Abstract
Free fatty acids (FFAs) exert both positive and negative effects on beta cell survival and insulin secretory function, depending on concentration, duration, and glucose abundance. Lipid signals are mediated not only through metabolic pathways, but also through cell surface and nuclear receptors. Toxicity is modulated by positive signals arising from circulating factors such as hormones, growth factors and incretins, as well as negative signals such as inflammatory mediators and cytokines. Intracellular mechanisms of lipotoxicity include metabolic interference and cellular stress responses such as oxidative stress, endoplasmic reticulum (ER) stress, and possibly autophagy. New findings strengthen an old hypothesis that lipids may also impair compensatory beta cell proliferation. Clinical observations continue to support a role for lipid biology in the risk and progression of both type 1 (T1D) and type 2 diabetes (T2D). This review summarizes recent work in this important, rapidly evolving field.
Keywords: Pancreatic beta cell, islet, lipotoxicity, glucolipotoxicity, lipid, triglyceride, free fatty acid, nonesterified fatty acid, growth factors, inflammation, metabolism, gpr40, FFAR1, fatty acid receptor, oxidative stress, endoplasmic reticulum stress, autophagy, PPAR, cell cycle, proliferation, insulin secretion, apoptosis
Introduction
The subject of toxic effects of lipid species on pancreatic beta cells is broad and growing. Early biochemical work illustrated how the intracellular metabolism of lipids can either promote or inhibit the insulin secretory response to glucose, depending on the context. Lipids are now known to act not only through biochemical nutrient pathways, but also through signaling via cell surface and nuclear receptors. Newer findings link lipotoxicity to inflammation, oxidative, nitrosative and endoplasmic reticulum (ER) stress pathways and autophagy. Many outstanding minds and teams have touched upon this field. Here we will cover recent advances specific to toxic effects of lipids on beta cell survival, insulin secretory function, and beta cell mass (Figure 1). As such, we do not discuss the well-established beneficial effects of lipids on insulin secretion via FFAR1 and other pathways. The review is organized from an outside-in perspective, beginning with extracellular factors, and from a physiological rather than a biochemical perspective. A section is devoted to the evolving new concept that lipotoxicity may impact beta cell mass by reducing beta cell proliferation, with an emphasis on how our own work integrates with the field. Given the broad nature of this topic, coverage of each concept is brief; the reader is encouraged to read the original sources. By design, the review is restricted to work from the past year or two.
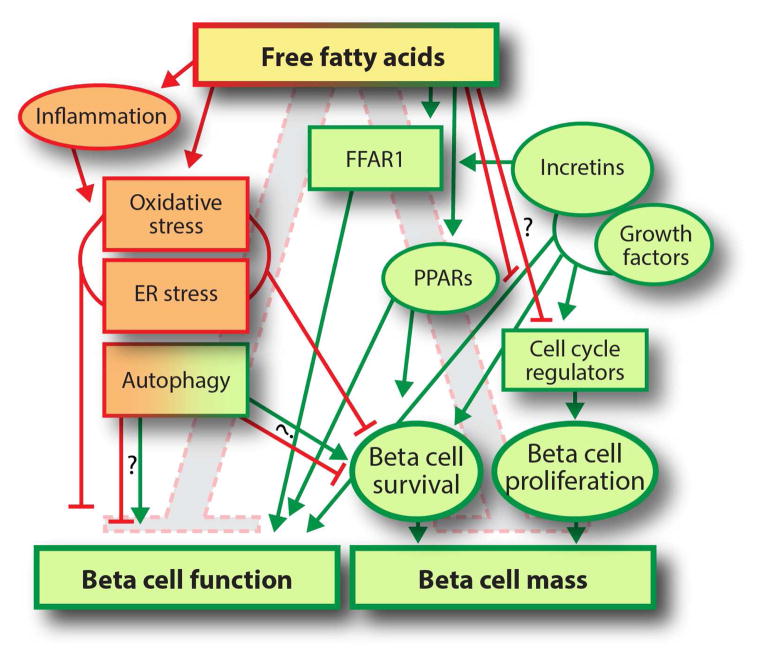
Figure 1.
Free fatty acids exert both positive (green) and negative (red) effects on beta cell mass and function. FFAs signal through receptors such as FFAR1 and PPARs, or through metabolic pathways as comprehensively reviewed in [1, 2]. Positive effects are mediated predominantly through FFAR1 and PPARs. Negative effects are mediated through inflammation, cellular stress mechanisms, and possibly inhibition of the cell cycle. Negative effects of FFAs are modulated by growth factors and incretins. Whether autophagy plays a net positive or net negative role is controversial.
Extracellular signals influencing lipotoxicity
Lipid effects on the beta cell are modulated by extracellular factors. Signals that promote beta cell mass and function, such as lactogens, estrogens, and incretins generally protect beta cells against lipotoxicity. As reviewed in detail [1, 2], toxic effects of lipids are usually manifested only when high glucose is also present. Extracellular beta cell toxins such as inflammatory cytokines synergize with lipotoxicity to further impair beta cell function and survival, as summarized below.
Growth factors and hormones
Circulating growth factors impact lipotoxicity in the beta cell. While lactogens protect beta cells against lipotoxic cell death via activation of Jak-Stat signaling [3], hepatocyte growth factor actually promotes lipotoxicity [4]. The insulin signaling nuclear factor FoxO1 mediates some aspects of lipotoxicity; a recent study mapped out which genes are regulated by FoxO1 in a beta cell line [5]. The female sex steroid hormone estradiol was found to regulate islet lipid synthesis; deletion of the ER-alpha receptor predisposed mice to lipotoxic beta cell dysfunction [6].
Significant recent effort has been directed towards understanding how incretin hormones, in particular Glp-1, interact with lipotoxicity. Increasing Glp-1 signaling is a new T2D therapeutic approach that has generated excitement because improved insulin secretion is accompanied by weight loss and, possibly, beta cell regeneration. Lipid exposure negatively impacts incretin signaling, both by downregulation of the Glp-1 receptor [7] and by interfering with downstream cAMP signaling [7, 8]. Treating diabetic mice with a combination of lipid-lowering therapy and Glp-1 agonist improved beta cell mass and function better than either alone [7]. Incretins were found to promote the interconnected network of beta cells in human islets, and exposure to lipids disrupted this connectivity, and impaired insulin secretion [9]. A number of teams have found that incretin signaling promotes insulin secretion and beta cell survival to counteract glucolipotoxicity in vitro, through effects on mitochondria [10], insulin signaling intermediates such as Akt and mTor [11–13], oxidative and endoplasmic reticulum stress [14], and the nuclear factor SREBP1 [13]. The protective effects of Glp-1 signaling against lipotoxicity have been extended to human islets [15].
Inflammatory mediators
Inflammation negatively impacts beta cell survival and function through multiple mechanisms [16]. Fatty acids can directly activate inflammatory pathways themselves, to potentiate inflammatory toxicity. In some cases inflammatory signals are propagated via intracellular lipid species as described below. Lipid exposure in vivo increases islet inflammation, and inflammatory cytokines and immune cells are present in the pancreas in T2D (recent examples: [17, 18]). Treating beta cells with palmitate increased chemokine production and recruitment of pro-inflammatory macrophages, via TLR4-Myd88 [19]. High fat feeding, and palmitate treatment in vitro, increased islet production of macrophage migration inhibitory factor (MIF); deletion of MIF protected beta cells against lipotoxic cell death [20]. Lipopolysaccharides (LPS) treatment reduced insulin secretion and expression of beta cell differentiation markers Pdx1 and MafA, though TLR4 and NF-kβ [21]. Lipids potentiated IL-1beta-induced endoplasmic reticulum stress through IRE1/Xbp activation [22]. Cytokines impaired insulin secretion and increased cell death via a pathway involving 12-lipoxygenase and its downstream lipid product, 12-hydroxyeicosatetraenoic acid (12-HETE) and Nox-1 and reactive oxygen species [23]. Additionally, blocking palmitoylation protected INS-1 cells against cytokine-induced oxidative and nitrosative stress [24]. Thus, lipotoxicity promotes and potentiates islet inflammation, supporting the concept that modulating inflammation might be a therapeutic approach for diabetes [18].
