Abstract
Methods for studying interactions of protein with lipids and detergents are described for representatives of two major classes of membrane proteins: (1) the α-helical heterooligomeric integral cytochrome b6f complex of oxygenic photosynthesis from cyanobacteria, and (2) the outer membrane β-barrel proteins BtuB and OmpF from Gram-negative Escherichia coli bacteria. Details are presented on the use of detergents for purification and crystallization of the b6f complex as well as a method for lipid exchange. The positions of detergent and lipid molecules, which define eight potential lipid-binding sites in the b6f complex, are described. Differences in detergent strategies for isolation and crystallization of β-barrel proteins relative to those for oligomeric helical membrane proteins are discussed, and purification and assessment of protein quality by circular dichroism (CD) is presented.
Keywords: BtuB, vitamin B12 receptor, cytochrome b6f complex, OmpF porin
INTRODUCTION
General and detailed methods for the use of detergents in the purification and crystallization of integral membrane proteins are the subject of an excellent review in this series (UNIT 4.8) and helpful discussions elsewhere (e.g., Ben-Shem et al., 2003; Hunte et al., 2003; Luckey, 2008). The present unit focuses on specific problems involving the use of detergents in structure-function studies of (1) the transmembrane α-helical hetero-oligomeric cyanobacterial cytochrome b6f complex (Cyt b6f; Kurisu et al., 2003b; Yamashita et al., 2007; Baniulis et al., 2009), for which general details of preparation and crystallization have been described (Baniulis et al., 2011); and (2) β-barrel proteins from the Escherichia coli outer membrane, which have been structurally characterized in the context of the cellular import mechanism of cytotoxic colicin. Import of these proteins across the outer membrane and entry into the cell is known to be cytotoxic (Kurisu et al., 2003a; Cherezov et al., 2006; Sharma et al., 2007; Yamashita et al., 2008). As discussed below (see Strategic Planning), the choice of detergents for purification and crystallization of β-barrel membrane proteins differs from that for multi-helical membrane proteins.
Basic Protocol 1 describes a method for screening detergents for efficiency of extraction of the α-helical hetero-oligomeric integral cytochrome b6f complex of oxygenic photosynthesis from cyanobacteria. Basic Protocol 2 presents a method for lipid exchange for purification and crystallization of the b6f complex. Basic Protocol 3 describes extraction and purification of two β-barrel proteins: vitamin B12-binding protein (BtuB) and the general porin outer membrane protein F (OmpF) from the outer membrane of E. coli.
STRATEGIC PLANNING
The Cytochrome b6f Complex
A rule of thumb for non-denaturing purification and crystallization of such multi-subunit, multi-helical membrane proteins is the requirement for mild detergents for extraction of the protein from the membrane as well as purification and crystallization. Mild detergents are characterized by an electrically neutral and large head group and a relatively long (e.g., C12) acyl chain. Frequently used mild detergents include the maltosides n-dodecyl-β-D-maltopyranoside (DDM) and n-undecyl-β-D-maltopyranoside (UDM), with acyl chains of twelve and eleven carbons, respectively. The latter is used frequently in the studies described here.
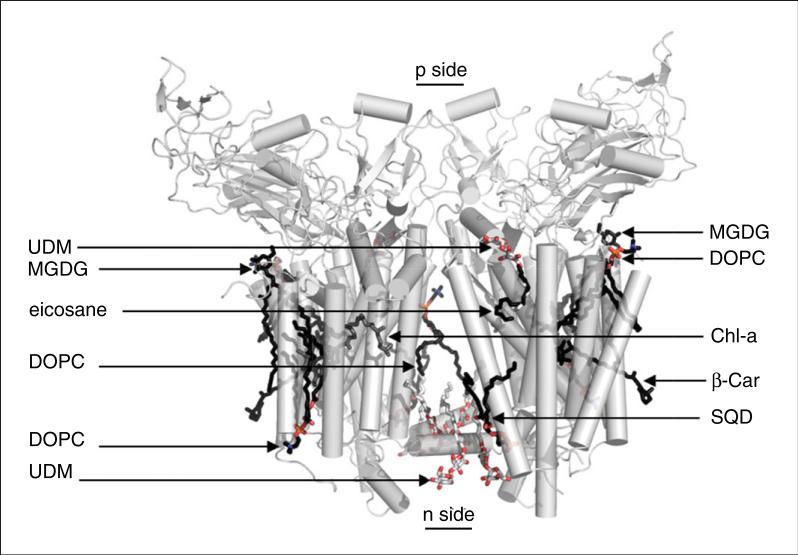
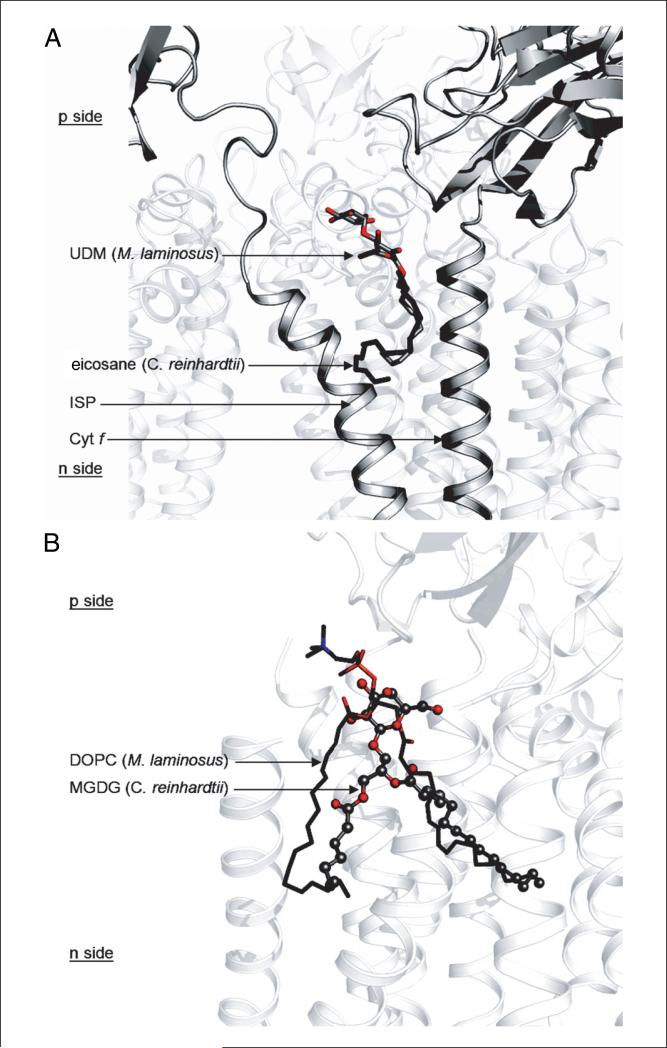
Maltoside detergents have become one of the most utilized classes of detergents for membrane protein purification and crystallization. Numerous membrane proteins have been successfully purified and crystallized in DDM, including the b6f complex (Kurisu et al., 2003b; Stroebel et al., 2003). However, DDM detergent micelles can reach a size of ~75 kDa (Anatrace). For membrane protein complexes of this size, large micelles may compromise the integrity of the complex during protein concentration, as the choice of the nominal molecular weight cut-off of the concentrator membrane (usually dictated by protein size) may result in accumulation of high detergent concentrations, leading to loss of protein integrity. A quantitative assay for dissociation of oligomeric structures is described (Harlan et al., 1995). It is expected that membrane proteins, in their native state, should have unique hydrodynamic properties. Based on the molecular weight of the polypeptides, prosthetic groups, lipids, and the detergent micelle, a stable membrane protein complex is expected to migrate on a size-exclusion chromatography column as a single Gaussian peak. However, loss of subunits and denaturation may change both the number of peaks as well as the profile of individual peaks. The presence of more than one peak for a hetero-oligomeric membrane protein in a size-exclusion chromatography profile may indicate the presence of multiple species that differ in composition. In this respect, detergents play a crucial role. Detergents with large micelle sizes may concentrate with the protein, as the large micelle size does not allow efficient exchange during protein concentration. A high detergent concentration has the potential to disrupt hydrophobic interactions that hold the subunits of the hetero-oligomeric membrane protein complex together, leading to subunit dissociation and loss of native structure. Specifically for the Cyt b6f complex, although the b6f dimer (MW 230 kDa) is much larger than the 75-kDa DDM micelle, the much smaller UDM micelle size (~35 kDa) allows exchange from the lower-quality Sol-grade detergent used for purification to the higher-quality analytical-grade UDM prior to crystallization. According to Anatrace, Sol-grade UDM has a purity of ≥97% as determined by HPLC analysis and contains less than 5% α-isomer of UDM, whereas analytical-grade UDM has purity of ≥99% with less than 0.2% α-UDM. The dependence of crystal quality on detergent purity has been described for the cyanobacterial photosystem I reaction center complex (Fromme et al., 2001). The b6f dimeric complex, depicted in a ribbon diagram with the positions of bound detergent used in purification and crystallization, and binding sites of lipids used in reconstitution and crystallization, is shown in Figure 29.7.1.
Figure 29.7.1.
Cytochrome b6f complex binding sites for lipids, detergents, and pigments. Three natural lipids, two molecules of the neutral monogalactosyldiacylglycerol (MGDG, seen in the green algal C. reinhardtii b6f complex), and one molecule of the acidic sulfoquinovosyldiacylglycerol (SQD, seen in b6f of C. reinhardtii, M. laminosus, and Nostoc PCC 7120) are observed per monomer. Three molecules of the synthetic lipid dioleoylphosphatidylcholine (DOPC) are resolved in the structure of the cyanobacterial b6f (M. laminosus and Nostoc PCC 7120), along with four n-undecyl-β-D-maltopyranoside (UDM) detergent molecules. Two pigment molecules, chlorophyll-a (Chl-a) and β-carotene (β-Car), are found in cyanobacterial and algal b6f, and third pigment, eicosane, is found in the algal b6f complex. Electrochemically negative (n) and positive (p) sides of the membrane are indicated. For the color version of this figure, go to http://www.currentprotocols.com/protocol/ps2907.
For solubilization of the b6f complex from the intramembrane system of cyanobacteria, Sol-grade UDM is typically used at a concentration ~20 times the critical micelle concentration (CMC), which is 0.029% (w/v) or 0.59 mM in water, and for crystallization, ~1.5-2.0 times the CMC. An important caveat is that the efficiency of extraction, at least in the case of cyanobacterial membranes, can be exquisitely sensitive to small differences in detergent concentration (Fig. 29.7.2). Greater extraction of Cyt b6f at 0.63% detergent concentration is detected as an increase in electron transfer activity from decylplastoquinol to cytochrome c in the extract (see description below). In addition, the range of detergent concentrations effective for membrane protein extraction in a particular experiment can vary by approximately ±0.1% in different cyanobacterial cultures ostensibly grown to the same cell density. In the procedure below, the efficiency of extraction is distinguished from denaturation of the complex by comparing specific activity of the thylakoid membrane preparation (M), the detergent extract of the membranes (D), and the insoluble membrane pellet (P) collected by centrifugation (see Basic Protocol 1, step 37).
