:4527–4534. doi: 10.1182/blood-2002-11-3359. [DOI] [PubMed] [Google Scholar]
- 17.Lancet JE, Gojo I, Gotlib J, et al. A phase 2 study of the farnesyltransferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood. 2007;109:1387–1394. doi: 10.1182/blood-2006-04-014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinelli G, Iacobucci I, Paolini S, Ottaviani E. Farnesyltransferase inhibition in hematologic malignancies: the clinical experience with tipifarnib. Clin Adv Hematol Oncol. 2008;6:303–310. [PubMed] [Google Scholar]
- 19.Reuter CWM, Wegner J, Morgan MA. Co-treating AML cells with FTI potentiates apoptosis induced by topoisomerase II inhibitors. Blood. 2002;100:542a. [Google Scholar]
- 20.Medeiros BC, Landau HJ, Morrow M, Lockerbie RO, Pitts T, Eckhardt SG. The farnesyltransferase inhibitor, tipifarnib, is a potent inhibitor of the MDR1 gene product, Pglycoprotein, and demonstrates significant cytotoxic synergism against human leukemia cell lines. Leukemia. 2007;21:739–746. doi: 10.1038/sj.leu.2404539. [DOI] [PubMed] [Google Scholar]
- 21.Korycka A, Smolewski P, Robak T. The influence of farnesyl protein transferase inhibitor R115777 (Zarnestra) alone and in combination with purine nucleoside analogs on acute myeloid leukemia progenitors in vitro. Eur J Haematol. 2004;73:418–426. doi: 10.1111/j.1600-0609.2004.00336.x. [DOI] [PubMed] [Google Scholar]
- 22.Yanamandra N, Colaco NM, Parquet NA, et al. Tipifarnib and bortezomib are synergistic and overcome cell adhesionmediated drug resistance in multiple myeloma and acute myeloid leukemia. Clin Cancer Res. 2006;12:591–599. doi: 10.1158/1078-0432.CCR-05-1792. [DOI] [PubMed] [Google Scholar]
- 23.Harousseau JL, Lancet JE, Reiffers J, et al. A phase 2 study of the oral farnesyltransferase inhibitor tipifarnib in patients with refractory or relapsed acute myeloid leukemia. Blood. 2007;109:5151–5156. doi: 10.1182/blood-2006-09-046144. [DOI] [PubMed] [Google Scholar]
- 24.Erba HP, Kopecky KJ, Kirschbaum MH, et al. Phase II studies of different schedules of the farnesyl transferase inhibitor tipifarnib for patients of age 70 or older with previously untreated acute myeloid leukemia (AML): a North American Intergroup study (S0432) Blood. 2007;11:136a. [Google Scholar]
- 25.Brandwein JM, Leber BF, Howson-Jan K, et al. A phase I study of tipifarnib combined with conventional induction and consolidation therapy for previously untreated patients with acute myeloid leukemia aged 60 years and over. Leukemia. 2009;23:631–634. doi: 10.1038/leu.2008.341. [DOI] [PubMed] [Google Scholar]
- 26.Harousseau J-L, Martinelli G, Jedrzejczak WW, et al. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009;114:1166–1173. doi: 10.1182/blood-2009-01-198093. [DOI] [PubMed] [Google Scholar]
- 27.Karp JE, Smith BD, Gojo I, et al. Phase II trial of tipifarnib as maintenance therapy in first complete remission in adults with acute myelogenous leukemia and poor-risk features. Clin Cancer Res. 2008;14:3077–3082. doi: 10.1158/1078-0432.CCR-07-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raponi M, Lancet JE, Fan H, et al. A 2-gene classifier for predicting response to the farnesyltransferase inhibitor tipifarnib in acute myeloid leukemia. Blood. 2008;111:2589–2596. doi: 10.1182/blood-2007-09-112730. [DOI] [PubMed] [Google Scholar]
- 29.Rolland D, Ribrag V, Haioun C, et al. Phase II trial and prediction of response of single agent tipifarnib in patients with relapsed/refractory mantle cell lymphoma: a Groupe d’Etude des Lymphomes de l’Adulte trial. Cancer Chemother Pharmacol. 2010;65:781–790. doi: 10.1007/s00280-009-1185-4. [DOI] [PubMed] [Google Scholar]