Abstract
Ca2+-triggered neurotransmitter release is characterized by two kinetically distinct components: a fast synchronous phase and a slow asynchronous phase. Yao et al. (2011) now report that double C2 domain (Doc2) proteins function as high-affinity Ca2+ sensors to specifically regulate the asynchronous component of neurotransmitter release.
Chemical synaptic transmission in the nervous system results from the fusion of synaptic vesicles with the presynaptic plasma membrane, which causes release of the neurotransmitter stored within. Vesicle fusion can either be spontaneous or driven by action potentials. The latter, known as evoked release, is the primary means of neuronal communication. When an action potential invades presynaptic terminals, the elevated intracellular Ca2+ entering through voltage-gated calcium channels triggers what is known as synchronous neurotransmitter release, which is then followed by a phase of vesicle fusion known as asynchronous release. Although spontaneous release occurs in the absence of action potentials, it also depends, in part, on Ca2+. How does the release apparatus detect the Ca2+ signal and translate it into vesicle fusion? What are the Ca2+ sensors for the different modes of release? In this issue, Yao et al. (2011) examine the function of double C2 domain (Doc2) proteins in vesicle fusion both in vitro and in vivo and provide evidence that Doc2 acts as a high-affinity Ca2+ sensor specifically for asynchronous release.
Ca2+-evoked synchronous release and asynchronous release exhibit different properties. Synchronous release only occurs during and immediately following an action potential, whereas asynchronous release occurs over a longer period of time following the termination of an action potential. The distinct properties of synchronous and asynchronous release most likely reflect the existence of at least two different Ca2+ sensors that couple the Ca2+ signals to the vesicle fusion machinery with different kinetics and Ca2+ sensitivities (Goda and Stevens, 1994). A fast and low-affinity sensor triggers synchronous release in response to the localized, high concentration of Ca2+ that only briefly exists around the voltage-gated calcium channels during the action potential. This so-called Ca2+ microdomain, or nanodomains, quickly collapses due to diffusion and Ca2+ buffering after the calcium channels close, resulting in a much lower concentration of Ca2+. A slow and perhaps high-affinity sensor continues to trigger asynchronous release in response to this residual Ca2+ signal. For the past two decades, numerous studies have demonstrated that synaptotagmin I is a Ca2+ sensor for synchronous release, functioning through its Ca2+-dependent interaction with phospholipid membranes and soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) (Figure 1) (Rizo and Rosenmund, 2008). Other members of the synaptotagmin family have naturally become the prime candidates for the Ca2+ sensors of asynchronous release, but so far, no evidence supports this hypothesis.
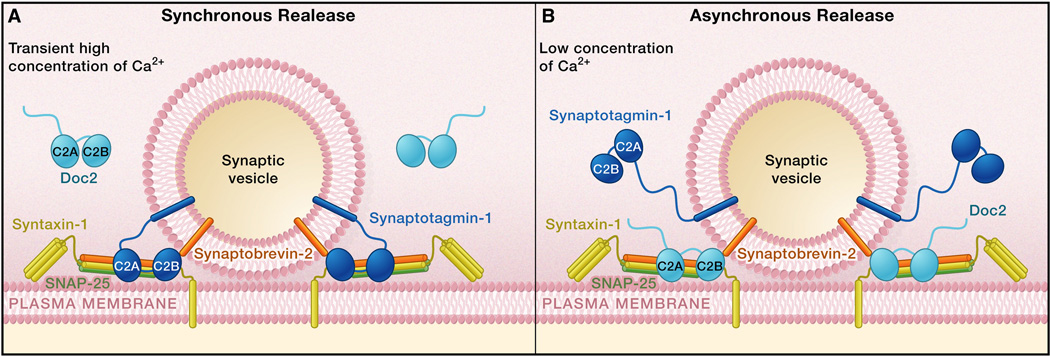
Figure 1.
Ca2+ Sensors Synaptotagmin I and Doc2 Trigger Evoked Neurotransmitter Release
(A) Action potentials open voltage-gated calcium channels, resulting in a brief and high-concentration influx of Ca2+. The C2A and C2B domains of synaptotagmin I bind to Ca2+ and interact with phospholipid membranes and SNAREs to trigger synchronous neurotransmitter release.
(B) Following the termination of action potentials, intracellular Ca2+ concentration decays to a much lower level. Doc2 binds to this residual Ca2+ and triggers asynchronous release, possibly through a mechanism similar to that of synaptotagmin I.
Among a large number of Ca2+-binding proteins is the Doc2 family (Doc2α, Doc2β, and Doc2γ), which contains two C2 domains (C2A and C2B) that are similar to those found in synaptotagmin I. The Doc2 proteins interact with phospholipids and some synaptic proteins involved in vesicle fusion, such as SNAREs, Munc18, and Munc13 (Friedrich et al., 2010). A recent study shows that Doc2 can stimulate SNARE-mediated fusion of reconstituted liposomes in a Ca2+-dependent manner and that Doc2α/β double-knockout mice exhibit reduce spontaneous release (Groffen et al., 2010). When two Ca2+ ligands (aspartic acid residues) in the C2A domain are substituted with asparagines to resemble a dominant-active Ca2+-bound state, this mutant Doc2 concurrently enhances spontaneous release. These results led to the proposal that Doc2 is a high-affinity Ca2+ sensor for spontaneous release (Groffen et al., 2010). Yao et al. (2011) now confirm the ability of Doc2 to stimulate SNARE-mediated membrane fusion. They further show that Doc2 binds to and dissociates from Ca2+ and phospholipid membranes more slowly than synaptotagmin I. Thus, the biochemical properties of Doc2 make it a good candidate for the Ca2+ sensor of asynchronous release.
