Abstract
Background
Preconditioning with volatile anaesthetic agents induces tolerance to focal cerebral ischaemia, although the underlying mechanisms have not been clearly defined. The present study analyses whether TREK-1, a two-pore domain K+ channel and target for volatile anaesthetics, plays a role in mediating neuroprotection by sevoflurane.
Methods
Differentiated SH-SY5Y cells were preconditioning with sevoflurane and challenged by oxygen–glucose deprivation (OGD). Cell viability and expression of caspase-3 and TREK-1 were evaluated. Rats that were preconditioned with sevoflurane were subjected to middle cerebral artery occlusion (MCAO), and the expression of TREK-1 protein and mRNA was analysed. Neurological scores were evaluated and infarction volume was examined.
Results
Sevoflurane preconditioning reduced cell death in differentiated SH-SY5Y cells challenged by OGD. Sevoflurane preconditioning reduced infarct volume and improved neurological outcome in rats subjected to MCAO. Sevoflurane preconditioning increased levels of TREK-1 mRNA and protein. Knockdown of TREK-1 significantly attenuated sevoflurane preconditioning-induced neuroprotective effects in vitro and in vivo.
Conclusions
Sevoflurane preconditioning-induced neuroprotective effects against transient cerebral ischaemic injuries involve TREK-1 channels. These results suggest a novel mechanism for sevoflurane preconditioning-induced tolerance to focal cerebral ischaemia.
Keywords: anaesthetics volatile, sevoflurane; brain, ischaemia; neuroprotection; preconditioning; TREK-1
Editor's key points.
Volatile anaesthetic preconditioning can provide protection from ischaemia–reperfusion injury.
Sevoflurane preconditioning reduced cell death, infarct size, and neurological injury in cellular and animal models of neuronal ischaemia.
Knockdown of TREK-1 reduced sevoflurane-induced neuroprotection, indicating a role for this ion channel in this effect.
Stroke is a major cause of disability and is responsible for 40% of severely disabled adults.1 Cerebral ischaemic events in the perioperative period increase mortality among surgical patients. The incidence of perioperative stroke is 0.08–0.7% in general surgery but up to 8–10% in heart valve surgery or aortic arch repair.2 There is an unmet need for developing more effective and safer approaches for reducing the risk of cerebral ischaemic/reperfusion injury during the perioperative period.
Ischaemic preconditioning-induced neuronal tolerance to ischaemia was first reported by Kitagawa and colleagues3 in 1990. However, ischaemic preconditioning is invasive and impractical for clinical practice. Several groups have found that pharmacological preconditioning methods such as volatile anaesthetics preconditioning can induce ischaemic tolerance in mice and rats.4–6 Furthermore, volatile anaesthetic preconditioning has been used in coronary arterial surgery for cardiac protection in a rat model.7 Volatile anaesthetic preconditioning is a promising strategy for perioperative neuroprotection that is less invasive, and sevoflurane preconditioning has been shown inducing tolerance against cerebral ischaemia insults in animal models.8,9 However, the underlying mechanisms for its neuroprotective effects are unclear.
Two-pore domain background potassium channels (K2P) are a diverse and highly regulated superfamily of ion channels that likely modulate membrane excitability in physiological functions.10 As a subfamily of K2P channels, TREK channels are predominantly expressed in the central nervous system.11 TREK channels can be activated by membrane stretch, temperature, and internal acidosis, and are regulated by various pharmacological agents and cellular lipids, including volatile anaesthetics. Recent studies have suggested that the activation of TREK channels including TREK-1 mediates neuroprotection.12 Moreover, researchers have demonstrated that TREK-1 plays an essential role for anaesthesia, neuroprotection, depression, and pain.13 We hypothesized that TREK-1 plays a crucial role in mediating the neuroprotective effect against cerebral ischaemia afforded by sevoflurane preconditioning.
Methods
Animals and cells
All experimental procedures were carried out in accordance with the protocols approved by the Ethics Committee for Animal Experimentation of the Fourth Military Medical University (Xi'an, China) and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and ARRIVE guidelines. SH-SY5Y cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Adult male Sprague–Dawley rats (280–320 g) were purchased from the Laboratory Animal Center of our university.
Experimental protocol
Role of TREK-1 in the neuroprotective effect of sevoflurane differentiated SH-SY5Y cells
SH-SY5Y human neuroblastoma cells were stimulated by all-trans-retinoic acid (RA) in cell culture for several days to stimulate differentiation into neurone-like cells, as identified by expressions of the neurone markers NeuN and βIII-tubulin. Differentiated cells were then subjected to various treatments. In the OGD group, cells were challenged by oxygen–glucose deprivation (OGD). In the Pre+OGD group, cells were exposed to 3.3 vol% sevoflurane for 2 h, and then after 1 h, they were subjected to OGD. In the siRNA group, small RNA interference (siRNA) was used to inhibit the expression of TREK-1 24 before sevoflurane preconditioning and OGD. In the siRNA-c group, control siRNA served as a reference to judge the effect of siRNA vector. Cells in the normal group were cultured without any treatments. After completion of treatments, cell viability was measured by the 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl-tetrazolium bromide (MTT) assay and caspase-3 expression was examined by western blot and immunofluorescence.
Role of TREK-1 in neuroprotection induced by sevoflurane in cerebral ischaemic rats
In order to investigate the effect of sevoflurane preconditioning against ischaemia–reperfusion injury, rats were randomly divided into three groups using an online randomization programme: sham, ischaemia (MCAO), and sevoflurane preconditioning plus ischaemia (Pre+MCAO) groups (n=8–9 each). Functional neurological outcomes were observed at 24, 48, 72 h, and 1 week after middle cerebral artery occlusion (MCAO) injury, and cerebral infarct volumes were measured at 72 h and 1 week. In addition, western blot and real-time polymerase chain reaction (PCR) analysis were used to detect levels of TREK-1 protein or in RNA at 4, 24, and 48 h after ischaemia–reperfusion injury in another set of animals.
In order to verify the role of TREK-1 in sevoflurane preconditioning-induced neuroprotection against ischaemia, we created an siRNA construct against TREK-1. The reliability of the siRNA was tested in normal rats before use. To examine the impact of siRNA on the neuroprotective effect of sevoflurane pretreatment, rats were randomly allocated to the following groups: sham, MCAO, sevoflurane preconditioning plus MCAO (Pre+MCAO), TREK siRNA plus sevoflurane preconditioning plus MCAO (siRNA+Pre+MCAO), and control siRNA plus sevoflurane preconditioning plus MCAO (siRNA-c+Pre+MCAO). The effect of siRNA on functional neurological outcomes was observed at 24, 48, and 72 after ischaemia, and cerebral infarct volume was compared at 72 h after ischaemia–reperfusion.
