Key Points
Depletion of host regulatory T cells with IL2DT improves efficacy of haploidentical NK cell therapy for refractory acute myeloid leukemia.
Depletion of Treg and persistence of NK cells for ≥7 days after NK cell adoptive transfer predicts beneficial clinical responses.
Abstract
Haploidentical natural killer (NK) cell infusions can induce remissions in some patients with acute myeloid leukemia (AML) but regulatory T-cell (Treg) suppression may reduce efficacy. We treated 57 refractory AML patients with lymphodepleting cyclophosphamide and fludarabine followed by NK cell infusion and interleukin (IL)-2 administration. In 42 patients, donor NK cell expansion was detected in 10%, whereas in 15 patients receiving host Treg depletion with the IL-2-diphtheria fusion protein (IL2DT), the rate was 27%, with a median absolute count of 1000 NK cells/μL blood. IL2DT was associated with improved complete remission rates at day 28 (53% vs 21%; P = .02) and disease-free survival at 6 months (33% vs 5%; P < .01). In the IL2DT cohort, NK cell expansion correlated with higher postchemotherapy serum IL-15 levels (P = .002), effective peripheral blood Treg depletion (<5%) at day 7 (P < .01), and decreased IL-35 levels at day 14 (P = .02). In vitro assays demonstrated that Tregs cocultured with NK cells inhibit their proliferation by competition for IL-2 but not for IL-15. Together with our clinical observations, this supports the need to optimize the in vivo cytokine milieu where adoptively transferred NK cells compete with other lymphocytes to improve clinical efficacy in patients with refractory AML. This study is registered at clinicaltrials.gov, identifiers: NCT00274846 and NCT01106950.
Introduction
Tumor lysis by natural killer (NK) cells is limited by inhibitory killer immunoglobulin receptors (KIRs) that mediate self-tolerance by engaging major histocompatibility complex class I antigens.1 In contrast, NK cells reconstituting after transplantation can overcome this major histocompatibility complex barrier by KIR ligand mismatching to mediate a potent anti-leukemia reaction by decreased triggering through inhibitory KIR.2 We have previously described the safety and preliminary efficacy of adoptive transfer of haploidentical NK cells.3 Patients were treated with lymphodepleting chemotherapy and received haploidentical NK cell infusions from siblings, parents, or children, followed by subcutaneous interleukin (IL)-2 to stimulate NK proliferation and activation. In that study, we found that 26% of poor prognosis acute myeloid leukemia (AML) patients achieved complete hematologic remission (CR) after NK cell adoptive transfer.
In subsequent applications of donor NK cell infusions to treat non-Hodgkin lymphoma, breast cancer, and ovarian cancer, we and others have found that host regulatory T cells (Tregs) are resistant to cytotoxic therapy and expand rapidly when IL-2 is administered after NK cell infusion.4,5 Tregs are phenotypically distinct CD4+CD25+Foxp3+ immunosuppressive lymphocytes residing in lymphoid organs and peripheral blood (PB). They prevent autoimmunity and mediate tolerance by restricting immune responses, including inhibition of NK-mediated cytotoxicity.6 In the setting of NK cell adoptive transfer, however, we hypothesize that host Tregs interfere with NK-cell proliferation and expansion. Because Tregs are uniquely dependent on the high affinity IL-2 receptor α chain (CD25) for their function and survival, IL-2 mediates the strongest proliferative signal for Tregs. We report here the results of in vitro tests to determine the effect of competition between Tregs and NK cells, which support the incorporation of Treg depletion into our adoptive transfer platform.
IL-2 diphtheria toxin (IL2DT, Denileukin diftitox; Ontak), is a recombinant cytotoxic fusion protein composed of the amino acid sequences for diphtheria toxin followed by truncated amino acid sequences for IL-2. Therefore, IL2DT should selectively deplete IL-2 receptor (CD25+)-expressing cells, including Tregs. IL2DT is 100 times more effective in killing cells bearing the IL-2 receptor α chain isoform (CD25) compared with cells expressing the lower-affinity IL-2 receptors (ie, CD122 and CD132).7 In murine AML models, depletion of Tregs by anti-IL-2 receptor α monoclonal antibody or IL-2 diphtheria toxin fusion protein dramatically improved the efficacy of adoptive NK or cytotoxic T-cell immunotherapy.8,9 IL2DT is a particularly attractive agent to test for the selective depletion of Tregs due to the short half-life, rapid internalization time, and induction of apoptosis, thus allowing for dosing regimens that will not affect adoptive immune therapy (ie, NK cells) infused just hours after IL2DT.10 Thus, we tested host Treg depletion with IL2DT in our platform of lymphodepleting chemotherapy to enhance in vivo NK cell expansion and induction of remissions in refractory AML after adoptive NK cell transfer.
Methods
Patient eligibility and clinical protocol
Patients with relapsed or primary refractory AML with adequate organ function who had failed ≥2 therapies were eligible for enrollment. The protocol and consent procedures were approved by the University of Minnesota institutional review board (clinicaltrials.gov NCT00274846 and NCT01106950), and informed consent was given by all patients and donors for treatment and prospective data collection in accordance with Declaration of Helsinki. Nonmobilized donor PB mononuclear cells (MNCs) were collected with the COBE Spectra Apheresis System (TerumoBCT, Lakewood, CO) for 3 (n = 31) or 5 hours (n = 26). The trial schema, chemotherapy, and trial end points are detailed in Figure 1, and standard definitions of response were used.11 The primary prospective end point of the study was successful in vivo donor NK cell expansion defined as measurement of >100 donor NK cells/μL of PB at day +14 after NK cell infusion [(absolute lymphocyte count/μL) × (% of lymphocyte gate that are CD56+/CD3− NK cells) × (% donor chimerism using standard short tandem repeat [STR] testing)].
Figure 1.
