Abstract
BACKGROUND
Understanding the physiology of pregnancy enables effective management of pregnancy complications that could otherwise be life threatening for both mother and fetus. A functional uterus (i) retains the fetus in utero during pregnancy without initiating stretch-induced contractions and (ii) is able to dilate the cervix and contract the myometrium at term to deliver the fetus. The onset of labour is associated with successful cervical remodelling and contraction of myometrium, arising from concomitant activation of uterine immune and endocrine systems. A large body of evidence suggests that actions of local steroid hormones may drive changes occurring in the uterine microenvironment at term. Although there have been a number of studies considering the potential role(s) played by progesterone and estrogen at the time of parturition, the bio-availability and effects of androgens during pregnancy have received less scrutiny. The aim of this review is to highlight potential roles of androgens in the biology of pregnancy and parturition.
METHODS
A review of published literature was performed to address (i) androgen concentrations, including biosynthesis and clearance, in maternal and fetal compartments throughout gestation, (ii) associations of androgen concentrations with adverse pregnancy outcomes, (iii) the role of androgens in the physiology of cervical remodelling and finally (iv) the role of androgens in the physiology of myometrial function including any impact on contractility.
RESULTS
Some, but not all, androgens increase throughout gestation in maternal circulation. The effects of this increase are not fully understood; however, evidence suggests that increased androgens might regulate key processes during pregnancy and parturition. For example, androgens are believed to be critical for cervical remodelling at term, in particular cervical ripening, via regulation of cervical collagen fibril organization. Additionally, a number of studies highlight potential roles for androgens in myometrial relaxation via non-genomic, AR-independent pathways critical for the pregnancy reaching term. Understanding of the molecular events leading to myometrial relaxation is an important step towards development of novel targeted tocolytic drugs.
CONCLUSIONS
The increase in androgen levels throughout gestation is likely to be important for establishment and maintenance of pregnancy and initiation of parturition. Further investigation of the underlying mechanisms of androgen action on cervical remodelling and myometrial contractility is needed. The insights gained may facilitate the development of new therapeutic approaches to manage pregnancy complications such as preterm birth.
Keywords: androgen, pregnancy, labour, cervix, myometrium
Introduction
Preterm birth (PTB), the outcome of preterm labour (PTL), is a major cause of maternal and perinatal morbidity and mortality, occurring in ∼11% of recorded live births worldwide ranging from 5% in developed countries to 18% in developing countries (Blencowe et al., 2012). The exact cause of PTL is unknown, although the concomitant activation of endocrine and immune pathways are believe to drive events, resulting in spontaneous initiation of uterine contractions and cervical ripening (CR) (Norman et al., 2007; Romero et al., 2007; Smith et al., 2012). There is no fully effective agent for prevention of PTL. Progesterone (P4) to maintain uterine quiescence, and cervical cerclage to prevent premature CR have some efficacy in terms of reducing PTB, but there is little evidence of long-term beneficial effects for the baby (Alfirevic et al., 2012; Likis et al., 2012). Similarly, short term, but not long-term, benefits were demonstrated in a recent network analysis of tocolytics (Haas et al., 2012). Thus, new strategies are needed.
Sex hormone steroids, including androgens, increase with normal pregnancy. The role of androgens in female physiology has been an active area of investigation for several decades. The major androgens synthesized in women are dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulphate (DHEAS), androstenedione (A4), testosterone (T) and dihydrotestosterone (DHT) (Burger, 2002; Labrie et al., 2011; Rothman et al., 2011) (Fig. 1). Although their impact on women's health has been investigated, particularly in the context of polycystic ovarian disease, the role they play during pregnancy remains less well described. A number of studies have explored androgenic actions in systems critical for pregnancy reaching term, and further understanding of their roles will facilitate management of potentially life-threatening complications for the mother and the fetus.
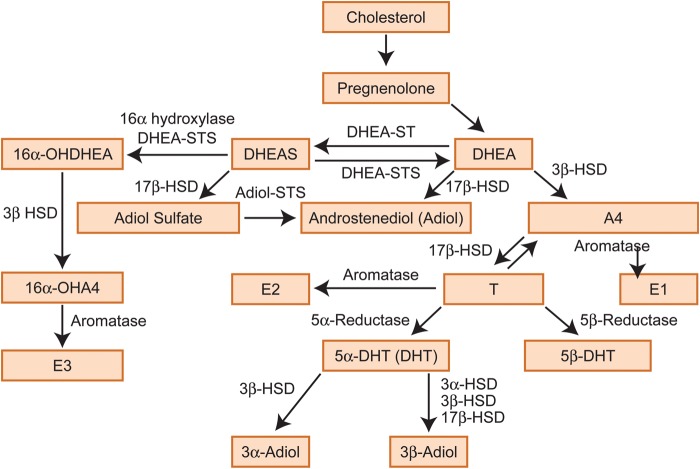
Figure 1.
Testosterone metabolism. DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulphate, A4, androstenedione; T, testosterone; E1, estrone; E2, estradiol; E3, estriol; 3α-HSD, 3-α-hydroxysteroid dehydrogenase/Δ-5-4 isomerase; 3β-HSD, 3-β-hydroxysteroid dehydrogenase/Δ-5-4 isomerase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; 3α-Adiol, 5α-androstane-3α, 17β-diol; 3β-Adiol, 5α- androstane-3β,17β-diol; DHT, dihydrotestosterone; STS, sulphatase; ST, sulfotransferase; 16α-OH, 16α-hydroxy.
Such key systems are cervical remodelling and myometrial contractility. In this review we discuss (i) the biosynthesis, clearance and availability of androgens during pregnancy, (ii) the associations of androgens with adverse pregnancy outcomes, (iii) the role of androgens in physiology of cervical remodelling and (iv) the roles of androgens in the physiology of the myometrial function with a focus on the crosstalk between androgens and myometrial contractile machinery.
Methods
Search strategy
We searched Pubmed using the keywords ‘androgens’ and ‘pregnancy’ to conduct to our knowledge the first narrative review decribing what is known about androgens during pregnancy. The initial Pubmed search identified 5976 manuscripts. Although the focus of this report was manuscripts published between 1980 and 2013, seminal references from 1930 to 1980 were included. For a study to be included in this review, it had to focus on potential roles for androgens in adverse pregnancy outcomes and processes involved in parturition, such as cervical remodelling and myometrial contractility. In addition, studies on androgen metabolism and papers documenting levels of androgens during pregnancy were included. Reports on the role of androgens on fetal gonadal development, fetal lung development and fetal immune system were not included. Following these inclusion and exclusion criteria, ∼500 studies were judged as relevant and used to form the basis of this review.
Results
Androgen biology during pregnancy
Herein we introduce androgens in female physiology focusing on androgen biosynthesis and androgen metabolic clearance at the time of pregnancy. Concentrations of androgens in the feto-maternal unit throughout gestation are reviewed. Afterwards, we highlight studies correlating androgen levels in the feto-maternal unit with adverse pregnancy outcomes.
Androgens in female physiology
Androgens are critically important for the development of the male reproductive tract during fetal life and the maintenance of male secondary sexual characteristics in adulthood (Scott et al., 2007; Welsh et al., 2008; Macleod et al., 2010). Androgens can also act as pro-hormones for biosynthesis of estrogens in both sexes (Rivas et al., 2002; Traish et al., 2011; Purohit and Foster, 2012). In women, androgens are synthesized by cells within the ovaries, the adrenal glands, and also in fat, acting in an endocrine, paracrine or intracrine manner. In addition to playing key roles in the regulation of reproductive tissues such as the ovary and endometrium, androgens have a functional impact on the liver, kidney, bone and muscle (Brenner et al., 2003; Walters et al., 2008; Sen and Hammes, 2010). An excess production of androgens in women, for example in association with conditions such as polycystic ovarian syndrome (PCOS), can result in acne, hirsutism and infertility as well as to an increase in the risk of other complications, such as high blood pressure, cardiovascular disease and type 2 diabetes mellitus (Rosmond, 2006; Dewailly, 2010; Escobar-Morreale et al., 2012). Since the 1960s, it has been generally hypothesized that, during pregnancy, androgens act as substrates for estrogen formation in the placenta (Pion et al., 1965; Siiteri and MacDonald, 1966; Edman et al., 1981; Smith, 2007). According to this hypothesis, DHEAS, produced by the fetal adrenals, and to a smaller extent from the maternal adrenals, enters the placenta, where it is metabolized to A4 and T, which is then converted into E1 (estrone) (Strauss et al., 1996). E1 is in turn converted into E2 (estradiol), which enters the fetal circulation where it is taken up by the liver and converted into E3 (estriol) (Hirano, 1961; Schwarzel et al., 1973). E3 can then pass to maternal circulation and be cleared in maternal urine (Willows, 1966). Androgen biosynthesis during pregnancy is extensively discussed later in this review and shown in Fig. 1.
Androgen-dependent signalling
The importance of the androgen receptor (AR) in mediating the effects of androgens is well documented. For example, mutations or deletions in the AR gene located on the X chromosome are associated in both man and mouse with failure to masculinize. This results in ‘testicular feminization’, a condition where a male with a 46XY karyotype is phenotypically female (Lubahn et al., 1988; Charest et al., 1991; Hughes et al., 2012). The AR is a member of a superfamily of ligand-activated transcription factors; only T and DHT bind AR with high specificity and affinity (Pereira de Jesus-Tran et al., 2006). Ligand binding within the cytoplasm, where the classic AR resides, results in a conformational change in receptor protein, dimerization, nuclear translocation, association with co-factors and ultimately interaction with specific regions of the genome known as androgen response elements (Denayer et al., 2010). The net result of these changes is an up- or down-regulation in gene expression. Such a response is termed ‘genomic’ as it involves gene transcription. In addition to their genomic effects, androgens are also able to exert their effects in a ‘non-genomic’ manner, independent of gene transcription or protein synthesis (Lang et al., 2013). Non-genomic effects of androgens involve interaction with the cell membrane via a number of signalling molecules, including membrane receptors, ion channels or enzyme-linked receptors and cytoplasmic regulatory proteins (Foradori et al., 2008). These non-genomic effects are rapid and can be observed within seconds, in contrast to genomic effects, which may take hours or even days.
Androgen biosynthesis
The biosynthetic conversion of cholesterol (Ch) to androgens involves several steps and enzymes. A fundamental step in this process is the synthesis of pregnenolone. Only certain cell types in humans are capable of pregnenolone manufacture, including testicular Leydig cells, ovarian theca and corpus luteum cells, placental trophoblast cells, adrenal cortex cells and some specific cells in the brain (reviewed in Ghayee and Auchus, 2007). Pregnenolone is metabolized to DHEA, which is further metabolized to A4, the precursor of T. T can be reduced to a number of androgens, such as 5α-DHT (DHT), 5β-DHT, 5β-androstandiol, 3α-Adiol, 3β-Adiol or further metabolized to estrogens. The reduction of T to DHT and the conversion of T to estrogens is catalysed by the metabolising enzymes 5α-reductase and aromatase (CYP19), respectively (Fig. 1). Androgen biosynthesis has been extensively reviewed by Penning (2010).
In the non-pregnant premenopausal woman, ∼50% of all DHEA is secreted by the adrenal glands, 20% from the ovaries and 30% from the peripheral tissues (Abraham, 1974). In contrast, the adrenals and ovaries produce equal amounts of A4 (Longcope, 1986). Regarding T, ∼50% is equally synthesized in the ovaries and adrenals and the other half is produced from A4 in the peripheral tissues (Piltonen et al., 2002). Finally, DHT is not secreted by the endocrine glands, and this androgen is synthesized from T in the peripheral androgen target tissues (Ito and Horton, 1971; Marchetti and Barth, 2013). During pregnancy, an additional source of androgens is the fetus and the placenta. In particular, the developing fetus is the source of placental 16α-hydroxy-DHEA, a product of 16α-hydroxylation of DHEAS in the fetal liver (Cantineau et al., 1985). In a similar way to its non-16α-hydroxylated form, 16α-hydroxy-DHEA can be metabolized to 16α-hydroxy-A4. The latter is the predominant source of E3 (Milewich et al., 1986) (Fig. 1).
Once synthesized, androgens enter the maternal circulation, where their levels can be detected. Usually T levels are reported as either total (tT) or ‘free’ (fT) with an important distinction between them; the latter are unbound to plasma proteins, such as sex-hormone-binding globulin (SHBG), and therefore able to pass freely across plasma membranes to interact with AR, whereas bound T is unable to penetrate the cell membrane (Mendel, 1989). The percentage binding of SHBG is greater for DHT (59%) than for T (44%) and E2 (20%) and is very low for A4, DHEA, E1 and E3 (Avvakumov et al., 2010). The affinity of each androgen for SHBG is dependent on their structural characteristics. For example, a 17β-OH group and a 5α-hydrogen moeity increase the affinity for SHBG binding, whereas androgens with either a double bond in ring A or an aromatic ring A are less SHBG potent (Siiteri and Simberg, 1986). Therefore, DHT, which has both the 17β-OH group and a 5α-hydrogen atom, has 2-fold greater affinity for SHBG than T, which has a double bond in ring A (Siiteri and Simberg, 1986).
Concentrations of androgens during pregnancy
A number of studies have reported elevated levels of some, but not all, circulating androgens during normal pregnancy (Mizuno et al., 1968; Rivarola et al., 1968; Saez et al., 1972; Dawood and Saxena, 1977; Buster et al., 1979; Bammann et al., 1980).
A notable increase in tT is observed from the first trimester of pregnancy and further elevations are reported towards term (Saez et al., 1972; Bammann et al., 1980; Berger et al., 1984). However, circulating fT levels only increase significantly at the third trimester of pregnancy (Dawood and Saxena, 1977). In addition, serum A4 is significantly elevated between 37–42 weeks of pregnancy compared with non-pregnant levels, but the relative increase of T during pregnancy is greater than the increase in A4 (Mizuno et al., 1968). In contrast, maternal circulating DHEAS levels fall across gestation to ∼50% of the non-pregnant levels (Milewich et al., 1978). Finally, SHBG levels increase during the first trimester of pregnancy and continue to increase dramatically throughout mid and late gestation (Wilke and Utley, 1987). Table I summarizes the maternal serum levels of different androgens during pregnancy and Fig. 2 is a graphical representation of this information.
Table I.
Levels of androgens in maternal serum.
| Androgen | Non-pregnant | First trimester | Second trimester | Third trimester | References |
|---|---|---|---|---|---|
| fT (pmol/l) | 6.2 | 11.1 | 7.5 | 13.3 | Wilke and Utley (1987) |
| tT (mmol/l) | 0.21–2.98 | 0.90–7.32 | 1.20–8.40 | 2.20–10.70 | O'Leary et al. (1991) |
| A4 (ng/ml) | 1.0–2.0 | 2.5–3.5 | 0.6–7.8 | 1.6–14.0 | Castracane et al. (1998), Carlsen et al. (2006) |
| DHT (ng/ml) | 0.022–0.107 | 0.113 | 0.18 | 0.1–0.3 | Buster et al. (1979), Dawood and Saxena (1977) |
| SHBG (nmol/l) | 42.2 | 68.1 | 279.3 | 246.1 | Wilke and Utley (1987) |
| DHEA (nmol/l) | 1.0–40.0 | 10.0–60.0 | 5.0–50.0 | 5.0–50.1 | Tagawa et al. (2004) |
| DHEAS (nmol/l) | 2000–4000 | 2000–4000 | 500–2000 | 500–200 | Tagawa et al. (2004) |
The table shows the mean concentrations of androgens as given in references quoted. tT, total testosterone; fT, free testosterone; SHBG, sex hormone binding globulin; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulphate; A4, androstenedione; DHT, dihydrotestosterone.
Figure 2.
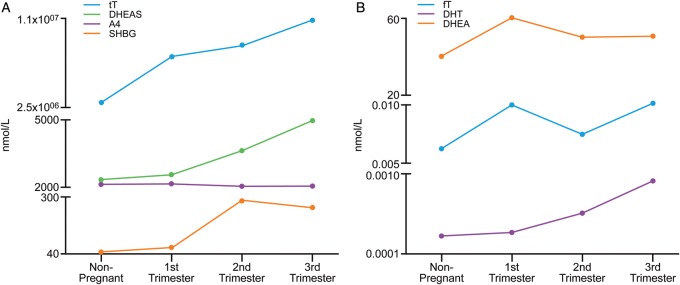
Graphical presentation of the highest (A) and lowest (B) levels of androgens in maternal serum throughout gestation. tT, total testosterone; fT, free testosterone; SHBG, sex hormone-binding globulin; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulphate; A4, androstenedione; DHT, dihydrotestosterone.
