Abstract
During the past three decades, the Wingless-type MMTV integration site (Wnt) signaling cascade has emerged as an essential system regulating multiple processes in developing and adult brain. Accumulating evidence points to a dysregulation of Wnt signaling in major neurodegenerative pathologies including Parkinson’s disease (PD), a common neurodegenerative disorder characterized by the progressive loss of midbrain dopaminergic (mDA) neurons and deregulated activation of astrocytes and microglia. This review highlights the emerging link between Wnt signaling and key inflammatory pathways during mDA neuron damage/repair in PD progression. In particular, we summarize recent evidence documenting that aging and neurotoxicant exposure strongly antagonize Wnt/β-catenin signaling in mDA neurons and subventricular zone (SVZ) neuroprogenitors via astrocyte–microglial interactions. Dysregulation of the crosstalk between Wnt/β-catenin signaling and anti-oxidant/anti-inflammatory pathways delineate novel mechanisms driving the decline of SVZ plasticity with age and the limited nigrostriatal dopaminergic self-repair in PD. These findings hold a promise in developing therapies that target Wnt/β-catenin signaling to enhance endogenous restoration and neuronal outcome in age-dependent diseases, such as PD.
Keywords: Wnt/β-catenin signaling, Parkinson’s disease, neuroinflammation, dopaminergic neurons, neurogenesis, neurodegeneration, neuroprotection
Introduction
The Wingless-type MMTV integration site (Wnt) pathway is a central player in a variety of biological processes. In the central nervous system (CNS), Wnt signaling cascades regulate and orchestrate all facets of neuronal functions including differentiation, neuronsurvival, axonal extension, synapse formation and plasticity, neurotrophin transcription, neurogenesis, and neuroprotection (Patapoutian and Reichardt, 2000; Ciani and Salinas, 2005; Lie et al., 2005; Inestrosa and Arenas, 2010; Zhang et al., 2011b; Harrison-Uy and Pleasure, 2012; Salinas, 2012). Midbrain dopaminergic (mDA) neurons play a pivotal role in controlling motor behaviors, cognitive, and affective brain functions. Compelling evidence also indicates a role of Wnt/β-catenin signaling pathway in mDA neuron development (Prakash and Wurst, 2006), but only recently, the expression of Wnt/β-catenin ligands and other components were characterized in intact and injured adult midbrain (L’Episcopo et al., 2011a, b).
Parkinson’s disease (PD) is a chronic movement disorder characterized by a progressive degeneration of mDA neurons in the ‘substantia nigra pars compacta’ (SNpc), an aggregation of α-synuclein (α-syn) into intraneuronal structures (called Lewy bodies and Lewy neurites), and a dysregulated immune activation in the SNpc (McGeer et al., 1988; Langston et al, 1999). As the disease advances, the progressive loss of dopamine storage in striatum results in decreased motor function with symptoms including resting tremors, rigidity, bradykinesia, and postural instability, accompanied by progressive impairment of autonomic, cognitive, and mood functions (Langston, 2006; Hirsch et al., 2013). Currently, there is no cure for PD, and available therapeutics only temporarily relieve PD symptoms (Olanow and Schapira, 2013).
Several genes have been identified in the rare familial cases (about 5%), whereas the majority of cases are sporadic, thus underlying a critical interplay between genetic susceptibility and environmental factors (Di Monte et al., 2002; Warner and Schapira, 2003; Gao and Hong, 2011; Gao et al., 2012; Cannon and Greenamyre, 2013) (Table 1). Aging and exposure to neurotoxic agents, in particular, represent critical contributing risk factors for mDA neuron demise (Betarbet et al., 2000; Marchetti et al., 2011; Villeda et al., 2011; Kamel, 2013). A multifactorial cascade of pathogenic events appears responsible for the selective and progressive SNpc neuronal cell death in PD and experimental parkinsonism (Hirsch et al., 2013). Among these culprits, oxidative stress and inflammation associated to molecular changes indicative of mitochondrial dysfunction and apoptosis have been defined in the parkinsonian brain (Abou-Sleiman et al., 2006). Importantly, astrocytes and microglial cells are key actors playing both beneficial and destructive roles. Their uncontrolled activation (i.e. under inflammatory/neurotoxic exposure or upon brain injury) may directly affect neurons by releasing various molecular mediators, such as pro-inflammatory cytokines, reactive oxygen (ROS), and nitrogen species (RNS), which in turn perpetuate/exacerbate glial activation, resulting in increased mDA neuron vulnerability and/or promoting SNpc cell death (Marchetti and Abbracchio, 2005; Whitton, 2007, 2010; Hu et al., 2008; McGeer and McGeer, 2008; Hirsch and Hunot, 2009; L’Episcopo et al., 2010a; Przedborski, 2010; Tansey and Goldberg, 2010). Specifically, both central and peripheral inflammation act in concert with genetic susceptibility and environmental factors to accelerate mDA neuron loss (Frank-Cannon et al., 2008; L’Episcopo et al., 2010a; Gao et al., 2011; Marchetti et al., 2011; Lastres-Becker et al., 2012), thereby pointing to glia and its mediators as a final common pathway toward neurodegeneration, neuroprotection, and neurorepair (Marchetti et al., 2005a, b, 2011, 2013; Morale et al., 2006).
Table 1. Gene–environment interactions in Parkinson’s disease.
| Environmental risk factors | Genes/products | Protective factors |
|---|---|---|
|
a,bOld age, a,bestrogen deficiency (in women); Rural living, a,bherbicides, pesticide exposure (paraquat, rotenone, organochlorines, carbamates); bMetal exposure, head injury, a,binfectious diseases during childhood; Maternal factors/early life events b(virus, drugs, endotoxins, hormone deficits); Drug-induced parkinsonism (bdrug abuse, neuroleptics, calcium-channel blockers) |
Familial PD
PARK1–PARK18 (aPARK2/Parkin, aPARK17/VPS35), a,bLRRK2, α-synuclein, UCH-L1, Tau |
cEstrogen replacement therapy (in post-menopausal women and OVX animals); cDietary factors/life style (tea, polyphenols, wine components, curcumin, drinking coffee, tobacco smoking); a,dEnvironment enrichment, exercise and social interactions; a,cChronic use of NSAIDs reduces risk by ~45% |
|
Dopaminergic DA receptors, DAT, TH, COMT, MAO a,bGSK-3p can contribute to PD risk | ||
|
Xenobiotic Metabolism/Detox CYP2D, CYP1A1, NAT, a,bHmox, GST, NQO2 | ||
|
Lipoprotein
Apolipoprotein E | ||
|
Survival/neurotrophic factors aNOR1, Nurr1, NGF, BDNF | ||
|
a,bInflammatory genes NOS, TNF-α, IL-1β, IL-6, ER-β |
DAT, dopamine transporter; TH, tyrosine hydroxylase; COMT, catechol-o-methyl-transferase; MAO, monoamino-oxidase; CYP2D, debrisoquine 4-hydroxylase; CYP1A1, cytochrome P450 1A1; NAT, N-acetyltransferase; Hmox, heme oxygenase 1; GST, glutathione transferase; NQO2, NAD(P)H:quinone oxidoreductase 2; NOR1, orphan nuclear receptor subfamily 4, group A, member 3; Nurr1, orphan nuclear receptor subfamily 4, group A, member 2; NGF, nerve growth factor; BDNF, brain derived neurotrophic factor.
Wnt/β-catenin dysregulation.
Activation of microglia and pro-inflammatory mediators in animal models of PD.
Mitigation/inhibition of microglial activation in animal models of PD.
Enhanced neurogenesis/synaptic plasticity and glial proliferation.
Wnts are important regulators of inflammation (Pereira et al., 2009; Neumann et al., 2010; Schaale et al., 2011; Valencia et al., 2011; Oderup et al., 2013). In the brain, both astrocyte and macrophage/microglia constitute a vital source of endogenous Wnt ligands; they harbor Wnt receptors and respond to Wnt in either pro- or anti-inflammatory manner (Halleskog et al., 2011; Kilander et al., 2011; L’Episcopo et al., 2011a, b, 2012, 2013; Hooper et al., 2012; Halleskog and Schulte, 2013; Marchetti and Pluchino, 2013). Neuroinflammation also modulates the regenerative capacity of the adult brain, acting on neural stem/progenitor cell (NPC) survival, proliferation, neurogenesis, and cell migration in both positive and negative fashions (Butovsky et al., 2006; Ekdahl et al., 2009; Ekdahl, 2012; Kokaia et al., 2012; L’Episcopo et al., 2012). Interestingly, aging, PD, and experimental PD models (induced by neurotoxins and genetic mutations) all impair NPC proliferation and differentiation in the subventricular zone (SVZ) of the adult mammalian brain (Freundlieb et al., 2006; Borta and Hoglinger, 2007; O’Keeffe et al., 2009; Winner et al., 2011; Desplats et al., 2012). The crosstalk between inflammatory and Wnt/β-catenin signaling cascades impacting on SVZ plasticity linked to nigrostriatal DA neuron injury/repair was recently high-lighted (L’Episcopo et al., 2012, 2013).
In this review, we introduce the Wnt/β-catenin pathway as a prosurvival signaling cascade in adult mDA neurons, the neuroinflammation and gene-environment interactions in PD, the role of Wnt signaling in bridging neuroinflammation and mDA neuron vulnerability/resistance, andpotential therapeutical implications for clinical treatment of PD.
The Wnt signaling cascade: a complex system for complex tasks
Wnts encode secretary glycoproteins to activate the Wnt signaling pathway. Wnt signals are context-dependently transduced to canonical and noncanonical pathways based on the expression profile of Wnt ligands, Wnt antagonists, the Frizzled (Fzd) family receptors, co-receptors, and the activity of cytoplasmic Wnt signaling regulators (Harrison-Uy and Pleasure, 2012; Salinas, 2012; Willert and Nusse, 2012). Wnt proteins are 39–46 kDa lipid-modified secreted glycoproteins that contain 350–400 amino acids. They share molecular and structural characteristics involving sequence identity with highly conserved 23–24 cysteine residues and several asparagines-linked glycosylation sites (Wnt homepage: http://www.stanford.edu/~rnusse/wntwindow.html).