Cell culture and characterization
The human neuroblastoma cell line SH-SY5Y is often stimulated by RA to differentiate into neurone-like cells to model the responses of neurones.14,15 Exponentially growing SH-SY5Y cells were grown in an airtight, integrated temperature-controlled cell culture chamber. Cells were kept in a 1:1 mixture of Ham's F12 nutrient and Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum, 0.28 μg ml−1 of gentamicin, and 250 μg ml−1 of amphotericin B, in a humidified atmosphere of 5% CO2 in air at 37°C until 90% confluence was reached. Then, 10 μM of RA (1% of the total volume, R2625, Sigma-Aldrich, St Louis, MO, USA) was added into the medium to stimulate the cells to differentiate. Every 2–3 days, the culture medium was replaced. Expression of the neuronal markers NeuN and βIII-tublin was examined by western blot at 1, 4, 7, and 10 days during the differentiation process. Since the western blot results showed that the highest level of neuronal marker expressions occurred at 7 days after RA stimulation, cells were used at this time point for further experiments. These experiments included the localization of the above-mentioned markers and TREK-1 by immunofluorescence.
Sevoflurane preconditioning and OGD in vitro
Preconditioning conditions were achieved by incubation of the cells in 3.3 vol% sevoflurane with 5% CO2 and 95% air for 2 h.16,17 Cells were then placed in the incubator filled with 5% CO2 95% air for 1 h before OGD.
To induce ischaemic injury, cells were subjected to OGD treatment. Cultures were first washed twice with DMEM without glucose and then put into pre-warmed OGD medium at 37°C for 120 min. The OGD buffer containing 154 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 1.0 mM NaH2PO4, 2.3 mM CaCl2, and 3.6 mM NaHCO3 was bubbled with 95% air and 5% CO2. OGD was terminated by returning to normal culture medium for an additional 24 h.
RNA interference
TREK-1 siRNA and negative control scrambled siRNAs were designed and purchased from Qiagen (Italy). The target sequence of the TREK1-siRNA used in this study was: 5′-CACGACCATTAATGTTATGAA-3′. To verify the effect of the siRNA, cells were divided into three groups: normal (without treatment), siRNA for TREK-1, and siRNA control. A total of 5×105 cells were plated into 35 mm plates, and at 1–2 days, 20 nM of siRNA was transfected using Hiperfect transfection reagent (Qiagen) according to the manufacturer's instructions. siRNA validation was carried out at 24 h by checking the expression of TREK-1 via western blot experiments.
Cell viability assay
Cell viability was measured by the MTT assay, a mitochondrial enzyme-dependent reaction. Briefly, MTT was added to cells in 96-well plates after completion of the experimental treatments. In metabolically active cells, the yellow tetrazolium MTT salt is cleaved into purple formazan crystals, which can be solubilized and the absorbance measured by a multi-plate reader at 490 nm.
Western blot
To assess the expression of neuronal markers, TREK-1, and apoptotic proteins, cells were seeded into 75 cm3 cell culture bottles at a density of 5×106, washed with ice-cold phosphate-buffered saline (PBS) twice, and resuspended in RIPA buffer (pH 7.0) containing protease inhibitors (F. Hoffmann-La Roche, Basel, Switzerland). After sonication, 20 µg of total protein extracts was subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane. Non-specific binding was blocked in 5% of milk in Tris-buffered saline Tween-20 (TBST) for 1 h, and probed with rabbit anti-beta III tubulin (1:5000, ab6046, Abcam, Cambridge, UK), mouse anti-NeuN (1:5000, MAB377, Millipore, MA, USA), rabbit anti-TREK-1 (1:800, sc-50412, Santa Cruz, CA, USA), or rabbit anti-caspase-3 (1:2000, ab32351, Abcam). After rinsing, membranes were probed with an anti-rabbit or anti-mouse secondary antibody for 2 h at room temperature. To examine the expressions of TREK-1 in the brain, animals were decapitated and the brains rapidly harvested. The ipsilateral forebrain to the ischaemic insult was dissected and homogenized.
Immunofluorescence staining
Cells grown on 22 mm sterilized glass coverslips were fixed with 4% (v/v) paraformaldehyde in PBS for 30 min and then thoroughly rinsed with PBS. Primary antibodies used were rabbit anti-βIII-tubulin (1:500), mouse anti-NeuN (1:2000), rabbit anti-TREK-1 (1:200), and rabbit anti-caspase-3 (1:1000). Subsequently, coverslips were washed with PBS and incubated with secondary antibodies conjugated with fluorescent probes for 2 h at room temperature. Nuclei were counterstained with DAPI. Coverslips were washed and mounted on glass slides with anti-fading medium for observation and analysis. Images were captured by using a Nikon E600 fluorescent microscope (Nikon, Tokyo, Japan).
Rat transient MCAO model
Cerebral ischaemia induced by transient MCAO in rats was performed as described previously.18,19 After an overnight fast, animals were anaesthetized by 3% sevoflurane for surgery the adequate depth of anaesthesia was ascertained by ensuring lack of response to toe pinch. After the skin of the neck was cut open and the carotid arteries were dissected out, a transient focal cerebral ischaemia model was induced by occlusion of the right middle cerebral artery using intraluminal 3-0 monofilament nylon suture (Ethicon, Inc., Osaka, Japan). After 120 min of ischaemia, the suture was removed from the internal carotid artery carefully. Rats in the sham-operated control group were subjected to the same surgical procedure except that the suture was not inserted. The rescue analgesia protocol included meloxicam i.p. 0.2 mg kg−1 body weight postoperatively on day 1 followed by 0.05 mg kg−1 per day for 3 days.
Sevoflurane preconditioning in vivo
Preconditioning of the rats was achieved by inhalation of 2.7 vol% sevoflurane+97% O2 for 1 h.8 The control group rats were treated for the same duration but were allowed to inhale 97% O2 only. After a washout period of 1 h, rats were subjected to MCAO surgery.
