Abstract
Background
Our long-term field survey revealed that the Cardinium infection rate in Bemisia tabaci Q (also known as biotype Q) population was low in Shandong, China over the past few years. We hypothesize that (1) the Cardinium-infected (C +) B. tabaci Q population cannot efficiently compete with the Cardinium-uninfected (C −) B. tabaci Q population; (2) no reproductive isolation may have occurred between C + and C −; and (3) the C − population has higher fitness than the C + population.
Methodology and Results
To reveal the differences in competitive ability and fitness between the two introduced populations (C + and C −), competition between C + and C − was examined over several generations. Subsequently, the reproductive isolation between C + and C − was studied by crossing C + with C − individuals, and the fitnesses of C + and C − populations were compared using a two-sex life table method. Our results demonstrate that the competitive ability of the C + whiteflies was weaker than that of C −. There is that no reproductive isolation occurred between the two populations and the C − population had higher fitness than the C + population.
Conclusion
The competitive ability and fitness differences of two populations may explain why C − whitefly populations have been dominant during the past few years in Shandong, China. However, the potential role Cardinium plays in whitefly should be further explored.
Introduction
The sweet potato whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodoidea), is a major crop pest [1], [2] and this species complex contains at least 31 cryptic species identified based on mitochondrial cytochrome oxidase I (mtCOI) sequences and crossing experiments [3], [4]. The best known species are MEAM1 (commonly known as biotype B, hereafter referred to as B. tabaci B or B) and MED (commonly known as biotype Q, hereafter referred to as B. tabaci Q or Q) because both are extremely invasive and globally distributed [3]. B. tabaci Q was first detected on the ornamental poinsettia (Euphorbia pulcherrima Willd.) in China in 2003 [5]. Since then, it has gradually displaced B. tabaci B which was introduced in the mid-1990s. Since 2008, B. tabaci Q has become the dominant whitefly species in most regions of China [6]–[9].
The symbiont Cardinium was first found in cell cultures established from the tick Ixodes scapularis Say [10] and was named Candidatus Cardinium hertigii by Zchori-Fein et al. [11]. Cardinium can alter the reproduction of its hosts by feminization [12], [13], parthenogenesis [14], or cytoplasmic incompatibility of infected hosts [15]–[17]. Prior studies showed that infection with this symbiont can improve the fitness of its host [18]–[20]. Harris et al. [21] reported that Cardinium infection frequency can increase greatly within the wasp Encarsia pergandiella population after nine generations. However, our long-term field survey revealed that the Cardinium infection rate in B. tabaci Q populations was low (7.6% to17.3%) in Shandong, China, during 2006–2009 [22], though the whitefly may have 10–12 generations per year in this area. Pan et al. [23] also reported that the infection frequency of Cardinium in B. tabaci Q was not very high (16%) in 61 localities in 19 provinces of China in 2009.
On the basis of these data, we hypothesize that (1) the Cardinium-infected (C +) B. tabaci Q population cannot efficiently compete with the Cardinium-uninfected (C −) B. tabaci Q population; (2) no reproductive isolation may have occurred between C + and C −, and if there had been reproductive isolation between them, C + would have been completely displaced by C − after long-term coexistence in the field; and (3) the C + population has higher fitness than the C − population.
To reveal the differences in competitive ability and fitness between the two introduced populations (C + and C −), competition between C + and C − was examined over several generations. Subsequently, the reproductive isolation between C + and C − was studied by crossing C + with C − individuals, and the fitnesses of C + and C − populations were compared using a two-sex life table method [24].
Materials and Methods
Ethics Statement
The research complies with all laws of the country (China) in which it was performed and was approved by the Department of Science and Technology of the Qingdao Agricultural University, China (permit number: 20110712).
Bemisia Tabaci Laboratory Population
The stock population of B. tabaci was obtained from laboratory colonies established from prior field collections. The C + and C − populations were provided cotton plants and maintained in isolated whitefly-proof screen cages in a greenhouse under controlled lighting and constant temperature (27±1°C) for about ten generations. The primary symbiont, Porteria, as well as secondary symbionts belonging to the genera Arsenophonus, Cardinium, Hamiltonella, Rickttisia, and Wolbachia, have been detected in B. tabaci Q [22], [25]–[27]. Using the specific primers of the primary symbiont, Portiera, and the secondary symbionts, Arsenophonus, Cardinium, Fritschea, Hamiltonella, Rickettsia, and Wolbachia, we found that the C + and C − populations were also infected with Portiera and Hamiltonella. Both C + and C − populations were maintained in separate cultures on potted cotton plants, Lu-Mian-Yan 21 cultivar. The purity of each of the cultures was monitored every 30 days by sampling 20 adults using PCR. Cotton plants were cultivated in 1.5 L plastic pots with nutritional soil and enclosed in whitefly-proof screen cages under controlled light and temperature in a screen house. Three pesticide-free, insect-free, young potted cotton plants were used in the large cages (40 cm×25 cm×50 cm). Plants were at the five to seven fully expanded true leaf stage. Plants were watered and replaced as necessary. All experiments were conducted in controlled climate chambers (27±1°C, 16L: 8D, and 60±5% RH).
Bemisia Tabaci Species Determination and Detection of Cardinium
Adult whiteflies were collected with a hand-held aspirator, preserved immediately in 95% ethanol, and stored at −20°C until processing. Genomic DNA was extracted from individual adult whiteflies of each collection using the DNAzol kit (Molecular Research Center, Inc., Cincinnati, OH) and stored at −20°C for subsequent use. The cleavage amplified polymorphic sequence (CAPS) of the mtCOI gene was used to determine the species of B. tabaci. The primers used for detection of the species were C1-J-2195 (5′-TTGATTTTTTGGTCATCCAGAAGT-3′) [28] and R-BQ-2819 (5′-CTGAATATCGRCGAGGCATTCC −3′) [29]. All PCR reactions were performed in 20 µl buffer containing 2 µl 10× buffer, 1.5 mM MgCl2, 0.2 µM of each primer, 0.2 µM dNTPs, 1 unit Taq DNA polymerase, and 2 µl template DNA. Reaction conditions were as follows: 1 cycle of 94°C for 5 min, 35 cycles of 94°C for 1 min, 52°C for 1 min, 72°C for 1 min, and final extension at 72°C for 10 min. The presence of mtCOI amplicons was visualized by electrophoresis in 1.0% agarose gel electrophoresis and ethidium bromide staining. The mtCOI fragment (623 bp) was cleaved using the restriction endonuclease VspI [30]. Aliquots of the PCR products (13 µl) were each digested with 5 U VspI (in 20 µl total reaction volume) at 37°C for 2 h. Specimens whose mtCOI fragments were cut by VspI were identified as B. tabaci Q.
