Abstract
The rapid release of prepared movements by a loud acoustic stimulus capable of eliciting a startle response has been termed the StartReact effect (Valls-Solé et al., 1999), and premotor reaction times (PMTs) of <70 ms are often observed. Two explanations have been given for these short latency responses. The subcortical storage and triggering hypothesis suggests movements that can be prepared in advance of a “go” signal are stored and triggered from subcortical areas by a startling acoustic stimulus (SAS) without cortical involvement. Alternatively, it has been hypothesized that the SAS can trigger movements from cortical areas through a faster pathway ascending from subcortical structures. Two experiments were designed to examine the possible role of primary motor cortex in the StartReact effect. In Experiment 1, we used suprathreshold transcranial magnetic stimulation (TMS) during the reaction time (RT) interval to induce a cortical silent period in the contralateral primary motor cortex (M1). Thirteen participants performed 20° wrist extension movements as fast as possible in response to either a control stimulus (82dB) or SAS (124 dB). PMTs for startle trials were faster than control trials, while TMS significantly delayed movement onset compared to No TMS or Sham TMS conditions. In Experiment 2, we examined the StartReact effect in a highly cortically represented action involving speech of a consonant-vowel (CV) syllable. Similar to previous work examining limb movements, a robust StartReact effect was found. Collectively, these experiments provide evidence for cortical (M1) involvement in the StartReact effect.
Keywords: motor cortex, motor preparation, speech, startle, StartReact, TMS
1. INTRODUCTION
The processes of movement preparation and initiation have been examined using methodology involving the use of a loud acoustic stimulus, capable of eliciting a startle reflex (for recent reviews see Valls-Sole et al., 2008, Carlsen et al., 2011, Carlsen et al., 2012). Using a wrist movement in response to a visual imperative stimulus (IS), Valls-Solé et al. (1995, 1999) sometimes presented a very loud (>130 dB) startling acoustic stimulus (SAS) at the same time as the IS. These “startle” trials produced fast premotor reaction times (PMT; time from the onset of the IS to the onset of the voluntary muscle activity) without changing the whole triphasic electromyographic (EMG) pattern or the movement kinematics (see also Carlsen et al., 2004b), a result known as the “StartReact” effect. The time course of such reactions following a SAS (<70ms; see Valls-Solé et al., 1999, Carlsen et al., 2004b) has been hypothesized to be too fast to invoke cortical activity leading these authors to propose a process of subcortical triggering. They suggested that, at least for simple movements, sufficient detail of the movement can be prepared and stored in advance, and then triggered by the startling stimulus from subcortical areas with limited involvement of the cortex. This subcortical triggering is thought to be mediated by the reticular formation, given this area in the brainstem is common to both the startle reflex and voluntary movement pathways (Yeomans and Frankland, 1995, Rothwell et al., 2002).
A number of recent experiments have provided data in support of subcortical involvement in the StartReact effect. One line of evidence has been through the examination of the StartReact effect for movements which are more dependent on cortico-motoneuronal connections such as finger abduction as compared to movements requiring muscles with a higher degree of innervation from reticulospinal pathways such as arm extension or coordinated grasp (e.g., Lawrence and Kuypers, 1968a, b, Davidson and Buford, 2006, Lemon, 2008, Baker, 2011). Although it is likely that both reticulospinal and corticospinal tracts are involved in a synergistic manner for all movements, movements more heavily dependent upon the corticospinal system would be less likely to show a reduction in reaction time in response to a SAS, if the StartReact effect is being mediated through a subcortical triggering mechanism. Indeed, both Carlsen et al. (2009) and Honeycutt et al. (2013) found a typical StartReact effect for the more reticulospinal based movements; however in the finger abduction task there was little if any reduction in PMT on startle trials when a startle response was observed in the sternocleidomastoid (SCM+) as compared to when no startle indicator was observed (SCM−), which the authors argued was due to the cortically-dependent nature of the task. Additionally, deep brain stimulation of the pedunculopontine nucleus has been shown to restore the StartReact effect in Parkinson’s disease patients (Thevathasan et al., 2011), suggesting subcortical involvement in the release of a prepared movement. Lastly, Nonnekes et al. (2014) examined the StartReact effect in patients with hereditary spastic paraplegia (HSP), a condition in which corticospinal tracts are degenerated. Although reaction time to a visual stimulus was delayed in HSP patients as compared to healthy controls, both groups exhibited a similar response to the SAS, confirming an intact StartReact effect. This result supported the notion of subcortical triggering of a stored response through the reticulospinal tract, although the authors acknowledged that subcortical motor preparation likely also involves some cortical processing.
While there has been growing indirect evidence for subcortical storage and triggering, contrasting research has implicated cortical involvement in the StartReact effect. For example, Alibiglou and MacKinnon (2012) questioned the viability of the subcortical triggering hypothesis by investigating whether a single, suprathreshold pulse of transcranial magnetic stimulation (TMS) delivered to the primary motor cortex (M1) could influence the rapid triggering of a movement by a SAS. The premise of the methodology used by these authors was that TMS can delay voluntary RT for a brief period of time due to inhibitory processes in cortical mechanisms (e.g., Day et al., 1989). When TMS was applied prior to the onset of a startle-induced movement there was a significant delay in the early release of the movement In addition, the onset of sternocleidomastoid (SCM) activity following a SAS, used as the indicator of startle, was not affected by TMS. The authors concluded that M1 does mediate the StartReact effect and response initiation was thus delayed when a cortical silent period was induced; however, the activity of the subcortical startle reflex pathway was not influenced. Furthermore, Marinovic et al. (2014) examined corticospinal excitability in response to a loud acoustic stimulus and found in addition to early response triggering, increased intracortical facilitation occurred during movement preparation, suggestive of a cortical role in the StartReact effect. Collectively, these observations may be explained through an activation model where the SAS increases cortical activation through an ascending reticulo-thalamo-cortical pathway (Maslovat et al., 2011, Carlsen et al., 2012). In this manner the SAS acts as a faster and non-voluntary trigger for a prepared movement; however, initiation occurs through the same cortical pathways involved in voluntary movement initiation.
In the following experiments we examined the involvement of cortical areas in the StartReact effect using two complementary experimental approaches. In Experiment 1, we extended and replicated the work of Alibiglou and MacKinnon (2012) but implemented a number of methodological changes to provide additional information pertaining to the involvement of cortical areas in the StartReact effect (see Methods, Experiment 1). In Experiment 2, we examined the StartReact effect for a highly cortically represented action involving speech of a consonant-vowel (CV) syllable. If movements are stored and triggered from subcortical structures by the SAS, it would be predicted that tasks requiring cortical involvement during movement initiation would not be subject to a StartReact effect.
2. METHODS – EXPERIMENT 1
In contrast to Alibiglou and Mackinnon (2012), who presented the SAS at 200 ms prior to the IS, we chose to replace the IS with the startling tone to avoid StartReact responses being triggered prior to the IS. In addition, we introduced a sham TMS condition to ensure participants were not affected by the audible and vibratory click that the TMS pulse produced, as recent evidence has shown that the sound click made by discharging the TMS coil may be sufficient to generate a response in reticular formation neurons (Fisher et al., 2012). We delivered TMS pulses at two time frames prior to EMG activation, 70 ms and 50 ms. We chose a time frame closer to the EMG onset than Alibiglou and MacKinnon as the closer the stimulation occurs relative to the overt response, the longer the RT delay (Day et al., 1989, Romaiguère et al., 1997, Hashimoto et al., 2004). We predicted the TMS pulse at 50 ms prior to the response would produce a longer RT delay as compared to the 70 ms pulse, providing additional evidence that the cortical silent period is mediating the RT delay and thus cortical involvement in the StartReact effect.
2.1 Participants
Eighteen participants with no obvious upper body abnormalities or sensory or motor dysfunctions volunteered to participate in this study. All participants gave written informed consent, and the study was conducted in accordance with the ethical guidelines set by the University of British Columbia. Participants were right handed based on a laterality quotient greater than .60 on the Edinburgh Handedness Inventory (Oldfield, 1971). However, only data from thirteen participants (6 male, 7 female; age 22±3 years) were employed in the final analysis. Five participants did not show activation in the SCM muscle during any startle trials (which is thought to be the most reliable indicator of a startle response), and thus were excluded from the analysis (see Carlsen et al., 2011 for more detail regarding the exclusion criteria for participants).
2.2 Experimental Task and Acoustic Stimuli
Participants were required to respond as quickly as possible to each presentation of an auditory stimulus by initiating a ballistic right wrist extension movement. The participants sat in a height-adjustable chair facing a table, with their right arm secured in a semi-prone position with the palm facing inward to a custom-made aluminum wrist manipulandum that moved in the transverse plane with an axis of rotation at the wrist joint. The arm portion of the manipulandum was oriented at an angle of 15º outward from the body midline, as this has been found to be a more comfortable position than an orientation parallel to the body midline. The wrist starting position was neutral (neither flexion nor extension) and was indicated by tactile feedback (using a magnet as a noncontact detent). In all experimental trials, the imperative stimulus (IS) followed the warning tone (100 ms, 1000 Hz, 80 dB) by a variable foreperiod between 1500 and 2500 ms, and could either consist of a control stimulus (80 +/−2 dB, 100 ms, 1000 Hz) or startling stimulus (124 +/−2 dB, 40 ms, 1000 Hz, <1 ms rise time). All auditory signals were generated by a customized computer program and were amplified and presented via a loudspeaker placed directly behind the head of the participant. The acoustic stimulus intensities were measured using a sound level meter (Cirrus Research model CR:252B) at a distance of 30 cm from the loudspeaker (approximately the distance to the ears of the participant).