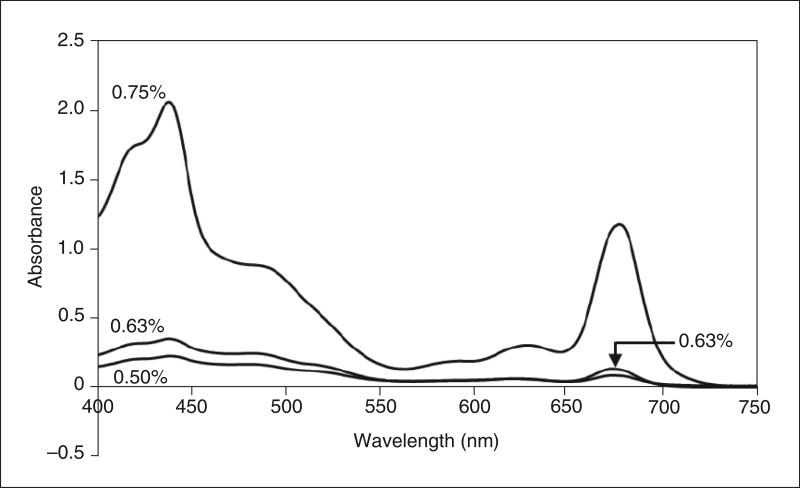
Figure 29.7.2.
Extraction efficiency of the detergent UDM. Thylakoid membranes of Nostoc PCC 7120 were suspended at a chlorophyll-a concentration of 1.6 mg/ml with UDM concentrations of 0.50%, 0.63%, and 0.75% (w/v). The mixtures were rocked gently for 30 min on ice and then centrifuged to remove unextracted material. Absorbance spectra of 20-fold dilutions of supernatant were recorded. At 0.50% UDM, ~10% of the total Cyt b6f is extracted from the membranes, whereas at higher UDM concentrations (0.63% and 0.75%), ~80% Cyt b6f is extracted from the membrane. However, 0.75% UDM also extracts a higher concentration of contaminating proteins, as measured by the larger amplitude of the Qy band (~670 nm).
Outer Membrane β-Barrel Proteins
The rule for use of mild detergents for purification and crystallization of multi-subunit integral helical membrane proteins does not apply to β-barrel proteins from the E. coli outer membrane, nor presumably to comparable outer membrane proteins (Omps) from other Gram-negative bacteria. The published purification scheme for the E. coli Omp BtuB, the vitamin B12-binding protein that is parasitized by bacteriophage and colicins for cellular import, employs the harsh (i.e., protein-denaturing) detergent lauryldiamine-N-oxide (LDAO), which has a small head group (Kurisu et al., 2003a; Taylor et al., 1998). A crystal structure of this Omp BtuB, done in meso in the lipid cubic phase, was obtained with a resolution of 1.95Å (Cherezov et al., 2006). Both the purification and in surfo (in detergent) crystallization of the E. coli OmpF porin, which resulted in a crystal structure with a resolution of 1.6Å (Fig. 29.7.3), utilized the harsh octyl-POE detergent. A similar strategy has been used for other outer membrane β-barrel proteins (Fairman et al., 2011).
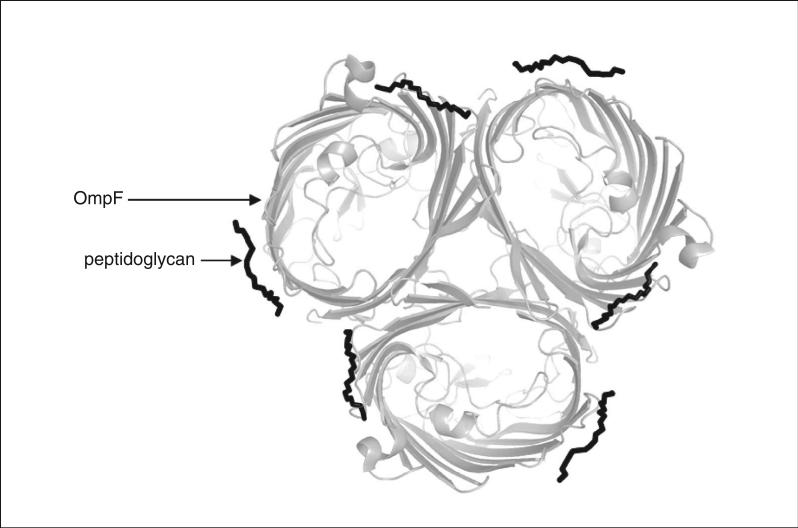
Figure 29.7.3.
Crystal structure (1.6Å resolution) of the trimeric E. coli OmpF porin, in a view normal to the plane of the membrane, showing two bound lipopolysaccharide molecules for each monomer (Yamashita et al., 2008).
The outer membrane of Gram-negative bacteria is significantly different in lipid composition and structure from the inner membrane (Nikaido, 2003). For purification and crystallization of β-barrel proteins, it is important to control the content of lipopolysaccharide (LPS), which is extracted with proteins by detergents because LPS-enriched porins do not crystallize.
SCREENING DETERGENTS TO DETERMINE MEMBRANE PROTEIN EXTRACTION EFFICIENCY
For stable preparation of isolated and purified membrane proteins, it is essential to identify the optimal conditions of detergent-mediated extraction. The type (ionic versus non-ionic) and concentration of the detergent are critical parameters that require screening and optimization. The use of harsh detergents may lead to denaturation, loss of subunits from hetero-oligomeric complexes, and compromised biological function. For membrane-bound enzymes such as the Cyt b6f complex, the use of enzymatic activity provides an important criterion for estimating the efficiency of a detergent-mediated extraction protocol. The cyanobacterial b6f complex catalyzes electron transfer from the physiological electron donor plastoquinol (dPQH2) to the small soluble protein plastocyanin (Cyt c6). Under the in vitro conditions described below, plastoquinol is replaced by decylplastoquinone (dPQ), and plastocyanin is replaced by Cyt c. Enzymatic activity in intact membranes is used as an estimate of the content of functional protein. Upon addition of detergent, the fraction of enzymatic activity obtained in the solubilized membrane-free fraction provides a measure of the efficiency of the extraction conditions and the stability of the extracted protein. Loss of total activity in the solubilized fraction and residual membrane pellet compared to the intact membranes is an indicator of protein instability.
Invoking the precedent set in studies on lipid-binding sites in the cytochrome oxidase of the bacterium Rhodobacter sphaeroides (Qin et al., 2006), and the common site of the sulfolipid in structures of b6f complexes from the green alga Chlamydomonas reinhardtii (Stroebel et al., 2003) and the cyanobacteria Mastigocladus laminosus (Yamashita et al., 2007) and Nostoc sp. PCC 7120 (Baniulis et al., 2009), it is assumed (Figs. 29.7.4 and 29.7.5) that the detergents used for purification and crystallization of the b6f complex bind to specific sites on the complex (Fig. 29.7.1) that are markers for natural lipid-binding sites.
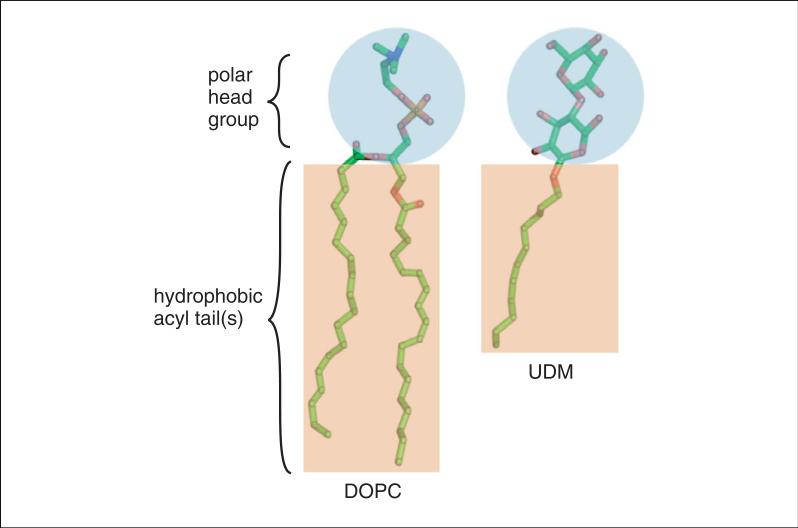
Figure 29.7.4.
Structures of the detergent UDM and the lipid DOPC. For the color version of this figure, go to http://www.currentprotocols.com/protocol/ps2907.
Figure 29.7.5.
Detergent and lipid used for reconstitution and crystallization can serve as markers for binding sites of natural lipids in the protein complex. (A) Detergent as a substitute of a natural ligand. UDM in the cyanobacterial Cyt b6f complex (M. laminosus and Nostoc PCC 7120) occupies the same niche as the photosynthetic pigment eicosane in the algal b6f complex. (B) Synthetic lipid as a lipid binding site marker. The acidic synthetic lipid DOPC used for crystallization of the cyanobacterial b6f complex (M. laminosus and Nostoc PCC 7120) occupies the same binding site as the natural neutral lipid MGDG in the b6f complex of the green alga C. reinhardtii. For the color version of this figure, go to http://www.currentprotocols.com/protocol/ps2907.
Materials
Nostoc PCC 7120 cells (ATCC)
BG11 medium (see recipe),
CO2 gas
Cell breakage buffer (see recipe)
Osmotic shock buffer (see recipe)
Membrane wash buffer (see recipe)
TNE buffer 1 (see recipe)
Acetone
Extraction buffer (see recipe)
10% (w/v) UDM-Sol (see recipe) in extraction buffer
25 mM decylplastoquinone (dPQ; Sigma-Aldrich) in 100% ethanol in a cuvette sealed with a rubber lid
Carbon-coated platinum catalyst (Sigma-Aldrich)
Hydrogen gas
Cyt b6f activity assay buffer (see recipe)
Ethanol
13-liter carboys
Gas regulators, glass and rubber tubing, and in-line barometer (Cole-Palmer) for CO2 supply
Spectrophotometer with thermostat and stirring control (Cary 4000 from Varian or equivalent)
3-ml glass cuvette and small magnetic bead for stirring
1-ml UV-transparent cuvette
Pellicon tangential flow filter (Millipore)
40-ml homogenizer with glass jacket and Teflon pestle, 4°C
300-ml glass beakers French pressure cell, 4°C
26.3-ml ultracentrifuge bottles (Beckman-Coulter)
Ultracentrifuge with Ti-70 and TLA-100.3 rotors (Beckman-Coulter)
Paint brush
Electric drill
1.5-ml polyallomer microcentrifuge tubes (Beckman-Coulter)
1-ml bullet-shaped interior cell vial with rubber lid
1- and 1.5-in. syringe needles
Parafilm
JA-10 rotor (Beckman-Coulter) with 500-ml Nalgene bottles
NOTE: Unless otherwise noted, all centrifugations are carried out at 4°C.
Grow and harvest cells
1. Grow Nostoc PCC 7120 cells in three 13-liter carboys each filled with 10 liters BG11 medium bubbling with ~1% CO2. Monitor cell growth by measuring absorbance at 730 nm until an absorbance of 1.0 is achieved.
The large-scale cyanobacterial culture is typically initiated in a 100-ml liquid culture. This serves as an inoculum for a 1-liter liquid culture, which in turn serves as the inoculum for a 10- to 12-liter liquid culture in a glass carboy. For all cultures, ~1 week is needed to reach an OD600 of 1.0 with CO2 bubbling.
2. Harvest cells using a Pellicon tangential flow filter, circulating the cell suspension through the filter at <15 psi, and concentrating cells to ~1 to 2 liters.
3. Transfer cells to 500-ml plastic centrifuge bottles and centrifuge 10 min at 2500 × g in a JA-10 rotor.
4. Decant supernatant, resuspend cells with 400 ml cold (4°C) cell breakage buffer, and centrifuge again.
5. Decant supernatant, resuspend cells with 100 ml cold cell breakage buffer, split into several aliquots in tared centrifuge tubes, and centrifuge again.
6. Decant supernatant, weigh cell pellets with tubes, and freeze.
Frozen pellets can be stored for at least 5 years at −80°C.
Lyse cells
7. Thaw 25-g aliquots of cells under cold running water and resuspend to 200 ml with cold cell breakage buffer.
8. Pour 10-15 ml of the cell suspension into a prechilled 40-ml glass homogenizer and homogenize until no clumps are visible. Pour into a 300-ml glass beaker on ice to keep cold, and repeat until the entire 200 ml cell suspension has been homogenized.
