Abstract
Concentrations of different gases and volatiles present or produced inside a fruit are determined by the permeability of the fruit tissue to these compounds. Primarily, surface morphology and anatomical features of a given fruit determine the degree of permeance across the fruit. Species and varietal variability in surface characteristics and anatomical features therefore influence not only the diffusibility of gases and volatiles across the fruits but also the activity and response of various metabolic and physiological reactions/processes regulated by these compounds. Besides the well-known role of ethylene, gases and volatiles; O2, CO2, ethanol, acetaldehyde, water vapours, methyl salicylate, methyl jasmonate and nitric oxide (NO) have the potential to regulate the process of ripening individually and also in various interactive ways. Differences in the prevailing internal atmosphere of the fruits may therefore be considered as one of the causes behind the existing varietal variability of fruits in terms of rate of ripening, qualitative changes, firmness, shelf-life, ideal storage requirement, extent of tolerance towards reduced O2 and/or elevated CO2, transpirational loss and susceptibility to various physiological disorders. In this way, internal atmosphere of a fruit (in terms of different gases and volatiles) plays a critical regulatory role in the process of fruit ripening. So, better and holistic understanding of this internal atmosphere along with its exact regulatory role on various aspects of fruit ripening will facilitate the development of more meaningful, refined and effective approaches in postharvest management of fruits. Its applicability, specially for the climacteric fruits, at various stages of the supply chain from growers to consumers would assist in reducing postharvest losses not only in quantity but also in quality.
Keywords: Endogenous volatiles, Gaseous exchange, Internal atmosphere, Fruit ripening, Storage, Postharvest, Postharvest management
Introduction
Fruits are an important source of energy, vitamins, minerals, dietary fibers, pigments (carotene, xanthophylls, anthocyanins etc.), flavonoids, phenolics and other phytochemicals in human diets. Fruits are also functional foods that are a source of nutraceuticals. It is through anti-oxidizing capacity along with anti-carcinogenic and anti-mutagenic activities that the pigments and other phytochemicals present in the fruit exhibit their protective effects against chronic disease states, different types of cancers, macular and cardiac vascular diseases and other age-related problems (Rao and Agarwal 1998; Chen et al. 2001; Powell and Bennett 2002; Giovannucci 2002; Mares-Perlman et al. 2002). These health benefits emphasise the need and importance of fruits in our daily diet. Increased awareness of these health benefits has led to a significant change in the food consumption habits of people. This has resulted in an increase in the demand for high quality, fresh and nutritious fruits with almost no residue level of any toxicant. Increased demand for fruits requires not only the higher production but also improved practices in quality management, storage, transport and processing. Presently, huge losses in quality and quantity occur between harvesting and consumption of fruits. Extent of postharvest losses for few fruits in developing countries and India are presented in Table 1. As per an estimate by Kader (2005), about one-third of all the fruits produced are never consumed by humans. Furthermore, losses occurring between production and retail sites are higher in the developing countries in comparison with developed countries. In India, about 30% of fruits and vegetables are lost after their harvest due to mismanagement (Pulamte 2008). This therefore emphasises the need for a massive thrust to reduce the postharvest losses (Pulamte 2008).
Table 1.
Estimated postharvest losses for some fruits in developing countries and India
| Fruit | Postharvest losses (% of production) | |
|---|---|---|
| In developing countries | In India | |
| Apple | 14 | 10–25 |
| Banana | 11–14 | 12–14 |
| Mango | 17–37 | 17–37 |
| Tomato | 13–16 | 10–20 |
| Citrus | 8–31 | 8–31 |
| Grapes | 27 | 23–30 |
Postharvest physiology, shelf-life and losses in fruits are interlinked and primarily governed by the last phase of fruit maturation referred to as ripening. Fruit ripening involves many physiological, biochemical and developmental changes occurring through a coordinated and genetically regulated programme (Stepanova and Alonso 2005; Barry and Giovannoni 2007; Bouzayen et al. 2010). Fruits, in general, show two distinctive respiratory patterns during the course of ripening and on this basis fruits are categorized into climacteric and non-climacteric groups (Biale 1964; McMurchie et al. 1972; Biale and Young 1981; Bufler 1986; Abeles et al. 1992; Lelievre et al. 1997; Yamane et al. 2007). Apple, mango, papaya, guava, kiwi, tomato, cherimoya, banana, pear, apricot, peach, plum, avocado and plantain etc. are climacteric fruits. On the other hand, citrus fruits (orange, grapefruit, lemon etc.), berries (cherry, strawberry, blackberry, cranberry etc.), pineapple, lychee, melon, loquat, pomegranate, cucumber and tamarillo etc. belong to non-climacteric group of fruits. Climacteric fruits show a dramatic increase in rate of respiration during ripening and this is referred as climacteric rise. The rise in respiration is either simultaneous or it is just followed after the rise in the rate of ethylene production (Burg and Burg 1962, 1965a; Lelievre et al. 1997). The process of ripening can be triggered and also accelerated by exogenous ethylene treatment in climacteric fruits (Tucker 1993). The plant hormone ethylene plays a major role in the ripening process of climacteric fruits and the presence of ethylene and its perception is required for the expression of ripening-related genes even at advance stages of fruit ripening (Hoeberichts et al. 2002; Alexander and Grierson 2002). On the other hand, respiration rates increase at least temporarily in non-climacteric fruits when treated with exogenous ethylene. These fruits also undergo senescence more rapidly in presence of ethylene. However, they do not undergo major changes in composition as found in climacteric fruits with an exception of degradation of chlorophyll in citrus fruits and pineapples (Goldschmidt et al. 1993; Noichinda 2000). Besides this, low levels of ethylene are involved in wound healing and responses to various infections in some fruits of either climacteric group or non-climacteric group (Saltveit 1999; Pech et al. 2003; Van Loon et al. 2006). In general, perishability of climacteric fruits is more rapid and severe than the non-climacteric fruits (Mishra and Gamage 2007). This is basically due to faster rate of ripening and ripening-related changes in climacteric fruits. In this way, ripening of fruits has direct implications for human diets and nutrition as well as for the agricultural industry (Giovannoni and El-Rakshy 2005).
Postharvest metabolic changes leading to increased respiratory activity and transpirational loss of water are the two basic aspects that determine the storage life and quality of fruits. Retardation of ripening and associated physiological and biochemical changes (transpiration, respiration, ethylene production, softening and compositional changes) have been achieved by the application of controlled atmosphere (CA), modified atmosphere (MA) or modified atmosphere packaging (MAP) (Kader 1986; Leshuk and Saltveit 1990; Kanellis et al. 1993; Kader and Saltveit 2003a, b; Yahia 2009; Kanellis et al. 2009; Mangaraj and Goswami 2009). These approaches have in fact become the established methods for extending the postharvest-life of fruits (Yahia 2009; Mangaraj and Goswami 2009; Kader 2009; Sharma et al. 2011; Ramayya et al. 2011). It has been observed that CA, MA and MAP basically modify the internal gaseous atmosphere of the fruits in favour of low O2 to CO2 ratio (Banks et al. 1993; Elyatem et al. 1994; Klieber et al. 1996; Baldwin et al. 1999; Amarante et al. 2001; Gil et al. 2002; Kader 2009; Berry and Sargent 2009; Mangaraj and Goswami 2009; Kanellis et al. 2009; Yahia 2009; Ramayya et al. 2011; Nath et al. 2011). These methods also regulate ethylene production and its response (Scully and Horsham 2008; Yahia 2009; Mangaraj and Goswami 2009; Kanellis et al. 2009). Today, different edible coatings with wide variations in their permeability to O2, CO2 and water vapours are also available for practical use (Mishra et al. 2010). Since, these findings have practical relevance for above storage methods and therefore further investigations on the composition of the internal atmosphere in fruits have been undertaken by various workers.
At ambient temperature the internal atmosphere in fruits comprises a mixture of many gases and volatiles including O2, CO2, water vapours, ethylene, alcohols, aldehydes, acetates, esters, ketones, aromatic hydrocarbons, terpenes, carboxylic acids, sulphur compounds, ammonia, jasmonate, salicylates etc. (Speirs et al. 1998; Baldwin et al. 2000; Pesis 2005; de Leon-Sanchez et al. 2009). Several workers have suggested that these gases and volatiles as mentioned above are involved in regulating ripening, senescence and related processes (Herregods 1977; Lougheed et al. 1987; Toivonen 1997). In light of this, Saltveit (2003) posed a question—Is it possible to find an optimal controlled atmosphere for storing fruits and vegetables? He emphasized that it is not only the external but internal environment of the commodity as well that determine its storability under a given external environment. This review investigates the range of gases and volatiles including ethylene present in the internal atmosphere of fruit and the factors controlling their production and diffusion through fruit tissue. The role of some of these gases and volatiles in regulating the ripening and ripening-related changes in fruit at individual and interactive levels are discussed with special reference to climacteric fruits.
Endogenous volatiles in fruits
Higher plants and plant parts produce a large and complex mixture of volatiles. They are considered volatile because they evaporate when exposed to air at room temperature and generally have low molecular weight (<250 Da) with distinctive odour. These compounds are formed via several biochemical pathways and they are generally found in very small amounts (Negre-Zakharov et al. 2009; Defilippi et al. 2009). Volatiles are produced in variable amounts at different times and in different tissues (Negre-Zakharov et al. 2009; Defilippi et al. 2009). Plants use various mechanisms to regulate the production and levels of these volatiles (Negre-Zakharov et al. 2009; Defilippi et al. 2009). The timing of release of many plant volatiles is closely tied to pollination and fruit dispersal and this has ecological and evolutionary significance (Vaughn 2007; Negre-Zakharov et al. 2009). Maturity or ripening stage of the fruit also influences eating quality and sensory quality of aroma (Lalel et al. 2003).
Discovery of the plant hormone ethylene brought the realization that at least some of the gaseous compounds produced and emitted by the plants may have important physiological roles. At present, more than 1,000 organic compounds have been reported to be emitted by plants (Dudareva et al. 2004). The aroma produced by various fruits during ripening was reviewed by Defilippi et al. (2009) and Pandit et al. (2009). Approximately 400 volatile compounds have been found in the ripening tomato fruit (Baldwin et al. 1991; de Leon-Sanchez et al. 2009). The volatiles present in fruits are as follows: ethylene, ethanol, acetaldehyde, methanol, acetone, butanol, ethane, hexanol, hexenol, 3-methyl butanal, ethyl acetate, propyl acetate, butyl acetate, propanol, acetate esters, ethyl butyrate, geraniol, octenal, octenol, citral, terpenes, carboxylic acids, sulphur compounds, ammonia, jasmonate, benzaldehyde and methyl salicylate besides other types of iso-, sec- or tert-alcohols, aromatic hydrocarbons, ketones, esters, aldehydes and higher carbon alcohols (Gustafson 1934; Petro-Turza 1987; Saltveit 1989; Baldwin et al. 1991; McDonald et al. 1996; Toivonen 1997; Frenkel et al. 1995; Speirs et al. 1998; Baldwin et al. 2000; Bai et al. 2003; Pesis 2005; Cadwallader 2005; Negre-Zakharov et al. 2009; Defilippi et al. 2009; de Leon-Sanchez et al. 2009).
Gaseous exchange and factors affecting the composition of the atmosphere in harvested fruit
Gaseous exchange across the fruit surface
Gaseous exchange occurring between a plant organ and its environment usually follows a specific path. Gas-filled intercellular spaces are considered as the predominant pathway for gas transport through bulky organs such as fruits (Ho et al. 2009). The rate of movement of a gas depends on the properties of that gas molecule, the concentration gradient and the physical attributes of the intervening barriers (Kader 1987). In general, the rate of release of a compound is a function of its volatility and the properties of cellular and intracellular membranes through which the compound has to diffuse (Dudareva et al. 2004). Comparative analysis of volatile compounds (emitted and present within the plant tissues) revealed that the emission of volatiles is not merely a function of their differential volatility but it also involves a cytologically organized excretory process (Gershenzon et al. 2000). The membranes of the storage compartment (where the compound exists) or epidermal cell wall might be differentially permeable to different volatile compounds. Little is known about metabolite trafficking between various subcellular compartments, the mechanism of the release process and how these processes contribute to the regulation of volatile emission (Dudareva et al. 2004). Usually, emission of a particular volatile compound into the atmosphere depends on the rate of its biosynthesis and the rate of its release (Dudareva and Pichersky 2000). Formation of volatile compounds is regulated at spatial (Pare and Tumlinson 1997) and developmental levels (Bouwmeester et al. 1998; Dudareva and Pichersky 2000). Further, environmental factors such as; light, temperature and moisture status can greatly influence the emission of volatiles (monoterpenes) from the leaves of plant (Staudt and Bertin 1998; Gershenzon et al. 2000).
In natural ecosystems, evolution of volatiles by the plant tissue is influenced to a larger extent by evapotranspiration (Charron and Cantliffe 1995). Emission rate of volatile organic compounds (VOCs) in Norway spruce (Picea abies) increased exponentially with increase in the temperature and temperature itself had a direct effect on the rate of transpiration (Filella et al. 2007). In the above study, correlation analysis indicated very clearly that the rate of emission of VOCs such as; acetic acid, acetaldehyde, methanol, acetone, ethanol, hexanal, hexanol, monoterpenes and methyl salicylate is directly associated with the rate of transpiration. This study also emphasised the role of partitioning of specific VOC between the gas and liquid phases (as described by the Henry’s law constant) in determining the rate of emission of volatiles from the plant surfaces. The release of volatiles from the plant organs with lower rates of transpiration (such as bulky fruits with well-developed diffusion barriers on the surface/peel/pericarp) will be less and therefore, the volatiles can accumulate in the tissues of such organs. Keeping in view the varietal variation in diffusion barriers and their characteristics for different plants and plant parts, the accumulation of volatiles would also be different and thereby their effect on various physiological processes.
In general, there are three major routes through which gaseous exchange take place for a harvested commodity 1. Outermost layers (cuticle, cuticular cracks and periderm), 2. Apertures (stomata and/or lenticels) and 3. Stem scar region (Solomos 1987; Ben-Yehoshua and Rodov 2003). In view of the presence of cracks on cuticular layer, exchange of gases and volatiles was reported through cuticular layer and it is primarily determined by extent of cracks present on cuticular layer (Ehret et al. 1993). For most of the horticultural produce, the skin represents the major barrier to gas exchange (Solomos 1987; Kader 1987). The diffusivity of gases for the fruit flesh is 10–20 times higher than the diffusivity from the skin (Solomos 1987; Banks and Nicholson 2000). In tomato, the stem scar (the place where the pedicel along with sepals connects the fruits to the stem) is the predominant site for gas exchange (Cameron and Yang 1982). It was also demonstrated that 85–90% of ethylene exchange occurs through this region of tomato fruit (de Vries et al. 1995).
Basis of gaseous exchange across the fruit boundary
Gas diffusion in fruits follows Fick’s law (Burg and Burg 1965b). This law states that the flux of a gas, diffusing normally through a barrier, is dependent on the diffusion coefficient and concentration gradient. Burg and Burg (1965b) and then Solomos (1987) developed relationships between rates of ethylene production by fruit and the internal concentrations and these relationships were surprisingly found similar across many fruit species. There is morphological and anatomical basis for gaseous exchange across the boundaries of harvested fruits (Cameron and Yang 1982; Solomos 1987; Kader and Saltveit 2003a, b). As per Kader and Morris (1977) anatomical features and not any alterations in biochemical pathways are the reasons for differences in the diffusion resistance. External (morphological) and internal (anatomical) features were reported to determine the keeping quality of grapes (Uys 1974), storability of tomato berries (Niemirow-Krizsai and Csillag 1994), firmness or mechanical resistance of tomato (Wann 1996) and olive fruits (Mulas 1994), O2 to CO2 ratio in tomato fruits (Yang and Shewfelt 1999; Kader and Saltveit 2003a, b; Pech et al. 2003) and ripening index, physiological loss in weight and rate of respiration in tomato fruits (Paul and Srivastava 2006). In a study by Bai et al. (2003), it was shown that ‘Granny Smith’ apples have relatively very few open pores (lenticels) in their peel surface and therefore they suffer from low rates of gas exchange and as a result these fruits are prone to develop off-flavours after they have been coated with waxes. In contrast, ‘Delicious’ apples have many open lenticels and they retain sufficient gas exchange even when coated with waxes. So, waxed ‘Delicious’ apples accumulate only low concentrations of ethanol and off-flavours (Bai et al. 2003). Likewise, it was reported that ‘Murcott’ mandarins are much more sensitive to anaerobic stress conditions than ‘Star Ruby’ grapefruit when exposed to N2 enriched atmosphere because they showed much more rapid and greater increase in respiration rates, internal CO2 concentrations, production of ethanol and acetaldehyde and development of off-flavour (Shi et al. 2005). When mandarin and grapefruits were compared by Shi et al. (2007), it emerged out that during postharvest storage or after exposure to anaerobic atmospheres, mandarin develops off-flavour much more rapidly than grapefruit. The occurrence of off-flavour was associated with increase in the levels of ethanol and acetaldehyde. Anatomical observations revealed that although the total thickness of the peel [comprising the albedo, the white inner layer and the flavedo (the coloured outer layer)] was greater in grapefruit but it was more condensed in mandarins. So, it was concluded that mandarins accumulate larger amount of acetaldehyde and ethanol after harvest than grapefruit because of higher activity of enzyme alcohol dehydrogenase (ADH) in the juice vesicles and lower permeability of their peel to gases (Shi et al. 2007). The extent of diffusibility of gases across the fruit boundaries therefore determines the internal atmosphere of the fruit in terms of the levels of O2 and CO2 (Nuevo et al. 1984; Ben-Yehoshua et al. 1983). Thus, the composition of the internal atmosphere of the fruit is always different to the external atmosphere in which it is kept (Dadzie et al. 1993). Peel of the fruit acts as a barrier due to different layers of plant tissues including aqueous, cuticular and waxy layers. Exchange of gases in fruits through the peel via diffusion from the openings (stomata and lenticels) is proportional to difference in the concentrations of gases across the barrier, total area of the peel, solubility of a gas in peel, solid state diffusion coefficient and total hole area available on the peel surface (as contributed by openings of stomata and/or lenticels) (Hagenmaier 2004, 2005). Gas transport in fruit tissue is governed by diffusion as well as by permeation. The permeation is basically caused by overall pressure gradient of a given gas (Ho et al. 2006b). So, permeation-diffusion–reaction model was applied to study gas transport in intact pear. Permeation was found to be minimum across skin and it gradually increased for cortex and vasculature tissues of pear fruit (Ho et al. 2006b).
Gas transport properties of fruits are important in understanding the internal atmosphere of fruits specially during their controlled atmosphere storage. Temperature had stronger effect on diffusivity of CO2 when compared with O2 (Ho et al. 2006a). For pear fruit, gas diffusibility in vertical axis was higher than the equatorial radius axis. Diffusivity was also found to be lesser in brown tissues of brown heart disorder of pear than the healthy tissue (Ho et al. 2006a). Gas exchange to a large extent depends on the arrangement pattern of cells and intercellular spaces (Mendoza et al. 2007). In view of this, a very comprehensive model of gas exchange of pear fruit was proposed for explaining the development of physiological disorder such as core breakdown and its role in long-term storage of this fruit (Franck et al. 2007). In the above study, the effect of the actual 3-D tissue structure of plant organs has been put forward but this could not be quantified for explaining gas exchange in plant tissues. Later on, Verboven et al. (2008) used high-resolution phase tomography (making use of synchrotron radiation) to explore the 3-D structure and cellular arrangements of pome fruit tissues in their natural state (i.e., with high water content) up to sub-micrometer resolution. For this study, pome fruits like; apple and pear were selected because their gas exchange properties have been shown to be very different and closely related to their storage lives (Schotsmans et al. 2004; Ho et al. 2006a, b; Franck et al. 2007; Ho et al. 2008). Results obtained by Verboven et al. (2008) revealed very clearly that the apple fruit had more voids than pear. The differences in void fraction (23% for apple cortex and only 5% for pear cortex) along with the extent of network architectures of voids explained the better ability of tissues to facilitate the gas exchange in apple fruit. This lower void volume in pear fruit compared to apples as shown by Verboven et al. (2008) was able to explain the earlier findings where pear fruit was found to be more sensitive to physiological disorder such as internal browning and its relation to gas exchange and the availability of internal O2 by Lammertyn et al. (2003), Franck et al. (2007) and Ho et al. (2008). Likewise, there is risk of developing physiological disorders in pear fruit during the course of ripening. This was shown to be due to increase in respiration resulting in anoxia at and near the center of the fruit even under the recommended storage conditions (Ho et al. 2010). Very recently, quantification of microporosity in apple and tomato fruits was done by magnetic resonance imaging (MRI) for the better understanding of relationship between gas transfer and various disorders in fruits during their postharvest-life (Musse et al. 2010).
Variability and causes of differences in internal atmosphere of fruits
There exists a large variability in the internal atmosphere of fruits belonging to different species, variety/cultivar and developmental stages. Resistance to diffusion of CO2 was found to vary with fruit, variety of a fruit and also size and maturity stage of fruit (Kader and Morris 1977; Zagory and Kader 1988). There is varietal variability in outer surface morphology as well as in internal anatomical features (distribution of trichomes, stomata and lenticels, thickness of cuticle and extent of cuticular cracks etc.) (Kader and Morris 1977; Zagory and Kader 1988; Paul and Srivastava 2006; Paul et al. 2007, 2010b). It is the combinations of all these features that determine the permeance or resistance of gaseous movement across the fruit (Saltveit 1999; Paul et al. 2007). Variations in the amount, composition and ultrastructure of cuticular/epicuticular wax among several apple cultivars were documented by Belding et al. (1998). Apart from this, morphological and mechanical properties of the cuticle as well as the epidermis were subjected to considerable change during growth and ripening of tomato fruit (Bargel and Neinhuis 2005). Number of stomata show significant difference among the cultivars of pear fruit (Kovacs et al. 1994) and sweet cherry (Peschel et al. 2003). Likewise, varietal variations in the number of lenticels, deposition pattern of cuticular/epicuticular wax, amount of cuticle and wax and internal anatomy of peel and exocarp regions were reported in mango fruits (Dietz et al. 1988; Paul et al. 2007). In tomato fruit, cuticle appeared to provide an excellent barrier (Thompson 2003) and as a result it may not contribute significantly for overall gaseous exchange across the fruit. However, instead of stomata, trichomes were observed on the surface of tomato fruits and trichome base cells are transformed into lenticels during maturation of the fruit (Clendenning 1941; Blanke 1986; Paul and Srivastava 2006). There are varietal differences in the number of trichomes, tendency of trichomes to get transformed into lenticels, density of lenticels and the dimension of stem scar portion of tomato fruits (Paul and Srivastava 2006).
