Abstract
The maintenance of blood pressure homeostasis is a complex process which is carefully regulated by a variety of inputs. We recently identified two sensory receptors (Olfactory receptor 78 and G protein couple receptor 41) as novel regulators of blood pressure. Both Olfr78 and Gpr41 are receptors for short chain fatty acids (SCFAs), and we showed that propionate (a SCFA) modifies blood pressure in a manner which is differentially modulated by the absence of either Olfr78 or Gpr41. In addition, propionate modifies renin release in an Olfr78-dependent manner. Our study also demonstrated that antibiotic treatment modulates blood pressure in Olfr78 null mice, indicating that SCFAs produced by the gut microbiota likely influence blood pressure regulation. In this addendum, we summarize the findings of our recent study and provide a perspective on the implications of the interactions between the gut microbiota and blood pressure control.
Keywords: short chain fatty acid, blood pressure, cardiovascular, renal, Gpr41, Gpr43, olfactory
Introduction
A recent paradigm in sensory physiology suggests that “sensory” receptors (taste receptors, olfactory receptors, and many other G-protein coupled receptors) play important roles in non-sensory tissues, where they serve as selective and sensitive chemoreceptors. For example, sour taste receptors function in the tongue to sense changes in pH as an indicator of sour taste1,2—in addition, these receptors are also found in neurons which contact the central canal of the spinal column, where they function to detect changes in pH in cerebrospinal fluid.1 In addition, olfactory receptors (ORs) participate in muscle cell migration3 and sperm chemotaxis,4 sweet taste receptors are found in the bladder,5 and bitter taste receptors mediate both bronchodilation and ciliary beat frequency in airways.6,7 G-protein coupled receptors of the GPR gene family are also known to act as sensors of metabolites.8-12 Intriguingly, ligands for these sensory receptors are often generated by physiological processes or metabolic pathways,1,8,12,13 suggesting that known metabolites or chemicals may have signaling functions beyond their traditional roles.8,12,13 Furthermore, sensory receptors such as ORs are expressed in a variety of tissues in mice, humans, and other primates, where their functional role has not yet been defined.14-16
Localization of Renal Olfactory Receptor Olfr78
We previously identified Olfactory Receptor 78 (Olfr78) as a “renal” olfactory receptor (OR),17 and in a recent study18 we set out to localize and to identify the ligand for this OR. We took advantage of a β-galactosidase reporter mouse model in order to localize Olfr78, and determined that Olfr78 is found in vascular resistance beds in a variety of tissues, as well in the renal afferent arteriole. The renal afferent arteriole is an incredibly specialized vessel: blood enters the renal glomerulus for filtration via the afferent arteriole, and this arteriole is the site where renin (the initial, rate-limiting step in the renin-angiotensin-aldosterone pathway) is stored for eventual release into the bloodstream. The renin-angiotensin-aldosterone pathway is a critical determinant of blood pressure, and therefore by storing and secreting renin, the afferent arteriole plays an important role in blood pressure control which involves a variety of feedback pathways. The localization of Olfr78 to vascular resistance beds and the afferent arteriole implied a potential role for Olfr78 in blood pressure control, autoregulation of tissue blood flow, and/or extracellular fluid volume regulation.