Yao et al. test the in vivo function of Doc2 in neurotransmitter release by suppressing Doc2α expression using a short hairpin RNA (shRNA) in cultured mouse hippocampal excitatory neurons. To specifically assess the impact on asynchronous release, they take advantage of knockout neurons that lack synaptotagmin I, which display a specific deficit in synchronous release (Geppert et al., 1994), and find that suppressing Doc2α expression reduces asynchronous release. In wild-type neurons, knockdown of Doc2α selectively decreases asynchronous release without affecting synchronous release. This result is also confirmed in the Doc2α knockout neurons. Conversely, overexpression of wild-type Doc2 proteins in neurons lacking synaptotagmin I and in wild-type neurons causes a specific increase in asynchronous release. Yao et al. also mutated two residues involved in Ca2+ binding in each C2 domain to asparagines, and overexpression of this gain-of-function mutant in neurons lacking synaptotagmin I enhances asynchronous release more than overexpression of the wild-type protein. Thus, Doc2 levels bidirectionally regulate Ca2+-evoked asynchronous release (Figure 1).
Is Doc2 a bona fide Ca2+ sensor for spontaneous release, evoked asynchronous release, or both? If so, loss-of-function mutations of the Ca2+-binding residues in the Doc2 C2 domains should abolish its Ca2+ sensor function for spontaneous and asynchronous release. A recent study tested this prediction by mutating three residues that are important for Ca2+ binding in each C2 domain to alanines to abolish Ca2+ binding. Unexpectedly, this mutant Doc2 fully rescues the decrease in spontaneous release induced by shRNA knockdown of Doc2 proteins in cultured mouse cortical inhibitory neurons, challenging the legitimacy of Doc2 as the Ca2+ sensor for spontaneous release (Pang et al., 2011). What is the effect of this mutant Doc2 in evoked asynchronous release? Unfortunately, Yao et al. do not report this. They also do not report how knockdown and overexpression of Doc2 affect the Ca2+ sensitivity of asynchronous release, another important parameter for assessing the Ca2+ sensor function.
Groffen et al., 2010 and Pang et al., 2011, and Yao et al. (2011) all agree that Doc2 does not affect evoked synchronous release. Groffen et al. (2010) and Pang et al. (2011), however, claim that Doc2 is not involved in asynchronous release either. What could account for this discrepancy? The three studies were based on different experimental approaches. Yao et al. (2011) use both knockdown and knockout approaches, and the results are consistent with each other, providing substantial strength to the data. In Pang et al. (2011), the knockdown efficiency is measured from the entire neuronal culture, but inhibitory neurons constitute only a small fraction of the neuronal population. Hence, it is not obvious if the Doc2 proteins were sufficiently suppressed in inhibitory neurons. Although spontaneous release is reduced in these neurons, asynchronous release may have a different sensitivity to Doc2 reduction. For example, shRNA-mediated knockdown of complexins affects excitatory neurons, but not inhibitory neurons (Maximov et al., 2009), whereas a full genetic knockout has the same effects on both neuronal types (Xue et al., 2008). Alternatively, other Ca2+ sensors may compensate for the loss of Doc2 proteins in cortical neurons assayed by Pang et al. (2011). It is also possible that Doc2 is the Ca2+ sensor for asynchronous release in excitatory neurons, but not inhibitory neurons. Finally, it is not obvious why Groffen et al. (2010) did not observe a defect in asynchronous release.
The study by Yao et al. is important because it provides a promising candidate for the Ca2+ sensor of asynchronous release in many cell types and raises interesting questions about their mechanism of action. It also raises an issue with respect to their counterparts in invertebrates, given that Doc2 proteins are not evolutionarily conserved in many species (Craxton, 2010). Future work may test whether rabphilin, a conserved C2 domain-containing protein that shares a high degree of homology with Doc2 proteins, subserves this role in invertebrates.
References
- 1.Craxton M. BMC Genomics. 2010;11:37. doi: 10.1186/1471-2164-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedrich R, Yeheskel A, Ashery U. Mol. Neurobiol. 2010;41:42–51. doi: 10.1007/s12035-009-8094-8. [DOI] [PubMed] [Google Scholar]
- 3.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 4.Goda Y, Stevens CF. Proc. Natl. Acad. Sci. USA. 1994;91:12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groffen AJ, Martens S, Díez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Bors JG, et al. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang ZP, Bacaj T, Yang X, Zhou P, Xu W, Südhof TC. Neuron. 2011;70:244–251. doi: 10.1016/j.neuron.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizo J, Rosenmund C. Nat. Struct. Mol. Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao J, Gaffaney JD, Kwon SE, Chapman ER. Cell. 2011;147:666–677. doi: 10.1016/j.cell.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue M, Stradomska A, Chen H, Brose N, Zhang W, Rosenmund C, Reim K. Proc. Natl. Acad. Sci. USA. 2008;105:7875–7880. doi: 10.1073/pnas.0803012105. [DOI] [PMC free article] [PubMed] [Google Scholar]