Transfection of siRNA
We tested the reliability of siRNA against TREK-1 in normal rats. The following target sequence was used: 5′-GGCUACGGGUGAUAUCUAATT-3′. The sense and antisense sequence of siRNA were 5′-GGCUACGGGUGAUAUCUAATT-3′and 3′-UUAGAUAUCACCCGUAGCCAG-5′, respectively. We carried out in vivo siRNA transfections according to the method of Luo and colleagues.20 Rats were randomly allocated to five groups: (i) normal (without intervention), (ii) siRNA (TREK-1 siRNA), (iii) siRNA-c (control scrambled siRNA was given), (iv) siRNA+Pre+MCAO (TREK-1 siRNA followed by sevoflurane preconditioning plus MCAO), and (v) siRNA-c+Pre+MCAO (control siRNA followed by sevoflurane preconditioning plus MCAO). Under pentobarbital anaesthesia (40 mg kg−1, i.p.), a stainless steel guide cannula was stereotactically fixed into the unilateral cerebral ventricle. The stereotaxic coordinate of the lateral cerebral ventricle was 1.0 mm posterior to the bregma and 1.2–1.5 mm lateral to the midsagittal line.21 A diluted mixture (5 ml) was microinjected into the ipsilateral lateral ventricle. After 24 h, animals were subjected to subsequent treatments.
Real-time PCR analysis for detection of TREK-1 mRNA expression in the brain
Total RNA was extracted from the forebrain using Trizol reagent (Invitrogen Technology) following the manufacturer's protocol. Forward and reverse primer sequences were: TREK-1 TGACCTCAGACAGTCGGTAT/CAAGCCTGCTATACCTCGT, β-actin GTGCCCATCTATGAGGGTTACGCG/GGAACCGCTCATTGCCGATGTG. Each 20 μl reaction system was mixed with 10 μl SYBR Premix Ex Taq II (2×) (Takara Bio Inc.), 0.8 μl PCR forward primer (10 μm), 0.8 μl PCR reverse primer (10 μm), 0.4 μl ROX Reference Dye (50×) (Takara Bio Inc.), 2 μl complementary DNA (cDNA), and 6 μl RNAase-free water (4°C). Conditions of the two-step PCR were as follows: 1 cycle at 95°C for 30 s, then 40 cycles at 95°C for 5 s, 55°C for 15 s, and 72°C for 31 s.
Physiological parameters
As sevoflurane inhalation can induce acute hypoxaemia, it was very necessary to rule out the neuroprotective effect of hypoxia-induced preconditioning. Therefore, blood gases of 10 additional animals were measured during sevoflurane preconditioning (n=5) or oxygen inhalation (n=5) in a separate experiment. A cannula (PE 10, Becton Dickinson, Rutherford, NJ, USA) connected to an amplifier was placed in the left femoral artery and attached to invasive arterial pressure monitoring equipment (Spacelabs Medical, Inc., Redmond, WA, USA). Arterial blood gas samples were continuously monitored at the beginning, during, and just before termination of preconditioning. Samples were then measured using the OMNI Modular System (AVL List GmbH Medizintechnik, Graz, Austria).
Since the surgical procedure of the MCAO model is very short (average time for intraluminal suture insertion is 5 min and the removal time is 1–2 min), the physiological parameters were not measured during the surgical procedure according to previous reports.22 However, a separate experiment was undertaken to measure physiological parameters, which showed no statistically significant differences between the sham and MCAO groups (data not shown). At the end of the study period, all animals were killed using i.p. sodium pentobarbital (120 mg kg−1).
Neurological scores and infarct volume
Neurological scores were evaluated using an 18-point scoring system reported by Garcia and colleagues23 by a blinded observer. The final score given to each animal was the summation of all six individual test scores. Cerebral infarct volume was measured using 2,3,5-triphenyltetrazolium chloride (TTC) staining (Sigma-Aldrich) as described.18 Infarct volume was calculated by Swanson's method to correct for oedema: 100×(contralateral hemisphere volume−non-lesioned ipsilateral hemisphere volume)/contralateral hemisphere volume.24
Statistical analysis
SPSS 13.0 was used to conduct statistical analyses. All values, except for neurological scores, are presented as mean (sem) and were analysed by one-way analysis of variance. The neurological scores were expressed as median (range), and were analysed with the Kruskal–Wallis test followed by the Mann–Whitney U-test with the Bonferroni correction. Values of P<0.05 were considered statistically significant.
Results
Characterization of differentiated SH-SY5Y cells
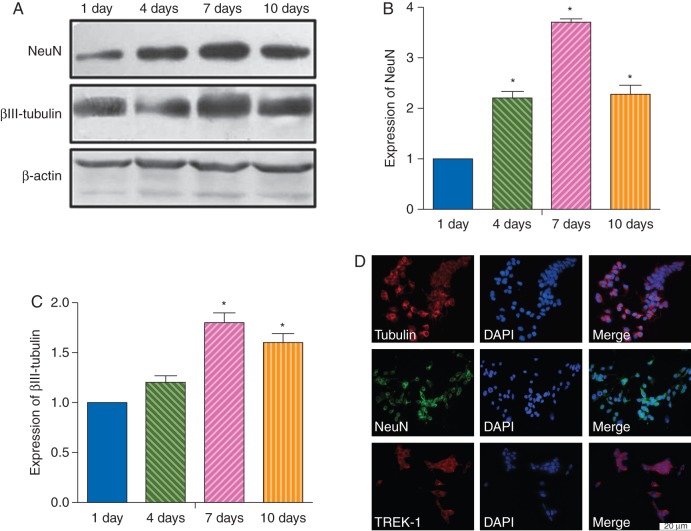
Expression of neuronal protein markers NeuN and βIII-tubulin was evaluated in RA-stimulated SH-SY5Y cells at various times during the differentiation process (Fig. 1a–c). Expression of NeuN and βIII-tubulin was found at 1, 4, 7, and 10 days after RA stimulation (Fig. 1b and c). The highest expression was observed at 7 days after stimulation. Immunofluorescent staining of 7 day RA-stimulated cells showed that the majority of the differentiated cells expressed NeuN and βIII-tubulin (Fig. 1d). Therefore, RA-stimulated SH-SY5Y cells incubated for 7 days were used in subsequent experiments. The differentiated cells also expressed a considerable amount of TREK-1 protein.
Fig 1.
Characterization of stimulated SH-SY5Y cells. (a–c) Western blot analysis for expression of the neuronal markers NeuN and βIII-tubulin in cultured SH-SY5Y cells stimulated by RA for 1, 4, 7, or 10 days. (a) Representative immunoblots. Histograms showing densitometric analyses of NeuN (b) and βIII-tubulin expression (c). Data presented as mean (sem). *P<0.05 compared with 1 day. (d) Photomicrographs of immunofluorescent staining showing expression of the two neuronal markers and TREK-1 in differentiated SH-SY5Y cells after RA stimulation for 7 days. Bar=20 μm.