PCR detection of Cardinium was performed using the primers Car-sp-F (5′-CGG CTT ATT AAG TCA GTT GTG AAA TCC TAG-3′) and Car-sp-R (5′-TCC TTC CTC CCG CTT ACA CG-3′). All PCR reactions were performed in 20 µl of reaction buffer containing 1×buffer, 0.16 mM of each dNTP, 0.5 mM of each primer, 0.5 unit Taq DNA polymerase, and 2 µl template DNA. Reaction conditions were as follows: 1 cycle of 95°C for 1 min, 35 cycles of 95°C for 30s, 57°C for 30 s, 72°C for 1 min, and final extension at 72°C for 5 min. All PCR reactions included a negative control (sterile water instead of DNA) to detect DNA contamination, and a positive control (DNA from previous sequencing) to prevent false negatives. The PCR products (544 bp) were electrophoresed in a 1.0% agarose gel in TAE [31].
Identification and Analysis of Orthologous Genes between C+ and C − Populations
To reveal genetic divergence between C+ and C− populations, orthologs of cytochrome P450 genes in the transcriptomes of these populations were analyzed. About 20 ug of total RNA (≥300 ng/ul) from each population (C+ or C−) was sent to the Shanghai Sangon Institute for library preparation and sequencing on an Illumina HiSeq2000. Raw reads was obtained and de novo transcriptome assembly was done with the short-read assembly program trinity [32]. Pairs of sequences longer than 1000 bp that mapped unambiguously to cytochrome P450 in the Swissprot database (E value<1e−5) were selected as cytochrome P450 genes. Some P450 genes such as P450 4C1 gene has high variation in B. tabaci cryptic species [33].
Competition between C + and C − Populations
To compare the competitive abilities of C + and C − populations, we conducted a cage experiment and followed the frequencies of Cardinium infection in whiteflies raised on cotton over ten generations. To observe changes in the relative proportion of C + and C − individuals, 30 pairs of C + and 30 pairs of C − newly emerged adults whitefly cultures were released into a cage and the infection frequency of Cardinium was monitored every 25 days (approximately one generation) by sampling 50 adults using PCR. Infection frequency monitoring of Cardinium started from the 75th day. Three replicates were carried out for this study.
Crossing Experiments
To examine the possibility of reproductive isolation between C + and C −, we carried out crossing experiments between Cardinium-infected and uninfected populations, ♀C −×♂C −, ♀C −×♂C +, ♀C +×♂C −, and ♀C +×♂C +, using virgin whiteflies. To obtain newly emerged unmated adults for experiments, adults were allowed to emerge in isolation and were kept individually before crossing. In the evening, cotton leaves with whitefly pupae (late 4th instar nymphs with red eyes) were cut from plants, and individual pupae with the attached portion of the leaf were placed into a Petri dish. To maintain humidity, a moist filter paper was put on the bottom of the Petri dish. The next morning (at 7∶00 am), the newly emerged adults were collected and sexed using a stereomicroscope [34].
For each cross, newly emerged females were individually transferred onto a cotton seedling as described by Li et al. [35] together with three adult males. Each female was allowed to lay eggs for 72 h. Adults were then collected using a small aspirator and stored at −20°C for later PCR confirmation of identity. To determine the sex ratio, the eggs laid by each female were observed daily until adult emergence and the newly emerged adults were collected and sexed using a stereomicroscope. Data were analyzed using a one-way analysis of variance (ANOVA) and means were compared using a least significant difference (LSD) test. To normalize the data, an arcsine transformation was used for sex ratio.
Fitness Assessment of C + and C − Population using the Two-Sex Life Table Method
To reveal differences in the fitnesses of C + and C −, we analyzed the demographic parameters of the population using the two-sex life table method. For the life table study, the rearing containers were made of plastic pots (11.5 cm top diameter, 7.8 cm bottom diameter, and 15.5 cm height), with inverted plastic cups (11.5 cm top diameter, 7.8 cm bottom diameter, and 15.5 cm height) used as covers. The bottom of the plastic cup was cut out and covered with fine mesh cloth for ventilation. A cotton seedling at the two-true-leaves stage was used in this study, only one true leaf was kept on the seedling and the other leaf was removed.
For the life table study, approximately 15 pairs of whiteflies were transferred into rearing containers with a cotton seedling. Eggs laid within 24 h were collected and 15 eggs were used for the life table study. Other eggs were removed. For the C + or C − populations, 15 replicates were carried out. The developmental stage and survival status of individual eggs were recorded daily starting at day 14, which corresponded to the day before adult emergence. Using this method, the survival rate of eggs was determined.
To determine fecundity, newly emerged whiteflies were collected and paired in egg-laying units [35]. The whiteflies were checked daily for survival and fecundity until the death of all individuals. Cotton seedlings were replaced daily.
For each population, the life history raw data of all individuals were analyzed based on the age-stage, two-sex life table theory [24] and the method described by Chi [36]. The software TWOSEX-MSChart [36] (available at http://140.120.197.173/Ecology/download/Twosex-MSChart.rar) was used for raw data analysis. The age-stage specific survival rate (sxj where x = age and j = stage), age-stage specific fecundity (fxj), age-specific survival rate (lx), age-special fecundity (mx), age-stage life expectancy (exj), reproductive value (vxj), preoviposition period of the adult female (APOP), and total preoviposition period of the female from birth (TPOP) were calculated. Among these parameters, sxj represents the probability that a newborn will survive to age x and stage j, and fxj represents the mean number of offspring produced by a female of age x. In the age-stage, two-sex life table, according to Chi and Liu [37], lx is estimated as 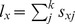 , and m
x is estimated as
, and m
x is estimated as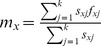 , where k is the number of stages. The age-stage life expectancy (exj) is the length of time that an individual of age x and stage j is expected to live. The life expectancy for an individual of age x and stage y was calculated as
, where k is the number of stages. The age-stage life expectancy (exj) is the length of time that an individual of age x and stage j is expected to live. The life expectancy for an individual of age x and stage y was calculated as 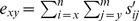 , where n is the number of age groups, m is the number of stages, and
, where n is the number of age groups, m is the number of stages, and 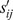 is the probability that an individual of age x and stage y will survive to age i and stage j and is calculated by assuming
is the probability that an individual of age x and stage y will survive to age i and stage j and is calculated by assuming 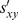 = 1 [37].
