Abstract
Homonuclear correlation NMR experiments are commonly used in the high-resolution structural studies of proteins. While 13C/13C chemical shift correlation experiments utilizing dipolar recoupling techniques are fully utilized under MAS, correlation of the chemical shifts of 15N nuclei in proteins has been a challenge. Previous studies have shown that the negligible 15N-15N dipolar coupling in peptides or proteins necessitates the use of a very long mixing time (typically several seconds) for effective spin diffusion to occur and considerably slows down a 15N/15N correlation experiment. In this study, we show that the use of mixing proton magnetization, instead of 15N, via the recoupled 1H-1H dipolar couplings enable faster 15N/15N correlation. In addition, the use of proton-detection under ultrafast MAS overcomes the sensitivity loss due to multiple magnetization transfer (between 1H and 15N nuclei) steps. In fact, less than 300 nL (~1.1 micromole quantity) sample is sufficient to acquire the 3D spectrum within 5 hours. Our results also demonstrate that a 3D 15N/15N/1H experiment can render higher resolution spectra that will be useful in the structural studies of proteins at ultrafast MAS frequencies. 3D 15N/15N/1H and 2D radio frequency-driven dipolar recoupling (RFDR)-based 1H/1H experimental results obtained from a powder sample of N-acetyla-L-15N-valyl-L-15N-leucine at 70 and 100 kHz MAS frequencies are presented.
Keywords: solid-state NMR, ultrafast MAS, proton-detection, RFDR, peptide
Introduction
Magic angle spinning (MAS) solid-state NMR spectroscopy has become an essential tool to obtain atomic-level structural and dynamic insights into folding/misfolding, aggregation, membrane interaction, ligand binding, and function of a variety of proteins.[1-8] In most such studies, like in a solution NMR approach, homonuclear chemical shift correlation is commonly used to assign peaks from multidimensional spectra of proteins.[9-12] While correlation of the chemical shifts of 13C nuclei is easily accomplished using dipolar recoupling techniques,[13-16] correlation of 15N chemical shifts in peptides and proteins has not been an easy task as the coherent dipolar coupling between 15N nuclei in a protein is very small.[17] In fact, the 15N-15N distance in a peptide backbone is conformation dependent: ~2.8 Å in an α-helix with a 15N-15N dipolar coupling of ~56 Hz and ~3.5 Å in a β-strand with a 15N-15N dipolar coupling of ~29 Hz. Though it is possible to use spin diffusion to accomplish this task, the required very long mixing time - typically on the order of seconds – considerably reduces the sensitivity of the technique due to spin-lattice (T1) relaxation of 15N.[17-19] Previous studies on static, oriented solids and MAS studies demonstrated that it is possible to shorten the very long mixing time using proton spin diffusion and also using proton-assisted approaches.[17, 20-23] Another study demonstrated the use of mixing proton magnetization to speed up the spin-diffusion process in static solids.[24] . A recent study demonstrated an approach 15N-BARE (Backbone REcoupling) that utilizes fpRFDR blocks to recouple 15N-15N dipolar couplings, and reported the use of the experimentally measured 15N-15N and 13C-13C dipolar couplings as restraints to improve the precision of the 3D structure of microcrystalline GB1 protein.[25]
In this study, we demonstrate a new proton-detected 3D experiment to overcome these difficulties – particularly at ultrafast (70 and 100 kHz) MAS frequencies. As shown in the 3D pulse sequence (Figure 1C), the 1H transverse magnetization is transferred to 15N nuclei by the first ramp-cross-polarization (ramp-CP) sequence [26] and then 15N chemical shift is expressed in the t1 period. The second ramp-CP sequence transfers the 15N magnetization back to 1H nuclei and the z-magnetization is allowed to spin-diffuse through the recoupled 1H-1H dipolar couplings. The 1H magnetization is then transferred to 15N nuclei by the third ramp-CP sequence to evolve under the 15N chemical shift in the t2 period. Then, the 15N magnetization is transferred to 1H magnetization by the fourth ramp-CP sequence. Finally, the 1H NMR spectrum is acquired in the t3 period. This method suffers from the magnetization loss during the four ramp-CP-based magnetization transfers between 1H and 15N nuclei. However, the ultrafast MAS, which has been demonstrated to be feasible to perform experiments up to spinning speed of 110 kHz [30-33], suppresses 1H-1H homonuclear dipolar interaction leading to narrow 1H spectral lines and enhances both sensitivity and resolution even though a very small amount of sample (<300 nL containing about 0.3 mg sample) is used. Although ultrafast MAS suppresses 1H-1H spin diffusion, RF driven 1H-1H zero-quantum recoupling by the finite-pulse-RFDR (radio frequency driven dipolar recoupling) pulse sequence ensures a rapid spin diffusion process in the mixing period of the pulse sequence.[34-38] The efficiency of this method is experimentally demonstrated on a powder sample of N-acetyl-15N-L-valyl-15N-L-leucine (NAVL).