Clinical trial schema. Patients received fludarabine 25 mg/m2/day intravenously (IV) daily (days −6 through −2) and cyclophosphamide 60 mg/kg/day IV (days −5 and −4) to lymphodeplete the recipient and facilitate homeostatic expansion of allogeneic NK cells. One (n = 11) or 2 doses (n = 4) of IL2DT, 12 (n = 11) or 18 mg/kg (n = 4) IV, were added at day −1 ± −2 to deplete Treg. NK cell products were administered by IV infusion on day 0 followed by subcutaneous IL-2 (9 × 106 units) starting 4 hours after NK cell infusion and given every other day for 6 doses to facilitate NK cell survival and expansion in vivo. Unseparated PB donor chimerism by STR and lymphocyte subsets were analyzed at days 7, 14, and 28. Bone marrow (BM) was analyzed for leukemia clearance at days 14 and 28 to assess disease status according to World Health Organization criteria. Toxicity and adverse events were classified according to National Cancer Institute Common Terminology Criteria for Adverse Events V 3.0. The primary prospective end point of the study was successful in vivo donor NK cell expansion defined as measurement of >100 donor NK cells/μL of PB at day +14 after NK cell infusion [(absolute lymphocyte count/μL) × (% of lymphocyte gate that are CD56+/CD3− NK cells) × (% donor chimerism using standard short tandem repeat testing)]. We evaluated BM at day 28 and used standard definitions of CR, CRp (<100 000 platelet count/μL), and CRi (<1000 absolute neutrophils/μL).
Preparation of the NK-enriched cell products
The apheresis products were T-cell (CD3) ± B-cell (CD19) depleted ± CD56 selected using the Miltenyi Biotec CliniMACS Cell Selection System and reagents (Miltenyi Biotec, Bergisch Gladbach, Germany) (Table 1) and cultured overnight with 1000 IU/mL IL-2 as previously published.12 An aliquot of this prepared cell product was analyzed by flow cytometry to determine the number of T, B, and NK cells by using fluorescein isothiocyanate, Ag-presenting cells, phycoerythrin, and Peridinin-Chlorophyll-Protein-Complex–conjugated antibodies against CD3, CD14, CD56, CD19 or CD20, KIR, and NKG2A (BD PharMingen, San Diego, CA) and tested in a 4-hour Cr-release cytotoxicity assay against the K562 cell line.13
Table 1.
Patients, treatment, and product characteristics
| Variable | Cohort 1 | Cohort 2 | Cohort 3 |
|---|---|---|---|
| No. of patients enrolled | 32 | 10 | 15 |
| Time period | 2003-2007 | 2005 | 2010-2011 |
| Patient age in years (range) | 46 (7-68 y) | 37 (5-65 y) | 51 (3-71 y) |
| Patient gender (male) | 19 (59%) | 5 (50%) | 8 (55%) |
| Marrow blasts (mean %) | 45% (range 2-98) | 36% (range 7-92) | 34% (range 8-69) |
| Number of prior therapies (mean) | 4 | 3 | 3 |
| Prior HCT | 2 auto/4 allo | 0 | 0 |
| Recipient CMV+ status | 14 (44%) | 6 (60%) | 7 (45%) |
| Conditioning | Cy/Flu | Cy/Flu | Cy/Flu |
| No. of IL-2 doses (mean) | 4 (range 3-6) | 4 (range 3-6) | 4 (range 1-6) |
| IL2DT received | No | No | Yes* |
| KIR mismatch in GVHD direction | 6 (17%) | 5 (50%) | 8 (53%) |
| Product processing method | CD3- | CD3-CD56+ | CD3-CD19- |
| Final product characteristics | |||
| Dose of NC/kg | 2.5 ± 0.8 x107 | 0.44 ± 0.09 × 107 | 4.7 ± 1.8 × 107 |
| Dose of NK cells/kg infused | 0.96 ± 0.3 × 107 | 0.34 ± 0.05 x107 | 2.6 ± 1.5 × 107 |
| Percentage NK cells | 39 ± 9% | 75 ± 6% | 54 ± 16% |
| Dose of T-cells/kg | 14 × 104 | 6.2 × 104 | 9.7 × 104 |
| Percentage T cells | 0.7% | 1.3% | 0.3% |
CD3−, CD3 depletion; CD3−CD56+, CD3 depletion followed by CD56-positive selection; CD19−, CD19 negative selection; CMV, cytomegalovirus; NC, nucleated cell dose.
Doses of 12 to 18 μg/kg × 1 (n = 11) or 2 doses (n = 4) were used.
Immunophenotyping and enzyme-linked immunosorbent assay
Patient PB was analyzed by flow cytometry before chemotherapy, days 0, 7, 14, and 28 after NK cell infusion. Lyphocytes were characterized by multicolor fluorescent antibodies directed against CD45, CD56, CD3, CD4, and CD20, Ki67 (BP PharMingen, San Diego, CA) and in selected patients CD25, CD127, Foxp3, Helios, 41BB, and CD40 ligands. Plasma IL-15, IL-7, IL-35, and proliferation assay supernatant for IL-15 and IL-2 concentrations were determined by commercial enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN).
5-Carboxyfluorescein diacetate succinimide ester assay to assess NK-cell suppression by Tregs
Umbilical cord blood (UCB)-derived Tregs were purified and expanded as previously reported.14 The effect of Tregs on NK cells (NK cell isolation kit, MACS; Miltenyi Biotec) or PBMNCs was tested after labeling with 5-carboxyfluorescein diacetate succinimide ester (CFSE) to assess proliferation induced by anti-CD3 mAb-coated beads (Dynal) or cytokines as indicated. We used the same suppression assay to evaluate patient PBMNCs collected at day 14 with healthy donor NK cells. Acquired data were analyzed using the proliferation platform in FlowJo (Treestar, Ashland, OR).
Statistical analysis
Disease-free survival (DFS) was estimated by Kaplan-Meier curves through 6 months after therapy.15 Cumulative incidence was used to estimate nonrelapse mortality (NRM), treating relapse and disease progression as competing risks.16 Simple proportions were used to describe in vivo donor NK-cell expansion and CR. Associations between NK-cell expansion and remission and Treg depletion were tested by the Fisher’s exact test and Wilcoxon rank-sum test, respectively. In vitro results were compared by paired 2-tailed t test.