In the fetus, levels of some androgens are dependent on fetal sex and gestation. In fetal blood, T levels are higher in males (Rodeck et al., 1985). This contrasts with DHT levels, which are similar in both sexes (Diez d'Aux and Pearson Murphy, 1974; Rodeck et al., 1985). In male fetuses, serum concentrations of T increase until the end of first trimester, reaching a peak of 150 ng/ dL at the end of week 12 of gestation, after which they decline by 70% to a nadir at 23 weeks (Rodeck et al., 1985). In female fetuses, T levels remain generally lower throughout first and second trimester (50 ng/dL) and decline rapidly at term (Diez d'Aux and Pearson Murphy, 1974). In addition to T, DHEA levels are higher in cord blood of male fetuses compared with females, whereas A4 levels are similar (Keelan et al., 2012). Labour is associated with increased levels of cord blood A4, DHEA and SHGB and decreased levels of tT and fT (Keelan et al., 2012). In amniotic fluid, T and A4 are higher in male-bearing pregnancies (Carson et al., 1982). On the other hand, there is no notable difference in amniotic fluid DHEA levels between male and female fetuses (Robinson et al., 1977). Although T levels are higher in the fetal compartment of male fetuses, there is no association between fetal sex and maternal serum concentrations of any androgen (Rivarola et al., 1968). The association between fetal sex and androgen levels, T or A4, in the fetal compartment, including serum and amniotic fluid, is consistent with androgen biosynthesis within Leydig cells of the fetal testis (Scott et al., 2009). Therefore, increased T and A4 levels in the amniotic fluid of male fetuses may reflect androgens excreted in fetal urine (Mitchell and Shackleton, 1969). The tissue origin and cause of the increase in androgens in maternal circulation during pregnancy remains uncertain but is likely to involve production from the ovary or placenta as discussed below.
Tissue origin of androgen synthesis during pregnancy
Within the ovary, androgens are synthesized in small luteal cells (SLC) of the corpus luteum (former theca cells of ovarian follicle) (Sanders et al., 1996). Once pregnancy occurs, it is plausible that SLC stimulation by human chorionic gonadotrophin (hCG), which is elevated in first trimester, results in the reported increase in T levels at this time (Braunstein et al., 1976; Liu and Hsueh, 1986). However, hCG levels reach a peak at the end of first trimester and then decline, in contrast to T, which increases steadily, suggesting either an alternative regulation of androgen production by the corpus luteum or an alternative source of androgen increase following the end of the first trimester (Braunstein et al., 1976). Studies on women with premature ovarian failure (POF) who become pregnant following in vitro fertilization (IVF) with donor oocyte transfers suggest that the ovary is the major contributor to circulating concentrations of T and A4 during pregnancy (Castracane and Asch, 1995). Such women were shown to have significantly lower levels of T and A4 when compared with pregnant women without POF. Perhaps not surprisingly, such studies are yet to be replicated by other research groups.
In addition to the maternal ovary, the maternal adrenal is an important source of androgen production throughout pregnancy. Indeed maternal and fetal virilization due to androgen excess have been reported in cases of adrenal adenomas (Fuller et al., 1983). Studies in baboons showed that the maternal adrenal production of DHEA and DHEAS is suppressed during pregnancy by E2; this is in line with a reported decrease in DHEAS in maternal circulation throughout pregnancy (Albrecht and Pepe, 1995; Umezaki et al., 2001; Tagawa et al., 2004). These studies demonstrated that removal of the fetus but not the placenta (fetectomy), in order to eliminate a fetal source of DHEA/DHEAS, induced a decline in E2 and an increase in DHEA/DHEAS levels in the maternal circulation (Albrecht et al., 1980; Albrecht and Pepe, 1995). The decrease in E2 is probably due to withdrawal of fetal precursors for E2 formation. Interestingly, the authors showed that exogenous E2 could inhibit the fetectomy-induced increase in DHEA/DHEAS. The observation that metabolic clearance rate of DHEA/DHEAS remained unchanged before and after E2 administration, led authors to conclude that the estrogen-induced decline in maternal DHEA/DHEAS levels reflected a decrease in maternal adrenal production of these androgens.
Despite the prevailing belief from studies from 1960s that the placenta does not hold the metabolic capacity to synthesize androgens de novo, but relies solely on fetal androgens, a recent study has demonstrated that placental syncytiotrophoblast (but not cytotrophoblast) can synthesize androgens (Pion et al., 1965; Siiteri and MacDonald, 1966; Escobar et al., 2011). Specifically, syncytiotrophoblast expresses both mRNA and protein of the metabolising enzyme CYP17, which converts C21 steroids (such as P4) to C19 steroids (such as T). The syncytiotrophoblast has been confirmed to have CYP17 activity in vitro (Escobar et al., 2011).
The non-pregnant human myometrium has been shown to possess the metabolic capacity to convert A4 to T and DHT in vitro, but whether this system is functional in pregnancy is unknown (Jasonni et al., 1982). Consistent with this, a more recent study has reported that myometrium, derived from non-pregnant and early pregnant pig uteri, can synthesize A4 and T in vitro (Franczak, 2008). Whether the cervix during pregnancy has the metabolic capacity to synthesize androgens is unknown.
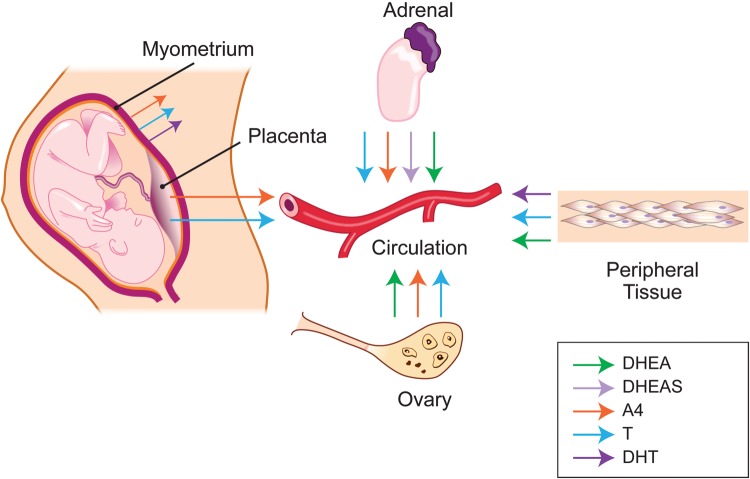
Figure 3 summarizes possible sites for androgen production in the materno-placental unit.
Figure 3.
Maternal sites of androgen synthesis. The maternal adrenal gland secretes DHEA, DHEAS, A4 and T to the maternal circulation, from which androgens get distributed to peripheral tissues. Placenta is an additional site for de novo synthesis of A4 and T synthesis. Ovaries can also produce and secrete T, A4 and DHEA into maternal circulation. Myometrium can also synthesize A4, T and DHT. Finally, DHEA and T can be generated from precursors by androgen metabolizing enzymes. DHEA, dehydroepiandrosterone; T, testosterone; DHT, dihydrotestosterone; DHEAS, dehydroepiandrosterone sulphate; A4, androstenedione.
Androgen clearance during pregnancy
The concentrations of androgens measurable in blood (Pc) are also influenced by their metabolic clearance rate (MCR), the volume of blood cleared of a steroid per unit time (Baird et al., 1969). The blood production rate of an androgen (Pb) is the amount of androgen entering circulation from all possible sources (endocrine glands and periphery). This can be calculated according to the formula Pb = MCR x Pc and it represents an approximation of daily production rate of the steroid (Gurpide, 1990). The MCR of androgens, which represents the summation of individual organ clearance rates, is highly influenced by the amounts of circulating SHBG, which reduces the peripheral breakdown of androgens (Vermeulen and Ando, 1979).
Soon after implantation, the production of maternal P4, E2 and androgens by the corpus luteum increases significantly (Elbaum and Keyes, 1976; Stouffer et al., 1977; Richardson and Masson, 1981; Laherty et al., 1985; Webley and Hearn, 1987; Fisch et al., 1989; Brannian and Stouffer, 1991; Stocco et al., 2007). Corpus luteum-derived E2 stimulates liver SHBG synthesis leading to an increase in SHBG levels (Wilke and Utley, 1987; Joseph, 1994). The increase in SHBG should decrease the availability of fT and therefore lower the clearance of T and DHT. Indeed, it has been reported that the MCR of T and DHT is lower during the first three months of pregnancy compared with the non-pregnant state; however, DHT and T blood production rates are unchanged (Saez et al., 1972). These finding are in agreement with a study in guinea-pigs, where pregnancy induced a 2-fold decrease in MCR of T (Despres et al., 1982).
In contrast to T and DHT, the MCR of DHEA and DHEAS increases 2- and 5-fold, respectively, starting at mid-pregnancy (Gant et al., 1971; Belisle et al., 1977; Belisle et al., 1980b). This phenomenon may reflect the metabolism of these steroids in the feto-placental unit, as both steroids are important as estrogen precursors (Goodyer and Branchaud, 1981; Longcope, 1996). In addition to the increase in the MCR of DHEAS, its production rate by maternal adrenals increases in pregnancy (Milewich et al., 1978). The net result is lower DHEAS levels in the maternal serum at the end of first trimester in comparison with the non-pregnant state (Tagawa et al., 2004). For A4, although pregnancy does not induce changes in the MCR, the production rate is increased 2-fold, explaining the observed increase in A4 in the blood (Belisle et al., 1980a, b; Castracane et al., 1998).
In conclusion from the above studies, it is understood that changes in the production of androgens (predominantly in the ovary and to a lesser extent in the adrenals and periphery) and the MCR occurring during pregnancy may all have an impact on androgen concentrations in the circulation. Of note, changes in concentrations of circulating androgens may not reflect the bioavailability of these steroids in target tissues and therefore their potential to play a role in the physiology of tissues such as the myometrium, cervix and placenta. The role of the increase in androgen levels during pregnancy has been understudied, although a number of adverse pregnancy outcomes have been correlated with impaired maternal androgen levels. These studies are discussed below.
Androgens and adverse pregnancy outcomes
Considering the adverse effects of increased circulating androgens in non-pregnant women, such as in PCOS, one would assume that the physiological pregnancy-induced increase in androgen levels could potentially have similar detrimental effects. However, it is generally accepted that pregnancy-specific mechanisms are activated to protect both the mother and fetus from pregnancy-induced androgen excess (Hensleigh et al., 1975). In the absence of these ‘protective’ mechanisms, high levels of androgens would cause hirsutism and/or virilization of both mother and female fetus.
Maternal ‘protective’ mechanisms include (i) the physiological increase of maternal circulating SHBG, which binds and inactivates elevated androgens (Hammond, 2011) (ii) the pregnancy-induced rapid elevation of P4, which competes for AR binding (Slayden et al., 2001; Birrell et al., 2007) and (iii) P4 having an affinity for 5α-reductase resulting in an inhibition of the conversion of T to the more potent DHT (Hodgins, 1982; Cabeza et al., 1999). Due to these mechanisms, pregnancy itself may reduce the clinical manifestation of pre-existing or pathological pregnancy-induced hyperandrogenic conditions (Phelan and Conway, 2011; Crisosto et al., 2012). The most common hyperandrogenic conditions are known as ‘non-tumour ovarian hyperandrogenism’ and include PCOS, hyperreactio luteinalis (HL) and pregnancy luteoma (Kanova and Bicikova, 2011). Numerous cases of HL, occurring predominantly during the second or third trimester and caused by high β-human chorionic gonadotrophin (β-hCG) levels, have been reported (Haimov-Kochman et al., 2004; Van Holsbeke et al., 2009; Amoah et al., 2011). These studies highlight that, in spite of the protective mechanisms, a small fraction of women with HL exhibit virilization and/or hirsutism, although their female fetuses are hardly ever virilized (Hensleigh et al., 1975; Foulk et al., 1997; Holt et al., 2005; Angioni et al., 2007; Van Holsbeke et al., 2009; Veleminsky, 2010; Abe et al., 2011; Amoah et al., 2011; Annamalai et al., 2011). Although most maternal hyperandrogenic symptoms of HL resolve post-partum, HL is highly associated with adverse pregnancy outcomes such as pre-eclampsia (PE) and PTB (Gatongi et al., 2006; Grgic et al., 2008; Masuyama et al., 2009; Atis et al., 2010; Haq, 2010; Simsek et al., 2012; Lynn et al., 2013). Other hyperandrogenic disorders, which are generally very rare, include fetal-induced hyperandrogenism, due to fetal aromatase deficiency (FAD), iatrogenic hyperandrogenism, caused by exogenous androgen administration and adrenal tumour hyperandrogenism (Dahl et al., 2008).
Fetal ‘protective’ mechanisms largely include the metabolism of androgens in the placenta (Siiteri and MacDonald, 1966; Simpson et al., 1994). Specifically, the aromatase complex within the placenta plays a critical role in the protection of fetus from the mother's androgens as this enzyme rapidly converts T or A4 to E1 and E2, respectively (Edman et al., 1981; Bardin et al., 1983; Hall et al., 1987; Pasanen and Pelkonen, 1994; Simpson et al., 1994). The efficacy of this placental barrier to androgens is evidenced by studies showing that women with hyperandrogenic disorders during pregnancy, such as PCOS, do not necessarily deliver virilized female fetuses (Sir-Petermann et al., 2002; Escobar-Morreale, 2010). On the other hand, women with rare FAD can deliver virilized fetuses, again demonstrating the crucial importance of placental aromatase (Shozu et al., 1991; Ludwig et al., 1998). Taken together, these studies show that pregnancy is associated with protective mechanisms, which ameliorate both maternal and fetal effects of the androgen increase associated with normal pregnancy physiology.
Besides the physiological androgen increase, numerous studies have explored the association of impaired androgen increases during pregnancy, and factors such as maternal age and sex of the baby, with pregnancy outcome. Women with PCOS, who have higher androgen levels than healthy pregnant women, are reported to have an ∼6% higher risk of delivering preterm than women without PCOS (Sir-Petermann et al., 2005; Yamamoto et al., 2012). However, androgen excess might not be the only reason for early birth in these women, as PCOS is also associated with an increase in many inflammatory mediators, which may contribute to PTL (Diamanti-Kandarakis et al., 2006). A study investigating the association of birth size of the offspring with maternal androgens has shown that elevated T is positively associated with in utero growth restriction (Carlsen et al., 2006). In addition, women pregnant with male fetuses are at greater risk of delivering preterm (McGregor et al., 1992; Zeitlin et al., 2002), a phenomenon sometimes attributed to the fact that pregnancies with male fetuses have higher levels of androgens in the fetal compartment compared with female fetuses. However, there is no evidence that the relationship between higher androgen levels in the fetal compartment and PTB is causal. In contrast to the positive association between androgen levels (at least in the fetal compartment) and the presence of a male fetus and PTB, increasing maternal age (which is negatively associated with maternal androgen levels in pregnancy) is known to be a risk factor for preterm deliveries (Astolfi and Zonta, 1999; Carlsen et al., 2003). Recently, a specific change in the sequence of the fetal AR gene has been associated with predisposition to PTB (Karjalainen et al., 2012). In particular, a linkage analysis study revealed that the exon-1 of AR of the offspring who was born preterm had more repetitions of a sequence encoding for a site in the domain involved in ligand activation (Karjalainen et al., 2012). Abnormal increase in androgens during pregnancy has been additionally correlated with the development of gestational diabetes, which is further associated with PTB (Espinos et al., 1992; Abbott et al., 2010; Vejrazkova et al., 2014; Ackerman et al., 2013; Morisset et al., 2013). This is probably due to the role of androgens in modulation of insulin secretion from the pancreas and inhibition of glucose uptake in fat and muscle cells (Corbould, 2008; Rao et al., 2013). The mechanism linking the two hormonal axes is poorly understood and, interestingly, has not been considered or explored during pregnancy. Increased T levels in the maternal circulation at third trimester have been associated with the incidence of PE, suggesting T as a predictive marker (Ghorashi and Sheikhvatan, 2008; Lorzadeh and Kazemirad, 2012; Sharifzadeh et al., 2012; Simsek et al., 2012). In this context, placentae from preeclamptic pregnancies have higher expression levels of AR in both male and female fetuses (Sathishkumar et al., 2012). Therefore, it is possible that AR signalling in the placenta contributes to the pathology of PE.
