Pathogenic brucellae are the agent of brucellosis, a disease that causes abortion and infertility in natural hosts such as sheep, goats, or cattle. Brucella melitensis, Brucella abortus, and Brucella suis are the most pathogenic Brucella species for humans and also the most relevant bacteria involved in economical loses and in animal and human health problems. In addition, these Brucella represent a zoonotic risk in low-income countries.1,2 Humans are infected through contacts with infected animals by aerosols or by ingestion of contaminated dairy products. Human brucellosis is a re-emerging febrile illness that may progress into a chronic phase characterized by the appearance of severe complications such as endocarditis, arthralgia, epididymitis, etc.1,3 If untreated, chronic brucellosis represents a threat, especially in endemic areas (Central and South America, the Middle East, Mediterranean countries, northern Africa, and countries of the Caucasus and Central Asia).4 A figure of 500 000 new cases/year is usually given as an estimate.3 Because of all these circumstances, brucellosis is classified according WHO among the top seven neglected zoonoses. These diseases in endemic area are considered as a human health problem with a direct link to poverty.
Brucella species are facultative gram-negative intracellular bacteria. In both humans and animals, Brucella first targets the respiratory epithelium, the conjunctiva, and sexual organs. Even nowadays, the cells targeted by the pathogen for entry remain uncharacterized and efforts have to be done to decipher where and how Brucella invade the body. However, what we do know is that bacteria internalized by phagocytes at the periphery move to regional lymph nodes, which may play a barrier to subsequent systemic dissemination. Brucella is capable of colonizing macrophages, monocytes, and dendritic cells and can be found in large numbers in the liver, the medulla, and the spleen.1 Therefore, it is not surprising that Brucella has engineered several devices to make its pathogenic life easier, defeating both innate and adaptive immunity. Brucella, like many other intracellular pathogenic bacteria, secretes effector proteins inside the host cytoplasm of infected cells in order to circumvent essential functions of the host defense, the final goal being the establishment of a long-lasting chronic infection beneficial for the invader.
The mechanisms involved in Brucella entry into host cells still remain to be characterized. Brucella can colonize macrophages and dendritic cells (DCs) as well as trophoblasts, fibroblasts, endothelial cells, and epithelial cells.
In both murine macrophages and human monocytes Brucella enters through lipid rafts.5,6 This event, also observed in DCs is dependent on the PI3-kinase and TLR4.7-9 Indeed, it has been shown that Brucella mutants lacking LPS O-chain do not use lipid rafts and are killed by the host cell suggesting that the Brucella O-chain plays an important role in early events of the Brucella-containing vacuole (BCV).10-12
Brucella entry into host cells also depends on the expression of BvrR/BvrS. This two component regulator system controls the expression of genes controlling the acylation of the Brucella LPS lipid A and the surface expression of several outer membrane proteins.13-15 Lipid raft-mediated Brucella internalization has been proposed to be under the control of the class A scavenger receptor16 and the cellular prion protein PrPc17 in macrophages. Brucella adhesion to macrophages and epithelial cells seems to be associated to the host surface expression of sialic acids, which bind the Brucella surface protein 41 encoded by the ugpB locus.18 Other Brucella proteins such as the product of the gene BMEI0216 have been implicated in adhesion and/or internalization into phagocytes.19 In addition, the B. abortus efp gene and the pathogenicity island Bab1_2009–2012 encoding a Brucella adhesin seem to play a role in Brucella uptake.20,21
In this issue of Virulence, Alva-Perez et al.22 investigated the role of a subfamily of (di)nucleoside oligophosphate molecules linked to other “X” molecules (NUDIX) enzymes. NUDIX have been described in other bacteria as invasins and are present in Brucella spp. The authors called this NUDIX enzyme InvA and generated a deletion mutant of the B. melitensis invA gene to understand its role in virulence. Such a mutant was attenuated during the first steps of invasion in HeLa cells and goat macrophages with a maximum attenuation at 2 h p.i. Interestingly, the mutant strain exhibited a low level of colocalization with cathepsin D, similar to the parental strain colocalization at 24 h p.i. showing that the invA gene is important during invasion but not for intracellular replication. The authors also showed that InvA was important for survival in vivo in mice. The major point here is that Brucella needs to turn off the power of oxidative stress. Under stress, increasing intracellular concentrations of alarmones (oligophosphate nucleotides) are sensed as a danger signal by the cell, which in turn will be ready to prepare for a stress adaptation. To inhibit the toxic effects of alarmone accumulation, bacterial NUDIX enzymes can hydrolyze alarmones, thereby promoting invasion and intracellular survival. This new mechanism of host response subversion highlights the adaptation of intracellular bacteria to their environment.