Sevoflurane preconditioning induced neuroprotection in vitro
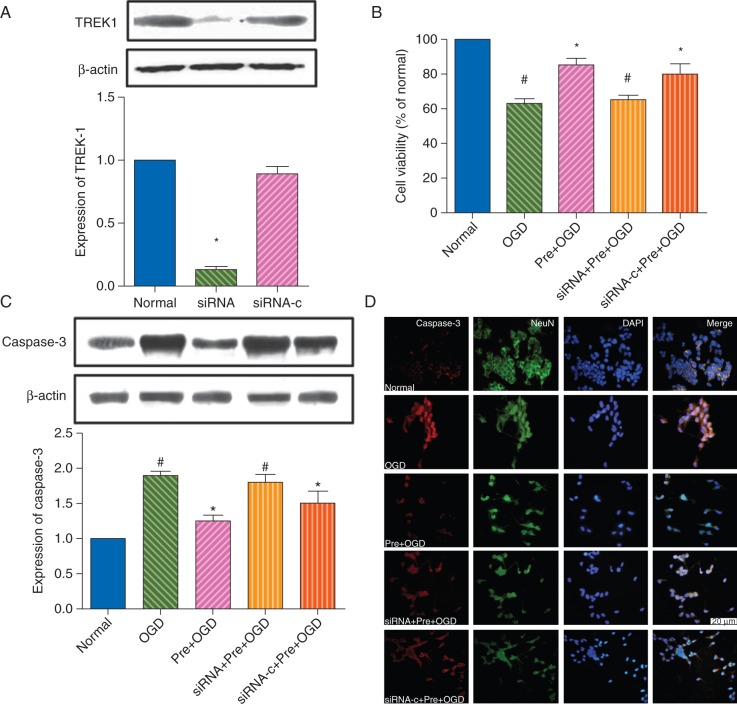
OGD reduced cell viability in differentiated SH-SY5Y cells, as evaluated by MTT and expression of Caspase-3. Preconditioning with sevoflurane (pre+OGD group) significantly attenuated the decrease in cell viability induced by OGD (P<0.05).
To clarify the role of TREK-1 in the neuroprotective effect of sevoflurane, siRNA knockdown was used (Fig. 2a). TREK-1 protein expression was down-regulated by TREK-1 siRNA [0.13 (0.03)], compared with control siRNA [0.89 (0.06); P<0.05]. TREK-1 siRNA [siRNA+Pre+OGD group; cell viability 65 (3%)] largely reversed the protective effect of sevoflurane against OGD [cell viability 85 (4%); compared with the Pre+OGD group, P<0.05], while the siRNA control [cell viability 80 (6%); siRNA-c+Pre+OGD group] was ineffective (P>0.05 vs the Pre+OGD group; Fig. 2b).
Fig 2.
TREK-1 mediates sevoflurane preconditioning-induced neuroprotection in vitro. (a) Western blot analysis showing the efficiency of TREK-1 siRNA knockdown of the expression of TREK-1 in differentiated SH-SY5Y cells. Normal, untreated control cells; siRNA, cells treated with TREK-1 siRNA; siRNA-c, cells treated with AllStars Negative Control siRNA. Note that protein expression of TREK-1 was inhibited by TREK-1 siRNA compared with either the normal or siRNA-c group. Data presented as mean (sem). *P<0.05 compared with the normal control group. (b) Histograms showing cell viabilities in the normal (control), OGD, sevoflurane preconditioning plus OGD (Pre+OGD), TREK-1 siRNA plus sevoflurane preconditioning plus OGD (siRNA+Pre+OGD), or control siRNA plus sevoflurane preconditioning plus OGD (siRNA−c+Pre+OGD) groups. *P<0.05 vs OGD group. #P<0.05 vs Pre+OGD group. (c) Western blot analysis for caspase-3 expression. Data presented as mean (sem). *P<0.05 vs the OGD group. #P<0.05 compared with the Pre+OGD group. (d) Representative immunofluorescence images showing activation of caspase-3 in SH-SY5Y cells at 24 h after OGD. Bar=20 μm.
Expression of the apoptosis-related protein, caspase-3, and the number of immunoreactive cells also markedly increased with OGD, as demonstrated by western blot [1.8 (0.1)-fold; P<0.05 vs normal control, Fig. 2c] and immunofluorescence (Fig. 2d). Pre-treatment with sevoflurane (Pre+OGD group) significantly attenuated the increase in caspase-3 protein expression induced by OGD [1.1 (0.1)-fold; P<0.05 vs normal control, Fig. 2c] and decreased the number of caspase-3 immunoreactive cells. Application of TREK-1 siRNA (siRNA+Pre+OGD group) reversed the effect of sevoflurane against OGD [1.6 (0.1)-fold; P<0.05 vs the Pre+OGD group, and P>0.05 vs the OGD groups], while control siRNA (siRNA-c+Pre+OGD group) did not [1.1 (0.1)-fold; P>0.05 vs the Pre+OGD group). Figure 2b and c shows similar results between western blot and immunofluorescence experiments.
