Abstract
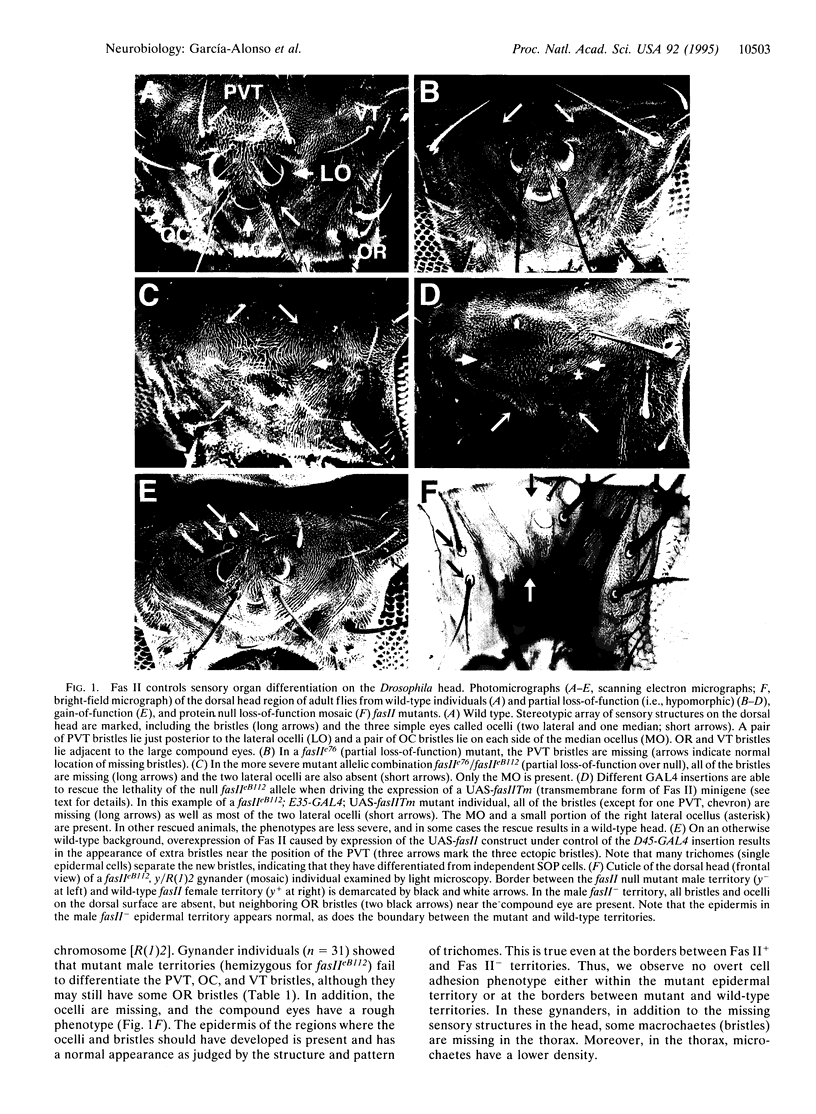
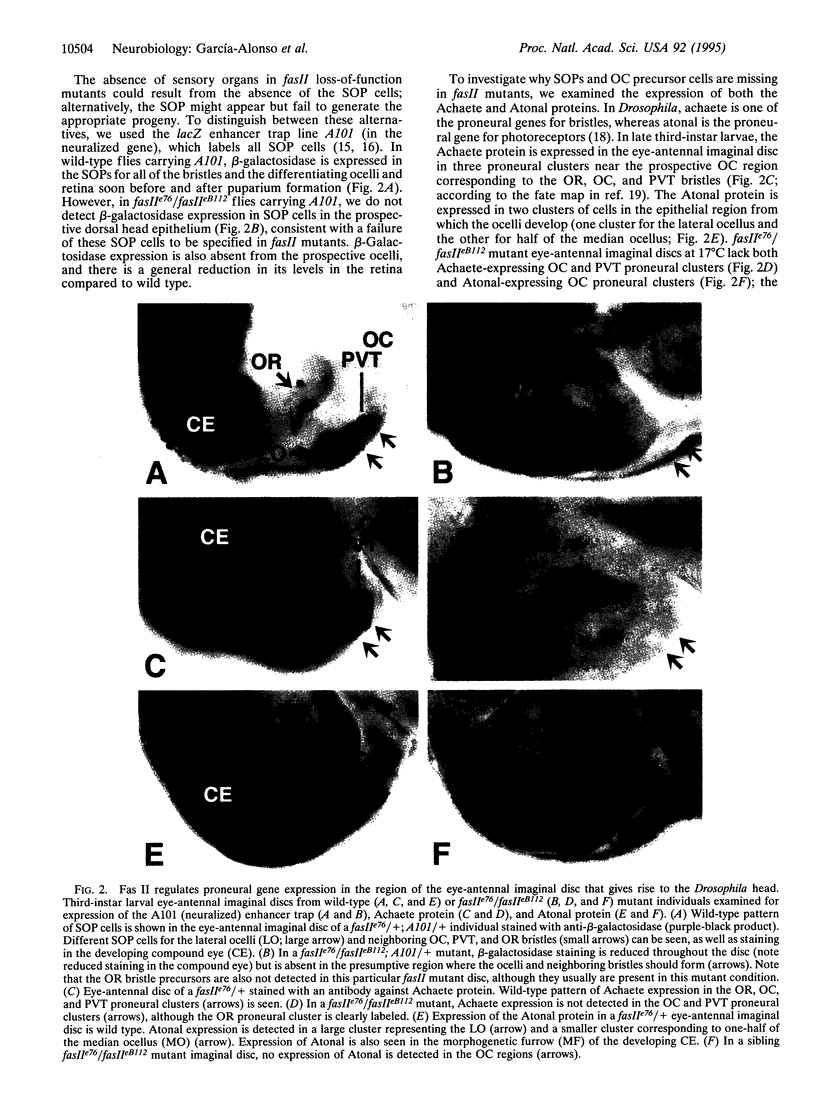
Fasciclin II (Fas II), an NCAM-like cell adhesion molecule in Drosophila, is expressed on a subset of embryonic axons and controls selective axon fasiculation. Fas II is also expressed in imaginal discs. Here we use genetic analysis to show that Fas II is required for the control of proneural gene expression. Clusters of cells in the eye-antennal imaginal disc express the achaete proneural gene and give rise to mechanosensory neurons; other clusters of cells express the atonal gene and give rise to ocellar photoreceptor neurons. In fasII loss-of-function mutants, the expression of both proneural genes is absent in certain locations, and, as a result, the corresponding sensory precursors fail to develop. In fasII gain-of-function conditions, extra sensory structures arise from this same region of the imaginal disc. Mutations in the Abelson tyrosine kinase gene show dominant interactions with fasII mutations, suggesting that Abl and Fas II function in a signaling pathway that controls proneural gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellen H. J., O'Kane C. J., Wilson C., Grossniklaus U., Pearson R. K., Gehring W. J. P-element-mediated enhancer detection: a versatile method to study development in Drosophila. Genes Dev. 1989 Sep;3(9):1288–1300. doi: 10.1101/gad.3.9.1288. [DOI] [PubMed] [Google Scholar]
- Boulianne G. L., de la Concha A., Campos-Ortega J. A., Jan L. Y., Jan Y. N. The Drosophila neurogenic gene neuralized encodes a novel protein and is expressed in precursors of larval and adult neurons. EMBO J. 1991 Oct;10(10):2975–2983. doi: 10.1002/j.1460-2075.1991.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Doherty P., Walsh F. S. Signal transduction events underlying neurite outgrowth stimulated by cell adhesion molecules. Curr Opin Neurobiol. 