Results
Patients and disease characteristics
A total of 57 patients with relapsed and refractory primary or secondary AML were treated from 2003 to 2011 (Table 1). All patients received lymphodepleting chemotherapy with fludarabine and cyclophosphamide, and the 15 patients in cohort 3 also received IL2DT (Figure 1). The outcomes of the first 19 patients in cohort 1 were published previously3 and are included here to compare the efficacy of different NK-cell products. Patient characteristics including age, percentage of marrow blasts at time of treatment, and the number of prior therapies were similar between cohorts. Six patients in cohort 1 had undergone prior hematopoietic stem cell transplantation (HCT; 2 autologous and 4 allogeneic). All NK cell donors were HLA-haploidentical relatives and about half were KIR ligand mismatched in the graft-versus-host disease (GVHD) direction. IL2DT-treated patients (Table 2) often had an antecedent hematologic malignancy with progression to AML (55%), and 40% had received prior hypomethylating therapy.
Table 2.
Treatment details, correlative analysis, and patient outcome
| No. | Age (years) | Prior disease | WBC* | Cytogenetics/FISH | Molecular | Marrow blasts* | No. of prior therapies | Disease status | KIR ligand mismatch | PB donor chimerism at day 7 | Donor PB NK cells at day 14 (cells/μL) | PB percentage of Tregs at day 14 | Disease response at day 28 | Time to death/time of survival (mo) | Survival status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | MDS | 82 | t(1;3),del 5q- | EGFR | 96% | 2† | PIF | No | 95% | 12390 | 0.02% | CRi | 1.1 | Dead |
| 2 | 59 | MDS | 0.2 | Complex, del 7p- | EGFR | 34% | 3 | PIF | No | 95% | 484 | 0.1% | PD | 0.9 | Dead |
| 3 | 49 | RAEB-2 | 55 | Normal | Flt3 ITD | 49% | 3† | PIF | No | 13% | 0 | 71% | CR‡ | 10 | Dead |
| 4 | 51 | ET/MDS | 10 | del 5q-, del 20q- | EGR1 | 40% | 3 | PIF | No | QNS | 0 | 49% | PD | 0.8 | Dead |
| 5 | 5 | Primary AML | 110 | t(9;11) | MLL | 95% | 5 | PIF | Yes | 0% | 0 | 27% | PD | 1.1 | Dead |
| 6 | 51 | Primary AML | 32 | Monosomy 7 | EVI1 | 81% | 4† | PIF | Yes | 34% | 24 | 1.1% | PD | 2.5 | Dead |
| 7 | 71 | MDS | 0.2 | Complex, del20q- | MYBL2 | 6% | 3† | Relapse | Yes | 68% | 0 | 17% | CR‡ | 9.4 | Alive |
| 8 | 64 | MPD | 53 | Monosomy 7 | EGFR | 9% | 5† | PIF | No | 92% | 532 | 0.06% | CRi‡ | 2.6 | Dead |
| 9 | 57 | MPD | 0.7 | Normal | Jak2 +, Flt3 ITD | 24% | 2† | PIF | Yes | QNS | No | 11% | CRi‡ | 12 | Alive |
| 10 | 37 | Primary AML | 2.2 | Normal | Flt3 ITD | 10% | 3 | PIF | No | 33% | No | 28% | CRp‡ | 12 | Alive |
| 11 | 13 | MDS | 225 | Trisomy 6 | Flt3 ITD | 7% | 3 | PIF | No | 42% | No | 10% | CR | 10.2 | Dead |
| 12 | 54 | Primary AML | 5.6 | t(12;14) | ETV6 | 36% | 4 | Relapse | Yes | 0% | No | NA | PD | 0.4 | Dead |
| 13 | 5 | Primary AML | NA | t(9;11) | MLL | 33% | 4 | Relapse | Yes | 0% | No | 16% | PD | 1.1 | Dead |
| 14 | 16 | Primary AML | 50 | t(3;22) del 9q | n/a | 85% | 6 | PIF | Yes | 99% | 1470 | 0% | PD | 1.2 | Dead |
| 15 | 73 | Primary AML | 2.5 | Trisomy 1 | PBX1 | 24% | 2 | Relapse | Yes | 70% | No | 20% | CRp‡ | 12 | Alive |
ET, essential thrombocythemia; MPD, myeloproliferative disorder; NA, not available; PD, progressive disease; PIF, primary induction failure; QNS, quantity not sufficient; RAEB, refractory anemia with excess blasts; WBC, white blood cell.
At time of therapy (in 106 cells/μL).
Includes prior demethylation agent.
Subsequent allogeneic donor stem cell transplant.
NK-cell products
Three different processing methods were used to prepare NK-cell products for infusion. These included CD3 depletion alone (cohort 1; n = 32), a CD3 depletion followed by CD56 selection (cohort 2; n = 10), and single step CD3/CD19 depletion (cohort 3; n = 15). Analysis of the cellular content of each product, summarized in Table 1, showed that the addition of CD56 selection resulted in threefold fewer NK cells per product compared with CD3 depletion alone. Lymphapheresis runs of 3 hours (n = 31) were well tolerated and were extended to 5 hours for donors in cohorts 2 and 3 to obtain higher NK cell doses. All clinical products were highly cytotoxic against K562 targets (data not shown). The highest NK cell doses (mean 2.6 ± 1.5 × 107 NK cells/kg) were obtained with the CD3/CD19 depletion method, due to the 5-hour collection time and reduced cell loss with the single GMP manipulation.
Infusion and long-term toxicities
All 57 patients tolerated NK-cell infusions well. Grade 1 to 2 nonhematologic toxicities (fever, chills, hypertension/hypotension, dyspnea, hypoxemia, headaches) were common, but there was only 1 grade 4 infusion-related hypersensitivity reaction, which promptly resolved with antihistamines and supportive care. We observed no infusional toxicity with IL2DT. Later grade 3 to 5 toxicities, which included infection or fevers (n = 5), cytomegalovirus viremia (n = 1), alveolar hemorrhage (n = 2), pulmonary events (n = 2), pleural effusion (n = 1), candidemia (n = 1), fungal pneumonia (n = 1), atrial fibrillation (n = 1), left ventricular dysfunction (n = 1), typhlitis (n = 1), meningitis (n = 1), and Epstein-Barr virus (EBV)-associated lymphoma (n = 1), were most likely related to prolonged cytopenias and immune suppression. One patient (cohort 1) died in remission from complications of EBV-associated lymphoproliferative disorder at day 116. We did not observe acute cytokine release syndrome or complications associated with tumor lysis, and no patients developed acute GVHD or autoimmune disorders.