Olfr78 as a Novel SCFA Receptor
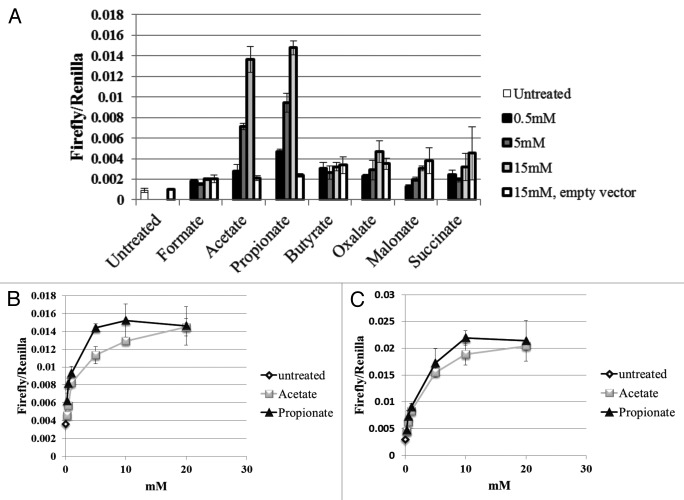
In order to better understand the physiological role of Olfr78 in the kidney, we set out to determine the ligand for this receptor. To do so, we employed a luciferase assay19 in which activation of the OR causes an increase in [cAMP]i, which can then be detected by a cAMP-dependent luciferase reporter. By employing an unbiased screen for potential ligands, we found18 that Olfr78 is a receptor for short chain fatty acids (SCFAs)—in particular, acetate and propionate. In addition, we confirmed a report by another group that the human homolog of Olfr78 also responds to SCFAs,20 implying that the function of this receptor may be conserved among multiple species. We found that the EC50 for Olfr78 was 920 µM for propionate, and 2.35mM for acetate (Fig. 1). In contrast, Gpr41 and Gpr43, well-studied SCFA receptors,9, 10,12,13, 21,23-26 have lower EC50 values both for propionate in a [35S]GTPγS binding assay (Gpr41 = 274 µM, Gpr43 = 259 µM22), and for acetate (Gpr41 = 1.3 mM, Gpr43 = 537 µM).22 Thus, Gpr41 and Gpr43 are significantly more sensitive to propionate and acetate, as compared with Olfr78. Interestingly, whereas both Gpr41 and Gpr43 can be activated by SCFAs in addition to acetate and propionate, Olfr78 responds solely to acetate and propionate.
Figure 1. Olfr78 was found to respond to acetate and propionate, but not any other ligands tested, including other mono- or di-carboxylic acids (A). Dose-response curves for Olfr78 (B) and it’s human ortholog, OR51E2 (C) indicate that both receptors are slightly more responsive to propionate than to acetate, with EC50 values in the high µM-low mM range (Olfr78: EC50 = 2.35 mM for acetate and 0.92 mM for propionate; OR51E2: EC50 = 2.93 mM for acetate and 2.16 mM for propionate). Figure modified from reference 18.
SCFAs: The Gut Microbiota
SCFAs are present in the bloodstream primarily as the result of metabolic production by the gut microbiota27; therefore, we wondered whether Olfr78 may be activated in response to changes in gut microbe metabolism. A recent and growing body of literature demonstrates that gut microbiota-derived metabolites play important roles in regulating the physiology of the host, and have been implicated in pathophysiological processes as varied as immune disorders,10,28-30 metabolism,31-33 atherosclerosis,34 irritable bowel syndrome,35,36 and chronic kidney disease.37,38 Gut microbes produce SCFAs (chiefly acetate, propionate and butyrate) such that the concentration in the colon is approximately 100mM.27 In the plasma, SCFAs have been found at 0.1–10mM.10,22,39 The identification of Olfr78 as a SCFA receptor,18 together with the localization of Olfr78,18 led to the novel hypothesis that Olfr78 may be responding to gut microbiota-derived SCFAs in order to regulate blood pressure.
Olfr78 and Renin Release
Because Olfr78 was found in tissues known to be important in blood pressure regulation, we hypothesized that SCFAs may signal via Olfr78 to modulate blood pressure. In particular, due to the localization of Olfr78 to the afferent arteriole, we investigated a potential role of Olfr78 in SCFA-mediated renin release. We labeled renin-containing granules with quinacrine, and then assessed the disappearance of quinacrine over time as an index of renin release. We found that when renin-containing JG cells were exposed to propionate, there was a decrease in quinacrine fluorescence (indicative of renin release), and that this effect was absent in Olfr78−/−. In line with these observations, we also found that Olfr78−/− had lower plasma renin levels (presumably due to the chronic absence of SCFA-mediated renin release), and lower baseline blood pressure (consistent with the lowered plasma renin). These data indicate that SCFA induce renin release, and that this effect is mediated via Olfr78.
Multiple SCFA Receptors and Blood Pressure Control
Because Olfr78 was also found in vascular resistance beds in a variety of tissues (skeletal muscle, diaphragm, etc.), we tested whether propionate may also have an acute, systemic effect on blood pressure. We found that an intravenous infusion of propionate resulted in dose-dependent drop in blood pressure which occurred in seconds and recovered over minutes. Due to the rapidity of the response, we reasoned that the response was likely mediated by either vasodilation or a change in heart rate. Based on the lack of evidence for changes in heart rate, and evidence in the literature that SCFAs cause vasodilation,40-42 we concluded that this is likely a vasodilatory effect.