The association of androgen levels with preterm delivery has been studied in a few primate studies. For example, continuous administration of A4 to pregnant rhesus monkeys from early gestation or for a period of 48 h during third trimester resulted in preterm initiation of myometrial contractions and cervical dilatation leading to preterm delivery (Figueroa et al., 1989; Mecenas et al., 1996). In these studies, preterm delivery was associated with an increase in E2 in maternal circulation, suggesting conversion of A4 to E2 in the placenta. Nathanielsz et al. (1998) later supported that notion by showing that the preterm phenotype could be rescued by administration of an aromatase inhibitor. However, in other studies, direct administration of E2 or E1 to pregnant primates failed to induce preterm delivery (Novy and Walsh, 1983; Albrecht et al., 1989). Therefore, it is likely that a physiological increase in androgen levels in maternal circulation from the start to end of pregnancy holds the key to term pregnancy. In support of this notion, abnormal changes in androgens levels at the start of pregnancy have been associated with spontaneous abortions or recurrent miscarriages. For example, spontaneous abortion was shown to occur in women whose androgen levels failed to increase at the beginning of pregnancy (Bammann et al., 1980). On the other hand, women with recurrent miscarriages had significantly higher levels of T, A4 and T/SHBG ratio compared with women without recurrent miscarriages (Okon et al., 1998).
Although these studies emphasize how the normal increase in androgen levels might be pivotal for maintenance of pregnancy and development of the fetus, they fail to show a consistent relationship between either androgen excess or androgen deficiency and pregnancy problems. What is more, the mechanism by which androgens impact pregnancy outcomes has not been fully understood. Below we discuss the effect of androgens on cervical remodelling and myometrial function, the key processes in determining the timing of delivery. Particular focus is paid to myometrial smooth muscle cell (MSMC) physiology and the ability of androgens to interact with the contractile machinery of the cell.
The role of androgens in CR
Structure of cervix during pregnancy
The cervical stroma is composed of fibroblasts, smooth muscle cells (SMCs), epithelial cells and immune cells (Leppert, 1995). These cells secrete an extracellular matrix (ECM), which consists primarily of collagen, glycosaminoglycans and proteoglycans. Hormones including progestogens and E2, relaxin and prostaglandin can alter ECM composition and affect the mechanical strength of the cervix (Ekman-Ordeberg et al., 2003; Simon and Einspanier, 2009; Ji et al., 2011; Ghule et al., 2012). The consequence of this process is remodelling of the cervix, which can be divided into four stages: softening (first trimester), ripening (second trimester), dilatation (third trimester) and reconstitution of the non-pregnant cervix post-partum (Read et al., 2007). Each stage has a characteristic collagen and proteoglycan composition as the tissue re-organizes and prepares for the next steps in the parturition process (Word et al., 2007). CR is characterized by a 60% decrease in collagen and proteoglycan levels and occurs in parallel with an increase in collagenase activity, which itself is involved in collagen catabolism and disturbance of collagen bundles (Ekman et al., 1986; Norman et al., 1993). However, collagen synthesis increases in the cervix at term due to collagen turnover being balanced between production and degradation (Ekman et al., 1986). Structural abnormalities of the cervix have been associated with an increased risk of PTB, thus a better understanding of the physiology of cervical remodelling is crucial (reviewed by Norman, 2007).
The role of androgens in CR
Numerous human and animal studies have explored the role of androgens in the cervix, particularly during pregnancy (Mochizuki et al., 1978a, b; Sasaki et al., 1982; Mochizuki and Maruo, 1985; Takahashi et al., 1984; Sakyo et al., 1986, 1987; Yamashita et al., 1991; El Maradny et al., 1996; Kanayama et al., 1998; Ji et al., 2008). These studies formed the basis of a hypothesis, whereby androgens regulate cervical remodelling, particularly CR at term. Table II summarizes key findings from these studies. Collectively, these reports provide enough evidence that the fetal androgen, DHEAS, promotes CR by enhancing collagenase activity and thus decreasing fibril collagen organization.
Table II.
Studies on the effect of androgen administration on CR.
| Androgen | Dose | Species/tissue | Key findings | Reference |
|---|---|---|---|---|
| DHEAS | 0.01 mg/ml 0.1 mg/ml |
Human In vitro: HCF derived from term pregnancy |
↑ IL-8 in conditioned medium ↑ IL receptor in HCF |
Kanayama et al. (1998) |
| DHEAS | 200 mg | Human In vivo: term pregnant women (>38 weeks) |
↑ Bishop score ↑ Collagenase activity |
Mochizuki et al. 1978a, b |
| DHEAS | 200 mg | Human In vivo: term pregnant women (38–42 weeks) |
↑ Bishop score ↑ Collagenase activity ↑ E2 in maternal serum |
Mochizuki and Maruo (1985) |
| DHEAS | 50–100 mg | Human In vivo: term pregnant women (38–42 weeks) |
↑ Bishop score ↓ Time to delivery No side effects |
Mochizuki et al. 1978a, b |
| DHEAS | 100 mg | Human In vivo: term pregnant multiparous and nulliparous women (>38 weeks) |
↑ Bishop score ↓ Duration of labour in nulliparous women ↓ Time to delivery in nulliparous No side effects |
Sasaki et al. (1982) |
| DHEAS | 50–200 mg | Human In vivo: term pregnant singleton and twin pregnancies (>39 weeks) |
↑ Bishop score (higher correlation of Bishop score with twin pregnancies) ↑ E2 levels in maternal serum (higher correlation of E2 levels with twin pregnancies) No change in E1, E3 levels in maternal serum. |
Takahashi et al. (1984) |
| DHEAS | 1 μM | Rabbit In vitro: CCs derived from term pregnancy |
↑ Collagenase levels No effect of E2 or DHEA treatment |
Sakyo et al. (1986) |
| DHEAS | 1 μM | Rabbit In vitro: CCs derived from term pregnancy |
↑ Collagenase levels No effect on collagen levels |
Sakyo et al. (1987) |
| DHEAS | 10 mg | Rabbit In vivo: term pregnancy |
↑ Collagenase activity ↓ Collagen content Combined DHEAS + IL-8 (100 ng) treatment-induced neutrophil infiltration Combined treatment produced maximal decrease in collagen content |
Maradny et al. (1996) |
| DHEAS | 100 mg | Rat In vivo: term pregnancy (day 16) |
↓ Collagen content ↑ Collagenase activity |
Yamashita et al. (1991) |
| DHEA | 0.01 mg/ml 0.1 mg/ml |
Human In vitro: HCF derived from term pregnancy |
Increase of IL-8 in conditioned medium Increase of IL receptor in HCF |
Kanayama et al. (1998) |
| DHT | 2 mg | Rat In vivo: term pregnancy (15 day) |
↓ Cervical resistance ↓ Proteoglycan mRNA Flutamide treatment (10 mg) ↑ Cervical resistance ↑ Proteoglycan mRNA |
Ji et al. (2008) |
DHEAS, dehydroepiandrosterone sulphate; DHT, dihydrotestosterone; HCF, human cervical fibroblast; CCs, cervical cells.
Mechanism of androgen effect on CR
The physiology or pharmacology of androgen effects on CR is not fully understood. In support of a physiological effect of DHEAS on CR, one study has reported that DHEAS levels are significantly correlated with favourable outcome according to the Bishop score, i.e. higher levels of DHEAS in women with successful (spontaneous) CR compared with women in need of induction of labour (Koyuncu et al., 1995). DHEAS administration has been additionally positively correlated with increases in E2 levels in maternal serum (Takahashi et al., 1984). Therefore, it has been argued that DHEAS is metabolized to estrogens in term cervix, which in turn initiates CR. However, studies examining such effects showed that administration of estrogens alone fails to ripen the cervix (Thiery et al., 1979; Larmon et al., 2002; Dasgupta and Singh, 2012). Notably DHT, which cannot be metabolized to estrogens, also promotes CR, implying an androgen-specific effect (Ji et al., 2008). Interestingly, mice with knockout (KO) of 5α-reductase type 1 develop an abnormal cervix that fails to ripen at labour (Mahendroo et al., 1999). 5α-reductase type 1 converts T to DHT and is the predominant enzyme expressed in cervix at term (Mahendroo and Russell, 1999). Administration of a 5α-reduced androgen, a metabolite of DHT, 5alpha-androstane-3alpha, 17β-diol, rescues the impaired phenotype of 5α-reductase type 1 KO animals, allowing normal CR (Mahendroo et al., 1996). The latter finding suggests local conversion of androgens to other (more potent) androgen metabolites as the underlying mechanism in the initiation of CR.
Although poorly explored, there is some evidence to suggest that the mechanism of DHEAS action on the collagenase increase is indirect and is mediated via DHEAS-stimulated neutrophil secretion of the proteolytic enzyme (El Maradny et al., 1996; Maymon et al., 2000). Indeed, DHEAS action on CR has been correlated with increased levels of IL-8 cytokine involved in chemotaxis of neutrophils recruited in cervix towards term (Kanayama et al., 1998; Maymon et al., 2000).
The involvement of AR in mediation of CR by androgens has been recently examined (Ji et al., 2008). In that study, antagonism of AR by flutamide inhibited events associated with CR, such as a decrease in collagen fibril organization, a decrease in cervical resistance and a decrease in proteoglycan synthesis in pregnant rats pre-treated with DHT on Day 16, suggesting genomic signalling (Ji et al., 2008). Although this study did not examine AR expression in the cervix, other immunohistochemical studies in non-pregnant and pregnant humans and canine cervix have demonstrated cervical expression of AR (Wilson and McPhaul, 1996; Vermeirsch et al., 2002; Ji et al., 2008; Vladic-Stjernholm et al., 2009). In addition, it has been reported that androgens up-regulate AR expression in the human cervix (van der Kwast et al., 1994).
All the above reports suggest that DHEA and DHT might promote CR via a pathway impacting collagenase synthesis and/or activity. Although the mechanism of this action is not well established, there is some evidence that it is possibly mediated via metabolism of androgens to reduced androgens and/or genomic signalling through AR. This notion needs more investigation. In addition, the role of androgens and androgen signalling in the cervical endothelial/epithelial cells and cervical SMCs in terms of cervical remodelling throughout pregnancy is surprisingly unexplored.
The role of androgens in myometrial function
MSMCs changes during pregnancy
MSMCs are specialized contractile cells that, in contrast to skeletal and SMCs, exhibit cellular plasticity, which enables reversible differentiation into different phenotypes (Gabbiani et al., 1981; Tomiyasu et al., 1988). Studies of myometrium during rat pregnancy have demonstrated that MSMCs undergo four phenotypic changes throughout gestation (Shynlova et al., 2009). Briefly, these are a proliferative stage, a synthetic stage, a contractile stage and, finally, a labour stage. In early pregnancy, following implantation of the conceptus, MSMCs proliferate rapidly and become hyperplastic. Hyperplasia is believed to be mediated by hormone signalling and growth factors, such as IGF-1 and EGF, have been shown to be involved in induction of the so-called proliferative phenotype (Shynlova et al., 2007). Following the proliferative phase, MSMCs undergo an intermediate synthetic phase starting on Day 14 of rodent pregnancy (Lye et al., 2001). The synthetic phenotype is characterized by increased synthesis of ECM and cellular hypertrophy of MSMCs (Shynlova et al., 2009). On Day 21 of rodent gestation, uterine myocytes switch to a contractile phenotype (Nishinaka and Fukuda, 1991; Fata et al., 2000; Shynlova et al., 2004). Differentiation into a contractile phenotype is largely attributed to mechanical stretch (Manabe et al., 1981; Loudon et al., 2004; Sooranna et al., 2004; Terzidou et al., 2005). MSMCs undergo a final phase of differentiation following initiation of labour, the so-called labour phenotype (Shynlova et al., 2009). At this point, the myometrium develops synchronous contractions and expresses a number of contraction-associated proteins (CAPs), such as connexin 43 (Con43), oxytocin receptor (OXTR) and prostaglandin F receptor (Fuchs et al., 1984; Slater et al., 1999; Sparey et al., 1999; Erkinheimo et al., 2000; Rehman et al., 2003). Returning MSMCs to a non-pregnant state involves rapid apoptosis (Roh et al., 2000; O'Brien et al., 2007; Shynlova et al., 2009).
A recent study, conducted in rats, reported that AR is highly expressed in MSCMs during the proliferative stage and progressively declines towards the end of pregnancy, suggesting a role (at least in this species) in establishment of myometrial growth at early stages of pregnancy (Liu et al., 2013). Liu et al. additionally showed that the AR decrease itself affects IGF-1 receptor (IGF-1R) stability and thus down-regulates downstream cascades that IGF-1 is involved in (including P13K/Akt, which is highly important in proliferative pathways) (Liu et al., 2013). Besides rats, a significant decrease in AR expression occurs in porcine myometrium 3 weeks prior to labour (Slomczynska et al., 2008). A human microarray study also showed AR gene down-regulation in the myometrium during preterm (0.4-fold) and term labour (0.3-fold) compared with non-labouring myometrium, but the protein expression was not determined (Bethin et al., 2003). Thus, androgen signalling in MSMCs is likely to be involved in proliferative pathways pivotal for ‘building’ of the myometrium in the early stages of pregnancy.
The role of androgens in myometrial contractility
Androgens have been demonstrated to relax SMCs, such as rabbit and pig trachea, rat mesenteric arterial bed, rat thoracic aorta and human coronary artery (Perusquia et al., 1991a, b; Chou et al., 1996; Costarella et al., 1996; Rosano et al., 1999; Tep-areenan et al., 2002; Perusquia et al., 2005; Kouloumenta et al., 2006; Bordallo et al., 2008; Montano et al., 2008; Chevalier et al., 2012). Robson (1937) was the first to suggest that androgens may have an inhibitory action on uterine muscle contraction. Following a 4-day administration of 12 mg of testosterone propionate (a small fast-acting ester of testosterone) to ovariectomized non-pregnant rabbits, he examined the contractile response of their uterus to 1 unit of injected oxytocin both in vivo and in vitro. The uterus of T-treated rabbits did not respond to oxytocin administration in contrast to the uterus of control rabbits. However, the spontaneous contractile activity of the uterus was only slightly affected (Robson, 1937). Following Robson, some other studies have demonstrated similar relaxing effects of various androgens including T, DHT, A4 and DHEAS on the myometrium of non-pregnant rats and non-pregnant and pregnant humans (Kubli-Garfias et al., 1980; Perusquia et al., 1991a, b; Perusquia et al., 2005). The findings from these studies are summarized in Table III. The relaxing effect occurs only in pharmacological concentrations (micromolar) and is rapid, suggesting non-genomic mechanism of action (Perusquia et al., 1990; Anderson et al., 2009; Tica et al., 2011). A logical assumption would be that the observed effect of androgens might not occur physiologically because physiological androgen concentrations are in a nanomolar range. However, understanding the molecular pathways employed by androgens in modulation of myometrial relaxation could help in the development of new tocolytic agents to manage preterm spontaneous contractions. We discuss below the current evidence for possible molecular mechanisms by which androgens might elicit relaxing effects in myometrium.
Table III.
Studies on the effect of androgen on myometrial contractions.
| Androgen | Dose | Species/tissue | Key findings | References |
|---|---|---|---|---|
| T, DHEA, androstanediol, androsterone, A4, 5α-DHT, 5β-DHT | 10–100 μM | Rat (non-pregnant) Ex vivo: myometrial strips |
Spontaneous contractions inhibited rapidly (<30 min) by all androgens Potency: androstanediol, androsterone >5β-DHT > T = A4 = DHEA = 5α-DHT |
Kubli-Garfias et al. (1980) |
| T, DHEA, androstanediol, androsterone, 5β-DHT | 3–100 μM | Rat (non-pregnant) Ex vivo: myometrial strips |
Tonic contractions (KCl induced) inhibited rapidly (<30 min) by all androgens Potency: androsterone = androstanediol >5β-DHT > T |
Perusquia et al. (1990) |
| androstanediol, androsterone, 5α-DHT, 5β-DHT | 3–100 μM | Rat (non-pregnant) Ex vivo: myometrial strips |
Serotonin-induced contractions inhibited rapidly (<30 min) by all androgens | Perusquia et al. (1991a, b) |
| androstanediol, androsterone, 5α-DHT, 5β-DHT | 3–100 μM | Rat (non-pregnant) Ex vivo: myometrial Strips |
Acetylcholine-induced contractions inhibited rapidly (<30 min) by all androgens | Perusquia et al. (1991a, b) |
| androstanediol, androsterone, 5α-DHT, 5β-DHT | 3–100 μM | Rat (non-pregnant) Ex vivo: myometrial strips |
Oxytocin-induced contractions inhibited rapidly (<30 min) by all androgens | Perusquia (1991) |
| T, DHEA, DHT, 5α-DHT, 5β-DHT, androstanediol, androsterone | 3–100 μM | Human (term pregnant, non-pregnant) Ex vivo: myometrial strips |
Spontaneous contractions and tonic contractions (KCl induced) inhibited rapidly (<30 min) by all androgens Potency: 5β-DHT > androsterone = DHEA = T > 5α-DHT = androstanediol |
Perusquia et al. (2005) |
DHEA, dehydroepiandrosterone; T, testosterone; DHT, dihydrotestosterone; DHEAS, dehydroepiandrosterone sulphate; A4, androstenedione.