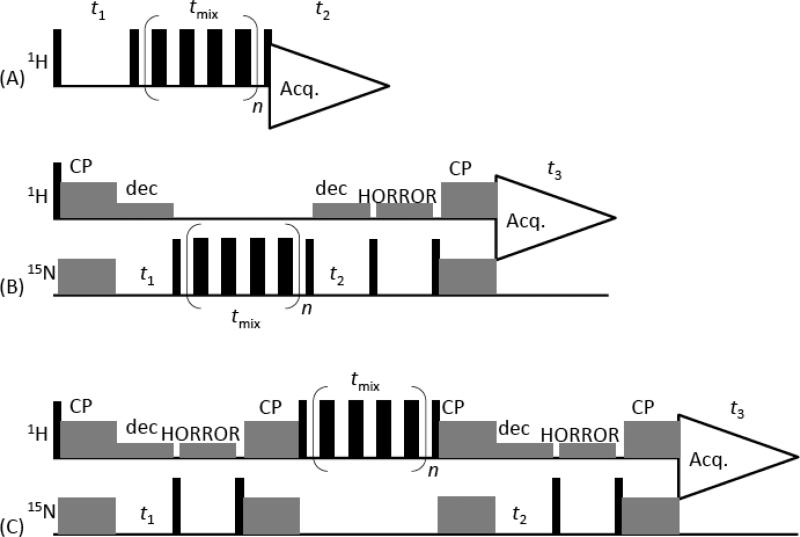
Figure 1. Proton-detected homonuclear correlation pulse sequences.
(A) 2D 1H/1H pulse sequence that correlates the isotropic chemical shifts of protons. In this method, the RFDR recoupling technique is used in the mixing time to recouple 1H-1H dipolar couplings. (B) Proton-detected 15N/15N chemical shift correlation experiment with the RFDR sequence in the mixing period to recouple 15N-15N dipolar couplings. In this pulse sequence, protons are prepared and detected, and ramp-CP [26] is employed to transfer the transverse magnetization from 1H to 15N or 15N to 1H as indicated. (C) Proton-detected 3D 15N/15N/1H experiment that correlates the isotropic chemical shifts of 15N, 15N and 1H nuclei. Chemical shifts of 15N nuclei are expressed during t1 and t2 periods, and z-magnetization of protons are exchanged during the mixing period via the 1H-1H dipolar couplings recoupled by RFDR. Protons are decoupled by a low-power CW decoupling [27] during the 15N chemical shift evolution periods t1 and t2, and the HORROR sequence [28] is used to destroy the proton magnetization remaining in the 1H channel [27, 29]. An echo sequence (tau-180°-tau = two MAS rotor period), before data acquisition, was used in (A) to suppress the background signal from the probe. Additional RFDR pulse sequences applied during the repetition time (not shown in the pulse schemes) considerably shorten the repetition delay (see the text). The XY414 phase cycling was used for all the RFDR schemes.
Experimental
All NMR experiments were performed on a 600 MHz JNM-ECA600II solid-state NMR spectrometer equipped using a 0.75 mm ultrafast MAS probe (JEOL RESONANCE Inc.). NAVL was prepared as explained elsewhere.[19] Samples were packed in a 0.75 mm zirconia rotor and all experiments were performed at room temperature. About 1.1 micromole sample was packed in the 0.75 mm MAS rotor (volume of 290 nL). The pulse sequence used to obtain homonuclear correlation spectra are shown in Figure 1. We also applied finite-pulse RFDR pulse sequence with the XY414 phase cycling during the mixing time to recouple zero-quantum dipolar interactions as shown in Figure 1.[38] We also utilized the RFDR pulse sequence to reduce the repetition delay as explained below. Since the 1H-1H spin diffusion is highly suppressed under ultrafast MAS condition, the T1 relaxation times of protons in NAVL are not uniform and varies from 0.98 to 8.3 s at 100 kHz MAS. The amide-protons have a T1 relaxation time of 4.5 s. Therefore, we normally need to provide a repetition delay of 5 to 6 s between successive scans to avoid signal saturation, however it can be shortened by applying the RFDR pulse sequence in the proton channel to recouple 1H-1H dipolar couplings during the repetition delay.[33] We have applied six RFDR trains with the XY414 phase cycling in which each RFDR train consists of 640 π pulses. This approach successfully reduced the required repetition delay to 2 s. The RFDR condition during the repetition delay was optimized to maximize the signal-to-noise (S/N) ratio per unit time for amide protons. The S/N of amide protons was improved by a factor of two if we compare the signal intensities observed with a repetition delay of 2 s and with/without RFDR. The πpulse durations used in the RFDR sequence were 1.6 μs for 1H and 6.5 μs for 15N. All other experimental conditions used in this study are given in the figure caption.