Membrane receptors influencing lipotoxicity
Initially, all lipid effects on the beta cell were thought to arise from effects on metabolic pathways. The finding that FFAs activate G-protein coupled receptors (GPCRs) has generated enormous excitement because of the biological and therapeutic implications [25]. FFAs activate GPR40 (now known as FFAR1), which is expressed on the surface of human and rodent beta cells, and is, remarkably, thought to mediate many of the positive effects of fatty acids on insulin secretion without negatively impacting beta cell function or survival [26–28]. Glucolipotoxicity may exert some negative effects by interfering with normal FFAR1 function. In one study hyperglycemia decreased, but hyperlipidemia increased, FFAR1 expression [29]. In INS-1 cells, saturated fatty acids decreased FFAR1 expression, whereas unsaturated fatty acids increased FFAR1 expression and protected against lipotoxicity [30]. Supporting a role for endogenous FFAR1 in insulin secretion, a single nucleotide polymorphism at the FFAR1 locus correlated with insulin secretory function in people; genotyping this locus may allow clinical prediction of which patients will respond to FFAR1 agonists [31].
FFAR1 agonists are under development as therapies for T2D. Preclinical studies suggest they potentiate insulin secretion, decrease beta cell apoptosis and maintain beta cell mass [25]. Like other GPCRs, careful ligand design may allow selective activation of positive functions without others that are less desirable [26, 32]. FFAR1 agonists potentiate glucose-dependent insulin secretion in vivo in diabetic rodents [33–36]. Although the majority of analyses suggest that FFAR1 activation doesn’t lead to lipotoxicity, in fact protects against lipotoxicity, a few studies have found that some lipotoxic effects may be mediated by FFAR1 signaling. Extended exposure of human islets to palmitate decreased insulin content and secretion, which was preventable by a FFAR1 antagonist [37]. In a mouse model of beta cell overload with failure, blocking FFAR1 reduced insulin secretion, circulating proinsulin, and beta cell apoptosis, suggesting the possibility that in the setting of beta cell failure increasing insulin secretion by FFAR1 agonism might increase stress by further overloading the beta cell [38, 39].
Intracellular mechanisms of lipotoxicity
Classically, lipid toxicity to beta cells was thought to arise from chronic alteration of biochemical substrate flux patterns, rendering the beta cell less responsive to glucose, and through protein kinase C signaling. More recently, lipid exposure has also been shown to activate cell stress responses including oxidative stress, endoplasmic reticulum stress, and autophagy. Much work remains to be done to identify how each of these mechanisms relates to human diabetes.
Metabolic pathways
Decades of research have illuminated many details of the biochemistry of lipid metabolism in beta cells. The roles of metabolic pathways in the positive and negative effects of fatty acids on insulin signaling, interaction between glucose and lipid metabolism, and lipotoxicity in general have been extensively and carefully reviewed [1, 2]. Upon entering the beta cell, free fatty acids are activated by acyl-CoA synthase, and then either oxidized or re-esterified for storage or glycerolipid cycling [2]. Glucose, fatty acids and amino acids interact at a biochemical level to influence many important cellular processes through the Krebs cycle, pyruvate cycling, and the glycerolipid-free fatty acid cycle [2, 40]. Over the past 1–2 years some new work has contributed to understanding of the role of FFA metabolism in lipotoxicity.
At the level of lipid entry into beta cells, one histological analysis has found that lipoprotein lipase (LPL), important for cleaving triglyceride to allow fatty acid entry into cells, is surprisingly not intravascular in mouse islets, but instead appears to be intracellular, where it would not have access to circulating triglyceride [41]. Islet expression of LPL was not altered by fasting-fed state, but was regulated by leptin [41]. Islet lipid uptake may be regulated by signals that change LPL location to extracellular; disruption of these signals may contribute to reduction of the lipid component of glucose stimulated insulin secretion (GSIS). A study seeking to determine which acyl-CoA synthase participates in GSIS determined that Acsl4 is required for fatty acid potentiation of GSIS in INS1 cells [42]. However, Acls4 was not required for metabolism of long chain FA, but instead appeared to be protective by sequestering a toxic fatty acid species, epoxyeicosatrienoic acids (EETs). Once inside the cell, fatty acid oxidation is thought to not be pathogenic, but instead protective against lipotoxicity as measured by ER stress [43, 44]. The insulin signaling intermediate mTor may impact rates of fatty acid storage versus utilization in human beta cells [45].
New findings regarding mechanisms of lipotoxicity include a pathway linking lipid exposure to altered glycosylation patterns in beta cells, resulting in impaired glucose transport [46]. Palmitate interferes with glucose uptake, calcium signaling, mitochondrial respiration and insulin content [47, 48]. Palmitate treatment altered calcium handling and insulin secretion through neprilysin, a secreted protease not previously known to impact beta cell function [49]. The metabolic lipotoxicity pathways underlying impaired insulin secretion and increased cell death were found to be mechanistically distinct in INS1 cells [48]. Also in INS1 cells, a comprehensive analysis of metabolite levels in the context of mRNA expression and histone modification cataloging is an important resource for the community [50]. Another mechanism of palmitate toxicity may be aberrant palmitoylation of proteins; blocking palmitoylation prevented lipotoxic beta cell death [51]. In a technical breakthrough, the interaction of glucose and fatty acids at the level of the electron transport chain was directly visualized using a sophisticated confocal-based imaging technique [52].
Other lipid-related species may also play a role in beta cell function and dysfunction. Ceramide and sphingolipids impact these processes [53]. Mice fed an isocaloric diet in which long chain FAs were replaced by medium chain FAs showed glucose intolerance and impaired insulin secretion [54]. The role of cholesterol in beta cell function and mass is unclear. The cholesterol transport protein ABCG1 was increased in insulinoma tissue, and correlated with insulin secretion [55]. The intracellular cholesterol transport protein Npc1, genetically associated with risk of obesity in humans, may also play a role in insulin secretion [56]. LXR alpha, a receptor for cholesterol-related compounds, is important for insulin secretion in vitro and in vivo by altering glucose metabolism, ATP production and calcium channel flux; modulating its activity deregulated lipid metabolism via SREBP [57].
Stress pathways: oxidative stress, ER stress and autophagy
Oxidative stress is caused by generation of reactive oxygen species (ROS) that exceeds reducing capacity. Beta cells have limited anti-oxidative defense mechanisms and are particularly susceptible to oxidative damage. Consequences of redox imbalance include lipid peroxidation, oxidation of proteins, DNA damage and interference of reactive species with signal transduction. Excesses of lipids and glucose induce oxidative and nitrosative stress in beta cells; short-term activation of ROS increases GSIS, but excessive ROS impairs insulin secretion [24, 58]. Human islets from both diabetic and nondiabetic individuals have detectable lipid peroxide protein adducts, suggesting oxidative damage [59]. In mice, lipid infusion increased islet ROS, and beta cell dysfunction was prevented by treatment with a reducing agent or inhibition of NADPH oxidase [60, 61]. Nicotinamide protected INS1 cells against lipotoxic cell death through sirtuins [62]. Reducing agents or antioxidants may improve beta cell function by protecting against oxidative stress.