The experimental task performed was an active right wrist extension as fast as possible to a target region located at 20º of angular displacement from the starting position. Instructions were to move “as fast and as accurately as possible” from the starting position and stop on the target and a monetary bonus of 10 cents (Canadian) was offered per trial for beating a prescribed minimum RT.
2.3 Instrumentation
Electromyographic (EMG) data were collected from the right wrist prime movers: the extensor carpi radialis longus (ECR) and the flexor carpi radialis (FCR), as well as bilaterally from the startle indicator SCM. The recording sites were prepared and cleansed to decrease electrical impedance, and bipolar preamplified Ag/AgCl surface EMG electrodes were attached over the middle of the muscle bellies parallel to muscle fibers. These electrodes were connected via shielded cabling to an external amplifier system (model 544, Therapeutics Unlimited). A grounding electrode was placed on the participants’ left radial styloid process. Wrist angular displacement data were collected using a potentiometer attached to the pivot point of the manipulandum. All raw data were digitally sampled for 2 s at 1 kHz (PCI-6023E, National Instruments) using a personal computer running a customized program written with LabVIEW® software (National Instruments, Texas, USA). Data collection was automatically initiated 500 ms before the IS.
2.4 Transcranial Magnetic Stimulation
Single-pulse TMS was delivered using a Magstim® 200 stimulator (Magstim Company, Dyfed, UK, maximum output intensity 2.0 T) through a figure-eight coil (each external diameter 7.0 cm). The coil was handheld and oriented tangentially to the scalp and perpendicular to the central sulcus so that the induced current flow was in a posterior to anterior direction (Werhahn et al., 1994). The optimal ‘hot spot’ for eliciting MEPs in the right ECR (agonist muscle for the response) was determined by stimulating over the contralateral M1. Because voluntary muscle contraction has been found to enhance corticospinal excitability, the participants were required to be relaxed with no muscular contraction and to fixate their eyes directly ahead (Hess et al., 1987, Thompson et al., 1991) while the ‘hot spot’ was found. Once a ‘hot spot’ was identified, the site was stimulated four times in order to confirm whether it was an optimal ‘hot spot’ where maximum MEPs could be evoked. This site was marked to ensure consistent coil placement. While at rest, motor threshold in the ECR was determined for each participant to the nearest 1% of maximum stimulator output where a minimum 50 μV MEP was evoked in 5 of 10 consecutive trials (Rossini et al., 1994), which was found to be an average of 43 ± 4% of maximum stimulator output. To set the experimental intensity of the TMS, participants maintained an isometric contraction in the ECR of 10% maximum voluntary contraction. The intensity of the magnetic stimulus was adjusted to produce a silent period (SP), defined as the time from the start of the MEP to the return of background EMG, in the ongoing voluntary EMG of approximately 150 ms (Ridding and Taylor, 2001, Smith et al., 2007). The mean TMS intensity used across participants was 67 ± 2% of maximum stimulator output (corresponding to a between-participant average of 157% of resting motor threshold).
2.5 Experimental Procedure
After all of the participant’s questions were answered and written informed consent was obtained, participants were seated 1.0 m from a 21-inch LCD monitor which displayed the RT from the previous trial, any monetary bonus gained, and the cumulative total of bonuses. Next, the participant’s right arm was strapped into the manipulandum and EMG sensors attached. Once TMS stimulation intensities were found, participants were first exposed to one startle trial while at rest (to obtain an indication of baseline startle reflexive activity), followed by one trial each of isometric maximum voluntary contraction for wrist extension and flexion (each lasting 2 s). To ensure consistent and fast RTs, participants then practiced the task for 50 trials. The criterion for consistent RTs was a stable mean RT and a SD < 15 ms for the previous 10 trials. More trials were given if the criteria were not met. Once RTs were consistent, participants performed a block of 25 trials consisting of 20 control tone (82 dB) trials and 5 startle (124 dB) trials. Mean PMT, defined as the time from the onset of the IS to the onset of ECR EMG activity, was calculated separately for control trials and startle trials in this block after each trial. The timing of TMS delivery was based on individual participants’ mean control and startle PMTs (see below).
2.6 Experimental Design
The experiment included seven conditions, including control and SAS stimuli as well as TMS and sham TMS delivery. Each condition commenced with the warning tone to ready participants for the upcoming trial. During the TMS Sham condition, the TMS coil was held perpendicular to the scalp such that the click of the TMS was heard and vibration felt but no neural tissue was stimulated. The seven conditions were:
Control Trials (40 trials): The IS for these trials was a control (82 dB) tone and there was no TMS stimulation.
Control + TMS (50 trials): TMS was delivered 50 (25 trials) or 70 (25 trials) ms before each participant’s mean control PMT.
Control + Sham TMS (30 trials): The TMS coil was held perpendicular to the scalp at the same site of stimulation and was delivered 50 (15 trials) or 70 (15 trials) ms before each participant’s mean control PMT.
SAS (8 trials): The IS for these trials was a SAS, and there was no TMS stimulation.
SAS + TMS (10 trials): The IS for these trials was a SAS, and TMS was delivered 50 (5 trials) or 70 (5 trials) ms before each participant’s mean control PMT.
SAS + Sham TMS (6 trials): The IS for these trials was a SAS, and the TMS coil was held perpendicular to the scalp. TMS was delivered 50 (3 trials) or 70 (3 trials) ms before each participant’s mean control PMT.
Catch (16 trials): Trials with no IS or TMS stimulation, two in each block.
160 movement trials were performed in total, separated across eight experimental blocks of 20 trials, which involved five blocks of TMS and three blocks of Sham TMS randomly determined. Each block contained five control trials, five control + TMS (TMS present or Sham) trials at each timing, one SAS trial, one SAS + TMS (TMS present or Sham) trial at each timing, and two catch trials. The trial order was pseudo-randomized such that SAS conditions could not be the first in a block or occur twice in a row. The approximate trial-to-trial interval was 10 s, although this varied because of the random foreperiod. The mean timing of TMS −50 was 98 ± 28 ms following the “go” signal in control trials and 41 ± 19 ms in startle trials, while the mean timing of TMS −70 was 78 ± 28 ms following the “go” signal in control trials and 21 ± 19 ms in startle trials.
2.7 Data Reduction
Analysis was restricted to the testing trials only (practice trials were not analyzed). A total of 100 of the 2080 trials were discarded (4.8%). Reasons for discarding trials included displacement RTs less than 80 ms (i.e., anticipation, 9 trials) or in excess of 400 ms (40 trials), incorrect movements (30 trials), and startle trials in which no detectable startle response (SCM activity) was observed (21 trials) (see Carlsen et al., 2011).
EMG data were analyzed off-line via a custom LabVIEW® program. EMG signals were rectified and then filtered using a first-order dual-pass elliptical filter with a low-pass frequency setting of 25 Hz. Movement onset was determined as the first change in angular displacement by 0.2 degrees following the go signal. Surface EMG burst onsets were defined as the point at which the EMG first began a sustained rise above baseline levels. The location of this point was determined by first displaying the EMG pattern on a computer monitor with a superimposed line indicating the point at which rectified, filtered EMG activity first increased on the EMG trace by more than 2 standard deviations based on the 100 ms immediately preceding the IS. This method allowed for the correction of potential errors caused by the algorithm. PMT was defined as EMG onset in the ECR muscle. PMT was the main measure of RT for this study because it represents an estimate of the total central processing time. EMG offsets were marked in a similar fashion, using mean EMG activity following the end of the movement as a baseline level to account for any residual time between bursts. These were also verified and manually adjusted, with the activity between EMG onset and EMG offset defined as a distinct activation burst.
Although the effect of TMS on startle trial RTs was the primary purpose of this study, we were also interested in how the TMS and SAS would affect the size of the first agonist (AG1) and startle indicator (SCM) EMG bursts (an analysis not performed by Alibiglou and MacKinnon, 2012). We predicted the SAS would increase the size of the AG1 burst due to increased activation associated with the loud acoustic stimulus, as has been shown in previous startle-based studies (Carlsen et al., 2004a, Maslovat et al., 2008, Maslovat et al., 2009). We also expected the TMS to facilitate the AG1 burst in both startle and control trials, as suprathreshold TMS of M1 has been shown to increase burst amplitude of centrally generated motor commands (Hashimoto et al., 2004). Conversely, we would not predict an effect of the TMS on SCM burst amplitude as this activation occurs via the startle reflex pathways, involving subcortical structures and reticulospinal pathways (Yeomans and Frankland, 1995, Rothwell et al., 2002), and thus would be unaffected by M1 disruption. Integrated EMG values for the initial burst of AG1 and SCM activation were computed by numerically integrating the rectified EMG for each participant for the first 30 ms (Q30) following burst onset.