Although the dose-response curve for this acute change in blood pressure was altered in Olfr78−/−, it was not absent. In fact, Olfr78−/− were hypersensitive to lower doses of propionate, demonstrating that Olfr78 does not promote a hypotensive response to propionate - but rather, opposes it! This indicates, then, that both in promoting renin secretion and in opposing the hypotensive response to propionate, Olfr78 is consistently acting to favor an increase in blood pressure. Because Olfr78 did not mediate the hypotensive response to propionate, we hypothesized that another receptor or pathway must be involved in mediating this response.
We focused on Gpr41 and/or Gpr43 as likely candidates for these other receptors, as Gpr41 and Gpr43 are previously characterized SCFA receptors known to respond to propionate.9,10,22 Gpr41 and Gpr43 are expressed in a variety of tissues,13,24-26 and respond to SCFAs produced by the gut microbiota to mediate physiological responses of the host, such as adiposity (Gpr419) and inflammatory responses (Gpr4310,22). To determine whether Gpr41 or Gpr43 might mediate the blood pressure response to SCFAs, we first used RT-PCR to establish that both Gpr41 and Gpr43 are expressed in blood vessels, along with Olfr78. To examine a potential functional role for Gpr41 in resistance vessels, we made use of Gpr41 KO mice. In wild-type mice, 10mM propionate produces a decrease in BP (13.9 mmHg18). This effect is absent in Gpr41−/−—in fact, the same propionate dose produced a modest hypertensive response in Gpr41−/− animals. These data indicate that Gpr41 contributes to the hypotensive effects of propionate, whereas, in opposition, Olfr78 antagonizes the hypotensive effects of propionate (please see ref. 43 for a more detailed review of this aspect of our work). Although there is much still to understand, it is clear that the BP response to propionate is quite complex, and involves (at a minimum) both Olfr78 and Gpr41 which act in opposition to one another (Fig. 2).
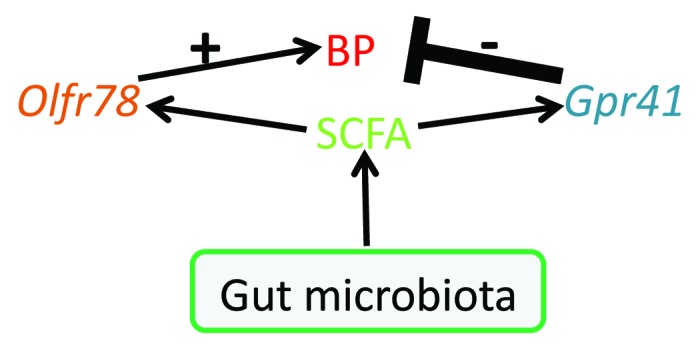
Figure 2. This diagram shows the roles of SCFAs, Olfr78, and Gpr41 in blood pressure (BP) regulation. Figure modified from reference 43.
Although our data supports the idea that propionate activation of Gpr41 leads to a hypotensive response and that propionate activation of Olfr78 favors a hypertensive response, it is natural to wonder “why” one ligand would activate two receptors, which then signal in opposition to each other. We believe a potential explanation for this lies in the EC50 values of Gpr41 and Olfr78. At basal levels of plasma propionate (reported to be between 0.1–10 mM10,22,39), we would expect Gpr41 (EC50 274 µM) but not Olfr78 (EC50 920 µM) to be somewhat active. As propionate in the circulating plasma increases, this would further activate Gpr41 and promote vasodilation—thereby explaining the predominant wild-type response of a drop in blood pressure upon propionate infusion. However, as plasma propionate levels continue to rise, Olfr78 would be activated as well, effectively serving as a “brake” on the pathway to prevent an inappropriate drop in blood pressure. Without the brake on the pathway, increases in plasma propionate could cause inappropriate (and potentially dangerous) drops in blood pressure, as evidenced by the increased sensitivity of the hypotensive response to propionate in Olfr78−/−.