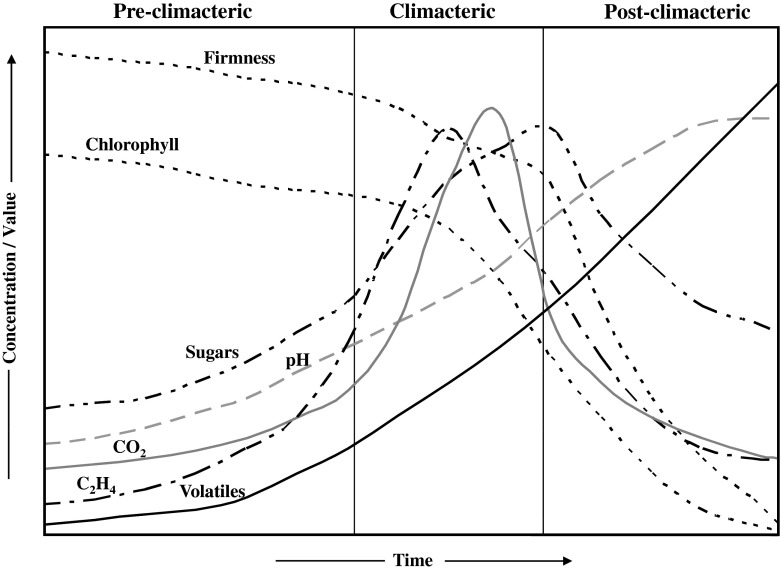
During the development, maturation and ripening—fruits undergo changes in texture, firmness, skin colour, composition of aroma and flavour volatiles, chemical composition (sugar content, acidity etc.), respiration and ethylene production (Fig. 1). Ratio of O2 to CO2 also gets altered with the growth/development/maturity of the fruit (Burg and Burg 1965a, b; Burg 1968; Kader and Morris 1977; Zagory and Kader 1988; Saltveit 1999). Gas exchange properties (specifically due to skin resistance) are not found to be consistent with the growth and maturity of the fruit. The reason for this may be the changes that occur in the anatomical properties and anatomical features of the fruit itself during its development and maturation (Zagory and Kader 1988; Longhurst et al. 1994; Kader and Saltveit 2003a; Bargel and Neinhuis 2005; Paul and Srivastava 2006; Paul et al. 2007; Ho et al. 2008; Paul et al. 2010b). In another study, selected dose of 1-methylcyclopropene (1-MCP) delayed the ripening of tomato fruits in a variety dependent manner (Paul et al. 2010a). Earlier, similar results were demonstrated in apple by Rupasinghe et al. (2000) and Watkins et al. (2000). Such differential effect might be due to the differences in the diffusion/absorption/retainability of 1-MCP by different varieties in view of the differences in surface morphology, anatomical features, contents of lipids and other cellular constituents of fruits (Kader and Saltveit 2003a, b; Dauny et al. 2003; Paul and Srivastava 2006; Nanthachai et al. 2007; Paul et al. 2010a). Physiological and biochemical differences and also the variations in the levels of ethylene, CO2 and O2 etc. for the plant-attached and plant-detached fruits for both the categories of fruits i.e., climacteric and non-climacteric were described along with their roles on the process of fruit ripening and in explaining the existing varietal variability in the rate of fruit ripening by Paul et al. (2011a).
Fig. 1.
Changes in various parameters during climacteric fruit ripening (Source: Nath et al. 2006)
Volatile composition of fruits and vegetables often shows wide range of variation. Such variations can partly be explained due to differences in the experimental procedures but, much of the variation is likely due to the varietal differences only. For example, wide differences in the concentrations of volatile were recorded in different varieties of apricot (Guichad and Souty 1988). The levels and proportions of different volatiles were also found to be responsible for the characteristic differences in flavour among the varieties of apricot (Guichad and Souty 1988). Similarly, Larsen and Poll (1990) found differences in flavour among the 10 raspberry cultivars due to variation in production of aroma volatiles. In apple cultivars, wide differences in susceptibility to scald were associated with the α-farnesene content (Huelin and Coggiola 1968). Gran and Beaudry (1993) observed wide variability in the threshold levels of oxygen required (at 0 °C) for the induction of anaerobic respiration in three apple varieties (0.7% for ‘Red Delicious’, 1.4% for ‘Red Fuji’ and about 1.9% for ‘McIntosh’). In this way, variety and storage condition can influence the degree of accumulation of products of anaerobic respiration such as; acetaldehyde and ethanol in tissues. Likewise, out of two varieties of raspberries (‘Meeker’ and ‘Qualicum’), when harvested at red-ripe stage and stored at 1 °C with 90% RH for 7 days, variety ‘Qualicum’ was found to be more susceptible to the accumulated acetaldehyde, ethanol and ethyl acetate in the MAP with high CO2 i.e., 10% CO2 with 5% O2 in comparison with other MAP conditions (6% CO2 with 10% O2 and 3% CO2 with 15% O2) (Toivonen et al. 1999). On the basis of available literature, it was concluded by Toivonen (1997) that conditions for induction of anaerobic metabolism were not consistent because factors such as; commodity characteristics, variety and temperature etc. are the important determinants of above said metabolic shift. In tomato fruit, differences in flavour among different varieties were in part due to the variation in production levels of aroma volatiles (Brauss et al. 1998). For mango fruits, volatiles (in different varieties and at different maturity stages) have been used as marker for identification of different maturity stages in different varieties. So, volatiles can thereby be used in determining the most optimum maturity stage for harvesting of mango fruits as this can result in attaining the best quality of harvested fruits on ripening (Lebrun et al. 2008; Pandit et al. 2009). In another study, discrimination of 28 apricot cultivars into four distinguishable aroma groups was achieved by analysing their volatile constituents (Aubert and Chanforan 2007).
Influence of the internal atmosphere on ripening and ripening-related changes
Ripening
During ripening of tomato fruits, rise in endogenous concentrations of CO2 and ethylene were reported along with the decrease in the concentration of O2 (Lyons and Pratt 1964). Significance of stem scar region as a major site for gaseous exchange of tomato fruit was exploited by Calbo et al. (1988) and Yang and Shewfelt (1999). They observed drastic reduction in the rate of ripening and thereby extension in the storage life of tomato fruits by sealing the stem scar region of fruits. The relationship between ripening behaviour and stem scar region of tomato fruit in different varieties was studied by blocking the stem scar region either completely or to different degrees (Paul et al. 2010b). In comparison to control, complete blockage of stem scar region of tomato fruits at green mature stage showed three-fold reduction in ripening index% (from 78.9 to 26.2 and from 45.3 to 15.1 in varieties ‘Pusa Ruby’ and ‘Pusa Gaurav’, respectively) at 14 days after treatment but with increased decay. It was also made clear in this study that it is the degree of blockage of the stem scar region that determines the extent to which the rate of respiration and ripening were suppressed. The extent of climacteric rise was reduced significantly in treated fruits. Suppressive effects of these treatments were however found to diminish with the advancement in the ripening stage of tomato fruits that were being treated (Paul et al. 2010b). Besides the major role of stem scar region, the lenticels on the surface of the tomato peel also appear to control the respiration and ripening by determining the overall exchange of gases and volatiles depending upon the developmental stage (green mature stage and onward) and variety of tomato (Paul and Srivastava 2006). In all the above studies, the basic change causing the delay in ripening or suppression of respiration was primarily due to the lower levels of ethylene and O2 to CO2 ratio within the fruit.
Role of ethylene in regulating the process of ripening, senescence and postharvest aspects of fruits and vegetables has been extensively reviewed by Kader et al. (1989), Abe and Watada (1991) Bouzayen et al. (2010) and Paul et al. (2011b). Earlier, Toivonen (1997) had reviewed the accumulation of non-ethylene and non-respiratory volatiles (alcohols, aldehydes, jasmonates, terpenes, carboxylic acids, sulphur compounds and ammonium) and discussed them in terms of their biological activity in harvested fruits and vegetables. It was pointed out by Toivonen (1997) that besides removing the ethylene, ethylene removing/absorbing agents can also remove the other organic volatiles from storage or package atmosphere (Kader et al. 1989; Matsumoto and Ogawa 1995). Therefore, at least some effects that were attributed due to the removal of ethylene may in fact be related to the removal of other volatiles which have not been measured or identified. So, there is a strong reason to evaluate the potential role and significance of volatiles other than the ethylene and their interaction with ethylene in postharvest situations and under different storage conditions (Lougheed et al. 1987; Toivonen 1997). Different factors and conditions affecting the exchange of volatiles (as already described and discussed above) can influence the accumulation and release of some important volatiles such as; ethylene, alcohols (mainly the ethanol), aldehydes (mainly the acetaldehyde) and methanol. These volatiles may be accumulated or released differentially and thereby influence the ripening process in fruits (Cadwallader 2005; Pesis 2005, 2006). This has been demonstrated for the ripening of climacteric fruits like; tomato (Kelly and Saltveit 1988; Saltveit and Sharaf 1992; McDonald et al. 1996) and apple (Pesis et al. 1994; Pesis 1995). Besides this, levels of volatiles are also found to be associated with storage disorders of apple fruit like; scald (Huelin and Coggiola 1968) and internal browning (Mendoza et al. 2007). The ripening and quality of non-climacteric fruits such as; grapes, orange and strawberries were also influenced by these volatiles (Saltveit and Ballinger 1983; Ke and Kader 1990; Ke et al. 1991).
Flavour and aroma
In tomato fruit, 17 volatiles have a significant impact on characteristic tomato-like aroma (Buttery 1993). Hexanal, cis-3-hexenal, trans-3-hexenal, trans-2-hexenal, cis-3-hexenol, 6-methyl-5-hepten-2-one, beta-ionone, 2-isobutylthiazole, 3-(methylthio)-1-propanol and 3-(methylthio)-1-propanal were important in imparting flavour to fresh red tomato (Tandon et al. 2000, 2001; Lewinsohn et al. 2001; Baldwin 2004). Hayata et al. (2002) reported that tomato-like flavour was correlated strongly with geranyl acetone, 2-methylbutanol, 3-methylbutanol and 6-methyl-5-hepten-2-one. Distinctive volatile components responsible for aroma and flavour in some important fruits are presented in Table 2.
Table 2.
Distinctive components of aroma for some fruits
| Fruit | Compound |
|---|---|
| Apple | ß-Damascenone, butyl hexanoate, isoamyl hexanoate, hexyl hexanoate, ethyl butanoate, propyl butanoate, hexyl butanoate, butyl acetate, hexanal, 2-hexenal, ethyl 2-methylbutyrate |
| Banana | 2-Hexenal, Eugenol, Isopentanol, decan-1-ol, 2-phenylethanol, 3-oxy-pentanoic acid, 3-methylbutanoic acid, 3-methylbutyl acetate, butanoate, 3-methylbutanoate, 5-methoxyeugenol, eugenol-methylether, elemicin |
| Mango | Ethyl butanoate, ethyl-2-butanoate, hexanal, cis-3-hexanal, trans-2-hexanal, γ-octalactone, γ-dodecalactone, furaneol, α-pinene, β-pinene, 3-carene, myrcenelimonene, p-cymene, terpinolene, α-copaene, caryophyllene |
| Tomato | Hexanal, trans-2-hexenal, cis-3-hexenal, cis-3-hexenol, β-ionone, β-damascenone, 1-penten-3-one, 3-methylbutanal, 3-methylbutanol, 2-isobutylthiazole, 3-(methylthio)-1-propanol, 3-(methylthio)-1-propanal, 1-nitro-phenylethane, trans-2-heptenal, phenylacetaldehyde, 6-methyl-5-hepten-2-one, methyl salicylate, geranylacetone |
| Peach | Benzaldehyde, benzyl alcohol, nonanol, linalool, ethyl hexanoate, 3-methylbutanoate, α-terpineol, γ-hexalactone, δ-decalactone, γ-undecalactone, δ-dodecalactone, α-pyrone, 6-pentyl-α-pyrone |
| Orange | Geranial, neral acetaldehyde, decanal, octanal, nonanal, ethyl acetate, ethyl propionate, ethyl butanoate, methyl butanoate, ethyl-2-methyl butanoate, ethyl-3-hydroxy hexanoate, linalool, α-terpineol, limonene, myrcene, α-pinene, valencene |
| Lemon | Citeral |
| Grapefruit | Acetaldehyde, decanal, ethyl acetate, methyl butanoate, ethyl butanoate, 1-p-menthene-8-thiol, nootkatone, limonene, naringin |
| Strawberry | Hexanal, cis-3-hexanal, trans-2-hexanal, furaneol, mesifuran, ethyl hexanoate, ethyl butanoate, methyl butanoate, ethyl-2-methyl propanoate, |
| Grape | Methyl anthranilate, o-aminoacetophenone, furaneol, methyl furaneol, β-damascenone, β-phenylethanol, butyl alcohol, hexyl alcohol, hexanal |
| trans-2-hexenal, isoamyl alcohol, acetaldehyde, isobutyraldehyde, ethyl acetate, ethyl propionate, butyl acetate, propyl acetate, 2-methylbutanol | |
| Linalool, geraniol, methoxyisobutylpyrazine | |
| Raspberry | 1-(π-Hydroxyphenyl)-3-butanone, α-ionone, β-ionone, geraniollinalool, benzyl alcohol, ethyl hexanoate, ethyl butanoate, methyl butanoate, γ-decalactone, 2-heptanone, cis-3-hexanal, β-damascenone |
Quality of the aroma is related to concentration and composition of volatiles present in the fruit. Negative effects of some of the above volatiles produced under anaerobic condition have been perceived on the quality of aroma (Forney et al. 1991; Hansen et al. 1992) but, positive effects of accumulation of some volatiles were also found to be influenced by volatiles such as; acetaldehyde and ethanol (Paz et al. 1981; Pesis et al. 1986, 1998; Saltveit and Mencarelli 1988; Frenkel et al. 1995). These positive or negative effects were largely found to be dependent on the concentrations of ethanol and acetaldehyde in strawberries and persimmon fruits (Prasad and Stadelbacher 1974; Pesis et al. 1986). Sweetness of tomato fruit was correlated not only with sucrose equivalents and pH but also with the volatiles including cis-3-hexenal, trans-2-hexenal, cis-3-hexanol, geranyl-acetone, 2-methylbutanol, 3-methylbutanol trans-2-heptenal, 6-methyl-5-hepten-2-one and 1-nitro-2-phenylethane. Likewise, sourness was correlated with soluble solids and pH along with the volatiles including acetaldehyde, acetone, 2-isobutylthiazole, geranyl-acetone, beta-ionone, hexanal and ethanol (Saltveit 2005).
Fruit decay
Fruit decay means any condition or sign, either physiological or pathological in origin, that makes the fruit unacceptable (Wills and Ku 2002). The role of low O2 to CO2 ratio or anaerobic condition is well known in determining the overall decay of fruits and vegetables (Banks 1984). Such situations were reported to suppress not only the biosynthesis but also the action of ethylene (Kanellis et al. 1989a, b). Ethylene is known to be involved in defense against pathogen as it stimulates phenylpropanoid pathway, synthesis of pathogenesis-related proteins and induces systemic resistance (Saltveit 1999). These findings explain the higher decay under the influence of blockage of stem scar region of tomato fruit as observed by Paul et al. (2010b). In tomato fruits, decay was up to 50% or more when the stem scar portion of fruits was sealed by coconut grease and it was primarily due to the anaerobic conditions (Calbo et al. 1988; Yang and Shewfelt 1999). Fruits of highbush blueberry, on the other hand, produce antimicrobial volatiles such as trans-2-hexenal that conferred resistance to anthracnose fruit decay (Polashock et al. 2007). Likewise, preharvest spray treatment of volatile compound like ethanol with calcium also reduced the gray mold development in table grapes (Chervin et al. 2009).
Role of some important endogenous volatiles in regulating fruit ripening
I. Ethylene
Ethylene is the main regulator of ripening in climacteric fruits
Ethylene (C2H4) is a naturally produced gaseous plant growth hormone with numerous effects on growth, development and storage-life of many fruits. As already stated earlier, this plant hormone plays a major role in the ripening process of climacteric fruits (Theologis et al. 1992; Yang 1995; Nagata et al. 1995; Lelievre et al. 1997; Saltveit 1999; Barry et al. 2000; Atta-Aly et al. 2000; Klee 2002; Alexander and Grierson 2002; Hoeberichts et al. 2002; Bouzayen et al. 2010; Paul et al. 2011b). Ethylene is thought to start a cascade of events leading to many interactive signaling and metabolic pathways for the progress of ripening in climacteric fruits (Fig. 2). The production of aroma during ripening of fruits also depends strongly on production and action of ethylene (Golding et al. 1998, 1999; Rupasinghe et al. 2000; Alexander and Grierson 2002; Lurie et al. 2002; Flores et al. 2002; Dandekar et al. 2004; Pech et al. 2008; Defilippi et al. 2009). But, Zhu et al. (2005) made it clear that production of aromatic volatiles may or may not be totally dependent on ethylene.
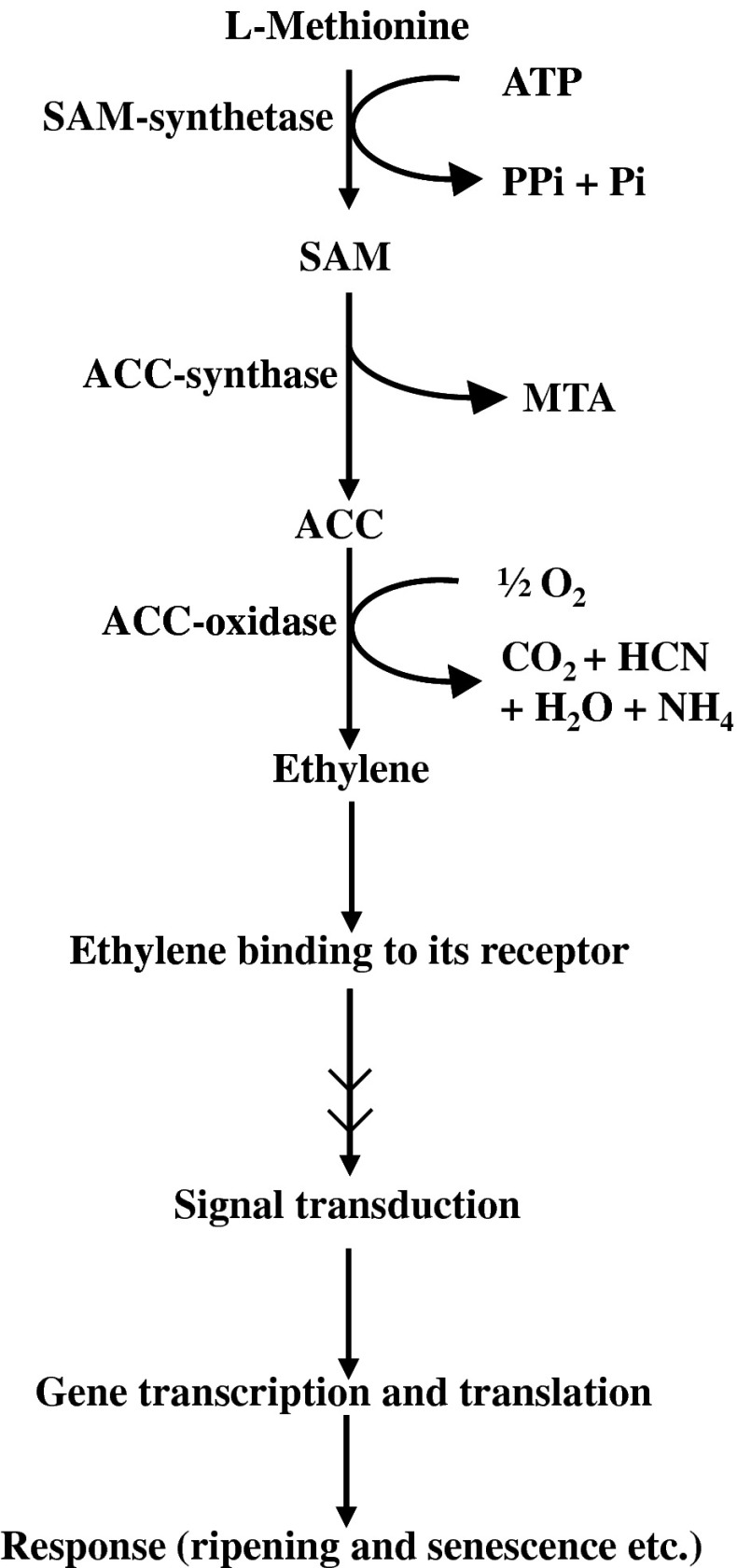
Fig. 2.