= 1 [37].
Population parameters, including the intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R
0), and mean generation time (T), were calculated as well. A bootstrap method [38] was used to estimate the standard errors of the population parameters. Among these parameters, the intrinsic rate of increase was estimated using the iterative bisection method from the Euler-Lotka equation (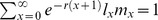 ) with age indexed from 0 [39]. The net reproduction rate (R0) is calculated as
) with age indexed from 0 [39]. The net reproduction rate (R0) is calculated as 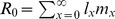 . The mean generation time is defined as the length of time that a population needs to increase R0 fold of its size (
. The mean generation time is defined as the length of time that a population needs to increase R0 fold of its size (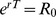 or
or 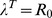 ) at a stable age distribution, and the mean generation time was calculated as
) at a stable age distribution, and the mean generation time was calculated as 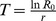 . Student’s t-test was used to determine differences in developmental times, fecundity, and population parameters between the C
+ and C
− populations.
. Student’s t-test was used to determine differences in developmental times, fecundity, and population parameters between the C
+ and C
− populations.
Results
Identification and Analysis of Orthologous Genes between C+ and C − Populations
In the preliminary analysis, 40 cytochrome P450 genes were identified (Table S1). Based on the cytochrome P450 genes identified in this study, the average identity between C+ and C− populations was 99.7% (range, 98.4% to 100.0%) (Table S1), indicating that the genetic backgrounds of these two populations are highly similar.
Competition between C + and C − Populations
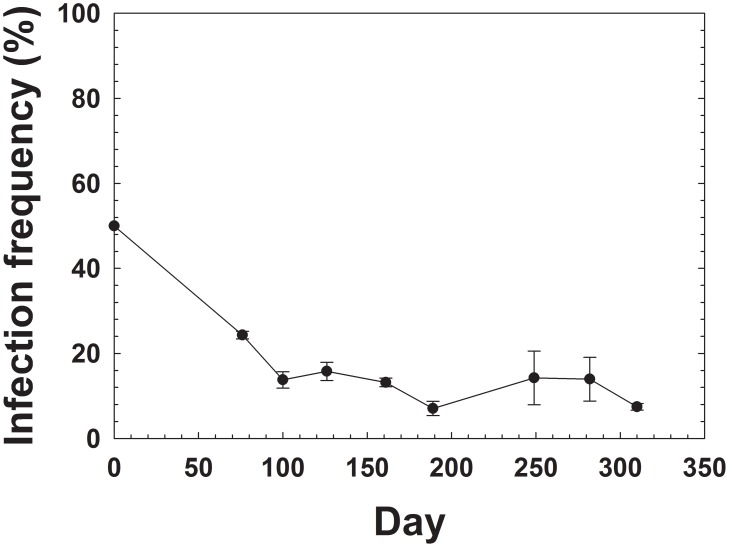
The changes in the relative ratio of C + and C − individuals within 310 days (approximately 11 generations) are shown in Fig. 1. The mixed cohort began with 50% C + and 50% C − individuals. The relative ratio of C − individuals reached 76% after 75 days and increased steadily over time, reaching 93% after 310 days. By contrast, the relative ratio of C + individuals decreased from the initial 50% to 24% after 75 days and remained between 7% and 20% thereafter.
Figure 1. Percentages of Cardinium-infected B. tabaci Q (C+) in a mixed cohort.
Sex Ratio among Crosses between C + and C − Populations
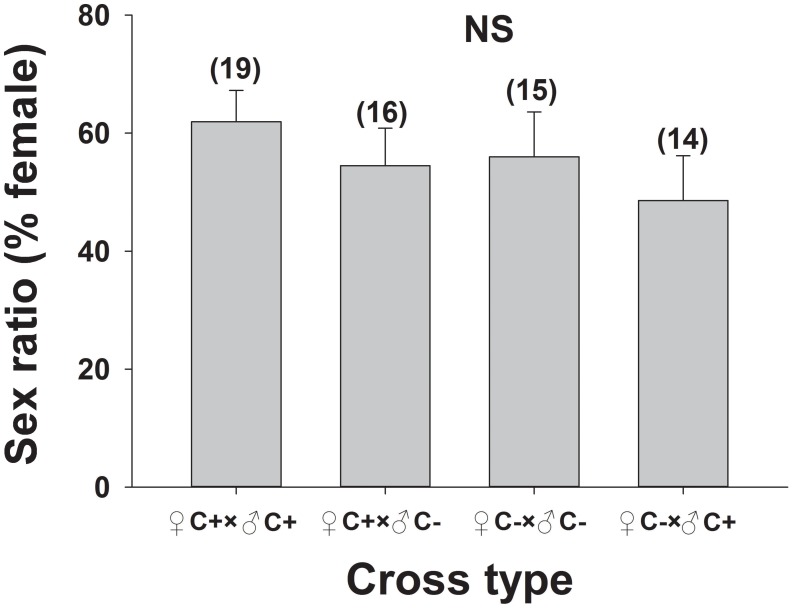
No significant differences were observed in the sex ratio of the F1 generation among the four possible crosses between C + and C − populations (P>0.05, ANOVA) (Fig. 2). These results suggest that no reproductive isolation or reproductive abnormalities occurred between C + and C − populations.
Figure 2. Sex ratio (proportion of females) in F1 offspring produced by crosses between Cardinium-infected (C+) and uninfected (C−) B.tabaci Q.
Results are mean percent sex ratio ± SE. Number of each of the cross types are shown in parentheses. NS represent not significant at the 5% level (LSD test, P>0.05).