9. Set a prechilled French pressure cell to 20,000 psi and pass the suspension through the pressure cell three times, keeping the cells on ice.
Prepare thylakoid membranes
NOTE: Perform all steps at 4°C in the dark (low light) to protect cyt b6f complex from chlorophyll pigment–mediated oxidative damage, which is induced by light.
10. Centrifuge broken cell mass 10 min at 1500 × g (using 500-ml plastic bottles in a JA-10 rotor) to remove cell debris and unbroken cells.
11. Carefully decant the supernatant into clean 26.3-ml ultracentrifuge bottles and centrifuge 45 min at 300,000 × g in a Ti-70 rotor.
12. Gently decant and discard the blue-red supernatant without disturbing the green membrane pellet. Keep the bottles on ice.
13. Resuspend the pellet in each bottle in ~5 ml cold osmotic shock buffer using a paint brush to dissolve the pellet.
14. Transfer 10-15 ml membrane suspension to a 40-ml glass homogenizer and homogenize with a Teflon homogenizer attached to an electric drill for 5-10 sec or until membrane fragments are evenly dispersed and no large clumps are visible.
15. Transfer homogenate to a 300-ml glass beaker on ice and adjust the volume to 200 ml with osmotic shock buffer.
16. Distribute to 26.3-ml ultracentrifuge bottles and centrifuge 45 min at 300,000 × g in a Ti-70 rotor.
17. Gently decant and discard the supernatant without disturbing the green pellet. Keep bottles on ice.
18. Resuspend pellet in 100 ml membrane wash buffer, homogenize as in step 14, and gently stir in an ice bath for 20 min.
19. Add 100 ml cold distilled water, transfer to 26.3-ml ultracentrifuge bottles, and centrifuge 45 min at 300,000 × g.
20. Gently decant and discard the supernatant without disturbing the green pellet, then repeat membrane washing (steps 18-19).
21. Gently decant and discard the supernatant and keep bottles on ice. Resuspend the membrane pellets to a total volume of 200 ml in TNE buffer 1 using a paint brush to dissolve the pellets.
22. Determine chlorophyll a concentration (μg/ml):
a. Add 10 μl membrane suspension to a 1.5-ml microcentrifuge tube containing 800 μl acetone (100%) and 190 μl distilled water. Vortex 30 sec.
b. Microcentrifuge 2 min at 16,000 × g, room temperature, to remove unextracted material.
c. Gently transfer the green supernatant to a 3-ml glass cuvette and record an absorbance spectrum at 646.6, 663.6, and 750.0 nm.
d. Determine chlorophyll a concentration in the organic phase extract using the equation:
[Chl a] = 17.76 × (A646.6 − A750.0) + 7.34 × (A663.6 − A750.0)
e. Apply a dilution correction of 100× to determine the chlorophyll a concentration in the membrane suspension.
For full details on this procedure, see Zhang and Cramer (2004).
23. Centrifuge the remaining membrane suspension 45 min at 300,000 × g.
24. Decant and discard the supernatant, and resuspend membrane pellets in a minimal volume of TNE using a paint brush.
25. Combine all fractions into one or two ultracentrifugation bottles and centrifuge 45 min at 300,000 × g.
26. Decant the supernatant and store the membrane pellet overnight at 0°C.
Screen conditions for detergent-mediated Cyt b6f extraction
27. Resuspend thylakoid membranes in extraction buffer at a [Chl a] of 2.4 mg/ml using a paint brush and keep on ice.
28. Transfer 670-μl aliquots to 1.5-ml microcentrifuge tubes labeled A, B, and C.
29. Add 280 μl extraction buffer to tube A, 267 μl to tube B, and 255 μl to tube C.
30. Add 50 μl of 10% UDM-Sol to tube A, 63 μl to tube B, and 75 μl to tube C.
Tubes A, B, and C contain final concentrations of 0.50%, 0.63%, and 0.75% UDM, respectively, and 1.6 mg/ml chlorophyll a.
31. Rock tubes gently on ice for 30 min in the dark.
32. Transfer the solution to 1.5-ml polyallomer ultracentrifuge tubes and centrifuge 45 min at 300,000 × g in a TLA 100.3 rotor.
Assay Cyt b6f extraction efficiency
CAUTION: Gaseous hydrogen is explosive. The following steps must be performed in a well-ventilated chemical hood, away from potential sources of fire.
33. Reduce dPQ to dPQH2 as follows:
a. Transfer 500 μl of 25 mM dPQ in ethanol to a 1-ml bullet-shaped interior cell vial.
b. Add carbon-coated platinum catalyst (an amount equivalent to tip of a spatula) and seal with the rubber lid.
c. Insert a 1-in. needle into the lid (without dipping it into the solution) to provide an exit for the hydrogen gas.
d. Attach a second 1.5-in. needle to the gaseous hydrogen supply, insert it into the dPQ solution in the vial, and gently bubble hydrogen gas for 10 min.
e. Turn off the hydrogen supply, remove both needles, and seal the rubber lid using Parafilm.
f. Dilute an aliquot 500-fold in ethanol and determine dPQH2 concentration by measuring absorbance at 290 nm and using an extinction coefficient (εM) of 4000 M–1 cm–1.
34. Initiate the assay reaction by combining the following in order in a 3-ml spectrophotometer cell with stirring:
assay buffer to give a total of 1.5 ml 20 μM dPQH2 thylakoid membranes equivalent to 3 μM Chl a.
Monitor the absorbance change at 551 nm in a Cary 4000 spectrophotometer.
35. Determine the activity of cytochrome c based on the observed change in A551 and εM = 21,000 M–1cm–1.
36. Calculate specific activity as electron transfer/sec/103 Chl a equivalent present in the thylakoid membranes.
See Fig. 29.7.6 for representative results.
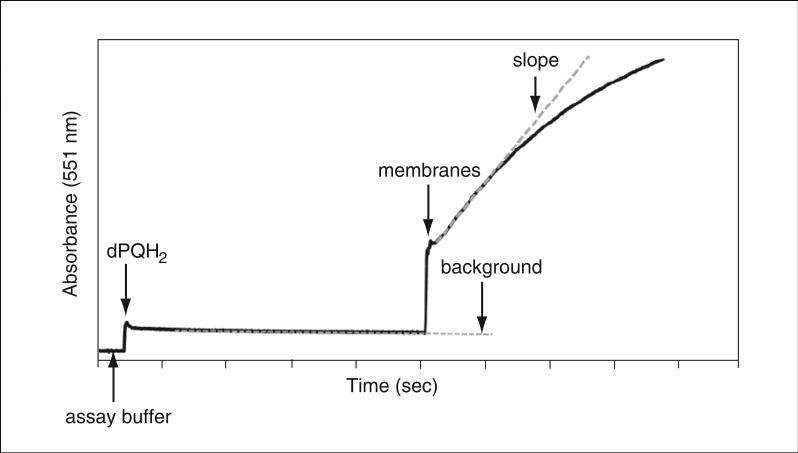
Figure 29.7.6.
Assay of Cyt b6f electron transfer activity. The Cyt b6f complex in cyanobacterial thylakoid membranes catalyzes electron transfer from the electron donor decylplastoquinol (dPQH2) to the electron acceptor cytochrome c (Cyt c). dPQH2 is added to the assay buffer and a baseline is recorded. The electron transfer reaction is initiated by addition of cyanobacterial membranes. Reduced Cyt c has an absorbance peak at 551 nm. As one electron is required to reduce one molecule of Cyt c, estimation of Cyt c reduction per unit time yields the catalytic activity of the Cyt b6f complex (calculations performed from initial electron transfer rate; slope shown as gray dotted line). Since Cyt b6f concentration cannot be determined in intact membranes, the specific rate is expressed in units of electrons transferred per 103 chlorophyll per second. The slow background rate of electron transfer from dPQH2 to Cyt c in the absence of Cyt b6f (gray dotted line marked background) is subtracted from the slope of b6f-catalyzed electron transfer.
37. Assess activity in the thylakoid membrane preparation (M), the detergent extract of the membranes (D), and the insoluble membrane pellet (P) collected by centrifugation (45 min at 300,000 × g, 4°C) and resuspended in the same volume of TNE buffer 1 as the volume of membrane suspension prior to extraction.
Efficiency of extraction is expressed as a percent of electron transfer activity in the extract relative to activity in intact membranes. Usually, an increase in specific activity of Cyt b6f complex is observed in the extract (M < D M). Therefore, to account for efficiency of extraction (E), the following formula is used:+E = (D/(D+M)) × 100%.
EXCHANGING MEMBRANE PROTEIN NATIVE LIPIDS
The dependence of integral membrane protein crystal structure on the presence of specific lipids has been demonstrated for a significant number of membrane proteins, such as the photosynthetic light-harvesting chlorophyll protein (Nussberger et al., 1993), aqua-porin (Hite et al., 2010), G-protein coupled receptors (GPCRs; Escriba et al., 2007; Hanson et al., 2008), and potassium ion channels (Lee et al., 2005). The structurally characterized integral membrane proteins with the largest number of distinct lipids and inferred lipid functions are photosynthetic reaction centers and electron transport complexes from energy-transducing membranes: the bacterial photosynthetic reaction center (Jones, 2007; Wohri et al., 2009), photosynthetic reaction centers II (Guskov et al., 2009; Umena et al., 2011) and I (Jordan et al., 2001; Kern et al., 2009), bovine (Shinzawa-Itoh et al., 2007) and bacterial cytochrome oxidase (Qin et al., 2007), and cytochrome bc1 (Palsdottir and Hunte, 2004) and b6f (Hasan et al., 2011) complexes.
In addition to the roles lipids play in creating and sustaining membrane protein structures, an important recent development in lipid-based methodology for studies of these protein structures has been the success of membrane protein crystallization using the lipid cubic phase, first proposed by Landau and Rosenbusch (1996). This method has recently been applied to α-helical membrane proteins, mainly single-subunit proteins such as bacteriorhodopsin and GPCRs (Caffrey, 2003; Lanyi, 2004; Cherezov et al., 2007).
As described below, a successful strategy for membrane protein purification involves replacement of native lipids in a membrane protein structure (Zhang et al., 2003). Purification of the cytochrome b6f complex of oxygenic photosynthesis from a cyanobacterial source requires removal of the abundant light-harvesting phycobiliproteins. Use of hydrophobic-interaction chromatography for partial removal of some phycobiliproteins (e.g., by passage through a propyl agarose column) also results in depletion of the native lipid in the complex. For additional details, see (Zhang et al., 2003). The native lipid can then be replaced by a lipid of choice during crystallization (Kurisu et al., 2003b; Yan et al., 2006; Yamashita et al., 2007; Baniulis et al., 2009), as described in detail elsewhere (Cramer et al., 2011).
A recent problem with the lipid exchange/replacement methodology described below is that the propyl agarose hydrophobic-interaction chromatography resin is apparently no longer being manufactured, and a suitable replacement material has not been found. Presently, purification of the cyanobacterial Cyt b6f complex for crystallographic studies is performed using a limited stock of propyl agarose (see Fig. 29.7.7 for an image of Cyt b6f crystals).
Figure 29.7.7.
Cyt b6f crystals from Nostoc PCC 7120. Brown, hexagonal, bipyramidal crystals are obtained by crystallization of the Cyt b6f complex purified from Nostoc PCC 7120 thylakoid membranes using the protocol described here. For the color version of this figure, go to http://www.currentprotocols.com/protocol/ps2907.