Ethylene biosynthesis, perception and response. SAM: S-Adenosyl-L-methionine, ACC: 1-Amino-cyclopropane-1-carboxylic acid, MTA: 5-Methylthioadenosine, HCN: Hydrogen cyanide
Regulation of ethylene production
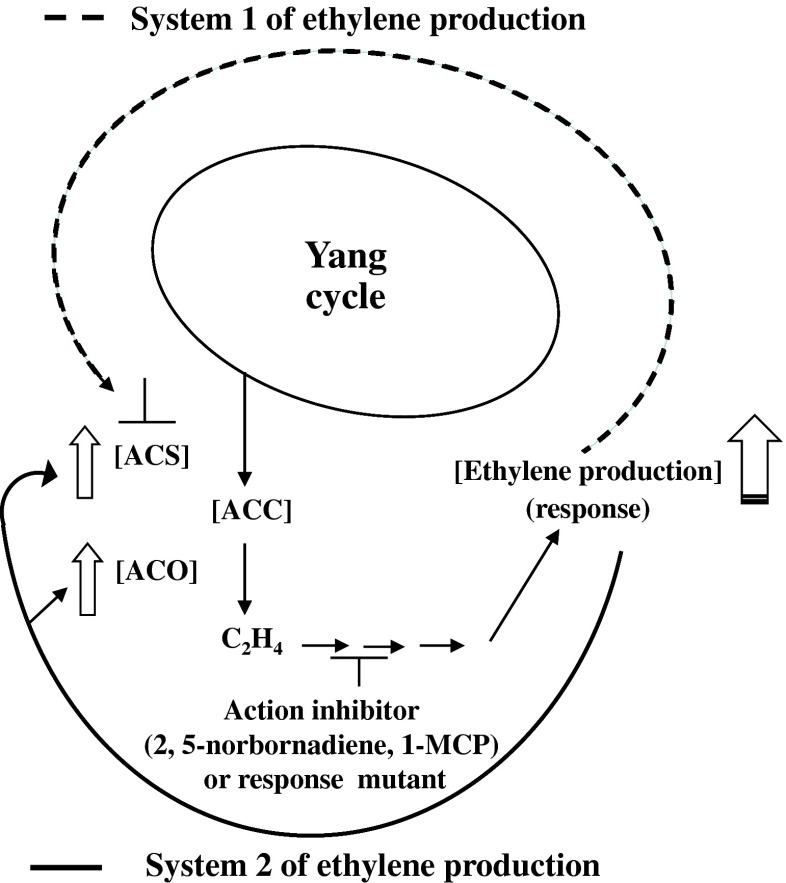
It is known that the rate of ethylene production in fruits undergoing ripening is controlled by the ability of the fruit to synthesize 1-aminocyclopropane-1-carboxylic acid (ACC) and to convert ACC to ethylene (Fig. 2). The two key enzymatic controls are at the levels of expression and activity of ACC-synthase and ACC-oxidase (Tucker 1993) as shown in Fig. 2. Two systems of ethylene production have been defined in plants (McMurchie et al. 1972). The first one is designated as system 1. System 1 operates and functions during normal growth and development and in response to various stresses. System 1 is responsible for the basal level of ethylene production in vegetative tissues and unripe fruits. This system is regulated in an auto-inhibitory manner (Fig. 3). This means that even the treatment of exogenous ethylene will not trigger any further synthesis of ethylene. The second system is system 2 and this operates during floral senescence and fruit ripening. This system is responsible for the large auto-inductive (auto-catalytic) increase in ethylene production during fruit ripening, specially in the climacteric fruits (Oetiker and Yang 1995; Lelievre et al. 1997; Nakatsuka et al. 1998; Inaba 2007) (Fig. 3). High genetic variability in the rate of production of ethylene has been reported for fruits such as; muskmelon, melon, peach and kiwifruit by Kendall and Ng (1988), Miccolis and Saltveit (1991), Klozenbucher et al. (1994) and Xu et al. (1998) respectively. It has been demonstrated that both these enzymes i.e., ACC-synthase and ACC-oxidase are encoded by multigene families in various plants and they are regulated by a number of regulating factors (Fluhr and Mattoo 1996; Lelievre et al. 1997; Nakatsuka et al. 1998; Barry et al. 2000; Alexander and Grierson 2002). Both of these enzymes are also regulated by the final product of the reaction i.e., ethylene (Lelievre et al. 1997) (Fig. 3). Lower ethylene production due to lower activity of ACC-oxidase was assigned as the cause for the formation of spongy tissue disorder in ‘Alphonso’ mango (Nagamani et al. 2010). The O2 and CO2 concentrations in the fruit play important role in the biosynthesis of ethylene and its action as well. This aspect has been described and discussed in detail in the subsequent part of this review.
Fig. 3.
Simplified pathway of ethylene biosynthesis in plants showing auto-inhibition (inhibiting its own production) and auto-induction of ethylene (inducing its own production). These two systems are referred as system 1 and system 2 of ethylene production respectively. In system 1, ethylene inhibits its own production by inhibiting ( ) ACS (ACC-synthase) expression/activity. It may be noted that the ACO (ACC-oxidase) activity is enhanced during system 1 but due to the absence of any enhancement in the activity of ACS there is no auto-induction. In system 2, ethylene induces more of its own production by stimulating (
) ACS (ACC-synthase) expression/activity. It may be noted that the ACO (ACC-oxidase) activity is enhanced during system 1 but due to the absence of any enhancement in the activity of ACS there is no auto-induction. In system 2, ethylene induces more of its own production by stimulating ( ) the expression/activity of both of the enzymes (ACS and ACO) simultaneously and this thereby enhances the overall ethylene production (
) the expression/activity of both of the enzymes (ACS and ACO) simultaneously and this thereby enhances the overall ethylene production ( ). 2, 4- norbornadiene and 1-MCP (1-methylcyclopropene) [action inhibitors of ethylene] and response mutants of ethylene block (
). 2, 4- norbornadiene and 1-MCP (1-methylcyclopropene) [action inhibitors of ethylene] and response mutants of ethylene block ( ) the action/response of the ethylene and thereby inhibit the system 2 of ethylene production (Source: Adapted and modified from Srivastava 2001)
) the action/response of the ethylene and thereby inhibit the system 2 of ethylene production (Source: Adapted and modified from Srivastava 2001)
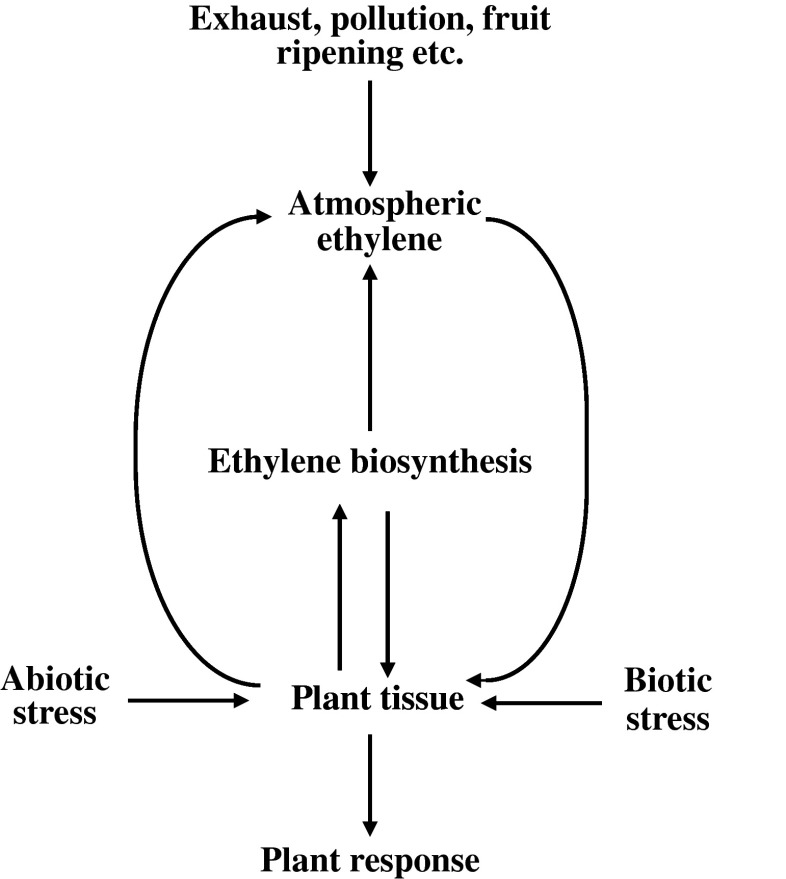
Besides the levels of ambient and internal concentrations of ethylene, production of ethylene is also governed by abiotic and biotic stresses. The interactive nature of ethylene at plant and at the level of its immediate environment is shown in Fig. 4. Plants and plant parts are not the only source of ethylene but; smoke, exhaust gases, ethylene releasing chemicals, catalytic production of ethylene from ethanol and analogs of ethylene (as produced by variety of processes within the plant system itself) are also important sources of ethylene or the other chemicals with ethylene-like activities. Such sources of ethylene and ethylene-like chemicals are common under storage/cold storage conditions. Although different analogs of ethylene (as listed in Table 3) have reduced activity or efficiency but, these analogs can elicit the same physiological effects as that of ethylene. So, presence of analogs of ethylene can also influence the fruit ripening specially under the storage conditions.
Fig. 4.
Interaction of ethylene with plant/plant parts and its immediate environment (Source: Adapted and modified from Saltveit 1999)
Table 3.
Relative activity of ethylene and its analogs in pea straight growth bioassay test
| Gases | Formula | ppm (μl l−1) in gas phase for half-maximum activity |
|---|---|---|
| Ethylene | C2H4 | 0.1 |
| Propylene | C3H6 | 10 |
| Vinyl chloride | C2H3Cl | 140 |
| Carbon monoxide | CO | 270 |
| Acetylene | C2H2 | 280 |
| Vinyl fluoride | C2H3F | 430 |
| Methyl acetylene (Propyne) | C3H4 | 800 |
| Vinyl bromide | C2H3Br | 1,600 |
| Allene (Propadiene) | C3H4 | 2,900 |
| Vinyl methyl ether | C3H6O | 10,000 |
| Ethyl acetylene (1-butyne) | C4H6 | 11,000 |
| 1-Butene | C4H8 | 27,000 |
| Vinyl ethyl ether | C4H8O | 30,000 |
| Carbon dioxide | CO2 | 30,000 |
| 1, 3-Butadiene | C4H6 | 500,000 |
Ethylene-diffusibility, its internal concentration and fruit ripening
In general, there is an inverse relationship between ethylene production and postharvest-life (Gussman et al. 1993; Zheng and Wolff 2000). Diffusion of ethylene to the external atmosphere follows Fick’s law from flat surfaces and it has often been applied to study the gas exchange in bulky organs such as fruits (Ben-Yehoshua and Cameron 1989). According to Lescourret et al. (2001), skin permeability can vary with fruit size. Permeability and skin area are highly variable among different species and also within the same species (Cameron and Reid 1982). Ethylene is diluted in fruit tissues and diffuses into the atmosphere and therefore according to Ben-Yehoshua and Cameron (1989), fruit volume and gaseous permeability of skin are important biophysical traits of fruits that need to be considered when analysing emission of ethylene.
Saltveit (1999) described that once the ripening of climacteric fruits has started, the internal ethylene concentration increases quickly to a much higher levels (even up to 100 μl l−1). This is due to the stronger diffusion resistance specially at later stages of fruit development. Ethylene concentration in tomato fruit was reported to surpass a threshold value of 4.9 ppm in order to induce ripening (Knegt et al. 1974). Work carried out by Sawamura et al. (1978) indicated that during the ripening of tomato fruits average ethylene concentration was unusually high in plant-attached fruits (5.4 ppm; on an average with a range from 2 to 13 ppm depending upon cultivars and seasonal conditions) in comparison to plant-detached tomato fruits (where the values for ethylene dropped to a lower level i.e., 1.4 ppm on an average). Since, such lower levels of ethylene are more commonly found in ethylene-induced processes and therefore the results suggested the possibility of presence of ethylene antagonizing factor in the plant-attached fruits (Sawamura et al. 1978). This concept might be true but, drastic reduction in the levels of ethylene in plant-detached fruits in comparison with plant-attached fruits might also be because of more effective gaseous exchange across the stem scar region of the harvested tomato fruits in comparison with the fruits that are still attached with the mother plant where the stem scar region is not exposed (as it is linked with pedicel). This explanation appears to be more relevant in view of the report by de Vries et al. (1995) where it was found that 85 to 90% of ethylene released by tomato fruit takes place through this stem scar region only.
Basic studies on the effect of ethylene on respiration have been done in climacteric as well as in non-climacteric fruits. Application of propylene (an analogue of ethylene) was reported to initiate an increase in respiration in climacteric fruits as well as in non-climacteric fruits but, this propylene-mediated induction or rise in the endogenous ethylene production occurred only in climacteric fruits (McMurchie et al. 1972; Yamane et al. 2007). In climacteric fruits like; banana, mango and tomato a substantial proportion of rise in rate of respiration is reported to be contributed by cyanide-insensitive or cyanide-resistant respiration (Kumar and Sinha 1992; Pandey et al. 1995; Reddy and Srivastava 1999). In contrast to climacteric fruits, cyanide-resistant respiration is present only to a limited extent in non-climacteric fruits. In these fruits, the upsurge in respiration and ethylene is either not observed or it is only transitory even after the application of exogenous ethylene (Lurie and Klein 1989; Kays and Paull 2004). A definite ethylene-mediated stimulation of respiration was however noticed in the peel of citrus fruits (non-climacteric) during their ripening (Goldschmidt 1997). Recently, it was pointed out by Paul et al. (2011a) that more information on the interactive and regulatory aspects of ethylene on the respiration (specially at biochemical and molecular levels) are required.
The concept of binding of ethylene to its receptor in plant system is widely accepted (Sisler and Yang 1984) and has also been proven beyond doubt (Sisler and Serek 1997; Sisler et al. 2006). But, usually, this aspect has not been taken into consideration in the studies dealing with resistance of the fruits to diffusion and exchange of gases (Ben-Yehoshua et al. 1985). So, more has to be learned about the retention and release of bound ethylene in plant tissues in relation to physiological activity of ethylene (Goldschmidt et al. 1993). In this direction, a theory of ethylene emission by tomato fruit was developed and used as a base to develop simulation model called ‘ETHY’ by Genard and Gouble (2005). This model was found to be highly sensitive to the parameters like; permeability of skin surface, internal concentration of O2, CO2 and ACC, change in fruit growth and temperature, activities of ACC-oxidase and ACC-synthase, concentration of ethylene itself and ATP production status. Besides this, changes in the levels of ethylene receptors and/or sensitivity towards the ethylene with the development and ripening have been reported not only in climacteric fruits like; tomato (Kevany et al. 2007, 2008), banana (Golding et al. 1999) and apple (Johnston et al. 2009) but also in non-climacteric fruits like; citrus (Goldschmidt 1997) and melon (Bower et al. 2002; Pech et al. 2008) and in Arabidopsis plant as well (Yoo et al. 2009).
II. Oxygen and carbon dioxide
Low oxygen
From outer to inner parts of plant organs (roots, tubers, seed and fruit etc.), levels of oxygen (O2) showed decreasing trend (Lammertyn et al. 2003). For fruits in general, same is being presented in Fig. 5. Difference in the depletion of internal O2 levels in different kind of fruits under MA condition was observed (Sornsrivichai et al. 1998). Yip et al. (1988) claimed that 50% reduction in ethylene production could be obtained at 1% level of O2. This is primarily because of the fact that O2 is itself a substrate for the reaction catalysed by the enzyme ACC-oxidase (Fig. 2). It has already been reported that O2 is required for the synthesis as well as the action of ethylene in fruits including tomato (Burg and Burg 1967). Fruits show reduction in respiration with lowering of O2 in the surrounding atmosphere and at specific reduced level of O2 there is induction of anaerobic respiration which leads to fast breakdown of sugars and this is named as the Pasteur effect (Kader 1986; Boersig et al. 1988). The Pasteur effect has practical importance in the modified atmosphere storage of fruits (Weichmann 1986). Oxygen concentrations must be managed so that aerobic respiration is minimised but anaerobic respiration, which leads to fast breakdown of sugars, is avoided (Kader 1986; Weichmann 1986; Boersig et al. 1988; Kubo et al. 1996). It was reported by Saltveit (2003) that the optimum level of O2 concentration needed to maintain the aerobic respiration is not only different for different commodities but it also shifts for a given commodity over a period of time during storage. In tomato fruits, low O2 caused not only an increase in the production of ethanol and acetaldehyde but it also delayed the ripening in comparison to control (Klieber et al. 1996). In bulky and dense storage organs (such as; apple fruit, potato tubers and legume seed pods), internal O2 concentration may fall to low levels of 8–10% near the surface and even to a very low levels of 2–5% in the center. Such conditions may enhance anaerobic respiration and trigger the accumulation of acetaldehyde and ethanol (Magness 1920; Rolletscheck et al. 2002). In tomato and pear fruits, hypoxia [the term loosely applies to any partial pressure of O2 that is less than 21 kPa Chervin et al. (1996)] can also result in increase in activity of pyruvate decarboxylase (PDC) and ADH (Nanos et al. 1992; Chen and Chase 1993). The activities of isoenzymes of ADH were found to be inversely related to the levels of O2 (Kanellis et al. 1991). Below a certain level of O2, the rise in CO2 production indicates a switch to fermentative metabolism. This O2 level has been called as the anaerobic compensation point (ACP) (Leshuk and Saltveit 1990). The ACP may vary for different fruits and for the same fruit at different maturity and at different storage temperatures besides being affected by different varieties of a given fruit (Boersig et al. 1988; Gran and Beaudry 1993; Kubo et al. 1996). McGlasson and Wills (1972) suggested that storage of green banana fruits at low O2 (3%) limits the operation of Krebs cycle at two steps 1. Between, either oxaloacetate or pyruvate and citrate and 2. Between 2-oxoglutarate and succinate. Besides this, this condition also caused the reduction in the activity of enzyme malate synthase which is involved in glyoxylate cycle.
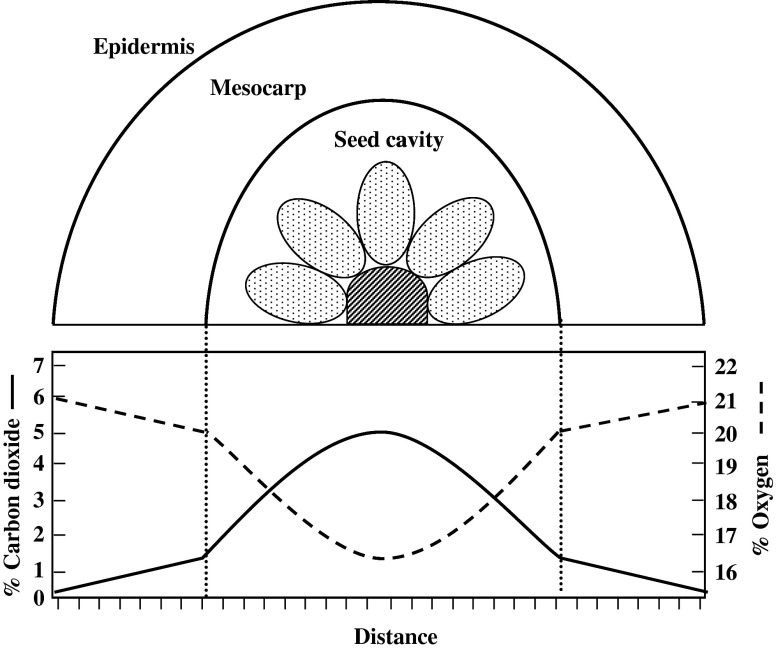
Fig. 5.
Cross section passing through a fruit showing how the concentration of O2 and CO2 can vary within different tissues of the fruit due to respiration and external and internal barriers to gas diffusion (Source: Kader and Saltveit 2003b)
Susceptibility of apple fruit to low O2 injury in CA storage was found to be positively correlated with resistance of the fruit towards the diffusion of gases. For a given strain/cultivar, resistance to gas diffusion was found to be affected by fruit’s maturity, duration of storage and whole fruit volume (Park et al. 1993). It was noticed that, ‘Marshall’ strain of McIntosh apple had higher resistance and thereby it showed more susceptibility to low O2 injury as this strain accumulated ten times higher ethanol when compared with ‘Rogers’ (another strain of McIntosh apple) (Park et al. 1993). This demonstrates the extent of varietal variability among the fruits for response towards their internal atmosphere and specially for the lower concentrations of O2.
Modified atmospheres with low concentrations of O2 can slow down the deterioration of fruits by decreasing respiration, ethylene production and tissue sensitivity to the ethylene (Kader et al. 1989). The extent of decrease in ethylene production therefore depends not only on the O2 concentration which is present in the fruit’s internal atmosphere but also on the sensitivity of ethylene production system under a prevailing concentration of O2. Sanders and de Wild (2003) reported lower partial pressure of O2 (lower than the external partial pressure of O2) due to rapid consumption of O2 by the tomato fruit and higher resistance of fruit to the diffusion of O2. Reduced O2 or elevated CO2 decreased the respiration rate (Smith et al. 1987a). In bulky storage organs such as fruits (where the length of the diffusion path may be considerable), hypoxia conditions have been demonstrated (Ho et al. 2008). So, low O2 stress may occur within the fruit as it grows and the resistance to the entry of O2 from the atmosphere into the fruit (via diffusion process through the skin and thickened cell layers of the cortex) becomes significant.
In avocado fruit, synthesis of cellulase and polygalacturonase was found to be directly related to the levels of O2. Insufficient availability of O2 can decrease the activities of cellulase and polygalacturonase (Knee 1982; Kanellis et al. 1989a, 1991). These two enzymes are involved in causing softening of fruits during ripening. In general, it has been observed that treatment to the fruits just prior to their storage with the condition like anaerobic or even exposure to the metabolites which are produced under such condition (like; acetaldehyde and/or ethanol) lead to improvement in fruit quality (Pesis 2006).