Addition of host Treg depletion with IL2DT significantly increases CR rate
Among the 42 patients in cohorts 1 and 2 who did not receive host Treg depletion with IL2DT, 9 (21%) achieved remissions (5 CR and 4 incomplete remission without neutrophil [<1000 cells/μL] and platelet recovery [CRi]) by day 35. The median duration of remission was 2.3 months (range, 1.8-15 months). This platform served as a bridging therapy for 4 patients who proceeded to allogeneic HCT between days 50 and 65 after NK-cell infusion. Among the other 5 patients who achieved remissions, 1 died of NRM (EBV event above), and 4 were ineligible for transplant due to comorbidities. They relapsed at 61, 67, 190, and 450 days after NK-cell infusion, demonstrating that this is not curative therapy. There was no improvement in the remission rate for the patients who received purified CD56-selected NK cell products (cohort 2) compared with those who received CD3-depleted products.
Augmented lymphocyte and Treg depletion with IL2DT (cohort 3) resulted in remissions at day 28 for 8 of 15 patients (53%), including CR (n = 3), CR without platelet recovery (CRp; n = 2), and CRi (n = 3), which was significantly better compared with strategies without IL2DT (CR rate, 21%; P = .02). Six patients in remission subsequently received allogeneic donor HCT 45 to 120 days after NK-cell therapy. One transplant-ineligible patient remained in CR until relapse at 8 months, and 1 patient who successfully expanded donor NK cells and cleared leukemia died of neutropenic sepsis at day 30. The median duration of remission in cohort 3 was 11.2 months (range, 1-32 months). Patients in CR and CRp attained neutrophil recovery (ANC > 1000 cells/μL) at a median of 20 days after NK-cell infusion (range, 15-22 days; n = 5).
There was no correlation between CR and AML blast burden, cytogenetics, number of prior therapies, KIR ligand mismatch, use of hypomethylating agent, or disease status at the time of enrollment in cohorts 1 and 2 (data not shown) or cohort 3 (Table 2). Considering relapse/progression as a competing risk, the overall NRM was similar between the groups: 12% (95% confidence interval [CI], 5-18%) in cohorts 1 and 2 and 13% (95% CI, 1-26%) in cohort 3. DFS at 6 months was 5% (95% CI, 1-14%) for cohorts 1 and 2 compared with 33% for cohort 3 (95% CI, 12-56%; P < .01). Thus, the addition of host Treg depletion with IL2DT into our previously published adoptive NK-cell therapy platform resulted in a remission rate >50% and superior DFS for patients with refractory/relapsed AML with no increased complications or toxicity.
Addition of Treg depletion with IL2DT enhances successful donor NK-cell expansion
In cohorts 1 and 2, 4 of 42 (10%) patients had detectable donor NK cells in vivo. In contrast, host Treg depletion with IL2TD was associated with a higher rate (4/15 [27%]) of successful donor NK-cell expansion. The magnitude of the NK-cell expansion was also higher after Treg depletion, with median absolute circulating donor-derived NK-cell counts at day +14 of 190 cells/μL (range, 110-240 cells/μL) and 1000 NK cells/μL (range, 480-12 390 cells/μL; P = .12), respectively. Failure to expand NK cells did not correlate with disease burden (WBC counts and percentage blasts) or prior therapy. Immunological parameters such as baseline PB Treg frequencies (measured in cohort 3) or number of IL-2 injections received did not influence NK-cell expansion (Table 2). In addition, there was no correlation between the incidence of NK-cell expansion and the method of NK-cell product manufacture (CD3-depleted and CD56-selected [cohort 2] vs CD3-depleted [cohort 1]) or the infused NK-cell dose (data not shown).
Magnitude of Treg depletion correlates with successful donor NK-cell expansion
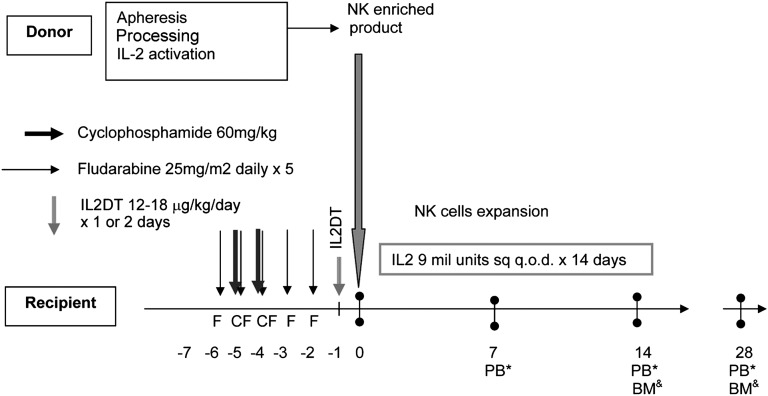