When BCVs are formed by interaction with early endosomes, they then loose early endosome markers and concomitantly acquire late endosomal/lysosomal membrane proteins such as LAMP1 and the small GTPase Rab7.23,24 Finally BCVs fuse with the endoplasmic reticulum as schematized in Figure 1.
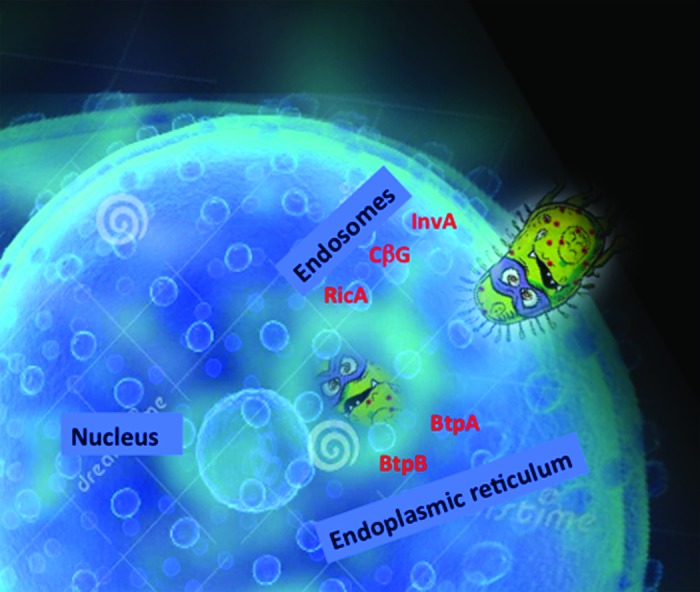
Figure 1. A schematic view of Brucella entry into a cell. Brucella uses different effector proteins that help the bacterium to travel from endosomes to finally hide inside the endoplasmic reticulum. We hypothesize that InvA protein acts at the level of entry during the first step of Brucella intracellular trafficking, followed by the action of the cyclic glucan (CbG), then RicA between late endosomes and the endoplasmic reticulum, and finally in the endoplasmic reticulum with BtpA and BtpB controlling host cell signaling.
The Brucella cyclic β-1,2-glucan (CβG) located in the periplasm of the bacteria, when released out of the bacterium is capable of modifying the composition of lipid rafts at the level of the BCV.25 In macrophages but not in DCs, CβG is a virulence factor.26 However, in contrast to other molecules expressed by Brucella such as the BtpA and BtpB, CβG appears to be a factor that mediates both macrophage and dendritic cell activation.27 In the case of Brucella spp. a TIR domain containing protein called BtpA/TcpB controls Toll-like receptor (TLR) signaling.26,28 BtpA is expressed by B. abortus and B. melitensis but seems to be absent from B. suis. BtpA was shown to interfere with TLR4 and TLR2 signaling through Myd88 interaction.26,28 Other reports proposed that BtpA targets the adaptor protein MAL/TIRAP.29,30 Direct comparison of the in vitro interaction between BtpA and either MyD88 or TIRAP showed a stronger interaction with MyD88.31 BtpA has been shown to bind phosphoinositides at the plasma membrane30 but also to induce ubiquitination of TIRAP.29 Another Btp family member was recently discovered, namely BtpB.32 BtpB is also translocated into host cell cytoplasm and interferes with the activation of dendritic cells. In vivo mouse studies revealed that BtpB contributes to virulence by controlling inflammatory responses. Together, Brucella TIR-containing proteins BtpA and BtpB modulate host inflammatory responses during infection.