C + and C − Population Parameters based on the Two-sex Life Table Method
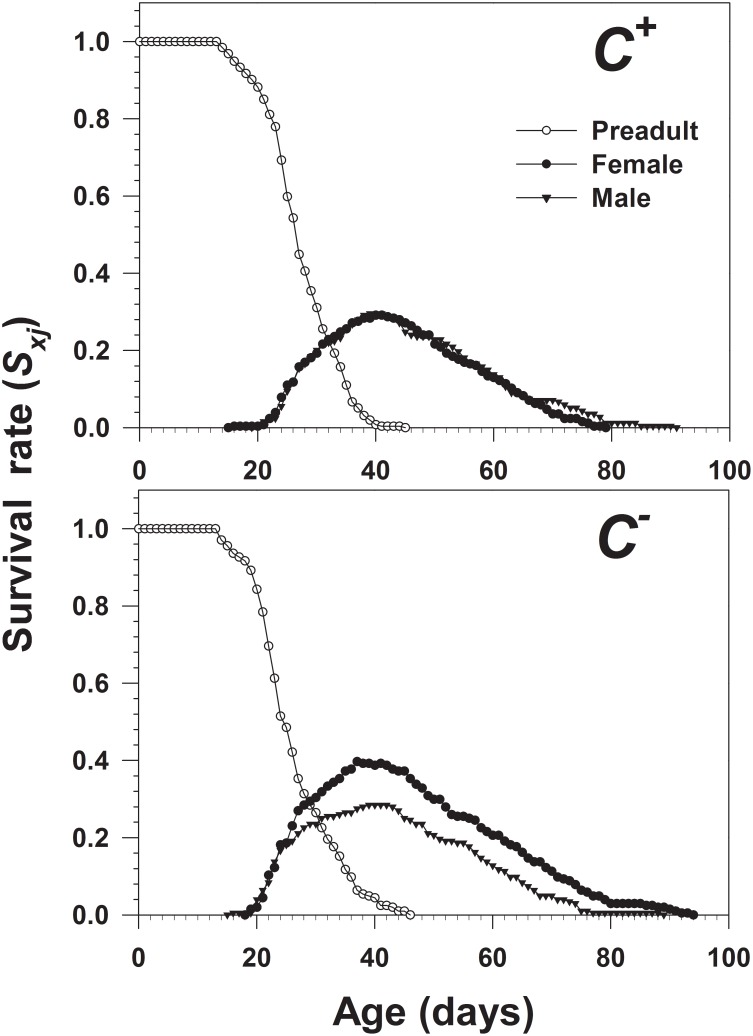
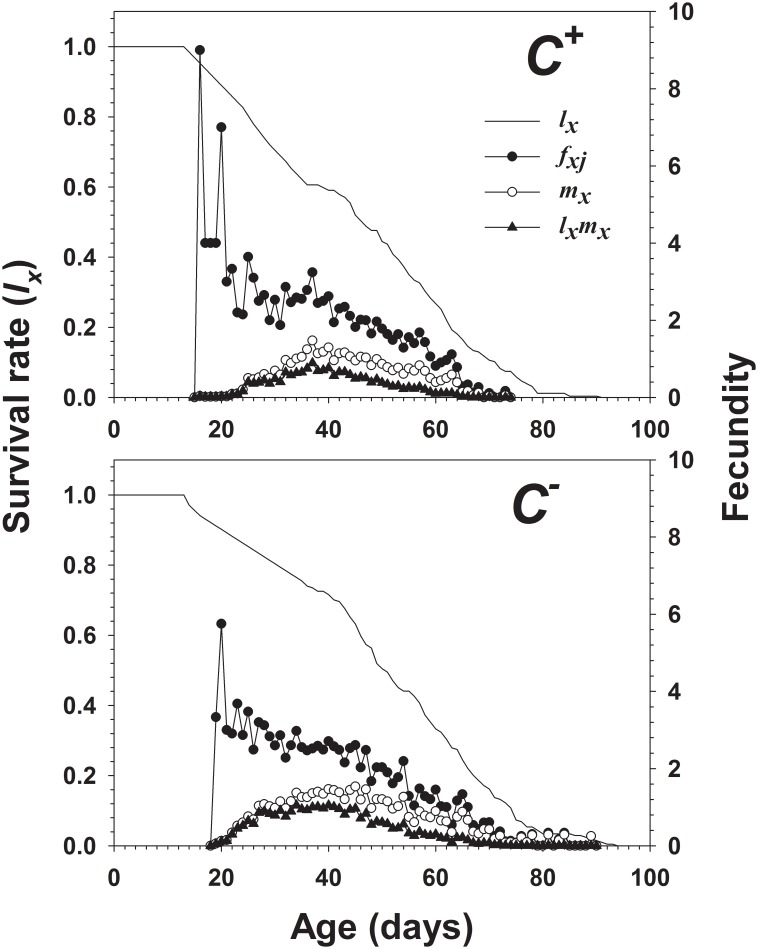
The age-stage survival rate (sxj) showed the probability that a newly deposited egg of B. tabaci Q would survive to age x and stage j (Fig. 3). The mean number of offspring produced by a female adult at age x relative to the age-stage specific fecundity (fxj) is shown in Fig. 4. The maximum lifelong fecundity was found to be 152 eggs per female for the C + population and 192 eggs per female for the C − population.
Figure 3. Age-stage specific survival rate (sxj) of Cardinium-infected (C+) and uninfected (C−) B. tabaci Q.
Figure 4. Age-specific survival rate (lx), female age-specific fecundity (fxj), age-specific fecundity of the total population (mx), and age-specific maternity (lxmx) of Cardinium-infected (C+) and uninfected (C−) B. tabaci Q.