2.8 Statistical Analysis
All dependent measures were analyzed with separate within-subjects analyses of variance (ANOVAs) for the TMS present condition and Sham TMS condition because the “No TMS” timing acts as a control for both conditions. PMT and integrated AG1 EMG (Q30) values were analyzed independently using a two (acoustic stimulus; control, startle) by three (TMS timing; No TMS, PMT −50 ms, PMT −70 ms) within-subjects ANOVA. SCM onsets, durations and Q30 values were analyzed using one-way (TMS timing; No TMS, PMT −50 ms, PMT −70 ms) within-subjects ANOVAs. Greenhouse-Geisser corrected degrees of freedom were used to correct for violations of the assumption of sphericity. Differences with a probability of < 0.05 were considered significant. Partial eta squared (ηp2) values are reported as a measure of effect size. Tukey’s honestly significant difference (HSD) post hoc tests were administered to determine the locus of the differences.
3. RESULTS – EXPERIMENT 1
A summary of the results for all dependent measures, including means and standard deviations, are provided in Table 1. The main results of the present study were that TMS delayed PMT and modulated the size of the first agonist burst in both control and startle trials, while TMS did not modulate the startle response (SCM activity).
Table 1.
Experiment 1 results for each TMS condition (TMS and Sham TMS), stimulus type and TMS timing, showing means and standard deviations (in brackets). PMT = premotor reaction time, AG1 = initial agonist burst (ECR), SCM = sternocleidomastoid (startle indicator).
| Variable | TMS Condition
|
|||||
|---|---|---|---|---|---|---|
| Control | Startle | |||||
|
| ||||||
| No TMS | TMS −50 | TMS −70 | No TMS | TMS −50 | TMS −70 | |
| PMT (ms) | 140.1 (26.8) | 212.6 (46.0) | 192.3 (41.4) | 84.6 (11.0) | 142.5 (37.6) | 125.4 (29.3) |
| AG1 Q30 (mV*ms) | 5.8 (2.8) | 7.6 (2.8) | 8.2 (3.2) | 6.5 (3.3) | 9.0 (3.3) | 9.0 (4.0) |
|
| ||||||
| SCM Onset (ms) | 68.3 (17.7) | 71.8 (19.0) | 64.3 (16.1) | |||
| SCM Duration (ms) | 48.3 (15.7) | 44.5 (16.2) | 43.1 (17.7) | |||
| SCM Q30 (mV*ms) | 0.44 (0.07) | 0.42 (0.04) | 0.42 (0.06) | |||
| Variable | Sham TMS Condition
|
|||||
|---|---|---|---|---|---|---|
| Control | Startle | |||||
|
| ||||||
| No TMS | TMS −50 | TMS −70 | No TMS | TMS −50 | TMS −70 | |
| PMT (ms) | 135.4 (23.4) | 140.8 (22.3) | 133.2 (23.8) | 84.1 (15.0) | 89.9 (12.1) | 84.3 (16.7) |
| AG1 Q30 (mV*ms) | 6.0 (2.5) | 7.1 (2.3) | 7.2 (2.3) | 7.9 (3.6) | 8.4 (4.1) | 8.0 (3.4) |
|
| ||||||
| SCM Onset (ms) | 64.5 (17.3) | 73.6 (18.9) | 71.0 (17.7) | |||
| SCM Duration (ms) | 45.3 (17.9) | 42.1 (13.3) | 43.4 (14.7) | |||
| SCM Q30 (mV*ms) | 0.42 (0.04) | 0.45 (0.14) | 0.42 (0.05) | |||
3.1 Premotor Reaction Time
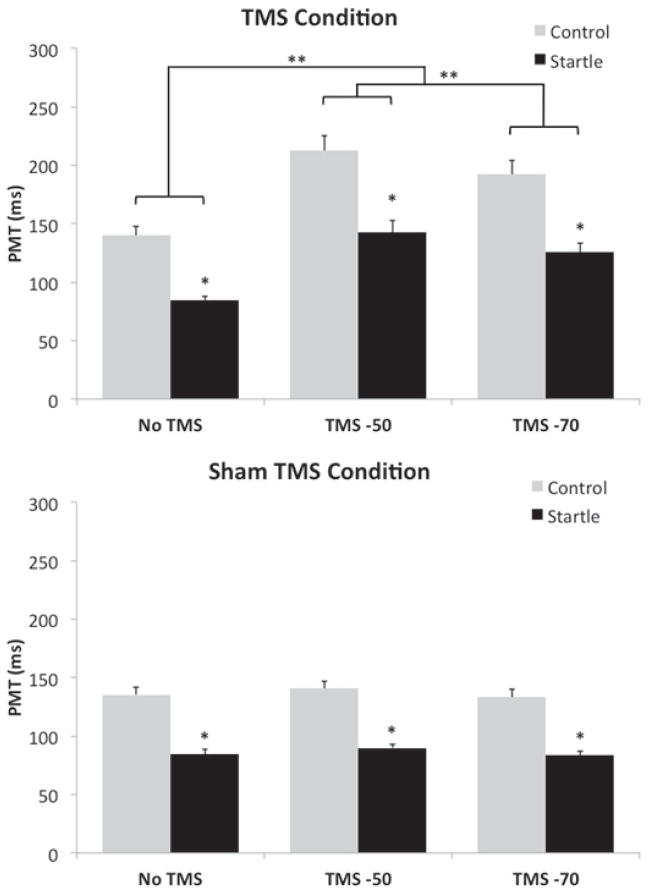
As predicted, PMTs for startle trials were faster than control trials for both the Sham (F(1,12) = 107.64, p < .001, ε = 1, ηp2 = .90) and TMS present (F(1,12) = 97.07, p < .001, ε = 1, ηp2 = .89) conditions. However, PMTs for both control and startle trials were delayed following suprathreshold TMS (F(2,24) = 48.77, p < .001, ε = 1, ηp2 = .80). Post-hoc analyses revealed that TMS delivered 50 ms and 70 ms prior to mean PMT significantly delayed PMT compared to trials without TMS, while TMS delivered 50 ms prior to mean EMG onset produced a significantly longer delay than TMS delivered 70 ms prior (Figure 1, top panel). This TMS-induced delay in PMT was similar for both startle and control trials, as shown by the lack of significant interaction effect between stimulus type and TMS timing (F(2,24) = 3.03, p = .067, ε = .53, ηp2 = .20). Conversely, the Sham TMS had no effect on control or startle PMTs as there was no main effect for TMS timing, nor was there a stimulus type by TMS timing interaction effect (Figure 1, bottom panel)
Figure 1.
Mean PMT values (SEM) for control and startle trials separated by TMS timing and TMS condition (top panel = TMS present, bottom panel = Sham TMS). Note the increase in PMT following TMS for both control and startle trials in the TMS present condition (top panel). * denotes an effect of the IS, ** denotes an effect of TMS.
3.2 Initial Agonist Burst
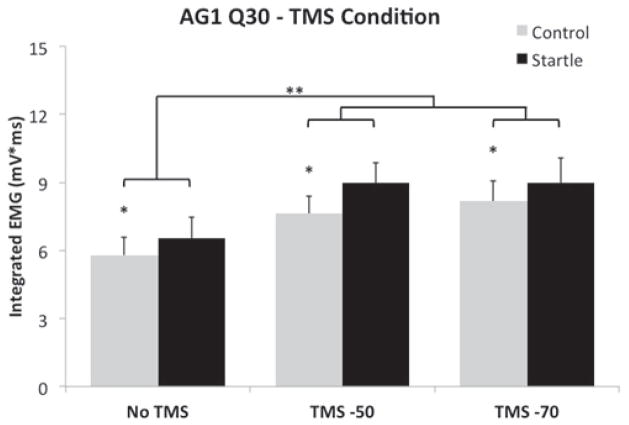
Mean AG1 integrated EMG (Q30) in the TMS condition was increased on startle trials, as compared to control trials (F(1,12) = 22.18, p = .005, ε = .99, ηp2 = .65). Independent of this modulation, there was also a main effect of TMS timing (F(2,24) = 11.47, p < .001, ε = .99, ηp2 = .49), due to increased AG1 Q30 when TMS was delivered 50 ms and 70 ms prior to mean PMT as compared to trials without TMS (Figure 2). There was no difference between TMS delivered at either −50 ms and TMS delivered at −70 ms. For the Sham TMS condition, the size of the first agonist burst was facilitated during startle trials, as compared to control trials (F(1,12) = 4.88, p = .047, ε = .53, ηp2 = .29); however, as expected, there was no effect of sham TMS timing on AG1 Q30 values (p = .187).
Figure 2.
Mean AG1 integrated EMG (Q30) values (SEM) for control and startle trials separated by TMS timing in the TMS present condition. Note the increase in integrated EMG in the TMS −50 and TMS −70 conditions for both control and startle trials. * denotes an effect of the IS, ** denotes an effect of TMS.
