Abstract
Invariant chain (Ii), a membrane glycoprotein, binds class II major histocompatibility complex (MHC) glycoproteins, probably via its class II-associated Ii peptide (CLIP) segment, and escorts them toward antigen-containing endosomal compartments. We find that a soluble, trimeric ectodomain of Ii expressed and purified from Escherichia coli blocks peptide binding to soluble HLA-DR1. Proteolysis indicates that Ii contains two structural domains. The C-terminal two-thirds forms an alpha-helical domain that trimerizes and interacts with empty HLA-DR1 molecules, augmenting rather than blocking peptide binding. The N-terminal one-third, which inhibits peptide binding, is proteolytically susceptible over its entire length. In the trimer, the N-terminal domains act independently with each CLIP segment exposed and free to bind an MHC class II molecule, while the C-terminal domains act as a trimeric unit.
Full text
PDF

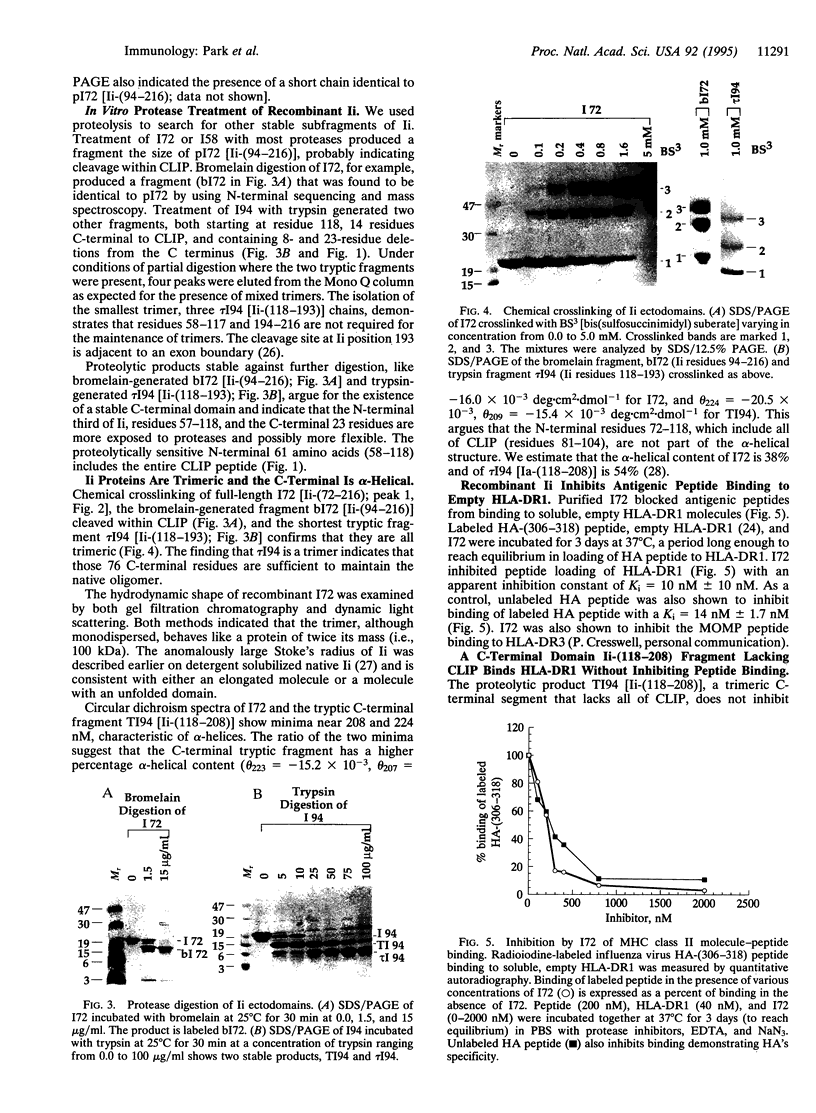


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amigorena S., Webster P., Drake J., Newcomb J., Cresswell P., Mellman I. Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J Exp Med. 1995 May 1;181(5):1729–1741. doi: 10.1084/jem.181.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avva R. R., Cresswell P. In vivo and in vitro formation and dissociation of HLA-DR complexes with invariant chain-derived peptides. Immunity. 1994 Dec;1(9):763–774. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- Bakke O., Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990 Nov 16;63(4):707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- Bikoff E. K., Huang L. Y., Episkopou V., van Meerwijk J., Germain R. N., Robertson E. J. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med. 1993 Jun 1;177(6):1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. S., Cresswell P. Role for intracellular proteases in the processing and transport of class II HLA antigens. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3975–3979. doi: 10.1073/pnas.85.11.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnerot C., Marks M. S., Cosson P., Robertson E. J., Bikoff E. K., Germain R. N., Bonifacino J. S. Association with BiP and aggregation of class II MHC molecules synthesized in the absence of invariant chain. EMBO J. 1994 Feb 15;13(4):934–944. doi: 10.1002/j.1460-2075.1994.tb06338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F., Germain R. N. Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 1995 Jan;2(1):73–88. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Ceman S., Rudersdorf R., Long E. O., Demars R. MHC class II deletion mutant expresses normal levels of transgene encoded class II molecules that have abnormal conformation and impaired antigen presentation ability. J Immunol. 1992 Aug 1;149(3):754–761. [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Gorga J. C., Vignali D. A., Lane W. S., Strominger J. L. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993 Jul 1;178(1):27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Lane W. S., Gorga J. C., Stern L. J., Vignali D. A., Strominger J. L. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992 Aug 27;358(6389):764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- Denzin L. K., Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995 Jul 14;82(1):155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- Ericson M. L., Sundström M., Sansom D. M., Charron D. J. Mutually exclusive binding of peptide and invariant chain to major histocompatibility complex class II antigens. J Biol Chem. 1994 Oct 21;269(42):26531–26538. [PubMed] [Google Scholar]
- Forood B., Feliciano E. J., Nambiar K. P. Stabilization of alpha-helical structures in short peptides via end capping. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):838–842. doi: 10.1073/pnas.90.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garboczi D. N., Hung D. T., Wiley D. C. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A. M., Pearson C., Quinn V., McDevitt H. O., Milburn P. J. Binding of an invariant-chain peptide, CLIP, to I-A major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):335–339. doi: 10.1073/pnas.92.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbury P. B., Zhang T., Kim P. S., Alber T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science. 1993 Nov 26;262(5138):1401–1407. doi: 10.1126/science.8248779. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Brown J. H., Gorga J. C., Stern L. J., Urban R. G., Chi Y. I., Stauffacher C., Strominger J. L., Wiley D. C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994 Apr 21;368(6473):711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- Jasanoff A., Park S. J., Wiley D. C. Direct observation of disordered regions in the major histocompatibility complex class II-associated invariant chain. