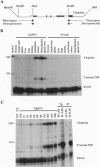
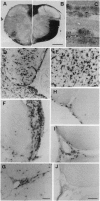
Abstract
Cytokines are now recognized to play important roles in the physiology of the central nervous system (CNS) during health and disease. Tumor necrosis factor alpha (TNF-alpha) has been implicated in the pathogenesis of several human CNS disorders including multiple sclerosis, AIDS dementia, and cerebral malaria. We have generated transgenic mice that constitutively express a murine TNF-alpha transgene, under the control of its own promoter, specifically in their CNS and that spontaneously develop a chronic inflammatory demyelinating disease with 100% penetrance from around 3-8 weeks of age. High-level expression of the transgene was seen in neurons distributed throughout the brain. Disease is manifested by ataxia, seizures, and paresis and leads to early death. Histopathological analysis revealed infiltration of the meninges and CNS parenchyma by CD4+ and CD8+ T lymphocytes, widespread reactive astrocytosis and microgliosis, and focal demyelination. The direct action of TNF-alpha in the pathogenesis of this disease was confirmed by peripheral administration of a neutralizing anti-murine TNF-alpha antibody. This treatment completely prevented the development of neurological symptoms, T-cell infiltration into the CNS parenchyma, astrocytosis, and demyelination, and greatly reduced the severity of reactive microgliosis. These results demonstrate that overexpression of TNF-alpha in the CNS can cause abnormalities in nervous system structure and function. The disease induced in TNF-alpha transgenic mice shows clinical and histopathological features characteristic of inflammatory demyelinating CNS disorders in humans, and these mice represent a relevant in vivo model for their further study.
Full text
PDF
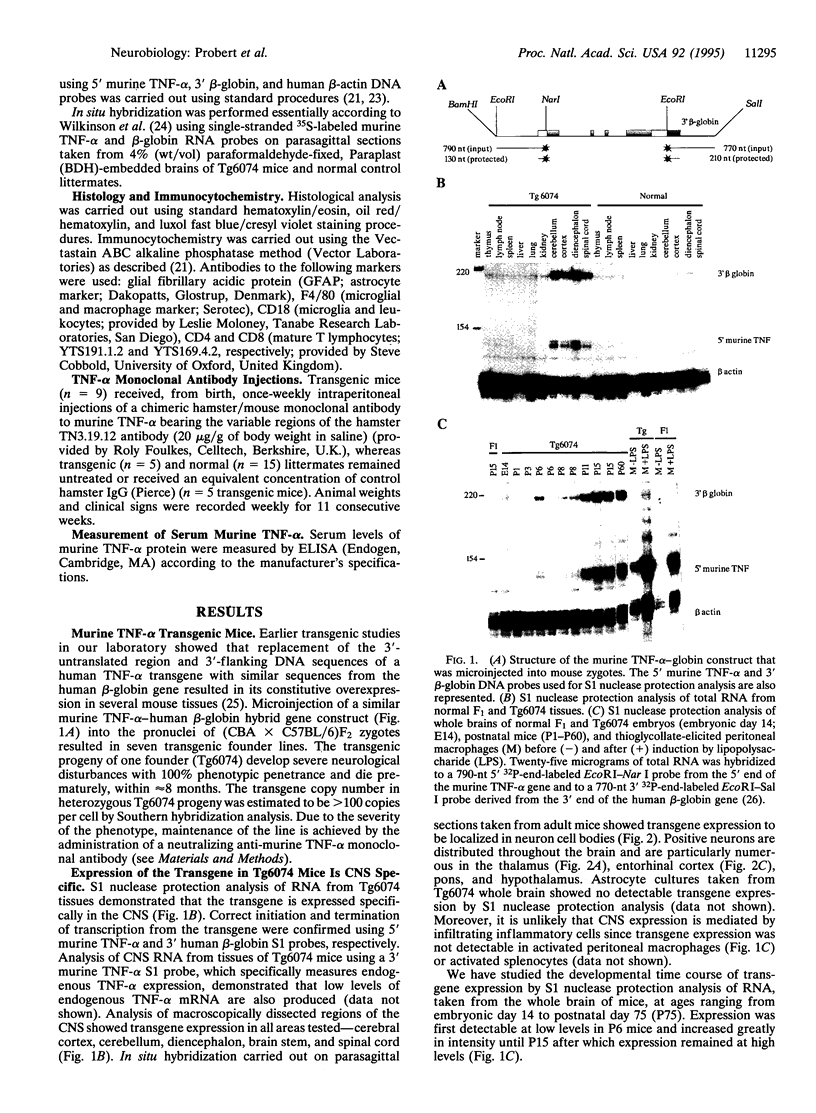
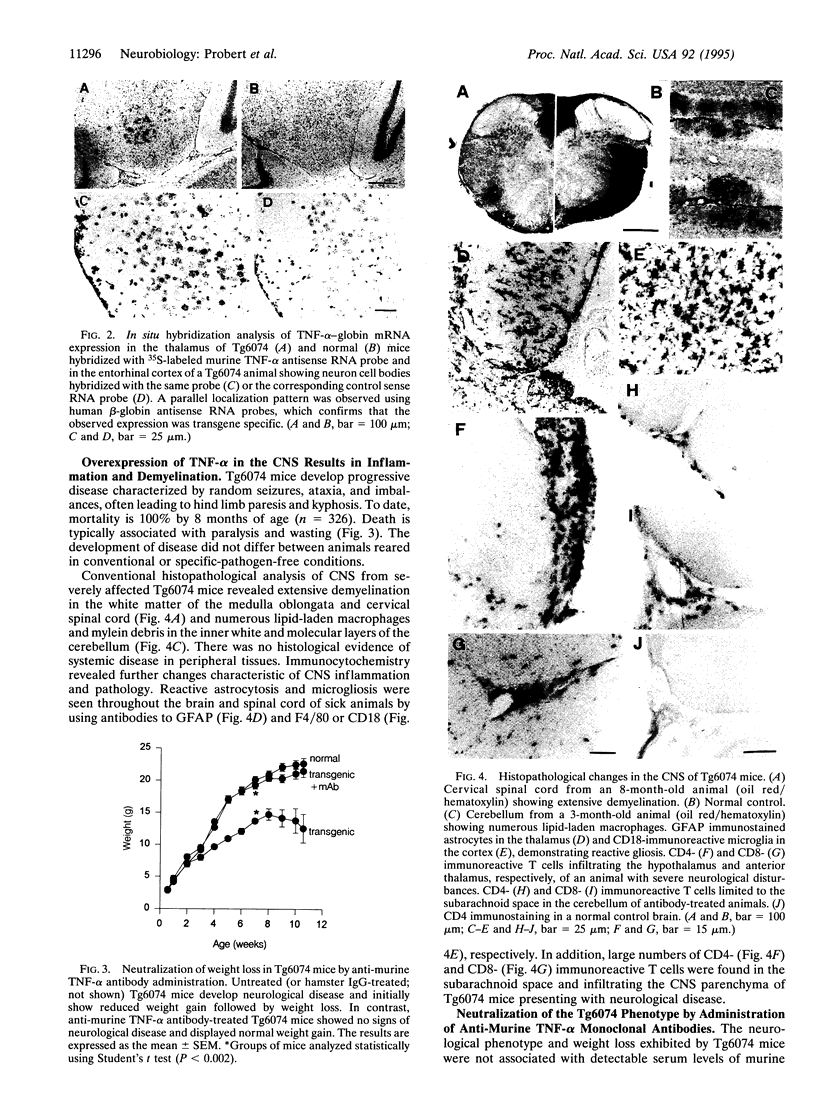


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archelos J. J., Jung S., Mäurer M., Schmied M., Lassmann H., Tamatani T., Miyasaka M., Toyka K. V., Hartung H. P. Inhibition of experimental autoimmune encephalomyelitis by an antibody to the intercellular adhesion molecule ICAM-1. Ann Neurol. 1993 Aug;34(2):145–154. doi: 10.1002/ana.410340209. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Baker D., Butler D., Scallon B. J., O'Neill J. K., Turk J. L., Feldmann M. Control of established experimental allergic encephalomyelitis by inhibition of tumor necrosis factor (TNF) activity within the central nervous system using monoclonal antibodies and TNF receptor-immunoglobulin fusion proteins. Eur J Immunol. 1994 Sep;24(9):2040–2048. doi: 10.1002/eji.1830240916. [DOI] [PubMed] [Google Scholar]
- Balasingam V., Tejada-Berges T., Wright E., Bouckova R., Yong V. W. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci. 1994 Feb;14(2):846–856. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breder C. D., Tsujimoto M., Terano Y., Scott D. W., Saper C. B. Distribution and characterization of tumor necrosis factor-alpha-like immunoreactivity in the murine central nervous system. J Comp Neurol. 1993 Nov 22;337(4):543–567. doi: 10.1002/cne.903370403. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan C. F., Litwak M. S., Schroeder C. E., Selmaj K., Raine C. S., Arezzo J. C. Preliminary studies of cytokine-induced functional effects on the visual pathways in the rabbit. J Neuroimmunol. 1989 Dec;25(2-3):227–239. doi: 10.1016/0165-5728(89)90141-0. [DOI] [PubMed] [Google Scholar]