Mechanism of MSMC contraction
MSMCs are myogenic, which means that they can contract spontaneously (Tomiyasu et al., 1988). The membrane potential in MSMCs is not stable. Spontaneous depolarization of membrane potential, fired by alterations in membrane currents and ion channels, occurs during pregnancy. A decrease in membrane potential triggers myocyte contractile activity and leads to spontaneous contractions; conversely, amplification of membrane potential to a resting −50 mV maintains the uterus in a quiescent state (Nakajima, 1971; Pressman et al., 1988). The upstroke of MSMCs membrane action potential (i.e. the initial decrease in membrane potential) is predominantly due to calcium (Ca2+) entry, whereas repolarization occurs as a result of blockage of Ca2+ channels (Nakajima, 1971). The key biochemical event in spontaneous MSMC contraction is an increase in intracellular Ca2+ ([Ca2+]) from 10−7 to 10−6 M (Horowitz et al., 1996). This increase is the outcome of extracellular influx of Ca2+ and/or release of Ca2+ from the sarcoplasmic reticulum (SR). The entry of Ca2+ is mediated via a variety of Ca2+ channels classified into voltage-operated Ca2+ channels (VOCCs) and voltage-independent Ca2+ channels (including receptor-operated Ca2+ channels, ROCCs) (Wray et al., 2003, 2005; Floyd and Wray, 2007; Noble et al., 2009). ROCCs are regulated by an agonist-receptor interaction (Guibert et al., 2008). Receptors that interact with ROCCs upon ligand binding are G protein coupled receptors (GPCRs), such as the OXTR. GPCRs can activate the inositol triphosphate (IP3) pathway leading to release of Ca2+ from SR via activation of phosphoinositide phospholipase C and hydrolysis of PIP2 (Meldrum et al., 1991). The resulting increase in [Ca2+] leads to formation of a complex between calmodulin and myosin light chain kinase, which then phosphorylates light chains on myocin (MLC) (Ito and Hartshorne, 1990). The latter event allows binding of actin to myocin leading to cross-bridge cycling, which then causes contraction (Dillon et al., 1981). Extrusion of [Ca2+] in MSMCs can occur by reverse processes and is mediated by two major transporters: plasma membrane Ca2+ ATPase (PMCA) and the Na+/Ca2+ exchange pump (NCX) (Shmigol et al., 1998). Studies examining mechanisms by which Ca2+ ions are removed from the cytoplasm in isolated rat MSMCs show that PMCA accounts for 85% of the Ca2+ efflux (Shmigol et al., 1998).
Besides VOCCs, ROCCs, PMCA and NCX, Ca2+ flux is highly influenced by MSMC membrane fluidity. A proton nuclear magnetic resonance (NMR) spectroscopy study investigating various parameters of myometrial membrane fluidity at pregnancy, including phospholipid double bonds, fatty acid chain length and the Ch/phospholipid ratio, reported that MSMC membrane fluidity is increased with labour (Pulkkinen et al., 1998). Cell membrane fluidity is modulated by changes in the concentration of membrane Ch (Shmygol et al., 2007). Ch is a component of the lipid bilayer, which integrates into the membrane by placing its steroid ring next to the hydrocarbon chains and locating its hydroxyl group close to the head of phospholipid (van Meer, 1989; Simons and Vaz, 2004; Ikonen, 2008). High Ch results in a stiff membrane and thus reduces membrane fluidity, whereas low Ch promotes membrane fluidity. Decreased membrane fluidity is characterized by inhibition of Ca2+ channels such as VOCCs and ROCCs (Jennings et al., 1999; Shmygol et al., 2007). Administration of Ch in vitro decreases [Ca2+] and inhibits the phasic contractions in MSMCs of rats and humans (Smith et al., 2005; Zhang et al., 2007). On the other hand, depletion of Ch from the rat MSMC membrane with methyl-beta-cyclodextrin treatment causes an increase in cell excitability and contraction (Jennings et al., 1999; Shmygol et al., 2007). High Ch has been correlated with increased activities of NCX and PMCA, which is consistent with their roles in promoting Ca2+ efflux (Ortega and Mas-Oliva, 1984; Kutryk and Pierce, 1988; Verbist et al., 1991).
Non-genomic interactions of androgens with the contractile machinery
Possible interactions of androgens with the MSMC contractile machinery, including interactions with VOCCs, ROCCs, the lipid bilayer, gap junctions and membrane receptors, which may result in the inhibition of contraction, are discussed below.
Androgens and VOCCs
High K+ in the extracellular space induces cell membrane depolarization, which in turn results in opening of Ca2+ channels (VOCCs) and an increase in [Ca2+]. In addition to the relaxing effect of androgens on spontaneous myometrial contractions, 5β-DHT rapidly relaxes tonic contractions (induced by high K+) and decreases single MSMC [Ca2+], while removal of the androgen reverses the effect (Perusquia et al., 2005). A logical explanation of the mechanism of the relaxing effect of the androgen in that case would be via inhibition of VOCCs. However, it is not clear whether the effect of androgens involves a physical interaction with VOCCs or is indirect.
Androgens and ROCCs
Besides VOCCs, there is some evidence that androgens may be able to block ROCCs. For example, androgens can relax rat uterine contractions induced by serotonin, oxytocin and acetylcholine in vitro (Perusquia, 1991; Perusquia et al., 1991a, b). Oxytocin, acetylcholine and serotonin receptors are GPCRs. Their activation leads to subsequent activation of extracellular Ca2+ influx via ROCCs and/or Ca2+ influx from SR via the IP3 pathway (Large, 2002; Thorneloe and Nelson, 2005). It is possible that androgens exert their effects on oxytocin-induced contraction via blockage of IP3 pathway and release of SR Ca2+. However, inhibition of the SR Ca2+ release is not sufficient for complete blockage of the overall Ca2+ influx (Kupittayanant et al., 2002). Therefore, a logical assumption would be that androgens block the ROCC-associated Ca2+ influx rather than components of IP3 pathway. This notion is supported by the inability of T in another study to inhibit both caffeine- and carbachol- induced Ca2+ release (activators of IP3-pathway) in coronary SMC (Murphy and Khalil, 1999). Identical findings were reported in isolated rat thoracic aortic strips and porcine coronary arteries, where T could inhibit K+-induced but not caffeine-induced contractions (Crews and Khalil, 1999a, b). There are no data on relaxing effect of androgens on a GPCR agonist-stimulated contractions (such as oxytocin and prostaglandin FP) induced contraction in human myometrium.
Androgens and the lipid bilayer
In a similar manner to Ch, it is plausible that Ch metabolites (including androgens) mediate non-genomic responses via direct penetration into the cell membrane and reduction in MSMC membrane fluidity. For example, hydrophobic androgens, such as DHT and T, have been shown to interact with the phospholipid bilayer of the negatively charged membrane (Duval et al., 1983; Van Bommel et al., 1987). Such interactions may impair Ca2+ homeostasis due to an increase in the activity of PMCA. Regulation of PMCA by androgens is documented in different tissues. For example, in synaptosomal plasma membranes, PMCA activity increases 95% following 10 μM of T treatment (Deliconstantinos, 1988). In addition, PMCA activity in kidney is increased following administration of 10 μM of T, as measured by in vitro assays where the ATP-dependent Ca2+ flux was determined by comparison of Ca2+ transport (implying PMCA activity) with and without exogenous addition of ATP (Dick et al., 2003). In line with the latter study, androgen deprivation induced by castration alters the immunochistochemical localization of PMCA in prostate epithelial cells with no changes in mRNA or protein levels (Coviello et al., 2006). Additionally, the regulation of Ca2+-ATPase in SR activity by T has been explored in ventricular muscle of orchidectomized (ORX) rats (Witayavanitkul et al., 2013). In that study, T treatment managed to prevent an ORX-induced decrease in Ca2+-ATPase activity. Collectively, an alternative mechanism for the non-genomic action of androgens in MSMC relaxation is proposed, whereby androgens penetrate the lipid bilayer and decrease membrane fluidity, resulting in an increase in PMCA activity and Ca2+ efflux. However, the effect of androgens on PMCA activity in the myometrium has not yet been explored and, thus, this hypothesis remains to be confirmed in this tissue.
Androgens and gap junctions
Increases in single SMC [Ca2+] initiates a synchronized increase in [Ca2+] in adjacent cells, a process mediated by gap junctions (Loch-Caruso et al., 2003; Brading and Brain, 2011; Boittin et al., 2013). Gap junction proteins increase the ability of MSMCs to generate synchronous contractions and mount a synchronous response to hormones or agents in the microenvironment of uterus at labour (Garfield and Hayashi, 1981; Ikeda et al., 1987). Connexins are gap junction trans-membrane proteins that assemble to form a gap junction; Con43 is the predominant connexin involved in the initiation of MSMC contractions at term (Chow and Lye, 1994). A study in pregnant rats on a high Ch diet showed that high Ch is associated with a decrease in Con43 protein in the myometrium, suggesting possible implications for uterine contractility (Elmes et al., 2011). A study in isolated rat cardiac myocytes demonstrated that gap junctional intracellular communication (GJIC) disturbance, by either silencing the gene for Con43 or by addition of specific drugs, such as heptanol, induced a significant reduction in Ca2+ transients, suggesting that Con43 may be involved in the regulation of basal Ca2+ signalling in these cells (Li et al., 2012). Interestingly, an estrogenic compound 4-OH-TCB has been shown to act in a similar manner to heptanol and significantly block GJIC in rat myometrium (Tsai et al., 1998). What is more, the same study showed that 4-OH-TCB could reduce contractions of rat myometrial strips, suggesting that the relaxing effect of 4-OH-TCB is mediated through blockage of GJIC. There is evidence that androgens can influence GJIC in isolated rat cardiac myocytes and Sertoli cells (Pluciennik et al., 1996). One study demonstrated a dose-dependent (1–25 μM) inhibitory effect of T propionate on GJIC, which was reversed by T withdrawal and was unaffected by pre-incubation with AR antagonist, again implying a non-genomic effect of T (Pluciennik et al., 1996). Based on these studies, it is tempting to hypothesize that androgens may induce relaxation of myometrium via direct interaction with the membrane proteolipidic structure, which might alter directly or indirectly the function of gap junction channels; however, more evidence in needed.
Androgens and ARs
A role for ARs in inhibition of myometrial contraction has been investigated (Perusquia et al., 2005). Flutamide-induced AR antagonism failed to inhibit the relaxing effect of 5β-DHT (Perusquia et al., 2005). Notably, in that study, flutamide was administered at a significantly lower concentration (10 μM) than the concentration of 5β-DHT (100 μM), thus it is not clear whether nuclear AR is involved in the relaxing effect. However, the rapid action of androgens on myometrial relaxation makes a genomic mechanism unlikely. On the other hand, a few reports have now demonstrated that AR can be recruited in non-genomic actions of androgens (reviewed in Hammes and Levin, 2011). This mechanism involves the largely unexplored membrane AR (mAR). The mAR is hypothesized to be either a GPCR or a classic AR lacking a DNA-binding site; the latter is thought to have greater affinity for androgens compared with nAR (Konoplya and Popoff, 1992; Foradori et al., 2008; Yang et al., 2011). A study on airway SMCs showed that in addition to T, treatments with membrane impermable T conjugated to BSA (TBSA) could inhibit contraction, suggesting that T exerts its effects on the outer aspect of the membrane rather than in the cytoplasm (Kouloumenta et al., 2006). However, the downstream mechanism of mAR activation in that study was unclear. The structure of mAR is yet to be characterized in mammals and it is unknown whether it is utilized by androgens to inhibit MSMC contraction (Thomas et al., 2006).
Collectively, the above studies allow us to hypothesize that androgen-induced MSMC relaxation is mediated via penetration of the androgen into the lipid bilayer where it affects molecules critical for generation of Ca2+ transients, including VOCCs, ROCCs, gap junctions and PMCA (Fig. 4).
Figure 4.
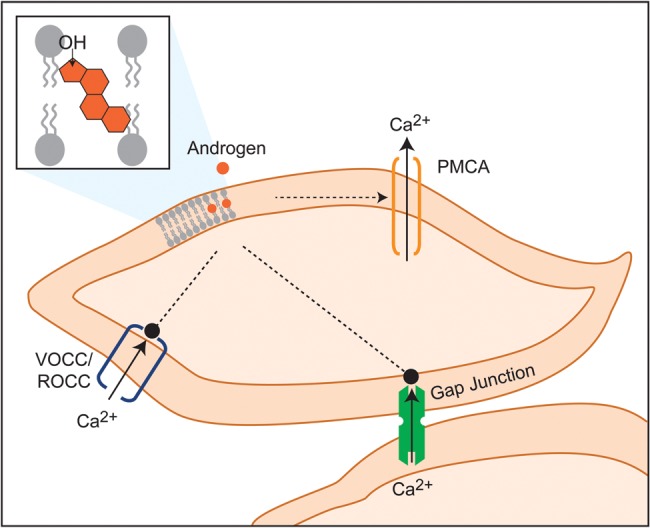
Hypothetical targets of androgens in modulation of MSMC relaxation. Androgens are hypothesized to interact with contractile machinery of MSMCs via penetration into the lipid bilayer. This might promote PMCA to induce rapid Ca2+ efflux, block VOCCs and ROCCs and impair IGJC via effects on the gap junctions. PMCA, Ca2+ ATPase; VOCC, voltage-operated Ca2+ channels; ROCC, receptor-operated Ca2+ channels; IGJC, intercellular gap junctional communication.
Conclusions
There is growing evidence that androgens, which increase throughout gestation, might play a functional role in the physiology of pregnancy in parallel with the well-established roles of progestins and estrogens. Herein we reviewed what is currently known about roles played by androgens in two critical systems in biology of pregnancy and parturition, cervical remodelling and myometrial function.
There is evidence from in vivo and in vitro studies that androgens promote CR and, thus, assist initiation of labour. On the other hand, androgens appear to be effective in in vitro tocolysis of myometrial contractions. Although the action of androgens on cervical remodelling is possibly mediated via classical activation of genomic signalling via AR, it is unlikely that the same mechanism mediates androgen effects in myometrial contractility. In contrast, there is more evidence that AR acting within the nucleus is involved in the proliferation of MSMCs and, thus, may be important for the growth of myometrium at the beginning of pregnancy. Further investigations are required to fully understand the molecular events regulated by androgens in cervix and myometrium and their interplay with other signalling pathways. These investigations should potentially utilize animal models of adverse pregnancy outcomes, where targeted administration of androgens to either cervix or myometrium could be highly informative. In conclusion, research into the roles of androgens in cervical and myometrial function during pregnancy and parturition has the potential to inform new therapeutic strategies for management of pregnancy complications, such as PTL and PTB.
Authors' roles
S.M., P.T.K.S. and J.E.N. made substantial contributions to the conception, design, analysis, drafting and revision of the article and approved the final version to be published. The authors declare that there are no conflicts of interest.
Funding
The authors are grateful for funding from the University of Edinburgh Principal's Career Development Research Fund and from the Albert McKern Bequest which jointly funded a PhD studentship to SM. Funding to pay the Open Access publication charges for this article was provided by the Albert McKern Bequest.
Conflict of interest
None declared.
Acknowledgement
We are grateful to Mr Ronnie Grant for graphical support.