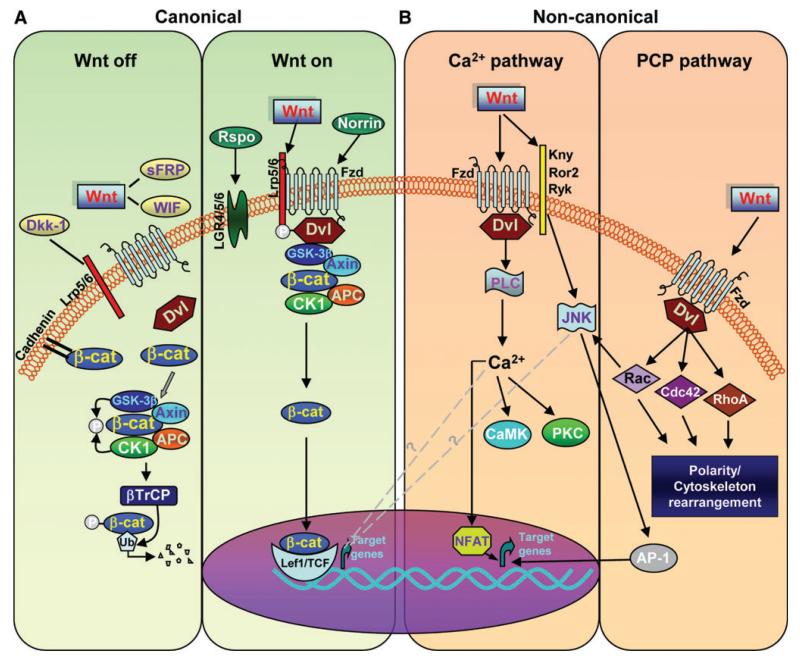
Wnt proteins are essentially described as Wnt1 (including Wnt2, Wnt3, Wnt3a, Wnt8, and Wnt8a) and Wnt5a (including Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, and Wnt11) classes by intracellular signaling pathways specifying Wnt signal transduction (Willert and Nusse, 2012). The Wnt1 class has been generally assumed to signal via the canonical Wnt/β-catenin pathway, whereas the Wnt5a class via the noncanonical Wnt/PCP and Wnt/Ca2+ pathways. However, this varies depending on the presence of receptors (Figure 1). In the absence of a Wnt ligand, i.e. in the ‘Wnt off’ state, cytoplasmic β-catenin protein is constantly degraded by the action of the Axin complex, which is composed of the scaffolding protein Axin, the tumor suppressor adenomatous polyposis coli gene product (APC), casein kinase 1 (CK1), and glycogen synthase kinase 3β (GSK-3β) (Figure 1A). CK1 and GSK-3β sequentially phosphorylate the amino terminal region of β-catenin, resulting in β-catenin recognition by β-Trcp, an E3 ubiquitin ligase subunit, and subsequent β-catenin ubiquitination and proteasomal degradation. The continual elimination of β-catenin prevents β-catenin from reaching the nucleus, and Wnt target genes are thereby repressed by proteins of the DNA-bound T cell factor/lymphoid enhancer factor (TCF/LEF) family (Figure 1A).
Figure 1. Wnt signaling cascades.
(A) In canonical Wnt/β-catenin pathway, binding of Wnts (‘Wnt on’) to a receptor complex composed of Fzd and LRP family members inhibits the APC/GSK-3β destruction complex and blockade of β-catenin (β-cat) by GSK-3β. β-cat then accumulates in the cytoplasm and translocates to the nucleus where it regulates target gene expression with TCF/LEF family of transcription factors. In the absence of Wnt ligand (‘Wnt off’), β-cat is targeted for proteolytic degradation by the APC/GSK-3β destruction complex. The proteins Norrin and R-spondin (Rspo) are unrelated to Wnt and act as Wnt agonists, whereas Dkk1, Wif, and sFRPs act as antagonists. (B) In noncanonical Wnt/Ca2+ pathway, the binding of Wnts promotes Fzd-mediated activation of G proteins, leading to the release of Ca2+ from intracellular stores and consequent activation of Ca2+-dependent effector molecules. Several Ca2+-sensitive targets, i.e. PKC, CamKII, and calcineurin, have been identified downstream of the Wnt/Ca2+ pathway. Targets of the Wnt/Ca2+ pathway appear to interact with the Wnt/β-catenin pathway at multiple points. Additionally, Fzd receptors in association with Kny, Ror2, or Ryk receptors can activate JNK, promoting target gene expression through AP-1. In noncanonical Wnt/PCP pathway, the binding of Wnts activates RhoA/B, Cdc42, or Rac1. Dvl activates Rac1, which can activate JNK to signal through the NFAT pathway.
GSK-3β is a serine/threonine proteinkinase known to regulate numerous cellular processes (Jope et al., 2007; Beurel et al., 2010; Kim and Snider, 2011) and its malfunction is also involved in the pathogenesis of human diseases, such as nervous system disorders, diabetes, and cancer (Takahashi-Yanaga, 2013; King et al., 2014). It was found that β-catenin is also trafficked out of the cells via exosomes, resulting in decreased levels of β-catenin independent of GSK-3β activity or proteasomal degradation (Chairoungdua et al., 2010). Therefore, pharmacological inhibitors of GSK-3β activity can induce β-catenin-dependent signaling in the absence of Wnt ligands.
The Wnt/β-catenin pathway is activated (i.e. in the ‘Wnt on’ state) when a Wnt ligand binds to a seven-transmembrane Fzd receptor (Janda et al., 2012) and its co-receptor, low-density lipoprotein receptor-related protein 6 (Lrp6) or the close relative Lrp5 (Figure 1A). The formation of a Wnt–Fzd–Lrp6 complex together with the recruitment of the scaffolding protein Dishevelled (Dvl) results in Lrp6 phosphorylation and activation and the recruitment of the Axin complex to the receptors. Wnt signaling inhibits GSK-3β activity, thus increasing the amount of β-catenin, which enters the nucleus and associates with TCF/LEF transcription factors, leading to the transcription of Wnt target genes for cell survival, proliferation, and differentiation (Behrens et al., 1996; Aberle et al., 1997; Gordon and Nusse, 2006).
In noncanonical Wnt/Ca2+ signaling pathway, the binding of Wnts promotes Fzd-mediated activation of pertussis Toxin-sensitive heterotrimeric guanine nucleotide-binding proteins (G proteins) (Figure 1B). This, inturn, stimulates the release of Ca2+ from intracellular stores, which leads to the activation of Ca2+-dependent effector molecules. Several Ca2+-sensitive targets—protein kinase C (PKC), Ca2+/calmodulin-dependent protein kinase II (CamKII), and the Ca2+/calmodulin-sensitive protein phosphatase calcineurin—have been identified downstream of the Wnt/Ca2+ pathway (Angers and Moon, 2009; van Ameronge, 2012). This leads to changes in cell movement and polarity and to the antagonism of the β-catenin pathway at multiple points (Figure 1A and B).
There are different endogenous regulators that modulate the Wnt/β-catenin signaling pathway. The Dickkopf (Dkk) family (Dkk1, Dkk2, Dkk3, Dkk4, and soggy) is a group of secreted glycoproteins, in which Dkk1 is the best characterized member. Secreted frizzled-related proteins (sFRPs) were considered Wnt signaling antagonists but recent studies show that specific family members can positively modulate Wnt signaling (Bovolenta et al., 2008; Angers and Moon, 2009; Esteve et al., 2011; van Amerongen, 2012). Fzd receptors can also bind to proteins from other families, such as Respondin and Norrin. Additionally, Wnt–Fzd binding and cooperation with particular co-receptors, such as Lrp5/6, receptor tyrosine kinase-like orphan receptor 1/2 (Ror1/2), and receptor-like tyrosine kinase (Ryk), can define downstream signal specificity (Figure 1B) (Ciani and Salinas, 2005; Clevers and Nusse, 2012; Salinas, 2012; van Amerongen, 2012).
Overall, multiple potential interactions among Wnt proteins, their receptors, and downstream effectors lead to various outcomes, thus increasing the complexity of the signal transduction network. The interruption of Wnt signaling leads to either hypo- or aberrant functioning that is associated with a variety of human hereditary diseases. Therefore, modulation of Wnt signaling is actively targeted for cancer, regenerative medicine, stem cell therapy, bone growth, and wound healing (Clevers and Nusse, 2012).
Wnt/β-catenin signaling in adult mDA neurons: a microenvironmental sensor balancing cell survival and death
The emerging ‘pro-survival role’ for the Wnt/β-catenin signaling pathway and the dysregulation of Wnt/Fzd cascade being accepted as critical determinant of major neurodegenerative disorders (Chong and Maiese, 2007; Varela-Nallar et al., 2009; Inestrosa and Arenas, 2010; Kim et al., 2011; Purro et al., 2012) associate the canonical Wnt/β-catenin pathway with the control of cell survival and death during injury. In a mouse model of basal ganglia injury by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), we conducted a wide gene expression analysis of 92 mRNA species involved in inflammation, immunity, stemness, self-renewal, DA neurodevelopment, and DA metabolism. A major upregulation of pro-inflammatory chemokines as well as Wnt1 during MPTP-induced mDA degeneration and self-recovery suggests Wnt signaling as an intrinsic response to mDA neuron injury (L’Episcopo et al., 2011a).
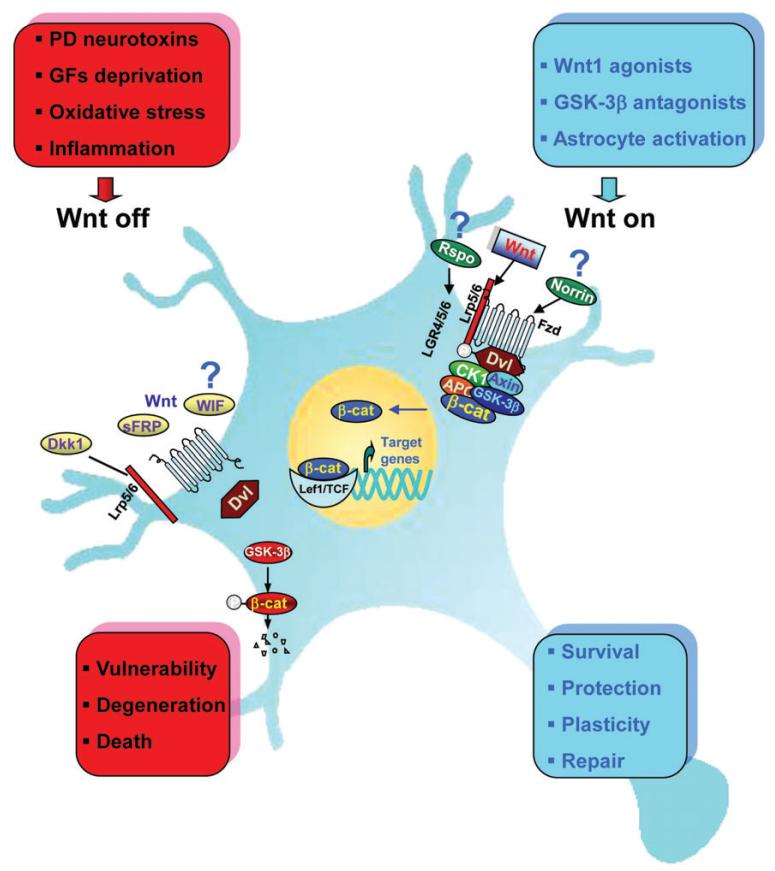
The neuroprotective effect of Wnt1 was reported in both mDA neurons treated with 6-hydroxydopamine (6-OHDA) and MPTP (L’Episcopo et al., 2011b) and the DA tumoral cell line SH-SY5Y exposed to 6-OHDA (Wei et al., 2013). Knocking down either β-catenin or Fzd-1 counteracts Wnt1 ligand-induced neuroprotection against various cytotoxic insults, identifying Wnt1/Fzd-1/β-catenin signaling pathway as a novel pro-survival mechanism (L’Episcopo et al., 2011b) (Figure 2). Stabilizing neuronal β-catenin was previously shown to render neurons ‘anti-apoptotic’ in cell cultures and transgenic mouse models (Li et al., 2007). Parkin, an E3 ubiquitin ligase linked to familial PD, regulates β-catenin protein levels in vivo and protects DA neurons by increased stabilization of β-catenin (Rawal et al., 2009; Inestrosa and Arenas, 2010), indicating that both prolonged and inactive/inefficient Wnt/β-catenin signaling may be detrimental to mDA neuronal populations. These further underscore that Fzd receptors and β-catenin are ‘physiological check-points’ for mDA neuron survival (Figure 2).