3.3 Activation of SCM during Startle Trials
The analyses of SCM EMG onset and duration did not reveal any significant differences due to TMS timing for either TMS present (onset, p = .198; duration, p = .258) or Sham TMS (onset, p = .075; duration, p = .608) conditions following startle trials. In addition, the analyses of SCM integrated EMG (Q30) did not reveal any differences due to TMS timing for either TMS present (p = .129) or Sham TMS (p = .543) conditions following startle trials. This indicates that TMS or Sham TMS did not influence SCM onset, duration, or the amplitude of SCM following the presentation of a SAS.
4. METHODS – EXPERIMENT 2
Speech execution has long been argued to be mediated by cortical connections (e.g., Jurgens, 2002, Simonyan and Horwitz, 2011, Tourville and Guenther, 2011), with fMRI studies confirming activation during speech production in such areas as the anterior cingulate gyrus, motor cortex, and supplementary as well as pre-supplementary motor areas (e.g., Riecker et al., 2005, Bohland and Guenther, 2006, Brendel et al., 2010, see Price, 2010 for a review). Additionally, TMS applied over the cortex delays the RT of vocalization (Terao et al., (2001) and the production of object naming (Schuhmann et al., 2009). There exists detailed evidence to suggest that the preparation of intended speech involves a wide range of cortical and subcortical networks and the initiation and execution of prepared speech depends on exerting cortical projections to subcortical areas via a descending cortico-subcortical pathway (Iwata et al., 1996, Jurgens, 2002, Simonyan and Horwitz, 2011). We believe the use of speech provides a novel alternative to cortically-dependent finger movements (i.e., Carlsen et al., 2009, Honeycutt et al., 2013), as speech requires a high degree of cortical and cognitive involvement (Grimme et al., 2011) and has been shown to be controlled in a different manner than non-speech facial movements (Tremblay et al., 2003). In the second experiment, we had participants vocalize the syllable “[ba]” in response to an auditory and visual stimulus, and on certain trials we delivered an unexpected SAS along with this stimulus. If there is cortical involvement in the StartReact effect, we would predict that the syllable “[ba]” would be subject to the StartReact effect and triggered early by the SAS.
4.1 Participants
Data were collected and analyzed from nine participants (different to those in Experiment 1; 3 male and 6 female; M = 23 years, SD = 4.2 years) who showed a consistent startle reflex in the SCM muscle on our baseline startle trial (a pretest startle trial prior to the testing session, no speech required) and more than 50% of the startle testing trials. Participants were all native speakers of North American English. Prior to testing, participants signed an informed consent form and were naïve to the hypothesis under investigation. The experiment was conducted following the ethical guidelines established by the University of British Columbia.
4.2 Apparatus, task, and procedures
Participants sat on an upright chair facing a computer monitor (Acer, X223W, 22″, 60 Hz refresh rate) at a distance of approximately 1.5 meters. Participants were instructed to look straight ahead at the monitor and respond to an acoustic stimulus by vocalizing the target syllable “[ba]” as quickly as possible. A visual display of the syllable “[ba]” was presented on the monitor concurrently with the acoustic stimulus. Throughout the testing session, participants were asked to start with their mouths closed in a relaxed posture.
All testing trials began with a warning tone (100 ms, 1000 Hz, 80 dB) played directly from the computer’s sound card. The acoustic imperative stimulus and visual “[ba]” followed the warning tone by a random foreperiod of between 1500 and 2500 ms. This auditory signal was either a control stimulus (80 ± 2 dB, 100ms, 1,000 Hz) or startling stimulus (124 ± 2 dB, 40ms, 1,000 Hz, < 1ms rise time), generated by a customized computer program. The acoustic stimuli were amplified (HiVi stereo power audio amplifier A180W) and then presented via a loudspeaker placed directly behind the head of the participant. The acoustic stimulus intensities were measured using a sound level meter (Cirrus Research model CR:252B, “A” weighted scale, impulse response mode) at a distance of 30 cm from the loudspeaker (approximately the distance to the ears of the participant).
Participants performed a single testing session of approximately 20 minutes. Prior to any RT trials, a baseline startle trial was introduced, in which an unexpected SAS was delivered to the seated participant who was waiting for testing to begin. Following this initial trial, participants performed lip movement and mouthing articulation tasks in order to become familiar with the testing procedure. Finally, participants performed a testing block consisting of twenty control trials and five startle trials. The five startle trials were presented pseudo-randomly such that the first trial was never a startle trial, nor were there two consecutive startle trials. Only results from the testing block were included in the analyses.
4.3 Recording equipment
Participants performed the tasks with three infrared light-emitting diodes placed on the center of the upper lip, the lower lip, and the bridge of the nose. 3D positions of these diodes were monitored using an OPTOTRAK (Northern Digital Inc., Waterloo, Ontario) motion analysis system (spatial resolution 0.01 mm). The data collected from the bridge of the nose were considered as a reference marker for the other two landmarks. The OPTOTRAK camera unit was placed above the computer monitor that was used to display the syllable “[ba]”. The 3D positions of the upper and lower lips were sampled at 500 Hz. Raw data from the OPTOTRAK were converted into 3D coordinates and digitally filtered using a second-order dual-pass Butterworth filter with a low-pass cutoff frequency of 10 Hz.
Bipolar surface EMG electrodes (Therapeutics Unlimited Inc., Iowa City, IA) were attached to four different locations: the skin above the upper vermilion border (labeled as “upper lip”), the skin below the lower vermilion border (labeled as “lower lip”), and the left and right SCM muscles. The EMG electrodes were placed centered between the midline and the right corner of the mouth, parallel to the muscle fibers. A ground electrode was placed on the right ulnar styloid process. A wired lapel microphone was pinned onto the collar of the participant in order to record the participant’s responses. Acoustic data were collected by the wired lapel microphone through a Preamp (USBPre Microphone Interface for Computer Audio, Sound Devices, LLC) before analyses. A customized LabVIEW® computer program controlled the stimulus presentation and the collection of EMG and acoustic data at a rate of 4 kHz (National Instruments, PC-MIO-16E-1). Data collection began 500 ms before the presentation of the stimulus and was terminated 2500 ms later.
4.4 Interpretation of EMG and lip displacement
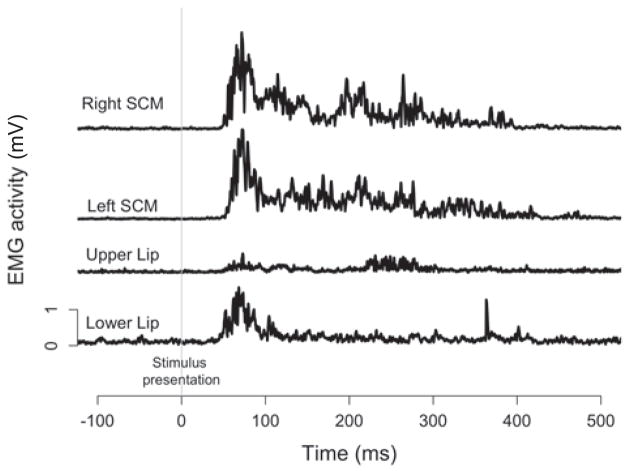
Mean rectified raw EMG traces of all baseline startle trials are displayed in Figure 3. EMG responses were observed in left and right SCM as well as the upper and lower lips at approximately the same time (upper lip M = 59 ms, SD = 9 ms; lower lip M = 54ms, SD = 13 ms, left SCM M = 60 ms, SD = 16 ms; right SCM M = 62 ms, SD = 17 ms). While it appears that all four muscles can be considered startle indicators, we chose to use SCM as our primary indicator for consistency with previous work (Carlsen et al., 2011). However, the involvement of the prime movers in the reflexive startle response did not allow for determination of voluntary EMG onset for vocalization during startle trials and thus our dependent measures included kinematic markers and acoustic burst onset (see below).
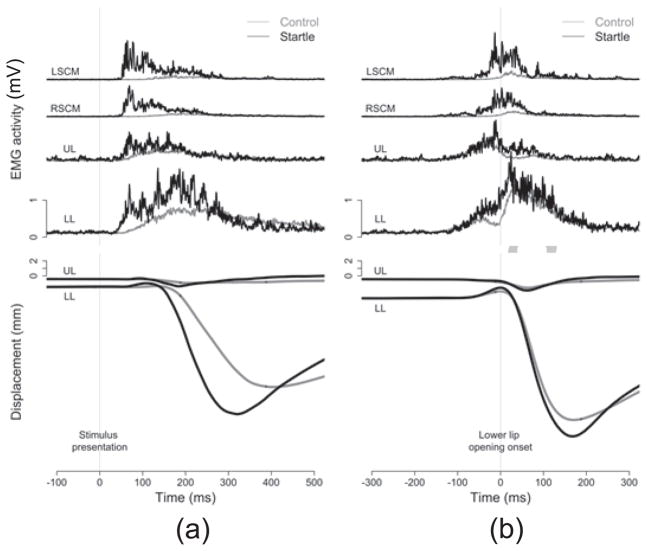
Figure 3.
Mean rectified raw EMG traces of baseline startle trial, including (from top) right SCM, left SCM, upper lip, and lower lip. EMG activity was plotted with respect to the imperative stimulus (the vertical grey line).