Results and Discussion
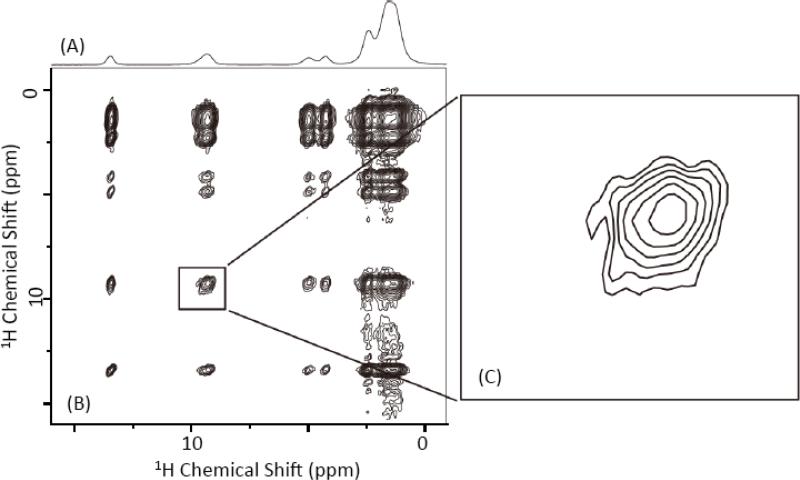
We have chosen NAVL as a model system to demonstrate the new solid-state NMR approach presented in this study. By spinning the powder sample at an ultrafast MAS frequency, all line broadening interactions - including the dipolar couplings between protons - are averaged out. As a result, a very high-resolution 1H chemical shift of NAVL is obtained (Figure 2A); spectral resolution achieved in this study is higher than that reported in a previous study on a uniformly-deuterated NAVL.[39] Then, we performed a 2D 1H/1H chemical shift correlation experiment using the pulse sequence given in Figure 1A. By using the finite-pulse RFDR pulse sequence with an efficient XY414 phase cycling to recouple 1H-1H dipolar couplings during the mixing time, an excellent 2D 1H/1H single-quantum correlation spectrum of NAVL was obtained as shown in Figure 2B. In spite of a very small amount of sample (290 nL) used for this experiment, the entire 2D 1H/1H spectrum was easily collected within 8.5 minutes by well utilizing the ultrafast MAS condition and proton-detection approach. The 2D spectrum of NAVL shown in Figure 2 is remarkable in that it is of a very high quality with excellent spectral resolution and a mixing time of 6.4 ms is sufficient to obtain total correlation of all proton resonances in the molecule; a series of 1H/1H 2D chemical shift correlation spectra of NAVL obtained at different mixing times given in Figure S1 (in the supporting information) indicate the fast spin diffusion process via the recoupled 1H-1H dipolar couplings. These results demonstrate the ability of the finite-pulse-RFDR-XY414 pulse sequence to efficiently recover 1H-1H dipolar couplings under ultrafast MAS conditions. We very recently reported a comprehensive analysis of the performance of XY-phase cycling based fp-RFDR for 2D 1H/1H chemical shift correlation experiments under ultrafast MAS conditions.[38] Even though the 2D 1H/1H spectrum shown in Figure 2B is well resolved, the resonances from two different amide-protons are not resolved (Figure 2C); spectra obtained at different mixing times are given in Figures S1 and S2 (in the supporting information). Reason for this could be the isotropic chemical shifts of amide-protons of Val and Leu residues in NAVL are quite similar within the achieved spectral resolution.
Figure 2. 2D chemical shift correlation of protons.