References
- Abbott DH, Bruns CR, Barnett DK, Dunaif A, Goodfriend TL, Dumesic DA, Tarantal AF. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab. 2010;299:E741–E751. doi: 10.1152/ajpendo.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Ono S, Igarashi M, Akira S, Watanabe A, Takeshita T. Conservative management of hyperreactio luteinalis: a case report. J Nippon Med Sch. 2011;78:241–245. doi: 10.1272/jnms.78.241. [DOI] [PubMed] [Google Scholar]
- Abraham GE. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab. 1974;39:340–346. doi: 10.1210/jcem-39-2-340. [DOI] [PubMed] [Google Scholar]
- Ackerman CM, Lowe LP, Dyer AR, Hayes MG, Metzger BE, Lowe WL, Urbanek M, Group HSCR. Maternal testosterone levels are associated with C-peptide levels in the Mexican American subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study cohort. Horm Metab Res. 2013;45:617–620. doi: 10.1055/s-0033-1347262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht ED, Haskins AL, Pepe GJ. The influence of fetectomy at midgestation upon the serum concentrations of progesterone, estrone, and estradiol in baboons. Endocrinology. 1980;107:766–770. doi: 10.1210/endo-107-3-766. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Crenshaw MC, Jr, Pepe GJ. The effect of estrogen on placental delivery after fetectomy in baboons. Am J Obstet Gynecol. 1989;160:237–241. doi: 10.1016/0002-9378(89)90128-2. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Pepe GJ. Suppression of maternal adrenal dehydroepiandrosterone and dehydroepiandrosterone sulfate production by estrogen during baboon pregnancy. J Clin Endocrinol Metab. 1995;80:3201–3208. doi: 10.1210/jcem.80.11.7593427. [DOI] [PubMed] [Google Scholar]
- Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2012;4:CD008991. doi: 10.1002/14651858.CD008991.pub2. [DOI] [PubMed] [Google Scholar]
- Amoah C, Yassin A, Cockayne E, Bird A. Hyperreactio luteinalis in pregnancy. Fertil Steril. 2011;95 doi: 10.1016/j.fertnstert.2011.03.060. 2429, e2421–2423. [DOI] [PubMed] [Google Scholar]
- Anderson L, Martin W, Higgins C, Nelson SM, Norman JE. The effect of progesterone on myometrial contractility, potassium channels, and tocolytic efficacy. Reprod Sci. 2009;16:1052–1061. doi: 10.1177/1933719109340926. [DOI] [PubMed] [Google Scholar]
- Angioni S, Portoghese E, Milano F, Melis GB, Fulghesu AM. Hirsutism and hyperandrogenism associated with hyperreactio luteinalis in a singleton pregnancy: a case report. Gynecol Endocrinol. 2007;23:248–251. doi: 10.1080/09513590701214513. [DOI] [PubMed] [Google Scholar]
- Annamalai AK, Hoveyda F, Williams RM, Patterson A, Simpson HL. Hyperreactio luteinalis. QJM. 2011;104:807–808. doi: 10.1093/qjmed/hcq141. [DOI] [PubMed] [Google Scholar]
- Astolfi P, Zonta LA. Risks of preterm delivery and association with maternal age, birth order, and fetal gender. Hum Reprod. 1999;14:2891–2894. doi: 10.1093/humrep/14.11.2891. [DOI] [PubMed] [Google Scholar]
- Atis A, Cifci F, Aydin Y, Ozdemir G, Goker N. Hyperreactio luteinalis with preeclampsia. J Emerg Trauma Shock. 2010;3:298. doi: 10.4103/0974-2700.66545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvakumov GV, Cherkasov A, Muller YA, Hammond GL. Structural analyses of sex hormone-binding globulin reveal novel ligands and function. Mol Cell Endocrinol. 2010;316:13–23. doi: 10.1016/j.mce.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Baird DT, Horton R, Longcope C, Tait JF. Steroid dynamics under steady-state conditions. Recent Prog Horm Res. 1969;25:611–664. doi: 10.1016/b978-0-12-571125-8.50017-x. [DOI] [PubMed] [Google Scholar]
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293–298. doi: 10.1016/0002-9378(80)90912-6. [DOI] [PubMed] [Google Scholar]
- Bardin CW, Brown T, Isomaa VV, Janne OA. Progestins can mimic, inhibit and potentiate the actions of androgens. Pharmacol Ther. 1983;23:443–459. doi: 10.1016/0163-7258(83)90023-2. [DOI] [PubMed] [Google Scholar]
- Belisle S, Osathanondh R, Tulchinsky D. The effect of constant infusion of unlabeled dehydroepiandrosterone sulfate on maternal plasma androgens and estrogens. J Clin Endocrinol Metab. 1977;45:544–550. doi: 10.1210/jcem-45-3-544. [DOI] [PubMed] [Google Scholar]
- Belisle S, Lehoux JG, Brault J. The metabolism of androstenedione in human pregnancy: the use of constant infusion of unlabeled steroid to assess its metabolic clearance rate, its production rate, and its conversion into androgens and estrogens. Am J Obstet Gynecol. 1980a;136:1030–1035. doi: 10.1016/0002-9378(80)90632-8. [DOI] [PubMed] [Google Scholar]
- Belisle S, Schiff I, Tulchinsky D. The use of constant infusion of unlabeled dehydroepiandrosterone for the assessment of its metabolic clearance rate, its half-life, and its conversion into estrogens. J Clin Endocrinol Metab. 1980b;50:117–121. doi: 10.1210/jcem-50-1-117. [DOI] [PubMed] [Google Scholar]
- Berger NG, Repke JT, Woodruff JD. Markedly elevated serum testosterone in pregnancy without fetal virilization. Obstet Gynecol. 1984;63:260–262. [PubMed] [Google Scholar]
- Bethin KE, Nagai Y, Sladek R, Asada M, Sadovsky Y, Hudson TJ, Muglia LJ. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol Endocrinol. 2003;17:1454–1469. doi: 10.1210/me.2003-0007. [DOI] [PubMed] [Google Scholar]
- Birrell SN, Butler LM, Harris JM, Buchanan G, Tilley WD. Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. FASEB J. 2007;21:2285–2293. doi: 10.1096/fj.06-7518com. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Alonso F, Le Gal L, Allagnat F, Beny JL, Haefliger JA. Connexins and M3 muscarinic receptors contribute to heterogeneous Ca(2+) signaling in mouse aortic endothelium. Cell Physiol Biochem. 2013;31:166–178. doi: 10.1159/000343358. [DOI] [PubMed] [Google Scholar]
- Bordallo J, de Boto MJ, Meana C, Velasco L, Bordallo C, Suarez L, Cantabrana B, Sanchez M. Modulatory role of endogenous androgens on airway smooth muscle tone in isolated guinea-pig and bovine trachea; involvement of beta2-adrenoceptors, the polyamine system and external calcium. Eur J Pharmacol. 2008;601:154–162. doi: 10.1016/j.ejphar.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Brading AF, Brain KL. Ion channel modulators and urinary tract function. Handb Exp Pharmacol. 2011;202:375–393. doi: 10.1007/978-3-642-16499-6_18. [DOI] [PubMed] [Google Scholar]
- Brannian JD, Stouffer RL. Progesterone production by monkey luteal cell subpopulations at different stages of the menstrual cycle: changes in agonist responsiveness. Biol Reprod. 1991;44:141–149. doi: 10.1095/biolreprod44.1.141. [DOI] [PubMed] [Google Scholar]
- Braunstein GD, Rasor J, Danzer H, Adler D, Wade ME. Serum human chorionic gonadotropin levels throughout normal pregnancy. Am J Obstet Gynecol. 1976;126:678–681. doi: 10.1016/0002-9378(76)90518-4. [DOI] [PubMed] [Google Scholar]
- Brenner RM, Slayden OD, Nayak NR, Baird DT, Critchley HO. A role for the androgen receptor in the endometrial antiproliferative effects of progesterone antagonists. Steroids. 2003;68:1033–1039. doi: 10.1016/s0039-128x(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl. 4):S3–S5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- Buster JE, Chang RJ, Preston DL, Elashoff RM, Cousins LM, Abraham GE, Hobel CJ, Marshall JR. Interrelationships of circulating maternal steroid concentrations in third trimester pregnancies. II. C18 and C19 steroids: estradiol, estriol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, delta 5-androstenediol, delta 4-androstenedione, testosterone, and dihydrotestosterone. J Clin Endocrinol Metab. 1979;48:139–142. doi: 10.1210/jcem-48-1-139. [DOI] [PubMed] [Google Scholar]
- Cabeza M, Gutierrez E, Miranda R, Heuze I, Bratoeff E, Flores G, Ramirez E. Androgenic and anti-androgenic effects of progesterone derivatives with different halogens as substituents at the C-6 position. Steroids. 1999;64:413–421. doi: 10.1016/s0039-128x(99)00018-5. [DOI] [PubMed] [Google Scholar]
- Cantineau R, Kremers P, De Graeve J, Gielen JE, Lambotte R. 15- and 16-hydroxylations of androgens and estrogens in the human fetal liver: a critical step in estetrol biosynthesis. J Steroid Biochem. 1985;22:195–201. doi: 10.1016/0022-4731(85)90112-8. [DOI] [PubMed] [Google Scholar]
- Carlsen SM, Jacobsen G, Bjerve KS. Androgen levels in pregnant women decrease with increasing maternal age. Scand J Clin Lab Invest. 2003;63:23–26. doi: 10.1080/00365510310000457. [DOI] [PubMed] [Google Scholar]
- Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol. 2006;155:365–370. doi: 10.1530/eje.1.02200. [DOI] [PubMed] [Google Scholar]
- Carson DJ, Okuno A, Lee PA, Stetten G, Didolkar SM, Migeon CJ. Amniotic fluid steroid levels. Fetuses with adrenal hyperplasia, 46,XXY fetuses, and normal fetuses. Am J Dis Child. 1982;136:218–222. [PubMed] [Google Scholar]
- Castracane VD, Asch RH. Testosterone and androstenedione in premature ovarian failure pregnancies: evidence for an ovarian source of androgens in early pregnancy. Hum Reprod. 1995;10:677–680. doi: 10.1093/oxfordjournals.humrep.a136010. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Stewart DR, Gimpel T, Overstreet JW, Lasley BL. Maternal serum androgens in human pregnancy: early increases within the cycle of conception. Hum Reprod. 1998;13:460–464. doi: 10.1093/humrep/13.2.460. [DOI] [PubMed] [Google Scholar]
- Charest NJ, Zhou ZX, Lubahn DB, Olsen KL, Wilson EM, French FS. A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol Endocrinol. 1991;5:573–581. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- Chevalier M, Gilbert G, Lory P, Marthan R, Quignard JF, Savineau JP. Dehydroepiandrosterone (DHEA) inhibits voltage-gated T-type calcium channels. Biochem Pharmacol. 2012;83:1530–1539. doi: 10.1016/j.bcp.2012.02.025. [DOI] [PubMed] [Google Scholar]
- Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94:2614–2619. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- Chow L, Lye SJ. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol. 1994;170:788–795. doi: 10.1016/s0002-9378(94)70284-5. [DOI] [PubMed] [Google Scholar]
- Corbould A. Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev. 2008;24:520–532. doi: 10.1002/dmrr.872. [DOI] [PubMed] [Google Scholar]
- Costarella CE, Stallone JN, Rutecki GW, Whittier FC. Testosterone causes direct relaxation of rat thoracic aorta. J Pharmacol Exp Ther. 1996;277:34–39. [PubMed] [Google Scholar]
- Coviello AD, Lakshman K, Mazer NA, Bhasin S. Differences in the apparent metabolic clearance rate of testosterone in young and older men with gonadotropin suppression receiving graded doses of testosterone. J Clin Endocrinol Metab. 2006;91:4669–4675. doi: 10.1210/jc.2006-0822. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Antagonistic effects of 17 beta-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol. 1999a;19:1034–1040. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol. 1999b;26:707–715. doi: 10.1046/j.1440-1681.1999.03110.x. [DOI] [PubMed] [Google Scholar]
- Crisosto N, Echiburu B, Maliqueo M, Perez V, Ladron de Guevara A, Preisler J, Sanchez F, Sir-Petermann T. Improvement of hyperandrogenism and hyperinsulinemia during pregnancy in women with polycystic ovary syndrome: possible effect in the ovarian follicular mass of their daughters. Fertil Steril. 2012;97:218–224. doi: 10.1016/j.fertnstert.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Dahl SK, Thomas MA, Williams DB, Robins JC. Maternal virilization due to luteoma associated with delayed lactation. Fertil Steril. 2008;90 doi: 10.1016/j.fertnstert.2008.01.055. 2006, e2017–2009. [DOI] [PubMed] [Google Scholar]
- Dasgupta E, Singh G. Vaginal misoprostol vs vaginal misoprostol with estradiol for labor induction: a prospective double blind study. J Obstet Gynaecol India. 2012;62:47–51. doi: 10.1007/s13224-012-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood MY, Saxena BB. Testosterone and dihydrotestosterone in maternal and cord blood and in amniotic fluid. Am J Obstet Gynecol. 1977;129:37–42. doi: 10.1016/0002-9378(77)90815-8. [DOI] [PubMed] [Google Scholar]
- Deliconstantinos G. Structure activity relationship of cholesterol and steroid hormones with respect to their effects on the Ca2+-stimulated ATPase and lipid fluidity of synaptosomal plasma membranes from dog and rabbit brain. Comp Biochem Physiol B. 1988;89:585–594. doi: 10.1016/0305-0491(88)90178-2. [DOI] [PubMed] [Google Scholar]
- Denayer S, Helsen C, Thorrez L, Haelens A, Claessens F. The rules of DNA recognition by the androgen receptor. Mol Endocrinol. 2010;24:898–913. doi: 10.1210/me.2009-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres G, Rigaudiere N, Delost P. Changes in the metabolic clearance rate and plasma production rate of testosterone in the pregnant guinea-pig. Steroids. 1982;39:703–710. doi: 10.1016/0039-128x(82)90141-6. [DOI] [PubMed] [Google Scholar]
- Dewailly D. Androgen excess in women: not just a cosmetic problem. Ann Endocrinol (Paris) 2010;71:1. doi: 10.1016/j.ando.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Alexandraki K, Piperi C, Protogerou A, Katsikis I, Paterakis T, Lekakis J, Panidis D. Inflammatory and endothelial markers in women with polycystic ovary syndrome. Eur J Clin Invest. 2006;36:691–697. doi: 10.1111/j.1365-2362.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- Dick IM, Liu J, Glendenning P, Prince RL. Estrogen and androgen regulation of plasma membrane calcium pump activity in immortalized distal tubule kidney cells. Mol Cell Endocrinol. 2003;212:11–18. doi: 10.1016/j.mce.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Diez d'Aux RC, Pearson Murphy BE. Androgens in the human fetus. J Steroid Biochem. 1974;5:207–210. doi: 10.1016/0022-4731(74)90133-2. [DOI] [PubMed] [Google Scholar]
- Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981;211:495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- Duval D, Durant S, Homo-Delarche F. Non-genomic effects of steroids. Interactions of steroid molecules with membrane structures and functions. Biochim Biophys Acta. 1983;737:409–442. doi: 10.1016/0304-4157(83)90008-4. [DOI] [PubMed] [Google Scholar]
- Edman CD, Toofanian A, MacDonald PC, Gant NF. Placental clearance rate of maternal plasma androstenedione through placental estradiol formation: an indirect method of assessing uteroplacental blood flow. Am J Obstet Gynecol. 1981;141:1029–1037. doi: 10.1016/s0002-9378(16)32694-1. [DOI] [PubMed] [Google Scholar]
- Ekman G, Malmstrom A, Uldbjerg N, Ulmsten U. Cervical collagen: an important regulator of cervical function in term labor. Obstet Gynecol. 1986;67:633–636. [PubMed] [Google Scholar]
- Ekman-Ordeberg G, Stjernholm Y, Wang H, Stygar D, Sahlin L. Endocrine regulation of cervical ripening in humans—potential roles for gonadal steroids and insulin-like growth factor-I. Steroids. 2003;68:837–847. doi: 10.1016/j.steroids.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Elbaum DJ, Keyes PL. Synthesis of 17beta-estradiol by isolated ovarian tissues of the pregnant rat: aromatization in the corpus luteum. Endocrinology. 1976;99:573–579. doi: 10.1210/endo-99-2-573. [DOI] [PubMed] [Google Scholar]
- El Maradny E, Kanayama N, Halim A, Maehara K, Sumimoto K, Terao T. Biochemical changes in the cervical tissue of rabbit induced by interleukin-8, interleukin-1beta, dehydroepiandrosterone sulphate and prostaglandin E2: a comparative study. Hum Reprod. 1996;11:1099–1104. doi: 10.1093/oxfordjournals.humrep.a019304. [DOI] [PubMed] [Google Scholar]