Sevoflurane preconditioning induced neuroprotection in vivo
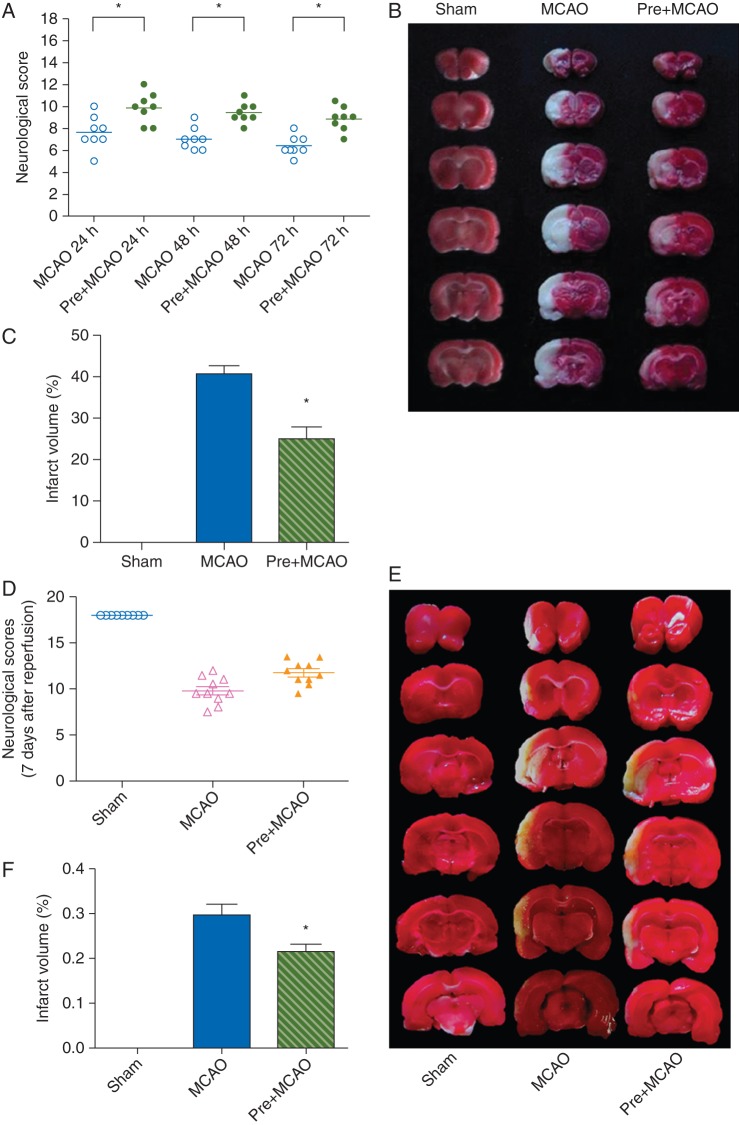
We further evaluated the role of TREK-1 in the induction of the neuroprotection of sevoflurane preconditioning in a focal cerebral ischaemia animal model. Changes of physiological parameters during oxygen inhalation or sevoflurane preconditioning are shown in Table 1. There were no significant differences among corresponding time points and between the sevoflurane and oxygen groups. Neuroprotection by sevoflurane preconditioning on ischaemia–reperfusion injury induced by MCAO was studied in the rat at 72 h and 1 week after reperfusion (Fig. 3). Sevoflurane preconditioning significantly attenuated neurological deficits, as judged by assessment of neurological scores, at 24 h [10 (8,12)], 48 h [9.25 (8,11)], 72 h [9 (7, 10.5)], and 1 week [11.75 (9.50, 13.50)] in the Pre+MCAO group, compared with the MCAO group at 24 h [7.5 (5,10)], 48 h [7.5 (6,9)], 72 h [6 (5,8)], and 1 week [9.5 (7.5,12.5)] (P<0.05 for each comparison). Sevoflurane preconditioning also reduced cerebral infarction volume, as examined by TTC staining of brain slices at 72 h [40 (2%) in the MCAO group vs 25 (3%) in the Pre+MCAO group] and 1 week [30 (2%) in the MCAO group vs 21 (2%) in the Pre+MCAO group] after MCAO (P<0.05 for each comparison).
Table 1.
Arterial blood gas anyalysis. Using separate animals, data for arterial blood gases were obtained before, during, and after 10 min of preconditioning in sevoflurane (Pre+MCAO) and oxygen control (MCAO) animals. There were no significant differences between the two groups. Values are expressed as mean (sem), n=5 in each group
| Before ischaemia |
During ischaemia |
After ischaemia |
||||
|---|---|---|---|---|---|---|
| MCAO | Pre+MCAO | MCAO | Pre+MCAO | MCAO | Pre+MCAO | |
| pH | 7.44 (0.02) | 7.45 (0.01) | 7.46 (0.01) | 7.45±0.03) | 7.44 (0.01) | 7.46 (0.01) |
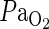 (kPa) (kPa) |
18.0 (0.8) | 17.8 (0.6) | 17.7 (0.7) | 18.0 (0.7) | 17.6 (0.7 | 17.8 (0.6) |
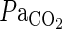 (kPa) (kPa) |
5.05 (0.20) | 4.8 (0.16) | 6.2 (0.2) | 4.8 (0.2) | 4.7 (0.2) | 4.9 (0.2) |
Fig 3.
Sevoflurane preconditioning induced neuroprotection in focal cerebral ischaemia. (a, d) Comparisons of neurobehavioral manifestations among the sham, MCAO, and pre+MCAO (sevoflurane preconditioning plus MCAO) groups at 24, 48, 72 h (a) and 7 days (d) after reperfusion. Each symbol represents the score for a single rat. A horizontal bar indicates the median value for each group (n=8–9 for each). *P<0.05 compared with the MCAO group. Representative photograph showing infarct volume in different groups 3 (b) and 7 days (e) after MCAO. Brain slices stained with TTC were from three representative rats subjected to sham, MCAO or sevoflurane preconditioning plus MCAO (Pre+MCAO). Histogram comparing infarct volume between the three groups at 3 (c) and 7 days (f) after reperfusion. Data presented as mean (sem) with individual values. *P<0.05 compared with the MCAO group (n=8–9).
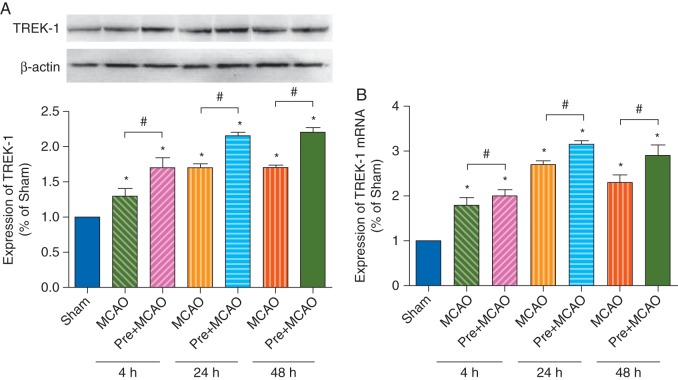
Sevoflurane preconditioning increased expression of TREK-1
Cerebral ischaemia–reperfusion injury, induced by MCAO, elevated expression of TREK-1 protein (Fig. 4a) and mRNA (Fig. 4b) levels at 4, 24, and 48 h after ischaemia. The increased TREK-1 expression in sevoflurane preconditioning groups was significantly higher than that of the MCAO group (P<0.05, Pre+MCAO vs MCAO).
Fig 4.
Sevoflurane preconditioning induced increase in TREK-1 protein and mRNA expression. (a) Western blot analysis of protein expression of TREK-1 in the sham, MCAO, and sevoflurane preconditioning plus MCAO (Pre+MCAO) groups at 4, 24, and 48 h after reperfusion. The upper panel shows TREK-1 and the corresponding β-actin bands. The lower panel histogram shows the results of the densitometric analysis. (b) mRNA expression of TREK-1 in the sham, MCAO, and sevoflurane preconditioning plus MCAO (Pre+MCAO) groups at 4, 24, and 48 h after reperfusion. *P<0.05 compared with the sham group. #P<0.05 compared with the MCAO group at the corresponding time point (n=8 for each).