It should be noted that although Gpr41 and Gpr43 were localized to blood vessels by PCR, they have not yet been localized to specific cell types within these blood vessels. Intriguingly, staining for Olfr78 (using b-galactosidase as a surrogate) revealed that in a given blood vessel profile, Olfr78 was expressed in only a subset of vascular smooth muscle cells (vSMC).18 Could it be that some vSMC express Olfr78, and others express Gpr41 or Gpr43? Or is there a subset of vSMC which express SCFA receptors, and a subset which do not? Although it is often assumed that all vSMCs in the same vessel have identical gene expression patterns, finding cell-specific patterns of expression within a single vessel leads to exciting questions of both gene expression regulation and downstream cell signaling pathways. For example, if Olfr78, Gpr41, and Gpr43 co-localize, this has important implications for future studies of downstream signaling pathways. Gpr41 and Gpr43 typically signal through an inhibitory G protein,44 whereas ORs typically signal through a stimulatory G protein.45 One possibility is that Gpr41 inhibits adenylate cyclase through Gi to promote vasorelaxation, and that Olfr78 activation in the same cell stimulates adenylate cyclase via Gs to oppose this effect (in support of this idea, an association has been reported between SCFA-induced vasorelaxation and cAMP levels46). However, the possibilities for how the downstream signaling of these receptors interact are completely altered if their expression is segregated to separate cells—in fact, we localized Gpr41 and Gpr43 to blood vessels by performing PCR on isolated vessels which had intact endothelium. Therefore, it is also possible that Gpr41 and/or Gpr43 localize to endothelial cells (and therefore, that the dilatory response could involve nitric oxide). Future studies will be required to understand the localization of each of these receptors in relation to each other, as this has important implications for the signaling pathway.
The Gut Microbiota and Blood Pressure Control
We recently published the results of gut microbiota reduction (using oral antibiotics) on the BP of Olfr78 mice.47 Analysis of fecal 16S rRNA sequences showed that oral antibiotics (vancomycin, ampicillin, and neomycin) reduced fecal micribioal biomass similarly in Olfr78 wild-type and Olfr78−/−. However, the addition of antibiotics to the drinking water increased BP in Olfr78−/−, but not Olfr78+/+, mice. According to our model (Fig. 2; reviewed in detail in ref. 43), when the ligand for both Olfr78 and Gpr41 is removed (via antibiotics), this has little effect in a wild-type animal because the mutually antagonistic actions of Olf78 and Gpr41 essentially cancel each other out. However, in an Olfr78−/− animal, propionate is acting solely via Gpr41 to affect BP; therefore removing the source of this ligand would be expected to reverse the unopposed hypotensive effect of propionate and thus produce a substantial increase in BP. Together with our data on the acute effects of SCFAs on both Olfr78 and Gpr41 KO, these data suggest that SCFAs generated by the gut microbiota modulate BP through effects on multiple receptors and pathways. According to our model, SCFA-mediated stimulation of Gpr41 decreases BP, while these same compounds act through Olfr78 to increase BP. These opposing responses may produce a “buffering” effect to prevent dramatic changes in BP in response to physiological variations in SCFA concentration.
Two Pathways to Regulate Blood Pressure
Our work implies that SCFAs have at least two separate effects on blood pressure: SCFAs act via Olfr78 in the afferent arteriole to modulate renin release (timescale of hours to days48), and can influence blood pressure by modulating peripheral resistance (vascular tone) through both Olfr78 and Gpr41 (timescale of seconds).
The rapid drop in blood pressure seen upon an iv infusion of propionate is likely due to an acute change in vascular tone. However, we also found that Olfr78−/− have lowered plasma renin levels. Is the basal hypotension see in Olfr78−/− due to altered vascular tone, or the absence of Olfr78-mediated renin release—or a combination of both? At this time, our data do not allow us to rule out either hypothesis. On the other hand, because SCFA effects on renin release appear to be mediated solely by Olfr78, the interpretation of any basal blood pressure differences in Gpr41−/− may be a bit clearer (since we would not expect the renin pathway to be altered in Gpr41−/−). In future studies, we will work to carefully localize Gpr41 as well as Gpr43 within the vasculature, as well as to further unravel the blood pressure phenotypes in these animals.
Implications
In recent years, it has become widely appreciated that SCFAs are naturally present in the plasma as a consequence of gut microbe metabolism, and that they play important roles in many aspects of host physiology.9,10,22,28,29,34-38 Our recent study highlights a novel area of influence for gut microbes and SCFAs—blood pressure control.