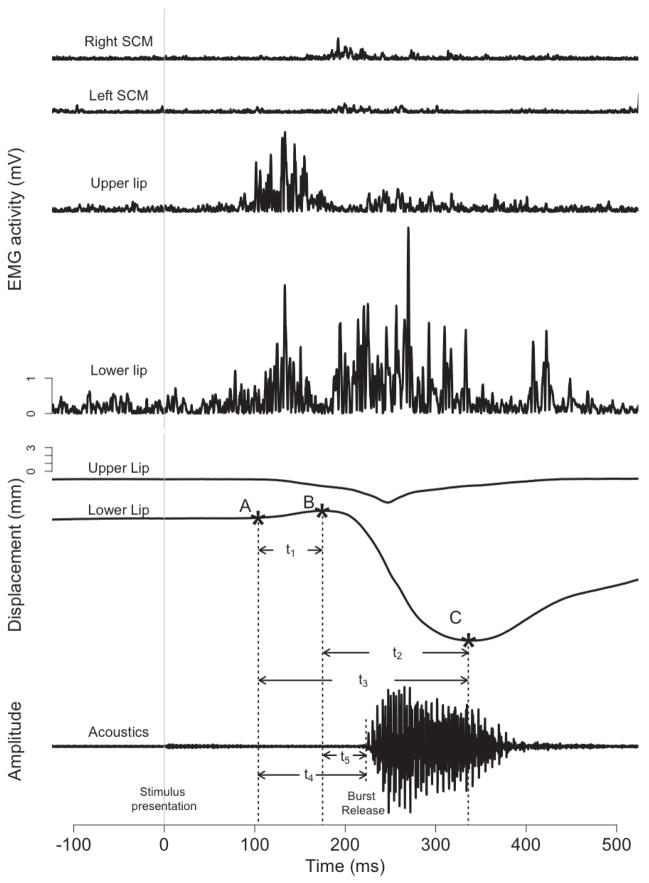
Typical profiles of EMG and lip displacement for control trials are displayed in Figure 4. In response to an imperative stimulus, the upper and lower lips first compress against each other (note the upward displacement of the lower lip from point A to B in Figure 4). This lip compression was evident in 91% of control trials and 87% of startle trials. Such compression between the lips is anticipated in order to contain the intraoral air pressure associated with the bilabial consonant [b]. As such, only the trials with lip compression were analyzed. Following lip compression, the opening phase of the vowel [a] was realized primarily through the downward movement of the lower lip (point B through C in Figure 4), with only minimal contribution from the upward movement of the upper lip. SCM muscles also showed activity during this opening phase, presumably associated with the vocal response (See Uemura et al. 2008 for similar findings).
Figure 4.
EMG activity, lip displacement and response acoustic waveforms of exemplar control trial. The EMG measures include (from top) right SCM, left SCM, upper lip, and lower lip. EMG activity was rectified and plotted with respect to the imperative stimulus (the vertical grey line). Upper and lower lip displacement and response acoustic waveforms are also plotted with respect to the imperative stimulus. Point A marks the beginning of voluntary movement; point B marks the time to the lower lip opening onset; point C marks the lowest position of the lower lip.
4.5 Data reduction, dependent measures, and statistical analyses
A total of 36 of the 225 trials (16%) were excluded from the analyses. Reasons for discarding trials included delayed lip movement onsets or voice onsets (defined as 2 SD away from the mean, 25 trials), startle trials in which no detectable SCM startle response or delayed response (>120 ms) was observed (10 trials), and trials with poor EMG data in which no obvious onsets could be identified (1 trial).
In each response, the initiation of lip compression was marked as the voluntary movement onset (point A on lower lip trace in Figure 4) which allowed us to measure the RT of the voluntary movement onset (time between the onset of imperative stimulus to the beginning of lip compression). Differences between RTs of voluntary movements in both control and startle responses were measured and analyzed using a paired Student’s t-test. After the lower lip reached its highest point (point B in Figure 4), the opening phase of the movement began, here termed the lower lip opening onset. The lower lip continued to move downward during the production of “[ba]”, until it reached its lowest position (point C in Figure 4). Thus, two kinematic events can be identified: lip compression and lip opening. The acoustic burst release time was defined as the latency from the presentation of the imperative stimulus to the onset of the acoustic burst of the bilabial consonant [b] (see acoustic signal, Figure 4). To measure the time of the initial acoustic burst release, the acoustic signals were first filtered (at a level of 10 Hz) using the PRAAT software (Boersma and Weenink, 2009). A narrow-band spectrogram (bandwidth of 43 Hz; wave-length of 30 ms) was displayed in PRAAT to mark the acoustic burst release. Syllable durations were also measured, marking the interval from the acoustic burst release to the end of periodicity of the vowel [a].
In order to examine whether the kinematic events in control responses are different from those in startle responses, we calculated the relative timing across kinematic markers A, B, and C by measuring the time between these markers, as well as the relative timing between the kinematic markers and acoustic burst onset (see Figure 4). The time between voluntary movement onset and lower lip opening onset is shown as t1 (duration of lip compression) whereas t2 marks the duration of the opening movement from the lower lip opening onset to the lower lip’s lowest position. The time frame for both of these events is t3 (time between point A and C in Figure 4), and the relative timing of each kinematic event with respect to the entire time course was calculated. To examine the relative timing between the kinematic events and acoustic signal, we calculated the time from movement onset to acoustic burst release (t4) and lower lip opening to acoustic burst release (t5). The ratios of t1/t3, t2/t3, t4/t3, t5/t3 for each trial (both Startle and Control) were calculated with the ratio data transformed by trial using the arcsine square root transformation (due to correlations between markers; McDonald, 2009). The transformed ratios were then aggregated by participant and analyzed via a paired Student’s t-test.
The peak-to-peak displacement of the lower lip was calculated as the vertical distance from the highest position (Figure 4, point B) to the lowest position (Figure 4, point C). The difference in peak-to-peak displacement between control and startle trials was analyzed using a paired Student’s t-test. Acoustic burst release times and syllable durations were both similarly analyzed using paired Student’s t-tests.
In order to confirm that the target syllable “[ba]” was successfully produced, the acoustics of the produced syllables in control and startle trials were analyzed. Acoustic formants F1 and F2 (the two lowest resonant frequencies of the vocal tract) are standard indicators of overall vocal tract shape for speech sounds, encoding information about articulator positions during vowels (Peterson and Barney, 1952, Fant, 1960) as well as about consonants and consonant-vowel transitions (Delattre et al., 1955). Frequency values for F1 and F2 were extracted throughout the entire duration of produced syllables using LPC formant tracker in PRAAT and normalized to their respective z-scores within each participant. Data were analyzed across normalized durations. For statistical comparison, formant data were submitted to smoothing spline analysis of variance (SSANOVA; see Davidson, 2006, Derrick and Schultz, 2013).
5. RESULTS – EXPERIMENT 2
5.1 Startle indicators
Mean EMG traces of every trial in each condition for the four muscles of interest (left SCM, right SCM, upper lip, and lower lip) are displayed in Figures 5a and 5b. When EMG traces are temporally aligned to the stimulus onset (Figure 5a, left panel), the muscles involved in the voluntary action of saying “[ba]” also show reflexive startle responses. These startle indicators are quite invariant and display typical reflexive patterns (as seen in Carlsen et al. 2009, Maslovat et al. 2009, 2011), followed by more diffuse EMG activity associated with the subsequent voluntary action. Figure 5a also shows that the onset latencies of the startle EMG responses in the “[ba]” trials were comparable with those in the baseline trials (Figure 3).
Figure 5.
Mean EMG traces (raw, rectified) and kinematic displacement data normalized to the imperative stimulus (a, left panel) and normalized to the lower lip opening onset (b, right panel). EMG traces are arranged in the following order (from top): left SCM (LSCM), right SCM (RSCM), upper lip (UL), and lower lip (LL). Kinematic trajectories depict mean vertical displacement of the lips, with the upper lip (UL) on top and the lower lip (LL) at bottom. Results from startle trials (black lines) were superimposed on those from control trials (grey lines). Note in panel (a), the speeding of the response relative to the imperative signal showing a StartReact effect. Note in panel (b) when normalized to the lower lip opening the movements appear near identical, confirming a similar movement was triggered by the SAS
As seen in Figure 5a, the more diffuse EMG activity for the voluntary movement was due to higher variability with regard to the onset of the voluntary action. Normalizing data to the lower lip opening onset allowed us to better examine the muscle activity that is associated with the voluntary action and to clearly compare the difference between control and startle trials. When the EMG traces are normalized to the onset of the lower lip opening movement (i.e., temporally aligned to the lower lip opening onset, as marked by the vertical grey line in Figure 5b, right panel) the EMG trace of voluntary action appears distinct (variability of time between onset of opening movement and EMG is low). As seen in Figure 5b, in control trials both upper and lower lips were engaged in the lip compression prior to the opening, whereas only the lower lip showed EMG activity during the voluntary opening movement. Similar patterns were also observed in startle trials. It is noted that stronger EMG activity in response to the SAS was observed in the lip muscles as well as in the SCM muscles. The SCM muscles showed both a startle response (activity before zero, marked by the grey line in Figure 5b) and EMG activity for the intended movement (activity after zero, marked by the vertical grey line in Figure 5b).