High carbon dioxide
There is a decreasing gradient in CO2 concentrations from the inner parts of the fruit to the surface, in reverse to the gradients observed for O2 (Fig. 5). High CO2 was reported to reduce the activity or synthesis of various enzymes of respiratory metabolism (Kerbel et al. 1988; Lange and Kader 1997a) including oxidative phosphorylation (Shipway and Bramlage 1973). Activation of enzymes of glyoxylate cycle was noticed in cucumber fruits when fruits were exposed to the environment of 60% CO2 (Yang et al. 1998). As per Lange and Kader (1997b), elevated CO2 could influence the respiration negatively by changing the intercellular pH. Studies on respiration and the factors influencing the respiration become important because the potential shelf-life of harvested plant parts (including fruits) was found to be closely related to the rate of respiration of the plant part (Uys 1974; Kader 1987; Varoquaux and Ozdemir 2005; Kader and Saltveit 2003b). Bufler (1984) reported that CO2 at high concentrations competitively inhibits the effects of ethylene by preventing the auto-induction of ACC-synthase, as shown in Fig. 6. The inhibitory effect of CO2 on auto-induced ethylene production in climacteric fruits could be due to competition between CO2 and ethylene for the same active site (Burg and Burg 1967; Mathooko et al. 1995). As per Burg and Burg (1967), the amount of CO2 in the intercellular spaces of fruits at pre-climacteric stage is low but this may approach to higher levels of around 10% during ripening and post-climacteric phase. This higher endogenous level of CO2 probably raises the threshold concentration of ethylene to higher levels for its action in fruits. It has been demonstrated that elevated CO2 (5–20%) inhibits ethylene production in climacteric fruits by inhibiting activities of ACC-synthase (Bufler 1984; Chavez-Franco and Kader 1993; Mathooko et al. 1995) and ACC-oxidase (Chavez-Franco and Kader 1993; Mathooko et al. 1995). CO2-mediated regulation of ACC-synthase and ACC-oxidase has also been reported by Kader (1986) and Yang (1987). In tomato, high CO2 induces the expression of stress-related genes and suppresses the transcription of ethylene-dependent and ethylene-independent ripening-associated genes (Rothan et al. 1997).
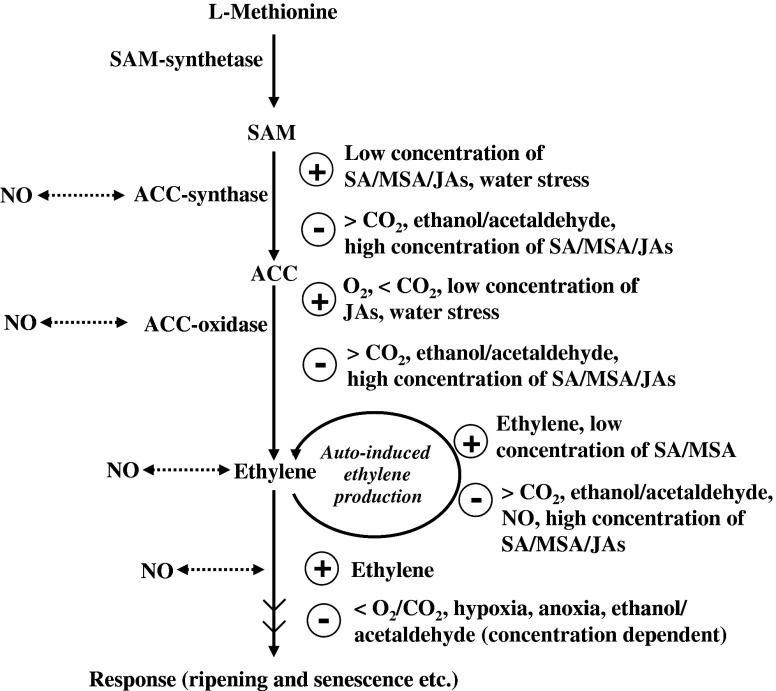
Fig. 6.
Different endogenous volatiles and other factors that play regulatory role in determining the production as well as the response of ethylene. The symbols  ,
,  , >, < and
, >, < and  indicate inducers, suppressors, higher, lower and possible interaction respectively. SAM: S-Adenosyl-L-methionine, ACC: 1-Amino-cyclopropane-1-carboxylic acid, SA: Salicylic acid, MSA: Methyl salicylic acid, JAs: Jasmonates, NO: Nitric oxide
indicate inducers, suppressors, higher, lower and possible interaction respectively. SAM: S-Adenosyl-L-methionine, ACC: 1-Amino-cyclopropane-1-carboxylic acid, SA: Salicylic acid, MSA: Methyl salicylic acid, JAs: Jasmonates, NO: Nitric oxide
Work carried out by Chaves and Tomas (1984) suggested that CO2 interferes with ethylene metabolism through a mass action effect. Besides this, displacing ethylene from its receptor site has also been proposed (Yang and Hoffman 1984). In comparison with control, production of ethylene in tomato and activities of ACC-synthase and ACC-oxidase were found to increase upon withdrawal of CO2 gas from the storage environment (Kubo et al. 1990; Mathooko et al. 1995). Levin et al. (1993) reported that CO2 concentration up to 10% stimulated in vivo activity of ACC-oxidase but, CO2 at 20% concentration had an inhibitory effect. Using a continuous flow through gas system, it has been demonstrated that 20% CO2 markedly decreases ethylene biosynthesis in ripening peaches by delaying and suppressing ACC-synthase at transcriptional level however, recovery occurs upon withdrawal of CO2 (Mathooko et al. 2001). At low concentrations (of about 1%), CO2 may promote ethylene production in climacteric fruits (Bufler 1986; Chavez-Franco and Kader 1993). At low levels, stimulatory effect of CO2 on the production of ethylene could be due to a balance between its stimulatory effect on the activity of ACC-oxidase and inhibitory effect on the activity of ACC-synthase wherein the contribution by the former being more significant (Mathooko 1996). All elevated levels of CO2 inhibit the activity of ACC-synthase while the activity of ACC-oxidase is differentially regulated by CO2 (it is stimulated at low CO2 levels but inhibited at high CO2 levels) (Mathooko 1996).
Tolerance of a commodity to elevated levels of CO2 depends on its physiological condition, maturity status, CO2 concentration within the tissue, duration of exposure, internal O2 concentration and storage temperature (Zagory and Kader 1988). It was reported by Mathooko et al. (1995) that CO2 itself could also act as an inducer of ACC-synthase depending on the commodity. Since the effect of CO2 was rapid and reversible so Kao and Yang (1982) proposed that CO2 exerts its effect by directly activating the ACC-oxidase (in vivo) rather than stimulating its synthesis. Later, Tian et al. (1994) proposed that the mechanism of CO2 stimulation of ACC-oxidase may be direct and probably through interaction with a non-substrate binding site on ACC-oxidase. They further stated that CO2 might combine reversibly with an ACC-oxidase-ACC complex to increase Vmax of the reaction. In tomato, interestingly, there are at least three forms of ACC-oxidase i.e., eth1, eth2 and eth3. These different forms of ACC-oxidase could probably be induced and/or synthesized in different tissues and at different developmental stages besides being a strong possibility that CO2 can also affect each of these forms of ACC-oxidase differentially (Bouzayen et al. 1993).
Ratio of O2 to CO2
As early as in 1936, Wardlaw and Leonard reported that respiratory climacteric is an anaerobic type of respiratory shift. Later on, climacteric rise was considered as a type of anaerobiosis because fruits naturally ripen from inside to outward (Leonard and Wardlaw 1941). Diagrammatic representation of fruit in Fig. 5 shows that how the concentrations of O2 and CO2 vary within the fruit. Respiration by the fruit tissues and barriers of diffusion/exchange of gases as posed by anatomical/morphological/physical/biochemical components (present either on surface or inside the fruit) are the responsible factors for the gradual lowering of O2 to CO2 ratio from outside to inside of the fruit.
As described above, conditions like; hypoxia or low O2/CO2 reduce the synthesis as well as the action of ethylene (Kanellis et al. 1991; Blanke 1991; Kanellis et al. 1993; Gorny and Kader 1996; Mathooko 1996). At the same time, hypoxia was also reported to reduce the expression of genes involved in the maturation process, which are regulated by ethylene (Kanellis et al. 1993). Production of volatiles has been shown to get altered in high CO2 or low O2 conditions (Mattheis et al. 1991; Ke et al. 1994; Larsen 1994). Besides this, high CO2 and/or low O2 within the atmosphere of the fruit can induce anaerobic metabolism resulting in enhanced accumulation of ethanol and acetaldehyde (Kader 1987). Ethanol and acetaldehyde were in fact reported to delay the ripening of tomato fruit (Kelly and Saltveit 1988; Beaulieu et al. 1997). This thereby explains the reason behind the retardation of ripening process due to short period of anaerobiosis treatment prior to the storage of tomato fruits as observed by Kelly and Saltveit (1988), Pesis and Marinansky (1993) and Paul and Srivastava (2006). CO2 at the level of 10 kPa in combination with 6 kPa of O2 was suggested to be suitable for cold storage for late and early harvested grapes up to 12 weeks and 4 weeks, respectively as this limits the losses due to gray mold (Crisosto et al. 2002).
Model based in silico analysis for the exchange of O2 and CO2 in pear fruit showed that O2 exchange takes place mainly through the intercellular spaces and the cell wall network and marginally through the intracellular liquid (cytoplasm). On the other hand, CO2 exchange occurs at similar rates through each of these phases (Ho et al. 2009). The biological variation in the apparent diffusivity of gases in tissue was related to the natural and random distribution of cells and pores in the cortex tissue (Ho et al. 2009). This thereby can have strong influence in deciding the available O2/CO2 ratio and subsequently the metabolic shifts and conditions created due to this. In this way, anatomical features can be considered to be responsible for already existing differences in the tolerance to reduced O2 and/or elevated CO2 levels among various fruits and vegetables. Promotive or inhibitory effects of O2 and/or CO2 levels, O2 to CO2 ratio, conditions like; hypoxia, anoxia and various volatile metabolites are being summarized for their effect on different steps of ethylene biosynthesis and ethylene response (or action) in Fig. 6. Regulatory role of above factors on the production of acetaldehyde and ethanol by modulating the expression and activities of PDC and ADH is presented in Fig. 7.
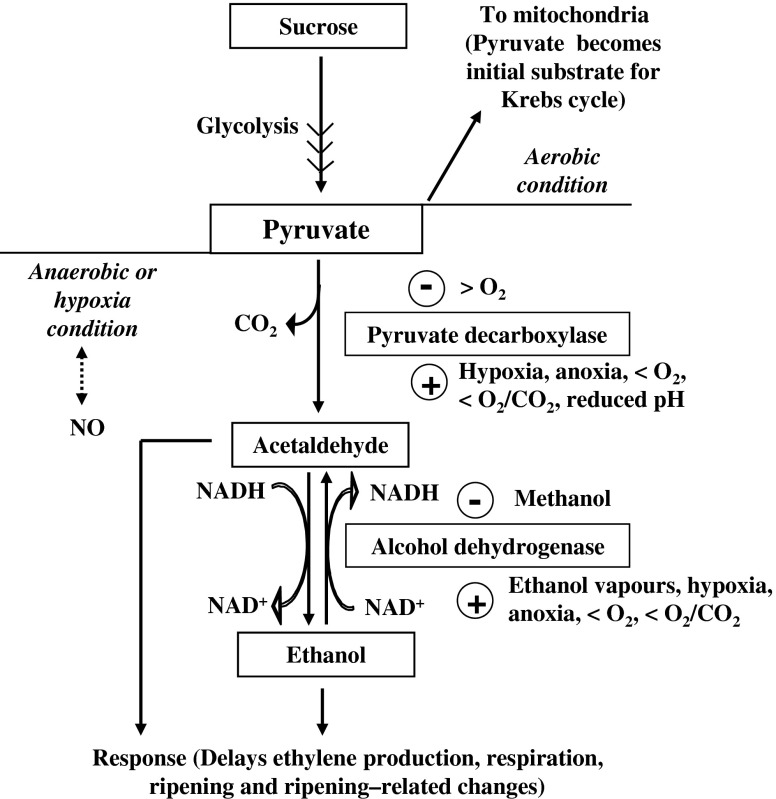
Fig. 7.
Pyruvate is produced during glycolysis. Under aerobic condition, this pyruvate enters into mitochondria for Krebs cycle. But under hypoxia or anaerobic condition, pyruvate is diverted for ethanolic glycolysis [where it is initially converted into acetaldehyde by the enzyme pyruvate decarboxylase (PDC) and then acetaldehyde is converted into ethanol by a reaction catalyzed by alcohol dehydrogenase (ADH)]. ADH basically catalyses bidirectional reaction for the inter-conversion of acetaldehyde and ethanol. The symbols  ,
,  , >, <, and
, >, <, and  indicate inducers, suppressors, higher, lower and possible interaction respectively. NO: Nitric oxide
indicate inducers, suppressors, higher, lower and possible interaction respectively. NO: Nitric oxide
III. Ethanol
Ethanol production in plants/fruits
Ethanol production (through anaerobic metabolism) generally results from low concentrations of O2 which can either be caused by reduced levels of external O2 or due to enhanced resistance to the diffusion of O2 into the plant parts/fruits (Jackson et al. 1982). Fruits undergoing the developmental/ripening process exhibit changes in the levels of O2 and CO2 inside them. These changes are usually in a direction that leads to a net reduction in O2 to CO2 ratio within the fruit and results in the accumulation of ethanol (Bufler and Bangerth 1982).
Ethanol-mediated inhibition of ripening
Loss of ethanol from fruit occurs predominantly by evaporation process and this is mainly determined by degree of diffusion resistance posed by the fruit in view of its surface/anatomical features as already described above. Effective ethanol concentrations for delaying the ripening were however found to be similar to that of endogenously built-up levels of ethanol as observed in following conditions 1. During ripening of fruit (Bufler and Bangerth 1982), 2. During anoxia (Jackson et al. 1982), 3. Under few days of anaerobiosis (Kelly and Saltveit 1988), 4. Under stress (Kimmerer and Kozlowski 1982) and 5. Fruits exposed directly to ethanol vapours (Kelly and Saltveit 1988). Ethanol appears to inhibit not only the synthesis of ethylene but its action as well (Ritenour et al. 1997; Saltveit and Mencarelli 1988; Pesis 2005; Asoda et al. 2009). Ethanol was also reported to delay ripening, production of ethylene and CO2, loss of chlorophyll and synthesis of lycopene (Kelly and Saltveit 1988; Yang and Shewfelt 1999; Podd et al. 2002; Pesis 2005). Promotive or inhibitory effect of O2 and/or CO2 levels, O2 to CO2 ratio, conditions like; hypoxia and anoxia and ethanol itself on the production of acetaldehyde (and thereby ethanol) by modulating the expression and activities of PDC and ADH are summarized in Fig. 7.
IV. Acetaldehyde
It has been proposed and demonstrated that ethanol-mediated delay in ripening is basically caused by acetaldehyde. Acetaldehyde is produced by conversion of ethanol into acetaldehyde via the reversible reaction catalyzed by the enzyme ADH (Pesis and Marinansky 1993; Burdon et al. 1996; Beaulieu et al. 1997; Podd et al. 2002) (Fig. 7). Since, acetaldehyde and ethanol are inter-convertible volatile compounds so they are being discussed together not only in relation to one another but also in terms of their final effect on fruit ripening.
Factors affecting the production of acetaldehyde and ethanol
Anaerobiosis leads to the production of acetaldehyde besides ethanol (Cossins 1978). Both, climacteric as well as non-climacteric fruits produce a lot of acetaldehyde and ethanol (Pesis 2005). For a given fruit, genetic variability was also seen in the levels of production of acetaldehyde and ethanol and also in the ability to survive under anaerobiosis (Pesis 2005).
Acetaldehyde in regulating the fruit ripening
Acetaldehyde inhibits the formation of ethylene by preventing the action of ACC-synthase and action and synthesis of ACC-oxidase (Pesis and Marinansky 1993; Burdon et al. 1996; Podd and van Staden 1998; Pesis et al. 1998). Exogenous ethanol application resulted in marked increase in acetaldehyde levels and this inhibited the ethylene production and ripening of tomato fruits (Pesis and Marinansky 1993). It was therefore suggested that it is the acetaldehyde and not the ethanol which is the causal agent for ethanol-induced inhibition of fruit ripening. Later on, it was in fact found to be true because it was only the level of acetaldehyde which was found to be associated with inhibition of ripening by Beaulieu et al. (1997). In light of above findings, it was concluded by Pesis (2005) that ethanol and acetaldehyde are natural compounds that are essential in governing the process of fruit ripening. These compounds are also associated with aroma production and removal of astringency. Various sites where acetaldehyde and ethanol can regulate the production and response of ethylene are presented in Fig. 6. Whereas in Fig. 7 regulatory effects of different gases, volatiles and conditions (that may prevail within the fruit’s internal atmosphere) are being presented on the activity of enzymes involved in the production of ethanol and acetaldehyde.
V. Water vapours/water status in fruit
Importance and diffusibility
Water is the most important component of plant tissues with unique physical and chemical properties. It plays a significant role in determining the quality of the fresh produce. Important functions of water in plant include; exchange of resources, medium for biochemical reactions, cell expansion and physical and chemical integrity of cell walls and other cellular constituents (Nilsen and Orcutt 1996). Fruit maintains vascular continuity with the mother plant and receives water till it remains attached to the plant. But, once detached, the fruit have no renewable source of water to compensate for the water that is being lost through transpiration. Detached fruit therefore experiences water stress. Water loss (transpiration) from freshly harvested fruit results in loss of salable weight, appearance (wilting and shriveling), textural quality (softening, flaccidity, limpness and crispness), juiciness and nutritional quality as well (Kader and Barrett 1996). Generally, it has been found true that fresh fruits become unacceptable when weight loss reaches more than 5% of harvest weight (Salunkhe and Desai 1984).
Transpiration rate of harvested fruit depends mainly on 1. The rate of cooling of fruit after its harvest, 2. Structure and condition of the fruit surface, 3. Surface to volume ratio of fruit, 4. Relative humidity and temperature during storage, 5. Air movement/circulation and 6. Atmospheric pressure in storage environment (Salunkhe and Desai 1984; Gamage and Rahman 1999). The main sites of transpiration in plant and its parts are the stomata, epidermal cells, lenticels, trichomes (hairs), stem scar, hydathodes and cuticular cracks (Ben-Yehoshua 1987). The surface characteristics such as; number of stomata on epidermis, type of surface, tissue underlying the skin and the structure, thickness and chemical composition of wax and cuticle play role in determining the water loss from fruit and these features vary greatly among the fruits and also with the developmental stages for a given fruit. Stem scar region is an important pathway for water loss in tomato fruit. In apple, lenticels account for up to 21% of the transpiration (Maquire et al. 2001). Complete coating of fruit was found to retard gaseous exchange by plugging the stomatal pores of citrus (Ben-Yehoshua et al. 1985). Such coatings reduced weight loss up to 20% in mandarin (Lawes and Prasad 1999), mango (Baldwin et al. 1999) and pear (Amarante et al. 2001). Variability in peel permeance, weight loss and internal atmosphere was recorded in different lines of mandarin fruit (Lawes and Prasad 1999). Size of the fruit is also an important determinant for extent of its weight loss because higher the ratio of surface area to unit volume the greater will be the loss of water by evaporation. As a result of this, a small size fruit or a tuber will lose weight faster than a bigger one (Salunkhe and Desai 1984). Likewise, under similar conditions, a leaf will lose its water (and thereby its weight) much faster than a fruit with similar surface area.
Influence of water status on ethylene production, ripening, senescence and shelf-life
It was reported by Grierson and Wardowski (1978) that water loss after harvesting causes reduction in fruit mass and it may also induce senescence. Water deficit in plant tissues also stimulate ethylene production (Fig. 6) and as a consequence there is an increase in the respiration of tissues (Yang and Pratt 1978). Several authors found that water stress decreases the pre-climacteric life of many fruits such as; banana, avocado, pear and plantain (Littmann 1972; Adato and Gazit 1974; George et al. 1982). It was found by Finger et al. (1995) that increase in respiration and evolution of ethylene was significantly higher when the fruits reached 5% of loss in terms of fresh mass. The increase in respiration was 70% and ethylene production was 50% more for such fruits when compared to the control fruits in banana. The results thereby confirmed that water stress (after the harvest of fruit) might affect the shelf-life depending upon the intensity of the water stress. So, alleviation of water stress has been proposed as an additional controlling factor for reducing the deterioration of fruits (Ben-Yehoshua et al. 1983). Increased ethylene production was found when fruit was detached (Hyodo 1991) and also after having experienced water stress/deficit during its development (Gelly et al. 2003). The level of transcription for the genes of ACC-synthase and ACC-oxidase responded positively to the water deficit in a tissue-specific and coordinated manner (Nakano et al. 2002). This resulted in enhanced activities of these two enzymes under water stress (Fig. 6). However, only little is known about the interaction of altered water status with other important volatiles that also have role in fruit ripening (Smith et al. 1987a, b). In this way, water status governs not only the broad changes but also the specific changes. Further studies are therefore necessary to understand the interactions and implications of altered water status with important volatile components of fruits.
Water content alone is not sufficient to describe the water status of different parts of fruits, its movement in fruits and also the associated physiological and biochemical changes occurring in the fruits (Nguyen et al. 2004). Water activity (aw) is considered as a more reliable parameter of water status. Water activity is defined as the ratio of the partial pressure of water in equilibrium with the food material to the partial pressure of pure water at the same temperature. It includes contribution of the pressure forces in the tissue (Nguyen et al. 2004). Water activity is important in the determination of the stability criteria for food stuffs in terms of microbial growth, browning, lipid oxidation, ripening, ripening-related changes and shelf-life. (Rockland and Stewart 1981; Labuza 1984; Joyce et al. 2002). Relationship between water content and aw of a given fruit gives water sorption isotherm. Since, different tissues of a fruit have different water status so, aw and water sorption isotherm for different tissues are also different. Besides this, aw and water sorption isotherm also change with changes in the storage conditions and storage durations (Hubinger et al. 1992; Joyce et al. 2002; Nguyen et al. 2004). In an attempt to relate the moisture distribution (water content) to the water status (water potential) for understanding the water movement in fresh fruit need was felt for a detailed modeling of cellular structural properties and intercellular spaces (Nguyen et al. 2004)
VI. Role of some other endogenous volatiles
Salicylic acid/methyl salicylate
Salicylic acid (SA) and its volatile derivatives [such as methyl salicylate (MeSA)] were characterized as inhibitors of ethylene biosynthesis (Leslie and Romani 1986). These volatiles were reported to inhibit the wound-induced expression ACC-synthase (Li et al. 1992). At low concentrations, MeSA appears to enhance the ripening processes in tomato (Ding et al. 2002). Dual effects of MeSA on ethylene metabolism (inhibitory and promotive) were suggested to be dependent upon the dose and developmental stage of fruits (Ding and Wang 2003). It was clearly demonstrated by Ding and Wang (2003) that low concentrations of MeSA have the potential to up-regulate ethylene biosynthesis by increasing the expression of ACC-synthase genes LE-ACS2 and LE-ACS4 (responsible for auto-induced production of ethylene during ripening of tomato fruit) and eliminating the transcription of LE-ACS6 (genes of ACC-synthase responsible for basal level of ethylene production in tomato fruit). On the other hand, high levels of MeSA in tomato fruit keep the ACC-synthase and ACC-oxidase genes repressed and thus inhibit the timely production of ethylene during ripening (Ding and Wang 2003). In this way, the endogenous concentration of MeSA could be critical in determining ethylene metabolism depending on the stage of the fruit.