Knockdown of TREK-1 by siRNA attenuated sevoflurane preconditioning-induced neuroprotection
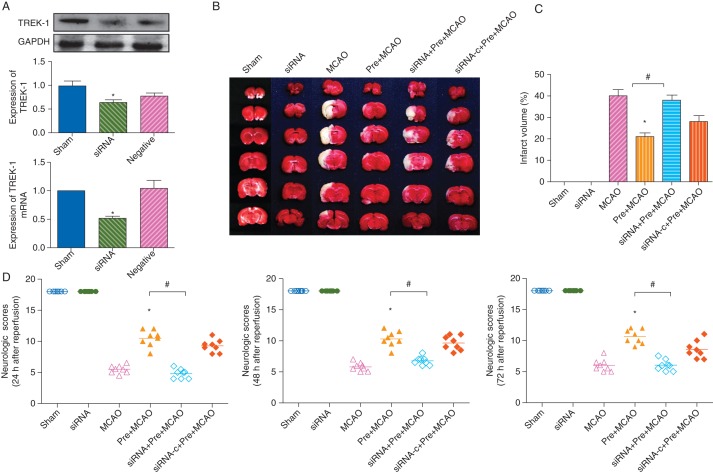
TREK-1 siRNA given intracerebroventrically (siRNA group) reduced expression of TREK-1 as demonstrated by western blot [0.58 (0.04)-fold in the siRNA group, P<0.05 vs control, Fig. 5a], and real-time PCR [0.52 (0.04)-fold in the siRNA group, P<0.05 vs control, Fig. 5a]. Administration of TREK-1 siRNA (siRNA+Pre+MCAO group) attenuated sevoflurane-induced improvement of neurological scores (Fig. 5d). Infarct volume examined at 72 h after ischaemia in this group was larger than that in the Pre+MCAO group [20 (6%) in the Pre+MCAO group vs 39 (5%) in the SiRNA+Pre+MCAO group, P<0.05, Fig. 5b and c]. There was no detectable difference between the MCAO group and the siRNA+Pre+MCAO group (P>0.05).
Fig 5.
Knockdown of TREK-1 expression attenuates the neuroprotective effect induced by sevoflurane preconditioning in the rat MCAO model. (a) Western blot and real-time PCR analysis showing the effect of siRNA on TREK-1 protein expression and mRNA in sham (untreated control rats), siRNA (rats injected with TREK-1 siRNA), and negative (rats treated with AllStars Negative Control siRNA). The upper panel shows the protein expression of TREK-1. The lower panel shows TREK-1 mRNA expression relative to GAPDH. *P<0.05 compared with the sham group. (b) Effect of TREK-1 siRNA on infarct volume. Brain slices stained with TTC from five representative rats chosen for each group. (c) Comparisons of infarct volumes. *P<0.05 compared with the MCAO group. #P<0.05 compared with the Pre+MCAO group. (d) Effect of TREK-1 siRNA on neurobehavioural indices. Each symbol represents the score for a single rat. A horizontal bar indicates the mean for each group. *P<0.05 compared with the MCAO group. #P<0.05 compared with the Pre+MCAO group (n=8 for each).
Discussion
The role of TREK-1, a two-pore domain potassium channel, in the neuroprotective effect induced by sevoflurane preconditioning against ischaemic events was studied in vitro and in vivo. The main findings are: (i) sevoflurane preconditioning improved cell viability and reduced apoptosis in neurone-like differentiated SH-SY5Y human neuroblastoma cells challenged by OGD. (ii) Sevoflurane preconditioning improved neurological outcomes and reduced infarction volume in rats subjected to transient focal cerebral ischaemia by MCAO. (iii) Sevoflurane preconditioning increased expression of TREK-1. (iv) Tolerance to ischaemia in the cells and rats induced by sevoflurane preconditioning is reduced by down-regulation of TREK-1 via RNA interference. These results indicate that TREK-1 plays a pivotal role in the induction of the neuroprotective effect by sevoflurane preconditioning.
Transient, non-lethal ischaemic preconditioning is the most effective method to protect neurones from subsequent severe ischaemic injury in surgery.25 Unfortunately, ischaemic preconditioning is invasive and therefore impractical for clinical practice. On the other hand, preconditioning using anaesthetic agents is less invasive and more amenable than preconditioning with ischaemia. Volatile anaesthetic agents such as sevoflurane and isoflurane can induce ischaemic tolerance to cerebral and spinal cord ischaemia in rabbits and rats.26–29 Repeated brief isoflurane anaesthesia induces focal cerebral ischaemia tolerance in rats in a dose–response manner.29 In this study, we confirmed that sevoflurane preconditioning significantly attenuated OGD-induced cell injury in vitro and reduced infarct volume and improved neurological deficits induced by cerebral ischaemia–reperfusion injury in vivo in a transient ischaemia rat model. In clinical practice, sevoflurane preconditioning has been applied before coronary arterial surgery with evidence of cardioprotection.30 Based on our findings, the neuroprotective effect of sevoflurane preconditioning in the perioperative period should be tested.
The precise mechanisms underlying the improvement of neurological function and reduction in brain damage by volatile anaesthetic preconditioning remain undefined. Isoflurane can inhibit glutamate release and reduce excitatory postsynaptic glutamate receptor-mediated responses.31,32 We have shown that volatile anaesthetic preconditioning induces neuroprotection by up-regulating antioxidant enzyme activities before ischaemic damage through generating reactive oxygen species.27 Free radicals might contribute to the endogenous antioxidant system after anaesthetic preconditioning. Yang and colleagues4 demonstrated that volatile anaesthetic preconditioning-induced protective effects against transient cerebral ischaemic injuries are mediated by activation of the Notch signalling pathway in mice. Ubiquitin-conjugated proteins also participate in cerebral ischaemic tolerance by isoflurane preconditioning.28 Inhibition of glycogen synthase kinase 3β (GSK3β), activation of which can lead to cell death, might play a key role in volatile anaesthetic postconditioning-induced neuroprotection.33 However, the relationships in the molecular network associated with the neuroprotective mechanism of volatile anaesthetics preconditioning are still unclear.