An alternative purification strategy for the cytochrome complex from cyanobacteria is affinity tagging (e.g., His6) and purification by nickel or cobalt affinity chromatography (Stroebel et al., 2003; Yan et al., 2008). A problem with this approach is the availability of appropriate strains. The genetically tractable cyanobacteria—i.e., strains that can be readily tagged for affinity chromatography—are single-cell strains such as Synechocystis sp. PCC 6803 or Synechococcus sp. PCC 7002. However, both these strains apparently contain a protease that cleaves the exposed p (lumen)—side exposed loop of the iron-sulfur protein (ISP; Zhang and Cramer, 2005), resulting in loss of the ISP and the dimer, and generating a monomeric product of ~110,000 kDa that does not have significant electron transport activity and is not crystallizable. The proteolysis of the ISP subunit is monitored by loss of electron transfer activity and SDS-PAGE analysis (Zhang et al., 2003). This protease is not inhibited by any conventional protease inhibitors (2-6 mM benzamidine, 2-6 mM 6-amino-hexanoic acid, 0.1-1 mM PMSF, 1-10 mM EDTA, 100 μM TPCK, or 100 μM 3,4-dichloro-isocoumarin). This interfering protease activity is less of a problem in filamentous cyanobacteria, two of which have been used for purification and crystallization of active cytochrome complex (M. laminosus: Kurisu et al., 2003b; Yan et al., 2006; Yamashita et al., 2007; and Nostoc PCC 7120 “Anabaena”: Baniulis et al., 2009). The latter study also presents a partial analysis of the possible identity of the protease. However, mutagenesis of filamentous cyanobacteria such as Nostoc PCC 7120 requires multiple steps of chromosomal segregation. M. laminosus has not been transformed successfully for genetic manipulation.
Materials
Propyl agarose chromatography resin (Sigma-Aldrich)
Chromatography wash buffer (see recipe)
Membrane suspension containing Cyt b6f complex from cyanobacterial membranes (see Basic Protocol 1)
10% (w/v) UDM-Sol (see recipe) in TNE/sucrose buffer
Pulverized ammonium sulfate
Elution buffer (see recipe)
Protein buffer for sucrose density gradient centrifugation: TNE buffer 1 (see recipe) with 0.05% UDM-Sol (prepare on day of experiment)
10% and 32% (w/v) sucrose gradient solutions (see recipe)
Desired lipids, e.g., neutral dioleoylphosphatidylcholine and acidic dioleoylphosphatidylglycerol (Avanti Polar Lipids)
Gaseous nitrogen
Desiccator
TNS buffer (see recipe)
10% (w/v) UDM-Ana (see recipe) in TNS buffer
20-ml, 1-cm-diameter glass chromatography column
Peristaltic pump for chromatography
5-ml fraction collection tubes
100- and 300-ml glass beakers
20-ml syringe and 0.45-μm syringe filter
100-kDa nominal molecular weight cut-off (MWCO) protein concentrators (Millipore)
SG15 gradient maker (Hoefer)
SW41-Ti rotor with canisters and tubes (Beckmann-Coulter), 4 °C
15-ml polycarbonate test tubes (Thermo Scientific Nunc)
16 × 150-mm glass test tubes (Sigma-Aldrich)
Parafilm
Liquid nitrogen
Sonicator with water bath
NOTE: Unless otherwise noted, all centrifugations are carried out at 4°C.
Prepare chromatography resin
1. Load ~20 ml propyl agarose (50% slurry in ethanol) into a 1-cm-diameter glass chromatography column under gravity.
2. Wash with up to 5 column volumes of distilled water under gravity to remove residual ethanol.
3. Equilibrate with 2 to 3 column volumes of chromatography wash buffer under gravity at 4°C.
The prepared column can be stored for up to 1 day at 4°C.
Purify and delipidate cyanobacterial Cyt b6f complex
4. From the extraction screening protocol (see Basic Protocol 1), determine the appropriate concentration of detergent required to extract 60% to 80% of Cyt b6f complex from cyanobacterial membranes.
5. Resuspend the thylakoid membrane preparation in extraction buffer at a chlorophyll a concentration of 3 mg/ml using a paint brush and keep on ice.
6. Determine the amount of UDM-Sol required to attain the concentration determined in step 4, and dissolve the detergent in a volume of extraction buffer equal to 0.5 volume of thylakoid membrane solution. Add the detergent solution to the membrane suspension dropwise while stirring, then continue stirring on ice for 30 min.
7. Transfer the membrane-detergent suspension to ultracentrifugation bottles and centrifuge 45 min at 300,000 × g in a Ti-70 rotor.
8. Carefully decant ~80 to 150 ml supernatant into a 300-ml beaker and determine its volume.
9. Calculate the amount of ammonium sulfate required to achieve 35% saturation in the supernatant (APPENDIX 3F). Add pulverized, clump-free ammonium sulfate to the solution over 2 to 3 min while stirring over ice, then continue stirring another 15 min.
10. Transfer solution to dry ultracentrifugation bottles and centrifuge 30 min at 160,000 × g in a Ti-70 rotor.
11. Carefully decant the green-brown supernatant to a dry glass beaker without disturbing the green-blue pellet.
12. Filter supernatant through a 0.45-μm syringe filter gently to avoid forming bubbles.
13. Load on the equilibrated propyl agarose column under gravity at a rate of ~0.5 ml/min.
14. Wash column with chromatography wash buffer at a rate of ~1-2 ml/min until the flowthrough fractions are clear.
This takes ~30 column volumes of buffer. A peristaltic pump may be used at this step.
15. Elute protein with elution buffer at ~1-2 ml/min, collecting 3-ml fractions.
A large fraction (~2/3) of Cyt b6f complex elutes with the early green fractions, whereas a smaller amount elutes with the later blue fractions.
16. Determine the concentration of Cyt b6f in each fraction using a redox difference spectrum of ascorbate minus ferricyanide. Collect fractions until cyt b6f cannot be detected (~30 ml).
Concentration of cyt b6f was determined based on the cytochrome f difference spectrum of ascorbate minus ferricyanide, using an extinction coefficient of 25 mM–1cm–1 at 556 nm (Metzger et al., 1997) relative to a baseline drawn between the spectral troughs at 538 and 568 nm (Baniulis et al., 2011).
17. Pool fractions containing >1 μM Cyt b6f and pool the remaining fractions separately.
18. Concentrate each pooled sample using a 100-kDa MWCO protein concentrator to exchange the buffer to protein buffer for sucrose density gradient centrifugation. Concentrate samples to 1.0-2.0 ml, add buffer to 15 ml, and concentrate again to 1.0-2.0 ml.
19. Using an SG15 gradient maker, pour linear 10% to 32% sucrose density gradients (~11 ml each) in six ultracentrifugation tubes for an SW41-Ti rotor.
Keep tubes at 4°C, away from vibrations and disturbance.
20. Mix the sample containing >1 μM Cyt b6f gently to prevent formation of clumps, then load on three sucrose gradients by gently dropping on the side of the tubes using a micropipet to avoid disturbing the gradient. Load the sample containing <1 μM Cyt b6f on the other three gradients.
21. Load gradients into canisters for the SW41-Ti rotor and balance the weight using protein buffer for sucrose density gradient centrifugation. Add buffer only to the sides of the tubes to avoid mixing the protein sample with the gradient.
22. Centrifuge 16 hr at 160,000 × g, using slow acceleration and deceleration to avoid rapid mixing of the gradient.
23. Gently remove the canisters and place upright in ice. Remove fractions very gently from the top using a micropipet and transfer to a 15-ml polycarbonate test tube on ice.
Rapid pipetting leads to mixing of fractions and must be avoided. See Fig. 29.7.8 for a representative sucrose density gradient profile.
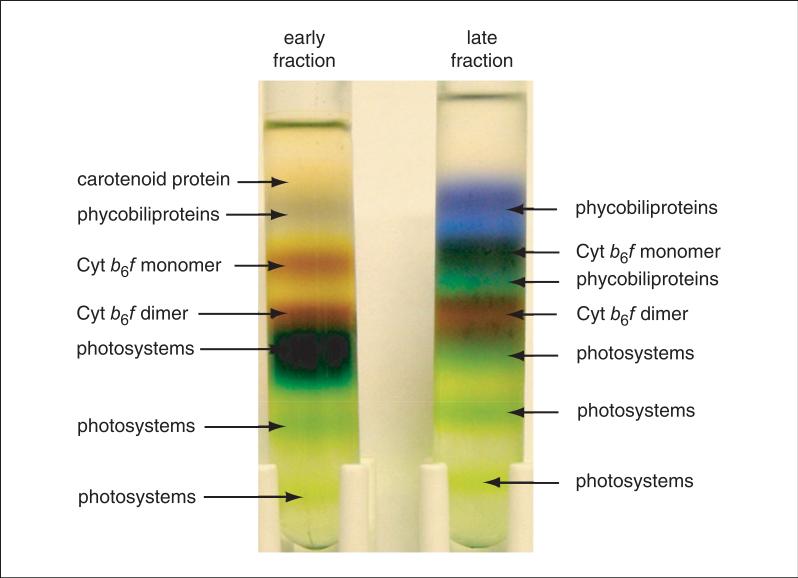
Figure 29.7.8.
Protein fractionation by sucrose density gradient centrifugation. Early protein fractions from the hydrophobic-interaction chromatography column are contaminated mainly with green photosystems, whereas late fractions have a significantly larger amount of phycobiliproteins. Dimeric Cyt b6f complex forms a brown band directly above the green photosystem band. A lower-density band of monomeric Cyt b6f complex is also observed. The dimeric Cyt b6f complex band is harvested into multiple fractions that are further purified by sucrose density gradient centrifugation to remove residual contaminants from the neighboring bands. The purified Cyt b6f is almost free of contaminants and is used for crystallographic studies. Figure originally published in the Journal of Biological Chemistry, Baniulis et al. (2009). For the color version, go to http://www.currentprotocols.com/protocol/ps2907.
Add lipids to purified Cyt b6f
24. Calculate the amount of lipid required to make 5 ml of a 1 mM lipid-containing buffer and transfer that amount of lipid to a clean 16 × 150–mm glass test tube.
25. Dry the lipids as a thin layer on the walls of the test tube under a gentle stream of nitrogen while vortexing. Leave overnight in a desiccator at room temperature to remove residual solvent.
Lipids are typically supplied in organic solvents, which must be removed.
26. Add 4.92 ml TNS buffer and vortex to suspend the dry lipid.
27. Add 75 μl of 10% UDM-Ana in TNS buffer (final 0.15% or 3 mM) and seal with Parafilm.
28. Freeze-thaw 15 times in liquid nitrogen.
29. Sonicate the lipid-detergent solution in a 20° to 25°C water bath for 20 min or until the solution becomes clear.
For neutral lipids, the solution may still be hazy even after 20 min. This solution can be used for crystallization.
30. Concentrate purified Cyt b6f complex to ~200 to 400 μl using a 100-kDa MWCO concentrator at 4°C.
31. Add 1 ml lipid-detergent solution to the concentrated protein and reconcentrate to 200 μl. Perform this step five times to exchange the Sol-grade detergent to Ana-grade detergent.
32. Concentrate the protein-lipid-detergent solution to 15 mg/ml Cyt b6f using a protein concentrator.
Cyt b6f from Nostoc PCC 7120 is crystallized at a concentration of 15 mg/ml (135 μM). This final solution contains 1 mM lipid and 3 mM UDM.
EXTRACTION AND PURIFICATION OF β-BARREL PROTEINS BtuB AND OmpF FROM THE OUTER MEMBRANE OF E. COLI
Efficient purification of outer membrane proteins is based on successful separation of the outer membranes from the inner membrane and soluble cytoplasmic proteins by selective detergent extraction. For this purpose, broken E. coli cells are first extracted several times with Triton X-100, which is efficient for solubilizing bacterial inner membranes but does not solubilize the outer membranes. β-Barrel proteins can then be extracted from the outer membranes using the detergent octylglucoside, which solubilizes outer membranes.