Jasmonic acid/jasmonates
Jasmonic acid (JA), its volatile ester i.e., methyl jasmonate (MJ) and other derivatives are collectively known as jasmonates (JAs). JAs are ubiquitous signaling molecules that mediate plant responses to environmental stress such as; wounding and insect/pathogen attack (Wasternack 2007). JAs also play role during developmental processes including pollen development, seed germination, root growth, and fruit growth and ripening (Pena-Cortes et al. 2005; Wasternack 2007). Multiple interferences and interactions between JAs and ethylene signaling pathways were studied by analyzing Arabidopsis mutants (Devoto and Turner 2005). The exact role of JA in fruit ripening and JA-ethylene interaction are still largely unclear. Both, delaying as well as promotive effects on ripening were recorded besides the response being the cultivar dependent.
Transient increases in concentrations of JAs occur during the onset of fruit ripening in apple and tomato suggest their involvement in modulation of the early steps of climacteric fruit ripening (Saniewski and Czapski 1983; Saniewski et al. 1987; Perez et al. 1993; Creelman and Mullet 1997). Furthermore, JAs were reported to regulate the early steps of climacteric fruit ripening by stimulating ethylene biosynthesis (Fan et al. 1998; Kondo et al. 2000). The application of exogenous JAs stimulates ethylene production and colour change in tomato (Saniewski and Czapski 1985). It was found that activities of ACC-synthase and ACC-oxidase were stimulated by JAs in a concentration range of 1–100 μM. But, continuous exposure at higher dose i.e., 1,000 μM inhibited both the activities (Fan et al. 1998). The convergence of ethylene and jasmonate pathways at transcriptional level of ethylene response factor 1 (ERF1) was proposed by Lorenzo et al. (2003). MJ induced the transcription of ACC-oxidase in tomato fruit (Imanishi and Nagata 2004; Imanishi et al. 2005). The mRNA levels of 81 genes were found to show elevation (by more than three-fold) in response to MJ in wild type tomato in comparison to nor mutant (Imanishi et al. 2005). The results indicate a strong interaction of jasmonate and ethylene and in this way endogenous status of jasmonate could regulate the ripening and ripening-related changes (Fig. 6). In a study by Ziosi et al. (2007), exogenous application of JAs led to alteration in ethylene biosynthesis, ethylene perception and polyamine accumulation. In another study involving transcriptome analysis of JAs treated peach fruit (at harvest) indicated that delayed ripening was due to interference in ripening- and stress/defense-related genes (Ziosi et al. 2008).
Nitric oxide
Nitric oxide (NO) has been reported to be emitted simultaneously with ethylene and in this way it showed a stoichiometric relationship with ethylene production (Leshem and Haramaty 1996). It was shown by Leshem et al. (1998) that exogenous NO extends the postharvest-life and delayed senescence in fruits, vegetables and flowers. Later, Leshem et al. (2000) made it clear that NO is natural senescence-delaying plant growth substance acting by down-regulating ethylene production. NO affects the ethylene production through its direct regulatory effect on ACC-synthase or ACC-oxidase enzymes as well as on their genes (Wills et al. 2000). Programmed cell death, which is considered to be caused by ethylene, is rather induced by NO as per Neil et al. (2003). In this context, some studies have shown that the aerenchyma formation (which is stimulated by hypoxia and ethylene) is instead caused by NO (Igamberdiev and Hill 2004; Borisjuk et al. 2007).
Application of NO to tomato delayed the burst in ethylene production and colour development at green mature and breaker stages of fruits but not at pink and full red stages (Eum et al. 2009). The results therefore implicate that NO might control the postharvest metabolism of fruits depending on its dose and the status of the commodity that is being treated (Eum et al. 2009). Borisjuk et al. (2007) proposed a key role of NO in immediate sensing and balancing the O2 status in seeds of the plant. NO was found to mediate reversible O2 balancing via its effect on respiratory activity and enabling the seed to avoid endogenous anoxia or extreme hypoxia. In this way, NO appears to control energy availability for ongoing biochemical and physiological activities. Depletion in the level of O2 and the conditions like; hypoxia or anoxia were already reported to prevail in fruit undergoing maturation and ripening (Lammertyn et al. 2003; Franck et al. 2007; Ho et al. 2008). So, NO-mediated sensing of O2 and balancing its levels in fruits cannot be ruled out. NO is also found to act as a regulatory factor for embryonic dormancy break in apple by stimulating biosynthesis of ethylene (Gniazdowska et al. 2007). It has been reported that NO can function as an endogenous mediator in diverse plant physiological processes but its production, effect and associated mechanisms are not known very precisely (Besson-Bard et al. 2008). Likewise, Wills et al. (2000) and Leshem et al. (2000), Eum et al. (2009) also reported that NO affects the ethylene production through its direct regulatory effect on the enzymes of ethylene biosynthesis (ACC-synthase and ACC-oxidase) and also on their genes during ripening of tomato.
The regulatory and interactive linkages of NO with biosynthesis and response of ethylene are presented in Fig. 6 beside this, interaction of NO with the anaerobic or hypoxia condition is shown in Fig. 7. Interactions of some of the volatiles like; C2H4, O2, CO2, SA/MeSA, JAs and NO on various components of respiratory metabolism was reported by Millar and Day (1996), Fan et al. (1997), Yang et al. (1998), Ederli et al. (2006), Leakey et al. (2009) and Wang et al. (2010). This shows that different volatiles and gases can affect the status of respiration which in turn is closely linked with the potential shelf-life of plant parts (including fruits) after their harvest (Kader 1987; Kader and Saltveit 2003b; Varoquaux and Ozdemir 2005; Paul and Srivastava 2006; Paul et al. 2010b).
Practical implications of the internal atmosphere in fruit
Permeability of different volatiles or gases is governed by diffusivity of these compounds in air, water and across the cellular microstructures of fruit. Microstructures of internal and surface tissues of fruit include their cellular details, porosity (up to micro level) and 3-D organization of cells. In this way, permeability changes in a dynamic way with changes in the metabolic activities of tissues, developmental stage, maturity and storage time. Besides this, the external factors like; temperature, relative humidity and gaseous composition of immediate storage environment also affect the permeability within and across the fruit. Fick’s law, as such cannot be applied directly to predict the gaseous concentration in fruits because fruit tissue does not represent a continuum. Several researchers have described different geometrical models with mathematical equations that also take into the consideration the tissue microstructures besides other factors (Nguyen et al. 2004; Verboven et al. 2008; Ho et al. 2008, 2009, 2010).
Today, efforts are being made for more effective use of some gases such as; carbon monoxide (CO), nitrous oxide (N2O), nitrogen (N2), sulphur dioxide (SO2), chlorine dioxide (ClO2) in the storage environment to reduce the microbial, insect and pest infestation. Besides this, ozone (O3) gas is also being exploited in delaying the ripening and improving the quality of the stored fruits (Scully and Horsham 2008; Mangaraj and Goswami 2009; Hoehn et al. 2009; Yahia 2009; Zambre et al. 2010; Rodoni et al. 2010). Ozone treated fruits are reported to show delay in ripening and decay, proper maintenance of quality, suppression in microbial contamination and reduction in the levels of ethylene in storage environment and within the fruits as well (Rice et al. 1982; Kim et al. 1999; Xu 1999; Zambre et al. 2010; Rodoni et al. 2010). Exposure of O3 also reduced the levels of pesticide in stored apples (Ong et al. 1996). Besides the practical applicability of above gases, required levels of O2, CO2, ethylene, humidity and condensation levels in the storage environment can also delay ripening and improve quality and storability of fruits (Scully and Horsham 2008; Mangaraj and Goswami 2009; Hoehn et al. 2009; Yahia 2009).
Developments in the field of plant volatiles and their roles further showed that the quality and quantity of volatiles in plant/plant part may also be linked with various types of abiotic and biotic stresses (Steindel et al. 2005; Karl et al. 2008; Loreto and Schnitzler 2010). Some specific examples are 1. Hexanal, trans-2-hexenal and hexyl acetate improve the safety of freshly sliced apple (Lanciotti et al. 2003), 2. Trans-2-hexenal confer resistance to anthracnose fruit decay in highbush blueberry (Polashock et al. 2007), 3. Hexanal also reduces infection of tomatoes by Botrytis cinerea (Utto et al. 2008), 4. Alpha-farnesene increases the suseptability of apple fruits to scald disorder (Huelin and Coggiola 1968; Watkins et al. 1993), 5. Different endogenous volatiles (aldehydes, alcohols and esters) and trans-2-hexenal exhibit antifungal properties against the Colletotrichum acutatum that causes anthracnose in strawberry fruits (Arroyo et al. 2007), 6. Several terpenoids (sesquiterpenes like; zingiberene and curcumene and the monoterpenes like; p-cymene, α-terpinene, and α-phellandrene) strongly repel whiteflies that infest tomatoes (Bleeker et al. 2009) and 7. Isoprene is reported to provide thermo tolerance in Arabidopsis (Sasaki et al. 2007) but, its role in fruits is yet to be studied. Strawberries treated with MJ alone or in combination with ethanol showed higher antioxidant capacity, total phenolics and anthocyanins along with longer postharvest-life and better quality and aromatic properties. Combined treatment also increased total volatile compounds during storage (although there were quantitative and qualitative changes depending upon the individual volatile compound) (Ayala-Zavala et al.2005). In view of the above reports, plant volatiles are important because fruits are reported to be accompanied by increase in the levels of reactive oxygen species during their maturation and ripening and this situation represents an oxidative stress like condition for fruits (Stanley 1991; Ferrie et al. 1994; Palma et al. 1995; Rogiers et al. 1998). These examples show that at least some of the volatiles in the internal atmosphere of fruit can provide protection against biotic and abiotic stresses during ripening and storage and reflect the potential practical applications of these volatiles in overall postharvest management of fruits.
Better understanding of the environment that prevails inside and also outside the fruit will allow us to further refine measures for improving the efficacy of storage environments for delaying the ripening and senescence, minimizing microbial or insect/pest-mediated contamination and improving the shelf-life and quality aspects of stored items. Future studies on storage technology should focus on 1. Defining and then generating optimum internal atmospheres in fruits, 2. Providing conditions which are cultivar specific, 3. Making use of high precision sensors for monitoring the levels of volatiles and gases 4. Developing and using a fruit specific and real time system to manipulate the levels of at least some of the most critical volatiles/gases during storage and 5. Exploiting the use of some of the fruit specific volatiles as exogenous treatments to tackle both a specific or a broad problems related to postharvest management.
Conclusion
Endogenous volatiles in fruits are usually known for their role in determining the flavour and aroma. Only a few studies show their importance and role in a specific plant process. It is rare that combined and interactive effects of different endogenous volatiles have been studied on postharvest physiology of fruits, ripening process and postharvest management practices that are being followed for fruits. Both, the external and the internal gaseous environments of the fruit play crucial role in regulating the process of ripening. So far, very little is being understood about the interactive mechanisms of different volatiles (other than ethylene) in regulating the process of ripening. Ethylene is of course a major controlling factor for the ripening and related changes in climacteric fruits but its biosynthesis, perception, sensitivity and even action are being influenced either directly or indirectly by other endogenous volatiles such as; acetaldehyde, ethanol, methyl salicylate, methyl jasmonate and NO besides the O2 to CO2 ratio and water status of the fruit. The internal atmospheres in fruit should be analysed and understood to enable effective manipulation of ripening and control of the quality of fruits. Efforts to decipher the dynamics of internal atmospheres in relation to the progress of fruit ripening could be of great practical importance. Such information could be used to more precisely manage the storage environment and to optimize the composition of the internal atmosphere in the fruit. This will help not only in achieving the best quality, aroma, flavour and shelf-life but also in minimizing the decay, pest infestation and microbial infection of fruits during storage.
References
- Abe K, Watada AE. Ethylene absorbent to maintain quality of lightly processed fruits and vegetables. J Food Sci. 1991;56:1589–1592. [Google Scholar]
- Abeles FB, Gahagan HE. Abscission: the role of ethylene, ethylene analogues, carbon dioxide, and oxygen. Plant Physiol. 1968;43:1255–1258. doi: 10.1104/pp.43.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles FB, Morgan PW, Saltveit ME. Ethylene in plant biology. Vol 15. 2. San Diego: Academic; 1992. [Google Scholar]
- Adato I, Gazit S. Water-deficit stress, ethylene production and ripening in avocado fruits. Plant Physiol. 1974;53:45–46. doi: 10.1104/pp.53.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- Amarante C, Banks NH, Ganesh S. Characterizing ripening behaviour of coated pears in relation to fruit internal atmosphere. Postharvest Biol Tech. 2001;23:51–59. [Google Scholar]
- Arroyo FT, Moreno J, Daza P, Boianova L, Romero F. Antifungal activity of strawberry fruit volatile compounds against Colletotrichum acutatum. J Agr Food Chem. 2007;55:5701–5707. doi: 10.1021/jf0703957. [DOI] [PubMed] [Google Scholar]
- Asoda T, Terai H, Kato M, Suzuki Y. Effects of postharvest ethanol vapor treatment on ethylene responsiveness in broccoli. Postharvest Biol Tech. 2009;2:216–220. [Google Scholar]
- Atta-Aly M, Brecht JK, Huber DJ. Ethylene feedback mechanisms in tomato and strawberry fruit tissues in relation to fruit ripening and climacteric patterns. Postharvest Biol Tech. 2000;20:151–162. [Google Scholar]
- Aubert C, Chanforan C. Postharvest changes in physicochemical properties and volatile constituents of apricot (Prunus armeniaca L.) characterization of 28 cultivars. J Agric Food Chem. 2007;55:3074–3082. doi: 10.1021/jf063476w. [DOI] [PubMed] [Google Scholar]
- Ayala-Zavala JF, Wang SY, Wang CY, Gonzalez-Aguilar GA. Methyl jasmonate in conjunction with ethanol treatment increases antioxidant capacity, volatile compounds and postharvest life of strawberry fruit. Eur Food Res Technol. 2005;221:731–738. [Google Scholar]
- Bai J, Hagenmaier RD, Baldwin EA. Coating selection for ‘Delicious’ and other apples. Postharvest Biol Tech. 2003;28:381–390. [Google Scholar]
- Baldwin EA (2004) Flavor. In: Gross KC, Wang CY, Saltveit M (eds) The commercial storage of fruits, vegetables and florist and nursery stocks. Agricultural research service, agriculture handbook number 66, Beltsville, MD. http://www.ba.ars.usda.gov/hb66/023flavor.pdf. Accessed 22 December 2010
- Baldwin EA, Burn JK, Kazokas W, Brecht JK, Hagenmaier RD, Bender RJ, Pesis E. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biol Tech. 1999;17:215–226. [Google Scholar]
- Baldwin EA, Nisperos-Carriedo MO, Moshonas MG. Quantitative analysis of flavour and other volatiles and for certain constituents of two tomato cultivars during ripening. J Am Soc Hort Sci. 1991;116:265–269. [Google Scholar]
- Baldwin EA, Scott JW, Shewmaker CK, Schuch W. Flavour trivia and tomato aroma: biochemistry and possible mechanisms for control of important aroma components. HortSci. 2000;35:1013–1022. [Google Scholar]
- Banks NH. Internal atmosphere modification in pro-long coated apples. Acta Hort. 1984;157:105–112. [Google Scholar]
- Banks NH, Cleland DJ, Yearsley CW, Kingsley M (1993) Internal atmosphere composition—a key concept in responses of fruits and vegetables to modified atmospheres. In: Proc Aust Postharvest Conf, University of Queensland, 19–23 Sept, p 137–143
- Banks NH, Nicholson SE. Internal atmosphere composition and skin permeance to gases of pepper fruit. Postharvest Biol Tech. 2000;18:33–41. [Google Scholar]
- Bargel H, Neinhuis C. Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J Exp Bot. 2005;56:1049–1060. doi: 10.1093/jxb/eri098. [DOI] [PubMed] [Google Scholar]
- Barry CS, Giovannoni JJ. Ethylene and fruit ripening. J Plant Growth Regul. 2007;26:143–159. [Google Scholar]
- Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JC, Peiser G, Saltveit ME. Acetaldehyde is a causal agent responsible for ethanol induced ripening inhibition in tomato fruits. Plant Physiol. 1997;113:431–439. doi: 10.1104/pp.113.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belding RD, Blankenship SM, Young E, Leidy RB. Composition and variability of epicuticular waxes in apple cultivars. J Am Soc Hort Sci. 1998;123:348–356. [Google Scholar]
- Ben-Yehoshua S. Transpiration, water stress and gas exchange. In: Weichman J, editor. Postharvest physiology of vegetables. New York: Marcel Dekker; 1987. p. 113. [Google Scholar]
- Ben-Yehoshua S, Burg SP, Young R. Resistance of citrus fruit to mass transport of water vapours and gases. Plant Physiol. 1985;79:1048–1053. doi: 10.1104/pp.79.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehoshua S, Cameron AC. Exchange determination of water vapour, carbon dioxide, oxygen, ethylene and other gases of fruits and vegetables. In: Linskens HF, Jackson JF, editors. Modern methods of plant analysis. New series, vol 9, gases in plant and microbial cells. Berlin: Springer; 1989. pp. 177–193. [Google Scholar]
- Ben-Yehoshua S, Rodov V. Transpiration and water stress. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetable. New York: Marcel Dekker; 2003. pp. 111–159. [Google Scholar]
- Ben-Yehoshua S, Shapiro B, Even-Chen Z, Lurie S. Mode of action of plastic film extending life of lemon and bell pepper fruit by alleviation of water stress. Plant Physiol. 1983;73:87–93. doi: 10.1104/pp.73.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AD, Sargent SA. Real-time microsensor measurement of internal oxygen partial pressure in tomato fruit under hypoxia conditions. Postharvest Biol Tech. 2009;52:240–242. [Google Scholar]
- Besson-Bard A, Pugin A, Wendeheme D. New insights into nitric oxide signaling in plants. Annu Rev Plant Biol. 2008;59:21–39. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- Biale JB. Growth, maturation and senescence in fruits. Science. 1964;146:880–888. doi: 10.1126/science.146.3646.880. [DOI] [PubMed] [Google Scholar]
- Biale JB, Young RE. Respiration and ripening in fruits–retrospective and prospect. In: Friend J, Rhodes MJC, editors. Recent advances in the biochemistry of fruits and vegetables. New York: Academic; 1981. pp. 1–39. [Google Scholar]
- Blanke MM. Comparative SEM study of stomata on developing quince, apple, grape and tomato fruit. Angewardte Botanik. 1986;60:209–214. [Google Scholar]
- Blanke MM. Respiration of apple and avocado fruits. Postharvest News Info. 1991;2:429–436. [Google Scholar]
- Bleeker PM, Diergaarde PJ, Ament K, Guerra J, Weidner M, Schutz S, de Both MTJ, Haring MA, Schuurink RC. The role of specific tomato volatiles in tomato–whitefly interaction. Plant Physiol. 2009;151:925–935. doi: 10.1104/pp.109.142661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersig MR, Kader AA, Romani RJ. Aerobic-anaerobic respiratory transition in pear fruit and cultured pear fruit cells. J Am Soc Hort Sci. 1988;111:869–873. [Google Scholar]