Figure 2. Schematic illustration of Wnt1/β-catenin signaling as a key player in mDA neuron survival/protection.
In the intact midbrain, canonical Wnt1-like agonists via activation of Fzd-1 receptors (‘Wnt on’) maintain the integrity of mDA neurons by blocking GSK-3β-induced phosphorylation (P) and proteosomal degradation of β-catenin. Stabilized β-catenin can translocate into the nucleus and associate with transcription factors to regulate the expression of Wnt target genes involved in DA neuron survival/plasticity. β-catenin may also function as a pivotal defense molecule against oxidative stress or as a coactivator for several nuclear receptors involved in the maintenance/protection of DA neurons (L’Episcopo et al., 2011b). Neurotoxic agents including PD neurotoxins (MPTP/MPP+, 6-OHDA), pesticides (rotenone), increased oxidative load as a result of growth factors (GFs) deprivation, or aging may antagonize Wnt/β-catenin signaling (‘Wnt off’) in DA neurons. Upregulation of active GSK-3β leads to β-catenin degradation and increased DA neuron vulnerability/degeneration/apoptosis. Various potential endogenous Wnt agonists (Respondin, Rspo, Norrin) or antagonists (Dkk1, Wif, sFRP) are also indicated.
GSK-3, in both mammalian isoforms GSK-3α and β, is another key actor in Wnt/β-catenin signaling and has been implicated in mDA neuron physiopathology during neurodegenerative and psychiatric diseases (Phukan et al., 2010; Takahashi-Yanaga, 2013). Particularly, GSK-3 inhibition has attracted widespread attention for its effects on mood stabilization, as PD patients experience severe psychiatric symptoms during the later stage of the disease (Phukan et al., 2010; Connolly and Fox, 2013; Takahashi-Yanaga, 2013; King et al., 2014). GSK-3β is a mitochondrial killer for ‘stressed’ mDA neuron and critically involved in oxidative stress-induced neuronal cell death (Figure 2). Hence, environmental toxins, such as rotenone and paraquat, neurotoxic compound 6-OHDA, as well as the active metabolite of MPTP, MPP+, are strong inducers of GSK-3β (Chen et al., 2004; Petit-Paitel et al., 2009; L’Episcopo et al., 2011a, b; Songin et al., 2011). In the absence of Wnt activity, the increased mitochondrial GSK-3β predisposes the degradation of β-catenin in parallel with caspase3 activation and DA neuron demise (Duka et al., 2009; Petit-Paitel et al., 2009). This increased GSK-3β was also reported in the striatum of post-mortem PD brains (Duka et al., 2009), and genetic screens revealed GSK-3β polymorphisms with altered transcription and splicing in PD (Kwok et al., 2005). As expected, blocking GSK-3β by systemic treatment with specific antagonists was shown to mitigate MPTP-induced nigrostriatal toxicity (Wang et al., 2007; L’Episcopo et al., 2011a, b).
Dkk1 is a prototypic Wnt/β-catenin antagonist that promotes degeneration of adult nigrostriatal DA neurons in the intact mid-brain (L’Episcopo et al., 2011b; Marchetti et al., 2013). Hence, the unilateral infusion of Dkk1 within the SNpc induced a time-dependent loss of mDA neuronal cell bodies in the ipsilateral but not contralateral uninfused SNpc, associated with the early and sharp downregulation of Fzd-1 and β-catenin proteins as well as a marked upregulation of active GSK-3β only in the ipsilateral SNpc (L’Episcopo et al., 2011b). Dun et al. (2012) recently reported that endogenous Dkk1 expression increased after lesioning the nigrostriatal DA system following a medial forebrain bundle 6-OHDA infusion in rodents. All together, these studies support the idea that an efficient Wnt/β-catenin signaling is required for mDA neuron survival, while the antagonism to this signaling pathway may lead to SNpc degeneration (Figure 2).
Previous evidence that Wnt signaling may be reinduced in the adult CNS after injury (Osakada et al., 2007) suggests a compensatory mechanism possibly implicated in mDA neuroprotection or neurorescue. Indeed, the ability of nigrostriatal DA neurons to respond to injury by triggering a panel of neurochemical, molecular, and functional compensatory mechanisms has been well documented, in both rodents and non-human primates (Hornykiewicz, 1993; Bezard and Gross, 1998; Collier et al., 2007; Zigmond et al., 2012). Activation of these endogenous compensatory mechanisms may mask early signs of PD before the appearance of clinical symptoms (Hornykiewicz, 1993; Bezard and Gross, 1998).
Neuroinflammation and gene–environment interactions in PD: a key role for neuron–glial crosstalk
Interactions between genetic and environmental factors are believed to play major roles in the onset and progression of PD (Table 1). Age has a causal relationship to PD, whereas female gender plays a neuroprotective role (Morale et al., 2008). Exercise, tobacco and caffeine consumption, and exposure to non-steroidal anti-inflammatory drugs (NSAIDs) are potential protective factors (Chen et al., 2003, 2005; Marchetti and Abbracchio, 2005; Powers et al., 2008; Gao et al., 2012). Environmental toxins have been proposed as potential risk factors for PD, especially exposure to neurotoxins and pesticides (Cannon and Greenamyre, 2013; Kamel, 2013; Goldman, 2014). Albeit the mechanisms whereby gene-environment interactions mediate the chronic progressive neurodegenerative processes in PD still remain elusive, accumulating findings from both epidemiological studies and animal models highlight neuroinflammation via glia as a potential common determinant in directing neurodegeneration/neuroprotection (Marchetti et al., 2005a, b, 2011, 2013; Morale et al., 2006).
Innate inflammatory processes associated with glial cell activation contribute to PD pathophysiology (McGeer et al., 1988; Whitton, 2007, 2010; Gao and Hong, 2008; McGeer and McGeer, 2008; Hirsch and Hunot, 2009; Przedborski, 2010; Taylor et al., 2013). The neuronal degeneration itself, particularly aggregated α-syn released from dying neurons or dead mDA neurons in neuropil, may promote a chronic inflammation, thus precipitating a vicious cycle of cell death (Zhang et al., 2005; Gao et al., 2011; Harms et al., 2013; Sanchez-Guajardo et al., 2013) and propagating mis-folded α-syn in a ‘prion-like’ fashion in PD (Lema Tome et al., 2012).
Neurons and glial cells communicate with each other by soluble factors as neurotransmitters, neuromodulators, and neuropeptides, or neuroimmune regulatory molecules that reduce or inhibit any exacerbated inflammatory response in the brain (Marchetti and Abbracchio, 2005; Morale et al., 2006; Belanger and Magistretti, 2009; Chavarria and Cardenas, 2013). A dysregulation of neuron–glia interactions may result in neuronal loss and/or reduced neurorepair capacity. In this respect, it should be mentioned that the SNpc contains almost 4.5 times of microglia compared with the cortex and other regions in the brain, which may predispose mDA neurons to immunological attacks (Tansey and Goldberg, 2010). On the other hand, mDA neurons have reduced anti-oxidant capacity compared with other cell types.
Astrocytes, microglia, and infiltrating monocyte-derived macrophages play major roles in mediating detrimental or neuroprotective effects via an array of growth/neurotrophic factors, pro-/anti-inflammatory cytokines, chemokines, and neurogenic transcription factors (Table 2). Microglial is maintained in quiescent state by various inhibitory receptors. CD200R through interaction with CD200, a transmembrane glycoprotein expressed on neurons, surveys glial activation state (Wang et al., 2011; Zhang et al., 2011a). A disruption of CD200–CD200R engagement can cause abnormal activation of microglia and consequent pathological changes. Glucocorticoid (GR) and estrogen (ER) receptors on microglia also restrain the excessive activation via blockade of major glial inflammatory pathways, including nuclear factor kappa B (NF-κB) signaling and iNOS-derived NO generation (Morale et al., 2004, 2006; Marchetti et al., 2005a, b). In addition, astrocytes can downregulate microglia activation. Reactive astrocytes upregulate molecules including glial fibrillary acidic protein (GFAP), S100, iNOS, and NF-κB and express Toll-like receptors (TLRs) involved in innate immune responses as well as other immune mediators (Table 2), thus participating in the regulation of astrocyte response to various stimuli (Dringen et al., 2000; Gennuso et al., 2004; Bélanger and Magistretti, 2009; Chen et al., 2009; Lee et al., 2009; Surh et al., 2009). In response to mDA neuron injury, reactive astrocytes and microglia, which proliferate and transform into active macrophage-like cells, produce a wide variety of molecules as immune mediators to combat pathology and provide neuroprotection (Kreutzberg, 1996; Aloisi, 1999) (Table 2). However, when chronically activated, some mediators can become detrimental to neuronal survival and/or increase mDA neuron vulnerability, particularly superoxide from plasma membrane NADPH oxidase (PHOX), cyclooxygenase 2 (COX2)-derived prostaglandin E2, inducible NO synthase (iNOS)-derived NO, and a plethora of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, and IFN-γ) (Sriram et al., 2002; Whitton, 2007, 2010; Gao and Hong, 2008; Hu et al., 2008; Hirsch and Hunot, 2009; L’Episcopo et al., 2010a; More et al., 2013; Taylor et al., 2013). The adaptive immune system has also been implicated in PD pathophysiology, as infiltration of CD4/CD8T cells were reported in the SNpc of PD patients and animal models of PD (McGeer et al., 1988; Brochard et al., 2009). Additionally, the peripheral T cell pool is also altered in PD (Baba et al., 2005; Sanchez-Guajardo et al., 2013). Increased inflammation and breakdown of the blood-brain barrier (BBB) facilitate the communication between the CNS and peripheral immune systems. Hence, peripheral immune responses can trigger inflammation and exacerbation of CNS degeneration via exaggerated pro-inflammatory mediators including TNF-α, IL-1β, ROS, and RNS and resultant mDA neuron death (Chen et al., 2008; Hu et al., 2008; Koprich et al., 2008; Pott Godoy et al., 2008; L’Episcopo et al., 2011a, b, c). With age, both central and peripheral neuroinflammation increase (Henry et al., 2009). Interestingly, microglia can become ‘primed’ with age, i.e. capable of adopting a potent neurotoxic and pro-inflammatory phenotype, with harmful consequences for mDA neuron health (Streit, 2010; L’Episcopo et al., 2011c; Njie et al., 2012).