- Campbell I. L., Abraham C. R., Masliah E., Kemper P., Inglis J. D., Oldstone M. B., Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M., Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993 May;54(1):15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry Z., Raine C. S., Hart M. N. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today. 1994 May;15(5):218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- Frei K., Siepl C., Groscurth P., Bodmer S., Schwerdel C., Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987 Sep;17(9):1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Corpuz M. Brain glia release factors with opposing actions upon neuronal survival. J Neurosci. 1993 Jan;13(1):29–37. doi: 10.1523/JNEUROSCI.13-01-00029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Woodward J., Young D. G., Krebs J. F., Lachman L. B. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988 Jul;8(7):2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Vassalli P., Lambert P. H. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol Rev. 1989 Dec;112:49–70. doi: 10.1111/j.1600-065x.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Hinton D. R., Johnson K., Merrill J. E. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989 Aug 1;170(2):607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991 Dec;10(13):4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G., Hurst J., deBoer E., Grosveld F. The human beta-globin gene contains a downstream developmental specific enhancer. Nucleic Acids Res. 1987 Jul 24;15(14):5739–5747. doi: 10.1093/nar/15.14.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G., Wrighton N., Hurst J., Grosveld F. Regulated expression of human A gamma-, beta-, and hybrid gamma beta-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell. 1986 Jul 4;46(1):89–94. doi: 10.1016/0092-8674(86)90862-7. [DOI] [PubMed] [Google Scholar]
- Kuroda Y., Shimamoto Y. Human tumor necrosis factor-alpha augments experimental allergic encephalomyelitis in rats. J Neuroimmunol. 1991 Nov;34(2-3):159–164. doi: 10.1016/0165-5728(91)90125-q. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Frei K., Kam-Hansen S., Zinkernagel R. M., Fontana A. Tumor necrosis factor alpha in cerebrospinal fluid during bacterial, but not viral, meningitis. Evaluation in murine model infections and in patients. J Exp Med. 1988 May 1;167(5):1743–1748. doi: 10.1084/jem.167.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A. P., Pitha P. M., Shin H. S., Shin M. L. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J. E., Ignarro L. J., Sherman M. P., Melinek J., Lane T. E. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993 Aug 15;151(4):2132–2141. [PubMed] [Google Scholar]