Acidification of BCVs is an essential step for Brucella intracellular survival33 and intracellular trafficking to the endoplasmic reticulum (ER).24 Acidic pH in BCVs promotes the expression of several genes required for virulence. This the case for the virB operon that encodes a type IV secretion system (T4SS) involved in the secretion of Brucella effector proteins.33
Getting to the proximity of the replication niche, namely the ER, the intermediate compartment is a target for Brucella. Communicating between the Golgi apparatus and the small GTPase Rab2 seems to be a host target and required for Brucella intracellular multiplication.34 Rab2 forms a complex of proteins with the glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the coat COPI complex and the protein kinase C (PKCλ). This complex controls the vesicular trafficking from the Golgi to the ER, via a vesicle/tubule cluster.35 Each protein of the GAPDH/COPI/Rab2/PKCλ complex is required for Brucella intracellular replication. This suggests that BCVs interact first with the vesicle/tubule cluster before reaching the ER. Recently, the Brucella translocated RicA effector protein was identified and characterized to recruit the small GTPase Rab2. This important finding highlighted the interaction at the molecular level between a Brucella protein (RicA) and a host protein complex controlled by a small GTPase (Rab2), thereby showing how pathogens subvert host intracellular trafficking.36
Following still uncharacterized membrane fusion events with the secretory pathway that require the small GTPase Sar1 at the level of ER exit sites37 BCVs finally fuse with the ER, a safe niche for Brucella replication.
Altogether, these results summarizing almost 20 years of research show that Brucella actually uses different angles to tackle host cell response and we are probably far away to know them all.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by institutional funding from Centre National de la Recherche Scientifique and Institut National de la Santé et de la Recherche Médicale. The author acknowledges Alexandre Gorvel for the excellent artwork in Figure 1.
References
- 1.Martirosyan A, Moreno E, Gorvel JP. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol Rev. 2011;240:211–34. doi: 10.1111/j.1600-065X.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 2.Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis RM. Interactions of the human pathogenic Brucella species with their hosts. Annu Rev Microbiol. 2011;65:523–41. doi: 10.1146/annurev-micro-090110-102905. [DOI] [PubMed] [Google Scholar]
- 3.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–9. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 4.Godfroid J, Scholz HC, Barbier T, Nicolas C, Wattiau P, Fretin D, Whatmore AM, Cloeckaert A, Blasco JM, Moriyon I, et al. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev Vet Med. 2011;102:118–31. doi: 10.1016/j.prevetmed.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Naroeni A, Porte F. Role of cholesterol and the ganglioside GM(1) in entry and short-term survival of Brucella suis in murine macrophages. Infect Immun. 2002;70:1640–4. doi: 10.1128/IAI.70.3.1640-1644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watarai M, Makino S, Fujii Y, Okamoto K, Shirahata T. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell Microbiol. 2002;4:341–55. doi: 10.1046/j.1462-5822.2002.00195.x. [DOI] [PubMed] [Google Scholar]
- 7.Pei J, Turse JE, Ficht TA. Evidence of Brucella abortus OPS dictating uptake and restricting NF-kappaB activation in murine macrophages. Microbes Infect. 2008;10:582–90. doi: 10.1016/j.micinf.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzmán-Verri C, Chaves-Olarte E, von Eichel-Streiber C, López-Goñi I, Thelestam M, Arvidson S, Gorvel JP, Moreno E. GTPases of the Rho subfamily are required for Brucella abortus internalization in nonprofessional phagocytes: direct activation of Cdc42. J Biol Chem. 2001;276:44435–43. doi: 10.1074/jbc.M105606200. [DOI] [PubMed] [Google Scholar]