Table 2. Astrocyte and microglial-derived factors and functions.
| Astrocyte | Macrophage/microglia |
|---|---|
|
Survival/differentiation NGF, BDNF, IGF-I, E2, basic fibroblast growth factor, ciliary neurotrophic factor, GDNF, NT-3, NT-4, Wnt1, Wnt5a |
Neurotrophic/survival NGF, BDNF, GDNF, NT-3, NT-4 |
|
Anti-oxidant Glutathione, Nrf2, Hmox1, E2, superoxide dismutase, MRP1 |
Pro-inflammatory Toll-like receptors, TNFa, IL1-p, IL-6, IL-2, IL-12, IL-18, IFNγ, PGE2, PGJ2, ROS, H2O2, RNS, arachonic acid, platelet activating factor, QA, GRs, ERs, Wnt2, Wnt5a, Wnt10 |
|
Metabolic support Glycogen, glycogen synthase enzymes, lactate |
Oxidative/neurotoxic PHOX, COX2, iNOS, O2, H2O2, OH2, NOO2 |
|
Immunoregulatory Cytokines, chemokines, Scavenger receptors, Toll-like receptors, hydrogen disulfide, GRs, ERs, Wnt1, Wnt5a |
Anti-inflammatory Transforming growth factor β, IL-10, Wnt3a |
|
Neuroprotective/regenerative Metallothioneins, E2, Wnt1, Wnt5a |
|
| Synapse clearance | |
| C1q, MRPs |
E2,17-β-estradiol; H2O2, hydrogen peroxide; MRP1, multidrug resistance protein 1; NT-3, neurotrophin-3.
Certain genetic mutations are associated with dysfunctional microglial response to SNpc injury and interact with environmental risk factors in mediating chronic PD progression (Table 1) (Gao and Hong, 2011; Gao et al., 2011, 2012; Lastres-Becker et al., 2012). A genetic dysfunction of α-syn coupled to increased neuroinflammation can potentiate each other, driving chronic progression of neurodegeneration (Gao et al., 2011; Harms et al., 2013; Sanchez-Guajardo et al., 2013). Likewise, α-syn dysfunction and deficiency of anti-oxidant systems, such as Nrf2-deficiency, co-operate to aggravate protein aggregation, neuronal death, and inflammation in early stage of PD (Lastres-Becker et al., 2012). Moreover, the loss of Parkin, the product of the PARK2 gene recently linked to Wnt signaling (Rawal et al., 2009), increases the vulnerability of mDA neurons to inflammation-related degeneration (Frank-Cannon et al., 2008). Of special interest, the PD-linked leucine-rich repeat kinase 2 (LRRK2) mutation, which has been linked to Wnt signaling (Berwick and Harvey, 2012a, b), increases pro-inflammatory cytokine release from activated primary microglial cells, resulting in mDA neurotoxicity (Gillardon et al., 2012), whereas LRRK2 inhibition attenuates microglial inflammatory responses (Kim et al., 2012). The PARK17 (encoding the vacuolar protein sorting 35 homolog gene, VPS35) mutation is linked to autosomal dominant late-onset of PD, showing an involvement in Wnt/β-catenin signaling and iron uptake (Deng et al., 2013), and further studies are required to unravel its potential consequences for glial cell compartment homeostasis in PD. Genetic factors may also interact with early life events (prenatal exposure to endotoxins, glucocorticoids, and neurotoxins) and alter innate glial responses, including Wnt signaling dysregulation, to influence mDA neuron vulnerability (Morale et al., 2004; Marchetti et al., 2005a, b, 2011; Gao and Hong, 2011; Kuypers et al., 2013).
Wnt signaling in regulation of neuroinflammation
Wnt signaling contributes to both lymphocyte development in the thymus and bone marrow (Gordon et al., 2005; Staal et al., 2008) and functions of mature peripheral immune cells (Blumenthal et al., 2006; Pereira et al., 2009; Neumann et al., 2010; Schaale et al., 2011). In the CNS, Wnt signaling may have both pro- and anti-inflammatory effects, with potential consequences for inflammation-driven brain damage and inflammation-directed brain repair, respectively (Marchetti and Pluchino, 2013).
Sources and functions of Wnt signaling in the immune system
In adult mammals, likely sources of Wnt ligands include T cells, dendritic cells (DCs), and monocytes/macrophages. Circulating (peripheral) T cells express high levels of TCF/LEF, which changes upon T cell activation (Staal et al., 2008). Activated CD4+ T cells (a subset involved in the adaptive immune system) produce Wnt5a in response to chemokine stimulation. This autocrine Wnt signaling augments chemokine-directed T cell migration independent of β-catenin (Ghosh et al., 2009). Regulatory T (CD4+CD25+Foxp3+, Treg) cells are another subset of T cells that suppress immune responses and induce self-tolerance. It was reported that enhanced Wnt/β-catenin signaling by GSK-3β inhibition prolonged Foxp3 expression, thus leading to increased survival and overall (tolerogenic) activity of Treg cells in vivo (Graham et al., 2010). Recent evidence, however, suggests that Wnt signaling directly and negatively modulates Foxp3 transcriptional activity and thereby impairing Treg cell function both in vitro and in vivo (van Loosdregt et al., 2013). Monocytes/macrophages are professional phagocytes in both innate and adaptive immunity of vertebrate animals. They phagocytose pathogens and also stimulate lymphocytes or other immune cells to respond to pathogens (via antigen presentation). As a source of Wnts, cultured macrophages express Wnt2 and Wnt10b, and in vivo macrophages use Wnt7b as a short-range paracrine signal to induce programmed cell death in vascular endothelial cells of the developing eye (Lobov et al., 2005). DCs are critical elements of adaptive immunity for acquiring and presenting antigens and regulate immune responses to various physiological and pathological stimuli. In humans, DCs as well as activated macrophages significantly upregulate Wnt5a in response to pathogens (e.g. parasites) (Chaussabel et al., 2003; Pereira et al., 2009), while tissue-healing macrophages produce Wnt7b to stimulate organ repair and regeneration (Lin et al., 2010). Additionally, the increase in Wnt5a signaling during monocyte differentiation activates noncanonical Ca2+/CamKII/NF-κB signaling that confers tolerogenic capabilities to Wnt5a-expressing DCs (Valencia et al., 2011). Importantly, higher levels of Wnt5a were found in patients with severe sepsis, demonstrating an active role of Wnt5a in the inflammatory response (Pereira et al., 2009).
Astrocyte-derived Wnt/β-catenin signaling bridges neuroinflammation to mDA neuroprotection/repair
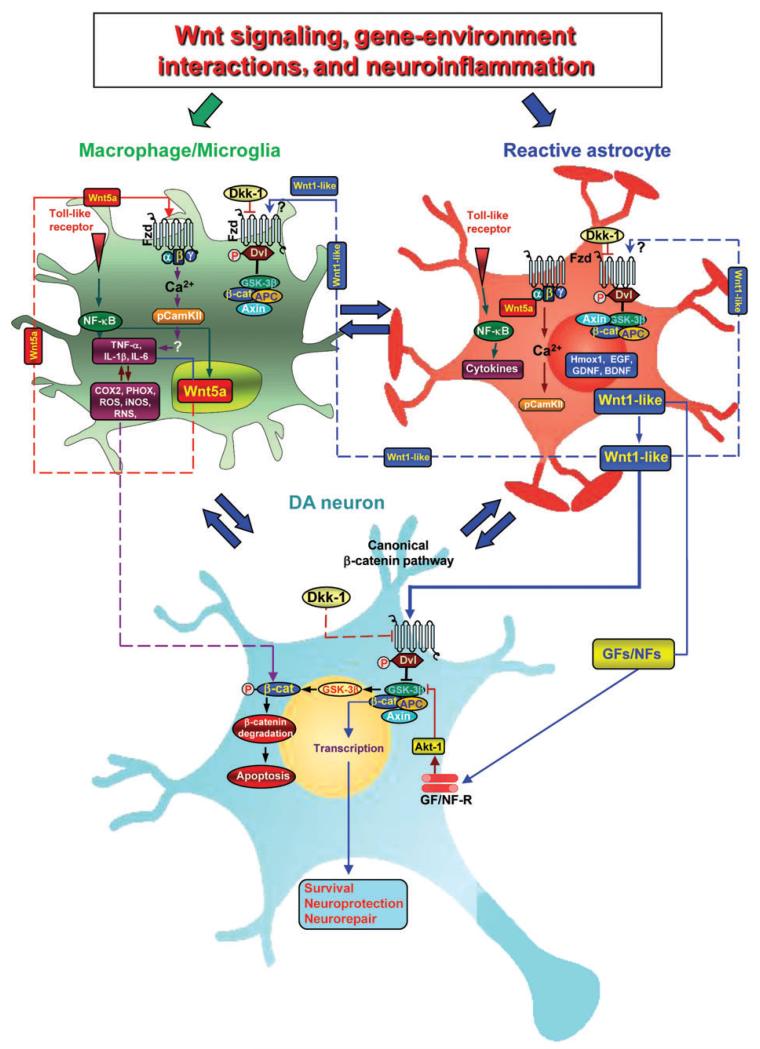
The recognized role of astrocytes as source of survival, neurotrophic, and neurogenic factors, including Wnt1 and Wnt5 (Table 2), prompted us to verify the hypothesis of an astrocyte-derived Wnt/β-catenin neuroprotective pathway. Astrocyte-derived factors including Wnt1 and Wnt5 are reported to regulate the proliferation and neurogenesis of DA progenitors during development (Castelo-Branco et al., 2006). Previous reports indicated that Wnt3a is expressed in adult hippocampal astrocytes (Lie et al., 2005; Kuwabara et al., 2009). Other studies reported that adult astrocytes are enriched with Wnt/β-catenin signaling components (Cahoy et al., 2008) that play a critical role in response during both injury and repair (Yang et al., 2012). In our recent study, increased Wnt1 mRNA transcription was detected in astrocytes derived ex vivo from MPTP-injured midbrain, and in situ hybridization demonstrated colocalization of Wnt1 with reactive GFAP+ astrocytes within the MPTP-injured midbrain, confirming that reactive astrocytes function via Wnt/β-catenin signaling during mDA injury/repair (L’Episcopo et al., 2011a). This was further supported by robust in vitro protective effects of astrocyte-derived factors against oxidative stress- and neurotoxin (6-OHDA and MPP+)-induced cell death (L’Episcopo et al., 2011a, b). This astrocyte-induced neuroprotection can be diminished by directly blocking Wnt/Fzd-1/β-catenin signaling with Dkk1 or magnified by exogenous activation of Wnt signaling with a specific GSK-3β antagonist and most importantly, is induced by addition of glial inserts to purified DA neurons just prior MPP+ insult (L’Episcopo et al., 2011b), thus corroborating the possibility that astroglial-derived Wnt1 might provide a compensatory mechanism to limit the degenerative process and/or activate the spontaneous SNpc self-repair program (Figure 3).