ER stress refers to failure to maintain homeostasis of the ER, the site of protein folding for all secretory peptides. The beta cell is a workhorse for insulin synthesis and secretion, and is sensitive to ER stress [16, 63]. Oxidative stress increases ER stress because redox state is important for proper ER function; however, agents that stress the beta cell ER may not alter its redox state [64]. Under conditions of moderate ER load the unfolded protein response (UPR) compensatory mechanism engages, but if the stress cannot be resolved cell death ensues. Fatty acids have long been known to increase ER load, by affecting protein processing, trafficking, Ca2+ regulation and oxidative stress [16, 63]. Saturated fatty acids such as palmitate induce ER stress, whereas unsaturated fatty acids exert protective effects; in INS1 cells, palmitate activated UPR via the PERK and IRE1 pathways, and the effect was prevented by co-treatment with oleate [65]. In bTC3 cells, palmitate activated all three UPR pathways, through store-activated calcium entry [66]. Palmitate also induced ER stress in a human beta cell line [67]. Protection by unsaturated fatty acids may be mediated by cellular inhibitor of apoptosis-1 (cIAP1), an E3 ligase for the cell death director CHOP [68]. A screen for effectors of palmitate-induced apoptosis discovered ER communication with the intrinsic mitochondrial apoptosis pathway, through BH3-only proteins DH5 and PUMA [69]. Another screen found ubiquitin C-terminal hydrolase L1 to be required for ER function, beta cell survival and insulin secretion when exposed to lipotoxicity [70]. ER morphological and functional distress caused by fatty acid treatment was traced to AMPK acting through a GTPase called dynamin related protein 1 (DRP1), known to regulate mitochondrial fission [71]. Another study found that palmitate influenced the makeup of ER lipid rafts, which determined the cellular response to ER stress [72]. The immediate early gene Npas4, rapidly induced by palmitate, promoted beta cell survival during ER stress [73]. Treatment with incretins protected beta cells against palmitate-induced ER stress [74]. Combined with the large body of clinical literature suggesting beta cell exhaustion contributes to T2D, and possibly T1D, management of ER stress may assist in preservation of beta cell mass and function.
Autophagy is used to recycle unnecessary or dysfunctional cellular components, and to ensure survival during starvation by maintaining cellular energy levels. Autophagy increases during nutrient stress; controversy exists as to whether autophagy is predominantly detrimental or protective to beta cells. Palmitate increases autophagy in rat and human beta cells and is associated with ER distension [75]. Atg7 overexpression sensitized cells to palmitate-induced autophagy, which was found to increase inflammatory mediators via cathepsin B and the NLRP3 inflammasome, linking cellular nutrient stress to inflammation [76]. On the other hand, mice deficient in beta cell autophagy due to deletion of Atg7 showed impaired ER adaptation and heightened sensitivity to ER stress, resulting in frank diabetes when mated onto a leptin deficient background [77]. Although fatty acids increase autophagosomes, suggesting increased autophagy, a dynamic study found that in fact autophagic flux, a measure of activity, was reduced in beta cells treated with oleate or palmitate [78]. Consistent with the concept that autophagy is activated by starvation, and fatty acid treatment represents nutrient excess, simulating starvation by rapamycin treatment partially restored autophagic flux [78]. The role of autophagy in beta cell failure in T2D requires further investigation.
Lipotoxicity effects in the nucleus
Some negative effects of lipids occur at the level of the nucleus. PPARs, classic nuclear lipid receptors, exert mostly positive effects on beta cell mass and function. Our own work has supported a new role for lipotoxicity in preventing beta cell proliferation, described in some detail in the cell cycle section below.
Lipid receptors
The peroxisome proliferator-activated receptor (PPAR) nuclear receptors are transcription factors regulating many genes involved in differentiation, development and metabolism. They play an important role in T2D because of their effects on circulating glucose and lipids, and both PPARα and PPARγ are targets of medications currently in use. PPARs are important for beta cell function. Overexpression of PPARα in obese mice preserved insulin secretion without affecting beta cell mass in one study [79]; in another study, however, treatment of obese mice with pan-PPAR agonist bezafibrate also maintained glucose homeostasis, but in this case by preventing weight gain [80]. Expansion of beta cell mass in leptin deficient mice is dependent on PPARγ [81]. PPARγ activation improved insulin secretion by increasing expression of FFAR1, as well as beta cell differentiation genes, in a pathway dependent on glucose transport, FFAR1, and phospholipase C [29, 82]. Thus, recent literature suggests that most of the cellular effects of lipids via PPARs are positive.
Cell cycle regulation: lipid effects on beta cell proliferation
A new, less well characterized form of lipotoxicity has been postulated: that in addition to impairment of insulin secretion and induction of beta cell death, lipid exposure may prevent beta cell mass expansion by inhibiting beta cell proliferation. Data supporting this hypothesis include cell culture studies showing that long chain FFAs reversibly blocked glucose induced beta cell proliferation in INS-1 cells; intriguingly, fatty acid oxidation was not required, and the dose of fatty acids used did not interfere with glucose metabolism [83, 84]. Palmitate has been shown to reduce proliferation in cultured human beta cells, an effect that was mitigated by co-incubation with oleate [85]. FFAR2, a receptor for short chain fatty acids, was upregulated in islets at a stage of pregnancy when beta cells proliferate [86]. In vivo, raising circulating levels of FFA by direct infusion of triglyceride with heparin prevented glucose-induced mouse beta cell proliferation [87]. FFA also reduced proliferation when directly applied to primary mouse beta cells in vitro, and the mechanism was traced to induction of cell cycle inhibitor proteins p16 and p18 [87].
Other studies contradict these findings, however, and FFAs have even been postulated to promote compensatory beta cell proliferation [88]. In seeming contradiction, beta cell proliferation increases in mice overfed with a high fat diet, but FFAs don’t increase until after beta cell proliferation begins, and proliferation may be driven by other changes associated with overnutrition [89]. In cultured rat islets, palmitic and oleic acids increased beta cell proliferation and insulin secretion synergistically with prolactin treatment [90]. A 1:1 mixture of oleic:palmitic acid stimulated tritiated thymidine incorporation in rat islets [45]. In Zucker fatty rats subjected to partial pancreatectomy, beta cell regeneration exceeded that of non-hyperlipidemic controls, with robust beta cell proliferation [91]. In seeming direct contradiction to the infusion study in mice, intravenous infusion of triglyceride and glucose into 6-month old rats resulted in increased beta cell mass and proliferation [92]. Many experimental differences may explain the seemingly contradictory findings, including the species, age, degree of hyperglycemia, insulin resistance and infusion procedure. A prior study of lipid infusion in rats also concluded that lipids increased beta cell mass and proliferation [93]. Thus, whether in vivo exposure to lipids promotes or prevents beta cell proliferation remains an open question, and how this relates to human biology is uncertain.
Evidence lipotoxicity is relevant to living human beings
Circulating lipids influence risk of developing T2D [94]. New data confirm prior findings that triglyceride levels positively correlate with risk of T2D, and specifically with beta cell dysfunction [95, 96]. In adolescents, acute elevation of triglyceride by overnight infusion reduced insulin secretion in response to hyperglycemic clamp; whether this effect is race-dependent remains unclear [97, 98]. Elevation of free fatty acids was strongly associated with reduced beta cell function in both children and adults, with, intriguingly, a more consistent effect seen on insulin secretory capacity than on insulin sensitivity [99]. However, the potentiation of acute glucose-stimulated insulin secretion by prior infusion of insulin was found to be independent of free fatty acids [100].