Although the idea of gut microbe metabolites regulating blood pressure is intriguing, it does beg several questions. For example: Why would gut microbe metabolites alter vascular resistance? One possibility is that this may function as a mechanism to facilitate absorption after a meal. Although most absorption occurs in the small intestine, a significant quantity of nutrients (including lactose, lactulose, and starches) are fermented into SCFA in the large intestine (in animals,27,49-57 as well as in humans58-66). We hypothesize that SCFA concentrations would peak in vessels serving the gut after a meal, and that this increase in SCFAs results in local vasodilation (as shown previously in ref. 67). This vasodilation may help to facilitate efficient absorption from the gut into the circulation, and thus ensure that nutrients are not lost in the stool. Although there is a paucity of data regarding to what extent plasma SCFA concentrations are modified by diet or by altering the gut flora, it is known that Gpr41−/− lose more SCFAs in the feces than their wild-type counterparts9). Although more work must be done to fully explain whether (or how) there may be a benefit to having a systemic change in BP after a meal, there is evidence that systemic BP does drop after a meal in humans—in fact, postprandial hypotension is quite common in the elderly.68
We also have wondered if some strains of gut microbes produce more SCFAs than others. Might it be that blood pressure is influenced not only by our own genetics, but also by the genetics of our gut microbiome? We do not yet have an answer to this, but look forward to future studies which we hope will illuminate the implications of this signaling pathway in human physiology. Indeed, although there are many potential clinical applications of this work, there is much we still do not yet understand. For example, is it possible to modulate blood pressure by altering the diet? Might one’s particular “blend” of microbiota help determine their resting blood pressure? Can purposeful altering one’s microbiota (i.e., antibiotics followed by probiotics) effect a change in blood pressure? Similarly, if a physician treats a patient with a high dose of antibiotics to treat an infection, is blood pressure control altered? Clearly, much remains to be understood about these pathways. We hope that by exploring the pathways by which SCFA receptors, SCFAs and gut microbes interact, we will better understood both blood pressure regulation and the unexpected ways in which gut microbes interact with our bodies.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This work was supported by NIH ROO DK081610 (to Pluznick JL) and a Gottschalk Award from the American Society of Nephrology. The author would like to thank Dr Michael Caplan and members of the Caplan and Pluznick laboratories for helpful discussions.
References
- 1.Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–8. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A. 2006;103:12569–74. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin CA, Kafadar KA, Pavlath GK. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell. 2009;17:649–61. doi: 10.1016/j.devcel.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–8. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 5.Elliott RA, Kapoor S, Tincello DG. Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J Urol. 2011;186:2455–62. doi: 10.1016/j.juro.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–4. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–93. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 9.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–72. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Li X, Ke Y, Lu Y, Wang F, Fan N, Sun H, Zhang H, Liu R, Yang J, et al. GPR48 increases mineralocorticoid receptor gene expression. J Am Soc Nephrol. 2012;23:281–93. doi: 10.1681/ASN.2011040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas SL, Toma I, Kang JJ, Meer EJ, Peti-Peterdi J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J Am Soc Nephrol. 2009;20:1002–11. doi: 10.1681/ASN.2008070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci U S A. 2011;108:8030–5. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Rogers M, Tian H, Zhang X, Zou DJ, Liu J, Ma M, Shepherd GM, Firestein SJ. High-throughput microarray detection of olfactory receptor gene expression in the mouse. Proc Natl Acad Sci U S A. 2004;101:14168–73. doi: 10.1073/pnas.0405350101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, De la Cruz O, Pinto JM, Nicolae D, Firestein S, Gilad Y. Characterizing the expression of the human olfactory receptor gene family using a novel DNA microarray. Genome Biol. 2007;8:R86. doi: 10.1186/gb-2007-8-5-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De la Cruz O, Blekhman R, Zhang X, Nicolae D, Firestein S, Gilad Y. A signature of evolutionary constraint on a subset of ectopically expressed olfactory receptor genes. Mol Biol Evol. 2009;26:491–4. doi: 10.1093/molbev/msn294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, et al. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci U S A. 2009;106:2059–64. doi: 10.1073/pnas.0812859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–5. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang H, Matsunami H. Evaluating cell-surface expression and measuring activation of mammalian odorant receptors in heterologous cells. Nat Protoc. 2008;3:1402–13. doi: 10.1038/nprot.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:124–33. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- 21.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–9. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 22.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–9. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 23.Kimura M, Mizukami Y, Miura T, Fujimoto K, Kobayashi S, Matsuzaki M. Orphan G protein-coupled receptor, GPR41, induces apoptosis via a p53/Bax pathway during ischemic hypoxia and reoxygenation. J Biol Chem. 2001;276:26453–60. doi: 10.1074/jbc.M101289200. [DOI] [PubMed] [Google Scholar]
- 24.Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149–56. doi: 10.2220/biomedres.30.149. [DOI] [PubMed] [Google Scholar]
- 25.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A. 2004;101:1045–50. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–60. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 27.Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B. 1987;86:439–72. doi: 10.1016/0305-0491(87)90433-0. [DOI] [PubMed] [Google Scholar]
- 28.Hwang JS, Im CR, Im SH. Immune disorders and its correlation with gut microbiome. Immune Netw. 2012;12:129–38. doi: 10.4110/in.2012.12.4.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–41. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 30.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol. 2011;8:523–31. doi: 10.1038/nrgastro.2011.133. [DOI] [PubMed] [Google Scholar]
- 36.Dahlqvist G, Piessevaux H. Irritable bowel syndrome: the role of the intestinal microbiota, pathogenesis and therapeutic targets. Acta Gastroenterol Belg. 2011;74:375–80. [PubMed] [Google Scholar]
- 37.Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol. 2013;37:1–6. doi: 10.1159/000345969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaziri ND. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens. 2012;21:587–92. doi: 10.1097/MNH.0b013e328358c8d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A. 2006;103:10011–6. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nutting CW, Islam S, Daugirdas JT. Vasorelaxant effects of short chain fatty acid salts in rat caudal artery. Am J Physiol. 1991;261:H561–7. doi: 10.1152/ajpheart.1991.261.2.H561. [DOI] [PubMed] [Google Scholar]
- 41.Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut. 1990;31:1391–4. doi: 10.1136/gut.31.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nutting CW, Islam S, Ye MH, Batlle DC, Daugirdas JT. The vasorelaxant effects of acetate: role of adenosine, glycolysis, lyotropism, and pHi and Cai2+ Kidney Int. 1992;41:166–74. doi: 10.1038/ki.1992.23. [DOI] [PubMed] [Google Scholar]
- 43.Pluznick JL. Renal and cardiovascular sensory receptors and blood pressure regulation. Am J Physiol Renal Physiol. 2013;305:F439–44. doi: 10.1152/ajprenal.00252.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown D, Sorscher EJ, Ausiello DA, Benos DJ. Immunocytochemical localization of Na+ channels in rat kidney medulla. Am J Physiol. 1989;256:F366–9. doi: 10.1152/ajprenal.1989.256.2.F366. [DOI] [PubMed] [Google Scholar]
- 45.Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244:790–5. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- 46.Daugirdas JT, Swanson V, Islam S, Nutting C, Kim DD, Wang XA, Fiscus RR. Acetate causes endothelium-independent increases in cyclic AMP in rat caudal artery. Am J Physiol. 1988;255:H1378–83. doi: 10.1152/ajpheart.1988.255.6.H1378. [DOI] [PubMed] [Google Scholar]