- 9.Billard E, Cazevieille C, Dornand J, Gross A. High susceptibility of human dendritic cells to invasion by the intracellular pathogens Brucella suis, B. abortus, and B. melitensis. Infect Immun. 2005;73:8418–24. doi: 10.1128/IAI.73.12.8418-8424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porte F, Naroeni A, Ouahrani-Bettache S, Liautard JP. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect Immun. 2003;71:1481–90. doi: 10.1128/IAI.71.3.1481-1490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittig MG, Kaufmann A, Robins A, Shaw B, Sprenger H, Gemsa D, Foulongne V, Rouot B, Dornand J. Smooth and rough lipopolysaccharide phenotypes of Brucella induce different intracellular trafficking and cytokine/chemokine release in human monocytes. J Leukoc Biol. 2003;74:1045–55. doi: 10.1189/jlb.0103015. [DOI] [PubMed] [Google Scholar]
- 12.Pei J, Ficht TA. Brucella abortus rough mutants are cytopathic for macrophages in culture. Infect Immun. 2004;72:440–50. doi: 10.1128/IAI.72.1.440-450.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman-Verri C, Manterola L, Sola-Landa A, Parra A, Cloeckaert A, Garin J, Gorvel JP, Moriyon I, Moreno E, Lopez-Goni I. The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc Natl Acad Sci U S A. 2002;99:12375–80. doi: 10.1073/pnas.192439399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamontagne J, Butler H, Chaves-Olarte E, Hunter J, Schirm M, Paquet C, Tian M, Kearney P, Hamaidi L, Chelsky D, et al. Extensive cell envelope modulation is associated with virulence in Brucella abortus. J Proteome Res. 2007;6:1519–29. doi: 10.1021/pr060636a. [DOI] [PubMed] [Google Scholar]
- 15.Manterola L, Moriyón I, Moreno E, Sola-Landa A, Weiss DS, Koch MH, Howe J, Brandenburg K, López-Goñi I. The lipopolysaccharide of Brucella abortus BvrS/BvrR mutants contains lipid A modifications and has higher affinity for bactericidal cationic peptides. J Bacteriol. 2005;187:5631–9. doi: 10.1128/JB.187.16.5631-5639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Watarai M, Suzuki H, Makino S, Kodama T, Shirahata T. Lipid raft microdomains mediate class A scavenger receptor-dependent infection of Brucella abortus. Microb Pathog. 2004;37:11–9. doi: 10.1016/j.micpath.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Watarai M, Kim S, Erdenebaatar J, Makino S, Horiuchi M, Shirahata T, Sakaguchi S, Katamine S. Cellular prion protein promotes Brucella infection into macrophages. J Exp Med. 2003;198:5–17. doi: 10.1084/jem.20021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castañeda-Roldán EI, Ouahrani-Bettache S, Saldaña Z, Avelino F, Rendón MA, Dornand J, Girón JA. Characterization of SP41, a surface protein of Brucella associated with adherence and invasion of host epithelial cells. Cell Microbiol. 2006;8:1877–87. doi: 10.1111/j.1462-5822.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- 19.Hernández-Castro R, Verdugo-Rodríguez A, Puente JL, Suárez-Güemes F. The BMEI0216 gene of Brucella melitensis is required for internalization in HeLa cells. Microb Pathog. 2008;44:28–33. doi: 10.1016/j.micpath.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Iannino F, Ugalde JE, Iñón de Iannino N. Brucella abortus efp gene is required for an efficient internalization in HeLa cells. Microb Pathog. 2012;52:31–40. doi: 10.1016/j.micpath.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Czibener C, Ugalde JE. Identification of a unique gene cluster of Brucella spp. that mediates adhesion to host cells. Microbes Infect. 2012;14:79–85. doi: 10.1016/j.micinf.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alva-Pérez J, Arellano-Reynoso B, Hernández-Castro R, Suárez-Güemes F. The invA gene of Brucella melitensis is involved in intracellular invasion and is required to establish infection in a mouse model. Virulence. 2014;5:563–74. doi: 10.4161/viru.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–56. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starr T, Ng TW, Wehrly TD, Knodler LA, Celli J. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic. 2008;9:678–94. doi: 10.1111/j.1600-0854.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 25.Arellano-Reynoso B, Lapaque N, Salcedo S, Briones G, Ciocchini AE, Ugalde R, Moreno E, Moriyón I, Gorvel JP. Cyclic beta-1,2-glucan is a Brucella virulence factor required for intracellular survival. Nat Immunol. 2005;6:618–25. doi: 10.1038/ni1202. [DOI] [PubMed] [Google Scholar]