- Borisjuk L, Macherel D, Benamar A, Wobus U, Rolletschek M. Low oxygen sensing and balancing in plant seeds—a role for nitric oxide. New Phytol. 2007;176:813–823. doi: 10.1111/j.1469-8137.2007.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester HJ, Gershenzon J, Konings MCJM, Croteau R. Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway. I. Demonstration of enzyme activities and their changes with development. Plant Physiol. 1998;117:901–912. doi: 10.1104/pp.117.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzayen M, Cooper W, Barry C, Zegzouti H, Hamilton AJ, Grierson D. EFF multigene family in tomato plants: expression and characterization. In: Pech JC, Latche A, Balague C, editors. Cellular and molecular aspects of the plant hormone ethylene. Dordrecht: Kluwer Academic Publishers; 1993. pp. 76–81. [Google Scholar]
- Bouzayen M, Latche A, Pech JC, Nath P. Mechanism of fruit ripening. In: Pua EC, Davey MR, editors. Plant developmental biology-biotechnological perspective. Vol 1. Berlin: Springer-Verlag, Heidelberg; 2010. pp. 319–339. [Google Scholar]
- Bower J, Holford P, Latche A, Pech JC. Culture conditions and detachment of the fruit influence the effect of ethylene on the climacteric respiration of the melon. Postharvest Biol Tech. 2002;26:135–146. [Google Scholar]
- Brauss MS, Linforth RST, Taylor AJ. Effect of variety, time of eating and fruit to fruit variation on volatile release during eating of tomato fruit (Lycopersicon esculentum) J Agric Food Chem. 1998;46:2287–2292. [Google Scholar]
- Bufler G. Ethylene enhanced 1-aminocyclopropane-1-carboxylic acid synthase activity in ripening apples. Plant Physiol. 1984;75:192–196. doi: 10.1104/pp.75.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufler G. Ethylene-promoted conversion of 1-aminocyclopropane-1-carboxylic acid to ethylene in peel of apple at various stages of development. Plant Physiol. 1986;80:539–543. doi: 10.1104/pp.80.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufler G, Bangerth F. Pyruvate decarboxylase in “Golden Delicious” apples; kinetics and relation to acetone and ethanol production in different storage atmospheres. Sci Hort. 1982;16:137–146. [Google Scholar]
- Burdon J, Dori S, Marinansky R, Pesis E. Acetaldehyde inhibition of ethylene biosynthesis in mango fruit. Postharvest Biol Tech. 1996;8:153–161. [Google Scholar]
- Burg SP. Ethylene, plant senescence and abscission. Plant Physiol. 1968;43:1503–1511. [PMC free article] [PubMed] [Google Scholar]
- Burg SP, Burg EA. The role of ethylene in fruit ripening. Plant Physiol. 1962;37:179–189. doi: 10.1104/pp.37.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP, Burg EA. Ethylene action and the ripening of fruits. Science. 1965;148:1190–1196. doi: 10.1126/science.148.3674.1190. [DOI] [PubMed] [Google Scholar]
- Burg SP, Burg EA. Gas exchange in fruits. Physiol Plant. 1965;18:870–886. [Google Scholar]
- Burg SP, Burg EA. Molecular requirement for the biological activity of ethylene. Plant Physiol. 1967;42:144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery R. Quantitative and sensory aspects of flavour of tomato and other vegetables and fruits. In: Acree TE, Teranishi R, editors. Flavour science: sensible principles and techniques. Washington: American Chemical Society; 1993. pp. 259–286. [Google Scholar]
- Cadwallader KR. Flavour and volatile metabolism in produce. In: Lamikanra O, Imam S, Ukaku D, editors. Produce degradation pathways and prevention. Boca Raton: CRC Press (Taylor and Francis Group); 2005. pp. 155–180. [Google Scholar]
- Calbo AG, Pererira AS, Horino Y (1988) Sealants for ripening control of tomatoes. In: Brazilian congress of vegetable crops. Proc SOB, Brazil, 6:28 (In Portuguese)
- Cameron AC, Reid SR. Diffusive resistance: importance and measurement in controlled atmosphere storage. In: Richardson DG, Meherink M, editors. Controlled atmospheres for storage transport of perishable agricultural commodities. Portland: Timber; 1982. pp. 171–180. [Google Scholar]
- Cameron AC, Yang SF. A simple method for the determination of resistance to gas diffusion in plant organs. Plant Physiol. 1982;70:21–23. doi: 10.1104/pp.70.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha KL, Pareek OP. Advances in horticulture: fruit crops, vol 3. New Delhi: Malhotra Publishing House; 1993. [Google Scholar]
- Charron CS, Cantliffe DJ. Volatile emissions from plants. Hort Rev. 1995;17:43–72. [Google Scholar]
- Chaves AR, Tomas JO. Effect of a brief CO2 exposure on ethylene production. Plant Physiol. 1984;76:88–91. doi: 10.1104/pp.76.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Franco SH, Kader AA. Effects of CO2 on ethylene biosynthesis in ‘Bartlett’ pears. Postharvest Biol Tech. 1993;3:183–190. [Google Scholar]
- Chen ARS, Chase T., Jr Alcohol dehydrogenase and pyruvate decarboxylase induction in ripening and hypoxia tomato fruit. Plant Physiol Biochem. 1993;31:875–885. [Google Scholar]
- Chen L, Stacewiez-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, Breemen R, van Ashton D, Bowen PE. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Canc Inst. 2001;93:1872–1879. doi: 10.1093/jnci/93.24.1872. [DOI] [PubMed] [Google Scholar]
- Chervin C, Brady CJ, Patterson BD, Faragher JD. Could studies on cell responses to low oxygen levels provide improved options for fruit storage and disinfestations? Postharvest Biol Tech. 1996;7:289–299. [Google Scholar]
- Chervin C, Lavigne DL, Westercamp P. Reduction in gray mold development in table grapes by preharvest sprays with ethanol and calcium chloride. Postharvest Biol Tech. 2009;54:115–117. [Google Scholar]
- Clendenning KA. Studies of the tomato in relation to its storage II. The effects of altered internal atmosphere upon the ripening and respiratory behaviour of tomato fruit stored at 12.5 °C. Can J Res. 1941;19:500–518. [Google Scholar]
- Cossins EA. Ethanol metabolism in plant. In: Hood DD, Crawford RMM, editors. Plant life in anaerobic environments. Ann Arbor: Science Publishers; 1978. pp. 169–202. [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Crisosto CH, Garner D, Crisosto G. Carbon dioxide-enriched atmosphere during cold storage limit losses from Botrytis but accelerate rachis browning of ‘Redglobs’ table grapes. Postharvest Biol Tech. 2002;26:181–189. [Google Scholar]
- Dadzie BK, Banks NH, Cleland DS, Hewett EW. Role of skin resistance to gas diffusion in the response of fruits to modified atmosphere. Acta Hort. 1993;343:129–134. [Google Scholar]
- Dandekar AM, Teo G, Defilippi BG, Uratsu SL, Passey AJ, Kader AA, Stow JR, Colgan RJ, James DJ. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res. 2004;13:373–384. doi: 10.1023/b:trag.0000040037.90435.45. [DOI] [PubMed] [Google Scholar]
- Dauny PT, Joyce DC, Gamby C. 1-Methylcyclopropene influx and efflux in ‘Cox’ apple and ‘Hass’ avocado fruit. Postharvest Biol Tech. 2003;29:101–105. [Google Scholar]
- de Leon-Sanchez FD, Pelayo-Zaldivar C, Rivera-Cabrera F, Ponce-Valadez M, Avila-Alejandre X, Fernandez FJ, Escalona-Buendia HB, Perez-Flores LJ. Effect of refrigerated storage on aroma and alcohol dehydrogenase activity in tomato fruit. Postharvest Biol Tech. 2009;54:93–100. [Google Scholar]
- de Vries HSM, Harren FJM, Voesenek LACJ, Blom CWPM, Woltering EJ, van der Valk HCPM, Reuss J. Investigation of local ethylene emission from intact cherry tomatoes by means of photothermal deflection and photoacoustic detection. Plant Physiol. 1995;107:1371–1377. doi: 10.1104/pp.107.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippi BG, Manriquez D, Luengwilai K, Gonzalez-Aguero M. Aroma volatiles: biosynthesis and mechanisms of modulation during fruit ripening. Adv Bot Res. 2009;50:1–37. [Google Scholar]
- Devoto A, Turner JG. Jasmonate-regulated Arabidopsis stress signalling network. Physiol Plant. 2005;123:161–172. [Google Scholar]
- Dietz TH, Timma-Raju KR, Joshi SS. Studies on loss of weight of mango fruits as influenced by cuticle and lenticels. Acta Hort. 1988;231:457–460. [Google Scholar]
- Ding CK, Wang C, Gross K, Smith D. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta. 2002;214:895–901. doi: 10.1007/s00425-001-0698-9. [DOI] [PubMed] [Google Scholar]
- Ding CK, Wang CY. The dual effects of methyl salicylate on ripening and expression of ethylene biosynthetic genes in tomato fruit. Plant Sci. 2003;164:589–596. [Google Scholar]
- Dudareva N, Picherky E, Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000;122:627–633. doi: 10.1104/pp.122.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, Ferranti F, Tosti N, Pasqualini S. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiol. 2006;142:595–608. doi: 10.1104/pp.106.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret DL, Helmer T, Hall JW. Cuticle cracking in tomato fruit. J Hort Sci. 1993;68:195–201. [Google Scholar]
- Elyatem SM, Banks NH, Cameron AC. Oxygen concentration effects on ethylene production by ripening banana tissue. Postharvest Biol Tech. 1994;4:343–351. [Google Scholar]
- Eum HL, Kim HB, Choi SB, Lee SK. Regulation of ethylene biosynthesis by nitric oxide in tomato (Solanum lycopersicum L.) fruit harvested at different ripening stages. Eur Food Res Technol. 2009;228:331–338. [Google Scholar]
- Fan X, Mattheis JP, Fellman JK. A role for jasmonates in climacteric fruit ripening. Planta. 1998;204:444–449. [Google Scholar]
- Fan X, Mattheis JP, Fellman JK, Patterson ME. Changes in jasmonic acid concentration during early development of apple fruit. Physiol Plant. 1997;101:328–332. [Google Scholar]
- Ferrie BJ, Beaudoin N, Burkhart W, Bowsher CG, Rothstein SJ. The cloning of two tomato lipoxygenase genes and their differential expression during fruit ripening. Plant Physiol. 1994;106:109–118. doi: 10.1104/pp.106.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filella I, Wilkinson MJ, Llusia J, Hewitt CN, Penuelas J. Volatile organic compounds emission in Norway spruce (Picea abies) in response to temperature changes. Physiol Plant. 2007;130:58–66. [Google Scholar]
- Finger FL, Puschmann R, Barros RS. Effects of water loss on respiration; ethylene production and ripening of banana fruit. Rev Bras Fisiol Veg. 1995;7:115–118. [Google Scholar]
- Flores F, El-Yahyaoui F, de Billerbeck G, Romojaro F, Latche A, Bouzayen M, Pech JC, Ambid C. Role of ethylene in the biosynthesis pathway of aliphatic ester aroma volatiles in Charentais Cantaloupe melons. J Exp Bot. 2002;53:201–206. doi: 10.1093/jexbot/53.367.201. [DOI] [PubMed] [Google Scholar]
- Fluhr R, Mattoo AK. Ethylene biosynthesis and perception. CRC Crit Rev Plant Sci. 1996;15:479–523. [Google Scholar]
- Forney CF, Mathheis JP, Austin RK. Volatile compounds produced by broccoli under anaerobic conditions. J Agric Food Chem. 1991;39:2257–2259. [Google Scholar]
- Franck C, Lammertyn J, Ho QT, Verboven P, Verlinden B, Nicolai BA. Browning disorders in pear: a review. Postharvest Biol Tech. 2007;43:1–13. [Google Scholar]
- Frenkel C, Erez A, Henninger MR (1995) Ethanol induced cold tolerance in chilling-sensitive crops. In: Harvest and post harvest technologies for fresh fruits and vegetables. Proc Int Conf Guanajuanta, Mexico, Feb. 20–24, 1995. ASAE, St. Joseph, MO, p 512–521
- Gamage TV, Rahman MS. Postharvest handling of foods of plant origin. In: Rahman MS, editor. Handbook of food preservation. NY: Marcel Dekker, Inc; 1999. pp. 11–46. [Google Scholar]
- Gelly M, Recasens I, Mata M, Arbones A, Rufat J, Girona J, Marsal J. Effect of water deficit during stage II of peach fruit development and post harvest on fruit quality and ethylene production. J Hort Sci Biotech. 2003;78:324–330. [Google Scholar]
- Genard M, Gouble B. ETHY. A theory of fruit climacteric ethylene emission. Plant Physiol. 2005;139:531–545. doi: 10.1104/pp.105.063339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JB, Marriott J, Palmer JM, Karikary SK. Sensitivity to water stress and ethylene of stored plantain fruits. J Exp Bot. 1982;33:1194–1201. [Google Scholar]
- Gershenzon J, McConkey ME, Croteau RB. Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol. 2000;122:205–213. doi: 10.1104/pp.122.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil MI, Conesa MA, Artes F. Quality changes in fresh cut tomato as affected by modified atmosphere packaging. Postharvest Biol Tech. 2002;25:199–207. [Google Scholar]
- Giovannoni JJ, El-Rakshy S. Genetic regulation of tomato fruit ripening and development and implementation of associated genomics tools. Acta Hort. 2005;682:63–72. [Google Scholar]
- Giovannucci E. Lycopene and prostate cancer risk. Methodological considerations in the epidemiologic literature. Pure Appl Chem. 2002;74:1427–1434. [Google Scholar]
- Gniazdowska A, Dobrzynska U, Babanczyk T, Bogatek R. Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production. Planta. 2007;225:1051–1057. doi: 10.1007/s00425-006-0384-z. [DOI] [PubMed] [Google Scholar]
- Golding JB, Shearer D, McGlasson WB, Wyllie SG. Relationships between respiration, ethylene and aroma production in ripening banana. J Agric Food Chem. 1999;47:1646–1651. doi: 10.1021/jf980906c. [DOI] [PubMed] [Google Scholar]
- Golding JB, Shearer D, Wyllie SG, McGlasson WB. Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol Tech. 1998;12:87–98. [Google Scholar]
- Goldschmidt EE. Ripening of citrus and other non-climacteric fruits: a role for ethylene. Acta Hort. 1997;463:335–340. [Google Scholar]
- Goldschmidt EE, Huberman M, Goren R. Probing the role of endogenous ethylene in degreening of citrus-fruit with ethylene antagonists. J Plant Growth Regul. 1993;12:325–329. [Google Scholar]
- Gorny JR, Kader AK. Controlled atmosphere suppression of ACC-synthase and ACC-oxidase in ‘Golden Delicious’ apple during long-term cold storage. J Am Soc Hort Sci. 1996;121:751–755. [Google Scholar]
- Gran CD, Beaudry RM. Determination of the low oxygen limit for several commercial apple cultivars by respiratory quotient break point. Postharvest Biol Tech. 1993;3:259–267. [Google Scholar]
- Grierson D, Wardowski WF. Relative humidity effects on the post harvest life of fruits and vegetable. HortSci. 1978;13:570–574. [Google Scholar]
- Guichad E, Souty M. Comparison of the relative quantities of aroma compounds found in fresh apricot (Prunus armeniaca) from six different varieties. Z Lebesm Unters Forsch. 1988;186:301–307. [Google Scholar]
- Gussman C, Goffredz J, Gianfagna T. Ethylene production and fruit softening rates in several apple fruit ripening variants. Hort Sci. 1993;28:135–137. [Google Scholar]
- Gustafson FG. Production of alcohol and acetaldehyde by tomatoes. Plant Physiol. 1934;9:359–367. doi: 10.1104/pp.9.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenmaier R. Method for measuring internal gases of citrus fruit and determining peel permeance. Proc Flo State Hort Soc. 2004;116:418–423. [Google Scholar]
- Hagenmaier RD. A comparison of ethane, ethylene and CO2 peel permeance for fruits with different coatings. Postharvest Biol Tech. 2005;37:56–64. [Google Scholar]
- Hansen M, Buttery RG, Stern DJ, Cantwell MI, Ling LC. Broccoli storage under low oxygen atmosphere: identification of higher boiling volatiles. J Agric Food Chem. 1992;40:850–852. [Google Scholar]
- Hayata Y, Maneerat C, Kozuka H, Sakamoto K, Ozajima Y. Flavor volatile analysis of ‘House Momotaro’ tomato fruit extract at different ripening stages by porapak Q column. J Jpn Soc Hort Sci. 2002;71:473–479. [Google Scholar]
- Herregods M. Disorders caused by some volatiles other than ethylene found in storage rooms. Acta Hort. 1977;62:247–256. [Google Scholar]
- Ho QT, Verboven P, Mebatsion HK, Verlinden BE, Vandewalle S, Nicolai BM. Microscale mechanisms of gas exchange in fruit tissue. New Phytol. 2009;182:162–174. doi: 10.1111/j.1469-8137.2008.02732.x. [DOI] [PubMed] [Google Scholar]
- Ho QT, Verboven P, Verlinden BE, Lammertyn J, Vandewalle S, Nicolai BM. A continuum model for metabolic gas exchange in pear fruit. PLoS Computational Biol. 2008;4:e1000023. doi: 10.1371/journal.pcbi.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho QT, Verboven P, Verlinden BE, Nicolai BM. A model for gas transport in pear fruit at multiple scales. J Exp Bot. 2010;61:2071–2081. doi: 10.1093/jxb/erq026. [DOI] [PubMed] [Google Scholar]
- Ho QT, Verlinden BE, Verboven P, Vandewalle S, Nicolai BM. A permeation-diffusion–reaction model of gas transport in cellular tissue of plant material. J Exp Bot. 2006;57:4215–4224. doi: 10.1093/jxb/erl198. [DOI] [PubMed] [Google Scholar]
- Ho QT, Verlinden BE, Verboven P, Nicolai BM. Gas diffusion properties at different positions in the pear. Postharvest Biol Tech. 2006;41:113–120. [Google Scholar]
- Hoeberichts FA, Van Der Plas LHW, Woltering EJ. Ethylene perception is required for the expression of tomato ripening related genes and associated physiological changes even at advanced stages of ripening. Postharvest Biol Tech. 2002;26:125–133. [Google Scholar]
- Hoehn E, Prange RK, Vigneault C. Storage technology and applications. In: Yahia EM, editor. Modified and controlled atmospheres for the storage, transportation, and packaging of horticultural commodities. Boca Raton: CRC Press, Taylor & Francis Group; 2009. pp. 17–50. [Google Scholar]
- Hubinger M, Menegalli FC, Aguerre RJ, Suarez C. Water vapor absorption isotherms of guava, mango and pine apple. J Food Sci. 1992;57:1405–1407. [Google Scholar]
- Huelin FE, Coggiola IM. Superficial scald, a functional disorder of stored apples. IV. Effect of variety, maturity, oiled wraps and diphenylamine on the concentration of α-farnesene in the fruit. J Sci Food Agric. 1968;19:297–301. doi: 10.1002/jsfa.2740190603. [DOI] [PubMed] [Google Scholar]
- Hui YH. In: Handbook of fruit and vegetable flavors. Hui YH, editor. New Jersey: Wiley; 2010. [Google Scholar]
- Hyodo H. Stress/wound ethylene. In: Mattoo AK, Suttle JS, editors. The plant hormone ethylene. Boca Raton: CRC; 1991. pp. 43–64. [Google Scholar]
- Igamberdiev AU, Hill RD. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot. 2004;55:2473–2482. doi: 10.1093/jxb/erh272. [DOI] [PubMed] [Google Scholar]
- Imanishi S, Nagata M. The effect of methyl jasmonate on the expression of ripening related genes in tomato fruits. Plant Cell Physiol. 2004;45(Suppl):78–84. [Google Scholar]
- Imanishi S, Noguchi A, Hatakeyama R, Nagata M. Monitoring the effect of jasmonates on the expression of ripening related genes in tomato fruit disks by cDNA macroarray. Acta Hort. 2005;682:149–153. [Google Scholar]
- Inaba A. Studies on the internal feedback regulation of ethylene biosynthesis and signal transduction during fruit ripening, and the improvement of fruit quality. J Jpn Soc Hort Sci. 2007;76:1–12. [Google Scholar]
- Jackson MB, Herman B, Goodenough A. An examination of the importance of ethanol in causing injury to flooded plants. Plant Cell Environ. 1982;5:163–172. [Google Scholar]
- Johnston JW, Gunaseelan K, Pidakala P, Wang M, Schaffer RJ. Co-ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. J Exp Bot. 2009;60:2689–2699. doi: 10.1093/jxb/erp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce DC, Hockings PD, Mazucco RA, Shorter AJ. H-1-Nuclear magnetic resonance imaging of ripening ‘Kensington Pride’ mango fruit. Funct Plant Biol. 2002;29:873–879. doi: 10.1071/PP01150. [DOI] [PubMed] [Google Scholar]
- Kader AA. Biochemical and physiological basis for effects of controlled and modified atmospheres on fruits and vegetables. Food Tech. 1986;40:99–104. [Google Scholar]
- Kader AA. Respiration and gas exchange of vegetable. In: Weichmann J, editor. Postharvest physiology of vegetable: food science and technology. New York: Marcel Dekker, Inc; 1987. pp. 25–43. [Google Scholar]
- Kader AA. Future research needs in post harvest biology and technology of fruits. Acta Hort. 2005;485:209–213. [Google Scholar]
- Kader AA. Future research and application needs. In: Yahia EM, editor. Modified and controlled atmospheres for storage, transpiration, packaging of horticultural commodities. Boca Raton: CRC Press, Taylor & Francis Group; 2009. pp. 569–575. [Google Scholar]
- Kader AA, Barrett DM. Classification, composition of fruits, and postharvest maintenance of quality. In: Somogyi LP, Ramaswamy HS, Hui YH, editors. Processing fruits: science and technology. Vol 1, biology, principles and applications. Lancaster: Technomic Publishing Company; 1996. pp. 1–24. [Google Scholar]
- Kader AA, Morris LL. Relative tolerance of fruits and vegetable to elevated CO2 and reduced O2 levels. Mich State Univ Hortic Rept. 1977;28:260–265. [Google Scholar]
- Kader AA, Saltveit ME. Respiration and gas exchange. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker, Inc; 2003. pp. 7–29. [Google Scholar]
- Kader AA, Saltveit ME. Atmosphere modification. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker, Inc; 2003. pp. 229–246. [Google Scholar]