- Elmes MJ, Tan DS, Cheng Z, Wathes DC, McMullen S. The effects of a high-fat, high-cholesterol diet on markers of uterine contractility during parturition in the rat. Reproduction. 2011;141:283–290. doi: 10.1530/REP-10-0378. [DOI] [PubMed] [Google Scholar]
- Erkinheimo TL, Saukkonen K, Narko K, Jalkanen J, Ylikorkala O, Ristimaki A. Expression of cyclooxygenase-2 and prostanoid receptors by human myometrium. J Clin Endocrinol Metab. 2000;85:3468–3475. doi: 10.1210/jcem.85.9.6809. [DOI] [PubMed] [Google Scholar]
- Escobar JC, Patel SS, Beshay VE, Suzuki T, Carr BR. The human placenta expresses CYP17 and generates androgens de novo. J Clin Endocrinol Metab. 2011;96:1385–1392. doi: 10.1210/jc.2010-2504. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF. Diagnosis and management of hirsutism. Ann N Y Acad Sci. 2010;1205:166–174. doi: 10.1111/j.1749-6632.2010.05652.x. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Carmina E, Dewailly D, Gambineri A, Kelestimur F, Moghetti P, Pugeat M, Qiao J, Wijeyaratne CN, Witchel SF, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146–170. doi: 10.1093/humupd/dmr042. [DOI] [PubMed] [Google Scholar]
- Espinos JJ, Corcoy R, Roca B, Rodriguez-Espinosa J, Calaf J. Serum insulin and androgen concentrations in women with histories of gestational diabetes. Med Clin (Barc) 1992;98:445–448. [PubMed] [Google Scholar]
- Fata JE, Ho AT, Leco KJ, Moorehead RA, Khokha R. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: functions of metalloproteinases and their inhibitors. Cell Mol Life Sci. 2000;57:77–95. doi: 10.1007/s000180050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa JP, Honnebier MB, Binienda Z, Wimsatt J, Nathanielsz PW. Effect of a 48-hour intravenous delta 4-androstenedione infusion on the pregnant rhesus monkey in the last third of gestation: changes in maternal plasma estradiol concentrations and myometrial contractility. Am J Obstet Gynecol. 1989;161:481–486. doi: 10.1016/0002-9378(89)90545-0. [DOI] [PubMed] [Google Scholar]
- Fisch B, Margara RA, Winston RM, Hillier SG. Cellular basis of luteal steroidogenesis in the human ovary. J Endocrinol. 1989;122:303–311. doi: 10.1677/joe.0.1220303. [DOI] [PubMed] [Google Scholar]
- Floyd R, Wray S. Calcium transporters and signalling in smooth muscles. Cell Calcium. 2007;42:467–476. doi: 10.1016/j.ceca.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulk RA, Martin MC, Jerkins GL, Laros RK. Hyperreactio luteinalis differentiated from severe ovarian hyperstimulation syndrome in a spontaneously conceived pregnancy. Am J Obstet Gynecol. 1997;176:1300–1302. doi: 10.1016/s0002-9378(97)70349-1. discussion 1302–1304. [DOI] [PubMed] [Google Scholar]
- Franczak A. Endometrial and myometrial secretion of androgens and estrone during early pregnancy and luteolysis in pigs. Reprod Biol. 2008;8:213–228. doi: 10.1016/s1642-431x(12)60013-8. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734–741. doi: 10.1016/0002-9378(84)90677-x. [DOI] [PubMed] [Google Scholar]
- Fuller PJ, Pettigrew IG, Pike JW, Stockigt JR. An adrenal adenoma causing virilization of mother and infant. Clin Endocrinol (Oxf) 1983;18:143–153. doi: 10.1111/j.1365-2265.1983.tb03197.x. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Schmid E, Winter S, Chaponnier C, de Ckhastonay C, Vandekerckhove J, Weber K, Franke WW. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci USA. 1981;78:298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant NF, Hutchinson HT, Siiteri PK, MacDonald PC. Study of the metabolic clearance rate of dehydroisoandrosterone sulfate in pregnancy. Am J Obstet Gynecol. 1971;111:555–563. doi: 10.1016/0002-9378(71)90472-8. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Hayashi RH. Appearance of gap junctions in the myometrium of women during labor. Am J Obstet Gynecol. 1981;140:254–260. doi: 10.1016/0002-9378(81)90270-2. [DOI] [PubMed] [Google Scholar]
- Gatongi DK, Madhvi G, Tydeman G, Hasan A. A case of hyperreactio luteinalis presenting with eclampsia. J Obstet Gynaecol. 2006;26:465–467. doi: 10.1080/01443610600759244. [DOI] [PubMed] [Google Scholar]
- Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord. 2007;8:289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- Ghorashi V, Sheikhvatan M. The relationship between serum concentration of free testosterone and pre-eclampsia. Endokrynol Pol. 2008;59:390–392. [PubMed] [Google Scholar]
- Ghule VV, Gray C, Galimberti A, Anumba DO. Prostaglandin-induced cervical remodelling in humans in the first trimester is associated with increased expression of specific tight junction, but not gap junction proteins. J Transl Med. 2012;10:40. doi: 10.1186/1479-5876-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer CG, Branchaud CL. Regulation of hormone production in the human feto-placental unit. Ciba Found Symp. 1981;86:89–123. doi: 10.1002/9780470720684.ch5. [DOI] [PubMed] [Google Scholar]
- Grgic O, Radakovic B, Barisic D. Hyperreactio luteinalis could be a risk factor for development of HELLP syndrome: case report. Fertil Steril. 2008;90 doi: 10.1016/j.fertnstert.2008.06.053. 2008, e2013–2006. [DOI] [PubMed] [Google Scholar]
- Guibert C, Ducret T, Savineau JP. Voltage-independent calcium influx in smooth muscle. Prog Biophys Mol Biol. 2008;98:10–23. doi: 10.1016/j.pbiomolbio.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Gurpide E. Experimental designs used to estimate rates of steroid production and metabolism in vivo and in vitro. Ann N Y Acad Sci. 1990;595:165–172. doi: 10.1111/j.1749-6632.1990.tb34290.x. [DOI] [PubMed] [Google Scholar]
- Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. BMJ. 2012;345:e6226. doi: 10.1136/bmj.e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov-Kochman R, Yanai N, Yagel S, Amsalem H, Lavy Y, Hurwitz A. Spontaneous ovarian hyperstimulation syndrome and hyperreactio luteinalis are entities in continuum. Ultrasound Obstet Gynecol. 2004;24:675–678. doi: 10.1002/uog.1759. [DOI] [PubMed] [Google Scholar]
- Hall PF, Chen S, Nakajin S, Shinoda M, Shively JE. Purification and characterization of aromatase from human placenta. Steroids. 1987;50:37–50. doi: 10.1016/0039-128x(83)90060-0. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Minireview: recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152:4489–4495. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod. 2011;85:431–441. doi: 10.1095/biolreprod.111.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq AN. Hyperreactio luteinalis associated with pregnancy induced hypertension. J Coll Physicians Surg Pak. 2010;20:137–139. [PubMed] [Google Scholar]
- Hensleigh PA, Carter RP, Grotjan HE., Jr Fetal protection against masculinization with hyperreactio luteinalis and virilization. J Clin Endocrinol Metab. 1975;40:816–823. doi: 10.1210/jcem-40-5-816. [DOI] [PubMed] [Google Scholar]
- Hirano M. Formation and biological activity of estriol in placenta. Tohoku J Exp Med. 1961;75:89–102. doi: 10.1620/tjem.75.89. [DOI] [PubMed] [Google Scholar]
- Hodgins MB. Binding of androgens in 5 alpha-reductase-deficient human genital skin fibroblasts: inhibition by progesterone and its metabolites. J Endocrinol. 1982;94:415–427. doi: 10.1677/joe.0.0940415. [DOI] [PubMed] [Google Scholar]
- Holt HB, Medbak S, Kirk D, Guirgis R, Hughes I, Cummings MH, Meeking DR. Recurrent severe hyperandrogenism during pregnancy: a case report. J Clin Pathol. 2005;58:439–442. doi: 10.1136/jcp.2004.018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996;76:967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- Hughes IA, Davies JD, Bunch TI, Pasterski V, Mastroyannopoulou K, MacDougall J. Androgen insensitivity syndrome. Lancet. 2012;380:1419–1428. doi: 10.1016/S0140-6736(12)60071-3. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Shibata Y, Yamamoto T. Rapid formation of myometrial gap junctions during parturition in the unilaterally implanted rat uterus. Cell Tissue Res. 1987;248:297–303. doi: 10.1007/BF00218196. [DOI] [PubMed] [Google Scholar]
- Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- Ito M, Hartshorne DJ. Phosphorylation of myosin as a regulatory mechanism in smooth muscle. Prog Clin Biol Res. 1990;327:57–72. [PubMed] [Google Scholar]
- Ito T, Horton R. The source of plasma dihydrotestosterone in man. J Clin Invest. 1971;50:1621–1627. doi: 10.1172/JCI106650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasonni VM, Bonavia M, Lodi S, Preti S, Bulletti C, Flamigni C. Androstenedione metabolism in human uterine tissues: endometrium, myometrium and leiomyoma. J Steroid Biochem. 1982;17:547–551. doi: 10.1016/0022-4731(82)90014-0. [DOI] [PubMed] [Google Scholar]
- Jennings LJ, Xu QW, Firth TA, Nelson MT, Mawe GM. Cholesterol inhibits spontaneous action potentials and calcium currents in guinea pig gallbladder smooth muscle. Am J Physiol. 1999;277:G1017–G1026. doi: 10.1152/ajpgi.1999.277.5.G1017. [DOI] [PubMed] [Google Scholar]
- Ji H, Dailey TL, Long V, Chien EK. Androgen-regulated cervical ripening: a structural, biomechanical, and molecular analysis. Am J Obstet Gynecol. 2008;198 doi: 10.1016/j.ajog.2007.11.012. 543, e541–549. [DOI] [PubMed] [Google Scholar]
- Ji H, Long V, Briody V, Chien EK. Progesterone modulates integrin {alpha}2 (ITGA2) and {alpha}11 (ITGA11) in the pregnant cervix. Reprod Sci. 2011;18:156–163. doi: 10.1177/1933719110382305. [DOI] [PubMed] [Google Scholar]
- Joseph DR. Structure, function, and regulation of androgen-binding protein/sex hormone-binding globulin. Vitam Horm. 1994;49:197–280. doi: 10.1016/s0083-6729(08)61148-6. [DOI] [PubMed] [Google Scholar]
- Kanayama N, El Maradny E, Goto J, Terao T. Effect of dehydroepiandrosterone sulfate on interleukin-8 receptor during cervical ripening. Eur J Endocrinol. 1998;138:587–593. doi: 10.1530/eje.0.1380587. [DOI] [PubMed] [Google Scholar]
- Kanova N, Bicikova M. Hyperandrogenic states in pregnancy. Physiol Res. 2011;60:243–252. doi: 10.33549/physiolres.932078. [DOI] [PubMed] [Google Scholar]
- Karjalainen MK, Huusko JM, Ulvila J, Sotkasiira J, Luukkonen A, Teramo K, Plunkett J, Anttila V, Palotie A, Haataja R, et al. A potential novel spontaneous preterm birth gene, AR, identified by linkage and association analysis of X chromosomal markers. PLoS One. 2012;7:e51378. doi: 10.1371/journal.pone.0051378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keelan JA, Mattes E, Tan H, Dinan A, Newnham JP, Whitehouse AJ, Jacoby P, Hickey M. Androgen concentrations in umbilical cord blood and their association with maternal, fetal and obstetric factors. PLoS One. 2012;7:e42827. doi: 10.1371/journal.pone.0042827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konoplya EF, Popoff EH. Identification of the classical androgen receptor in male rat liver and prostate cell plasma membranes. Int J Biochem. 1992;24:1979–1983. doi: 10.1016/0020-711x(92)90294-b. [DOI] [PubMed] [Google Scholar]
- Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. Non-genomic effect of testosterone on airway smooth muscle. Br J Pharmacol. 2006;149:1083–1091. doi: 10.1038/sj.bjp.0706936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu F, Aksoy S, Karanfil C, Demir N, Bozkurt K, Tuncay G. [The effect of dehydroepiandrosterone sulfate on cervical maturation during pregnancy. J Gynecol Obstet Biol Reprod (Paris) 1995;24:630–633. [PubMed] [Google Scholar]
- Kubli-Garfias C, Lopez-Fiesco A, Pacheco-Cano MT, Ponce-Monter H, Bondani A. In vitro effects of androgens upon the spontaneous rat uterine contractility. Steroids. 1980;35:633–641. doi: 10.1016/0039-128x(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Kupittayanant S, Luckas MJ, Wray S. Effect of inhibiting the sarcoplasmic reticulum on spontaneous and oxytocin-induced contractions of human myometrium. BJOG. 2002;109:289–296. doi: 10.1111/j.1471-0528.2002.01110.x. [DOI] [PubMed] [Google Scholar]
- Kutryk MJ, Pierce GN. Stimulation of sodium-calcium exchange by cholesterol incorporation into isolated cardiac sarcolemmal vesicles. J Biol Chem. 1988;263:13167–13172. [PubMed] [Google Scholar]
- Labrie F, Martel C, Balser J. Wide distribution of the serum dehydroepiandrosterone and sex steroid levels in postmenopausal women: role of the ovary? Menopause. 2011;18:30–43. doi: 10.1097/gme.0b013e3181e195a6. [DOI] [PubMed] [Google Scholar]
- Laherty RF, Rotten D, Yamamoto M, Jaffe RB. Effects of oestradiol and prolactin on progesterone production by rhesus monkey luteal cells in vitro. Acta Endocrinol (Copenh) 1985;108:266–272. doi: 10.1530/acta.0.1080266. [DOI] [PubMed] [Google Scholar]
- Lang F, Alevizopoulos K, Stournaras C. Targeting membrane androgen receptors in tumors. Expert Opin Ther Targets. 2013;17:951–963. doi: 10.1517/14728222.2013.806491. [DOI] [PubMed] [Google Scholar]
- Large WA. Receptor-operated Ca2(+)-permeable nonselective cation channels in vascular smooth muscle: a physiologic perspective. J Cardiovasc Electrophysiol. 2002;13:493–501. doi: 10.1046/j.1540-8167.2002.00493.x. [DOI] [PubMed] [Google Scholar]
- Larmon JE, Magann EF, Dickerson GA, Morrison JC. Outpatient cervical ripening with prostaglandin E2 and estradiol. J Matern Fetal Neonatal Med. 2002;11:113–117. doi: 10.1080/jmf.11.2.113.117. [DOI] [PubMed] [Google Scholar]
- Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38:267–279. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- Li C, Meng Q, Yu X, Jing X, Xu P, Luo D. Regulatory effect of connexin 43 on basal Ca2+ signaling in rat ventricular myocytes. PLoS One. 2012;7:e36165. doi: 10.1371/journal.pone.0036165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likis FE, Edwards DR, Andrews JC, Woodworth AL, Jerome RN, Fonnesbeck CJ, McKoy JN, Hartmann KE. Progestogens for preterm birth prevention: a systematic review and meta-analysis. Obstet Gynecol. 2012;120:897–907. doi: 10.1097/AOG.0b013e3182699a15. [DOI] [PubMed] [Google Scholar]
- Liu YX, Hsueh AJ. Synergism between granulosa and theca-interstitial cells in estrogen biosynthesis by gonadotropin-treated rat ovaries: studies on the two-cell, two-gonadotropin hypothesis using steroid antisera. Biol Reprod. 1986;35:27–36. doi: 10.1095/biolreprod35.1.27. [DOI] [PubMed] [Google Scholar]
- Liu L, Li Y, Xie N, Shynlova O, Challis JR, Slater D, Lye S, Dong X. Proliferative action of the androgen receptor in human uterine myometrial cells—a key regulator for myometrium phenotype programming. J Clin Endocrinol Metab. 2013;98:218–227. doi: 10.1210/jc.2012-2451. [DOI] [PubMed] [Google Scholar]
- Loch-Caruso RK, Criswell KA, Grindatti CM, Brant KA. Sustained inhibition of rat myometrial gap junctions and contractions by lindane. Reprod Biol Endocrinol. 2003;1:62. doi: 10.1186/1477-7827-1-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab. 1986;15:213–228. doi: 10.1016/s0300-595x(86)80021-4. [DOI] [PubMed] [Google Scholar]
- Longcope C. Dehydroepiandrosterone metabolism. J Endocrinol. 1996;150(Suppl.):S125–S127. [PubMed] [Google Scholar]
- Lorzadeh N, Kazemirad S. The effects of fetal gender on serum human chorionic gonadotropin and testosterone in normotensive and preeclamptic pregnancies. J Pregnancy. 2012;2012:874290. doi: 10.1155/2012/874290. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Loudon JA, Sooranna SR, Bennett PR, Johnson MR. Mechanical stretch of human uterine smooth muscle cells increases IL-8 mRNA expression and peptide synthesis. Mol Hum Reprod. 2004;10:895–899. doi: 10.1093/molehr/gah112. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240:327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Beck A, Wickert L, Bolkenius U, Tittel B, Hinkel K, Bidlingmaier F. Female pseudohermaphroditism associated with a novel homozygous G-to-A (V370-to-M) substitution in the P-450 aromatase gene. J Pediatr Endocrinol Metab. 1998;11:657–664. doi: 10.1515/jpem.1998.11.5.657. [DOI] [PubMed] [Google Scholar]