1994 Feb;4(1):49–55. doi: 10.1016/0959-4388(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Elkins T., Zinn K., McAllister L., Hoffmann F. M., Goodman C. S. Genetic analysis of a Drosophila neural cell adhesion molecule: interaction of fasciclin I and Abelson tyrosine kinase mutations. Cell. 1990 Feb 23;60(4):565–575. doi: 10.1016/0092-8674(90)90660-7. [DOI] [PubMed] [Google Scholar]
- Ferreiro B., Kintner C., Zimmerman K., Anderson D., Harris W. A. XASH genes promote neurogenesis in Xenopus embryos. Development. 1994 Dec;120(12):3649–3655. doi: 10.1242/dev.120.12.3649. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Bieber A. J., Rehm E. J., Snow P. M., Traquina Z. R., Hortsch M., Patel N. H., Goodman C. S. Molecular genetics of neuronal recognition in Drosophila: evolution and function of immunoglobulin superfamily cell adhesion molecules. Cold Spring Harb Symp Quant Biol. 1990;55:327–340. doi: 10.1101/sqb.1990.055.01.034. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rehm E. J., Goodman C. S. Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell. 1991 Oct 4;67(1):45–57. doi: 10.1016/0092-8674(91)90571-f. [DOI] [PubMed] [Google Scholar]
- Harrelson A. L., Goodman C. S. Growth cone guidance in insects: fasciclin II is a member of the immunoglobulin superfamily. Science. 1988 Nov 4;242(4879):700–708. doi: 10.1126/science.3187519. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M. J., Gertler F. B., Goodman W., Hoffmann F. M. The Drosophila Abelson proto-oncogene homolog: identification of mutant alleles that have pleiotropic effects late in development. Cell. 1987 Dec 4;51(5):821–828. doi: 10.1016/0092-8674(87)90105-x. [DOI] [PubMed] [Google Scholar]