(A) A 1D 1H NMR spectrum of a powder sample of NAVL obtained at 100 kHz MAS. (B) A 2D 1H/1H chemical shift correlation spectrum of NAVL powder sample obtained using the pulse sequence given in Figure 1(A) with a 6.4 ms RFDR mixing time and XY414 phase cycling at 100 kHz MAS. 64 t1 points with a recycle delay of 2 s were used. The total measurement time was 8.5 minutes. (C) An expanded amide-1H chemical shift region of the 2D spectrum is shown (right). 2D spectra recorded at RFDR different mixing times are given in Figures S1 and S2 in the Supporting Information.
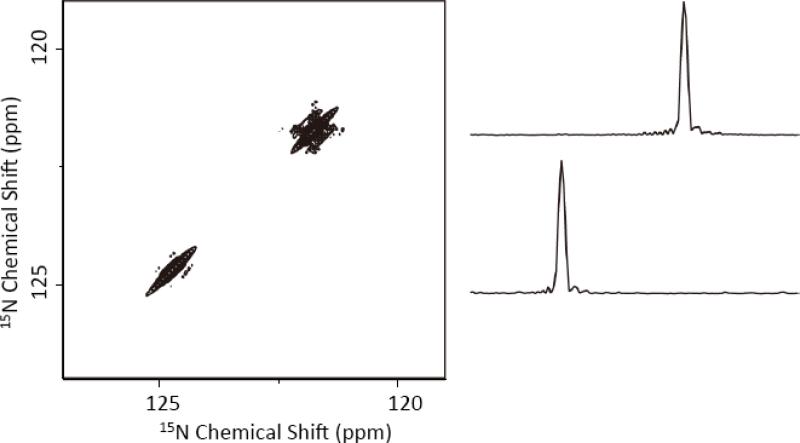
We then performed a proton-detected 3D 15N/15N/1H experiment on the NAVL sample to quickly obtain the 2D 15N/15N chemical shift correlation spectrum using the pulse sequence given in Figure 1B. This pulse sequence utilized the XY414 phase cycling based finite-pulse RFDR pulse sequence to recouple 15N-15N dipolar couplings in the mixing time. However, we observed no cross peaks in the resultant spectrum as shown in Figure 3. The 15N chemical shift values are in agreement with previous studies on NAVL.[19] The absence of cross peaks confirmed that there is no significant dipolar coupling between 15N nuclei in NAVL, which is in complete agreement with previous studies.[17-19] These results further confirmed that the recoupling pulse sequences are not effective in achieving chemical shift correlation of 15N nuclei – this is unlike the successful use of recoupling techniques to correlate the chemical shifts of 13C nuclei in peptides and proteins. It may be noted that we recently demonstrated that XY414 phase cycling based finite-pulse-RFDR pulse sequence provides an optimum performance under various experimental conditions.[38]
Figure 3. Proton-detected 15N/15N chemical shift correlation obtained with an RFDR mixing in the 15N channel.
A proton-detected 2D 15N/15N chemical shift correlation spectrum of NAVL powder sample obtained using the pulse sequence given in Figure 1(B) with 32 ms RFDR mixing time and XY414 phase cycling at 100 kHz MAS. 32 t1 and 32 t2 points were observed with a recycle delay of 2 s. The measurement time was 18.2 hour. The 2D spectrum is a project of the 3D 15N/15N/1H spectrum on to the 15N/15N plane. 1D spectral slices extracted from the 2D 15N/15N spectrum are shown (right). A contact time of 1 ms was used for both CP transfers.