- 26.Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O, Alexopoulou L, et al. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathog. 2008;4:e21. doi: 10.1371/journal.ppat.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martirosyan A, Pérez-Gutierrez C, Banchereau R, Dutartre H, Lecine P, Dullaers M, Mello M, Salcedo SP, Muller A, Leserman L, et al. Brucella β 1,2 cyclic glucan is an activator of human and mouse dendritic cells. PLoS Pathog. 2012;8:e1002983. doi: 10.1371/journal.ppat.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cirl C, Wieser A, Yadav M, Duerr S, Schubert S, Fischer H, Stappert D, Wantia N, Rodriguez N, Wagner H, et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008;14:399–406. doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- 29.Sengupta D, Koblansky A, Gaines J, Brown T, West AP, Zhang D, Nishikawa T, Park SG, Roop RM, 2nd, Ghosh S. Subversion of innate immune responses by Brucella through the targeted degradation of the TLR signaling adapter, MAL. J Immunol. 2010;184:956–64. doi: 10.4049/jimmunol.0902008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radhakrishnan GK, Yu Q, Harms JS, Splitter GA. Brucella TIR Domain-containing Protein Mimics Properties of the Toll-like Receptor Adaptor Protein TIRAP. J Biol Chem. 2009;284:9892–8. doi: 10.1074/jbc.M805458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhary A, Ganguly K, Cabantous S, Waldo GS, Micheva-Viteva SN, Nag K, Hlavacek WS, Tung CS. The Brucella TIR-like protein TcpB interacts with the death domain of MyD88. Biochem Biophys Res Commun. 2012;417:299–304. doi: 10.1016/j.bbrc.2011.11.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salcedo SP, Marchesini MI, Degos C, Terwagne M, Von Bargen K, Lepidi H, Herrmann CK, Santos Lacerda TL, Imbert PR, Pierre P, et al. BtpB, a novel Brucella TIR-containing effector protein with immune modulatory functions. Front Cell Infect Microbiol. 2013;3:28. doi: 10.3389/fcimb.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boschiroli ML, Ouahrani-Bettache S, Foulongne V, Michaux-Charachon S, Bourg G, Allardet-Servent A, Cazevieille C, Liautard JP, Ramuz M, O’Callaghan D. The Brucella suis virB operon is induced intracellularly in macrophages. Proc Natl Acad Sci U S A. 2002;99:1544–9. doi: 10.1073/pnas.032514299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fugier E, Salcedo SP, de Chastellier C, Pophillat M, Muller A, Arce-Gorvel V, Fourquet P, Gorvel JP. The glyceraldehyde-3-phosphate dehydrogenase and the small GTPase Rab 2 are crucial for Brucella replication. PLoS Pathog. 2009;5:e1000487. doi: 10.1371/journal.ppat.1000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tisdale EJ, Kelly C, Artalejo CR. Glyceraldehyde-3-phosphate dehydrogenase interacts with Rab2 and plays an essential role in endoplasmic reticulum to Golgi transport exclusive of its glycolytic activity. J Biol Chem. 2004;279:54046–52. doi: 10.1074/jbc.M409472200. [DOI] [PubMed] [Google Scholar]
- 36.de Barsy M, Jamet A, Filopon D, Nicolas C, Laloux G, Rual JF, Muller A, Twizere JC, Nkengfac B, Vandenhaute J, et al. Identification of a Brucella spp. secreted effector specifically interacting with human small GTPase Rab2. Cell Microbiol. 2011;13:1044–58. doi: 10.1111/j.1462-5822.2011.01601.x. [DOI] [PubMed] [Google Scholar]
- 37.Celli J, Salcedo SP, Gorvel JP. Brucella coopts the small GTPase Sar1 for intracellular replication. Proc Natl Acad Sci U S A. 2005;102:1673–8. doi: 10.1073/pnas.0406873102. [DOI] [PMC free article] [PubMed] [Google Scholar]