- 47.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–5. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood JM, Schnell CR, Cumin F, Menard J, Webb RL. Aliskiren, a novel, orally effective renin inhibitor, lowers blood pressure in marmosets and spontaneously hypertensive rats. J Hypertens. 2005;23:417–26. doi: 10.1097/00004872-200502000-00025. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong DG, Beever DE. Post-abomasal digestion of carbohydrate in the adult ruminant. Proc Nutr Soc. 1969;28:121–31. doi: 10.1079/PNS19690023. [DOI] [PubMed] [Google Scholar]
- 50.Demigne C, Remesy C. Influence of unrefined potato starch on cecal fermentations and volatile fatty acid absorption in rats. J Nutr. 1982;112:2227–34. doi: 10.1093/jn/112.12.2227. [DOI] [PubMed] [Google Scholar]
- 51.Demigné C, Rémésy C, Rayssiguier Y. Effect of fermentable carbohydrates on volatile fatty acids, ammonia and mineral absorption in the rat caecum. Reprod Nutr Dev. 1980;20(4B):1351–9. doi: 10.1051/rnd:19800726. [DOI] [PubMed] [Google Scholar]
- 52.Hintz HF, Hogue DE, Walker EF, Jr., Lowe JE, Schryver HF. Apparent digestion in various segments of the digestive tract of ponies fed diets with varying roughage-grain ratios. J Anim Sci. 1971;32:245–8. doi: 10.2527/jas1971.322245x. [DOI] [PubMed] [Google Scholar]
- 53.Keys JE, Jr., DeBarthe JV. Site and extent of carbohydrate, dry matter, energy and protein digestion and the rate of passage of grain diets in swine. J Anim Sci. 1974;39:57–62. doi: 10.2527/jas1974.39157x. [DOI] [PubMed] [Google Scholar]
- 54.Orskov ER, Fraser C, Kay RN. Dietary factors influencing the digestion of starch in the rumen and small and large intestine of early weaned lambs. Br J Nutr. 1969;23:217–26. doi: 10.1079/BJN19690029. [DOI] [PubMed] [Google Scholar]
- 55.Orskov ER, Fraser C, McDonald I. Digestion of concentrates in sheep. 3. Effects of rumen fermentation of barley and maize diets on protein digestion. Br J Nutr. 1971;26:477–86. doi: 10.1079/bjn19710053. [DOI] [PubMed] [Google Scholar]
- 56.Orskov ER, Fraser C, Mason VC, Mann SO. Influence of starch digestion in the large intestine of sheep on caecal fermentation, caecal microflora and faecal nitrogen excretion. Br J Nutr. 1970;24:671–82. doi: 10.1079/BJN19700068. [DOI] [PubMed] [Google Scholar]
- 57.Topping DL, Illman RJ, Taylor MN, McIntosh GH. Effects of wheat bran and porridge oats on hepatic portal venous volatile fatty acids in the pig. Ann Nutr Metab. 1985;29:325–31. doi: 10.1159/000176989. [DOI] [PubMed] [Google Scholar]
- 58.Anderson IH, Levine AS, Levitt MD. Incomplete absorption of the carbohydrate in all-purpose wheat flour. N Engl J Med. 1981;304:891–2. doi: 10.1056/NEJM198104093041507. [DOI] [PubMed] [Google Scholar]
- 59.Bond JH, Levitt MD. Quantitative measurement of lactose absorption. Gastroenterology. 1976;70:1058–62. [PubMed] [Google Scholar]
- 60.Englyst HN, Cummings JH. Digestion of the polysaccharides of some cereal foods in the human small intestine. Am J Clin Nutr. 1985;42:778–87. doi: 10.1093/ajcn/42.5.778. [DOI] [PubMed] [Google Scholar]
- 61.McNeil NI, Bingham S, Cole TJ, Grant AM, Cummings JH. Diet and health of people with an ileostomy. 2. Ileostomy function and nutritional state. Br J Nutr. 1982;47:407–15. doi: 10.1079/BJN19820052. [DOI] [PubMed] [Google Scholar]
- 62.Perman JA, Modler S. Role of the intestinal microflora in disposition of nutrients in the gastrointestinal tract. J Pediatr Gastroenterol Nutr. 1983;2(Suppl 1):S193–6. doi: 10.1097/00005176-198300201-00028. [DOI] [PubMed] [Google Scholar]
- 63.Pomare EW, Branch WJ, Cummings JH. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J Clin Invest. 1985;75:1448–54. doi: 10.1172/JCI111847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandberg AS, Andersson H, Kivistö B, Sandström B. Extrusion cooking of a high-fibre cereal product. 1. Effects on digestibility and absorption of protein, fat, starch, dietary fibre and phytate in the small intestine. Br J Nutr. 1986;55:245–54. doi: 10.1079/BJN19860031. [DOI] [PubMed] [Google Scholar]
- 65.Saunders DR, Wiggins HS. Conservation of mannitol, lactulose, and raffinose by the human colon. Am J Physiol. 1981;241:G397–402. doi: 10.1152/ajpgi.1981.241.5.G397. [DOI] [PubMed] [Google Scholar]
- 66.Wiggins HS. Nutritional value of sugars and related compounds undigested in the small gut. Proc Nutr Soc. 1984;43:69–75. doi: 10.1079/PNS19840029. [DOI] [PubMed] [Google Scholar]
- 67.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 68.Van Orshoven NP, Jansen PA, Oudejans I, Schoon Y, Oey PL. Postprandial hypotension in clinical geriatric patients and healthy elderly: prevalence related to patient selection and diagnostic criteria. J Aging Res. 2010;2010:243752. doi: 10.4061/2010/243752. [DOI] [PMC free article] [PubMed] [Google Scholar]