Figure 3. Gene-environment interactions and crosstalk among astrocytes, microglia, and DA neuronsvia Wnt signaling.
Upon nigrostriatal injury, at the neuron–astrocyte interface, astrocyte-derived Wnt1 via Fzd-1 receptor likely transmits pro-survival signals into mDA neurons by inhibition of GSK-3β to incite cytoprotection/neurorepair. Aging, MPTP exposure, and various gene/environmental risk factors can impair astrocyte neuroprotection in the face of microglia exacerbation, also via inhibition of Wnt1 expression and downregulation of anti-oxidant/anti-inflammatory cytoprotective proteins in astrocytes, for mDA neuron death/survival. At the microglial-astrocyte interface, upon activation by neurotoxins, endotoxins, or brain injury, macrophage/microglia produce a panel of pro-inflammatory cytokines (including TNF-α and IL-1β), chemokines, and Wnt5a (Pereira et al., 2009). Wnt5a may act via both autocrine Wnt5a/CamKII activation and paracrine stimulation via Fzd-5 to further stimulate pro-inflammatory cytokine production. Upregulation of microglial ROS, RNS, and GSK-3β further exacerbate microglia reaction. In this scenario, astrocytes may respond to microglial-derived chemokines with increased Wnt1-like proteins, activation of canonical Wnt/β-catenin signaling in microglia, inhibition of GSK-3β, and consequent decrease of the pro-inflammatory status. In addition, astrocyte-derived growth/neurotrophic and anti-oxidant factors can mitigate the inflammatory milieu and favor a progressive neurorescue program for mDA neurons.
Aging and neurotoxin exposure antagonize Wnt/β-catenin signaling
Aging is associated with increased inflammation both at peripheral and central levels (Henry et al., 2009), exaggerated production of pro-inflammatory mediators, and decreased ability of the nigrostriatal DA system to recover after neurotoxic challenge (Ho and Blum, 1998). Indeed, there was a lack of Wnt1 expression in mid-brain astrocytes in response to MPTP injury. Hence, two critical risk factors, aging and MPTP exposure, promoted a long-lasting decrease in β-catenin and Fzd-1 receptor accompanying upregulation of active GSK-3β, supporting a dysfunction of Wnt signaling in the aged injured midbrain (L’Episcopo et al., 2011a). Therefore, aging-induced decline of Wnt/β-catenin signaling in astrocytes and their crosstalk with DA neurons may underlie increased DA neuron vulnerability with age.
Wnt signaling and astrocyte–microglia crosstalk regulate the pro-inflammatory status in the MPTP-injured midbrain
When microglia is activated in vivo by MPTP exposure, expression levels of cytokines (TNF-α and IL-1β) and chemokines, together with the concurrent generation of ROS and RNS, are rapidly and robustly upregulated (L’Episcopo et al., 2010b, 2011a, c, 2012), and a major increase is observed at the peak of mDA neurodegeneration. Microglia in such pro-inflammatory status produce Wnt5a, which may constitute one part of a self-perpetrating cycle via noncanonical autocrine Wnt5a/CamKII activation and paracrine regulation for further stimulation of pro-inflammatory cytokines, such as iNOS andCOX2 (Figure 3).The pro-inflammatory role of Wnt5a in microglia was recently underlined by Halleskog and coworkers, reporting increased expression of iNOS, COX2, and chemokines and enhanced invasive and proliferative capacity of microglia exposed to Wnt5a in vitro (Halleskog et al., 2011; Kilander et al., 2011; Halleskog and Schulte, 2013).
MPTP also induces upregulation of the pro-inflammatory GSK-3β that further exacerbates microglial reaction (Jope et al., 2007; Beurel et al., 2010; Beurel, 2011; L’Episcopo et al., 2011a). There is ample evidence that the NF-κB pathway and the Wnt/β-catenin pathway interact to differentially regulate inflammation, with GSK-3β playing a central role in between. While GSK-3β is a negative regulator of β-catenin, it positively regulates NF-κB by targeting IkB, the major inhibitor of NF-κB, to proteasomal degradation (Wang et al., 2004; Beurel, 2011; Lin et al., 2012). On the other hand, β-catenin itself can form a complex with the p50 subunit of NF-κB, thereby preventing NF-κB transcriptional activity (Lin et al., 2012).
Astrocyte–microglia crosstalk represents one possible mean to repress the pro-inflammatory status via chemokine-induced Wnt1-like proteins in astrocytes, activation of canonical Wnt/β-catenin signaling in microglia, and inhibition of GSK-3β, with consequent anti-inflammatory effects (Chong and Maiese, 2007; Beurel, 2011; L’Episcopo et al., 2011a, b; Schaale et al., 2011) (Figure 3). Likewise, pharmacologic GSK-3β antagonism can restrain inflammatory microglial activation via inhibition of pro-inflammatory cytokines downstream of the NF-κB pathway (Beurel et al., 2010; L’Episcopo et al., 2011a). Altogether, astrocyte-derived Wnt1-like proteins coupled to a panel of growth/neurotrophic and anti-oxidant factors can mitigate the inflammatory milieu and favor a progressive neurorescue program for mDA neurons (Figure 3). At the neuron-glial interface, astrocyte-derived Wnt1 via Fzd-1 receptor likely transmits pro-survival signals in mDA neurons, possibly via blocking GSK-3β-induced degradation of β-catenin, which in turn incite cytoprotection/neurorepair (L’Episcopo et al., 2011a,b). Notably, exaggerated microglial pro-inflammatory status can still impair astrocyte anti-inflammatory functions and mDA neurorescue via inhibition of Wnt1 expression and downregulation of anti-oxidant/anti-inflammatory cytoprotective proteins in astrocytes (L’Episcopo et al., 2013).
Wnt signaling, neuroinflammation, and astrocyte–microglia–neuroprogenitor crosstalk: a complete regulatory loop orchestrating mDA neuron plasticity
The SVZ is the predominant region for adult neurogenesis in the brain. Different growth/neurotrophic factors, morphogens, NO, cytokines, and other key molecules exist within the SVZ and contribute to SVZ regulation (Butovsky et al., 2006; Freundlieb et al., 2006; Adachi et al., 2007). Particularly, the DA input to the SVZ arising from the nigrostriatal innervation plays a significant positive role (Freundlieb et al., 2006; Borta and Hoglinger, 2007; O’Keeffe et al., 2009; Winner et al., 2011). Activation of Wnt/β-catenin signaling is sufficient to increase the percentage of dividing cells that give rise to new neurons in the SVZ (Adachi et al., 2007; L’Episcopo et al., 2012). Recent findings support the implication of the immunesystem in the modulation of adult neurogenesis in the injured brain (Ekdahl et al., 2009; Martino et al., 2011; Tepavcevic et al., 2011; Cusimano et al., 2012; Ekdahl, 2012; Kokaia et al., 2012; L’Episcopo et al., 2012, 2013), which prompted a further investigation in the inflammatory modulation of the SVZ niche during MPTP-induced nigrostriatal plasticity.
Astrocyte- and microglial-derived mediators regulate the response of SVZ neuroprogenitors to MPTP
A profound inhibition of SVZ NPC proliferation with loss of the neurotransmitter dopamine is observed in human PD brains and non-human primate and rodent PD models (Freundlieb et al., 2006; Borta and Hoglinger, 2007; O’Keeffe et al, 2009). Time-course studies in MPTP-induced in vivo NPC impairment indicated a biphasic response. Between 1 and 14 days post-MPTP, a reduced proliferation of SVZ NPCs and a severe decrease of doublecortin-positive (DCX+) neuroblasts were associated with the mDA neuron degeneration. Between 14 and 28 days, a significant recovery of NPC proliferation and DCX+ cells to pre-MPTP levels was observed, coincided with a progressive striatal DA neuron re-innervation (L’Episcopo et al., 2012). The analysis of the spatio–temporal correlation documented that inhibition of both proliferation and neuron differentiation were preceded and accompanied by microglial activation with increased inflammatory mediators including PHOX-derived ROS and iNOS/NO and astrocyte nitration, possibly indicating astrocyte dysfunction both in striatum and SVZ (L’Episcopo et al., 2012). Further study indicated that MPTP-induced impairment of neurogenic potential was transient and attributed to environmental factors rather than intrinsic properties of NPC progeny (L’Episcopo et al., 2012). Hence, we unveiled both beneficial (pro-neurogenic) and harmful (inhibitory) effects of glial-derived factors in the regulation of NPC proliferation and neuron formation capacity during MPTP-induced nigrostriatal injury and recovery in young mice. Specifically, while astrocytes were endowed with protective and neurogenic capabilities both in the absence and presence of MPTP/MPP+, microglia increased NPC neurogenic potential in basal conditions but further impaired NPC survival in exposure to MPTP/MPP+, thus prompting future investigation of specific factors and signaling mechanisms involved in microglial and astrocyte responses.