- Kader AA, Zagory D, Kerbel EL. Modified atmosphere packaging of fruits and vegetables. CRC Crit Rev Food Sci Nutr. 1989;28:1–30. doi: 10.1080/10408398909527490. [DOI] [PubMed] [Google Scholar]
- Kanellis AK, Loulakakis KA, Hassan M, Roubelakis-Angelakis KA. Biochemical and molecular aspects of the low oxygen action on fruit ripening. In: Pech JC, Latche A, Balaque C, editors. Cellular and molecular aspects of biosynthesis and action of the plant hormone ethylene. Dordrecht: Kluwer Academic Publishers; 1993. pp. 117–122. [Google Scholar]
- Kanellis AK, Solomos T, Mattoo AK. Hydrolytic enzyme activities and protein pattern of avocado fruit ripened in air and in low oxygen with and without ethylene. Plant Physiol. 1989;90:259–266. doi: 10.1104/pp.90.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellis AK, Solomos T, Mattoo AK. Changes in sugar, enzymatic activities and acid phophatse isozyme profile of bananas ripened in air or stored in 2.5% O2 with and without ethylene. Plant Physiol. 1989;90:251–258. doi: 10.1104/pp.90.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellis AK, Solomos T, Roubelakis-Angelakis KA. Suppression of cellulase and polygalacturonase and induction of alcohol dehydrogenase isoenzymes of avocado fruit mesocarp subjected to low oxygen stress. Plant Physiol. 1991;96:269–274. doi: 10.1104/pp.96.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellis AK, Tonutti P, Perata P. Biochemical and molecular aspects of modified and controlled atmospheres. In: Yahia EM, editor. Modified and controlled atmospheres for storage, transpiration and packaging of horticultural commodities. Boca Raton: CRC Press, Taylor & Francis Group; 2009. pp. 553–567. [Google Scholar]
- Kao CH, Yang SF. Light inhibition of the conversion of 1-aminocyclopropane-1-carboxylic acid to ethylene is mediated through carbon dioxide. Planta. 1982;155:251–266. doi: 10.1007/BF00392725. [DOI] [PubMed] [Google Scholar]
- Karl T, Guenther A, Turnipseed A, Patton EG, Jardine K. Chemical sensing of plant stress at the ecosystem scale. Biogeosci. 2008;5:1287–1294. [Google Scholar]
- Kays SJ, Paull RE (2004) Metabolic process in harvested products. In: Postharvest biology. Exon Press, Athens, p 79–136
- Ke D, Kader AA. Tolerance of Valencia oranges to controlled atmospheres as determined by physiological responses and quality attributes. J Am Soc Hort Sci. 1990;115:779–783. [Google Scholar]
- Ke D, Zhou L, Kader AA. Mode of oxygen and carbon dioxide action on strawberry ester biosynthesis. J Am Soc Hort Sci. 1994;119:971–975. [Google Scholar]
- Ke DL, Goldstein L, O’Mohony M, Kader AA. Effect of short-term exposure to low O2 and high CO2 atmospheres on quality attributes of strawberries. J Food Sci. 1991;56:50–54. [Google Scholar]
- Kelly MO, Saltveit ME. Effect of endogenously synthesized and exogenously applied ethanol on tomato fruit ripening. Plant Physiol. 1988;88:143–147. doi: 10.1104/pp.88.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall SA, Ng TJ. Genetic variation of ethylene production in harvested muskmelon fruit. HortSci. 1988;23:759–761. [Google Scholar]
- Kerbel EL, Keder AA, Romani RS. Effect of elevated CO2 concentrations on glycolysis in intact ‘Bartlet’ pear fruit. Plant Physiol. 1988;86:1205–1209. doi: 10.1104/pp.86.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Taylor MG, Klee H. Fruit-specific suppression of the ethylene receptor LeETR4 results in early-ripening tomato fruit. Plant Biotechnol J. 2008;6:295–300. doi: 10.1111/j.1467-7652.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Kevany BM, Tieman DM, Taylor MG, Cin VD, Klee H. Ethylene receptor degradation controls the timing of ripening in tomato fruit. The Plant J. 2007;51:446–458. doi: 10.1111/j.1365-313X.2007.03170.x. [DOI] [PubMed] [Google Scholar]
- Kim JG, Yousef AE, Dave S. Application of ozone for enhancing the microbiological safety and quality of foods: a review. J Food Prot. 1999;62:1071–1087. doi: 10.4315/0362-028x-62.9.1071. [DOI] [PubMed] [Google Scholar]
- Kimmerer TW, Kozlowski TT. Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress. Plant Physiol. 1982;69:840–847. doi: 10.1104/pp.69.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ. Control of ethylene mediated processes in tomato at the level of receptors. J Exp Bot. 2002;53:2057–2063. doi: 10.1093/jxb/erf062. [DOI] [PubMed] [Google Scholar]
- Klieber A, Ratanachinakorn B, Simone DH. Effect of low oxygen and high carbon dioxide on tomato cultivar ‘Bermuda’ fruit physiology and composition. Sci Hort. 1996;65:251–261. [Google Scholar]
- Klozenbucher KA, Altman SA, McIntosh MS, Walsh CS. Effect of cultivar on endogenous ethylene evolution and its relationship to increase of soluble protein in peach mesocarp tissue. Fruit Var J. 1994;48:20–26. [Google Scholar]
- Knee M. Fruit softening III. Requirement for oxygen and pH effects. J Exp Bot. 1982;33:1263–1269. [Google Scholar]
- Knegt E, Kramer SJ, Bruinsma J. Pectin changes and internal ethylene concentrations in ripening tomato fruit. Colloques Internationaux CNRS. 1974;238:355–358. [Google Scholar]
- Kondo S, Tomiyama A, Seto H. Changes of endogenous jasmonic acid and methyl jasmonate in apples and sweet cherries during fruit development. J Am Soc Hort Sci. 2000;125:282–287. [Google Scholar]
- Kovacs E, Kovacs J, Zackel E, Genova J. Structural and chemical changes of pear skin. Acta Hort. 1994;368:243–250. [Google Scholar]
- Kubo Y, Inaba A, Nakamura R. Respiration and C2H4 production in various harvested crops held in CO2-enriched atmospheres. J Am Soc Hort Sci. 1990;115:975–978. [Google Scholar]
- Kubo Y, Inaba A, Nakamura R. Extinction point and critical oxygen concentration in various fruits and vegetables. J Jap Hort Sci. 1996;65:397–402. [Google Scholar]
- Kumar S, Sinha SK. Alternative respiration and heat production in ripening banana fruits (Musa paradisiacal var. Mysore Kadali) J Exp Bot. 1992;43:1639–1642. [Google Scholar]
- Labuza TP. Moisture sorption: practical aspects of isotherm measurement and use. St Paul Minnesota: American Association of Cereal Chemistry; 1984. [Google Scholar]
- Lalel HJD, Singh Z, Tan SC. Maturity stage at harvest affects fruit ripening, quality and biosynthesis of aroma volatile compounds in ‘Kensington Pride’ mango. J Hort Sci Biotech. 2003;78:225–233. [Google Scholar]
- Lammertyn J, Scheerlinck N, Jancsok P, Verlinden BE, Nicolai BM. A respiration-diffusion model for ‘Conference’ pears II: stimulation and relation to core breakdown. Postharvest Biol Tech. 2003;30:43–55. [Google Scholar]
- Lanciotti R, Belletti N, Patrignani F, Gianotti A, Gardini F, Guerzoni ME. Application of hexanal, (E)-2-hexenal, and hexyl acetate to improve the safety of fresh-sliced apples. J Agric Food Chem. 2003;51:2958–2963. doi: 10.1021/jf026143h. [DOI] [PubMed] [Google Scholar]
- Lange DL, Kader AA. Effects of elevated carbon dioxide on key mitochondrial respiratory enzymes in ‘Hass’ avocado fruit and fruit disks. J Am Soc Hort Sci. 1997;122:228–244. [Google Scholar]
- Lange DL, Kader AA. Elevated carbon dioxide exposure alters intracellular pH and energy charge in avocado fruit tissue. J Am Soc Hort Sci. 1997;122:253–257. [Google Scholar]
- Larsen M. Volatile compounds formed in strawberries under anaerobic conditions and their influence on self-flavour formation. In: Maarse H, van der Heij DG, editors. Trends in flavour research. Amsterdam: Elsevier; 1994. pp. 421–424. [Google Scholar]
- Larsen M, Poll L. Odour threshold of some important aroma compounds in raspberries. Z Lebesm Unters Forsch. 1990;191:129–131. [Google Scholar]
- Lawes GS, Prasad L. Peel permeance and storage changes in internal atmosphere composition of surface-coated mandarin. Acta Hort. 1999;485:249–254. [Google Scholar]
- Leakey ADB, Xu F, Gillespie KM, McGrath JM, Ainsworth EA, Ort DR. Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. PNAS. 2009;106:3597–3602. doi: 10.1073/pnas.0810955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun M, Plotto A, Goodner K, Ducamp M-N, Baldwin E. Discrimination of mango fruit maturity by volatiles using the electronic nose and gas chromatography. Postharvest Biol Tech. 2008;48:122–131. [Google Scholar]
- Lelievre JM, Latche A, Jones B, Bouzayen M, Peach JC. Ethylene and fruit ripening. Physiol Plant. 1997;101:727–739. [Google Scholar]
- Leonard ER, Wardlaw CW. Studies in tropical fruit. XII. The respiration of banana during storage at 53F and ripening at controlled temperatures. Ann Bot (NS) 1941;5:379–423. [Google Scholar]
- Lescourret F, Genard M, Habib R, Fishman S. Variation in surface conductance to water vapour diffusion in peach fruit and its effects on fruit growth assessed by a simultaneous model. Tree Physiol. 2001;21:735–741. doi: 10.1093/treephys/21.11.735. [DOI] [PubMed] [Google Scholar]
- Leshem YY, Haramaty E. The characterization and controlling effects of the nitric oxide free radical in vegetative stress and senescence of Pisum sativum Linn. J Plant Physiol. 1996;148:258–263. [Google Scholar]
- Leshem YY, Huang JS, Tzeng DS, Chou CC. Nitric oxide gas as an endogenous regulator of fruit, vegetable and flower maturation and senescence. In: Ledhem YY, editor. Nitric oxide in plants: occurrence, fuction and use. Dordrecht: Kluwer Academic Publishers; 2000. pp. 33–62. [Google Scholar]
- Leshem YY, Wills RBH, Ku VVV. Evidence for the function of the radical gas nitric oxide (NO) as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem. 1998;36:825–833. [Google Scholar]
- Leshuk JA, Saltveit ME., Jr . Controlled atmosphere storage requirement and recommendations for vegetables. In: Calderon M, Barkai-Golan R, editors. Food preservation by modified atmosphere. Boca Raton: CRC; 1990. pp. 315–352. [Google Scholar]
- Leslie CA, Romani RJ. Salicylic acid: a new inhibitor of ethylene biosynthesis. Plant Cell Rep. 1986;5:144–146. doi: 10.1007/BF00269255. [DOI] [PubMed] [Google Scholar]
- Levin A, Sonego L, Zutkhi Y, Ben-Arie R. Effects of CO2 on ethylene production by apples at low and high O2 concentrations. In: Pech JC, Latche A, Balague C, editors. Cellular and molecular aspects of the plant hormone ethylene. Dordrecht: Kluwer Academic Publishers; 1993. pp. 150–151. [Google Scholar]
- Lewinsohn E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Nam KH, Amar O, Lastochkin E, Larkov O, Ravid U, Hiatt W, Gepstein S, Pichersky E. Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol. 2001;127:1256–1265. [PMC free article] [PubMed] [Google Scholar]
- Li N, Parsons BL, Liu D, Mattoo AK. Accumulation of wound-inducible ACC-synthase transcript in tomato fruit is inhibited by salicylic acid and polyamines. Plant Mol Biol. 1992;18:477–487. doi: 10.1007/BF00040664. [DOI] [PubMed] [Google Scholar]
- Littmann MD. Effect of water stress on the ripening of climacteric fruits. Queensl J Agr Anim Sci. 1972;29:103–113. [Google Scholar]
- Longhurst TJ, Lee E, Hinde R, Brady CJ, Speirs J. Structure of the tomato Adh2 gene and Adh2 pseudogenes and a study of Adh2 gene expression in fruit. Plant Mol Biol. 1994;26:1073–1084. doi: 10.1007/BF00040690. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. Ethylene response factor 1 integrates signal from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Schnitzler JP. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Lougheed EC, Murr DP, Toivonen PMA. Ethylene and non-ethylene volatiles. In: Weichmann J, editor. Postharvest physiology of vegetables. New York: Marcel Dekker, Inc; 1987. pp. 255–276. [Google Scholar]
- Lurie S, Klein JD. Cyanide metabolism in relation to ethylene production and cyanide-insensitive respiration in climacteric and non-climacteric fruits. J Plant Physiol. 1989;135:518–521. [Google Scholar]
- Lurie S, Pre-Aymard C, Ravid U, Larkov O, Fallik E. Effect of 1-methylcyclopropene on volatile emission and aroma in cv. Anna apples. J Agric Food Chem. 2002;50:4251–4256. doi: 10.1021/jf0200873. [DOI] [PubMed] [Google Scholar]
- Lyons JM, Pratt HK. Effect of stage of maturity and ethylene treatment on respiration and ripening of tomato fruits. J Am Soc Hort Sci. 1964;84:491–500. [Google Scholar]
- Magness JR. Composition of gases in intercellular spaces of apples and potatoes. Bot Gaz. 1920;70:308–316. [Google Scholar]
- Mangaraj S, Goswami TK. Modified atmosphere packaging—an ideal food preservation technique. J Food Sci Tech. 2009;46:399–410. [Google Scholar]
- Maquire KM, Banks NH, Opara LU. Factors affecting weight loss of apples. Hortic Rev. 2001;25:197–233. [Google Scholar]
- Mares-Perlman JA, Millen AE, Ficek TL, Hankinson SE. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. J Nutr. 2002;132:5185–5245. doi: 10.1093/jn/132.3.518S. [DOI] [PubMed] [Google Scholar]
- Mathooko FM. Regulation of ethylene biosynthesis in higher plants by carbon dioxide. Postharvest Biol Tech. 1996;7:1–26. [Google Scholar]
- Mathooko FM, Kubo Y, Inaba A, Nakamura R. Characterization of the regulation of ethylene biosynthesis in tomato fruit by carbon dioxide and diazocyclopentadiene. Postharvest Biol Tech. 1995;5:221–233. [Google Scholar]
- Mathooko FM, Tsunasima Y, Owino W, Kubo Y, Inaba A. Regulation of genes encoding ethylene biosynthesis in peach fruit by carbon dioxide and 1-methylcyclopropene. Postharvest Biol Tech. 2001;21:265–281. [Google Scholar]
- Matsumoto M, Ogawa M (1995) Ethylene removal agents, postharvest preservation agent and deodorant. Canadian Patent No. 1334628
- Mattheis JP, Buchanan DA, Fellman JK. Change in apple fruit volatiles after storage in atmosphere inducing anaerobic metabolism. J Agric Food Chem. 1991;39:1602–1605. [Google Scholar]
- McDonald RE, McCollum TG, Baldwin EA. Prestorage heat treatments influence, free sterols and flavour volatiles of tomatoes stored at chilling temperature. J Am Soc Hort Sci. 1996;121:531–536. [Google Scholar]
- McGlasson WB, Wills RBH. Effects of oxygen and carbon dioxide on respiration, storage life, and organic acids of green bananas. Aust J Biol Sci. 1972;25:35–42. [Google Scholar]
- McMurchie EJ, McGlasson WB, Eaks IL. Treatment of fruit with propylene gives information about biogenesis of ethylene. Nature. 1972;237:235–236. doi: 10.1038/237235a0. [DOI] [PubMed] [Google Scholar]
- Mendoza F, Verboven P, Mebatsion HK, Kerckhofs G, Wevers M, Nicolai B. Three-dimensional pore space quantification of apple tissue using X-ray computed microtomography. Planta. 2007;226:559–570. doi: 10.1007/s00425-007-0504-4. [DOI] [PubMed] [Google Scholar]
- Miccolis V, Saltveit ME., Jr Morphological and physiological changes during fruit growth and maturation of seven melon cultivars. J Am Soc Hort Sci. 1991;116:1025–1029. [Google Scholar]
- Millar AH, Day DA. Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS Lett. 1996;398:155–158. doi: 10.1016/s0014-5793(96)01230-6. [DOI] [PubMed] [Google Scholar]
- Mishra B, Khatkar BS, Garg MK, Wilson LA. Permeability of edible coating. J Food Sci Tech. 2010;47:109–113. doi: 10.1007/s13197-010-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra VK, Gamage TV. Postharvest physiology of fruits and vegetables. In: Rahman MS, editor. Handbook of food preservation. 2. Boca Raton: CRC Press (Taylor and Francis Group); 2007. pp. 19–48. [Google Scholar]
- Mulas M. Genetic variability of histological characteristics in olive fruits. Acta Hort. 1994;356:70–73. [Google Scholar]
- Musse M, Guio FD, Quellec S, Cambert M, Challois S, Davenel A. Quantification of microporosity in fruit by MRI at various magnetic fields: comparison with X-ray microtomography. Magn Reson Imag (MRI) 2010;28:1525–1534. doi: 10.1016/j.mri.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Nagamani JE, Shivashankara KS, Roy TK. Role of oxidative stress and activity of ethylene biosynthetic enzymes on formation of spongy tissue in ‘Alphonso’ mango. J Food Sci Tech. 2010;47:295–299. doi: 10.1007/s13197-010-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata M, Mori H, Tabei Y, Sato T, Hirai M, Imaseki H. Modification of tomato fruit ripening by transformation with sense or antisense chimeric 1-aminocyclopropane-1-carboxylate synthase gene. Acta Hort. 1995;394:213–218. [Google Scholar]
- Nakano R, Inoue S, Kubo Y, Inaba A. Water stress-induced ethylene in the calyx trigger autocatalytic ethylene production and fruit softening in ‘Tone-wase’ persimmon grown in a heated plastic-house. Postharvest Biol Tech. 2002;25:293–300. [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of l-aminocyclopropane-l-carboxylate synthase, 1-aminocyclopropane-l-carboxylate oxidase and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanos GD, Romani RJ, Kader AA. Metabolic and other response of ‘Bartlett’ pear fruit and suspension cultured ‘Passe Crassane’ pear fruit cells held in 0.25% O2. J Am Soc Hort Sci. 1992;117:934–940. [Google Scholar]
- Nanthachai N, Ratanachinakorn B, Kosittrakun M, Beaudry RM. Absorption of 1-MCP by fresh produce. Postharvest Biol Tech. 2007;43:291–297. [Google Scholar]
- Nath P, Trivedi PK, Sane VA, Sane AP. Role of ethylene in fruit ripening. In: Khan NA, editor. Ethylene action in plants. Heidelberg: Springer; 2006. pp. 151–184. [Google Scholar]
- Nath A, Deka BC, Singh A, Patel RK, Paul D, Mishra LK, Ojha H (2011) Extension of shelf life of pear fruits using different packaging materials. J Food Sci Tech. doi:10.1007/s13197-011-0305-4 [DOI] [PMC free article] [PubMed]
- Negre-Zakharov F, Long MC, Dudareva N. Floral scents and fruit aromas inspired by nature. In: Osbourn AE, Lanzotti V, editors. Plant-derived natural products: synthesis, function and application. Dordrecht: Springer; 2009. pp. 405–434. [Google Scholar]
- Neil SJ, Desikan R, Hancock JT. Nitric oxide signaling in plants. New Phytol. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TA, Verboven P, Daudin JD, Vandewalla S, Nicolai BM. Effect of picking date, time and temperature on water sorption of ‘Conference’ pear tissue. Postharvest Biol Tech. 2004;33:243–253. [Google Scholar]
- Niemirow-Krizsai B, Csillag A. Anatomical reference to storability in tomato berries. Acta Hort. 1994;368:310–315. [Google Scholar]
- Nilsen ET, Orcutt DM (1996) Water dynamics in plants (chapter 7). In: Physiology of plant under stress: abiotic factors. Wiley, New York
- Noichinda S. Respiration rate and ethylene production of lime fruit. Natl J King Mongkut’s Inst Tech (North Bangkok) 2000;7:27–31. [Google Scholar]
- Nuevo PA, Lizada MCC, Pantastico Er B. Gas diffusion factors in fruits III. Oxygen and carbon dioxide. Postharvest Res Notes. 1984;1:86–89. [Google Scholar]
- Oetiker JH, Yang SF. The role of ethylene in fruit ripening. Acta Hort. 1995;398:167–178. [Google Scholar]
- Ong KC, Cash JN, Zabik MJ, Siddiq M, Jones AL. Chlorine and ozone washes for pesticide removal from apples and processed apple sauce. Food Chem. 1996;55:153–160. [Google Scholar]
- Palma T, Marangoni AG, Stanely DW. Environmental stresses effect tomato microsomal membrane function differently than natural ripening and senescence. Postharvest Biol Tech. 1995;6:257–273. [Google Scholar]
- Pandey M, Zeng Y, Prasad NK, Srivastava GC. Alternate respiration during ripening of tomato fruits. Indian J Plant Physiol. 1995;3:94–96. [Google Scholar]
- Pandit SS, Kulkarni RS, Chidley HG, Giri AP, Pujari KH, Kollner TG, Degenhardt J, Gershenzon J, Gupta VS. Changes in volatile composition during fruit development and ripening of ‘Alphonso’ mango. J Sci Food Agric. 2009;89:2071–2081. [Google Scholar]
- Pare PW, Tumlinson JH. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 1997;114:1161–1167. doi: 10.1104/pp.114.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YM, Blanpied GD, Jozwiak Z, Liu FW. Postharvest studies of resistance to gas diffusion in McIntosh apples. Postharvest Biol Tech. 1993;2:329–339. [Google Scholar]
- Paul V, Malik SK, Srivastava GC. Intervarietal differences in the surface morphology and anatomy of mango (Mangifera indica L.) fruits. Phytomorphol. 2007;57:211–220. [Google Scholar]
- Paul V, Pandey R, Srivastava GC. Ripening of tomato (Solanum lycopersicum L.). Part I: 1-methylcyclopropene mediated delay at higher storage temperature. J Food Sci Tech. 2010;47:519–526. doi: 10.1007/s13197-010-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul V, Pandey R, Srivastava GC. Ripening of tomato (Solanum lycopersicum L.). Part II: regulation by its stem scar region. J Food Sci Tech. 2010;47:527–533. doi: 10.1007/s13197-010-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul V, Pandey R, Srivastava GC (2011a) The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene—an overview. J Food Sci Tech. doi:10.1007/s13197-011-0293-4 [DOI] [PMC free article] [PubMed]
- Paul V, Pandey R, Srivastava GC. Tomato fruit ripening: regulation of ethylene production and its response. Indian J Plant Physiol. 2011;16:117–131. [Google Scholar]