The link between cholesterol metabolism and diabetes is an active area of investigation. HMG-CoA reductase inhibitors (statin-class medications) are now known to slightly increase the risk of new onset T2D, but the mechanism remains unknown [101]. Seemingly contradictory to this, atorvastatin preserves beta cell function in some patients with early T1D; the reason behind this observation is equally unclear [102]. The cardioprotective high-density lipoprotein (HDL) cholesterol particle, which transports cholesterol out of tissues and back to the liver for clearance, appears to be protective against development of T2D [103]. Protection was correlated with larger particle size, possibly implicating flux of cholesterol transport in diabetes prevention. Blood taken from subjects treated with a CETP inhibitor, which elevates HDL levels, increased insulin secretion from a beta cell line, an effect that may have been related to efflux of cholesterol from the cultured cells [104]. In diabetic mice, chronic infusion of HDL improved blood glucose and pancreatic islet architecture [105]. HDL protected beta cells against ER stress-mediated cell death [106].
A novel concept based on observations of diabetes remission after bariatric surgery links beta cell dysfunction in T2D with fat accumulation in the pancreas itself [107]. Consistent with this hypothesis, pancreatic steatosis in people with genetic ATGL deficiency was associated with impaired insulin secretory function without impairment in insulin sensitivity [108]. On the other hand, an imaging-based study found that in nondiabetic individuals with mild obesity, pancreatic lipid content varied by ethnicity, and in both African American and Caucasian subjects pancreatic triglyceride was positively correlated with first phase insulin secretion after IV glucose challenge [109]. Whether pancreatic lipid content is a marker of a global process, such as insulin resistance or failure of the adipocyte storage system, or has direct effects on beta cell function, remains unknown.
Conclusion
Beta cell lipotoxicity takes many forms, with respect to lipid species, cellular location of action, pathways involved, and end effects on beta cell survival, mass, and function. Since lipid biology clearly interacts with diabetes risk and complications in people, and lipid signaling is amenable to therapeutic intervention, this is an important field of study that directly impacts human health. Much work needs to be done to identify rational basis for new therapies that target lipotoxicity to prevent and treat beta cell failure in diabetes.
Acknowledgments
Funding supporting this work includes NIH: DK095140 (LCA).
Footnotes
Conflict of Interest
Rohit B. Sharma and Laura C. Alonso declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Poitout V, Robertson RP. Glucolipotoxicity: Fuel Excess and Beta-Cell Dysfunction. Endocrine reviews. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prentki M, Matschinsky FM, Madiraju SR. Metabolic Signaling in Fuel-Induced Insulin Secretion. Cell metabolism. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Kondegowda NG, Mozar A, Chin C, et al. Lactogens Protect Rodent and Human Beta Cells against Glucolipotoxicity-Induced Cell Death through Janus Kinase-2 (Jak2)/Signal Transducer and Activator of Transcription-5 (Stat5) Signalling. Diabetologia. 2012;55:1721–1732. doi: 10.1007/s00125-012-2501-9. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Pertusa JA, Dube J, Valle SR, et al. Novel Proapoptotic Effect of Hepatocyte Growth Factor: Synergy with Palmitate to Cause Pancreatic {Beta}-Cell Apoptosis. Endocrinology. 2010;151:1487–1498. doi: 10.1210/en.2009-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin HY, Yin Y, Zhang JX, et al. Identification of Direct Forkhead Box O1 Targets Involved in Palmitate-Induced Apoptosis in Clonal Insulin-Secreting Cells Using Chromatin Immunoprecipitation Coupled to DNA Selection and Ligation. Diabetologia. 2012;55:2703–2712. doi: 10.1007/s00125-012-2643-9. [DOI] [PubMed] [Google Scholar]
- 6•.Tiano JP, Delghingaro-Augusto V, Le May C, et al. Estrogen Receptor Activation Reduces Lipid Synthesis in Pancreatic Islets and Prevents Beta Cell Failure in Rodent Models of Type 2 Diabetes. The Journal of clinical investigation. 2011;121:3331–3342. doi: 10.1172/JCI44564. Important work linking lipid metabolism to estradiol signaling in beta cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang ZF, Deng Y, Zhou Y, et al. Pharmacological Reduction of Nefa Restores the Efficacy of Incretin-Based Therapies through Glp-1 Receptor Signalling in the Beta Cell in Mouse Models of Diabetes. Diabetologia. 2013;56:423–433. doi: 10.1007/s00125-012-2776-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimple ME, Keller MP, Rabaglia MR, et al. Prostaglandin E2 Receptor, Ep3, Is Induced in Diabetic Islets and Negatively Regulates Glucose- and Hormone-Stimulated Insulin Secretion. Diabetes. 2013;62:1904–1912. doi: 10.2337/db12-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodson DJ, Mitchell RK, Bellomo EA, et al. Lipotoxicity Disrupts Incretin-Regulated Human Beta Cell Connectivity. The Journal of clinical investigation. 2013;123:4182–4194. doi: 10.1172/JCI68459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Stanojevic V, Brindamour LJ, Habener JF. Glp1-Derived Nonapeptide Glp1(28–36)Amide Protects Pancreatic Beta-Cells from Glucolipotoxicity. The Journal of endocrinology. 2012;213:143–154. doi: 10.1530/JOE-11-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao XY, Gu ZY, Liu P, et al. The Human Glucagon-Like Peptide-1 Analogue Liraglutide Regulates Pancreatic Beta-Cell Proliferation and Apoptosis Via an Ampk/Mtor/P70s6k Signaling Pathway. Peptides. 2013;39:71–79. doi: 10.1016/j.peptides.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang HW, Mizuta M, Saitoh Y, et al. Glucagon-Like Peptide-1 and Candesartan Additively Improve Glucolipotoxicity in Pancreatic Beta-Cells. Metabolism: clinical and experimental. 2011;60:1081–1089. doi: 10.1016/j.metabol.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Hong SW, Lee J, Park SE, et al. Repression of Sterol Regulatory Element-Binding Protein 1-C Is Involved in the Protective Effects of Exendin-4 in Pancreatic Beta-Cell Line. Molecular and cellular endocrinology. 2012;362:242–252. doi: 10.1016/j.mce.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Shimoda M, Kanda Y, Hamamoto S, et al. The Human Glucagon-Like Peptide-1 Analogue Liraglutide Preserves Pancreatic Beta Cells Via Regulation of Cell Kinetics and Suppression of Oxidative and Endoplasmic Reticulum Stress in a Mouse Model of Diabetes. Diabetologia. 2011;54:1098–1108. doi: 10.1007/s00125-011-2069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah P, Ardestani A, Dharmadhikari G, et al. The Dpp-4 Inhibitor Linagliptin Restores Beta-Cell Function and Survival in Human Isolated Islets through Glp-1 Stabilization. The Journal of clinical endocrinology and metabolism. 2013;98:E1163–1172. doi: 10.1210/jc.2013-1029. [DOI] [PubMed] [Google Scholar]