Two-pore domain K+ channels have received considerable attention in neuroprotection in recent years. TREK-1 is expressed throughout the brain and spinal cord and activated by membrane stretch, intracellular acidification, and lysophospholipids.34 Lysophospholipids possess a potent protective effect against global cerebral ischaemia injury or glutamate excitotoxicity in neuronal cultures.35 Since extracellular lysophospholipids open TREK-1 channels, it is likely that the K2P channels might also have a neuroprotective role. Heurteaux and colleagues13 showed that TREK1−/− mice have increased sensitivity to ischaemia and epilepsy. However, mice that lack TREK-1 expression did not show increased damage after traumatic brain injury.36 These controversial results indicate that the role of TREK-1 in neuroprotection requires further investigation.
TREK-1 is activated by a variety of volatile anaesthetics such as isoflurane, sevoflurane, and halothane in transfected mammalian cells.37 However, other structurally and functionally different K2P channels, such as TRAAK, are insensitive to volatile anaesthetics.38 Accumulated evidence supports that TREK-1 is activated by volatile anaesthesia agents.39 We postulated that volatile anaesthetic preconditioning induces cerebral ischaemic tolerance through activation of TREK-1. The neuroprotective effect of sevoflurane preconditioning was partially reversed by down-regulation of TREK-1 with siRNA, as supported by the deleterious effect on neurological function and increases in infarct volume after MCAO. Sevoflurane preconditioning apparently activates TREK-1 in differentiated SH-SY5Y cells and rat brain, and leads to tolerance against ischaemia. These findings strengthen the idea that neuroprotection induced by sevoflurane preconditioning against ischaemia is related to its action on TREK-1 and open the way for a novel neuroprotective strategy. Nevertheless, the mechanism of neuroprotection by TREK-1 activation induced by volatile anaesthetics preconditioning needs to be further elucidated. Westphalen and colleagues40 found that deletion of TREK-1 significantly reduced the inhibitory effect of halothane on glutamate release from cerebrocortical nerve terminals, which implies that reduced excitatory transmission is a possible mechanism of neuroprotective effect of anaesthetic preconditioning. The intracellular signal transduction events after activation of TREK-1 by sevoflurane are still unclear.
In conclusion, preconditioning with sevoflurane protects neurones from ischaemic injury and this effect is affected by TREK-1. The mechanistic link between TREK-1 activation by sevoflurane and improved neurological outcome needs to be explored in further detail.
Authors' contributions
H.D. and L.T. were responsible for study conception and design. M.C. and Z.L. participated in establishing the animal and cell models and in behavioural testing. L.T. and H.Z. performed immunofluorescence staining and molecular biological detection. B.S. and Y.H. performed the data analysis and interpretation. H.D. and Y.H. obtained funding and provided administrative support. L.T. and M.C. were responsible for drafting the manuscript. L.T. and H.D. made critical revisions. H.D. supervised the study.
Declaration of interest
None declared.
Funding
This work was supported in part by the National Natural Science Foundation of China (no. 81128005 and no. 81371510 to H.D., no. 81200948 to Y.H.).
References
- 1.Wechsler LR. Intravenous thrombolytic therapy for acute ischaemic stroke. N Engl J Med. 2011;364:2138–46. doi: 10.1056/NEJMct1007370. doi:10.1056/NEJMct1007370. [DOI] [PubMed] [Google Scholar]
- 2.Selim M. Perioperative stroke. N Engl J Med. 2007;356:706–13. doi: 10.1056/NEJMra062668. doi:10.1056/NEJMra062668. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa K, Matsumoto M, Tagaya M, et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–4. doi: 10.1016/0006-8993(90)90189-i. doi:10.1016/0006-8993(90)90189-I. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Dong H, Deng J, et al. Sevoflurane preconditioning induces neuroprotection through reactive oxygen species-mediated up-regulation of antioxidant enzymes in rats. Anesth Analg. 2011;112:931–7. doi: 10.1213/ANE.0b013e31820bcfa4. doi:10.1213/ANE.0b013e31820bcfa4. [DOI] [PubMed] [Google Scholar]
- 5.Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in neonatal rats. Anesthesiology. 2004;101:695–703. doi: 10.1097/00000542-200409000-00018. doi:10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Kapinya KJ, Lowl D, Futterer C, et al. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–98. doi: 10.1161/01.str.0000020092.41820.58. doi:10.1161/01.STR.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- 7.Fradorf J, Huhn R, Weber NC, et al. Sevoflurane-induced preconditioning: impact of protocol and aprotinin administration on infarct size and endothelial nitric-oxide synthase phosphorylation in the rat heart in vivo. Anesthesiology. 2010;113:1289–98. doi: 10.1097/ALN.0b013e3181f97fec. doi:10.1097/ALN.0b013e3181f97fec. [DOI] [PubMed] [Google Scholar]
- 8.Codaccioni JL, Velly LJ, Moubarik C, Bruder NJ, Pisano PS, Guillet BA. Sevoflurane preconditioning against focal cerebral ischemia: inhibition of apoptosis in the face of transient improvement of neurological outcome. Anesthesiology. 2009;110:1271–8. doi: 10.1097/ALN.0b013e3181a1fe68. doi:10.1097/ALN.0b013e3181a1fe68. [DOI] [PubMed] [Google Scholar]
- 9.Ding Q, Wang Q, Deng J, et al. Sevoflurane preconditioning induces rapid ischemic tolerance against spinal cord ischemia/reperfusion through activation of extracellular signal-regulated kinase in rabbits. Anesth Analg. 2009;109:1263–72. doi: 10.1213/ane.0b013e3181b2214c. doi:10.1213/ane.0b013e3181b2214c. [DOI] [PubMed] [Google Scholar]
- 10.Siegelbaum SA, Camardo JS, Kandel ER. Serotonin and cyclic AMP close single K+ channels in aplysia sensory neurones. Nature. 1982;299:413–7. doi: 10.1038/299413a0. doi:10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- 11.Mirkovic K, Palmersheim J, Lesage F, Wickman K. Behavioral characterization of mice lacking Trek channels. Front Behav Neurosci. 2012;6:60. doi: 10.3389/fnbeh.2012.00060. doi:10.3389/fnbeh.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franks NP, Honore E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci. 2004;25:601–8. doi: 10.1016/j.tips.2004.09.003. doi:10.1016/j.tips.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Heurteaux C, Guy N, Laigle C, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. Embo J. 2004;23:2684–95. doi: 10.1038/sj.emboj.7600234. doi:10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes FM, Schroder R, Da FMJ, et al. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 2010;1337:85–94. doi: 10.1016/j.brainres.2010.03.102. doi:10.1016/j.brainres.2010.03.102. [DOI] [PubMed] [Google Scholar]