We evaluated the magnitude of Treg depletion achieved with IL2DT to determine whether it correlated with successful NK-cell expansion and attainment of complete remission. Prior to lymphodepleting chemotherapy, the median absolute lymphocyte count in patients was 800 cells/μL (range, 0-1300 cells/μL), and Tregs (CD4+FoxP3+CD25hi) comprised 4% (range, 0.3-7.5%, n = 15) of PB lymphocytes (median absolute Treg count, 34/μL; range, 0-60 cells/μL). Following the cyclophosphamide and fludarabine (day −2), patients became lymphopenic (median WBC, 350 cells/μL; range, 0-760 cells/μL). At that time, most residual lymphocytes were T cells (median, 79%; range, 23-95%), predominantly CD3+CD4+ (median, 89% of T cells; range, 74-97%), with very few CD3+CD8+ effectors surviving (median, 7%; range, 1-9%). Although Tregs comprised 9% of all lymphocytes (range, 0.03-27%; absolute count, 31 cells/μL; range, 10-94 cells/μL), suggesting relative Treg chemoresistance, they were not proliferating based on undetectable Ki67 expression (data not shown). Treg levels were measured on day 7 following the infusion of IL2DT and the first 3 doses of IL-2. Tregs were depleted in some patients (n = 6; median PB Tregs, 1%; range, 0.05-4.7%]), but more often, host Tregs accumulated (n = 9; median Tregs, 28%; range, 9-52%]). We observed a strong inverse correlation between day 7 absolute PB Treg count and successful in vivo donor NK-cell expansion. Of the 6 patients with <5% Tregs at day 7 (median absolute Treg of 3 cells/μL; range, 0-6 cells/μL), 5 successfully expanded donor NK cells. In contrast, none of the 9 patients with higher Treg counts expanded NK cells (median Treg, 32; range, 10-69 cells/μL; P < .01; Figure 2A). Treg proliferation measured by the proportion of cells expressing Ki67 was significantly higher in patients without successful in vivo donor NK expansion compared with those who did expand (90 ± 2% vs 65 ± 5%; P = .004), suggesting that host Tregs were responding to the IL-2 administration given to the patient after NK-cell infusion. The ability of IL2DT to deplete Tregs is further supported by reductions in serum IL-35 concentrations 14 days after adoptive transfer in patients who received IL2DT compared with those who did not (88.2 [range, 5-186] pg/mL, n = 12 vs 30 [range, 10-83] pg/mL, n = 15; P = .02). Tregs collected 14 days from adoptive transfer from 4 patients who did not expand were further characterized by immunophenotyping. They were all CD4+CD25+CD127lowFoxp3+Helioshigh, and of those, 29 ± 5.5% were 41BB+CD40L−, consistent with an activated Treg phenotype.17 NK cells from 2 patients who had Treg >25% 41BB+CD40L− after thawing exhibited >90% suppression of healthy donor NK cells (supplemental Figure 1, available on the Blood Web site).
Figure 2.
Treg depletion leads to NK-cell persistence and expansion that correlates with remission. (A) Successful in vivo donor NK cell expansion was observed in 80% of patients with Treg depletion (shown as percentage of PB lymphocytes; n = 6) compared with 0% in patients with high levels of Tregs (n = 9). (B) Rates of complete remission in patients with or without detectable donor NK cells in PB at day 7. (C) PB flow cytometry plot of selected subjects who demonstrated in vivo NK expansion at days 7 and 14.
Early donor NK persistence correlates with AML clearance
To determine whether the presence of low levels of donor NK cells enhances clinical efficacy, we compared rates of AML clearance in patients with and without detectable persistence of donor NK cells at day 7 after infusion. Although all patients were leukopenic (<100 WBC cells/μL) at day 7, donor chimerism was measurable by STR. Although only 4 patients met the end point of successful in vivo donor NK-cell expansion (at day 14 after adoptive transfer), 10 of 15 (66%) had donor NK cells detectable 7 days after infusion (4 patients shown in Figure 2C). As NK cells were the predominant population circulating in blood, they accounted for the majority of donor chimerism in these samples. For the 10 patients with detectable donor NK cells (mean donor STR chimerism, 49.5% [range, 13-99%]), 7 (70%) attained CR by day 28 (Table 2; Figure 2B-C). In contrast, only 1 of 5 patients (20%) who lacked detectable donor NK cells (no donor chimerism) at day 7 achieved CR (P = .05; Figure 2B-C).
In vivo expanded donor NK cells are cytotoxic and up-regulate inhibitory receptors
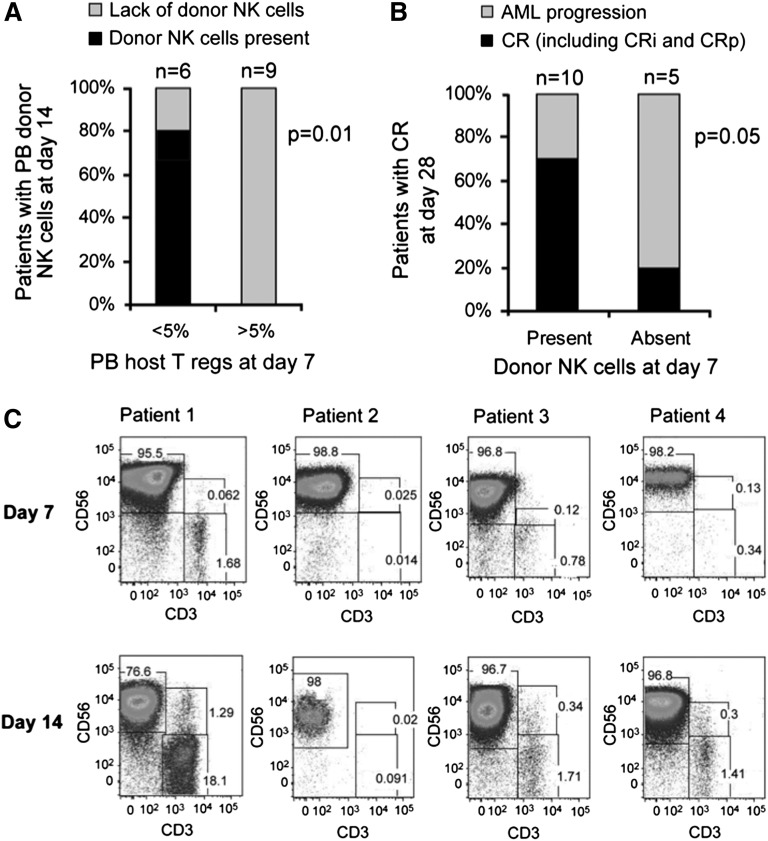
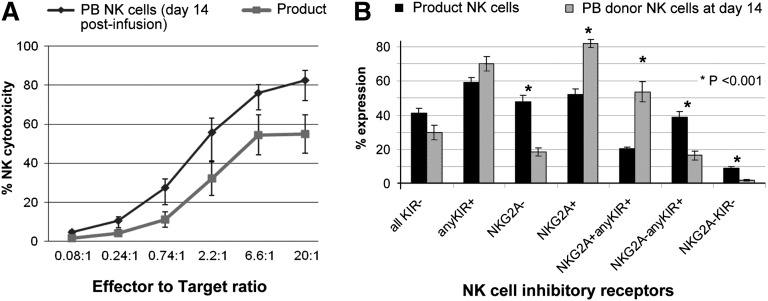
The cytotoxicity of in vivo expanded donor NK cells collected from the PB of patients on day 14 against K562 targets was higher than that of NK cells from the IL-2-activated products (Figure 3A). Although this may be explained in part by the composition of NK cells in the PBMNC product (mean 53% NK cells in the product vs 99% in the PB of the patients), it is evident that the cells had potent activity. A comparison of the phenotype profiles of the 2 demonstrated that in vivo expanded NK cells expressed higher levels of the inhibitory receptor NKG2A (P = .001), with no change in KIR expression (Figure 3B).