5.2 The StartReact effect
If there was cortical involvement in the StartReact effect, it would be predicted that a SAS would induce faster onset of movements of a prepared syllable. Our results support this prediction. Shorter RTs of voluntary movement onsets (time from stimulus presentation to point A in Figure 4) were observed for startle trials (M = 75 ms) as compared to control trials (M = 116 ms), (t(8) = 4.07, p < 0.01). The latency of the acoustic burst release was also accelerated in startle trials (M = 204 ms) compared to control trials (M = 268 ms), (t(8) = 2.97, p = 0.018). In addition to shorter latencies of the kinematic markers, the SAS triggered increased muscle activity (see Figure 5), yielding a significantly increased range of lower lip peak-to-peak displacement (M = 21.2 mm) than control trials (M = 18.5 mm), (t(8) = −3.83, p < 0.01).
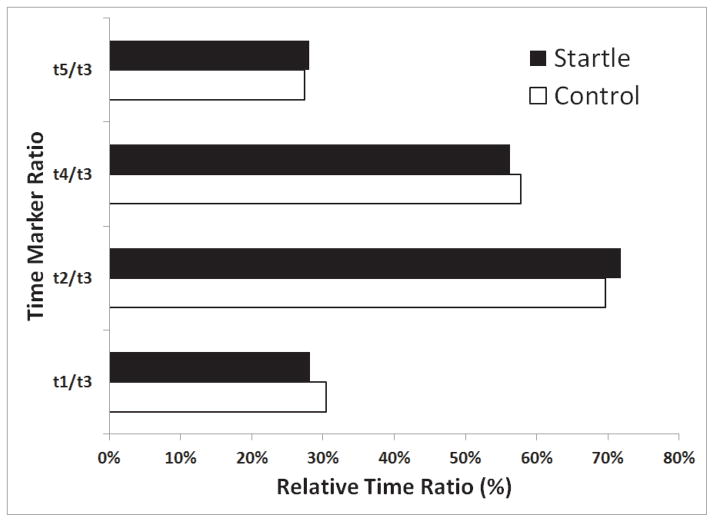
However, the relative timing relationship of the kinematic and acoustic events remained unaffected. No significant difference was found between control and startle values for the arcsine square root transformed relative time data between voluntary movement onset and lower lip opening onset (t1 in Figure 4; control M = 0.58, startle M = 0.57; p = 0.68), lower lip opening to peak displacement (t2 in Figure 4; control M = 0.99, startle M = 1.00; p = 0.68), movement onset to acoustic burst release (t4 in Figure 4; control M = 0.86, startle M = 0.85; p = 0.65) and lower lip opening to acoustic burst release (t5 in Figure 4; control M = 0.27, startle M = 0.28; p = 0.78), suggesting that the relative timing of the kinematic and acoustic markers was not altered during the startle trials. These relative time frames for control and startle trials are shown in Figure 6, with the relative time intervals representing untransformed data.
Figure 6.
Relative timing ratio (non-transformed) of various kinematic and acoustic time markers for startle and control trials. Time intervals include the time between: voluntary movement onset and lower lip opening onset (t1), lower lip opening onset to lowest lower lip position (t2), voluntary movement onset to acoustic burst release (t4) and lower lip opening to acoustic burst release (t5), each as a ratio of the time between voluntary movement onset and lower lip lowest position (t3) (see also Figure 4). Note the similarity in ratios between startle and control trials (all p > 0.5), confirming similar relative timing of the produced movement kinematics and voice onset.
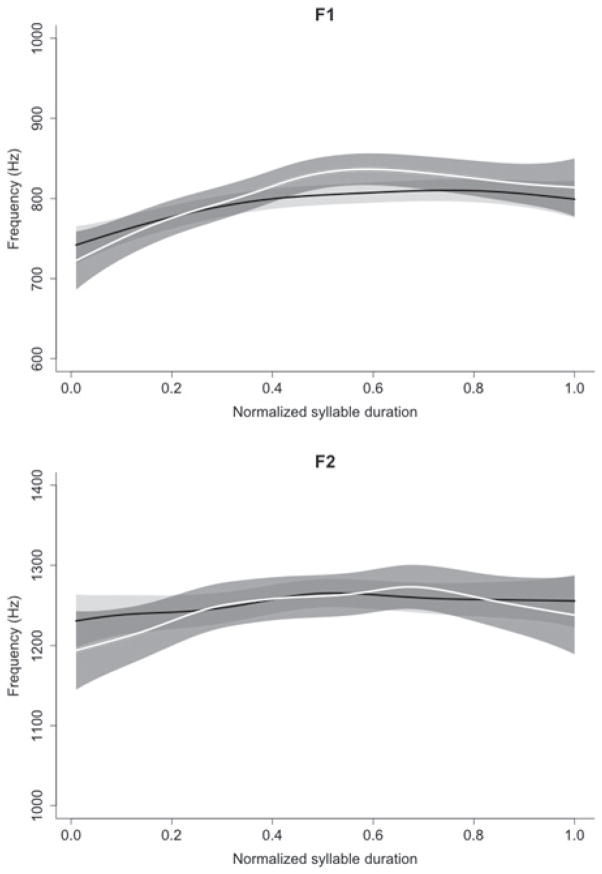
The SSANOVA results of formants are displayed in Figure 7, including the fit predicted by the SSANOVA model and shading of a 95% confidence intervals. For F1 and F2, frequency profiles between control and startle responses were within a 95% confidence interval (as indicated by overlapping confidence interval bands in Figure 7). No difference in syllable duration was observed between control (M = 176 ms) and startle trials (M = 168 ms), (t(8) = − 0.56, p = 0.59).
Figure 7.
SSANOVA comparison of formant frequencies F1 (top panel) and F2 (bottom panel) over syllable “[ba]” durations. Shading around the black (control responses) and white (startle responses) lines represents a 95% confidence interval. Note the similarity of the format frequencies, confirming a similar acoustic response in both control and startle trials.
6. DISCUSSION
It has been well established that the presentation of a SAS can trigger the release of a prepared motor response at a latency much shorter than is observed during a voluntary RT task (i.e., <70 ms have been observed). Because the magnitude of RT shortening is well beyond what would be expected by intersensory facilitation or stimulus intensity, it is believed that the SAS acts through an alternate, atypical initiation pathway. There is current disagreement, however, as to the neural structures involved in this initiation process, with some researchers suggesting the SAS causes release of the stored response through subcortical structures (Valls-Solé et al., 1999, Carlsen et al., 2004b, Nonnekes et al., 2014), while others contest the movement is triggered from the motor cortex via a subcortical pathway (Alibiglou and MacKinnon, 2012, Carlsen et al., 2012, Marinovic et al., 2014). We examined the involvement of cortical areas in the StartReact effect by using a suprathreshold TMS protocol in an arm extension task and by examining the effect of the SAS on a cortically-dependent speech response. The results of both experiments provide converging evidence for cortical (M1) involvement in the StartReact effect. The TMS-invoked cortical silent period (Experiment 1) delayed voluntary response latency in both non-startle and startle trials (Figure 1), yet the startle reflexive activity was unaffected by the TMS pulse. These results are most consistent with the explanation that the SAS results in a startle reflex via a subcortical, reticulospinal pathway but the prepared response involves cortical structures. Additionally, the SAS produced a typical StartReact effect during speech production of a CV syllable (Experiment 2), a task which relies on cortical involvement (Tourville and Guenther, 2011) and thus would be unlikely to be initiated exclusively via subcortical structures.
A number of methodological differences between Experiment 1 and that conducted by Alibiglou and Mackinnon (2012) allowed for additional confirmation of M1 involvement in the StartReact effect. Consistent with previous research involving a cortical SP (Day et al., 1989, Romaiguère et al., 1997, Hashimoto et al., 2004), we found that the closer the stimulation to the actual overt response in both control and startle trials, the longer the delay in RT. When TMS was delivered 50 ms versus 70 ms prior to the expected EMG onset, RT was slowed by approximately 20 ms in both the startle and control trials (Table 1 & Figure 1), providing added verification that the inducement of a cortical SP was responsible for the RT delay. Further support that the TMS-induced cortical SP delayed RT was provided by the Sham TMS condition, where no neural tissue was stimulated. During the Sham TMS condition the audible click and vibration emitted by the TMS coil had no impact on PMT, regardless of stimulation timing. As previous research has shown that the sound click of a TMS coil may be sufficient to generate a neural response (Fisher et al., 2012), the use of a sham TMS condition confirmed our effects were due to the stimulation of cortical inhibitory pathways rather than any unrelated side effects of the TMS discharge. Furthermore, by presenting the SAS as the “go” signal (rather than 200 ms prior), we ensured participants were only presented with a single tone on each trial, and removed the confound of the TMS pulse triggering the movement prior to the imperative signal.