Therapeutical window of opportunity for modulating SVZ to incite neurorestoration in PD: further evidence for a Wnt–glial neuroimmune connection
The critical role of microglia in adult neurogenesis and the potential for anti-inflammatory drug treatment to modulate this system have been emphasized by earlier and more recent studies (Butovsky et al., 2006; Ehninger et al., 2011; Biscaro et al., 2012; Ekdahl, 2012; L’Episcopo et al., 2012, 2013). Different lines of evidences point to age-dependent dysregulation of Wnt signaling as causal factor in aging-induced impairment of neurogenic potential of NPCs within both the SVZ and the hippocampus subgranular zone (SGZ). In the SVZ, aging and MPTP antagonize Wnt/β-catenin signaling leading to neurogenic impairment via crosstalk between inflammatory pathways at least in part mediated by upregulation of microglial pro-inflammatory mediator-induced downregulation of Wnt/β-catenin signaling (L’Episcopo et al., 2013). In SGZ, decreased Wnt3 release from aged hippocampal astrocytes regulates the age-associated decline of adult neurogenesis (Okamoto et al., 2011). In addition, the aging brain microenvironment was shown to decrease hippocampal neurogenesis through Wnt-mediated survivin signaling (Miranda et al., 2012). Specifically, the endogenous Wnt antagonist Dkk1 increases with age, resulting in the suppression of adult neurogenesis and proliferation, whereas Dkk1 knockout mice show increased Wnt signaling, resulting in enhanced neurogenesis and improved spatial memory (Seib et al., 2013).
Hence, aging-induced perturbations of the redox/inflammatory balance in the SVZ was uncovered. A two hit model was presented (Supplemetary Figure S1). The age-related impairment in astrocyte–microglia crosstalk serves as the first hit, which in turn drives SVZ impairment with reduced ability of the master redox modulator Nrf2 to mediate SVZ adaptation to other risk factors including MPTP (the second hit), leading to a long-lasting SVZ impairment that is possibly implicated in the failure of aged rodent to recover upon SNpc injury (Rojo et al., 2010; Kaidery et al., 2013; L’Episcopo et al., 2013). The phosphatidylinositol 3-kinase (PI3K)/Akt-mediated Wnt/β-catenin signaling cascade was revealed as a key target of microglial inflammatory modulation of NPCs both in vivo and in vitro (L’Episcopo et al., 2013). Thus, interruption of PI3K/Akt and Wnt/Fzd/β-catenin signaling cascades, switching on/off GSK-3β activation, were causally related to the impairment of SVZ NPCs. Importantly, HCT1026, a novel mixed cyclooxygenase inhibitor endowed with a safe profile, may rescue aging-induced SVZ impairment by normalization of aging-related Nrf2 and Wnt/β-catenin pathways and contribute to a long-lasting neuroprotection against MPTP-induced nigrostriatal DA toxicity and motor impairment in aged mice (L’Episcopo et al., 2010b, 2011c, 2012, 2013) (Supplemetary Figure S1). Together, these findings indicate that pharmacological manipulation of this endogenous system, either directly or indirectly via inflammation-dependent SVZ modulation, may have therapeutical implications for PD.
Declines in neurogenic output of the SVZ and olfactory capacity, as well as PD, occur throughout aging. The inflammation-dependent impairment of SVZ niche may contribute to some non-motor PD symptoms, suggesting a potential window of opportunity for therapeutical strategies for mitigating the inflammatory microenvironment, upregulating endogenous neurogenesis, and/or favoring integration of new neurons to incite neurorepair in age-dependent neurodegenerative disorders, such as PD (Rueger et al., 2012; Sakata et al., 2012; Wallenquist et al., 2012).
Concluding remarks and therapeutical perspectives
Wnt signaling at the neuroimmune interface plays a pivotal role in the regulation of neuroprogenitors, post-mitotic neurons, and central immune cell functions in PD. A ‘Wnt-continuum’ may emerge, underlying the control of genetic programs for DA neuron homeostasis under health and disease states. In the healthy brain without injury or neurotoxic exposure, in vivo active canonical Wnt/β-catenin signaling may patrol the more vulnerable CNS regions including the SVZ neurogenic niche and the more susceptible neuronal populations, such as the nigral DA neurons, to limit ongoing inflammatory responses and protect the quiescent neuroprogenitors and post-mitotic neurons from harmful attacks. In contrast, when environmental stimuli (i.e. aging, neurotoxin, viral or inflammatory exposure, brain injury) interact with genetic susceptibility factors to overcome a certain threshold, the homeostatic Wnt/neuroinflammatory response may fail to increase the vulnerability to degeneration, reduce self-repairability, and limit neurogenic potential, as observed in PD.
The Wnt pathway it self is protective in different tissues; however, the specific receptor–ligand binding, in the absence or presence of endogenous inhibitors/activators, within a particular inflammatory context, may mediate various tissue-/cell-specific responses of canonical Wnt signaling, with implications for the connection between Wnts and neurogenesis/neuroprotection and use of Wnt agonists or antagonists (Choi et al., 2012; Shruster et al., 2012).
Pharmacological manipulation of microglial oxidative and nitrosative status, either in vitro or in vivo, induces a successful neurogenesis rescue. The inflammation-dependent SVZ modulation in consistent with DA neuroprotection in MPTP-lesioned mice implicates a therapeutical window of opportunity for anti-inflammatory drug strategies. The inter-relationships among inflammatory, survival, and Wnt/β-catenin signaling cascades uncover a complete regulatory loop impacting in both SVZ plasticity and neuronal outcome in PD. Thus, harnessing inflammatory responses through targeted modulation of neuroimmune components and Wnt/β-catenin signaling for DA neuroprotection and repair have therapeutic potential for PD.
Supplementary Material
Acknowledgments
Funding
This work was supported by the Italian Ministry of Health (GR08-7), the Italian Ministry of Research, the Italian Multiple Sclerosis Association (AISM, grant 2010/R/31), Banca Agricola Popolare di Ragusa (BAPR), the European Research Council (ERC) under the ERC-2010-StG Grant agreement no. 260511-SEM_SEM, the International Foundation for Research in Paraplegia (IRP, RG 69318), the European Community (EC) 7th Framework Program (FP7/2007-2013) under Grant Agreement no. 280772-iONE, the OASI Institute for Research and Care on Mental Retardation and Brain Aging (IRCCS), Troina (EN), Italy, and the Evelyn Trust (RG 69865).
Footnotes
Conflict of interest: none declared.
References
- Aberle H, Bauer A, Stappert J, et al. β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Adachi K, Mirzadeh Z, Sakaguchi M, et al. β-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Aloisi F. The role of microglia and astrocytes in CNS immune surveillance and immunopathology. Adv. Exp. Med. Biol. 1999;468:123–133. doi: 10.1007/978-1-4615-4685-6_10. [DOI] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Baba Y, Kuroiwa A, Uitti R, et al. Alterations of T-lymphocyte populations in Parkinson’s disease. Parkinsonism Relat. Disord. 2005;11:493–498. doi: 10.1016/j.parkreldis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Magistretti PJ. The role of astroglia in neuroprotection. Dialogues Clin. Neurosci. 2009;11:281–295. doi: 10.31887/DCNS.2009.11.3/mbelanger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DC, Harvey K. LRRK2 functions as a Wnt signaling scaffold, bridging cytosolic proteins and membrane-localized LRP6. Hum. Mol. Genet. 2012a;21:4966–4979. doi: 10.1093/hmg/dds342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DC, Harvey K. The importance of Wnt signalling for neurodegeneration in Parkinson’s disease. Biochem. Soc. Trans. 2012b;40:1123–1128. doi: 10.1042/BST20120122. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Beurel E. Regulation by glycogen synthasekinase-3 of inflammation and T cells in CNS diseases. Front. Mol. Neurosci. 2011;4:18. doi: 10.3389/fnmol.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3(GSK3) Trends Immunol. 2010;31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog. Neurobiol. 1998;55:93–116. doi: 10.1016/s0301-0082(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Biscaro B, Lindvall O, Tesco G, et al. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer’s disease. Neurodegener. Dis. 2012;9:187–198. doi: 10.1159/000330363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal A, Ehlers S, Lauber J, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- Borta A, Hoglinger GU. Dopamine and adult neurogenesis. J. Neurochem. 2007;100:587–595. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, et al. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadière B, Prigent A, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. Gene-environment interactions in Parkinson’s disease: specific evidence in humans and mammalian models. Neurobiol. Dis. 2013;57:38–46. doi: 10.1016/j.nbd.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco G, Sousa KM, Bryja V, et al. Ventral midbrain glia express region-specific transcription factors and regulate dopaminergic neurogenesis through Wnt-5a secretion. Mol. Cell. Neurosci. 2006;31:251–262. doi: 10.1016/j.mcn.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Chairoungdua A, Smith L, Pochard P, et al. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussabel D, Semnani RT, McDowell MA, et al. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–681. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- Chavarría A, Cárdenas G. Neuronal influence behind the central nervous system regulation of the immune cells. Front. Integr. Neurosci. 2013;7:64. doi: 10.3389/fnint.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Herman MA, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch. Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- Chen G, Bower KA, Ma C, et al. Glycogen synthasekinase 3β (GSK3β) mediates 6-hydroxy dopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- Chen H, Jacobs EJ, Schwarzschild MA, et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann. Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- Chen H, O’Reilly EJ, Schwarzschild MA, et al. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am. J. Epidemiol. 2008;167:90–95. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, et al. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. Proc. Natl Acad. Sci. USA. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JI, Jee MK, Im YB, et al. Novel GSK-3β inhibitors and CBM-1078 guide hATSCs’ deaging via Oct4 and β-catenin activation. Antioxid. Redox Signal. 2012 doi: 10.1089/ars.2011.4422. doi: 10.1089/ars.2011.4422. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Cellular demise and inflammatory microglial activation during β-amyloid toxicity are governed by Wnt1 and canonical signalling pathways. Cell Signal. 2007;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Lipton J, Daley BF, et al. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol. Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B, Fox SH. Treatment of cognitive, psychiatric, and affective disorders associated with Parkinson’s disease. Neurotherapeutics. 2014 doi: 10.1007/s13311-013-0238-x. doi: 10.1007/s13311-013-0238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusimano M, Biziato D, Brambilla E, et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Gao K, Jankovic J. The VPS35 gene and Parkinson’s disease. Mov. Disord. 2013;28:569–575. doi: 10.1002/mds.25430. [DOI] [PubMed] [Google Scholar]
- Desplats P, Patel P, Kosberg K, et al. Combined exposure to Maneb and Paraquat alters transcriptional regulation of neurogenesis-related genes in mice models of Parkinson’s disease. Mol. Neurodegener. 2012;7:49. doi: 10.1186/1750-1326-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson’s disease. Neurotoxicology. 2002;23:487–502. doi: 10.1016/s0161-813x(02)00099-2. [DOI] [PubMed] [Google Scholar]