Figure 3.
In vivo expanded NK cells are potent killers, express high levels of NKG2A, and correlate with endogenous IL-15 prior to NK cell infusion. (A) Cytotoxicity of in vitro IL-2 activated NK cell products compared with PB NK cells isolated at day 14 after in vivo expansion. Cytotoxicity assay against K526 targets at various effector to target ratios. Cytotoxicity of the NK cell product (in gray; 15 infusion products; mean ± standard error of the mean [SEM]) prior to infusion compared with NK cells isolated from the PB at day 14 of those that expanded (black line; 4 subjects; showed mean ± SEM). (B) Expression of inhibitory receptors on NK cells in product and in vivo expanded NK cells at day 14. (C) Serum IL-15 levels at various time points after NK cell infusion. Comparison of IL2DT cohort patients with donor NK expansion (n = 4) vs no NK cell expansion (n = 11) and all patients not treated with IL2DT (n = 42, mean and standard deviation shown).
Endogenous serum IL15 levels correlate with NK-cell proliferation and expansion
Because lymphodepleting chemotherapy affects levels of homeostatic cytokines, we measured serum concentrations of IL-15 and IL-7 at baseline, after chemotherapy, and at day 14.3 In cohort 3, serum IL-15 and IL-7 levels were low at baseline (mean, 11.8 pg/mL [range, 1.5-44] and 3 pg/mL [range, 0-52], respectively). They increased eightfold after chemotherapy (mean, 93 pg/mL [range, 30-221]; P = .002) and 91 pg/dL [range, 11-151]; P < .001, respectively). In the patients receiving host Treg depletion with IL2DT, serum IL-15 levels at the time of NK-cell infusion (day 0) correlated with successful donor NK-cell expansion (148 pg/mL [range, 74-221] in expanders vs 33 pg/mL [range, 21-46] in nonexpanders, P = .002; supplemental Figure 2). In contrast, cohorts 1 and 2 without Treg depletion both had low IL-15 levels on day 0 (mean, 35 vs 32 pg/dl), and there was no correlation with expansion. By day 7, the IL-15 levels in cohort 3 had declined to levels similar to cohorts 1 and 2 (32 pg/mL [range, 19-61] in all patients) and had returned to close to baseline at day 14, suggesting a transient window for IL-15-driven homeostatic NK-cell expansion after lymphodepleting chemotherapy. This is supported by high NK-cell proliferation rate at day 7 (mean Ki67 expression, 98.2 ± 2%), which fell by day 14 (mean, 46 ± 24%; P = .05).
Tregs suppress in vitro NK-cell proliferation in the presence of IL-2 but not IL-15
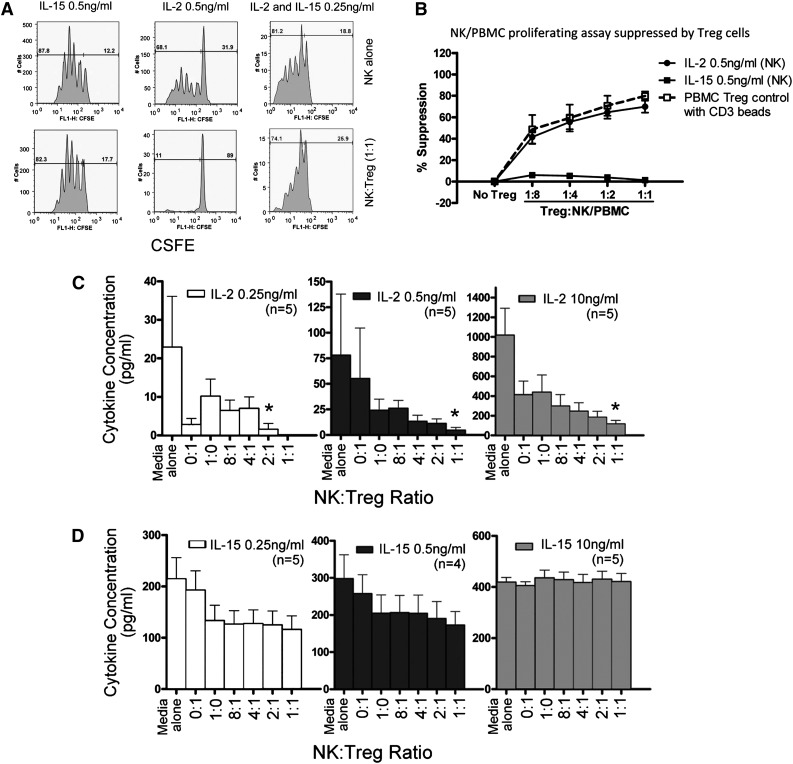
To better understand the influence of IL-15 and IL-2 on Treg and NK-cell proliferation, we analyzed cell and cytokine interactions in vitro. CFSE-labeled NK cells or T-effector cells were purified from healthy donors and incubated with allogeneic UCB-derived Tregs. Tregs were potent inhibitors of CD3 bead-stimulated PB T-effector proliferation, as we have previously demonstrated.14 Resting NK cells in media without cytokines did not proliferate (data not shown), and the addition of IL-2 (0.5 ng/mL) or IL-15 (0.5 ng/mL) or the combination (both, 0.25 ng/mL) was required to stimulate NK-cell proliferation after 5 days of culture (Figure 4A). Without added Tregs, both cytokines induced equivalent NK-cell proliferation at 5 days in a concentration-dependent manner. Proliferation of IL-2- but not IL-15-activated NK cells was potently suppressed by Tregs in a concentration-dependent manner (Figure 4A-B). The proliferation of IL-2-stimulated NK cells (0.5 ng/mL) was inhibited 50% to 85% by Tregs at ratios from 1:8 to 1:1. In contrast, proliferation of IL-15-stimulated NK cells was not inhibited by Tregs (<10%), even at a 1:1 ratio. The addition of Tregs at a 1:1 ratio induced potent suppression of IL-2-stimulated NK proliferation, which was not observed with IL-15 or the combination of IL-2 and IL-15, supporting a model where competition with Tregs that consume and deplete IL-2 is 1 potential mechanism for the observed limited NK-cell proliferation. High concentrations of IL-2 (10 ng/mL) could partially overcome Treg inhibition (data not shown).