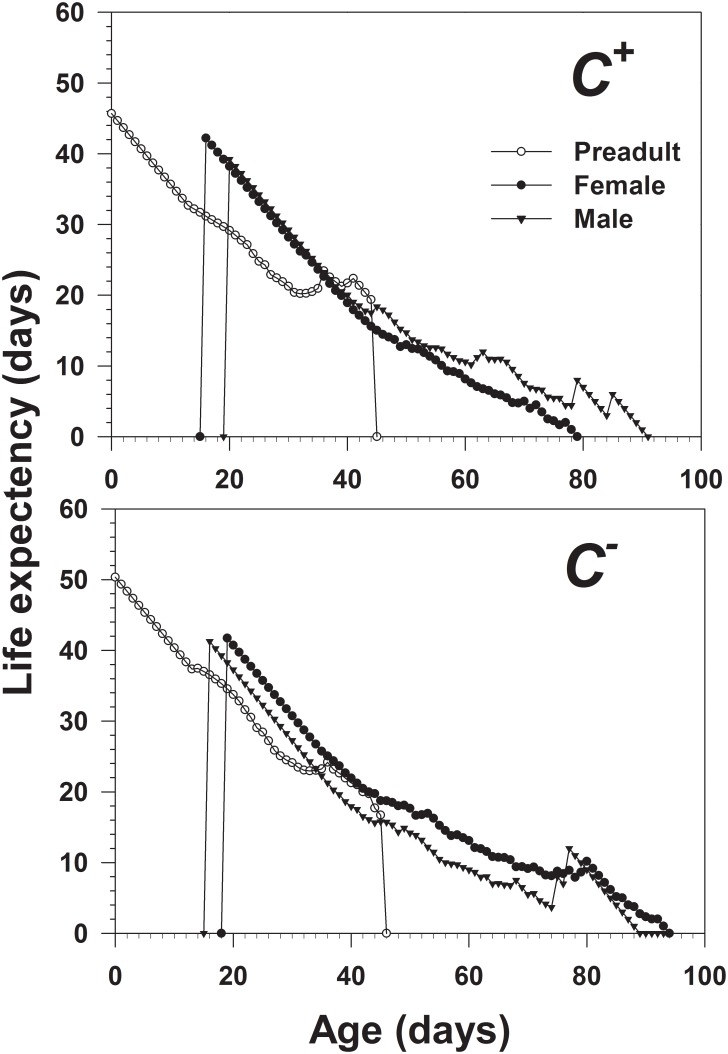
The age-specific survival rate (lx) curve is the age-specific survival rate, including all individuals of the cohort and ignoring the stage of differentiation, while the age-specific fecundity (mx) is the mean fecundity of all individuals in the total population. The product of lx and mx is the age-specific maternity (lx mx) of the C + and C − populations. Higher peaks of mx and lxmx were observed in the C − population. The age-stage specific life expectancy (exj) of B. tabaci is shown in Fig. 5. The maximum life expectancy was 45.75 days for the C + population and 50.48 days for the C − population. The age-stage life expectancy of the C + population was significantly shorter than the C − population.
Figure 5. Age-stage specific life expectancies (exj) of Cardinium-infected (C+) and uninfected (C−) B. tabaci Q.
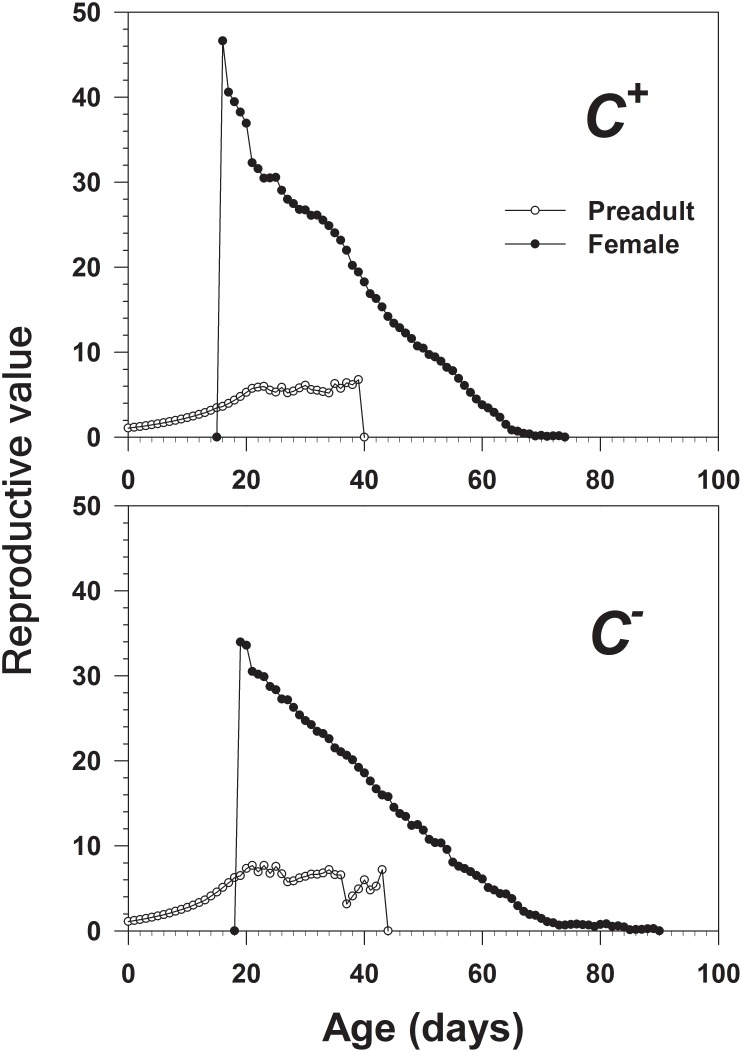
The age-stage reproductive value (vxj) (Fig. 6) delineates the contribution of an individual to age x and stage j of the future population [40]. In our study, the reproductive values increased sharply to 47.23 in the C + population when females started to emerge at day 19. The corresponding reproductive values increased sharply to 33.96 in the C − population when females started to emerge at day 16, 3 days earlier than the C + population.
Figure 6. Age-stage specific reproductive value (vxj) of Cardinium-infected (C+) and uninfected (C−) B. tabaci Q.
The fecundity and mean developmental time of each life stage, including pre-adult duration, adult (female, male) longevity, APOP (adult preoviposition period), TPOP (total preoviposition period), and oviposition period, are given in Table 1. The pre-adult duration of the C − population was significantly shorter than that of the C + population (P<0.05). However, differences in the other parameters between the two populations were not significant (P>0.05).