In addition to TMS delaying the onset of movement, the size of the initial agonist burst in both control and startle trials was facilitated compared to No TMS or Sham TMS conditions (Figure 2). Hashimoto et al. (2004) also found a facilitation of integrated response EMG following suprathreshold TMS delivered in the premotor RT period at timings where PMT was delayed. They argued that, because peripheral electrical stimulation to the muscles decreased the size of response EMG (Day et al., 1989), suprathreshold TMS of M1 centrally disrupts motor commands. The facilitation in AG1 EMG in both control and startle trials in the present study implicates the involvement of M1 in the initiation of movements in both conditions. The TMS-induced facilitation was independent of the observed facilitation in AG1 EMG following startle compared to control trials, which has been reported in other recent studies involving the use of a SAS (Carlsen et al., 2004b, Maslovat et al., 2008, Maslovat et al., 2009). These authors attributed an increase in AG1 burst size to the increased neural activation associated with being startled.
One possible alternative explanation for the delay in movement onset and modulation of the initial agonist burst in startle trials is that the TMS could have directly influenced the subcortical (reticulospinal) pathways, which have been hypothesized to mediate the rapid release of movement by a SAS (Valls-Solé et al., 1999, Carlsen et al., 2004b). Recent evidence suggests that suprathreshold TMS may evoke responses in the brainstem. For example, Fisher et al. (2012) found that at high TMS intensities to M1, responses were frequently observed by single unit recordings in the reticular formation of anaesthetized macaques monkeys at a latency of 1–3 ms. Although this possibility exists, it seems an unlikely explanation of the present data since the startle response was unaffected by TMS. The characteristics of SCM muscle activity (onset, duration and Q30), used as an indicator as to whether or not participants were startled (Carlsen et al., 2011), were no different when TMS was present compared to No TMS and Sham TMS conditions. This result would indicate that the subcortical (reticular formation) startle pathways were not influenced by the TMS. Alibiglou and Mackinnon (2012) also found that the onset of SCM activity following a SAS was not affected by TMS, whereas movement onset was delayed. The authors concluded that the StartReact effect is mediated, in part by cortical pathways and that these pathways differ from the pathways that mediate the startle reflex. This dissociation between the pathways involved in the StartReact effect and those involved in the startle reflex are consistent with studies examining prepulse inhibition (PPI). The startle response can be significantly suppressed when the SAS is preceded by a low intensity electrical stimulus to the fingers or 82 dB acoustic tone, but this PPI does not affect the RT for the prepared response (Valls-Solé et al., 2005, Maslovat et al., 2012).
In the present study, a TMS-evoked cortical SP delayed both control and startle trials, consistent with previous suggestions that cortically-mediated pathways may contribute to the StartReact effect (Alibiglou and MacKinnon, 2012, Carlsen et al., 2012). However, startle trials were still initiated faster than control trials following the presentation of a SAS indicating that startle trials were initiated via a faster mechanism than control trials despite the cortical disruption by TMS. It is possible that an involuntary subcortical trigger caused by activation from the startle response could release cortically prepared and stored movements at fast latencies. The site for this trigger could be the giant neurons of the nucleus reticularis pontis caudalis (nRPC) in the reticular formation of the brainstem. The nRPC receives input from the cochlear nucleus and acts as a control center for the startle response (Yeomans and Frankland, 1995). Descending pathways from the nRPC have been shown to mediate the startle response (Yeomans and Frankland, 1995), while ascending pathways to M1 from the nRPC could be responsible for mediating the StartReact effect (Carlsen et al., 2012). Therefore, if a movement is triggered by a SAS via the brainstem but the M1 is in an inhibited state due to a cortical SP created by TMS, the trigger must wait for termination of the SP before the movement can be initiated. However, if the M1 is not inhibited then cortically prepared and stored movements can be involuntarily triggered at much shorter latencies than the normal voluntary trigger (control trials). The suggestion that the reticular formation is a divergence point for the startle response and StartReact effect is indirectly supported by the previously summarized research by Thevathasan et al. (2011), who found deep brain stimulation of the pedunculopontine nucleus (PPN) restored the StartReact effect in Parkinson’s disease patients but did not restore the startle reflex. Activity in the PPN region has been shown to change during movement preparation and execution and the PPN has strong connections to the supplementary motor area and cerebellum (Tsang et al., 2010). Thus, it is possible that stimulation of the PPN may restore or facilitate motor and premotor pathways involved in the StartReact effect, but have no effect on the descending pathway which involves the startle reflex. Alternatively, it is possible that motor preparation at the subcortical level depends on cortical input through the PPN.
Experiment 2 used a different approach to examine cortical involvement in the StartReact effect, by determining the effect of a SAS on a cortically-dependent task, namely speech production. Based on Experiment 1, we predicted that both lip movements and the acoustic burst would be subject to rapid release by a SAS in a similar manner to limb movements, thus providing additional evidence for cortical involvement in the StartReact effect. Our results confirmed this prediction as both the kinematics and prepared syllable were initiated significantly earlier in startle trials. In addition, the timing of the kinematic and acoustic markers (Figure 6), syllable duration, and acoustic formant profiles of SAS-induced syllables (Figure 7), matched those of the voluntary responses. As both temporal and acoustic properties of the movement kinematics and syllable remained intact, it is clear that it is not simply the initial lip movements triggered early but rather the SAS effects rapid release of the entire prepared syllable. Similar to and in support of the results of Experiment 1, data from Experiment 2 can be explained by the model which posits that the SAS causes an accelerated cortical trigger of a prepared response via the same execution pathways as are used for non-startle trials (Carlsen et al., 2012).
As a secondary finding, our data indicated that the upper and lower lip can also act as robust startle indicators, as activity in these two muscles was present at short latency on startle trials, even when a response was not required (Figure 3, see also Figure 5a). While not surprising [previous research has shown startle-related activation in lips and orofacial muscles (Brown et al., 1991, Valls-Sole et al., 2008)], reflex activation in the lips presented a confound in determining EMG onset. The startle indicator also functioned as a prime mover for the required speech response (see Siegmund et al., 2001 for a similar issue with SCM activation). This confound required us to use kinematic and acoustic markers as our indicators of response latency, rather than EMG onset. However, given the magnitude of response speeding due to the SAS (41 ms for movement onset, 64 ms for acoustic burst release time), combined with the short latency of voluntary movement on startle trials (75 ms), we are confident that this StartReact effect is similar to that observed in past studies examining the effect of SAS on limb and body movements.
While the results of both experiments provide strong support in favor of cortical involvement in the StartReact effect, it is worthwhile to examine how these data can be understood in conjunction with previous data supporting subcortical triggering of a prepared movement. While both Carlsen et al. (2009) and Honeycutt et al. (2013) found a lack of StartReact effect in a finger abduction task, it is possible that the choice of intrinsic hand muscles may have confounded the data recorded from startle trials, as examination of the reflexive startle activation in the finger abduction muscles has been found to be disproportionately long, as compared to other startle indicators (Brown et al., 1991, see Table 1). In fact, the difference in median latency of startle reflex in the triceps (71 ms) as compared to the first dorsal interosseous (FDI; 99 ms) was approximately the same as that observed by Carlsen et al. for RT values on startle trials (85 ms for triceps versus 106 ms for FDI). Thus it is possible that the RT of 106 ms for the FDI may represent a valid StartReact effect that produces longer RTs than typically seen in other muscles. The work by Nonnekes et al. (2014) showing an intact StartReact effect in patients with degenerated corticospinal tracts does indicate reticulospinal involvement in response triggering. However, it is difficult to determine the degree of neuroplasticity in the reticulospinal tract of these patients in order to bypass the dysfunctional corticospinal system.
Thus, while we believe our data provide compelling support for cortical involvement in motor preparation and the StartReact effect, it does not preclude the involvement of subcortical structures and pathways. In line with the conclusions of previous authors, it is possible that both systems are involved in the rapid triggering of prepared movements in response to a SAS (Marinovic et al., 2014, Nonnekes et al., 2014) and further research is necessary to determine the specific pathways involved in the StartReact effect. This knowledge may have important ramifications as use of a SAS is becoming more popular as a methodology used to investigate various aspects of movement control in special populations such as Parkinson’s disease (Carlsen et al., 2013, Fernandez-Del-Olmo et al., 2013) and stroke patients (Honeycutt and Perreault, 2012), with conclusions drawn about whether specific neural circuitry is intact or damaged.
Highlights.