Figure 4.
Cytokine-induced NK-cell proliferation is suppressed by allogeneic Treg cells. (A) Healthy donor purified NK cells were CSFE labeled and cultured alone or with UCB-derived Tregs at a ratio of 1:1. Suppression of proliferation of NK cells incubated with IL-2 (0.5 ng/mL), IL-15 (0.5 ng/mL), or a combination of IL-2 + IL-15 (0.25 ng/mL) was measured. Shown is 1 representative donor of 6 experiments. (B) Healthy donor NK cells and UCB-derived Tregs were coincubated with 0.5 ng/mL of IL-2 or IL-15 and cocultured at various Treg:NK cell ratios. Percent suppression of NK proliferation by Tregs was evaluated by CFSE dilution. Treg suppression of CD3 bead-stimulated PBMNC effector T-cell proliferation was measured as a control. Data are an aggregate of 5 separate experiments. NK cells and Tregs were coincubated with IL-2 or IL-15 for 4 days, and (C) IL-2 or (D) IL-15 in the supernatant was measured by enzyme-linked immunosorbent assay. Various cytokine concentrations (0.2, 0.5, and 10 ng/mL) and Treg:NK cell ratios (1:1, 2:1, 4:1, 8:1) were compared. Results from 5 NK cells donors are shown (mean and SEM). *P = .05 compared with NK cells alone.
To determine whether Tregs inhibit NK-cell proliferation by competition for cytokines, we measured Treg utilization of IL-2 or IL-15 by incubating NK cells (1 × 105 NK cells) with or without Treg (1 × 105 Tregs) and 0.25, 0.5, or 10 ng/mL of cytokine for 4 days (Figure 4C). When starting with 0.5 ng/mL, after 4 days of culture, the mean remaining concentrations of IL-2 were 78 pg/mL with no cells, 24 pg/mL with NK cells alone, 55 pg/dL with Treg alone, and 4.5 pg/mL with a 1:1 ratio of NK and Treg cells (P = .05 compared with NK cells alone) . Similar IL-2 consumption by Tregs was observed using low (0.25 ng/mL) or high (10 ng/mL) dose IL-2 in the starting media. In contrast, NK consumed IL-15 (concentrations, 0.25, 0.5, and 10 ng/mL), but the addition of Tregs did not affect residual IL-15 levels (Figure 4D). This suggests that Tregs compete for or consume IL-2, a finding that may be directly relevant to in vivo platforms where the tolerability of pharmacologic IL-2 dosing limits the achievable serum blood concentrations.
Discussion
Adoptive therapy using haploidentical NK cells can induce remissions in patients with relapsed and refractory AML. Successful in vivo expansion of donor NK cells is correlated with attainment of remission, and thus methods to enhance NK-cell expansion are critical to overall clinical efficacy. The strategy tested here, augmenting the lymphodepleting platform with an immunotoxin (IL2DT) to deplete Tregs, led to improvements in rates of in vivo NK-cell expansion and AML remission in 27% and 53% of patients, respectively, compared with cohorts without IL2DT.3,18 Expanded donor NK cells maintained proliferative and cytotoxic effector function in vivo with the setting of sustained Treg depletion and elevated serum levels of IL-15 and IL-7. In humans, IL-15 is secreted by monocytes, macrophages, and dendritic cells in response to PB lymphopenia and signals through common IL-2/IL-15 receptor β (CD122) and γ chains (CD132) expressed on lymphocytes.19 IL2DT may potentiate IL-15 release by more profound blood or tissue lymphodepletion by elimination of activated (postchemotherapy) lymphocytes expressing CD25, although future study is needed to understand these interactions.
Our clinical observations are compatible with preclinical studies from Zhou et al showing that a brief course of IL2DT restored the proliferation of transferred cytotoxic T lymphocytes and reduced the leukemia burden in the liver and spleen of mice, markedly increasing survival.8 Hallet et al analyzed the efficacy of NK cells in a murine adoptive transfer model in which Tregs were depleted with an anti-CD25 antibody.9 NK cell-mediated killing and the survival of leukemia-bearing mice cotreated with IL-2 and anti-CD25 antibody were markedly improved compared with either treatment alone. Although CD8+ T-cell depletion did not change these outcomes, NK-cell depletion completely abrogated all antitumor effects, indicating the essential role of NK cells in mediating these antitumor responses.
Our studies show that lack of in vivo NK-cell expansion correlates with high numbers of host-derived Tregs with an activated phenotype and suppressive function. This suggests that the Treg-mediated suppressive environment may, in part, blunt the efficacy of adoptive NK-cell therapy. Our in vitro data demonstrate that this is mediated in part through Treg competition for IL-2, as has been reported in animal models.20,21 However, we acknowledge that the absence of donor NK cells in patients is likely the consequence of a variety of rejection mechanisms.8,22-25 Direct NK-cell inhibition via Treg membrane-bound transforming growth factor-β has been reported and may also contribute suppression of in vivo NK-cell expansion.26 Targeting some proposed inhibitory mechanisms such as blockade of cytotoxic T-lymphocyte antigen 4, the programmed cell death protein 1 pathway, or inhibitors of indoleamine 2,3-dioxygenase may further unleash suppressed NK-mediated immunity.22-25 Ultimately, the relative influence of these mechanisms may be depend on the specific tumor type and needs further study.