- 15.Cheung YT, Lau WK, Yu MS, et al. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009;30:127–35. doi: 10.1016/j.neuro.2008.11.001. doi:10.1016/j.neuro.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Lin D, Feng C, Cao M, Zuo Z. Volatile anesthetics may not induce significant toxicity to human neuron-like cells. Anesth Analg. 2011;112:1194–8. doi: 10.1213/ANE.0b013e3181fdf69d. doi:10.1213/ANE.0b013e3181fdf69d. [DOI] [PubMed] [Google Scholar]
- 17.Bantel C, Maze M, Trapp S. Neuronal preconditioning by inhalational anesthetics: evidence for the role of plasmalemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2009;110:986–95. doi: 10.1097/ALN.0b013e31819dadc7. doi:10.1097/ALN.0b013e31819dadc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hata R, Mies G, Wiessner C, et al. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:367–75. doi: 10.1097/00004647-199804000-00004. doi:10.1097/00004647-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Gou X, Xiong L, et al. Trans-activator of transcription-mediated delivery of NEP1–40 protein into brain has a neuroprotective effect against focal cerebral ischemic injury via inhibition of neuronal apoptosis. Anesthesiology. 2008;108:1071–80. doi: 10.1097/ALN.0b013e318173f66b. doi:10.1097/ALN.0b013e318173f66b. [DOI] [PubMed] [Google Scholar]
- 20.Luo J, Wang Y, Chen X, et al. Increased tolerance to ischemic neuronal damage by knockdown of Na+–Ca2+ exchanger isoform 1. Ann N Y Acad Sci. 2007;1099:292–305. doi: 10.1196/annals.1387.016. doi:10.1196/annals.1387.016. [DOI] [PubMed] [Google Scholar]
- 21.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–44. doi: 10.1038/nm964. doi:10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 22.Sun J, Tong L, Luan Q, et al. Protective effect of delayed remote limb ischemic postconditioning: role of mitochondrial K(ATP) channels in a rat model of focal cerebral ischemic reperfusion injury. J Cereb Blood Flow Metab. 2012;32:851–9. doi: 10.1038/jcbfm.2011.199. doi:10.1038/jcbfm.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–34. doi: 10.1161/01.str.26.4.627. 635 doi:10.1161/01.STR.26.4.627. [DOI] [PubMed] [Google Scholar]
- 24.Swanson RA, Sharp FR. Infarct measurement methodology. J Cereb Blood Flow Metab. 1994;14:697–8. doi: 10.1038/jcbfm.1994.88. doi:10.1038/jcbfm.1994.88. [DOI] [PubMed] [Google Scholar]
- 25.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. doi:10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Xiong L, Chen S, Wang Q. Isoflurane tolerance against focal cerebral ischemia is attenuated by adenosine A1 receptor antagonists. Can J Anaesth. 2006;53:194–201. doi: 10.1007/BF03021827. doi:10.1007/BF03021827. [DOI] [PubMed] [Google Scholar]
- 27.Sang H, Cao L, Qiu P, Xiong L, Wang R, Yan G. Isoflurane produces delayed preconditioning against spinal cord ischemic injury via release of free radicals in rabbits. Anesthesiology. 2006;105:953–60. doi: 10.1097/00000542-200611000-00016. doi:10.1097/00000542-200611000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Zhang HP, Yuan LB, Zhao RN, et al. Isoflurane preconditioning induces neuroprotection by attenuating ubiquitin-conjugated protein aggregation in a mouse model of transient global cerebral ischemia. Anesth Analg. 2010;111:506–14. doi: 10.1213/ANE.0b013e3181e45519. doi:10.1213/ANE.0b013e3181e45519. [DOI] [PubMed] [Google Scholar]
- 29.Xiong L, Zheng Y, Wu M, et al. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg. 2003;96:233–7. doi: 10.1097/00000539-200301000-00047. [DOI] [PubMed] [Google Scholar]
- 30.Lee MC, Chen CH, Kuo MC, Kang PL, Lo A, Liu K. Isoflurane preconditioning-induced cardio-protection in patients undergoing coronary artery bypass grafting. Eur J Anaesthesiol. 2006;23:841–7. doi: 10.1017/S0265021506000354. doi:10.1017/S0265021506000354. [DOI] [PubMed] [Google Scholar]
- 31.Kimbro JR, Kelly PJ, Drummond JC, Cole DJ, Patel PM. Isoflurane and pentobarbital reduce AMPA toxicity in vivo in the rat cerebral cortex. Anesthesiology. 2000;92:806–12. doi: 10.1097/00000542-200003000-00024. doi:10.1097/00000542-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Harada H, Kelly PJ, Cole DJ, Drummond JC, Patel PM. Isoflurane reduces N-methyl-d-aspartate toxicity in vivo in the rat cerebral cortex. Anesth Analg. 1999;89:1442–7. doi: 10.1097/00000539-199912000-00022. [DOI] [PubMed] [Google Scholar]
- 33.Lin D, Li G, Zuo Z. Volatile anesthetic post-treatment induces protection via inhibition of glycogen synthase kinase 3beta in human neuron-like cells. Neuroscience. 2011;179:73–9. doi: 10.1016/j.neuroscience.2011.01.055. doi:10.1016/j.neuroscience.2011.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–61. doi: 10.1038/nrn2117. doi:10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 35.Blondeau N, Lauritzen I, Widmann C, Lazdunski M, Heurteaux C. A potent protective role of lysophospholipids against global cerebral ischemia and glutamate excitotoxicity in neuronal cultures. J Cereb Blood Flow Metab. 2002;22:821–34. doi: 10.1097/00004647-200207000-00007. doi:10.1097/00004647-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Namiranian K, Brink CD, Goodman JC, Robertson CS, Bryan RJ. Traumatic brain injury in mice lacking the K channel, TREK-1. J Cereb Blood Flow Metab. 2011;31:e1–6. doi: 10.1038/jcbfm.2010.223. doi:10.1038/jcbfm.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel AJ, Honore E, Maingret F, et al. A mammalian two pore domain mechano-gated S-like K+ channel. Embo J. 1998;17:4283–90. doi: 10.1093/emboj/17.15.4283. doi:10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–6. doi: 10.1038/8084. doi:10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 39.Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. Embo J. 2000;19:1784–93. doi: 10.1093/emboj/19.8.1784. doi:10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westphalen RI, Krivitski M, Amarosa A, Guy N, Hemmings HJ. Reduced inhibition of cortical glutamate and GABA release by halothane in mice lacking the K+ channel, TREK-1. Br J Pharmacol. 2007;152:939–45. doi: 10.1038/sj.bjp.0707450. doi:10.1038/sj.bjp.0707450. [DOI] [PMC free article] [PubMed] [Google Scholar]