- Dringen J, Gutterer M, Hirrlinger J. Glutathione metabolism in brain. Metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- Duka T, Duka V, Joyce JN, et al. α-synuclein contributes to GSK-3β-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009;23:2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun Y, Li G, Yang Y, et al. Inhibition of the canonical Wnt pathway by Dickkopf-1 contributes to the neurodegeneration in 6-OHDA-lesioned rats. Neurosci. Lett. 2012;525:83–88. doi: 10.1016/j.neulet.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Wang LP, Klempin F, et al. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011;345:69–86. doi: 10.1007/s00441-011-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT. Microglial activation: tuning and pruning adult neurogenesis. Front. Pharmacol. 2012;3:41. doi: 10.3389/fphar.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Esteve P, Sandonìs A, Ibañez C, et al. Secreted frizzled-related proteins are required for Wnt/β-catenin signalling activation in the vertebrate optic cup. Development. 2011;138:4179–4184. doi: 10.1242/dev.065839. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, et al. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J. Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlieb N, François C, Tandé D, et al. Dopaminergic subtantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J. Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Gene-environment interactions: key to unraveling the mystery of Parkinson’s disease. Prog. Neurobiol. 2011;94:1–19. doi: 10.1016/j.pneurobio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhang F, Zhou H, et al. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ. Health Perspect. 2011;119:807–814. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Nalls MA, Shi M, et al. An exploratory analysis on gene-environment interactions for Parkinson disease. Neurobiol. Aging. 2012;33:2528.e1–2528.e6. doi: 10.1016/j.neurobiolaging.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennuso F, Fernetti C, Tirolo C, et al. Bilirubin protects astrocytes from its own toxicity inducing up-regulation and translocation of multidrug resistance-associated protein 1 (Mrp 1) Proc. Natl Acad. Sci. USA. 2004;101:2470–2475. doi: 10.1073/pnas.0308452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh MC, Collins GD, Vandanmagsar B, et al. Activation of Wnt5A signaling is required for CXC chemokine ligand 12-mediated T-cell migration. Blood. 2009;114:1366–1373. doi: 10.1182/blood-2008-08-175869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillardon F, Schmid R, Draheim H. Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience. 2012;208:41–48. doi: 10.1016/j.neuroscience.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Goldman SM. Environmental toxins and Parkinson’s disease. Annu. Rev. Pharmacol. Toxicol. 2014;54:141–164. doi: 10.1146/annurev-pharmtox-011613-135937. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Dionne MS, Schneider DS, et al. WntD is a feedback inhibitor of Dorsal/NF-κB in Drosophila development and immunity. Nature. 2005;437:746–749. doi: 10.1038/nature04073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JA, Fray M, de Haseth S, et al. Suppressive regulatory T-cell activity is potentiated by glycogen synthase kinase 3β inhibition. J. Biol. Chem. 2010;285:32852–32859. doi: 10.1074/jbc.M110.150904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleskog C, Schulte G. WNT-3A and WNT-5A counteract lipopolysaccharide-induced pro-inflammatory changes in mouse primary microglia. J. Neurochem. 2013;125:803–808. doi: 10.1111/jnc.12250. [DOI] [PubMed] [Google Scholar]
- Halleskog C, Mulder J, Dahlström J, et al. WNT signaling in activated microglia is proinflammatory. Glia. 2011;59:119–131. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Cao S, Rowse AL, et al. MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J. Neurosci. 2013;33:9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison-Uy SJ, Pleasure SJ. Wnt signaling and forebrain development. Cold Spring Harb. Perspect. Biol. 2012;4:a008094. doi: 10.1101/cshperspect.a008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, et al. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Jenner P, Przedborski S. Pathogenesis of Parkinson’s disease. Mov. Disord. 2013;28:24–30. doi: 10.1002/mds.25032. [DOI] [PubMed] [Google Scholar]
- Ho A, Blum M. Induction of interleukin-1 associated with compensatory dopaminergic sprouting in the denervated striatum of young mice: model of aging and neurodegenerative disease. J. Neurosci. 1998;18:5614–5629. doi: 10.1523/JNEUROSCI.18-15-05614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C, Sainz-Fuertes R, Lynham S, et al. Wnt3a induces exosome secretion from primary cultured rat microglia. BMC Neurosci. 2012;13:144. doi: 10.1186/1471-2202-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Parkinson’s disease and the adaptive capacity of the nigrostriatal dopamine system: possible neurochemical mechanisms. Adv. Neurol. 1993;60:140–147. [PubMed] [Google Scholar]
- Hu X, Zhang D, Pang H, et al. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J. Immunol. 2008;181:7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging role of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, et al. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem. Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidery NA, Banerjee R, Yang L, et al. Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson’s disease. Antioxid. Redox Signal. 2013;18:139–157. doi: 10.1089/ars.2011.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel F. Paths from pesticides to Parkinson’s. Science. 2013;341:722–723. doi: 10.1126/science.1243619. [DOI] [PubMed] [Google Scholar]
- Kilander MB, Halleskog C, Schulte G. Recombinant WNTs differentially activate β-catenin-dependent and -independent signalling in mouse microglia-like cells. Acta Physiol. 2011;203:363–372. doi: 10.1111/j.1748-1716.2011.02324.x. [DOI] [PubMed] [Google Scholar]
- Kim WY, Snider WD. Functions of GSK-3 signaling in development of the nervous system. Front. Mol. Neurosci. 2011;4:44. doi: 10.3389/fnmol.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Won S, Hwang DY, et al. Downregulation of Wnt/β-catenin signaling causes degeneration of hippocampal neurons in vivo. Neurobiol. Aging. 2011;32:2316.e1–2316.e15. doi: 10.1016/j.neurobiolaging.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Kim B, Yang MS, Choi D, et al. Impaired inflammatory responses in murine Lrrk2-knockdown brain microglia. PLoS One. 2012;7:e34693. doi: 10.1371/journal.pone.0034693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MK, Pardo M, Cheng Y, et al. Glycogen synthase kinase-3 inhibitors: rescuers of cognitive impairments. Pharmacol. Ther. 2014;141:1–12. doi: 10.1016/j.pharmthera.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia Z, Martino G, Schwartz M, et al. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat. Neurosci. 2012;15:1078–1087. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Reske-Nielsen C, Mithal P, et al. Neuroinflammation mediated by IL-1β increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J. Neuroinflammation. 2008;5:8. doi: 10.1186/1742-2094-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers E, Willems MG, Collins JJ, et al. Altered canonical Wingless-Int (Wnt) signaling in the ovine fetal lung after exposure to intra-amniotic lipopolysaccharide (LPS) and antenatal betamethasone. Pediatr. Res. 2013 doi: 10.1038/pr.2013.226. doi: 10.1038/pr.2013.226. [DOI] [PubMed] [Google Scholar]
- Kwok JB, Hallupp M, Loy CT, et al. GSK3β polymorphism alter transcription and splicing in Parkinson’s disease. Ann. Neurol. 2005;58:829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann. Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, et al. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Ulusoy A, Innamorato NG, et al. α-synuclein expression and Nrf2-deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson’s disease. Hum. Mol. Genet. 2012;21:3173–3192. doi: 10.1093/hmg/dds143. [DOI] [PubMed] [Google Scholar]
- Lee M, Schwab C, Yu S, et al. Astrocytes produce the anti-inflammatory and neuroprotective agent hydrogen sulphide. Neurobiol. Aging. 2009;30:1523–1534. doi: 10.1016/j.neurobiolaging.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Lema Tomé CM, Tyson T, Rey LN, et al. Inflammation and α-synuclein’s prion-like behavior in Parkinson’s disease--is there a link? Mol. Neurobiol. 2013;47:561–574. doi: 10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, et al. Glia as a turning point in the therapeutic strategy of Parkinson’s disease. CNS Neurol. Disord. Drug Targets. 2010a;9:349–372. doi: 10.2174/187152710791292639. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Caniglia S, et al. Combining nitric oxide release with anti-inflammatory activity preserves nigrostriatal dopaminergic innervation and prevents motor impairment in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J. Neuroinflammation. 2010b;7:83. doi: 10.1186/1742-2094-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, et al. Reactive astrocytes and Wnt/β-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiol. Dis. 2011a;41:508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Serapide MF, Tirolo C, et al. A Wnt1 regulated Frizzled-1/β-catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: therapeutical relevance for neuron survival and neuroprotection. Mol. Neurodegener. 2011b;6:49. doi: 10.1186/1750-1326-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, et al. Switching the microglial harmful phenotype promotes lifelong restoration of subtantia nigra dopaminergic neurons from inflammatory neurodegeneration in aged mice. Rejuvenation Res. 2011c;14:411–424. doi: 10.1089/rej.2010.1134. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, et al. Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease involves crosstalk between inflammatory and Wnt/β-catenin signaling pathways: functional consequences for neuroprotection and repair. J. Neurosci. 2012;32:2062–2085. doi: 10.1523/JNEUROSCI.5259-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, et al. Aging-induced Nrf2-ARE pathway disruption in the subventricularzone (SVZ) drives neurogenic impairment in parkinsonian mice via PI3K-Wnt/β-catenin dysregulation. J. Neurosci. 2013;33:1462–1485. doi: 10.1523/JNEUROSCI.3206-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Wang LL, Liu SJ, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing β-catenin, a mechanism involved in Alzheimer’s degeneration. Proc. Natl Acad. Sci. USA. 2007;104:3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HG, et al. Wnt signaling regulates adult hippocampal neurogenesis. Nature. 2005;473:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lin SL, Li B, Rao S, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl Acad. Sci. USA. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Kuo HC, Wang JS, et al. Regulation of inflammatory response by 3-methyladenine involves the coordinative actions on Akt and glycogen synthase kinase 3β rather than autophagy. J. Immunol. 2012;189:4154–4164. doi: 10.4049/jimmunol.1102739. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti B, Abbracchio MP. To be or not to be (inflamed) is that the question in anti-inflammatory drug therapy of neurodegenerative diseases? Trends Pharmacol. Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Pluchino S. Wnt your brain be inflamed? Yes, it Wnt! Trends Mol. Med. 2013;19:144–156. doi: 10.1016/j.molmed.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti B, Serra PA, L’Episcopo F, et al. Hormones are key actors in gene x environment interactions programming the vulnerability to Parkinson’s disease: glia as a common final pathway. Ann. NY Acad. Sci. 2005a;1057:296–318. doi: 10.1196/annals.1356.023. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Serra PA, Tirolo C, et al. Glucocorticoid receptor-nitric oxide crosstalk and vulnerability to experimental Parkinsonism: pivotal role for glia-neuron interactions. Brain Res. Rev. 2005b;48:302–321. doi: 10.1016/j.brainresrev.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Marchetti B, L’Episcopo F, Tirolo C, et al. Vulnerability to Parkinson’s disease: towards an unifying theory of disease etiology. In: Nriagu JO, editor. Encyclopedia of Environmental Health. Elsevier BV; Amsterdam: 2011. pp. 690–704. [Google Scholar]