- 16.Mirmira RG. Saturated Free Fatty Acids: Islet Beta Cell “Stressers”. Endocrine. 2012;42:1–2. doi: 10.1007/s12020-012-9713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang C, Naassan AE, Chamson-Reig A, et al. Susceptibility to Fatty Acid-Induced Beta-Cell Dysfunction Is Enhanced in Prediabetic Diabetes-Prone Biobreeding Rats: A Potential Link between Beta-Cell Lipotoxicity and Islet Inflammation. Endocrinology. 2013;154:89–101. doi: 10.1210/en.2012-1720. [DOI] [PubMed] [Google Scholar]
- 18.Donath MY, Dalmas E, Sauter NS, Boni-Schnetzler M. Inflammation in Obesity and Diabetes: Islet Dysfunction and Therapeutic Opportunity. Cell metabolism. 2013;17:860–872. doi: 10.1016/j.cmet.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 19••.Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated Fatty Acid and Tlr Signaling Link Beta Cell Dysfunction and Islet Inflammation. Cell metabolism. 2012;15:518–533. doi: 10.1016/j.cmet.2012.01.023. This paper shows the critical role inflammation plays in lipotoxicity. [DOI] [PubMed] [Google Scholar]
- 20.Saksida T, Stosic-Grujicic S, Timotijevic G, et al. Macrophage Migration Inhibitory Factor Deficiency Protects Pancreatic Islets from Palmitic Acid-Induced Apoptosis. Immunology and cell biology. 2012;90:688–698. doi: 10.1038/icb.2011.89. [DOI] [PubMed] [Google Scholar]
- 21.Amyot J, Semache M, Ferdaoussi M, et al. Lipopolysaccharides Impair Insulin Gene Expression in Isolated Islets of Langerhans Via Toll-Like Receptor-4 and Nf-Kappab Signalling. PloS one. 2012;7:e36200. doi: 10.1371/journal.pone.0036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miani M, Colli ML, Ladriere L, et al. Mild Endoplasmic Reticulum Stress Augments the Proinflammatory Effect of Il-1beta in Pancreatic Rat Beta-Cells Via the Ire1alpha/Xbp1s Pathway. Endocrinology. 2012;153:3017–3028. doi: 10.1210/en.2011-2090. [DOI] [PubMed] [Google Scholar]
- 23.Weaver JR, Holman TR, Imai Y, et al. Integration of Pro-Inflammatory Cytokines, 12-Lipoxygenase and Nox-1 in Pancreatic Islet Beta Cell Dysfunction. Molecular and cellular endocrinology. 2012;358:88–95. doi: 10.1016/j.mce.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Mohammed AM, Syeda K, Hadden T, Kowluru A. Upregulation of Phagocyte-Like Nadph Oxidase by Cytokines in Pancreatic Beta-Cells: Attenuation of Oxidative and Nitrosative Stress by 2-Bromopalmitate. Biochemical pharmacology. 2013;85:109–114. doi: 10.1016/j.bcp.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Poitout V, Lin DC. Modulating Gpr40: Therapeutic Promise and Potential in Diabetes. Drug discovery today. 2013;18:1301–1308. doi: 10.1016/j.drudis.2013.09.003. Interesting review of progress towards therapeutic use of FFAR1 agonists. [DOI] [PubMed] [Google Scholar]
- 26.Mancini AD, Poitout V. The Fatty Acid Receptor Ffa1/Gpr40 a Decade Later: How Much Do We Know? Trends in endocrinology and metabolism: TEM. 2013;24:398–407. doi: 10.1016/j.tem.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Ferdaoussi M, Bergeron V, Zarrouki B, et al. G Protein-Coupled Receptor (Gpr)40-Dependent Potentiation of Insulin Secretion in Mouse Islets Is Mediated by Protein Kinase D1. Diabetologia. 2012;55:2682–2692. doi: 10.1007/s00125-012-2650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Y, Swaminath G, Cao Q, et al. Activation of Ffa1 Mediates Glp-1 Secretion in Mice. Evidence for Allosterism at Ffa1 Molecular and cellular endocrinology. 2013;369:119–129. doi: 10.1016/j.mce.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Meidute Abaraviciene S, Muhammed SJ, Amisten S, et al. Gpr40 Protein Levels Are Crucial to the Regulation of Stimulated Hormone Secretion in Pancreatic Islets. Lessons from Spontaneous Obesity-Prone and Non-Obese Type 2 Diabetes in Rats Molecular and cellular endocrinology. 2013;381:150–159. doi: 10.1016/j.mce.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Tuo Y, Feng DD, Wang DF, et al. Long-Term in Vitro Treatment of Ins-1 Rat Pancreatic Beta-Cells by Unsaturated Free Fatty Acids Protects Cells against Gluco- and Lipotoxicities Via Activation of Gpr40 Receptors. Clinical and experimental pharmacology & physiology. 2012;39:423–428. doi: 10.1111/j.1440-1681.2012.05691.x. [DOI] [PubMed] [Google Scholar]
- 31.Wagner R, Kaiser G, Gerst F, et al. Reevaluation of Fatty Acid Receptor 1 as a Drug Target for the Stimulation of Insulin Secretion in Humans. Diabetes. 2013;62:2106–2111. doi: 10.2337/db12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin DC, Guo Q, Luo J, et al. Identification and Pharmacological Characterization of Multiple Allosteric Binding Sites on the Free Fatty Acid 1 Receptor. Molecular pharmacology. 2012;82:843–859. doi: 10.1124/mol.112.079640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yabuki C, Komatsu H, Tsujihata Y, et al. A Novel Antidiabetic Drug, Fasiglifam/Tak-875, Acts as an Ago-Allosteric Modulator of Ffar1. PloS one. 2013;8:e76280. doi: 10.1371/journal.pone.0076280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito R, Tsujihata Y, Matsuda-Nagasumi K, et al. Tak-875, a Gpr40/Ffar1 Agonist, in Combination with Metformin Prevents Progression of Diabetes and Beta-Cell Dysfunction in Zucker Diabetic Fatty Rats. British journal of pharmacology. 2013;170:568–580. doi: 10.1111/bph.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou HY, Wu HT, Hung HC, et al. Multiple Mechanisms of Gw-9508, a Selective G Protein-Coupled Receptor 40 Agonist, in the Regulation of Glucose Homeostasis and Insulin Sensitivity. American journal of physiology Endocrinology and metabolism. 2013;304:E668–676. doi: 10.1152/ajpendo.00419.2012. [DOI] [PubMed] [Google Scholar]
- 36.Gowda N, Dandu A, Singh J, et al. Treatment with Cnx-011–67, a Novel Gpr40 Agonist, Delays Onset and Progression of Diabetes and Improves Beta Cell Preservation and Function in Male Zdf Rats. BMC pharmacology & toxicology. 2013;14:28. doi: 10.1186/2050-6511-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kristinsson H, Smith DM, Bergsten P, Sargsyan E. Ffar1 Is Involved in Both the Acute and Chronic Effects of Palmitate on Insulin Secretion. Endocrinology. 2013;154:4078–4088. doi: 10.1210/en.2013-1352. [DOI] [PubMed] [Google Scholar]
- 38.Sun P, Wang T, Zhou Y, et al. Dc260126: A Small-Molecule Antagonist of Gpr40 That Protects against Pancreatic Beta-Cells Dysfunction in Db/Db Mice. PloS one. 2013;8:e66744. doi: 10.1371/journal.pone.0066744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Sun P, Zhang X, et al. Inhibition of Gpr40 Protects Min6 Beta Cells from Palmitate-Induced Er Stress and Apoptosis. Journal of cellular biochemistry. 2012;113:1152–1158. doi: 10.1002/jcb.23450. [DOI] [PubMed] [Google Scholar]