- Paul V, Srivastava GC. Role of surface morphology in determining the ripening behaviour of tomato (Lycopersicon esculentum Mill.) fruits. Sci Hort. 2006;110:84–92. [Google Scholar]
- Paz O, Jones HW, Prevost BA, Frenkel C. Enhancement of fruit sensory quality by postharvest applications of acetaldehyde and ethanol. J Food Sci. 1981;47:270–274. [Google Scholar]
- Pech JC, Bouzayen M, Latche A. Climacteric fruit ripening: ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 2008;175:114–120. [Google Scholar]
- Pech JC, Bouzayen M, Latche A, Sanmartin M, Aggelis A, Kanellis AK. Physiological, biochemical and molecular aspects of ethylene biosynthesis and action. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker, Inc; 2003. pp. 247–285. [Google Scholar]
- Pena-Cortes H, Barrios P, Dorta F, Polanco V, Sanchez C, Sanchez E, Ramirez Z. Involvement of jasmonic acid and derivatives in plant responses to pathogens and insects and in fruit ripening. J Plant Growth Regul. 2005;23:246–260. [Google Scholar]
- Perez AG, Sanz C, Richardson DG, Olias JM. Methyl jasmonate vapours promote α-carotene synthesis and chlorophyll degradation in Golden delicious apple peel. J Plant Growth Regul. 1993;12:163–167. [Google Scholar]
- Peschel S, Beyer M, Knoche M. Surface characteristics of sweet cherry fruit: stomata number, distribution, functionality and surface wetting. Sci Hort. 2003;97:265–278. [Google Scholar]
- Pesis E. Induction of fruit aroma and quality by post harvest application of natural metabolites or anaerobic conditions. In: Linsken HF, Jackson JF, editors. Fruit analysis, modern methods of plant analysis. Vol 18. Berlin: Springer; 1995. pp. 19–36. [Google Scholar]
- Pesis E. The role of the anaerobic metabolites, acetaldehyde and ethanol in fruit ripening, enhancement of fruit quality and fruit deterioration. Postharvest Biol Tech. 2005;37:1–19. [Google Scholar]
- Pesis E. Postharvest treatments prior storage with anaeribiosis or anaerobic metabolites to improve fruit quality. In: Noureddine B, Norio S, editors. Advances in postharvest technology for horticultural crops. Trivandrum: Research Signpost; 2006. pp. 251–274. [Google Scholar]
- Pesis E, Ampunpong C, Shusiri B, Hewett EW. Enhancement of ethylene and CO2 production in apple fruit following short term exposure to high CO2. Postharvest Biol Tech. 1994;4:309–317. [Google Scholar]
- Pesis E, Faiman D, Dori S. Postharvest effects of acetaldehyde vapour on ripening related enzyme activity in avocado fruit. Postharvest Biol Tech. 1998;13:245–253. [Google Scholar]
- Pesis E, Levi A, Ben-Arie R. Deastringency of persimmon fruits by creating a modified atmosphere in polyethylene bags. J Food Sci. 1986;51:1014–1016. [Google Scholar]
- Pesis E, Marinansky R. Inhibition of tomato ripening by acetaldehyde vapour or anaerobic conditions prior to storage. J Plant Physiol. 1993;142:717–721. [Google Scholar]
- Petro-Turza M. Flavour of tomato and tomato products. Food Rev Int. 1987;2:309–351. [Google Scholar]
- Podd LA, Hill PN, van Staden J. Physiological response and extension of vase life of cut carnation flowers treated with ethanol and acetaldehyde II. Protein content and enzyme activity. Plant Growth Regul. 2002;38:107–117. [Google Scholar]
- Podd LA, van Staden J. The role of ethanol and acetaldehyde in flower senescence and fruit ripening—a review. Plant Growth Regul. 1998;26:183–189. [Google Scholar]
- Polashock JJ, Saftner RA, Kramer M. Postharvest highbush blueberry fruit antimicrobial volatile profiles in relation to anthracnose fruit rot resistance. J Am Soc Hort Sci. 2007;132:859–868. [Google Scholar]
- Powell ALT, Bennett AB. Tomato. In: Valpuestra V, editor. Fruit and vegetable biotechnology. Cambridge: Woodhead Publisher Limited; 2002. pp. 185–221. [Google Scholar]
- Prasad K, Stadelbacher GJ. Effect of acetaldehyde vapour on post harvest decay and market quality of fresh strawberries. Phytopathol. 1974;64:948–951. [Google Scholar]
- Pulamte L (2008) Key issues in post harvest management of fruits and vegetables in India. India Sci Technol. http://www.nistads.res.in/indiasnt2008/t6rural/t6rur14.htm. Accessed 23 December 2010
- Ramayya N, Niranjan K, Duncan E (2011) Effects of modified atmosphere packaging on quality of ‘Alphonso’ mangoes. J Food Sci Tech. doi:10.1007/s13197-010-0215-x [DOI] [PMC free article] [PubMed]
- Rao AV, Agarwal S. Bioavailability and in vitro antioxidant properties of lycopene from tomato products and their role in prevention of cancer. Nutr Canc. 1998;31:199–203. doi: 10.1080/01635589809514703. [DOI] [PubMed] [Google Scholar]
- Reddy YV, Srivastava GC. Ethylene biosynthesis and respiration in mango fruits during ripening. Indian J Plant Physiol. 1999;4:32–35. [Google Scholar]
- Rice RG, Frquhar W, Bollyky LJ. Review of the application of ozone for increasing storage time for perishable foods. Ozone Sci Eng. 1982;4:47–163. [Google Scholar]
- Ritenour MA, Mangrich ME, Beaulieu JC, Rab A, Saltveit ME. Ethanol effects on ripening of climacteric fruit. Postharvest Biol Tech. 1997;12:35–42. [Google Scholar]
- Rockland LB, Stewart GF. Water activity: influences on food quality. New York: Academic; 1981. [Google Scholar]
- Rodoni L, Casadei N, Concellon A, Alicia ARC, Vicente AR. Effect of short-term ozone treatments on tomato (Solanum lycopersicum L.) fruit quality and cell wall degradation. J Agric Food Chem. 2010;58:594–599. doi: 10.1021/jf9029145. [DOI] [PubMed] [Google Scholar]
- Rogiers SY, Kumar GNM, Knowles NR. Maturation and ripening of fruit of Amelanchier alnifolia Nutt. are accompanied by increasing oxidative stress. Ann Bot. 1998;81:203–211. [Google Scholar]
- Rolletscheck H, Borisjuck L, Koschorreck M, Wobus U, Weber H. Legume embryos develop in a hypoxic environment. J Exp Bot. 2002;53:1099–1107. doi: 10.1093/jexbot/53.371.1099. [DOI] [PubMed] [Google Scholar]
- Rothan C, Duret S, Chevallier C, Raymond P. Suppression of ripening associated gene expression in tomato fruits subjected to high CO2 concentration. Plant Physiol. 1997;114:255–263. doi: 10.1104/pp.114.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupasinghe HPV, Murr DP, Paliyath G, Skog L. Inhibitory effect of 1-MCP on ripening and superficial scald development in ‘McIntosh’ and ‘Delicious’ apples. J Hort Sci Biotech. 2000;75:271–276. [Google Scholar]
- Saltveit ME. Effect of ethylene on quality of fresh fruit and vegetables. Postharvest Biol Tech. 1999;15:279–292. [Google Scholar]
- Saltveit ME. Is it possible to find an optimal controlled atmosphere? Postharvest Biol Tech. 2003;27:3–10. [Google Scholar]
- Saltveit ME (2005) Fruit ripening and fruit quality. In: Heuvelink E (ed) Tomatoes. CAB International, p 145–170
- Saltveit ME, Sharaf AA. Ethanol inhibit ripening of tomato fruit harvested at various degrees of ripeness without affecting subsequent quality. J Am Soc Hort Sci. 1992;117:793–798. [Google Scholar]
- Saltveit ME., Jr Effect of alcohols and their interaction with ethylene on the ripening of epidermal pericarp discs of tomato fruit. Plant Physiol. 1989;90:167–174. doi: 10.1104/pp.90.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltveit ME, Jr, Mencarelli F. Inhibition of ethylene synthesis and action in ripening tomato fruit by ethanol vapours. J Am Soc Hort Sci. 1988;113:572–576. [Google Scholar]
- Saltveit ME, Ballinger WE. Effect of anaerobic nitrogen and carbon dioxide atmosphere on ethanol production and postharvest quality of “Carlos” grapes. J Am Soc Hort Sci. 1983;108:462–465. [Google Scholar]
- Salunkhe DK, Desai BB. Postharvest biotechnology of vegetables. Boca Raton: CRC; 1984. [Google Scholar]
- Salunkhe DK, Do JY. Bioenergetics of aroma constituents of fruits and vegetables. CRC Crit Rev Food Sci Nutr. 1977;8:161–190. doi: 10.1080/10408397609527221. [DOI] [PubMed] [Google Scholar]
- Sanders MG, de Wild HPJ. The relation between in vivo ethylene production and oxygen partial pressure. Postharvest Biol Tech. 2003;30:143–151. [Google Scholar]
- Saniewski M, Czapski J. The effect of methyl jasmonate on lycopene and beta-carotene accumulation in ripening red tomatoes. Experientia. 1983;39:1373–1374. [Google Scholar]
- Saniewski M, Czapski J. Stimulatory effect of methyl jasmonate on the ethylene production in tomato fruits. Experientia. 1985;41:256–257. [Google Scholar]
- Saniewski M, Czapski J, Nowacki J, Lange E. The effect of methyl jasmonate on ethylene and 1-aminocyclopropane-1-carboxylic acid production in apple fruits. Biol Plant. 1987;29:199–203. [Google Scholar]
- Sasaki K, Saito T, Lamsa M, Caldentey KMO, Suzuki M, Ohyama K, Muranaka T, Ohara K, Yazaki K. Plants utilize isoprene emission as a thermotolerance mechanism. Plant Cell Physiol. 2007;48:1254–1262. doi: 10.1093/pcp/pcm104. [DOI] [PubMed] [Google Scholar]
- Sawamura M, Knegt E, Bruinsma J. Levels of endogenous ethylene, carbon dioxide, and soluble pectin and activities of pectin methylesterase and polygalacturonase in ripening tomato fruits. Plant Cell Physiol. 1978;19:1061–1069. [Google Scholar]
- Schotsmans SW, Verlinden BE, Lammertyn J, Nicolai BM. The relationship between gas transport properties and histology of apple. J Sci Food Agric. 2004;84:1131–1140. [Google Scholar]
- Scully AD, Horsham MA (2008) Active packaging for fruits and vegetables. In: Wilson CL (ed) Intelligent and active packaging for fruits and vegetables. CRC Press, p 57–71
- Sharma RR, Pal RK, Singh D, Samuel DVK, Sethi S, Kumar A (2011) Evaluation of heat shrinkable films for shelf life and quality of individually wrapped Royal Delicious apples under ambient conditions. J Food Sci Tech. doi:10.1007/s13197-011-0332-1 [DOI] [PMC free article] [PubMed]
- Shi JX, Goldschmidt EE, Goren R, Porat R. Molecular, biochemical and anatomical factors governing ethanol fermentation metabolism and accumulation of off-flavours in mandarins and grapefruit. Postharvest Biol Tech. 2007;46:242–251. [Google Scholar]
- Shi JX, Pora R, Goren R, Goldschmidt EE. Physiological responses of ‘Murcott’ mandarins and ‘Star Ruby’ grapefruits to anaerobic stress conditions and their relation to fruit taste, quality, and emission of off-flavour volatiles. Postharvest Biol Tech. 2005;38:99–105. [Google Scholar]
- Shipway MR, Bramlage WJ. The effect of carbon dioxide on activity of apple mitochondria. Plant Physiol. 1973;51:1095–1098. doi: 10.1104/pp.51.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisler EC, Grichko VP, Serek M. Interaction of ethylene and other compounds with the ethylene receptor: agonists and antagonists. In: Khan NA, editor. Ethylene action in plants. Berlin: Springer Verlag; 2006. pp. 1–34. [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at receptor level: recent developments. Physiol Plant. 1997;100:577–582. [Google Scholar]
- Sisler EC, Yang SF. Antiethylene effect of cis-butene and cyclic olefine. Phytochem. 1984;23:2765–2768. [Google Scholar]
- Smith S, Geeson J, Stow J. Production of modified atmospheres in deciduous fruits by the use of films and coatings. HortSci. 1987;22:772–777. [Google Scholar]
- Smith SM, Geeson JD, Browne KM, Genge PM, Evesson HP. Modified atmosphere retail packaging of discovery apples. J Sci Food Agric. 1987;40:165–178. [Google Scholar]
- Solomos T. Principles of gas exchange in bulky plant tissue. HortSci. 1987;22:766–771. [Google Scholar]
- Sornsrivichai J, Yantarasri T, Gemma H. Respiration kinetics of mangoes and Asian pear fruits under MA conditions and its relation to the ripening behaviour. Acta Hort. 1998;464:339–344. [Google Scholar]
- Speirs J, Lee E, Holt K, Kim YD, Scott NS, Loveys B, Schuch W. Genetic manipulation of alcohol dehydrogenase levels in ripening tomato fruit affects the balance of some flavour aldehydes and alcohols. Plant Physiol. 1998;117:1047–1058. doi: 10.1104/pp.117.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava LM (2001) Structure and metabolism of plant hormones In: Plant growth and development: hormones and environment. Academic Press, San Diego, California, USA, p 233–250
- Stanley DW. Biological membrane deterioration and associated quality losses in food tissues. Crit Rev Food Sci Nutr. 1991;30:487–553. doi: 10.1080/10408399109527554. [DOI] [PubMed] [Google Scholar]
- Staudt M, Bertin N. Light and temperature dependence of the emission of cyclic and acyclic monoterpenes from holk oak (Quercus ilex L.) leaves. Plant Cell Environ. 1998;21:85–395. [Google Scholar]
- Steindel F, Beauchamp J, Hansel A, Kesselmeier J, Kleist E, Kuhn U, Wisthaler A, Wildt J (2005) Stress induced VOC emissions from mildew infested oak. Geophys Res Abstr 7:EGU05-A-03010
- Stepanova AN, Alonso JM. Ethylene signaling and response pathway a unique signaling cascade with a multitude of inputs and outputs. Physiol Plant. 2005;123:195–206. [Google Scholar]
- Tandon KS, Baldwin EA, Shewfelt RL. Aroma perception of individual volatile compounds in fresh tomatoes (Lycopersicon esculentum Mill.) as affected by the medium of evaluation. Postharvest Biol Tech. 2000;20:261–268. [Google Scholar]
- Tandon KS, Jordan M, Goodner KL, Baldwin EA. Characterization of fresh tomato aroma volatiles using GC-olfactometry. Proc Fla State Hort Soc. 2001;114:142–144. [Google Scholar]
- Theologis A, Zarembinski TI, Oeller PW, Liang X, Abel S. Modification of fruit ripening by suppressing gene expression. Plant Physiol. 1992;100:549–551. doi: 10.1104/pp.100.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK. Postharvest technology of fruits and vegetables. In: Thompson AK, editor. Fruits and vegetables: harvesting handling and storage. Oxford: Blackwell Publishing Ltd; 2003. p. 354. [Google Scholar]
- Tian MS, Hewett EW, Lill RE. Effects of carbon dioxide on ethylene forming enzyme in Japanese pear and apple. Postharvest Biol Tech. 1994;4:1–12. [Google Scholar]
- Toivonen PMA. Non-ethylene, non-respiratory volatiles in harvested fruits and vegetables: their occurrence, biological activity and control. Postharvest Biol Tech. 1997;12:109–125. [Google Scholar]
- Toivonen PMA, Escobar S, Emona JP. Response of three raspberry cultivars to different modified atmosphere conditions. Acta Hort. 1999;505:33–38. [Google Scholar]
- Tucker GA. Introduction. In: Seymour G, Talor J, Tucker G, editors. Biochemistry of fruit ripening. London: Chapman & Hall; 1993. pp. 1–51. [Google Scholar]
- Utto W, Mawson AJ, Bronlund JE. Hexanal reduces infection of tomatoes by Botrytis cinerea whilst maintaining quality. Postharvest Biol Tech. 2008;47:434–437. [Google Scholar]
- Uys DC. Keeping quality of grapes with special reference to skin characteristics. South Africa: Dept. of Agricultural Technical Services, Agricultural Research; 1974. p. 229. [Google Scholar]
- Van Loon LC, Geraats BPJ, Linthorst HJM. Ethylene as a modulator of disease resistance in plants. Tr Plant Sci. 2006;11:185–191. doi: 10.1016/j.tplants.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Varoquaux P, Ozdemir IS. Packaging and produce degradation. In: Lamikanra O, Imam S, Ukuku D, editors. Produce degradation pathways and prevention. Boca Raton: CRC Press, Taylor & Francis Group; 2005. pp. 117–153. [Google Scholar]
- Vaughn SF. Plant volatiles. In: Roberts K, editor. Handbook of plant science. Vol 2. West Sussex: Wiley; 2007. pp. 1054–1060. [Google Scholar]
- Verboven P, Kerckhofs G, Mebatsion HK, Ho QT, Temst K, Wevers M, Cloetens P, Nicolai BM. Three-dimensional gas exchange pathways in pome fruit characterized by synchrotron X-ray computed tomography. Plant Physiol. 2008;147:518–527. doi: 10.1104/pp.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma LR, Joshi VK. Post-harvest technology of fruits and vegetables. New Delhi: Indus Publishing Co; 2000. pp. 3–4. [Google Scholar]
- Wang H, Liang X, Huang J, Zhang D, Lu H, Liu Z, Bi Y. Involvement of ethylene and hydrogen peroxide in induction of alternative respiratory pathway in salt-treated Arabidopsis calluses. Plant Cell Physiol. 2010;51:1754–1765. doi: 10.1093/pcp/pcq134. [DOI] [PubMed] [Google Scholar]
- Wann EV. Physical characteristics of mature green and ripe tomato fruit tissue of normal and firm genotypes. J Am Soc Hort Sci. 1996;121:380–383. [Google Scholar]
- Wardlaw CW, Leonard ER. Studies in tropical fruits. Ann Bot. 1936;50:622–653. [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins CB, Barden CL, Bramlage WJ. Relationship between alpha-farnesene, ethylene production and superficial scald development of apples. Acta Hort. 1993;343:155–160. [Google Scholar]
- Watkins CB, Nock JF, Whitaker BD. Responses of early, mid and late season apple cultivars to postharvest application of 1-methylcyclopropene (1-MCP) under air and controlled atmosphere storage conditions. Postharvest Biol Tech. 2000;19:17–32. [Google Scholar]
- Weichmann J. The effect of controlled atmosphere storage on the sensory and nutritional quality of fruits and vegetables. Hort Rev. 1986;8:101–127. [Google Scholar]
- Wills RBH, Ku VVV. Use of 1-MCP to extend the time to ripen the green mature tomatoes and postharvest life of ripe tomatoes. Postharvest Biol Tech. 2002;26:86–90. [Google Scholar]
- Wills RBH, Ku VVV, Leshem YY. Fumigation with nitric oxide to extend the postharvest life of strawberries. Postharvest Biol Tech. 2000;18:75–79. [Google Scholar]
- Xu L. Use of ozone to improve the safety of fresh fruits and vegetables. Food Tech. 1999;53:58–61. [Google Scholar]
- Xu ZC, Ikoma Y, Yano M, Ogawa K, Hyodo H. Varietal differences in the potential to produce ethylene and gene expression of ACC-synthase and ACC-oxidase between ‘Kuimi’ and ‘Hong Xin’ of Chinese kiwifruit. J Jpn Soc Hort Sci. 1998;67:204–209. [Google Scholar]
- Yahia EM. Introduction. In: Yahia EM, editor. Modified and controlled atmospheres for the storage, transportation and packaging of horticultural commodities. Boca Raton: CRC Press, Taylor & Francis Group; 2009. pp. 1–16. [Google Scholar]
- Yamane M, Abe D, Yasui S, Yokotani N, Kimata W, Ushijima K, Nakano R, Kubo Y, Inaba A. Differential expression of ethylene biosynthetic genes in climacteric and non-climacteric Chinese pear fruit. Postharvest Biol Tech. 2007;44:220–227. [Google Scholar]
- Yang CX, Shewfelt RL. Effects of sealing of stem scar on ripening rate and internal ethylene, oxygen and carbon dioxide concentrations of tomato fruits. Acta Hort. 1999;485:399–404. [Google Scholar]
- Yang SF. Regulation of biosynthesis and action of ethylene. Acta Hort. 1987;201:53–59. [Google Scholar]
- Yang SF. The role of ethylene in fruit ripening. Acta Hort. 1995;398:167–178. [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- Yang SF, Pratt HK. The physiology of ethylene in wounded plant tissue. In: Kaul G, editor. Biochemistry of wounded plant tissue. Berlin: Walter de Gruyter; 1978. pp. 595–622. [Google Scholar]
- Yang Y, Hideki M, Fukushima T. Activation of glyoxylate cycle enzymes in cucumber fruits exposed to CO2. Plant Cell Physiol. 1998;39:533–539. [Google Scholar]
- Yip WK, Jiao XZ, Yang SF. Dependence of in vitro ethylene production rate on 1-aminocyclopropane-1-carboxylic acid content and oxygen concentrations. Plant Physiol. 1988;88:553–558. doi: 10.1104/pp.88.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-D, Cho Y, Sheen J. Emerging connections in the ethylene signaling network. Trends Plant Sci. 2009;14:270–279. doi: 10.1016/j.tplants.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagory D, Kader AA. Modified atmosphere packaging of fresh produce. Food Tech. 1988;42:70–77. [Google Scholar]
- Zambre SS, Venkatesh KV, Shah MG. Tomatoes redness for assessing ozone treatment to extend the shelf life. J Food Eng. 2010;96:463–468. [Google Scholar]
- Zheng XY, Wolff DW. Ethylene production, shelf-life and evidence of RFLP-polymorphisms linked to ethylene genes in melon (Cucumis melo L.) Theor Appl Genet. 2000;101:613–624. [Google Scholar]
- Zhu HL, Zhu BZ, Fu DQ, Xie YH, Hao YL, Luo YB. Role of ethylene in biosynthetic pathways of aroma volatiles in ripening fruit. Russ J Plant Physiol. 2005;52:691–695. [Google Scholar]
- Ziosi V, Bonghi C, Bregoli AM, Trainotti L, Biondi S, Sutthiwal S, Kondo S, Costa G, Torrigiani P. Jasmonate-induced transcriptional changes suggest a negative interference with the ripening syndrome in peach fruit. J Exp Bot. 2008;59:563–573. doi: 10.1093/jxb/erm331. [DOI] [PubMed] [Google Scholar]
- Ziosi V, Bregoli AM, Bonghi C, Rasori A, Biondi S, Costa G, Torrigiani P. Jasmonates delay ripening by interfering with ethylene biosynthesis and perception and with polyamine accumulation in peach fruit. In: Ramina A, Chang C, Giovannoni J, Klee H, Perata P, Wolterinig E, editors. Advances in plant ethylene research. Wageningen: Springer; 2007. pp. 109–110. [Google Scholar]