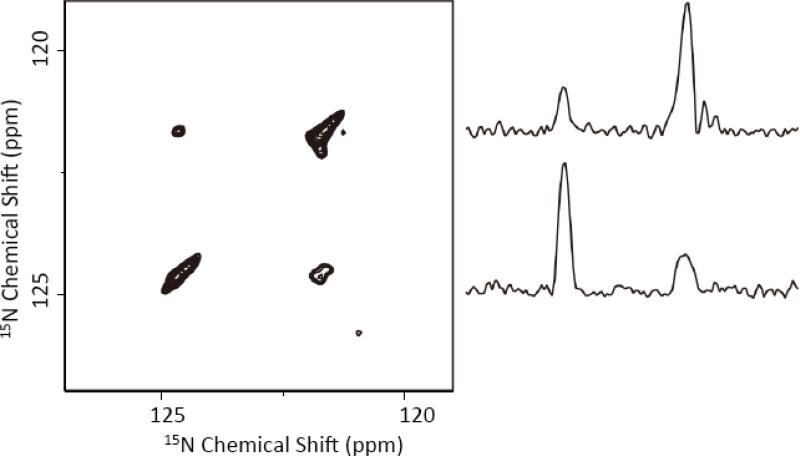
To accomplish 15N/15N chemical shift correlation, we implemented a new 3D 15N/15N/1H pulse sequence that is shown in Figure 1C. As mentioned earlier, this proton-detected 3D pulse sequence expresses the chemical shift of 15N nuclei in the incrementable t1 period, transfers the 15N transverse magnetization to protons via CP, utilizes the finite-pulse RFDR with an efficient XY414 phase cycling to recouple the 1H-1H dipolar couplings during the mixing time, transfers the 1H transverse magnetization to 15N via CP, expresses the chemical shift of 15N nuclei in the incrementable t2 period, then finally transfers the 15N transverse magnetization via cross-polarization to protons for detection. It is highly impressive that any loss of magnetization due to several magnetization transfer steps, between the 1H and 15N channels, in the sequence, is well compensated by the higher sensitivity gained by the proton-detection approach. In fact, 31% of the original signal intensity remains after the first two CP periods of the pulse sequence. Assuming the same transfer magnetization efficiency, there will be 10% of the original signal intensity before signal acquisition (i.e., after the four CP periods of the 3D pulse sequence). The 1H-1H spin diffusion process could further reduce the magnetization. Therefore, our experimental results show a survival of about 1.6% of the original signal intensity for detection. Most importantly, this amount of signal is strong enough to measure the 3D spectrum obtained with 8 scans in less than 5 hrs. The 2D 15N/15N correlation spectrum of NAVL obtained at 100 kHz using this 3D sequence is given in Figure 4. It is remarkable that the 2D spectrum clearly reveals the cross peaks and the connectivity of 15N nuclei in NAVL. Unlike the long spin diffusion based mixing (typically several seconds), our experiment needed only 6.4 ms to accomplish the correlation of 15N nuclei. In fact, a mixing time 3.2 ms was sufficient to obtain the 2D spectrum but the observed cross peak intensity is smaller than that obtained using a 6.4 ms mixing time. This 3D experiment was also performed at 70 kHz MAS and the resultant 2D 15N/15N spectrum is given in Figure S3. It is remarkable that the 2D 15N/15N spectra obtained at two different spinning speeds, 70 and 100 kHz, are very similar. This observation suggests that the proposed 3D 15N/15N/1H pulse sequence could also be useful for studies at a lower spinning speed (like 70 kHz) with an added advantage of utilizing a large MAS rotor (1 or 1.3 mm) with a relatively more sample volume (1 to 3 μL). While performing this experiment at 70 kHz could potentially beneficial for samples like proteins, proton spectral resolution could depend on the nature of the sample. For example, experiments performed on a highly homogeneous sample at 100 kHz or higher MAS frequencies could provide a better proton spectral resolution and therefore sensitivity than that obtained with a lower spinning speed (for example, 70 kHz).
Figure 4. Proton-detected 15N/15N chemical shift correlation obtained with an RFDR mixing in the 1H channel.
A proton-detected 2D 15N/15N chemical shift correlation spectrum of NAVL powder sample obtained using the 3D 15N/15N/1H pulse sequence given in Figure 1(C) with a 6.4 ms RFDR mixing time and XY414 phase cycling at 100 kHz MAS. 16 t1 and 16 t2 points were observed with a recycle delay of 2 s. The measurement time was 4.6 hour. The 2D spectrum is a project of the 3D 15N/15N/1H spectrum on to the 15N/15N plane. 1D spectral slices extracted from the 2D 15N/15N spectrum are shown (right). A contact time of 1 ms was used for all the CP transfers.
These results demonstrate the power of ultrafast MAS, proton detection, and RFDR-based 1H-1H dipolar recoupling utilized in this new method. The correlation in the new method is based on the spin diffusion driven by the coherent 1H-1H dipolar couplings; thus, this method is not restricted by the homonuclear dipolar interactions between the nuclei of interest (15N nuclei in the present case). Therefore, this method can be applied to low-γ nuclei that exhibit a very small homonuclear dipolar coupling as demonstrated for 15N in this study, as long as the two-way heteronuclear magnetization transfer efficiency is efficient.