BtuB and OmpF are purified from the resulting extracts by anion-exchange chromatography, which serves not only as a main purification step, but also to exchange the detergent used for extraction with detergents suitable for crystallization. For BtuB, a second purification is performed using size-exclusion chromatography. During purification, special attention must be paid to removal of non-proteinacious contamination by LPSs. The presence of the chelating agent EDTA is important for LPS removal.
Following purification, protein concentration can be determined from the absorbance at 280 nm using appropriate extinction coefficients. This method, however, can be used only for solutions of pure protein and is sensitive to light scattering. In the procedure outlined below, a spectrum is measured across a wavelength range of 240 to 400 nm, which allows observation and estimation of the contribution of light scattering to the absorbance at 280 nm. As proteins do not absorb light significantly above 320 nm, the absorbance from 320 to 400 nm can be used to estimate and correct for light scattering. The 240 to 270 nm interval can also show the presence of nucleic acids, which have an absorbance peak at 260 nm.
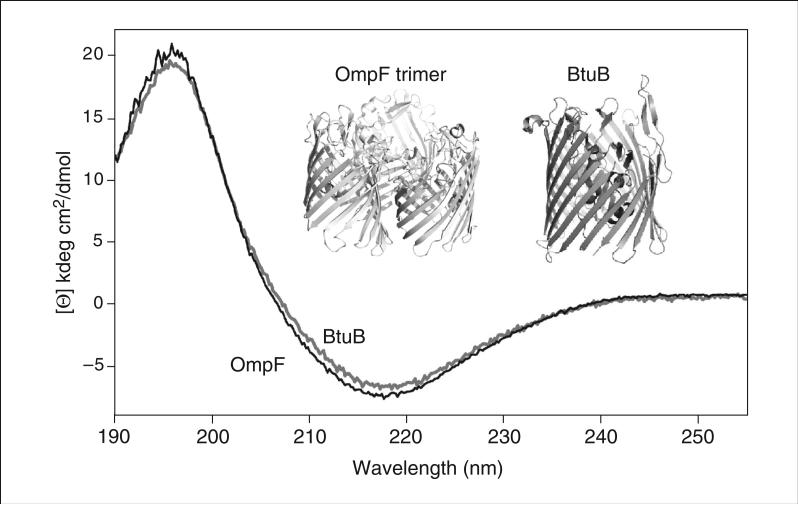
Finally, a far UV circular dichroism (CD) spectrum is used as a valuable tool to test the quality of isolated membrane proteins. A distinct characteristic of bacterial outer membrane proteins is the β-barrel structure of the integral membrane core (Fig. 29.7.11, inset). In contrast, inner membrane protein complexes have integral membrane cores that formed by transmembrane α-helices. The far UV CD spectrum of β strands has a minimum at 218 nm and a maximum at 195 nm.
Figure 29.7.11.
Far UV circular dichroism (CD) spectra of BtuB (black) and OmpF (gray). Secondary structure of both proteins consists of 60% β-strand and 6% α-helix in BtuB or 3% α-helix in OmpF. Spectra were measured in a quartz cuvette with a 0.1-mm path length. Inset: Crystal structures of OmpF trimer (PDB ID 2ZFG) and BtuB (PDB ID 2GUF).
Materials
LB medium
0.5 M ampicillin or 0.25 M kanamycin (for BtuB or OmpF, respectively)
E. coli strain TNE012 (pJC3) AmpR or MH225 (pPR272) KanR (for BtuB or OmpF, respectively)
Buffer A: 50 mM Tris, pH 8.0, with 2 mM EDTA
100× protease inhibitor solution (see recipe)
2 M MgSO4
DNase 1
Triton X-100
1.5% or 3.0% (w/v) β-octylglucoside in buffer A (for BtuB or OmpF, respectively)
1% (w/v) β-octylglucoside in buffer A
0.1% (w/v) lauryldiamine-N-oxide (LDAO, 1-dodecanamine-N,N-dimethyloxide) or 0.8% (w/v) N-octylpolyoxyethylene (octyl-POE) in buffer A (for BtuB or OmpF, respectively)
LiCl
0.1% LDAO in TNE buffer 2 (see recipe), degassed
Nitrogen gas
18 × 150–mm culture tubes with caps
2-liter flasks
Sterile toothpicks
Incubator shaker (e.g., Innova 4430, New Brunswick Scientific)
Spectrophotometer with 1-cm cuvette (for determining cell density)
Avanti J-E centrifuge with JA-10 rotor and appropriate bottles (Beckman-Coulter) or equivalent
Teflon homogenizer with glass jacket
Continuous-flow French press
JA-25.5 rotor and appropriate bottles (Beckman-Coulter) or equivalent
Optima LE-80K centrifuge with Ti70 rotor and appropriate bottles
(Beckman-Coulter) or equivalent
Anion-exchange column(s):
DEAE-Sepharose and Q-agarose columns (HiPrep FF 16/10, GE Healthcare Life Sciences; for BtuB)
FPLC system (Acta-FPLC, GE Healthcare Life Sciences; for OmpF)
CentriPrep 50 concentrators (Millipore)
Superdex 200 column (FF 10/300, GE Healthcare Life Sciences)
Cary Bio 300 UV Vis spectrophotometer (Varian) and quartz cuvettes with 1-mm or 1-cm optical path length
CD spectrophotometer (e.g., Charascan, Applied Photophysics) and demountable quartz cuvette with 0.1-mm path length (Starna Cells or Hellma Cells)
Additional reagents and equipment for SDS-PAGE (UNIT 10.1)
CAUTION: PMSF is highly toxic. Wear gloves and work in a chemical fume hood when using PMSF.
NOTE: Unless otherwise noted, all centrifugations are carried out at 4°C.
Grow and harvest cells
1. Autoclave four 18 × 150–mm culture tubes containing 5 ml LB medium each and four 2-liter flasks containing 1 liter LB medium each. Allow to cool to room temperature.
2. Add 5 μl each of 0.5 M ampicillin or 0.25 M kanamycin to each tube, and 1 ml of the same stocks to each flask.
3. Using toothpicks, scrape frozen cells and inoculate medium in the 5-ml tubes. Cover with caps and shake overnight in a 30oC incubator shaker.
4. Transfer culture from tubes to 2-liter flasks and incubate at 37oC, shaking at a rate of 220 rpm, until the optical density at 600 nm in a 1-cm cuvette reaches 1.0 to 1.2.
5. Harvest cells by centrifuging 10 min at 11,000 × g in a JA-10 rotor.
6. Decant supernatant, resuspend pellets in 400 ml buffer A, and repeat centrifugation in one tared bottle in the JA-10 rotor.
7. Determine yield of harvested cells by measuring weight of bottle with pellet and subtracting weight of empty bottle.
The yield of cells is ~10 g from 4 liter culture for both BtuB- and OmpF-producing strains. The cell pellet may be stored up to 1 year in a –80°C freezer.
Prepare outer membranes
8. Resuspend cell pellet in 100 ml buffer A and homogenize for 20 to 30 sec using a Teflon homogenizer with glass jacket.
9. Add the following:
1 ml 100× protease inhibitor solution (final 1 mM PMSF, 0.2 mM TPCK)
1 ml 2 M MgSO4 (final 20 mM)
20 μg DNase 1.
10. Break cells by passing the suspension three times through a continuous-flow French press at 20,000 lb./in.2.
11. Remove unbroken cells by centrifuging 5 min at 1200 × g.
12. Harvest broken cells and membrane fragments by centrifuging the supernatant 30 min at 35,000 × g in a JA-25.5 rotor.
13. Resuspend pellet in 200 ml buffer A with 2% Triton X-100 and add 2 ml of 100× protease inhibitor solution. Incubate 20 min on ice with magnetic stirring.
14. Centrifuge 30 min at 35,000 × g.
15. Repeat Triton X-100 extraction and centrifugation one more time.
The outer membrane pellet may be stored up to 1 year in a −80°C freezer.
Extract BtuB or OmpF from outer membranes
16. Resuspend outer membrane pellet in 200 ml buffer A containing 1.5% or 3.0% β-octylglucoside (for BtuB or OmpF, respectively) and incubate with magnetic stirring for 1 hr at room temperature.
17. Centrifuge 30 min at 300,000 × g in a Ti70 rotor (centrifuge Optima LE-80K, Beckman-Coulter). Collect supernatant and place on ice.
18. For OmpF, re-extract the pellet and combine the two supernatants on ice.
Combined detergent extracts can be stored overnight on ice.
Perform all subsequent steps in ice or a cold box.
Purify by anion-exchange chromatography (BtuB and OmpF)
19. Equilibrate the appropriate column with 40 ml buffer A containing 1% β-octylglucoside.
20. Load supernatant at a flow rate of 0.5 to 1.0 ml/min.
21. Wash column with 4 column volumes (80 ml) buffer A containing 0.1% LDAO (for BtuB) or 0.8% octyl-POE (for OmpF) at a flow rate of 0.5 ml/min.
In addition to purification, this step is important for exchange of detergents.
22. Elute protein by applying a linear salt gradient (4 column volumes) of 0 to 0.8 M LiCl in buffer A containing 0.1% LDAO or 0.8% octyl-POE.
23. Analyze polypeptide composition of eluent fractions by SDS-PAGE and combine fractions containing relatively pure protein.
Sample results for purification of BtuB and OmpF are shown in Figures 29.7.9A and 29.7.10, respectively.
Figure 29.7.9.
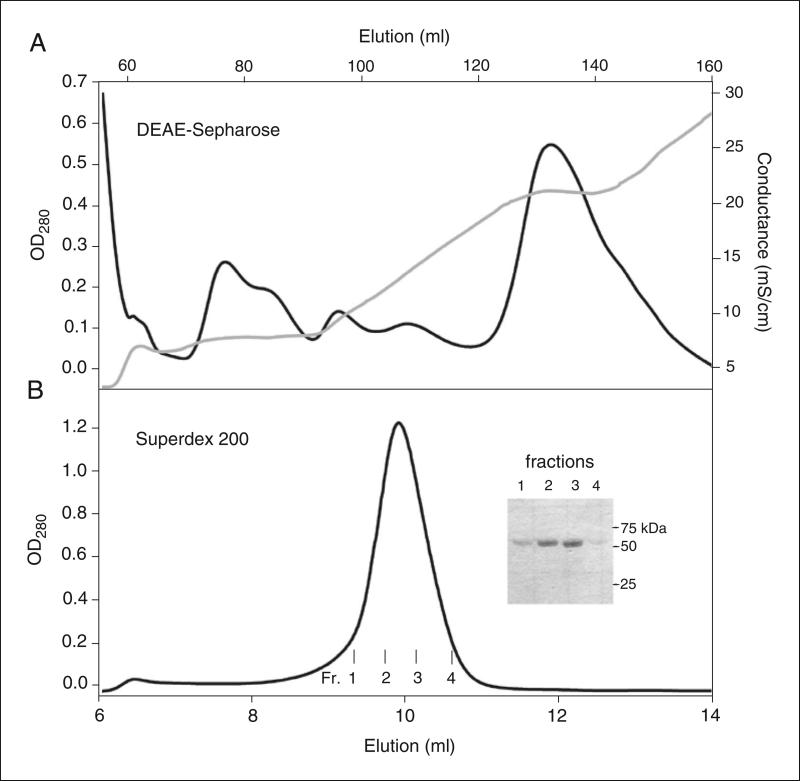
Chromatography profiles from two-step purification of BtuB. (A) Anion-exchange chromatography was performed using a DEAE-Sepharose column (HiPrep, 16/10). Loading buffer, 50 mM Tris, pH 8.0, 5 mM EDTA, 1.5% β-octylglucoside. Washing buffer, 50 mM Tris, pH 8.0, 5 mM EDTA, 0.1% LDAO. LiCl gradient (0-0.6 M) applied to elute BtuB in three segments: (i) 2 column volumes (CV) linear gradient, 0-0.2 M; (ii) 1 CV, 0.2 M; (iii) 4 CV, linear gradient 0.2-0.6 M LiCl. BtuB eluted at 0.2 M LiCl. (B) Size-exclusion chromatography of BtuB from the DEAE-Sepharose column was performed using a Superdex 200 column (10/300). Elution buffer: 20 mM Tris, pH 8.0, 0.1 M NaCl, 0.5 mM EDTA, 0.1 % LDAO. Inset: SDS-PAGE (12% polyacrylamide) of BtuB peak fractions eluted from Superdex column. Records of absorbance at 280 nm (black) and conductivity (gray) are presented.