- Lye SJ, Mitchell J, Nashman N, Oldenhof A, Ou R, Shynlova O, Langille L. Role of mechanical signals in the onset of term and preterm labor. Front Horm Res. 2001;27:165–178. doi: 10.1159/000061025. [DOI] [PubMed] [Google Scholar]
- Lynn KN, Steinkeler JA, Wilkins-Haug LE, Benson CB. Hyperreactio luteinalis (enlarged ovaries) during the second and third trimesters of pregnancy: common clinical associations. J Ultrasound Med. 2013;32:1285–1289. doi: 10.7863/ultra.32.7.1285. [DOI] [PubMed] [Google Scholar]
- Macleod DJ, Sharpe RM, Welsh M, Fisken M, Scott HM, Hutchison GR, Drake AJ, van den Driesche S. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl. 2010;33:279–287. doi: 10.1111/j.1365-2605.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Russell DW. Male and female isoenzymes of steroid 5alpha-reductase. Rev Reprod. 1999;4:179–183. doi: 10.1530/ror.0.0040179. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Cala KM, Russell DW. 5 alpha-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10:380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981–992. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Sakaguchi M, Nakajima A. Initiation of uterine contractions by purely mechanical stretching of the uterus at midpregnancy. Int J Biol Res Pregnancy. 1981;2:63–69. [PubMed] [Google Scholar]
- Maradny E, Kanayama N, Maehara K, Kobayashi T, Terao T. Dehydroepiandrosterone sulfate potentiates the effect of interleukin-8 on the cervix. Gynecol Obstet Invest. 1996;42:191–195. doi: 10.1159/000291952. [DOI] [PubMed] [Google Scholar]
- Marchetti PM, Barth JH. Clinical biochemistry of dihydrotestosterone. Ann Clin Biochem. 2013;50:95–107. doi: 10.1258/acb.2012.012159. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Tateishi Y, Matsuda M, Hiramatrsu Y. Hyperreactio luteinalis with both markedly elevated human chorionic gonadotropin levels and an imbalance of angiogenic factors subsequently developed severe early-onset preeclampsia. Fertil Steril. 2009;92 doi: 10.1016/j.fertnstert.2009.04.002. 393, e391–393. [DOI] [PubMed] [Google Scholar]
- Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, Yoon BH. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–99. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- McGregor JA, Leff M, Orleans M, Baron A. Fetal gender differences in preterm birth: findings in a North American cohort. Am J Perinatol. 1992;9:43–48. doi: 10.1055/s-2007-994668. [DOI] [PubMed] [Google Scholar]
- Mecenas CA, Giussani DA, Owiny JR, Jenkins SL, Wu WX, Honnebier BO, Lockwood CJ, Kong L, Guller S, Nathanielsz PW. Production of premature delivery in pregnant rhesus monkeys by androstenedione infusion. Nat Med. 1996;2:443–448. doi: 10.1038/nm0496-443. [DOI] [PubMed] [Google Scholar]
- Meldrum E, Parker PJ, Carozzi A. The PtdIns-PLC superfamily and signal transduction. Biochim Biophys Acta. 1991;1092:49–71. doi: 10.1016/0167-4889(91)90177-y. [DOI] [PubMed] [Google Scholar]
- Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- Milewich L, Gomez-Sanchez C, Madden JD, Bradfield DJ, Parker PM, Smith SL, Carr BR, Edman CD, MacDonald PC. Dehydroisoandrosterone sulfate in peripheral blood of premenopausal, pregnant and postmenopausal women and men. J Steroid Biochem. 1978;9:1159–1164. doi: 10.1016/0022-4731(78)90006-7. [DOI] [PubMed] [Google Scholar]
- Milewich L, MacDonald PC, Guerami A, Midgett WT, Lassiter WL, Carr BR. Human fetal liver estrogen 16 alpha-hydroxylase: precursor specificity, kinetic parameters, and in vitro regulation. J Clin Endocrinol Metab. 1986;63:180–191. doi: 10.1210/jcem-63-1-180. [DOI] [PubMed] [Google Scholar]
- Mitchell FL, Shackleton CH. The investigation of steroid metabolism in early infancy. Adv Clin Chem. 1969;12:141–215. doi: 10.1016/s0065-2423(08)60259-0. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Lobotsky J, Lloyd CW, Kobayashi T, Murasawa Y. Plasma androstenedione and testerone during pregnancy and in the newborn. J Clin Endocrinol Metab. 1968;28:1133–1142. doi: 10.1210/jcem-28-8-1133. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Maruo T. Effect of dehydroepiandrosterone sulfate on uterine cervical ripening in late pregnancy. Acta Physiol Hung. 1985;65:267–274. [PubMed] [Google Scholar]
- Mochizuki M, Honda T, Deguchi M, Morikawa H, Tojo S. A study on the effect of dehydroepiandrosterone sulfate on so-called cervical ripening. Acta Obstet Gynecol Scand. 1978a;57:397–401. doi: 10.3109/00016347809156518. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Honda T, Tojo S. Collagenolytic activity and steroid levels after administration of dehydroepiandrosterone sulfate. Int J Gynaecol Obstet. 1978b;16:248–253. doi: 10.1002/j.1879-3479.1978.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Montano LM, Calixto E, Figueroa A, Flores-Soto E, Carbajal V, Perusquia M. Relaxation of androgens on rat thoracic aorta: testosterone concentration dependent agonist/antagonist L-type Ca2+ channel activity, and 5beta-dihydrotestosterone restricted to L-type Ca2+ channel blockade. Endocrinology. 2008;149:2517–2526. doi: 10.1210/en.2007-1288. [DOI] [PubMed] [Google Scholar]
- Morisset AS, Dube MC, Drolet R, Pelletier M, Labrie F, Luu-The V, Tremblay Y, Robitaille J, John Weisnagel S, Tchernof A. Androgens in the maternal and fetal circulation: association with insulin resistance. J Matern Fetal Neonatal Med. 2013;26:513–519. doi: 10.3109/14767058.2012.735725. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Khalil RA. Decreased [Ca(2+)](i) during inhibition of coronary smooth muscle contraction by 17beta-estradiol, progesterone, and testosterone. J Pharmacol Exp Ther. 1999;291:44–52. [PubMed] [Google Scholar]
- Nakajima A. Action potential of human myometrial fibers. Am J Obstet Gynecol. 1971;111:266–269. doi: 10.1016/0002-9378(71)90900-8. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Jenkins SL, Tame JD, Winter JA, Guller S, Giussani DA. Local paracrine effects of estradiol are central to parturition in the rhesus monkey. Nat Med. 1998;4:456–459. doi: 10.1038/nm0498-456. [DOI] [PubMed] [Google Scholar]
- Nishinaka K, Fukuda Y. Changes in extracellular matrix materials in the uterine myometrium of rats during pregnancy and postparturition. Acta Pathol Jpn. 1991;41:122–132. doi: 10.1111/j.1440-1827.1991.tb02507.x. [DOI] [PubMed] [Google Scholar]
- Noble K, Matthew A, Burdyga T, Wray S. A review of recent insights into the role of the sarcoplasmic reticulum and Ca entry in uterine smooth muscle. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl. 1):S11–S19. doi: 10.1016/j.ejogrb.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Norman JE. Preterm labour. Cervical function and prematurity. Best Pract Res Clin Obstet Gynaecol. 2007;21:791–806. doi: 10.1016/j.bpobgyn.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Norman M, Ekman G, Malmstrom A. Changed proteoglycan metabolism in human cervix immediately after spontaneous vaginal delivery. Obstet Gynecol. 1993;81:217–223. [PubMed] [Google Scholar]
- Norman JE, Bollapragada S, Yuan M, Nelson SM. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth. 2007;7(Suppl. 1):S7. doi: 10.1186/1471-2393-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novy MJ, Walsh SW. Dexamethasone and estradiol treatment in pregnant rhesus macaques: effects on gestational length, maternal plasma hormones, and fetal growth. Am J Obstet Gynecol. 1983;145:920–931. doi: 10.1016/0002-9378(83)90841-4. [DOI] [PubMed] [Google Scholar]
- O'Brien M, O'Shaughnessy D, Ahamide E, Morrison JJ, Smith TJ. Differential expression of the metalloproteinase MMP3 and the alpha5 integrin subunit in human myometrium at labour. Mol Hum Reprod. 2007;13:655–661. doi: 10.1093/molehr/gam047. [DOI] [PubMed] [Google Scholar]
- Okon MA, Laird SM, Tuckerman EM, Li TC. Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil Steril. 1998;69:682–690. doi: 10.1016/s0015-0282(98)00007-7. [DOI] [PubMed] [Google Scholar]
- O'Leary P, Boyne P, Flett P, Beilby J, James I. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin Chem. 1991;37:667–672. [PubMed] [Google Scholar]
- Ortega A, Mas-Oliva J. Cholesterol effect on enzyme activity of the sarcolemmal (Ca2+ + Mg2+)-ATPase from cardiac muscle. Biochim Biophys Acta. 1984;773:231–236. doi: 10.1016/0005-2736(84)90086-5. [DOI] [PubMed] [Google Scholar]
- Pasanen M, Pelkonen O. The expression and environmental regulation of P450 enzymes in human placenta. Crit Rev Toxicol. 1994;24:211–229. doi: 10.3109/10408449409021606. [DOI] [PubMed] [Google Scholar]
- Penning TM. New frontiers in androgen biosynthesis and metabolism. Curr Opin Endocrinol Diabetes Obes. 2010;17:233–239. doi: 10.1097/MED.0b013e3283381a31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira de Jesus-Tran K, Cote PL, Cantin L, Blanchet J, Labrie F, Breton R. Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity. Protein Sci. 2006;15:987–999. doi: 10.1110/ps.051905906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusquia MC. G. Inhibitory effect of androgens and progestins on the contraction induced by oxytocin in the rat myometrium. Med Sci Res. 1991;19:177–179. [Google Scholar]
- Perusquia M, Garcia-Yanez E, Ibanez R, Kubli-Garfias C. Non-genomic mechanism of action of delta-4 and 5-reduced androgens and progestins on the contractility of the isolated rat myometrium. Life Sci. 1990;47:1547–1553. doi: 10.1016/0024-3205(90)90183-r. [DOI] [PubMed] [Google Scholar]
- Perusquia M, Campos G, Corona JL, Kubli-Garfias C. Antagonism by 5-reduced steroids of the tonic and phasic contractions induced by serotonin in the isolated rat uterus. Proc West Pharmacol Soc. 1991a;34:395–398. [PubMed] [Google Scholar]
- Perusquia M, Corona JL, Kubli-Garfias C. Inhibitory effect of 5-reduced androgens and progestins on the uterine contraction induced by acetylcholine. Proc West Pharmacol Soc. 1991b;34:89–92. [PubMed] [Google Scholar]
- Perusquia M, Navarrete E, Jasso-Kamel J, Montano LM. Androgens induce relaxation of contractile activity in pregnant human myometrium at term: a nongenomic action on L-type calcium channels. Biol Reprod. 2005;73:214–221. doi: 10.1095/biolreprod.104.036954. [DOI] [PubMed] [Google Scholar]
- Phelan N, Conway GS. Management of ovarian disease in pregnancy. Best Pract Res Clin Endocrinol Metab. 2011;25:985–992. doi: 10.1016/j.beem.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Piltonen T, Koivunen R, Morin-Papunen L, Ruokonen A, Huhtaniemi IT, Tapanainen JS. Ovarian and adrenal steroid production: regulatory role of LH/HCG. Hum Reprod. 2002;17:620–624. doi: 10.1093/humrep/17.3.620. [DOI] [PubMed] [Google Scholar]
- Pion R, Jaffe R, Eriksson G, Wiqvist N, Diczfalusy E. Studies on the metabolism of c-21 steroids in the human foeto-placental unit. I. Formation of a beta-unsaturated 3-ketones in midterm placentas perfused in situ with pregnenolone and 17-alpha-hydroxypregnenolone. Acta Endocrinol (Copenh) 1965;48:234–248. [PubMed] [Google Scholar]
- Pluciennik F, Verrecchia F, Bastide B, Herve JC, Joffre M, Deleze J. Reversible interruption of gap junctional communication by testosterone propionate in cultured Sertoli cells and cardiac myocytes. J Membr Biol. 1996;149:169–177. doi: 10.1007/s002329900017. [DOI] [PubMed] [Google Scholar]
- Pressman EK, Tucker JA, Jr, Anderson NC, Jr, Young RC. Morphologic and electrophysiologic characterization of isolated pregnant human myometrial cells. Am J Obstet Gynecol. 1988;159:1273–1279. doi: 10.1016/0002-9378(88)90463-2. [DOI] [PubMed] [Google Scholar]
- Pulkkinen MO, Nyman S, Hamalainen MM, Mattinen J. Proton NMR spectroscopy of the phospholipids in human uterine smooth muscle and placenta. Gynecol Obstet Invest. 1998;46:220–224. doi: 10.1159/000010038. [DOI] [PubMed] [Google Scholar]
- Purohit A, Foster PA. Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers. J Endocrinol. 2012;212:99–110. doi: 10.1530/JOE-11-0266. [DOI] [PubMed] [Google Scholar]
- Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol. 2013;9:479–493. doi: 10.1038/nrendo.2013.122. [DOI] [PubMed] [Google Scholar]
- Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction. 2007;134:327–340. doi: 10.1530/REP-07-0032. [DOI] [PubMed] [Google Scholar]
- Rehman KS, Yin S, Mayhew BA, Word RA, Rainey WE. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. Mol Hum Reprod. 2003;9:681–700. doi: 10.1093/molehr/gag078. [DOI] [PubMed] [Google Scholar]
- Richardson MC, Masson GM. Stimulation by human chorionic gonadotrophin of oestradiol production by dispersed cells from human corpus luteum: comparison with progesterone production; utilization of exogenous testosterone. J Endocrinol. 1981;91:197–203. doi: 10.1677/joe.0.0910197. [DOI] [PubMed] [Google Scholar]
- Rivarola MA, Forest MG, Migeon CJ. Testosterone, androstenedione and dehydroepiandrosterone in plasma during pregnancy and at delivery: concentration and protein binding. J Clin Endocrinol Metab. 1968;28:34–40. doi: 10.1210/jcem-28-1-34. [DOI] [PubMed] [Google Scholar]
- Rivas A, Fisher JS, McKinnell C, Atanassova N, Sharpe RM. Induction of reproductive tract developmental abnormalities in the male rat by lowering androgen production or action in combination with a low dose of diethylstilbestrol: evidence for importance of the androgen-estrogen balance. Endocrinology. 2002;143:4797–4808. doi: 10.1210/en.2002-220531. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Judd HL, Young PE, Jones OW, Yen SS. Amniotic fluid androgens and estrogens in midgestation. J Clin Endocrinol Metab. 1977;45:755–761. doi: 10.1210/jcem-45-4-755. [DOI] [PubMed] [Google Scholar]
- Robson Reactions of the uterine muscle and endometrium of rabbit to testosterone. Quart J Exp Physiol. 1937;35:355–363. [Google Scholar]
- Rodeck CH, Gill D, Rosenberg DA, Collins WP. Testosterone levels in midtrimester maternal and fetal plasma and amniotic fluid. Prenat Diagn. 1985;5:175–181. doi: 10.1002/pd.1970050303. [DOI] [PubMed] [Google Scholar]
- Roh CR, Oh WJ, Yoon BK, Lee JH. Up-regulation of matrix metalloproteinase-9 in human myometrium during labour: a cytokine-mediated process in uterine smooth muscle cells. Mol Hum Reprod. 2000;6:96–102. doi: 10.1093/molehr/6.1.96. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano GM, Leonardo F, Pagnotta P, Pelliccia F, Panina G, Cerquetani E, della Monica PL, Bonfigli B, Volpe M, Chierchia SL. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670. doi: 10.1161/01.cir.99.13.1666. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Androgen excess in women—a health hazard? Med Hypotheses. 2006;67:229–234. doi: 10.1016/j.mehy.2005.09.057. [DOI] [PubMed] [Google Scholar]
- Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, Goh VH, Ridgway EC, Wierman ME. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids. 2011;76:177–182. doi: 10.1016/j.steroids.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JM, Forest MG, Morera AM, Bertrand J. Metabolic clearance rate and blood production rate of testosterone and dihydrotestosterone in normal subjects, during pregnancy, and in hyperthyroidism. J Clin Invest. 1972;51:1226–1234. doi: 10.1172/JCI106917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakyo K, Ito A, Mori Y. Effects of dehydroepiandrosterone sulphate on the production of collagenase and gelatinolytic metalloproteinase by rabbit uterine cervical cells in primary cultures. J Pharmacobiodyn. 1986;9:276–286. doi: 10.1248/bpb1978.9.276. [DOI] [PubMed] [Google Scholar]