- Mogi M., Harada M., Riederer P., Narabayashi H., Fujita K., Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994 Jan 3;165(1-2):208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Molleston M. C., Thomas M. L., Hickey W. F. Novel major histocompatibility complex expression by microglia and site-specific experimental allergic encephalomyelitis lesions in the rat central nervous system after optic nerve transection. Adv Neurol. 1993;59:337–348. [PubMed] [Google Scholar]
- Mucke L., Eddleston M. Astrocytes in infectious and immune-mediated diseases of the central nervous system. FASEB J. 1993 Oct;7(13):1226–1232. doi: 10.1096/fasebj.7.13.8405808. [DOI] [PubMed] [Google Scholar]
- Peunova N., Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature. 1995 May 4;375(6526):68–73. doi: 10.1038/375068a0. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C. R., Oomura Y., Kai Y. Tumor necrosis factor and interleukin-1 beta: suppression of food intake by direct action in the central nervous system. Brain Res. 1988 May 10;448(1):106–114. doi: 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- Probert L., Keffer J., Corbella P., Cazlaris H., Patsavoudi E., Stephens S., Kaslaris E., Kioussis D., Kollias G. Wasting, ischemia, and lymphoid abnormalities in mice expressing T cell-targeted human tumor necrosis factor transgenes. J Immunol. 1993 Aug 15;151(4):1894–1906. [PubMed] [Google Scholar]
- Probert L., Plows D., Kontogeorgos G., Kollias G. The type I interleukin-1 receptor acts in series with tumor necrosis factor (TNF) to induce arthritis in TNF-transgenic mice. Eur J Immunol. 1995 Jun;25(6):1794–1797. doi: 10.1002/eji.1830250647. [DOI] [PubMed] [Google Scholar]
- Racke M. K., Bonomo A., Scott D. E., Cannella B., Levine A., Raine C. S., Shevach E. M., Röcken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994 Nov 1;180(5):1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine C. S. Membrane specialisations between demyelinated axons and astroglia in chronic EAE lesions and multiple sclerosis plaques. Nature. 1978 Sep 28;275(5678):326–327. doi: 10.1038/275326a0. [DOI] [PubMed] [Google Scholar]
- Raine C. S. Multiple sclerosis: a pivotal role for the T cell in lesion development. Neuropathol Appl Neurobiol. 1991 Aug;17(4):265–274. doi: 10.1111/j.1365-2990.1991.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Robbins D. S., Shirazi Y., Drysdale B. E., Lieberman A., Shin H. S., Shin M. L. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987 Oct 15;139(8):2593–2597. [PubMed] [Google Scholar]
- Rothwell N. J., Hopkins S. J. Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci. 1995 Mar;18(3):130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K. W., Farooq M., Norton W. T., Raine C. S., Brosnan C. F. Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol. 1990 Jan 1;144(1):129–135. [PubMed] [Google Scholar]