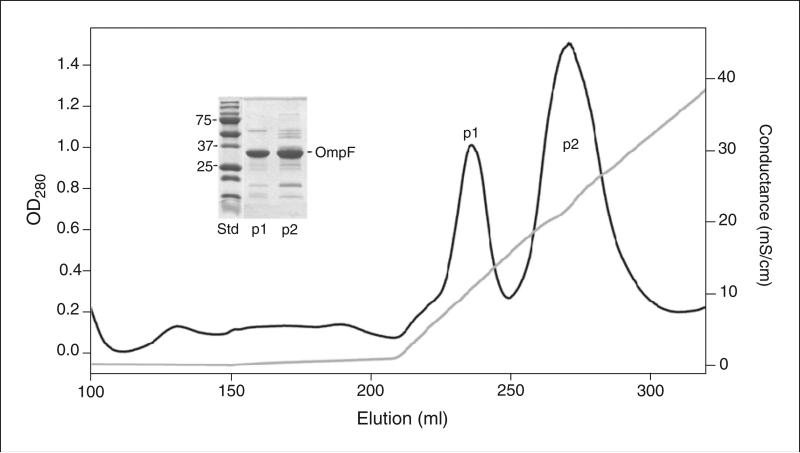
Figure 29.7.10.
Purification of OmpF by anion-exchange chromatography using a Q-agarose column (HiPrep, 16/10). Loading buffer, 50 mM Tris, pH 8.0, 5 mM EDTA, 3% β-octylglucoside. Washing buffer, (7 CV) 50 mM Tris, pH 8.0, 5 mM EDTA, 1% octyl-POE. Linear LiCl gradient applied to elute OmpF, (6 CV) 0-0.6 M. OmpF eluted at 0.1 M (peak 1) and 0.25 M (peak 2) LiCl. Inset: SDS-PAGE (12% polyacrylamide) of OmpF samples from peaks 1 and 2. Ladder above main OmpF band implies OmpF heterogeneity caused by different amounts of bound LPS. Records of absorbance at 280 nm (black) and conductivity (gray) are presented.
OmpF elutes in two peaks (Fig. 29.7.10). The first peak contains LPS-free OmpF, which has been successfully crystallized. The second peak contains LPS-enriched OmpF, which does not crystallize. To obtain additional LPS-free OmpF, the protein from the second peak can be desalted by dilution in buffer A with 2 mM EDTA and 1.0 % octyl-POE, then concentrated two to three times with a Centriprep 50 concentrator, and run again through the Q-column.
24. Concentrate protein by centrifuging at 1500 × g in a CentriPrep 50 concentrator according to manufacturer's instructions.
Concentrated proteins after anion-exchange chromatography can be stored overnight on ice.
Purify by size-exclusion chromatography (BtuB only)
25. Equilibrate a Superdex 200 column with 2 column volumes (48 ml) degassed 0.1% LDAO in TNE buffer 2.
26. Load 0.5 ml concentrated BtuB on the column and elute at a rate of 0.3 to 0.5 ml/min.
The elution peak of BtuB is centered at 9.9 ml.
27. Combine fractions containing BtuB and concentrate using a CentriPrep 50 concentrator.
Determine protein concentration
28. Set the spectrophotometer measurement program for baseline correction.
29. Fill a 1-mm or 1-cm cuvette with the detergent solution in which the protein is dissolved and measure baseline. Use a 1-mm cuvette if absorbance at 280 nm is expected to be >2.0.
The filtrate obtained during concentration is suitable for this step.
30. Measure the absorbance spectrum of the protein solution from 240 to 400 nm.
31. Analyze the spectrum for the presence of light scattering. If light scattering is detected, estimate its value at 280 nm by extrapolating from the spectrum in the region of 320-400 nm.
Light scattering increases monotonically with decreasing wavelength.
32. Correct the protein absorbance at 280 nm for the contribution of light scattering.
33. Calculate the protein concentration using extinction coefficients of 144.5 mM–1cm–1 and 2.2 ml/mg for BtuB or 54.2 mM–1cm–1 and 1.4 ml/mg for OmpF.
Obtain far UV CD spectrum
34. Purge a CD spectrophotometer with nitrogen gas for 2 hr and turn on instrument.
It is extremely important to purge the instrument with inert gas to remove oxygen before the UV lamp is ignited (instrument is turned on). Far UV light can produce ozone, which can damage instrument optics.
35. Load 40 μl sample buffer in a cuvette with a 0.1-mm path length, being very careful not to introduce air bubbles in the sample.
Due to the presence of detergents and other buffer components that have a large absorbance in this spectral region, it is important to use optical cuvettes with a small path length. In general, the use of a 1-mm cuvette will not allow measurement of a spectrum below 200 nm due to excessive absorbance. For samples with protein concentration of 10 mg/ml, a cuvette with an optical path length of 0.01 to 0.02 mm (sample volume 10 to 20 μl) is recommended.
36. Measure the baseline CD spectrum from 260 to 185 nm.
37. Rinse and dry both plates and load protein solution.
In general, 1 mg/ml protein is an optimal concentration in a 0.1-mm cuvette. Far UV CD spectra of proteins prepared for crystallization (~10 mg/ml) can also be measured in a demountable cuvette with a 0.01-mm path length. In this case, 10 μl sample is sufficient.
38. Measure the CD spectrum over the same spectral range used for baseline (Fig. 29.7.11).
To reduce noise, it is more efficient to measure and average three to five spectra with short integration times (~0.2 sec) than to measure the spectrum using an integration time >1 sec.
Initially, baseline spectrum is recorded for solution in which protein is contained, and this spectrum is automatically subtracted. If for some reason baseline was not measured and buffer was measured later, the buffer spectrum could be subtracted from the sample spectrum using the “Maths” operation in the Cary spectrophotometer software.
39. Normalize the CD spectrum for protein concentration and cuvette path length.
The far UV CD spectra of β-barrel proteins exhibit a minimum at 218 nm and a maximum between 190 and 200 nm. α-Helical proteins have two minima at 208 and 222 nm.
Contamination by helical proteins would result in a broadening of the 218-nm minimum and the appearance of shoulders in respective regions of the spectrum
BtuB and OmpF have a molar ellipticity (amplitude of CD signal normalized for optical path length and protein concentration) at 218 nm of approximately − 10 ± 0.8 kdeg cm2 dmol–1.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2E; for suppliers, see SUPPLIERS APPENDIX.
BG11 medium
For 1 liter:
1500 mg NaNO3
30 mg K2HPO4
75 mg MgSO4·7H2O
36 mg CaCl2·2H2O
1 mg EDTA 20 mg Na2CO3
6 mg ferric ammonium citrate
0.5 mg HEPES
2.9 mg H3BO3
1.8 mg MnCl2·4H2O
0.22 mg ZnSO4·7H2O
0.39 mg NaMoO4·5H2O
0.08 mg CuSO4·5H2O
0.05 mg Co(NO3)2·6H2O
Adjust pH to 7.5
Store autoclaved medium indefinitely at room temperature
This medium is prepared according to the recipe described by Rippka et al. (1979).
Cell breakage buffer
25 mM HEPES-KOH, pH 7.5 at 4°C
0.4 M sucrose
10 mM MgCl2
10 mM CaCl2
Store up to 1 day at 4°C
Before use add:
2 mM benzamidine
2 mM amino caproic acid
0.25 mM phenylmethylsulfonyl fluoride (PMSF, from 200 mM stock in 100% ethanol)
CAUTION: PMSF is highly toxic. Wear gloves and work in a chemical fume hood when using PMSF.
Chromatography wash buffer
TNE buffer 1 (see recipe)
35% saturated ammonium sulfate (at 4°C; APPENDIX 3F)
Store up to 1 week at 4°C
On day of experiment add:
0.05% UDM-Sol (see recipe)
1× protease inhibitor solution (see recipe)
CAUTION: PMSF is highly toxic. Wear gloves and work in a chemical fume hood when using PMSF.
Cyt b6f activity assay buffer
50 mM Tris-Cl, pH 7.5 (APPENDIX 2E)
0.05% (w/v) UDM-Sol (see recipe)
50 μM cytochrome c (from equine heart, Sigma-Aldrich)
Prepare fresh
Cytochrome c can be stored as a 25 mM stock solution in 50 mM Tris-Cl, pH 7.5, for up to 1 year at −20°C.
Elution buffer
TNE buffer 1 (see recipe)
20% saturated ammonium sulfate (at 4°C; APPENDIX 3F)
Store up to 1 week at 4°C
On day of experiment add:
0.05% UDM-Sol (see recipe)
1× protease inhibitor solution (see recipe)
CAUTION: PMSF is highly toxic. Wear gloves and work in a chemical fume hood when using PMSF.
Extraction buffer
10% sucrose in TNE buffer 1 (see recipe)
Store up to 1 day at 4°C
Membrane wash buffer
2 M NaBr
10 mM tricine, pH 8.0 at 4°C
0.3 M sucrose
Store up to 1 day at 4°C
Before use add:
2 mM benzamidine
2 mM amino caproic acid
0.25 mM PMSF (from 200 mM stock in 100% ethanol)
CAUTION: PMSF is highly toxic. Wear gloves and work in a chemical fume hood when using PMSF.
Osmotic shock buffer
20 mM tricine, pH 8.0 at 4°C
Store up to 1 week at 4°C
Before use add:
2 mM benzamidine
2 mM amino caproic acid
0.25 mM PMSF (from 200 mM stock in 100% ethanol)
CAUTION: PMSF is highly toxic. Wear gloves and work in a chemical fume hood when using PMSF.
Protease inhibitor solution, 100×
Prepare 100 mM phenylmethylsulfonyl fluoride (PMSF) and 20 mM tosyl phenylalanyl chloromethyl ketone (TPCK) in ethanol. Store frozen in aliquots.
CAUTION: PMSF is highly toxic. Wear gloves and work in a chemical fume hood when using PMSF.
Sucrose gradient solutions, 10% and 32% (w/v)
TNE buffer 1 (see recipe)
10% or 32% (w/v) sucrose
Store up to 1 week at 4°C
On day of experiment add:
0.05% UDM-Sol (see recipe)
TNE buffer 1
30 mM Tris, pH 7.5 at 4°C (APPENDIX 2E)
50 mM NaCl
1 mM EDTA
Store up to 1 week at 4°C
Before use add:
2 mM benzamidine
2 mM amino caproic acid
0.25 mM PMSF (from 200 mM stock in 100% ethanol)
CAUTION: PMSF is highly toxic. Wear gloves and work in a chemical fume hood when using PMSF.