Conclusions
While the need for high-resolution multidimensional spectra and proton-detected experiments for structural studies on biological solids have been fully realized, the chemical shift correlation of amide-15N nuclei is not easy to achieve. In this study, we demonstrated a new 3D 15N/15N/1H chemical shift correlation technique that can resolve amide-proton resonances and also quickly correlate the chemical shifts of 15N nuclei under ultrafast MAS conditions. Our experimental results demonstrate the use of recoupled 1H-1H dipolar couplings to mix protons and hence to enable faster 15N/15N single quantum correlation. Though the use of multiple steps to transfer magnetization between 1H and 15N nuclei in the pulse sequence results in a loss of the net magnetization and reduces the overall sensitivity, about 31% magnetization survives after the first two CP periods of the 3D pulse sequence due to the high magnetization transfer efficiency and proton-detection under ultrafast MAS frequency utilized in this study. Therefore, we believe that this method would be valuable in the development of higher dimensional techniques to correlate 13C, 15N and 1H chemical shifts for resonance assignment in the structural studies of proteins. While the proposed 3D 15N/15N/1H method is complementary to other methods that are used in the assignment of resonances from a uniformly-15N-labeled protein, like the 15N-BARE based experiments [25], our approach would particularly be beneficial in the development of 15N-based solid-state MAS experiments.
Supplementary Material
Highlights.
A new 3D 15N/15N/1H chemical shift correlation MAS technique is demonstrated.
1H-mixing via the recoupled 1H-1H dipolar couplings enable faster 15N/15N correlation.
1H-detection under ultrafast MAS renders fast acquisition of the 3D spectrum.
Acknowledgment
This research was supported by funds from NIH (GM084018 and GM095640 to A.R.) and JEOL Resonance Inc. (Tokyo, Japan). We would like to thank the JEOL Resonance scientists for help with the spectrometer and ultrafast MAS probe.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information Available
High-resolution 2D 1H/1H chemical shift correlation spectra of a powder sample of NAVL obtained at different RFDR mixing times under ultrafast MAS conditions.
References
- 1.Tang M, Comellas G, Rienstra CM. Advanced Solid-State NMR Approaches for Structure Determination of Membrane Proteins and Amyloid Fibrils. Acc. Chem. Res. 2013;46:2080–2088. doi: 10.1021/ar4000168. [DOI] [PubMed] [Google Scholar]
- 2.Tycko R. Molecular structure of amyloid fibrils: insights from solid-state NMR. Q. Rev. Biophys. 2006;39:1–55. doi: 10.1017/S0033583506004173. [DOI] [PubMed] [Google Scholar]
- 3.McDermott A. Structure and Dynamics of Membrane Proteins by Magic Angle Spinning Solid-State NMR. Ann. Rev. Biophys. 2009;38:385–403. doi: 10.1146/annurev.biophys.050708.133719. [DOI] [PubMed] [Google Scholar]
- 4.Linser R, Dasari M, Hiller M, Higman V, Fink U, Lopez del Amo JM, Markovic S, Handel L, Kessler B, Schmieder P, Oesterhelt D, Oschkinat H, Reif B. Proton-Detected Solid-State NMR Spectroscopy of Fibrillar and Membrane Proteins. Angew. Chem. 2011;50:4508–4512. doi: 10.1002/anie.201008244. [DOI] [PubMed] [Google Scholar]
- 5.Ward ME, Shi L, Lake E, Krishnamurthy S, Hutchins H, Brown LS, Ladizhansky V. Proton-Detected Solid-State Nmr Reveals Intramembrane Polar Networks in a Seven-Helical Transmembrane Protein Proteorhodopsin. J. Am. Chem. Soc. 2011;133:17434–17443. doi: 10.1021/ja207137h. [DOI] [PubMed] [Google Scholar]
- 6.Yan S, Suiter CL, Hou GH, Zhang T. Polenova, Probing structure and dynamics of protein assemblies by magic angle spinning NMR spectroscopy. Acc Chem Res. 2013;46:2047–2058. doi: 10.1021/ar300309s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weingarth M, Prokofyev A, van der Cruijsen EA, Nand AMD, Bonvin O, Pongs M. Baldus,Structural determinants of specific lipid binding to potassium channels. J Am Chem Soc. 2013;135:3983–3988. doi: 10.1021/ja3119114. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Park YB, Caporini MA, Rosay M, Zhong L, Cosgrove DJ, M. Hong M. Sensitivity-enhanced solid-state NMR detection of expansin's target in plant cell walls. Proc Natl Acad Sci U S A. 2013;110:16444–16449. doi: 10.1073/pnas.1316290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comellas G, Rienstra CM. Protein structure determination by magic-angle spinning solid-state NMR, and insights into the formation, structure, and stability of amyloid fibrils. Annu. Rev. Biophys. 2013;42:515–536. doi: 10.1146/annurev-biophys-083012-130356. [DOI] [PubMed] [Google Scholar]