- Huang F., Dambly-Chaudière C., Ghysen A. Position-reading and the emergence of sense organ precursors in Drosophila. Prog Neurobiol. 1994 Feb;42(2):293–297. doi: 10.1016/0301-0082(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. Neuronal cell fate specification in Drosophila. Curr Opin Neurobiol. 1994 Feb;4(1):8–13. doi: 10.1016/0959-4388(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Jarman A. P., Grell E. H., Ackerman L., Jan L. Y., Jan Y. N. Atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994 Jun 2;369(6479):398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- Jiménez F., Modolell J. Neural fate specification in Drosophila. Curr Opin Genet Dev. 1993 Aug;3(4):626–632. doi: 10.1016/0959-437x(93)90099-b. [DOI] [PubMed] [Google Scholar]
- Klämbt C., Jacobs J. R., Goodman C. S. The midline of the Drosophila central nervous system: a model for the genetic analysis of cell fate, cell migration, and growth cone guidance. Cell. 1991 Feb 22;64(4):801–815. doi: 10.1016/0092-8674(91)90509-w. [DOI] [PubMed] [Google Scholar]
- Lin D. M., Fetter R. D., Kopczynski C., Grenningloh G., Goodman C. S. Genetic analysis of Fasciclin II in Drosophila: defasciculation, refasciculation, and altered fasciculation. Neuron. 1994 Nov;13(5):1055–1069. doi: 10.1016/0896-6273(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Lin D. M., Goodman C. S. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994 Sep;13(3):507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Papalopulu N., Kintner C. R. Molecular genetics of neurulation. Ciba Found Symp. 1994;181:90–102. doi: 10.1002/9780470514559.ch6. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Carroll S. B. The achaete-scute complex: generation of cellular pattern and fate within the Drosophila nervous system. FASEB J. 1994 Jul;8(10):714–721. doi: 10.1096/fasebj.8.10.8050670. [DOI] [PubMed] [Google Scholar]