TNE buffer 2
20 mM Tris·Cl, pH 8.0 (APPENDIX 2E)
100 mM NaCl
0.1 mM EDTA
Store up to 1 week at 4°C
TNS buffer
30 mM Tris·Cl, pH 7.5 (APPENDIX 2E)
50 mM NaCl
10% (w/v) sucrose
Store up to 1 week at 4°C
UDM-Sol or UDM-Ana, 10% (w/v)
Equilibrate n-undecyl-β-D-maltopyranoside, Sol-grade or Ana-grade (UDM-Sol or UDM-Ana, Anatrace), to room temperature prior to weighing. Dissolve in the indicated buffer at room temperature or 4°C with gentle rocking to avoid foaming. Prepare fresh or store up to 1 day at –20°C in a sealed bottle in a desiccator.
COMMENTARY
Background Information
The Cyt b6f complex of oxygenic photosynthesis is central to energy transduction in prokaryotic cyanobacteria and eukaryotic algae and plants. Biochemical, biophysical, and structural information have been derived from complexes purified in the enzymatically active state. The protocols described here yield crystallization-grade Cyt b6f from thylakoid membranes of the mesophilic filamentous cyanobacterium Nostoc PCC 7120. As the complex is located in a lipid bilayer, detergents are required for membrane disruption and protein solubilization. Basic Protocol 1 describes the optimization of detergent-mediated extraction conditions. Purification of solubilized complex by hydrophobic-interaction chromatography leads to extensive loss of native lipids that are crucial for structural stability. Basic Protocol 2 describes methods of Cyt b6f purification, delipidation, and augmentation with synthetic lipids.
Critical Parameters and Troubleshooting
Cyt b6f complex
The hetero-oligomeric nature of the b6f complex makes the use of mild purification conditions absolutely essential. Rapid purification is required to ensure that the complex does not undergo proteolysis. Specific issues are addressed below and in Table 29.7.1.
Table 29.
7.1 Troubleshooting for Cyt b6f Complex
| Problem | Cause | Solution |
|---|---|---|
| Poor cell growth | Low nutrient availability | Increase light and CO2 supply; add filtered phosphate solution to 1× concentration after 2-3 days of growth in carboy |
| Contamination | Cultivate cyanobacteria on nitrate-free BG11 agar plates and select single colonies to remove contaminants | |
| Uneven/uncontrolled flow of cell suspension through French press during lysis, poor cell lysis | Cell clumping, viscosity | Re-homogenize sample to remove clumps and dilute further with cell lysis buffer |
| Deformation of French pressure cell nylon ball | Change nylon ball | |
| Poor protein extraction from cyanobacterial membranes | Incorrect detergent concentration | Use dry, desiccated detergent to make fresh stock; perform detergent extraction screening |
| Altered membrane composition with possibly reduced Cyt b6f content | Measure dPQH2 to Cyt c electron transfer activity standardized against Chl | |
| Low dPQH2-to-Cyt c enzymatic activity in membranes (significantly less than 200 electrons/103 Chl/sec at 25°C) | Poor quality Cyt c | Prepare fresh stock of Cyt c from new vial |
| Incomplete reduction of dPQH2 | Reduce dPQH2 and determine concentration | |
| Monomerization/proteolysis of Cyt b6f | Perform ammonium sulfate precipitation, then measure electron transfer activity and Cyt b6f concentration by redox difference spectra; if <200 electrons/Cyt f/sec at 25 °C, complex may be inactive due to denaturation or proteolysis | |
| Large amount of blue-green proteins bound to chromatography column | Over-extraction of proteins by detergent from membranes | Wash column until flowthrough fractions are colorless, carefully separate fractions with > 1 μM b6f from those with lower b6f concentration, and perform multiple sucrose density gradient steps with careful fractionation of bands |
| Sample sinks in sucrose gradient upon loading | Residual ammonium sulfate in protein buffer | Exchange protein buffer to remove ammonium sulfate |
| Sample smearing on sucrose gradient | Air bubble in sucrose density gradient tube | Repeat sucrose density gradient centrifugation with carefully prepared gradient |
| Rapid rotor acceleration and deceleration | Use slow acceleration and deceleration | |
| Poor separation of protein bands on sucrose gradient (bands very close to each other without sharp edges) | Overloading of sample | Reduce amount of protein loaded |
| Physical agitation of gradient tubes during handling | Do not shake tubes | |
| Slow concentration of protein in lipid buffer | Lipid insufficiently dissolved by freeze-thaw and sonication | Centrifuge protein-lipid sample to remove insoluble lipid fraction, then repeat concentration using fresh lipid buffer solution |
Cell lysis and membrane preparation
Cell lysis must be performed in a 200-ml suspension volume. This dilution ensures that clumps do not form and the viscosity of the solution does not increase appreciably during cell lysis to interfere with French pressure cell.
Thylakoid membrane preparation and washing must be performed rigorously to remove contaminating, peripherally bound proteins.
Chlorophyll estimation must be performed in triplicate to rule out pipetting errors. This is important, as the chlorophyll-to-detergent ratio is one of the most important factors in the extraction of both Cyt b6f and contaminating proteins.
Detergent-mediated extraction
Addition of the detergent must be performed dropwise over 2-3 min to avoid formation of zones with high detergent concentrations. Such zones may cause over-extraction of Cyt b6f and contaminants, leading to problems during fractionation by sucrose density gradient centrifugation.
Ammonium sulfate precipitation and chromatography
Once detergent-mediated extraction has been performed, ammonium sulfate precipitation must be carried out gently, with continuous stirring. It is essential to remove clumps of ammonium sulfate, as their addition to the extract may cause local increases in ammonium sulfate concentration, causing precipitation and removal of Cyt b6f from the extract.
The clarified supernatant obtained after precipitation and centrifugation must be further clarified by passing through a syringe filter. This step is crucial to subsequent chromatography, as the presence of precipitated protein pellets results in accumulation of debris in the propyl agarose column. The layer of debris reduces the speed of chromatography and causes uneven flow, leading to poor separation between the early green fractions and the late blue fractions.
Once the protein has been eluted, buffer exchange must be performed to remove ammonium sulfate. In the presence of ammonium sulfate, the protein sample sinks to the bottom of the sucrose gradient and does not separate into individual protein bands.
Sucrose density gradient centrifugation
Sucrose density gradients must be prepared without any air bubbles. If even a single air bubble is observed within a gradient at any stage, the gradient tube must be discarded. Air bubbles cause turbulent mixing of the gradient, which leads to spreading of the protein sample across the gradient without separation.
The protein sample must be concentrated to a volume that does not exceed 1/10th the volume of the sucrose density gradient. Larger sample volumes cause dilution, which leads to poor resolution of individual protein fractions.
Protein must be loaded very gently, in drops along the side of the ultracentrifugation tube, using a 200-μl micropipet. It is very important to minimize physical agitation of the gradients, which can result from poor handling during gradient formation or storage, sample loading, weight balancing, centrifugation, storage on ice prior to fractionation, and elution. Physical disturbance at any stage may potentially cause failure due to sample and gradient mixing.
The sample must be removed from the gradient very gently. The blue and orange bands at the top of the gradient may be removed in 500-μl fractions without disturbing the underlying bands. Typically, a 100- or 200-μl micropipet set at 75 μl is used to elute the brown Cyt b6f band. This small volume allows removal of the purest b6f fraction with minimal contamination from the neighboring green band. The b6f band must not be eluted into a single fraction. Rather, it must be further fractionated into three to four bands. The top and middle fractions of the b6f band, which are relatively well separated from the neighboring green band, must be eluted first, with care. These bands provide the purest fraction of b6f complex for crystallization. The fractions closer to the green band are typically contaminated with other proteins, and should be purified further on another sucrose density gradient.
Harvesting of the Cyt b6f fraction must be performed in small volumes. Protein bands tend to wet the walls of the ultracentrifuge tube. As a result, after harvesting a small volume, the tube must be incubated on ice for a few minutes to allow the b6f sample on the tube walls to settle down into the gradient.
Lipid exchange
Lipids such as dioleoylphosphatidylcholine and dioleoylphosphatidylglycerol are typically supplied in organic solvents. Overnight drying in a desiccator is essential to remove residual traces of solvents, which may cause protein denaturation. Once dried, the lipid must be suspended in TNS buffer without detergent. As the lipid suspension is vortexed to remove the lipid from the test tube walls, the presence of detergent will cause foaming, leading to lipid loss. Hence, detergent is added only after the lipid has been resus-pended. No vortexing should be performed once the detergent has been added to the buffer.
Both freeze-thawing and sonication must be performed to efficiently dissolve the lipid. Once the lipid is dissolved, the buffer is gradually clarified. Acidic lipids such as dioleoylphosphatidylglycerol tend to form very clear solution prior to sonication. However, sonication must still be performed to reduce the size of lipid-detergent complexes, which would otherwise occlude the pores of the protein concentrator membranes and interfere with crystallization.
Anticipated Results
Cyt b6f complex
Cell growth and lysis
The aim of cell growth is to obtain cell mass that is adequate for large-scale protein isolation (4 to 6 mg Cyt b6f). Typically, 0.8 to 1.0 g wet cell mass is obtained per liter of Nostoc PCC 7120 culture. During lysis, >80% cell breakage is expected to take place, leaving behind only a very small pellet of cell debris at 1000 × g.
Thylakoid membrane preparation
Following lysis, ultracentrifugation separates membrane vesicles from the soluble fraction. The color of the soluble fraction (supernatant) is bluish-red. Membrane washing reduces the amount of contaminating blue-red protein significantly, and the final wash with TNE buffer 1 is expected to yield a very light shade of blue to almost clear supernatant.
Extraction screening and assay
The objective of extraction screening is to determine the optimal detergent concentration for Cyt b6f extraction from cyanobacterial membranes. Extraction screening constitutes a crucial step in the purification procedure to avoid sample loss due to incomplete extraction of b6f or over-extraction of contaminants. As contaminating proteins interfere with estimation of extraction efficiency by redox difference spectroscopy, an enzymatic assay of Cyt b6f electron transfer activity provides a quantitative measure to estimate the fraction of Cyt b6f that is extracted out of the membranes.
Purification and delipidation
In addition to Cyt b6f and photosystems, the propyl agarose hydrophobic-interaction chromatography resin binds phycobiliproteins such as the blue phycocyanin. Purification involves removal of the weakly bound orange and yellow carotenoid proteins and partial removal of green photosystems. Lipids are removed from the Cyt b6f complex due to hydrophobic interactions with propyl agarose. During elution, the delipidated Cyt b6f complex co-migrates with the green photosystem fraction and with the blue phycocyanin fraction. The brown color of the Cyt b6f complex is not observed on the chromatography column or in the eluted fractions. Final separation of the Cyt b6f protein from the contaminants is achieved by sucrose density gradient centrifugation.
The dimeric protein fraction obtained from Nostoc represents 70% to 80% of the total purified b6f complex. The final yield of purified b6f complex is ~7 mg pure dimer from 25 g wet cell pellet. The heme ratio of the isolated b6f complex (cyt b6/cyt f) is 2.1 ± 0.07 by redox difference spectrophotometry using extinction coefficients described previously (Metzger, 1997). The decylplastoquinolplastocyanin oxidoreductase activity of the b6f complex is 277 ± 14 sec–1 cyt f–1.
Lipid exchange
Addition of lipids is required to obtain highly diffracting crystals of the Cyt b6f complex. It has previously been shown that delipidated Cyt b6f complex from the moderately thermophilic cyanobacterium M. laminosus yields poorly diffracting crystals. Addition of lipids leads to drastic improvement in crystal quality.
β-Barrel proteins
The described protocols for protein extraction and purification allow preparation of 2 mg BtuB and 4 mg LPS-free OmpF per liter of cell culture. BtuB (Cherezov et al., 2006) and OmpF (Yamashita et al., 2008) have been crystallized in 0.1% LDAO and 1% octyl-POE, respectively. The resolution of the crystal structures of BtuB (PDB ID 2GUF) and OmpF (PDB ID 2ZFG) were 1.95Å and 1.59 Å, respectively.