Table 1. Basic statistics of the life history of Cardinium-infected and uninfected Bemisia tabaci Q.
| Statistic | Stage or sex | C+ | C− | P | |||
| n | Mean ± SE | n | Mean ± SE | ||||
| Pre-adult duration (d) | Pre-adult | 155 | 28.97±0.43* | 152 | 26.30±0.53 | 0.017 | |
| Adult longevity (d) | Female | 75 | 29.79±1.06 | 87 | 33.37±1.51 | 0.05 | |
| Male | 80 | 29.94±1.24 | 65 | 30.37±1.29 | 0.81 | ||
| TPOP (d) | Female | 75 | 29.23±0.65 | 87 | 28.06±0.69 | 0.23 | |
| APOP (d) | Female | 75 | 0.68±0.10 | 87 | 0.56±0.10 | 0.44 | |
| Oviposition (d) | Female | 75 | 18.25±0.85 | 87 | 20.19±0.98 | 0.18 | |
| Fecundity (eggs per female) | Female | 75 | 60.36±3.37 | 87 | 68.12±4.43 | 0.15 | |
All P values are calculated using Student’s t-test. APOP (adult preoviposition period) and TPOP (total preoviposition period) were calculated using females that produced fertile eggs.
*Significant difference (Student’s t-test, P<0.05).
Based on the two-sex life table method (Table 2), the intrinsic rate of increase (r), net reproductive rate (R 0), and finite rate of increase (λ) of the C − population (0.09192 d−1, 29.06 offspring, and 1.096 d−1, respectively) were significantly higher than that of the C + population (0.07561 d−1, 17.82 offspring, and 1.078 d−1, respectively). The mean generation time (T) of the C + population (38.09 d) was significantly longer than that of C − population (36.67 d).
Table 2. Population parameters for Cardinium-infected and uninfected Bemisia tabaci Q.
| Population parameters | C+ (SE) | C− (SE) |
| r (d−1) | 0.07561 (0.0035) | 0.09192 (0.0036)* |
| λ | 1.078 (0.0037) | 1.096 (0.012)* |
| R0 | 17.82 (2.04) | 29.06 (3.04)* |
| T (d) | 38.09 (0.82)* | 36.67 (0.81) |
*Significant difference (Student’s t-test, P<0.05)
Discussion
Our results showed that the percentage of C − whitefly decreased after three generations, indicating that the C + whitefly has weak competitive ability. The results proved the initial hypothesis that the competitive ability of the infected host whiteflies was weaker than that of uninfected ones, which is in agreement with results of previous field surveys in China [8], [9]. Our results also revealed that no reproductive isolation occurred between the two populations and the C − population had higher fitnesses than the C + population. The C − whiteflies had higher r values (because they developed faster), higher survivorship of immature stages, and a higher net reproductive rate than those of C + whiteflies at 27°C. These differences may explain why C − whitefly populations have been dominant during the past few years in Shandong province, China.
For whiteflies, they possess a haplo-diploid sex determination system, in which unfertilized eggs developed into males and fertilized eggs develop into females [41]. In our study, we did not observe parthenogenesis or feminization in the experimental crosses. Although an analysis of cytochrome P450 genes in the two whitefly populations revealed that they were highly similar, the effect of Cardinium on whitefly reproduction should be examined in more detail in future studies. In recent decades, multiple roles of bacterial symbionts in arthropods have been revealed [42], [43]. Bacterial symbionts can manipulate the reproductive biology of hosts or affect the host fitness to increase transmission of the symbiont [18], [21], [43]–[54]. Most studies have shown that Cardinium can alter the reproduction of its hosts, which, in turn, is helpful to the spread of the symbiont within the population [12]–[20], [41]. For instance, embryonic mortality resulting from cytoplasmic incompatibility is the most common effect associated with endosymbiont infection [50]; consequently, the symbionts can maximize their spread. Cytoplasmic incompatibility induced by Cardinium has been widely reported in arthropods, such as the parasitoid wasp Encarsia pergandiella [15], spider mite Eotetranychus suginamensis [16], sexual spider mite Bryobia sarothamni [55], and whitebacked planthopper Sogatella furcifera [17]. If Cardinium does not alter the reproduction of the whitefly host, then this symbiont may play a different role in the whitefly than observed in previous studies that showed it induces feminization [12], [13], parthenogenesis [14], and cytoplasmic incompatibility of infected hosts [15]–[17]. Our results are similar to the observations of White et al. [56], [57] and Stefanini & Duron [58], which showed there was no cytoplasmic incompatibility or progeny sex ratio distortion in Cardinium-infected individuals of the parasitic wasp Encarsia inaron [56], [57] or the spider Holocnemus pluchei [58].
Based on the two-sex life table method in this study, we revealed that the C − population had higher fitness than the C + population (Tables 1 and 2), which might indicate that Cardinium may have cryptic negative effects on the fitness of the host. The role Cardinium plays in changing the fitness cost of the whitefly should be examined further in future studies. Many studies have suggested that Cardinium can increase the fitness of the hosts [13], [18]–[20]. For example, Weeks & Stouthamer [18] found that the fecundity advantage of infected females was approximately 1.6 fold higher than that of uninfected females over a 6-day egg-laying period in the predatory mite, Metaseiulus occidentailis, and Giorgini et al. [13] showed that the antibiotic removal of Cardinium reduced offspring production by adult Encarsia hispida females. If it is the case that Cardinium has cryptic negative effects on the fitness of the whitefly host, then this would be in disagreement with previous studies that showed that Cardinium has no detectable effect on either reproduction or development of the host [56]–[59]. Similar negative effects of symbiont on host insect have been revealed for Wolbachia in Drosophila, in which the infection drastically reduces life span [60], and causes widespread degeneration of tissues, culminating in early death of Drosophila melanogaster [61]. On the other hand, because Cardinium still exists in the field, there might be a benefit to the whitefly. The evolution of this symbiont strain needs to be further explored.
Finally, the two-sex life table gives the most comprehensive description and analysis of the survival and reproduction of a population and, thus, this method may be highly beneficial in revealing the difference in the two populations. This method takes into account the male population and the variable developmental rate occurring among individuals and can overcome the shortcoming of the traditional female-based, age-specific life table method, which ignores the male individuals, the stage of differentiation, and variable developmental rates among individuals.
Supporting Information
The cytochrome P450 genes identified in this study.
(XLS)
Funding Statement
This research was supported by the High-Level Talents Fund of Qingdao Agricultural University (631212), the National Natural Science Foundation of China (31272105), the Science and Technology Development Planning Program of Qingdao (13-1-3-108-nsh), and the Taishan Mountain Scholar Constructive Engineering Foundation of Shandong to D. Chu. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Oliveira M, Henneberry T, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci . Crop Prot 20: 709–723. [Google Scholar]
- 2.Czosnek H, Ghanim M (2011) Bemisia tabaci-Tomato yellow leaf curl virus interaction causing worldwide epidemics. In: Winston M.O. Thompson. The whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) interaction with geminivirus-infected host plants. Springer 51–67.