- 40.Prentki M, Madiraju SR. Glycerolipid/Free Fatty Acid Cycle and Islet Beta-Cell Function in Health, Obesity and Diabetes. Molecular and cellular endocrinology. 2012;353:88–100. doi: 10.1016/j.mce.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Nyren R, Chang CL, Lindstrom P, et al. Localization of Lipoprotein Lipase and Gpihbp1 in Mouse Pancreas: Effects of Diet and Leptin Deficiency. BMC physiology. 2012;12:14. doi: 10.1186/1472-6793-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Klett EL, Chen S, Edin ML, et al. Diminished Acyl-Coa Synthetase Isoform 4 Activity in Ins 832/13 Cells Reduces Cellular Epoxyeicosatrienoic Acid Levels and Results in Impaired Glucose-Stimulated Insulin Secretion. The Journal of biological chemistry. 2013;288:21618–21629. doi: 10.1074/jbc.M113.481077. Insight into role of acyl-CoA synthases in beta cell function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sargsyan E, Sol ER, Bergsten P. Upr in Palmitate-Treated Pancreatic Beta-Cells Is Not Affected by Altering Oxidation of the Fatty Acid. Nutrition & metabolism. 2011;8:70. doi: 10.1186/1743-7075-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi SE, Jung IR, Lee YJ, et al. Stimulation of Lipogenesis as Well as Fatty Acid Oxidation Protects against Palmitate-Induced Ins-1 Beta-Cell Death. Endocrinology. 2011;152:816–827. doi: 10.1210/en.2010-0924. [DOI] [PubMed] [Google Scholar]
- 45.Vernier S, Chiu A, Schober J, et al. Beta-Cell Metabolic Alterations under Chronic Nutrient Overload in Rat and Human Islets. Islets. 2012;4:379–392. doi: 10.4161/isl.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohtsubo K, Chen MZ, Olefsky JM, Marth JD. Pathway to Diabetes through Attenuation of Pancreatic Beta Cell Glycosylation and Glucose Transport. Nature medicine. 2011;17:1067–1075. doi: 10.1038/nm.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somesh BP, Verma MK, Sadasivuni MK, et al. Chronic Glucolipotoxic Conditions in Pancreatic Islets Impair Insulin Secretion Due to Dysregulated Calcium Dynamics, Glucose Responsiveness and Mitochondrial Activity. BMC cell biology. 2013;14:31. doi: 10.1186/1471-2121-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barlow J, Affourtit C. Novel Insights into Pancreatic Beta-Cell Glucolipotoxicity from Real-Time Functional Analysis of Mitochondrial Energy Metabolism in Ins-1e Insulinoma Cells. The Biochemical journal. 2013;456:417–426. doi: 10.1042/BJ20131002. [DOI] [PubMed] [Google Scholar]
- 49.Zraika S, Koh DS, Barrow BM, et al. Neprilysin Deficiency Protects against Fat-Induced Insulin Secretory Dysfunction by Maintaining Calcium Influx. Diabetes. 2013;62:1593–1601. doi: 10.2337/db11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malmgren S, Spegel P, Danielsson AP, et al. Coordinate Changes in Histone Modifications, Mrna Levels, and Metabolite Profiles in Clonal Ins-1 832/13 Beta-Cells Accompany Functional Adaptations to Lipotoxicity. The Journal of biological chemistry. 2013;288:11973–11987. doi: 10.1074/jbc.M112.422527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baldwin AC, Green CD, Olson LK, et al. A Role for Aberrant Protein Palmitoylation in Ffa-Induced Er Stress and Beta-Cell Death. American journal of physiology Endocrinology and metabolism. 2012;302:E1390–1398. doi: 10.1152/ajpendo.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Lam AK, Silva PN, Altamentova SM, Rocheleau JV. Quantitative Imaging of Electron Transfer Flavoprotein Autofluorescence Reveals the Dynamics of Lipid Partitioning in Living Pancreatic Islets. Integrative biology : quantitative biosciences from nano to macro. 2012;4:838–846. doi: 10.1039/c2ib20075a. Impressive technique for visualizing metabolic pathways. [DOI] [PubMed] [Google Scholar]
- 53.Boslem E, Meikle PJ, Biden TJ. Roles of Ceramide and Sphingolipids in Pancreatic Beta-Cell Function and Dysfunction. Islets. 2012;4:177–187. doi: 10.4161/isl.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marcal AC, Camporez JP, Lima-Salgado TM, et al. Changes in Food Intake, Metabolic Parameters and Insulin Resistance Are Induced by an Isoenergetic, Medium-Chain Fatty Acid Diet and Are Associated with Modifications in Insulin Signalling in Isolated Rat Pancreatic Islets. The British journal of nutrition. 2013;109:2154–2165. doi: 10.1017/S0007114512004576. [DOI] [PubMed] [Google Scholar]
- 55.Zhou H, Li C, Li J, et al. Associations of Atp-Binding Cassette Transporter A1 and G1 with Insulin Secretion in Human Insulinomas. Pancreas. 2012;41:934–939. doi: 10.1097/MPA.0b013e318243a5e3. [DOI] [PubMed] [Google Scholar]
- 56.Jelinek D, Castillo JJ, Garver WS. The C57bl/6j Niemann-Pick C1 Mouse Model with Decreased Gene Dosage Has Impaired Glucose Tolerance Independent of Body Weight. Gene. 2013;527:65–70. doi: 10.1016/j.gene.2013.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng ZX, Yin Y, Lv JH, et al. Aberrant Activation of Liver X Receptors Impairs Pancreatic Beta Cell Function through Upregulation of Sterol Regulatory Element-Binding Protein 1c in Mouse Islets and Rodent Cell Lines. Diabetologia. 2012;55:1733–1744. doi: 10.1007/s00125-012-2516-2. [DOI] [PubMed] [Google Scholar]
- 58.Graciano MF, Valle MM, Kowluru A, et al. Regulation of Insulin Secretion and Reactive Oxygen Species Production by Free Fatty Acids in Pancreatic Islets. Islets. 2011;3:213–223. doi: 10.4161/isl.3.5.15935. [DOI] [PubMed] [Google Scholar]
- 59.MacDonald MJ, Langberg EC, Tibell A, et al. Identification of Atp Synthase as a Lipid Peroxide Protein Adduct in Pancreatic Islets from Humans with and without Type 2 Diabetes Mellitus. The Journal of clinical endocrinology and metabolism. 2013;98:E727–731. doi: 10.1210/jc.2012-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koulajian K, Ivovic A, Ye K, et al. Overexpression of Glutathione Peroxidase 4 Prevents Beta-Cell Dysfunction Induced by Prolonged Elevation of Lipids in Vivo. American journal of physiology Endocrinology and metabolism. 2013;305:E254–262. doi: 10.1152/ajpendo.00481.2012. [DOI] [PubMed] [Google Scholar]