- 10.Hou G, Yan S, Sun S, Han Y, Byeon IJ, Ahn J, Concel J, Samoson A, Gronenborn AM, Polenova T. Spin diffusion driven by R-symmetry sequences: applications to homonuclear correlation spectroscopy in MAS NMR of biological and organic solids. J Am Chem Soc. 2011;133:3943–3953. doi: 10.1021/ja108650x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber M, Bockmann A, Hiller S, Meier BH. 4D Solid-State NMR for Protein Structure Determination. Phys. Chem. Chem. Phys. 2012;14:5239–5246. doi: 10.1039/c2cp23872a. [DOI] [PubMed] [Google Scholar]
- 12.Pauli J, Baldus M, van Rossum B, de Groot H, Oschkinat H. Backbone and side-chain 13C and 15N signal assignments of the alpha-spectrin SH3 domain by magic angle spinning solid-state NMR at 17.6 Tesla. Chembiochem. 2001;2:272–281. doi: 10.1002/1439-7633(20010401)2:4<272::AID-CBIC272>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Griffin RG. Dipolar recoupling in MAS spectra of biological solids. Nat Struct Biol. 1998;(Suppl):508–12. doi: 10.1038/749. [DOI] [PubMed] [Google Scholar]
- 14.De Paëpe G, Bayro MJ, Lewandowski J, Griffin RG. Broadband homonuclear correlation spectroscopy at high magnetic fields and MAS frequencies. J Am Chem Soc. 2006;128:1776–1777. doi: 10.1021/ja0550430. [DOI] [PubMed] [Google Scholar]
- 15.De Paepe G. Dipolar Recoupling in Magic Angle Spinning Solid-State Nuclear Magnetic Resonance. Ann. Rev. Phys. Chem. 2012;63:661–684. doi: 10.1146/annurev-physchem-032511-143726. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen AB, Bjerring M, Nielsen JT, Nielsen NC. Symmetry-based dipolar recoupling by optimal control: band-selective experiments for assignment of solid-state NMR spectra of proteins. J Chem Phys. 2009;131:025101. doi: 10.1063/1.3157737. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Struppe J, Ramamoorthy A. Two-dimensional homonuclear chemical shift correlation established by the cross-relaxation driven spin diffusion in solids. J Chem Phys. 2008;128:052308. doi: 10.1063/1.2826323. [DOI] [PubMed] [Google Scholar]
- 18.Ramamoorthy A, Gierasch LM, Opella SJ. Four-dimensional solid-state NMR experiment that correlates the chemical-shift and dipolar-coupling frequencies of two heteronuclei with the exchange of dilute-spin magnetization. J Magn Reson B. 1995;109:112–116. doi: 10.1006/jmrb.1995.1157. [DOI] [PubMed] [Google Scholar]
- 19.Wei Y, Ramamoorthy A. 2D 15N–15N isotropic chemical shift correlation established by 1H–1H dipolar coherence transfer in biological solids. Chem. Phys. Lett. 2001;34:312–316. [Google Scholar]
- 20.Lewandowski JR, De Paëpe G, Eddy MT, Griffin RG. 15N-15N proton assisted recoupling in magic angle spinning NMR. J Am Chem Soc. 2009;131:5769–76. doi: 10.1021/ja806578y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khitrin AK, Xu J, Ramamoorthy A. Cross-correlations between low-γ nuclei in solids via a common dipolar bath. J Magn Reson. 2011;212:95–101. doi: 10.1016/j.jmr.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang W, Nevzorov AA. Repetitive cross-polarization contacts via equilibration-re equilibration of the proton bath: Sensitivity enhancement for NMR of membrane proteins reconstituted in magnetically aligned bicelles. J Magn Reson. 2011;212:245–248. doi: 10.1016/j.jmr.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Giffard M, Hediger S, Lewandowski JR, Bardet M, Simorre JP, Griffin RG, De Paëpe G. Compensated second-order recoupling: application to third spin assisted recoupling. Phys Chem Chem Phys. 2012;14:7246–7255. doi: 10.1039/c2cp40406k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramamoorthy A, Gierasch LM, Opella SJ. Three-dimensional solid-state NMR correlation experiment with 1H homonuclear spin exchange. J Magn Reson B. 1996;111:81–84. doi: 10.1006/jmrb.1996.0063. [DOI] [PubMed] [Google Scholar]