- 3. De Barro PJ, Liu SS, Boykin LM, Dinsdale AB (2011) Bemisia tabaci: a statement of species status. Annu Rev Entomol 56: 1–19. [DOI] [PubMed] [Google Scholar]
- 4. Wang HL, Yang J, Boykin LM, Zhao QY, Li Q, et al. (2013) The characteristics and expression profiles of the mitochondrial genome for the Mediterranean species of the Bemisia tabaci complex. BMC Genomics 14: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu D, Zhang YJ, Brown JK, Cong B, Xu BY, et al. (2006) The introduction of the exotic Q biotype of Bemisia tabaci from the Mediterranean region into China on ornamental crops. Fla Entomol 89: 168–174. [Google Scholar]
- 6. Chu D, Wan FH, Zhang YJ, Brown JK (2010) Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environ Entomol 39: 1028–1036. [DOI] [PubMed] [Google Scholar]
- 7. Teng X, Wan FH, Chu D (2010) Bemisia tabaci biotype Q dominates other biotypes across China. Fla Entomol 93: 363–368. [Google Scholar]
- 8. Pan HP, Chu D, Ge DQ, Wang SL, Wu QJ, et al. (2011) Further spread of and domination by Bemisia tabaci biotype Q on field crops in China. J Econ Entomol 104: 978–985. [DOI] [PubMed] [Google Scholar]
- 9. Chu D, Gao CS, De Barro P, Zhang YJ, Wan FH (2011) Investigation of the genetic diversity of an invasive whitefly in China using both mitochondrial and nuclear DNA markers. Bull Entomol Res 101: 467–475. [DOI] [PubMed] [Google Scholar]
- 10. Kurtti TJ, Munderloh UG, Andreadis TG, Magnarelli LA, Mather TN (1996) Tick cell culture isolation of an intracellular prokaryote from the tick Ixodes scapularis. . J Invertebrate Pathol 67: 318–321. [DOI] [PubMed] [Google Scholar]
- 11. Zchori-Fein E, Perlman SJ, Kelly SE, Katzir N, Hunter MS (2004) Characterization of a ‘Bacteroidetes’ symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): proposal of ‘Candidatus Cardinium hertigii’. Int J Syst Evol Microbiol 54: 961–968. [DOI] [PubMed] [Google Scholar]
- 12. Weeks AR, Marec F, Breeuwer JA (2001) A mite species that consists entirely of haploid females. Science 292: 2479–2482. [DOI] [PubMed] [Google Scholar]
- 13. Giorgini M, Monti MM, Caprio E, Stouthamer R, Hunter MS (2009) Feminization and the collapse of haplodiploidy in an asexual parasitoid wasp harboring the bacterial symbiont Cardinium . Heredity 102: 365–371. [DOI] [PubMed] [Google Scholar]
- 14. Provencher LM, Morse GE, Weeks AR, Normark BB (2005) Parthenogenesis in the Aspidiotus nerii complex (Hemiptera: Diaspididae): a single origin of a worldwide, polyphagous lineage associated with Cardinium bacteria. Ann Entomol Soc Amer 98: 629–635. [Google Scholar]
- 15. Hunter MS, Perlman SJ, Kelly SE (2003) A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella . Proc R Soc Lond Ser B-Biol Sci 270: 2185–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gotoh T, Noda H, Ito S (2007) Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity 98: 13–20. [DOI] [PubMed] [Google Scholar]
- 17. Zhang XF, Zhao DX, Hong XY (2012) Cardinium-the leading factor of cytoplasmic incompatibility in the planthopper Sogatella furcifera doubly infected with Wolbachia and Cardinium . Environ Entomol 41: 833–840. [Google Scholar]
- 18. Weeks AR, Stouthamer R (2004) Increased fecundity associated with infection by a cytophaga-like intracellular bacterium in the predatory mite, Metaseiulus occidentalis . Proc R Soc Lond Ser B 271: S193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zchori-Fein E, Gottlieb Y, Kelly S, Brown J, Wilson J, et al. (2001) A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proc Natl Acad Sci USA 98: 12555–12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kenyon S, Hunter M (2007) Manipulation of oviposition choice of the parasitoid wasp, Encarsia pergandiella, by the endosymbiotic bacterium Cardinium . J Evol Biol 20: 707–716. [DOI] [PubMed] [Google Scholar]
- 21. Harris L, Kelly S, Hunter M, Perlman S (2010) Population dynamics and rapid spread of Cardinium, a bacterial endosymbiont causing cytoplasmic incompatibility in Encarsia pergandiella (Hymenoptera: Aphelinidae). Heredity 104: 239–246. [DOI] [PubMed] [Google Scholar]
- 22. Chu D, Gao C, De Barro P, Zhang Y, Wan F, et al. (2011) Further insights into the strange role of bacterial endosymbionts in whitefly, Bemisia tabaci: Comparison of secondary symbionts from biotypes B and Q in China. Bull Entomol Res 101: 477–486. [DOI] [PubMed] [Google Scholar]
- 23. Pan H, Li X, Ge D, Wang S, Wu Q, et al. (2012) Factors affecting population dynamics of maternally transmitted endosymbionts in Bemisia tabaci . PLoS ONE 7: e30760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chi H (1988) Life-table analysis incorporating both sexes and variable development rates among individuals. Environ Entomol 17: 26–34. [Google Scholar]
- 25. Nirgianaki A, Banks GK, Frohlich DR, Veneti Z, Braig HR, et al. (2003) Wolbachia infections of the whitefly Bemisia tabaci. . Curr Microbiol 47: 93–101. [DOI] [PubMed] [Google Scholar]
- 26. Zchori-Fein E, Brown J (2002) Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann Entomol Soc Am 95: 711–718. [Google Scholar]
- 27. Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, et al. (2007) Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci . Bull Entomol Res 97: 407–413. [DOI] [PubMed] [Google Scholar]
- 28. Simon C, Frati F, Beckenbach A, Crespi B, Liu H, et al. (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87: 651–701. [Google Scholar]
- 29. Chu D, Hu XS, Gao CS, Zhao HY, Nichols RL, et al. (2012) Use of mtCOI PCR-RFLP for identifying subclades of Bemisia tabaci Mediterranean group. J Econ Entomol 105: 242–251. [DOI] [PubMed] [Google Scholar]