Subjects performed a simple reaction time (RT) task in response to an auditory cue
Transcranial magnetic stimulation (TMS) was applied on control and startle trials
TMS induced a cortical silent period and delayed both control and startle RTs
For a cortically dependent speech RT task, startling stimulus also sped response
Results indicate cortical involvement in response triggering by startling stimulus
Acknowledgments
Acknowledgements for this study go to separate Natural Sciences and Engineering Research Council of Canada grants awarded to Ian M. Franks and to Romeo Chua, and to US National Institutes of Health (NIH) Grant DC-02717 to Haskins Laboratories.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alibiglou L, MacKinnon CD. The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over motor cortex. J Physiol. 2012;590:919–936. doi: 10.1113/jphysiol.2011.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat: Doing Phonetics by Computer. 2009. (version 5.1.15) [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Brendel B, Hertrich I, Erb M, Lindner A, Riecker A, Grodd W, Ackermann H. The contribution of mesiofrontal cortex to the preparation and execution of repetitive syllable productions: an fMRI study. Neuroimage. 2010;50:1219–1230. doi: 10.1016/j.neuroimage.2010.01.039. [DOI] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain. 1991;114:1891–1902. doi: 10.1093/brain/114.4.1891. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Almeida QJ, Franks IM. Using a startling acoustic stimulus to investigate underlying mechanisms of bradykinesia in Parkinson’s disease. Neuropsychologia. 2013;51:392–399. doi: 10.1016/j.neuropsychologia.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp Brain Res. 2004a;159:301–309. doi: 10.1007/s00221-004-1924-z. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. Journal of Motor Behavior. 2004b;36:253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Differential effects of startle on reaction time for finger and arm movements. J Neurophysiol. 2009;101:306–314. doi: 10.1152/jn.00878.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Franks IM. Preparation for voluntary movement in healthy and clincial populations: Evidence from startle. Clin Neurophysiol. 2012;123:21–33. doi: 10.1016/j.clinph.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev. 2011;35:366–376. doi: 10.1016/j.neubiorev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res. 2006;173:25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- Davidson L. Comparing tongue shapes from ultrasound imaging using smmothing spline analysis of variance. J Acoust Soc Am. 2006;120:407–415. doi: 10.1121/1.2205133. [DOI] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Maertens de Noordhout A, Nakashima K, Shannon K, Marsden CD. Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain. Brain. 1989;112:649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- Delattre PC, Liberman AM, Cooper FS. Acoustic loci and transitional cues for consonants. J Acoust Soc Am. 1955:27. [Google Scholar]
- Derrick D, Schultz B. Acoustic correlates of flaps in North American English. Proceedings of Meetings on Acoustics. 2013;19:060260. [Google Scholar]
- Fant G. Acoustic theory of speech production. The Hague, Netherlands: Mouton & Co; 1960. [Google Scholar]
- Fernandez-Del-Olmo M, Bello O, Lopez-Alonso V, Marquez G, Sanchez JA, Morenilla L, Valls-Solé J. The effects of startle and non-startle auditory stimuli on wrist flexion movement in Parkinson’s disease. Neurosci Lett. 2013;548:56–60. doi: 10.1016/j.neulet.2013.05.069. [DOI] [PubMed] [Google Scholar]
- Fisher KM, Zaaimi B, Baker SN. Reticular formation responses to magnetic brain stimulation of primary motor cortex. J Physiol. 2012;590:4045–4060. doi: 10.1113/jphysiol.2011.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimme B, Fuchs S, Perrier P, Schoner G. Limb versus speech motor control: a conceptual review. Motor Control. 2011;15:5–33. doi: 10.1123/mcj.15.1.5. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Inaba D, Matsumura M, Naito E. Two different effects of transcranial magnetic stimulation to the human motor cortex during the pre-movement period. Neurosci Res. 2004;50:427–436. doi: 10.1016/j.neures.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Responses in small hand muscles from magnetic stimulation of the human brain. J Physiol. 1987;388:397–419. doi: 10.1113/jphysiol.1987.sp016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Kharouta M, Perreault EJ. Evidence for reticulospinal contributions to coordinated finger movements in humans. J Neurophysiol. 2013;110:1476–1483. doi: 10.1152/jn.00866.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Perreault EJ. Planning of ballistic movement following stroke: insights from the startle reflex. PLoS ONE. 2012;7:e43097. doi: 10.1371/journal.pone.0043097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K, Yagi J, Tsuboi Y, Koshikawa N, Sumino R, Cools AR. Anatomical connections of the ventral but not the dorsal part of the striatum with the parvicellular reticulation formation: Implications for the anatomical substrate of oral movements. Neuroscience Research Communications. 1996;18:71–78. [Google Scholar]
- Jurgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968a;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968b;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Tresilian JR, de Rugy A, Sidhu S, Riek S. Corticospinal modulation induced by sounds depends on action preparedness. J Physiol. 2014;592:153–169. doi: 10.1113/jphysiol.2013.254581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslovat D, Carlsen AN, Chua R, Franks IM. Response preparation changes during practice of an asynchronous bimanual movement. Exp Brain Res. 2009;195:383–392. doi: 10.1007/s00221-009-1801-x. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Carlsen AN, Ishimoto R, Chua R, Franks IM. Response preparation changes following practice of an asymmetrical bimanual movement. Exp Brain Res. 2008;190:239–249. doi: 10.1007/s00221-008-1467-9. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Hodges NJ, Chua R, Franks IM. Motor preparation and the effects of practice: evidence from startle. Behav Neurosci. 2011;125:226–240. doi: 10.1037/a0022567. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Kennedy PM, Forgaard CJ, Chua R, Franks IM. The effects of prepulse inhibition timing on the startle reflex and reaction time. Neurosci Lett. 2012;513:243–247. doi: 10.1016/j.neulet.2012.02.052. [DOI] [PubMed] [Google Scholar]
- McDonald JH. Handbook of biological statistics. Baltimore, MD: Sparky House Publishing; 2009. [Google Scholar]
- Nonnekes J, Oude Nijhuis LB, de Niet M, de Bot ST, Pasman JW, van de Warrenburg BP, Bloem BR, Weerdesteyn V, Geurts AC. StartReact restores reaction time in HSP: evidence for subcortical release of a motor program. J Neurosci. 2014;34:275–281. doi: 10.1523/JNEUROSCI.2948-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peterson GE, Barney HL. Control methods used in a study of the vowels. J Acoust Soc Am. 1952;24:175–184. [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL. Mechanisms of motor-evoked potential facilitation following prolonged dual peripheral and central stimulation in humans. J Physiol. 2001;537:623–631. doi: 10.1111/j.1469-7793.2001.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64:700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Romaiguère P, Possamaï CA, Hasbroucq T. Motor cortex involvement during choice reaction time: A transcranial magnetic stimulation study in man. Brain Res. 1997;755:181–192. doi: 10.1016/s0006-8993(97)00095-4. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, MacKinnon CD, Valls-Solé J. Role of brainstem-spinal projections in voluntary movement. Mov Disord. 2002;17:S27–S29. doi: 10.1002/mds.10054. [DOI] [PubMed] [Google Scholar]
- Schuhmann T, Schiller NO, Goebel R, Sack AT. The temporal characteristics of functional activation in Broca’s area during overt picture naming. Cortex. 2009;45:1111–1116. doi: 10.1016/j.cortex.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Inglis JT, Sanderson DJ. Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol-London. 2001;535:289–300. doi: 10.1111/j.1469-7793.2001.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Horwitz B. Laryngeal motor cortex and control of speech in humans. Neuroscientist. 2011;17:197–208. doi: 10.1177/1073858410386727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Mennemeier M, Bartel T, Chelette KC, Kimbrell T, Triggs W, Dornhoffer JL. Repetitive transcranial magnetic stimulation for tinnitus: a pilot study. Laryngoscope. 2007;117:529–534. doi: 10.1097/MLG.0b013e31802f4154. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Enomoto H, Furubayashi T, Shiio Y, Machii K, Hanajima R, Nishikawa M, Iwata NK, Saito Y, Kanazawa I. Hemispheric lateralization in the cortical motor preparation for human vocalization. J Neurosci. 2001;21:1600–1609. doi: 10.1523/JNEUROSCI.21-05-01600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Bogdanovic M, Coyne TJ, Silburn PA, Aziz TZ, Brown P. A block to pre-prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain. 2011;134:2085–2095. doi: 10.1093/brain/awr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PD, Day BL, Rothwell JC, Dressler D, Maertens de Noordhout A, Marsden CD. Further observations on the facilitation of muscle responses to cortical stimulation by voluntary contraction. Electroencephalogr Clin Neurophysiol. 1991;81:397–402. doi: 10.1016/0168-5597(91)90029-w. [DOI] [PubMed] [Google Scholar]
- Tourville JA, Guenther FH. The DIVA model: A neural theory of speech acquisition and production. Lang Cogn Process. 2011;26:952–981. doi: 10.1080/01690960903498424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Shiller DM, Ostry DJ. Somatosensory basis of speech production. Nature. 2003;423:866–869. doi: 10.1038/nature01710. [DOI] [PubMed] [Google Scholar]
- Tsang EW, Hamani C, Moro E, Mazzella F, Poon YY, Lozano AM, Chen R. Involvement of the human pedunculopontine nucleus region in voluntary movements. Neurology. 2010;75:950–959. doi: 10.1212/WNL.0b013e3181f25b35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura N, Tanaka M, Kawazoe T. Study on motor learning of sternocleidomastoid muscles during ballistic voluntary opening. Journal of the Japan Prosthodontic Society. 2008;52:494–500. doi: 10.2186/jjps.52.494. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Kofler M, Kumru H, Castellote JM, Sanegre MT. Startle-induced reaction time shortening is not modified by prepulse inhibition. Exp Brain Res. 2005;165:541–548. doi: 10.1007/s00221-005-2332-8. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp Brain Res. 2008;187:497–507. doi: 10.1007/s00221-008-1402-0. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Rothwell JC, Goulart FR, Cossu G. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Solé A, Valldeoriola F, Muñoz E, Gonzalez LE, Tolosa ES. Reaction time and acoustic startle in normal human subjects. Neurosci Lett. 1995;195:97–100. doi: 10.1016/0304-3940(94)11790-p. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer B-U, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: Neurons and connections. Brain Research Reviews. 1995;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]