- 61.Koulajian K, Desai T, Liu GC, et al. Nadph Oxidase Inhibition Prevents Beta Cell Dysfunction Induced by Prolonged Elevation of Oleate in Rodents. Diabetologia. 2013;56:1078–1087. doi: 10.1007/s00125-013-2858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee SJ, Choi SE, Jung IR, et al. Protective Effect of Nicotinamide on High Glucose/Palmitate-Induced Glucolipotoxicity to Ins-1 Beta Cells Is Attributed to Its Inhibitory Activity to Sirtuins. Archives of biochemistry and biophysics. 2013;535:187–196. doi: 10.1016/j.abb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Back SH, Kaufman RJ. Endoplasmic Reticulum Stress and Type 2 Diabetes. Annual review of biochemistry. 2012;81:767–793. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schuiki I, Zhang L, Volchuk A. Endoplasmic Reticulum Redox State Is Not Perturbed by Pharmacological or Pathological Endoplasmic Reticulum Stress in Live Pancreatic Beta-Cells. PloS one. 2012;7:e48626. doi: 10.1371/journal.pone.0048626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sommerweiss D, Gorski T, Richter S, et al. Oleate Rescues Ins-1e Beta-Cells from Palmitate-Induced Apoptosis by Preventing Activation of the Unfolded Protein Response. Biochemical and biophysical research communications. 2013;441:770–776. doi: 10.1016/j.bbrc.2013.10.130. [DOI] [PubMed] [Google Scholar]
- 66.Cui W, Ma J, Wang X, et al. Free Fatty Acid Induces Endoplasmic Reticulum Stress and Apoptosis of Beta-Cells by Ca2+/Calpain-2 Pathways. PloS one. 2013;8:e59921. doi: 10.1371/journal.pone.0059921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vasu S, McClenaghan NH, McCluskey JT, Flatt PR. Effects of Lipotoxicity on a Novel Insulin-Secreting Human Pancreatic Beta-Cell Line, 1. 1b4. Biological chemistry. 2013;394:909–918. doi: 10.1515/hsz-2013-0115. [DOI] [PubMed] [Google Scholar]
- 68.Qi Y, Xia P. Cellular Inhibitor of Apoptosis Protein-1 (Ciap1) Plays a Critical Role in Beta-Cell Survival under Endoplasmic Reticulum Stress: Promoting Ubiquitination and Degradation of C/Ebp Homologous Protein (Chop) The Journal of biological chemistry. 2012;287:32236–32245. doi: 10.1074/jbc.M112.362160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69•.Cunha DA, Igoillo-Esteve M, Gurzov EN, et al. Death Protein 5 and P53-Upregulated Modulator of Apoptosis Mediate the Endoplasmic Reticulum Stress-Mitochondrial Dialog Triggering Lipotoxic Rodent and Human Beta-Cell Apoptosis. Diabetes. 2012;61:2763–2775. doi: 10.2337/db12-0123. Novel pathway implicated in lipotoxic beta cell death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu KY, Li H, Wada K, Johnson JD. Ubiquitin C-Terminal Hydrolase L1 Is Required for Pancreatic Beta Cell Survival and Function in Lipotoxic Conditions. Diabetologia. 2012;55:128–140. doi: 10.1007/s00125-011-2323-1. [DOI] [PubMed] [Google Scholar]
- 71.Wikstrom JD, Israeli T, Bachar-Wikstrom E, et al. Ampk Regulates Er Morphology and Function in Stressed Pancreatic Beta-Cells Via Phosphorylation of Drp1. Molecular endocrinology. 2013;27:1706–1723. doi: 10.1210/me.2013-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boslem E, Weir JM, MacIntosh G, et al. Alteration of Endoplasmic Reticulum Lipid Rafts Contributes to Lipotoxicity in Pancreatic Beta-Cells. The Journal of biological chemistry. 2013;288:26569–26582. doi: 10.1074/jbc.M113.489310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabatini PV, Krentz NA, Zarrouki B, et al. Npas4 Is a Novel Activity-Regulated Cytoprotective Factor in Pancreatic Beta-Cells. Diabetes. 2013;62:2808–2820. doi: 10.2337/db12-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oh YS, Lee YJ, Kang Y, et al. Exendin-4 Inhibits Glucolipotoxic Er Stress in Pancreatic Beta Cells Via Regulation of Srebp1c and C/Ebpbeta Transcription Factors. The Journal of endocrinology. 2013;216:343–352. doi: 10.1530/JOE-12-0311. [DOI] [PubMed] [Google Scholar]
- 75.Martino L, Masini M, Novelli M, et al. Palmitate Activates Autophagy in Ins-1e Beta-Cells and in Isolated Rat and Human Pancreatic Islets. PloS one. 2012;7:e36188. doi: 10.1371/journal.pone.0036188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li S, Du L, Zhang L, et al. Cathepsin B Contributes to Autophagy-Related 7 (Atg7)-Induced Nod-Like Receptor 3 (Nlrp3)-Dependent Proinflammatory Response and Aggravates Lipotoxicity in Rat Insulinoma Cell Line. The Journal of biological chemistry. 2013;288:30094–30104. doi: 10.1074/jbc.M113.494286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quan W, Hur KY, Lim Y, et al. Autophagy Deficiency in Beta Cells Leads to Compromised Unfolded Protein Response and Progression from Obesity to Diabetes in Mice. Diabetologia. 2012;55:392–403. doi: 10.1007/s00125-011-2350-y. [DOI] [PubMed] [Google Scholar]
- 78.Las G, Serada SB, Wikstrom JD, et al. Fatty Acids Suppress Autophagic Turnover in Beta-Cells. The Journal of biological chemistry. 2011;286:42534–42544. doi: 10.1074/jbc.M111.242412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hogh KL, Uy CE, Asadi A, et al. Overexpression of Peroxisome Proliferator-Activated Receptor Alpha in Pancreatic Beta-Cells Improves Glucose Tolerance in Diet-Induced Obese Mice. Experimental physiology. 2013;98:564–575. doi: 10.1113/expphysiol.2012.068734. [DOI] [PubMed] [Google Scholar]
- 80.da Silva Faria T, Correia-Junior AL, Dos Anjos TL, et al. Adverse Association between Obesity and Menopause in Mice Treated with Bezafibrate, a Pan Peroxisome Proliferator-Activated Receptor Agonist. Menopause. 2013;20:1264–1274. doi: 10.1097/GME.0b013e31828f5e3c. [DOI] [PubMed] [Google Scholar]
- 81.Vivas Y, Martinez-Garcia C, Izquierdo A, et al. Early Peroxisome Proliferator-Activated Receptor Gamma Regulated Genes Involved in Expansion of Pancreatic Beta Cell Mass. BMC medical genomics. 2011;4:86. doi: 10.1186/1755-8794-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim HS, Hwang YC, Koo SH, et al. Ppar-Gamma Activation Increases Insulin Secretion through the up-Regulation of the Free Fatty Acid Receptor Gpr40 in Pancreatic Beta-Cells. PloS one. 2013;8:e50128. doi: 10.1371/journal.pone.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhodes CJ. Igf-I and Gh Post-Receptor Signaling Mechanisms for Pancreatic Beta-Cell Replication. Journal of molecular endocrinology. 2000;24:303–311. doi: 10.1677/jme.0.0240303. [DOI] [PubMed] [Google Scholar]
- 84.Cousin SP, Hugl SR, Wrede CE, et al. Free Fatty Acid-Induced Inhibition of Glucose and Insulin-Like Growth Factor I-Induced Deoxyribonucleic Acid Synthesis in the Pancreatic Beta-Cell Line Ins-1. Endocrinology. 2001;142:229–240. doi: 10.1210/endo.142.1.7863. [DOI] [PubMed] [Google Scholar]
- 85.Maedler K, Oberholzer J, Bucher P, et al. Monounsaturated Fatty Acids Prevent the Deleterious Effects of Palmitate and High Glucose on Human Pancreatic Beta-Cell Turnover and Function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 86.Layden BT, Durai V, Newman MV, et al. Regulation of Pancreatic Islet Gene Expression in Mouse Islets by Pregnancy. The Journal of endocrinology. 2010;207:265–279. doi: 10.1677/JOE-10-0298. [DOI] [PubMed] [Google Scholar]