- 25.Hu K, Qiang W, Bermejo GA, Schwieters CD, Tycko R. Restraints on backbone conformations in solid state NMR studies of uniformly labeled proteins from quantitative amide 15N–15N and carbonyl 13C–13C dipolar recoupling data. J. Magn. Reson. 2012;218:115–127. doi: 10.1016/j.jmr.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metz G, Wu X, Smith SO. Ramped-Amplitude Cross olarization in magic-angle-spinning NMR. J. Magne. Reson. A. 1994;110:219–227. [Google Scholar]
- 27.Ishii Y, Tycko R. Sensitivity Enhancement in Solid State 15N NMR by Indirect Detection with High-Speed Magic Angle Spinning. J. Magn. Reson. 2000;142:199–204. doi: 10.1006/jmre.1999.1976. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen NC, Bildsoe H, Jakobsen HJ, Levitt MH. Double-quantum homonuclear rotary resonance: Efficient dipolar recovery in magic-angle spinning nuclear magnetic resonance. J. Chem. Phys. 1994;101:1805–1812. [Google Scholar]
- 29.Wiench JW, Bronnimann CE, Lin VS-Y, Pruski M. Chemical shift correlation NMR spectroscopy with indirect detection in fast rotating solids: studies of organically functionalized mesoporous silicas. J. Am. Chem. Soc. 2007;129:12076–12077. doi: 10.1021/ja074746+. [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama Y, Malon M, Gan Z, Endo Y, Nemoto T. Proton-nitrogen-14 overtone two-dimensional correlation NMR spectroscopy of solid-sample at very fast magic angle sample spinning. J. Magn. Reson. 2013;230:160–164. doi: 10.1016/j.jmr.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Parthasarathy S, Nishiyama Y, Ishii Y. Sensitivity and resolution enhanced solid-state NMR for paramagnetic systems and biomolecules under very fast magic angle spinning. Acc. Chem. Res. 2013;46:2127–2135. doi: 10.1021/ar4000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi T, Mao K, Paluch P, Nowak-Król A, Sniechowska J, Nishiyama Y, Gryko Daniel T., Potrzebowski Marek J., Pruski Marek. Study of Intermolecular Interactions in the Corrole Matrix by Solid-State NMR under 100 kHz MAS and Theoretical Calculations. Angew. Chem. Int. Ed. 2013;52:14108–14111. doi: 10.1002/anie.201305475. [DOI] [PubMed] [Google Scholar]
- 33.Ye Yue Qi, Malon Michal, Martineau Charlotte, Taulelle Francis, Nishiyama Yusuke. Rapid measurement of multidimensional 1H solid-state NMR Spectra at ultra-fast MAS frequencies. J. Magn. Reson. 2014;239:75–80. doi: 10.1016/j.jmr.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Bennett AE, Ok JH, Griffin RG, Vega S. Chemical-Shift Correlation Spectroscopy in Rotating Solids - Radio Frequency-Driven Dipolar Recoupling and Longitudinal Exchange. J. Chem. Phys. 1992;96:8624–8627. [Google Scholar]
- 35.Bennett AE, Rienstra CM, Griffiths JM, Zhen WG, Lansbury PT, Griffin RG. Homonuclear Radio Frequency-Driven Recoupling in Rotating Solids. J. Chem. Phys. 1998;108:9463–9479. [Google Scholar]
- 36.Ishii Y. C-13-C-13 Dipolar Recoupling under Very Fast Magic Angle Spinning in Solid-State Nuclear Magnetic Resonance: Applications to Distance Measurements, Spectral Assignments, and High-Throughput Secondary-Structure Determination. J. Chem. Phys. 2001;114:8473–8483. [Google Scholar]
- 37.Shen M, Hu B, Lafon O, Trébosc J, Chen Q, Amoureux J-P. Broadband finite-pulse radio-frequency-driven recoupling (fp-RFDR) with (XY8)41 super-cycling for homo-nuclear correlations in very high magnetic fields at fast and ultra-fast MAS frequencies. J. Magn. Reson. 2012;223:107–119. doi: 10.1016/j.jmr.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama Y, Zhang R, Ramamoorthy A. Pulse-Finiteio Radio Frequency-Driven Recoupling with Phase Cycling for 2D 1H/1H Correlation at Ultrafast MAS Frequencies. J Magn Reson. doi: 10.1016/j.jmr.2014.03.004. (in press; DOI: http://dx.doi.org/10.1016/j.jmr.2014.03.004) [DOI] [PMC free article] [PubMed]
- 39.Reif B, Jaroniec CP, Rienstra CM, Hohwy M, Griffin RGRG. 1H-1H MAS correlation spectroscopy and distance measurements in a deuterated peptide. J Magn Reson. 2001;151:320–327. doi: 10.1006/jmre.2001.2354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.