- Marchetti B, L’Episcopo F, Morale MC, et al. Uncovering novel actors in astrocyte-neuron crosstalk in Parkinson’s disease: the Wnt/β-catenin signaling cascade as the common final pathway for neuroprotection and self-repair. Eur. J. Neurosci. 2013;37:1550–1563. doi: 10.1111/ejn.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G, Pluchino S, Bonfanti L, et al. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol. Rev. 2011;91:1281–1304. doi: 10.1152/physrev.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov. Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, et al. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Miranda CJ, Braun L, Jiang Y, et al. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging Cell. 2012;11:542–552. doi: 10.1111/j.1474-9726.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morale MC, Serra P, Delogu MR, et al. Glucocorticoid receptor deficiency increases vulnerability of the nigrostriatal dopaminergic system: critical role of glial nitric oxide. FASEB J. 2004;18:164–166. doi: 10.1096/fj.03-0501fje. [DOI] [PubMed] [Google Scholar]
- Morale MC, Serra PA, L’Episcopo F, et al. Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience. 2006;138:869–878. doi: 10.1016/j.neuroscience.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Morale MC, L’Episcopo F, Tirolo C, et al. Loss of aromatase cytochrome P450 function as a risk factor for Parkinson’s disease? Brain Res. Rev. 2008;57:431–443. doi: 10.1016/j.brainresrev.2007.10.011. [DOI] [PubMed] [Google Scholar]
- More SV, Kumar H, Kim IS, et al. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators Inflamm. 2013;2013:952375. doi: 10.1155/2013/952375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Schaale K, Farhat K, et al. Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. FASEB J. 2010;24:4599–4612. doi: 10.1096/fj.10-160994. [DOI] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, et al. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol. Aging. 2012;33:195.e1–195.e12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oderup C, LaJevic M, Butcher EC. Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. J. Immunol. 2013;190:6126–6134. doi: 10.4049/jimmunol.1203002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Inoue K, Iwamura H, et al. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J. 2011;25:3570–3582. doi: 10.1096/fj.11-184697. [DOI] [PubMed] [Google Scholar]
- O’Keeffe GO, Barker RA, Caldwell MA. Dopaminergic modulation of neurogenesis in the subventricular zone of the adult brain. Cell Cycle. 2009;8:18. doi: 10.4161/cc.8.18.9512. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Schapira A. Therapeutic prospects for Parkinson’s disease. Ann. Neurol. 2013;74:337–347. doi: 10.1002/ana.24011. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, et al. Wnt signalling promotes regeneration in the retina of adult mammals. J. Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr. Opin. Neurobiol. 2000;10:392–399. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira CP, Bachli EB, Schoedon G. The wnt pathway: a macrophage effector molecule that triggers inflammation. Curr. Atheroscler. Rep. 2009;11:236–242. doi: 10.1007/s11883-009-0036-4. [DOI] [PubMed] [Google Scholar]
- Petit-Paitel A, Brau F, Cazareth J, et al. Involvement of cytosolic and mitochondrial GSK-3β in mitochondrial dysfunction and neuronalcell death of MPTP/MPP-treated neurons. PLoS One. 2009;4:e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phukan S, Babu VS, Kannoji A, et al. GSK3β: role in therapeutic landscape and development of modulators. Br. J. Pharmacol. 2010;160:1–19. doi: 10.1111/j.1476-5381.2010.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott Godoy MC, Tarelli R, Ferrari CC, et al. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain. 2008;131:1880–1894. doi: 10.1093/brain/awn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KM, Kay DM, Factor SA, et al. Combined effects of smoking, coffee, and NSAIDs on Parkinson’s disease risk. Mov. Disord. 2008;23:88–95. doi: 10.1002/mds.21782. [DOI] [PubMed] [Google Scholar]
- Prakash N, Wurst W. Genetic networks controlling the development of midbrain dopaminergic neurons. J. Physiol. 2006;575:403–410. doi: 10.1113/jphysiol.2006.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S. Inflammation and Parkinson’s disease pathogenesis. Mov. Disord. 2010;25:S55–S57. doi: 10.1002/mds.22638. [DOI] [PubMed] [Google Scholar]
- Purro SA, Dickins EM, Salinas PC. The secreted Wnt antagonist Dickkopf-1 is required for amyloid β-mediated synaptic loss. J. Neurosci. 2012;32:3492–3498. doi: 10.1523/JNEUROSCI.4562-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal N, Corti O, Sacchetti P, et al. Parkin protects dopaminergic neurons from excessive Wnt/β-catenin signalling. Biochem. Biophys. Res. Commun. 2009;388:473–478. doi: 10.1016/j.bbrc.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Innamorato NG, Martín-Moreno AM, et al. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia. 2010;58:588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- Rueger MA, Muesken S, Walberer M, et al. Effects of minocycline on endogenous neural stem cells after experimental stroke. Neuroscience. 2012;215:174–183. doi: 10.1016/j.neuroscience.2012.04.036. [DOI] [PubMed] [Google Scholar]
- Sakata H, Niizuma K, Yoshioka H, et al. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb. Perspect. Biol. 2012;4:a008003. doi: 10.1101/cshperspect.a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Barnum CJ, Tansey MG, et al. Neuroimmunological processes in Parkinson’s disease and their relation to α-synuclein: microglia as the referee between neuronal processes and peripheral immunity. ASN Neuro. 2013;5:113–139. doi: 10.1042/AN20120066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaale K, Neumann J, Schneider D, et al. Wnt signaling in macrophages: augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur. J. Cell Biol. 2011;90:553–559. doi: 10.1016/j.ejcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Seib DR, Corsini NS, Ellwanger K, et al. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 2013;12:204–214. doi: 10.1016/j.stem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Shruster A, Ben-Zur T, Melamed E, et al. Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PLoS One. 2012;7:e40843. doi: 10.1371/journal.pone.0040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songin M, Strosznajder JB, Fital M, et al. Glycogen synthase kinase 3β and its phosphorylated form (Y216) in the paraquat-induced model of parkinsonism. Neurotox. Res. 2011;19:162–171. doi: 10.1007/s12640-010-9153-7. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, et al. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. FASEB J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial activation and neuroinflammation in Alzheimer’s disease: a critical examination of recent history. Front. Aging Neurosci. 2010;2:22. doi: 10.3389/fnagi.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Li MH, et al. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch. Pharm. Res. 2009;32:1163–1176. doi: 10.1007/s12272-009-1807-8. [DOI] [PubMed] [Google Scholar]
- Takahashi-Yanaga F. Activator or inhibitor? GSK-3 as a new drug target. Biochem. Pharmacol. 2013;86:191–199. doi: 10.1016/j.bcp.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Main BS, Crack PJ. Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem. Int. 2013;62:803–819. doi: 10.1016/j.neuint.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Tepavcevic V, Lazarini F, Alfaro-Cervello C, et al. Inflammation-induced subventricular zone dysfunction leads to olfactory deficits in a targeted mouse model of multiple sclerosis. J. Clin. Invest. 2011;121:4722–4734. doi: 10.1172/JCI59145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia J, Hernández-López C, Martínez VG, et al. Wnt5askews dendritic cell differentiation to an unconventional phenotype with tolerogenic features. J. Immunol. 2011;187:4129–4139. doi: 10.4049/jimmunol.1101243. [DOI] [PubMed] [Google Scholar]
- van Amerongen R. Alternative Wnt pathways and receptors. Cold Spring Harb. Perspect. Biol. 2012;4:a007914. doi: 10.1101/cshperspect.a007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdregt J, Fleskens V, Tiemessen MM, et al. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity. 2013;39:298–310. doi: 10.1016/j.immuni.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Grabowski CP, Alfaro IE, et al. Role of the Wnt receptor Frizzled-1 in presynaptic differentiation and function. Neural Dev. 2009;4:41. doi: 10.1186/1749-8104-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenquist U, Holmqvist K, Hånell A, et al. Ibuprofen attenuates the inflammatory response and allows formation of migratory neuroblasts from grafted stem cells after traumatic brain injury. Restor. Neurol. Neurosci. 2012;30:9–19. doi: 10.3233/RNN-2011-0606. [DOI] [PubMed] [Google Scholar]
- Wang X, Adhikari N, Li Q, et al. The role of β-transducin-repeat-containing protein (β-Trcp) in the regulation of NF-κB in vascular smooth muscle cells. Atherioscler. Thromb. Vasc. Biol. 2004;24:85–90. doi: 10.1161/01.ATV.0000104012.40720.c4. [DOI] [PubMed] [Google Scholar]
- Wang W, Yang Y, Ying C, et al. Inhibition of glycogensynthase kinase-3β protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52:1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Zhang S, Yan ZQ, et al. Impaired CD200-CD200R-mediated microglia silencing enhances midbrain dopaminergic neurodegeneration: roles of aging, superoxide, NADPH oxidase, and p38 MAPK. Free Radic. Biol. Med. 2011;50:1094–1106. doi: 10.1016/j.freeradbiomed.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Warner TT, Schapira AH. Genetic and environmental factors in the cause of Parkinson’s disease. Ann. Neurol. 2003;53(Suppl 3):S16–S23. doi: 10.1002/ana.10487. [DOI] [PubMed] [Google Scholar]
- Wei L, Sun C, Lei M, et al. Activation of Wnt/β-catenin pathway by exogenous Wnt1 protects SH-SY5Y cells against 6-hydroxydopamine toxicity. J. Mol. Neurosci. 2013;49:105–115. doi: 10.1007/s12031-012-9900-8. [DOI] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br. J. Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton PS. Neuroinflammation and the prospects for anti-inflammatory treatment of Parkinson’s disease. Curr. Opin. Investig. Drugs. 2010;11:788–794. [PubMed] [Google Scholar]
- Willert K, Nusse R. Wnt proteins. Cold Spring Harb. Perspect. Biol. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur. J. Neurosci. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- Yang C, Iyer RR, Yu AC, et al. β-catenin signaling initiates the activation of astrocytes and its dysregulation contributes to the pathogenesis of astrocytomas. Proc. Natl Acad. Sci. USA. 2012;109:6963–6968. doi: 10.1073/pnas.1118754109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, et al. Aggregated α-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang XJ, Tian LP, et al. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J. Neuroinflammation. 2011a;8:154. doi: 10.1186/1742-2094-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang X, Yang S, et al. The Wnt/β-catenin signaling pathway in the adult neurogenesis. Eur. J. Neurosci. 2011b;33:1–8. doi: 10.1111/j.1460-9568.2010.7483.x. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Cameron JL, Hoffer BJ, et al. Neurorestorationby physical exercise: moving forward. Parkinsonism Relat. Disord. 2012;18:S147–S150. doi: 10.1016/S1353-8020(11)70046-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.