- Sakyo K, Ito A, Mori Y. Dehydroepiandrosterone sulfate stimulates collagenase synthesis without affecting the rates of collagen and noncollagen protein syntheses by rabbit uterine cervical fibroblasts. Biol Reprod. 1987;36:277–281. doi: 10.1095/biolreprod36.2.277. [DOI] [PubMed] [Google Scholar]
- Sanders SL, Stouffer RL, Brannian JD. Androgen production by monkey luteal cell subpopulations at different stages of the menstrual cycle. J Clin Endocrinol Metab. 1996;81:591–596. doi: 10.1210/jcem.81.2.8636273. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Nakano R, Kadoya Y, Iwao M, Shima K, Sowa M. Cervical ripening with dehydroepiandrosterone sulphate. Br J Obstet Gynaecol. 1982;89:195–198. doi: 10.1111/j.1471-0528.1982.tb03612.x. [DOI] [PubMed] [Google Scholar]
- Sathishkumar K, Balakrishnan M, Chinnathambi V, Chauhan M, Hankins GD, Yallampalli C. Fetal sex-related dysregulation in testosterone production and their receptor expression in the human placenta with preeclampsia. J Perinatol. 2012;32:328–335. doi: 10.1038/jp.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzel WC, Kruggel WG, Brodie HJ. Studies on the mechanism of estrogen biosynthesis. 8. The development of inhibitors of the enzyme system in human placenta. Endocrinology. 1973;92:866–880. doi: 10.1210/endo-92-3-866. [DOI] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, De Gendt K, Verhoeven G, O'Shaughnessy P, Sharpe RM. Role of androgens in fetal testis development and dysgenesis. Endocrinology. 2007;148:2027–2036. doi: 10.1210/en.2006-1622. [DOI] [PubMed] [Google Scholar]
- Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24:1393–1403. doi: 10.1210/me.2010-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifzadeh F, Kashanian M, Fatemi F. A comparison of serum androgens in pre-eclamptic and normotensive pregnant women during the third trimester of pregnancy. Gynecol Endocrinol. 2012;28:834–836. doi: 10.3109/09513590.2012.683061. [DOI] [PubMed] [Google Scholar]
- Shmigol A, Eisner DA, Wray S. Carboxyeosin decreases the rate of decay of the [Ca2+]i transient in uterine smooth muscle cells isolated from pregnant rats. Pflugers Arch. 1998;437:158–160. doi: 10.1007/s004240050761. [DOI] [PubMed] [Google Scholar]
- Shmygol A, Noble K, Wray S. Depletion of membrane cholesterol eliminates the Ca2+-activated component of outward potassium current and decreases membrane capacitance in rat uterine myocytes. J Physiol. 2007;581:445–456. doi: 10.1113/jphysiol.2007.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shozu M, Akasofu K, Harada T, Kubota Y. A new cause of female pseudohermaphroditism: placental aromatase deficiency. J Clin Endocrinol Metab. 1991;72:560–566. doi: 10.1210/jcem-72-3-560. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Mitchell JA, Tsampalieros A, Langille BL, Lye SJ. Progesterone and gravidity differentially regulate expression of extracellular matrix components in the pregnant rat myometrium. Biol Reprod. 2004;70:986–992. doi: 10.1095/biolreprod.103.023648. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Tsui P, Dorogin A, Langille BL, Lye SJ. Insulin-like growth factors and their binding proteins define specific phases of myometrial differentiation during pregnancy in the rat. Biol Reprod. 2007;76:571–578. doi: 10.1095/biolreprod.106.056929. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl. 1):S2–10. doi: 10.1016/j.ejogrb.2009.02.044. [DOI] [PubMed] [Google Scholar]
- Siiteri PK, MacDonald PC. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab. 1966;26:751–761. doi: 10.1210/jcem-26-7-751. [DOI] [PubMed] [Google Scholar]
- Siiteri PK, Simberg NH. Changing concepts of active androgens in blood. Clin Endocrinol Metab. 1986;15:247–258. doi: 10.1016/s0300-595x(86)80023-8. [DOI] [PubMed] [Google Scholar]
- Simon C, Einspanier A. The hormonal induction of cervical remodeling in the common marmoset monkey (Callithrix jacchus) Reproduction. 2009;137:517–525. doi: 10.1530/REP-08-0417. [DOI] [PubMed] [Google Scholar]
- Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- Simsek Y, Celen S, Ustun Y, Danisman N, Bayramoglu H. Severe preeclampsia and fetal virilization in a spontaneous singleton pregnancy complicated by hyperreactio luteinalis. Eur Rev Med Pharmacol Sci. 2012;16:118–121. [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburu B, Gazitua R, Recabarren S, Cassorla F. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122–2126. doi: 10.1093/humrep/dei009. [DOI] [PubMed] [Google Scholar]
- Slater DM, Dennes WJ, Campa JS, Poston L, Bennett PR. Expression of cyclo-oxygenase types-1 and -2 in human myometrium throughout pregnancy. Mol Hum Reprod. 1999;5:880–884. doi: 10.1093/molehr/5.9.880. [DOI] [PubMed] [Google Scholar]
- Slayden OD, Nayak NR, Burton KA, Chwalisz K, Cameron ST, Critchley HO, Baird DT, Brenner RM. Progesterone antagonists increase androgen receptor expression in the rhesus macaque and human endometrium. J Clin Endocrinol Metab. 2001;86:2668–2679. doi: 10.1210/jcem.86.6.7606. [DOI] [PubMed] [Google Scholar]
- Slomczynska M, Duda M, Burek M, Knapczyk K, Czaplicki D, Koziorowski M. Distribution of androgen receptor in the porcine uterus throughout pregnancy. Reprod Domest Anim. 2008;43:35–41. doi: 10.1111/j.1439-0531.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- Smith R. Parturition. N Engl J Med. 2007;356:271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- Smith RD, Babiychuk EB, Noble K, Draeger A, Wray S. Increased cholesterol decreases uterine activity: functional effects of cholesterol alteration in pregnant rat myometrium. Am J Physiol Cell Physiol. 2005;288:C982–C988. doi: 10.1152/ajpcell.00120.2004. [DOI] [PubMed] [Google Scholar]
- Smith R, Paul J, Maiti K, Tolosa J, Madsen G. Recent advances in understanding the endocrinology of human birth. Trends Endocrinol Metab. 2012;23:516–523. doi: 10.1016/j.tem.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR. Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod. 2004;10:109–113. doi: 10.1093/molehr/gah021. [DOI] [PubMed] [Google Scholar]
- Sparey C, Robson SC, Bailey J, Lyall F, Europe-Finner GN. The differential expression of myometrial connexin-43, cyclooxygenase-1 and -2, and Gs alpha proteins in the upper and lower segments of the human uterus during pregnancy and labor. J Clin Endocrinol Metab. 1999;84:1705–1710. doi: 10.1210/jcem.84.5.5644. [DOI] [PubMed] [Google Scholar]
- Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Nixon WE, Gulyas BJ, Hodgen GD. Gonadotropin-sensitive progesterone production by rhesus monkey luteal cells in vitro: a function of age of the corpus luteum during the menstrual cycle. Endocrinology. 1977;100:506–512. doi: 10.1210/endo-100-2-506. [DOI] [PubMed] [Google Scholar]
- Strauss JF, 3rd, Martinez F, Kiriakidou M. Placental steroid hormone synthesis: unique features and unanswered questions. Biol Reprod. 1996;54:303–311. doi: 10.1095/biolreprod54.2.303. [DOI] [PubMed] [Google Scholar]
- Tagawa N, Hidaka Y, Takano T, Shimaoka Y, Kobayashi Y, Amino N. Serum concentrations of dehydroepiandrosterone and dehydroepiandrosterone sulfate and their relation to cytokine production during and after normal pregnancy. Clin Chim Acta. 2004;340:187–193. doi: 10.1016/j.cccn.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ikeno N, Hoshiai H, Suzuki M. The effect of dehydroepiandrosterone sulfate on serum steroid concentration in singleton and twin pregnancies and its effect on cervical canal-ripening in singleton pregnancies. Tohoku J Exp Med. 1984;142:289–298. doi: 10.1620/tjem.142.289. [DOI] [PubMed] [Google Scholar]
- Tep-areenan P, Kendall DA, Randall MD. Testosterone-induced vasorelaxation in the rat mesenteric arterial bed is mediated predominantly via potassium channels. Br J Pharmacol. 2002;135:735–740. doi: 10.1038/sj.bjp.0704522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzidou V, Sooranna SR, Kim LU, Thornton S, Bennett PR, Johnson MR. Mechanical stretch up-regulates the human oxytocin receptor in primary human uterine myocytes. J Clin Endocrinol Metab. 2005;90:237–246. doi: 10.1210/jc.2004-0277. [DOI] [PubMed] [Google Scholar]
- Thiery M, De Gezelle H, Van Kets H, Voorhoof L, Verheugen C, Smis B, Gerris J, Derom R, Martens G. The effect of locally administered estrogens on the human cervix. Z Geburtshilfe Perinatol. 1979;183:448–452. [PubMed] [Google Scholar]
- Thomas P, Dressing G, Pang Y, Berg H, Tubbs C, Benninghoff A, Doughty K. Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids. 2006;71:310–316. doi: 10.1016/j.steroids.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83:215–242. doi: 10.1139/y05-016. [DOI] [PubMed] [Google Scholar]
- Tica AA, Dun EC, Tica OS, Gao X, Arterburn JB, Brailoiu GC, Oprea TI, Brailoiu E. G protein-coupled estrogen receptor 1-mediated effects in the rat myometrium. Am J Physiol Cell Physiol. 2011;301:C1262–C1269. doi: 10.1152/ajpcell.00501.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyasu BA, Chen CJ, Marshall JM. Comparison of the activity of circular and longitudinal myometrium from pregnant rats: co-ordination between muscle layers. Clin Exp Pharmacol Physiol. 1988;15:647–656. doi: 10.1111/j.1440-1681.1988.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone (DHEA)—a precursor steroid or an active hormone in human physiology. J Sex Med. 2011;8:2960–2982. doi: 10.1111/j.1743-6109.2011.02523.x. quiz 2983. [DOI] [PubMed] [Google Scholar]
- Tsai ML, Cesen-Cummings K, Webb RC, Loch-Caruso R. Acute inhibition of spontaneous uterine contractions by an estrogenic polychlorinated biphenyl is associated with disruption of gap junctional communication. Toxicol Appl Pharmacol. 1998;152:18–29. doi: 10.1006/taap.1998.8516. [DOI] [PubMed] [Google Scholar]
- Umezaki H, Hess DL, Valenzuela GJ, Ducsay CA. Fetectomy alters maternal pituitary-adrenal function in pregnant rhesus macaques. Biol Reprod. 2001;65:1616–1621. doi: 10.1095/biolreprod65.5.1616. [DOI] [PubMed] [Google Scholar]
- Van Bommel T, Marsen T, Bojar H. Effects of high-dose medroxyprogesterone acetate and various other steroid hormones on plasma membrane lipid mobility in CAMA-1 mammary cancer cells. Anticancer Res. 1987;7:1217–1223. [PubMed] [Google Scholar]
- van der Kwast TH, Dommerholt HB, van Vroonhoven CC, Chadha S. Androgen receptor expression in the cervix of androgen-treated female-to-male transsexuals: association with morphology and chain-specific keratin expression. Int J Gynecol Pathol. 1994;13:133–138. doi: 10.1097/00004347-199404000-00006. [DOI] [PubMed] [Google Scholar]
- Van Holsbeke C, Amant F, Veldman J, De Boodt A, Moerman P, Timmerman D. Hyperreactio luteinalis in a spontaneously conceived singleton pregnancy. Ultrasound Obstet Gynecol. 2009;33:371–373. doi: 10.1002/uog.6325. [DOI] [PubMed] [Google Scholar]
- van Meer G. Lipid traffic in animal cells. Annu Rev Cell Biol. 1989;5:247–275. doi: 10.1146/annurev.cb.05.110189.001335. [DOI] [PubMed] [Google Scholar]
- Vejrazkova D, Vcelak J, Vankova M, Lukasova P, Bradnova O, Halkova T, Kancheva R, Bendlova B. Steroids and insulin resistance in pregnancy. J Steroid Biochem Mol Biol. 2014;139:122–129. doi: 10.1016/j.jsbmb.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Veleminsky M., Jr Hyperreactio luteinalis in spontaneous singleton pregnancy. Neuro Endocrinol Lett. 2010;31:304–305. [PubMed] [Google Scholar]
- Verbist J, Gadella TW, Jr, Raeymaekers L, Wuytack F, Wirtz KW, Casteels R. Phosphoinositide-protein interactions of the plasma-membrane Ca2(+)-transport ATPase as revealed by fluorescence energy transfer. Biochim Biophys Acta. 1991;1063:1–6. doi: 10.1016/0005-2736(91)90345-9. [DOI] [PubMed] [Google Scholar]
- Vermeirsch H, Van den Broeck W, Coryn M, Simoens P. Immunohistochemical detection of androgen receptors in the canine uterus throughout the estrus cycle. Theriogenology. 2002;57:2203–2216. doi: 10.1016/s0093-691x(02)00908-1. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Ando S. Metabolic clearance rate and interconversion of androgens and the influence of the free androgen fraction. J Clin Endocrinol Metab. 1979;48:320–326. doi: 10.1210/jcem-48-2-320. [DOI] [PubMed] [Google Scholar]
- Vladic-Stjernholm Y, Vladic T, Blesson CS, Ekman-Ordeberg G, Sahlin L. Prostaglandin treatment is associated with a withdrawal of progesterone and androgen at the receptor level in the uterine cervix. Reprod Biol Endocrinol. 2009;7:116. doi: 10.1186/1477-7827-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biol Reprod. 2008;78:380–389. doi: 10.1095/biolreprod.107.064089. [DOI] [PubMed] [Google Scholar]
- Webley GE, Hearn JP. Local production of progesterone by the corpus luteum of the marmoset monkey in response to perfusion with chorionic gonadotrophin and melatonin in vivo. J Endocrinol. 1987;112:449–457. doi: 10.1677/joe.0.1120449. [DOI] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118:1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke TJ, Utley DJ. Total testosterone, free-androgen index, calculated free testosterone, and free testosterone by analog RIA compared in hirsute women and in otherwise-normal women with altered binding of sex-hormone-binding globulin. Clin Chem. 1987;33:1372–1375. [PubMed] [Google Scholar]
- Willows RL. Urinary estriol estimations in normal and abnormal pregnancies. Can Med Assoc J. 1966;94:1098–1101. [PMC free article] [PubMed] [Google Scholar]
- Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol. 1996;120:51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- Witayavanitkul N, Woranush W, Bupha-Intr T, Wattanapermpool J. Testosterone regulates cardiac contractile activation by modulating SERCA but not NCX activity. Am J Physiol Heart Circ Physiol. 2013;304:H465–H472. doi: 10.1152/ajpheart.00555.2012. [DOI] [PubMed] [Google Scholar]
- Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- Wray S, Jones K, Kupittayanant S, Li Y, Matthew A, Monir-Bishty E, Noble K, Pierce SJ, Quenby S, Shmygol AV. Calcium signaling and uterine contractility. J Soc Gynecol Investig. 2003;10:252–264. doi: 10.1016/s1071-5576(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium. 2005;38:397–407. doi: 10.1016/j.ceca.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Feigenbaum SL, Crites Y, Escobar GJ, Yang J, Ferrara A, Lo JC. Risk of preterm delivery in non-diabetic women with polycystic ovarian syndrome. J Perinatol. 2012;32:770–776. doi: 10.1038/jp.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Oshima S, Matsuo K, Ito K, Ito A, Mori Y. Pharmacological studies of intravaginally applied dehydroepiandrosterone sulfate (DHA-S) Nihon Yakurigaku Zasshi. 1991;98:31–39. doi: 10.1254/fpj.98.1_31. [DOI] [PubMed] [Google Scholar]
- Yang X, Guo Z, Sun F, Li W, Alfano A, Shimelis H, Chen M, Brodie AM, Chen H, Xiao Z, et al. Novel membrane-associated androgen receptor splice variant potentiates proliferative and survival responses in prostate cancer cells. J Biol Chem. 2011;286:36152–36160. doi: 10.1074/jbc.M111.265124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin J, Saurel-Cubizolles MJ, De Mouzon J, Rivera L, Ancel PY, Blondel B, Kaminski M. Fetal sex and preterm birth: are males at greater risk? Hum Reprod. 2002;17:2762–2768. doi: 10.1093/humrep/17.10.2762. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bricker L, Wray S, Quenby S. Poor uterine contractility in obese women. BJOG. 2007;114:343–348. doi: 10.1111/j.1471-0528.2006.01233.x. [DOI] [PubMed] [Google Scholar]