- 87•.Pascoe J, Hollern D, Stamateris R, et al. Free Fatty Acids Block Glucose-Induced Beta-Cell Proliferation in Mice by Inducing Cell Cycle Inhibitors P16 and P18. Diabetes. 2012;61:632–641. doi: 10.2337/db11-0991. Infusion of lipids into mice prevents glucose-induced beta cell proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prentki M, Madiraju SR. Glycerolipid Metabolism and Signaling in Health and Disease. Endocrine reviews. 2008;29:647–676. doi: 10.1210/er.2008-0007. [DOI] [PubMed] [Google Scholar]
- 89.Stamateris RE, Sharma RB, Hollern DA, Alonso LC. Adaptive Beta-Cell Proliferation Increases Early in High-Fat Feeding in Mice, Concurrent with Metabolic Changes, with Induction of Islet Cyclin D2 Expression. American journal of physiology Endocrinology and metabolism. 2013;305:E149–159. doi: 10.1152/ajpendo.00040.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brelje TC, Bhagroo NV, Stout LE, Sorenson RL. Beneficial Effects of Lipids and Prolactin on Insulin Secretion and Beta-Cell Proliferation: A Role for Lipids in the Adaptation of Islets to Pregnancy. The Journal of endocrinology. 2008;197:265–276. doi: 10.1677/JOE-07-0657. [DOI] [PubMed] [Google Scholar]
- 91.Delghingaro-Augusto V, Nolan CJ, Gupta D, et al. Islet Beta Cell Failure in the 60% Pancreatectomised Obese Hyperlipidaemic Zucker Fatty Rat: Severe Dysfunction with Altered Glycerolipid Metabolism without Steatosis or a Falling Beta Cell Mass. Diabetologia. 2009;52:1122–1132. doi: 10.1007/s00125-009-1317-8. [DOI] [PubMed] [Google Scholar]
- 92•.Fontes G, Zarrouki B, Hagman DK, et al. Glucolipotoxicity Age-Dependently Impairs Beta Cell Function in Rats Despite a Marked Increase in Beta Cell Mass. Diabetologia. 2010;53:2369–2379. doi: 10.1007/s00125-010-1850-5. Infusion of lipids and glucose together into rats results in greatly increased beta cell proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steil GM, Trivedi N, Jonas JC, et al. Adaptation of Beta-Cell Mass to Substrate Oversupply: Enhanced Function with Normal Gene Expression. American journal of physiology Endocrinology and metabolism. 2001;280:E788–796. doi: 10.1152/ajpendo.2001.280.5.E788. [DOI] [PubMed] [Google Scholar]
- 94.Fazio S, Linton MF. Killing Two Birds with One Stone, Maybe: Cetp Inhibition Increases Both High-Density Lipoprotein Levels and Insulin Secretion. Circulation research. 2013;113:94–96. doi: 10.1161/CIRCRESAHA.113.301832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imamura F, Mukamal KJ, Meigs JB, et al. Risk Factors for Type 2 Diabetes Mellitus Preceded by Beta-Cell Dysfunction, Insulin Resistance, or Both in Older Adults: The Cardiovascular Health Study. American journal of epidemiology. 2013;177:1418–1429. doi: 10.1093/aje/kws440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng T, Gao Y, Tian H. Relationship between Blood Lipid Profiles and Pancreatic Islet Beta Cell Function in Chinese Men and Women with Normal Glucose Tolerance: A Cross-Sectional Study. BMC public health. 2012;12:634. doi: 10.1186/1471-2458-12-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hughan KS, Bonadonna RC, Lee S, et al. Beta-Cell Lipotoxicity after an Overnight Intravenous Lipid Challenge and Free Fatty Acid Elevation in African American Versus American White Overweight/Obese Adolescents. The Journal of clinical endocrinology and metabolism. 2013;98:2062–2069. doi: 10.1210/jc.2012-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michaliszyn SF, Bonadonna RC, Sjaarda LA, et al. Beta-Cell Lipotoxicity in Response to Free Fatty Acid Elevation in Prepubertal Youth: African American Versus Caucasian Contrast. Diabetes. 2013;62:2917–2922. doi: 10.2337/db12-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salgin B, Ong KK, Thankamony A, et al. Higher Fasting Plasma Free Fatty Acid Levels Are Associated with Lower Insulin Secretion in Children and Adults and a Higher Incidence of Type 2 Diabetes. The Journal of clinical endocrinology and metabolism. 2012;97:3302–3309. doi: 10.1210/jc.2012-1428. [DOI] [PubMed] [Google Scholar]
- 100•.Lopez X, Cypess A, Manning R, et al. Exogenous Insulin Enhances Glucose-Stimulated Insulin Response in Healthy Humans Independent of Changes in Free Fatty Acids. The Journal of clinical endocrinology and metabolism. 2011;96:3811–3821. doi: 10.1210/jc.2011-0627. Potentiation of insulin secretion by insulin is not due to suppression of lipolysis and reduced FFAs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colbert JD, Stone JA. Statin Use and the Risk of Incident Diabetes Mellitus: A Review of the Literature. The Canadian journal of cardiology. 2012;28:581–589. doi: 10.1016/j.cjca.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 102.Strom A, Kolb H, Martin S, et al. Improved Preservation of Residual Beta Cell Function by Atorvastatin in Patients with Recent Onset Type 1 Diabetes and High Crp Levels (Diator Trial) PloS one. 2012;7:e33108. doi: 10.1371/journal.pone.0033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abbasi A, Corpeleijn E, Gansevoort RT, et al. Role of Hdl Cholesterol and Estimates of Hdl Particle Composition in Future Development of Type 2 Diabetes in the General Population: The Prevend Study. The Journal of clinical endocrinology and metabolism. 2013;98:E1352–1359. doi: 10.1210/jc.2013-1680. [DOI] [PubMed] [Google Scholar]
- 104.Siebel AL, Natoli AK, Yap FY, et al. Effects of High-Density Lipoprotein Elevation with Cholesteryl Ester Transfer Protein Inhibition on Insulin Secretion. Circulation research. 2013;113:167–175. doi: 10.1161/CIRCRESAHA.113.300689. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Q, Wan R, Guo R, et al. Long-Term High Density Lipoprotein Infusion Ameliorates Metabolic Phenotypes of Diabetic Db/Db Mice. Diabetes/metabolism research and reviews. 2013;29:130–138. doi: 10.1002/dmrr.2372. [DOI] [PubMed] [Google Scholar]
- 106.Petremand J, Puyal J, Chatton JY, et al. Hdls Protect Pancreatic Beta-Cells against Er Stress by Restoring Protein Folding and Trafficking. Diabetes. 2012;61:1100–1111. doi: 10.2337/db11-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor R. Banting Memorial Lecture 2012: Reversing the Twin Cycles of Type 2 Diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2013;30:267–275. doi: 10.1111/dme.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Natali A, Gastaldelli A, Camastra S, et al. Metabolic Consequences of Adipose Triglyceride Lipase Deficiency in Humans: An in Vivo Study in Patients with Neutral Lipid Storage Disease with Myopathy. The Journal of clinical endocrinology and metabolism. 2013;98:E1540–1548. doi: 10.1210/jc.2013-1444. [DOI] [PubMed] [Google Scholar]
- 109.Szczepaniak LS, Victor RG, Mathur R, et al. Pancreatic Steatosis and Its Relationship to Beta-Cell Dysfunction in Humans: Racial and Ethnic Variations. Diabetes care. 2012;35:2377–2383. doi: 10.2337/dc12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]