- Selmaj K. W., Raine C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988 Apr;23(4):339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Papierz W., Glabiński A., Kohno T. Prevention of chronic relapsing experimental autoimmune encephalomyelitis by soluble tumor necrosis factor receptor I. J Neuroimmunol. 1995 Feb;56(2):135–141. doi: 10.1016/0165-5728(94)00139-f. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cannella B., Brosnan C. F. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991 Mar;87(3):949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Farooq M., Norton W. T., Brosnan C. F. Cytokine cytotoxicity against oligodendrocytes. Apoptosis induced by lymphotoxin. J Immunol. 1991 Sep 1;147(5):1522–1529. [PubMed] [Google Scholar]
- Sharief M. K., Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991 Aug 15;325(7):467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- Shin T., Kojima T., Tanuma N., Ishihara Y., Matsumoto Y. The subarachnoid space as a site for precursor T cell proliferation and effector T cell selection in experimental autoimmune encephalomyelitis. J Neuroimmunol. 1995 Feb;56(2):171–178. doi: 10.1016/0165-5728(94)00144-d. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Somera F. P., Eng L. F. Immunocytochemical staining for glial fibrillary acidic protein and the metabolism of cytoskeletal proteins in experimental allergic encephalomyelitis. Brain Res. 1983 Apr 4;264(2):241–253. doi: 10.1016/0006-8993(83)90822-3. [DOI] [PubMed] [Google Scholar]
- Sommer N., Löschmann P. A., Northoff G. H., Weller M., Steinbrecher A., Steinbach J. P., Lichtenfels R., Meyermann R., Riethmüller A., Fontana A. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat Med. 1995 Mar;1(3):244–248. doi: 10.1038/nm0395-244. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B. Heterogeneity of microglial and perivascular cell populations: insights gained from the facial nucleus paradigm. Glia. 1993 Jan;7(1):68–74. doi: 10.1002/glia.440070112. [DOI] [PubMed] [Google Scholar]
- Tyor W. R., Glass J. D., Griffin J. W., Becker P. S., McArthur J. C., Bezman L., Griffin D. E. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992 Apr;31(4):349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Ulvestad E., Williams K., Bø L., Trapp B., Antel J., Mørk S. HLA class II molecules (HLA-DR, -DP, -DQ) on cells in the human CNS studied in situ and in vitro. Immunology. 1994 Aug;82(4):535–541. [PMC free article] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Whittemore S. R., Sanon H. R., Wood P. M. Concurrent isolation and characterization of oligodendrocytes, microglia and astrocytes from adult human spinal cord. Int J Dev Neurosci. 1993 Dec;11(6):755–764. doi: 10.1016/0736-5748(93)90064-k. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., Champion J. E., McMahon A. P. A molecular analysis of mouse development from 8 to 10 days post coitum detects changes only in embryonic globin expression. Development. 1987 Apr;99(4):493–500. doi: 10.1242/dev.99.4.493. [DOI] [PubMed] [Google Scholar]
- Williams K. C., Ulvestad E., Hickey W. F. Immunology of multiple sclerosis. Clin Neurosci. 1994;2(3-4):229–245. [PubMed] [Google Scholar]
- Yong V. W., Moumdjian R., Yong F. P., Ruijs T. C., Freedman M. S., Cashman N., Antel J. P. Gamma-interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7016–7020. doi: 10.1073/pnas.88.16.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]