- 30. Khasdan V, Levin I, Rosner A, Morin S, Kontsedalov S, et al. (2005) DNA markers for identifying biotypes B and Q of Bemisia tabaci (Hemiptera: Aleyrodidae) and studying population dynamics. Bull Entomol Res 95: 605–613. [DOI] [PubMed] [Google Scholar]
- 31. Nakamura Y, Kawai S, Yukuhiro F, Ito S, Gotoh T, et al. (2009) Prevalence of Cardinium bacteria in planthoppers and spider mites and taxonomic revision of "Candidatus Cardinium hertigii" based on detection of a new Cardinium group from biting midges. Appl Environ Microbiol 75: 6757–6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang XW, Luan JB, Li JM, Su YL, Xia J, et al. (2011) Transcriptome analysis and comparison reveal divergence between two invasive whitefly cryptic species. BMC Genomics 12: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luan JB, Ruan YM, Zhang L, Liu SS (2008) Pre-copulation intervals, copulation frequencies, and initial progeny sex ratios in two biotypes of whitefly, Bemisia tabaci. . Entomol Exp Appl 129: 316–324. [Google Scholar]
- 35. Li X, Degain BA, Harpold VS, Marçon PG, Nichols RL, et al. (2012) Baseline susceptibilities of B-and Q-biotype Bemisia tabaci to anthranilic diamides in Arizona. Pest Manag Sci 68: 83–91. [DOI] [PubMed] [Google Scholar]
- 36.Chi H (2013) TWOSEX-MsChart: a computer program for the age-stage, two-sex life table analysis. http://140.120.197.173/Ecology/download/Twosex-MSChart.rar.
- 37. Chi H, Su HY (2006) Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ Entomol 35: 10–21. [Google Scholar]
- 38.Efron B, Tibshirani R (1993) An introduction to the bootstrap. London: Chapman & hall.
- 39.Goodman D (1982) Optimal life histories, optimal notation, and the value of reproductive value. Am Nat 803–823.
- 40.Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford, United Kingdom.
- 41. Blackman RL, Cahill M (1998) The karyotype of Bemisia tabaci (Hemiptera: Aleyrodidae). Bull Entomol Res 88: 213–215. [Google Scholar]
- 42. Werren JH (1997) Biology of Wolbachia . Annu Rev Entomol 42: 587–609. [DOI] [PubMed] [Google Scholar]
- 43. Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6: 741–751. [DOI] [PubMed] [Google Scholar]
- 44. Hagimori T, Abe Y, Date S, Miura K (2006) The first finding of a Rickettsia bacterium associated with parthenogenesis induction among insects. Curr Microbiol 52: 97–101. [DOI] [PubMed] [Google Scholar]
- 45. Haine ER (2008) Symbiont-mediated protection. Proc R Soc B 275: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang JJ, Dong P, Xiao LS, Dou W (2008) Effects of removal of Cardinium infection on fitness of the stored-product pest Liposcelis bostrychophila (Psocoptera: Liposcelididae). J Econ Entomol 101: 1711–1717. [DOI] [PubMed] [Google Scholar]
- 47. Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ (2010) Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329: 212–215. [DOI] [PubMed] [Google Scholar]
- 48. Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, et al. (2011) Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332: 254–256. [DOI] [PubMed] [Google Scholar]
- 49. Vanthournout B, Swaegers J, Hendrickx F (2011) Spiders do not escape reproductive manipulations by Wolbachia . BMC Evol Biol 11: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rousset F, Bouchon D, Pintureau B, Juchault P, Solignac M (1992) Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc R Soc Lond Ser B-Biol Sci 250: 91–98. [DOI] [PubMed] [Google Scholar]
- 51. Stouthamer R, Breeuwer JA, Hurst GD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53: 71–102. [DOI] [PubMed] [Google Scholar]
- 52. Stouthamer R, Breeuwer J, Luck R, Werren J (1993) Molecular identification of microorganisms associated with parthenogenesis. Nature 361: 66–68. [DOI] [PubMed] [Google Scholar]
- 53. Hurst GD, Jiggins FM, von der Schulenburg JHG, Bertrand D, West SA, et al. (1999) Male-killing Wolbachia in two species of insect. Proc R Soc Lond Ser B 266: 735–740. [Google Scholar]
- 54. Breeuwer J, Stouthamer R, Barns S (1992) Phylogeny of cytoplasmic incompatibility micro-organisms in the parasitoid wasp genus Nasonia (Hymenoptera: Pteromalidae) based on 16S ribosomal DNA sequences. Insect Mol Biol 1: 25–36. [DOI] [PubMed] [Google Scholar]
- 55. Ros VID, Breeuwer JAJ (2009) The effects of, and interactions between, Cardinium and Wolbachia in the doubly infected spider mite Bryobia sarothamni . Heredity 102: 413–422. [DOI] [PubMed] [Google Scholar]
- 56. White JA, Kelly SE, Perlman SJ, Hunter MS (2009) Cytoplasmic incompatibility in the parasitic wasp Encarsia inaron: disentangling the roles of Cardinium and Wolbachia symbionts. Heredity 102: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. White JA, Kelly SE, Cockburn SN, Perlman SJ, Hunter MS (2011) Endosymbiont costs and benefits in a parasitoid infected with both Wolbachia and Cardinium. . Heredity 106: 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stefanini A, Duron O (2012) Exploring the effect of the Cardinium endosymbiont on spiders. J Evol Biol 25: 1521–1530. [DOI] [PubMed] [Google Scholar]
- 59.Bull JJ (1983) Evolution of sex determining mechanisms. Menlo Park (California): Benjamin/Cummings.
- 60. Brownstein J, Hett E, O’Neill S (2003) The potential of virulent Wolbachia to modulate disease transmission by insects. J Invertebrate Pathol 84: 24–29. [DOI] [PubMed] [Google Scholar]
- 61. Min KT, Benzer S (1997) Genetics Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci 94: 10792–107961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The cytochrome P450 genes identified in this study.
(XLS)