Time Considerations
Cyt b6f complex
Preparation of large-scale cyanobacterial cell cultures may take up to 15 to 20 days. Once this is done, cell lysis and membrane preparation are typically performed on the same day. Homogenization of the cyanobacterial cell pellet to remove clumps and cell breakage in the French pressure cell may take up to 1.5 to 2.0 hr, and membrane preparation from the lysate may take up to 6 hr.
Protein extraction from cyanobacterial membranes and removal of impurities by selective ammonium sulfate precipitation may take up to 3 hr. Hydrophobic-interaction chromatography may require up to 6 to 8 hr. Preparation of sucrose gradients and exchange of protein buffer may need an additional 2 to 3 hr. Sucrose density gradient centrifugation requires 16 hr, and elution of the protein from the gradients may take 2 hr.
Preparation of the lipid-containing buffer involves freeze-thawing, which may take up to 45 min, followed by sonication, which requires an additional 20 min. Protein concentration and buffer exchange may require up to 1 to 2 hr.
β-Barrel proteins
Cell growth and harvesting take 2 days, outer membrane preparation takes 1 day, and protein extraction and purification take 2 to 3 days.
Acknowledgments
These studies have been supported by NIH grants to WAC (GM–038323, cytochrome complex; GM-018457, outer membrane proteins, colicin import), a Purdue University Fellowship to SSH, and infrastructure support from the Purdue University Cancer Center.
Literature Cited
- Baniulis D, Yamashita E, Whitelegge JP, Zatsman AI, Hendrich MP, Hasan SS, Ryan CM, Cramer WA. Structure-function, stability, and chemical modification of the cyanobacterial cytochrome b6f complex from Nostoc sp. PCC 7120. J. Biol. Chem. 2009;284:9861–9869. doi: 10.1074/jbc.M809196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniulis D, Zhang H, Yamashita E, Zakharova T, Hasan SS, Cramer WA. Purification and crystallization of the cyanobacterial cytochrome b6f complex. In: Carpentier R, editor. Methods in Molecular Biology, Photosynthesis Research Protocols. Humana Press; Totowa, N.J.: 2011. pp. 65–77. [DOI] [PubMed] [Google Scholar]
- Ben-Shem A, Nelson N, Frolow F. Crystallization and initial X-ray diffraction studies of higher plant photosystem I. Acta Crystallogr. D Biol. Crystallogr. 2003;59:1824–1827. doi: 10.1107/s0907444903016056. [DOI] [PubMed] [Google Scholar]
- Caffrey M. Membrane protein crystallization. J. Struct. Biol. 2003;142:108–132. doi: 10.1016/s1047-8477(03)00043-1. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Yamashita E, Liu W, Zhalnina M, Cramer WA, Caffrey M. In meso structure of the cobalamin transporter, BtuB, at 1.95Å resolution. J. Mol. Biol. 2006;364:716–734. doi: 10.1016/j.jmb.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer WA, Yamashita E, Baniulis D, Hasan SS. The cytochrome b6f complex of oxygenic photosynthesis. In: Messerschmidt A, editor. Handbook of Metalloproteins. John Wiley & Sons; Chichester, U.K.: 2011. pp. 16–28. [Google Scholar]
- Escriba PV, Wedegaertner PB, Goni FM, Vogler O. Lipid-protein interactions in GPCR-associated signaling. Biochim. Biophys. Acta. 2007;1768:836–852. doi: 10.1016/j.bbamem.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Fairman JW, Noinaj N, Buchanan SK. The structural biology of β-barrel membrane proteins: A summary of recent reports. Curr. Opin. Struct. Biol. 2011;21:523–531. doi: 10.1016/j.sbi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme P, Jordan P, Krauss N. Structure of photosystem I. Biochim. Biophys. Acta. 2001;1507:5–31. doi: 10.1016/s0005-2728(01)00195-5. [DOI] [PubMed] [Google Scholar]
- Guskov A, Kern J, Gabdulkhakov A, Broser M, Zouni A, Saenger W. Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat. Struct. Mol. Biol. 2009;16:334–342. doi: 10.1038/nsmb.1559. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, Velasquez J, Kuhn P, Stevens RC. A specific cholesterol binding site is established by the 2.8 Å structure of the human β2-adrenergic receptor. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JE, Picot D, Loll PJ, Garavito RM. Calibration of size-exclusion chromatography: Use of a double Gaussian distribution function to describe pore sizes. Anal. Biochem. 1995;224:557–563. doi: 10.1006/abio.1995.1087. [DOI] [PubMed] [Google Scholar]
- Hasan SS, Yamashita E, Ryan CM, Whitelegge JP, Cramer WA. Conservation of lipid functions in cytochrome bc complexes. J. Mol. Biol. 2011;414:145–162. doi: 10.1016/j.jmb.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hite RK, Li ZL, Walz T. Principles of membrane protein interactions with annular lipids deduced from aquaporin-0 2D crystals. EMBO J. 2010;29:1652–1658. doi: 10.1038/emboj.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunte C, Jagow GV, Schaegger H, editors. Membrane Protein Purification and Crystallization: A Practical Guide. 2nd ed. Academic Press; Amsterdam: 2003. [Google Scholar]
- Jones MR. Lipids in photosynthetic reaction centres: Structural roles and functional holes. Prog. Lipid Res. 2007;46:56–87. doi: 10.1016/j.plipres.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N. Three-dimensional structure of cyanobacterial photosystem I at 2.5Å resolution. Nature. 2001;411:909–917. doi: 10.1038/35082000. [DOI] [PubMed] [Google Scholar]
- Kern J, Zouni A, Guskov A, Krauss N. Lipids in the structure of the photosystem I, photosystem II, and the cytochrome b6f complex. In: Wada H, Murata N, editors. Lipids in Photosynthesis: Essential and Regulatory Functions. Springer Science; Dordrecht, The Netherlands: 2009. pp. 203–242. [Google Scholar]
- Kurisu G, Zakharov SD, Zhalnina MV, Bano S, Eroukova VY, Rokitskaya TI, Antonenko YN, Wiener MC, Cramer WA. The structure of BtuB with bound colicin E3 R-domain implies a translocon. Nat. Struct. Biol. 2003a;10:948–954. doi: 10.1038/nsb997. [DOI] [PubMed] [Google Scholar]
- Kurisu G, Zhang H, Smith JL, Cramer WA. Structure of the cytochrome b6f complex of oxygenic photosynthesis: Tuning the cavity. Science. 2003b;302:1009–1014. doi: 10.1126/science.1090165. [DOI] [PubMed] [Google Scholar]
- Landau EM, Rosenbusch JP. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14532–14535. doi: 10.1073/pnas.93.25.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi JK. Bacteriorhodopsin. Annu. Rev. Physiol. 2004;66:665–688. doi: 10.1146/annurev.physiol.66.032102.150049. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee A, Chen JY, MacKinnon R. Structure of the KvAP voltage-dependent K+ channel and its dependence on the lipid membrane. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15441–15446. doi: 10.1073/pnas.0507651102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M. Membrane Structural Biology. Cambridge University Press; New York: 2008. [Google Scholar]
- Metzger SU, Cramer WA, Whitmarsh J. Critical analysis of the extinction coefficient of chloroplast cytochrome f. Biochim. Biophys. Acta. 1997;1319:233–241. doi: 10.1016/s0005-2728(96)00164-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussberger S, Dorr K, Wang DN, Kuhlbrandt W. Lipid-protein interactions in crystals of the plant light-harvesting complex. J. Mol. Biol. 1993;234:347–356. doi: 10.1006/jmbi.1993.1591. [DOI] [PubMed] [Google Scholar]
- Palsdottir H, Hunte C. Lipids in membrane protein structures. Biochim. Biophys. Acta. 2004;1666:2–18. doi: 10.1016/j.bbamem.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Qin L, Hiser C, Mulichak A, Garavito RM, Ferguson-Miller S. Identification of conseved lipid/detergent binding site in a high resolution structure of the membrane protein, cytochrome c oxidase. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16117–16122. doi: 10.1073/pnas.0606149103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Sharpe MA, Garavito RM, Ferguson-Miller S. Conserved lipid-binding sites in membrane proteins: A focus on cytochrome c oxidase. Curr. Opin. Struct. Biol. 2007;17:444–450. doi: 10.1016/j.sbi.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979;111:1–61. [Google Scholar]
- Sharma O, Yamashita E, Zhalnina MV, Zakharov SD, Datsenko KA, Wanner BL, Cramer WA. Structure of the complex of the colicin E2 R-domain and its BtuB receptor. The outer membrane colicin translocon. J. Biol. Chem. 2007;282:23163–23170. doi: 10.1074/jbc.M703004200. [DOI] [PubMed] [Google Scholar]
- Shinzawa-Itoh K, Aoyama H, Muramoto K, Terada H, Kurauchi T, Tadehara Y, Yamasaki A, Sugimura T, Kurono S, Tsujimoto K, Mizushima T, Yamashita E, Tsukihara T, Yoshikawa S. Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 2007;26:1713–1725. doi: 10.1038/sj.emboj.7601618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebel D, Choquet Y, Popot J-L, Picot D. An atypical haem in the cytochrome b6f complex. Nature. 2003;426:413–418. doi: 10.1038/nature02155. [DOI] [PubMed] [Google Scholar]
- Taylor R, Burgner JW, Clifton J, Cramer WA. Purification and characterization of monomeric Escherichia coli vitamin B12 receptor with high affinity for colicin E3. J. Biol. Chem. 1998;273:31113–31118. doi: 10.1074/jbc.273.47.31113. [DOI] [PubMed] [Google Scholar]
- Umena Y, Kawakami K, Shen JR, Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature. 2011;473:55–60. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- Wohri AB, Wahlgren WY, Malmerberg E, Johansson LC, Neutze R, Katona G. Lipidic sponge phase crystal structure of a photosynthetic reaction center reveals lipids on the protein surface. Biochemistry. 2009;48:9831–9838. doi: 10.1021/bi900545e. [DOI] [PubMed] [Google Scholar]
- Yamashita E, Zhang H, Cramer WA. Structure of the cytochrome b6f complex: Quinone analogue inhibitors as ligands of heme cn. J. Mol. Biol. 2007;370:39–52. doi: 10.1016/j.jmb.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita E, Zhalnina MV, Zakharov SD, Sharma O, Cramer WA. Crystal structures of the OmpF porin: Function in a colicin translocon. EMBO J. 2008;27:2171–2180. doi: 10.1038/emboj.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Kurisu G, Cramer WA. Intraprotein transfer of the quinone analogue inhibitor 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone in the cytochrome b6f complex. Proc. Natl. Acad. Sci. U.S.A. 2006;103:69–74. doi: 10.1073/pnas.0504909102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Dashdorj N, Baniulis D, Yamashita E, Savikhin S, Cramer WA. On the structural role of the aromatic residue environment of the chlorophyll a in the cytochrome b6f complex. Biochemistry. 2008;47:3654–3661. doi: 10.1021/bi702299b. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cramer WA. Purification and crystallization of the cytochrome b6f complex in oxygenic photosynthesis. Methods Mol. Biol. 2004;274:67–78. doi: 10.1385/1-59259-799-8:067. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cramer WA. Problems in obtaining diffraction-quality crystals of hetero-oligomeric integral membrane proteins. J. Struct. Funct. Genomics. 2005;6:219–223. doi: 10.1007/s10969-005-1912-y. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kurisu G, Smith JL, Cramer WA. A defined protein-detergent-lipid complex for crystallization of integral membrane proteins: The cytochrome b6f complex of oxygenic photosynthesis. Proc. Nat. Acad. Sci. U.S.A. 2003;100:5160–5163. doi: 10.1073/pnas.0931431100. [DOI] [PMC free article] [PubMed] [Google Scholar]