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9900–9904. doi: 10.1073/pnas.92.21.9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N., Lauer W., Habicht J., Dobberstein B. Primary structure of the gene for the murine Ia antigen-associated invariant chains (Ii). An alternatively spliced exon encodes a cysteine-rich domain highly homologous to a repetitive sequence of thyroglobulin. EMBO J. 1987 Jun;6(6):1677–1683. doi: 10.1002/j.1460-2075.1987.tb02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. A., Yewdell J. W., Bennink J. R., Cresswell P. Invariant chain targets HLA class II molecules to acidic endosomes containing internalized influenza virus. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5998–6002. doi: 10.1073/pnas.88.14.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcherek G., Gnau V., Jung G., Rammensee H. G., Melms A. Supermotifs enable natural invariant chain-derived peptides to interact with many major histocompatibility complex-class II molecules. J Exp Med. 1995 Feb 1;181(2):527–536. doi: 10.1084/jem.181.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marić M. A., Taylor M. D., Blum J. S. Endosomal aspartic proteinases are required for invariant-chain processing. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2171–2175. doi: 10.1073/pnas.91.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. S., Blum J. S., Cresswell P. Invariant chain trimers are sequestered in the rough endoplasmic reticulum in the absence of association with HLA class II antigens. J Cell Biol. 1990 Sep;111(3):839–855. doi: 10.1083/jcb.111.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. S., Cresswell P. Invariant chain associates with HLA class II antigens via its extracytoplasmic region. J Immunol. 1986 Apr 1;136(7):2519–2525. [PubMed] [Google Scholar]
- Mellins E., Smith L., Arp B., Cotner T., Celis E., Pious D. Defective processing and presentation of exogenous antigens in mutants with normal HLA class II genes. Nature. 1990 Jan 4;343(6253):71–74. doi: 10.1038/343071a0. [DOI] [PubMed] [Google Scholar]
- Neefjes J. J., Ploegh H. L. Inhibition of endosomal proteolytic activity by leupeptin blocks surface expression of MHC class II molecules and their conversion to SDS resistance alpha beta heterodimers in endosomes. EMBO J. 1992 Feb;11(2):411–416. doi: 10.1002/j.1460-2075.1992.tb05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters P. J., Neefjes J. J., Oorschot V., Ploegh H. L., Geuze H. J. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991 Feb 21;349(6311):669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Reyes V. E., Lu S., Humphreys R. E. Cathepsin B cleavage of Ii from class II MHC alpha- and beta-chains. J Immunol. 1991 Jun 1;146(11):3877–3880. [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J Immunol. 1990 Mar 1;144(5):1849–1856. [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990 Jun 14;345(6276):615–618. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- Romagnoli P., Germain R. N. The CLIP region of invariant chain plays a critical role in regulating major histocompatibility complex class II folding, transport, and peptide occupancy. J Exp Med. 1994 Sep 1;180(3):1107–1113. doi: 10.1084/jem.180.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., Stern L. J., Wiley D. C., Germain R. N. MHC class II function preserved by low-affinity peptide interactions preceding stable binding. Nature. 1994 Aug 25;370(6491):647–650. doi: 10.1038/370647a0. [DOI] [PubMed] [Google Scholar]
- Schaiff W. T., Hruska K. A., Jr, McCourt D. W., Green M., Schwartz B. D. HLA-DR associates with specific stress proteins and is retained in the endoplasmic reticulum in invariant chain negative cells. J Exp Med. 1992 Sep 1;176(3):657–666. doi: 10.1084/jem.176.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Southwood S., Miller J., Appella E. Binding of major histocompatibility complex class II to the invariant chain-derived peptide, CLIP, is regulated by allelic polymorphism in class II. J Exp Med. 1995 Feb 1;181(2):677–683. doi: 10.1084/jem.181.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan V. S., Cameron P., Porter G., Gammon M., Amaya M., Mellins E., Zaller D. M. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995 Jun 29;375(6534):802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Wiley D. C. The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide. Cell. 1992 Feb 7;68(3):465–477. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- Teyton L., O'Sullivan D., Dickson P. W., Lotteau V., Sette A., Fink P., Peterson P. A. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990 Nov 1;348(6296):39–44. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- Viville S., Neefjes J., Lotteau V., Dierich A., Lemeur M., Ploegh H., Benoist C., Mathis D. Mice lacking the MHC class II-associated invariant chain. Cell. 1993 Feb 26;72(4):635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- West M. A., Lucocq J. M., Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature. 1994 May 12;369(6476):147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]