Although a single dose of IL2DT seems insufficient to entirely deplete host Tregs, the decreased IL-35 levels (made by Tregs) 14 days after adoptive transfer suggest a partial effect. Given the profound absolute lymphopenia at the time of IL2DT infusion (day −1) and the fact that sampling is limited to PB, which may miss effects related to Tregs trafficking to and from lymphoid tissues, the immediate depleting effect of IL2TD in tissues is not directly measurable. In some patients, we observed rebound in the CD4+Foxp3+CD127− cell compartment and Treg proliferation (measured by Ki67 expression), which was likely induced by the pharmacologic IL-2 given with the intent of promoting in vivo expansion of donor NK cells. This is consistent with the observed proliferative effect of in vivo low dose IL-2 on Tregs.27
The IL2DT cohort demonstrated that lack of host Tregs is associated with improved in vivo donor NK-cell expansion and remission induction. However, in addition to IL2DT, cohort 3 also received products with higher NK cells doses, which may have contributed to the better clinical results. Higher numbers of infused cells may be needed to overwhelm any residual T cell-mediated rejection of the partially HLA-mismatched NK cells. Although the lymphodepleting chemotherapy was efficient in inducing significant circulating lymphopenia, it is more difficult to assess secondary lymphoid tissues, such as the lymph nodes and spleen, where such rejection might occur. Antitumor effects of lymphodepleting chemotherapy also should be considered, although the Flu/Cy regimen was identical in 3 cohorts with widely different response rates. IL2DT could provide an additional anti-leukemia effect by targeting CD25 expressed on AML blasts and leukemia stem cells.28-30
Several groups are actively investigating methods to expand NK cells ex vivo using genetically engineered antigen-expressing cells with membrane-bound IL-15, IL-21, and 41BB ligand expression to overcome the limitations of in vivo NK-cell expansion.31-33 These NK cell products should be tested clinically against the fresh activated NK cell products described here.
In summary, we demonstrated that adoptively transferred haploidentical adult NK cells that expand in vivo are associated with promising clinical efficacy in patients with refractory or relapsed AML. High NK-cell doses are obtained after depletion of CD3+ and CD19+ cells from a 5-hour donor apheresis collection, and there does not seem to be a negative effect of co-infused monocytes. It is also possible that IL-15 receptor α on monocytes may facilitate trans-presentation of endogenous IL-15 seen early after lymphodepleting chemotherapy enhanced by IL2DT. Our data suggest that the detection of donor NK cells as early as 7 days after infusion may serve as a surrogate biomarker for clinical response, a method that should be further tested and validated in future cellular trials.34 Interrupting pathways that maintain the immunosuppressive environment and supplementing IL-15 should be incorporated to further improve NK-cell expansion rates and to increase clinical benefit.
Acknowledgments
The authors thank the technical and quality assurance staff at Molecular & Cellular Therapeutics, Cytokine Reference Laboratory, University of Minnesota cGMP facility, and our dedicated research staff in Center of Experimental Therapeutics at University of Minnesota, in particular Dixie Lewis, Tim Krepski, Judy Witte, and Jill Aughey for outstanding support and invaluable contributions in conducting the trial. We want to acknowledge the University of Minnesota Masonic Cancer Center Translational Therapy Core Laboratory for excellent assistance.
This work is supported by National Institutes of Health, National Cancer Institute grants P01 CA65493 (J.S.M., S.C., M.R.V., P.B.M., and B.R.B.), P01 CA111412 (J.S.M., S.C., T.E.D., and D.J.W.), and R01 CA72669 (B.R.B.). Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award (UL1TR000114). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Partial funding of correlative assays and agent Denileukin Diftitox were provided by Eisai Inc.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.B., S.C., M.R.V., P.M., D.J.W., B.R.B., and J.S.M. designed and performed the research, analyzed and interpreted the data, and wrote the manuscript; T.E.D. performed statistical analysis; B.Z., J.C., A.P.-M. and K.H. performed correlative studies; D.H.M. was responsible for GMP cell processing and manuscript preparation; and D.L. performed the patient research and was responsible for toxicity assessments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey S. Miller, Blood and Marrow Transplant Program, University of Minnesota, Mayo Mail Code 480, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: mille001@umn.edu.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 3.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 4.Bachanova V, Burns LJ, McKenna DH, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59(11):1739–1744. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geller MA, Cooley S, Judson PL, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13(1):98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasteiger G, Kastenmuller W. Foxp3+ regulatory T-cells and IL-2: the Moirai of T-cell fates? Frontiers Immunol. 2012;3:179. doi:10:3389/fimmu.2012.00179. eCollection 2012. [DOI] [PMC free article] [PubMed]
- 7.Foss FM. DAB(389)IL-2 (denileukin diftitox, ONTAK): a new fusion protein technology. Clin Lymphoma. 2000;1(Suppl 1):S27–S31. [PubMed] [Google Scholar]
- 8.Zhou Q, Bucher C, Munger ME, et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. 2009;114(18):3793–3802. doi: 10.1182/blood-2009-03-208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallett WH, Ames E, Alvarez M, et al. Combination therapy using IL-2 and anti-CD25 results in augmented natural killer cell-mediated antitumor responses. Biol Blood Marrow Transplant. 2008;14(10):1088–1099. doi: 10.1016/j.bbmt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada Y, Aoyama A, Tocco G, et al. Differential effects of denileukin diftitox IL-2 immunotoxin on NK and regulatory T cells in nonhuman primates. J Immunol. 2012;188(12):6063–6070. doi: 10.4049/jimmunol.1200656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Döhner H, Estey EH, Amadori S, et al. European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 12.McKenna DH, Kadidlo DM, Miller JS, Orchard PJ, Wagner JE, McCullough J. The Minnesota Molecular and Cellular Therapeutics Facility: a state-of-the-art biotherapeutics engineering laboratory. Transfus Med Rev. 2005;19(3):217–228. doi: 10.1016/j.tmrv.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80(9):2221–2229. [PubMed] [Google Scholar]
- 14.Hippen KL, Harker-Murray P, Porter SB, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112(7):2847–2857. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan EL. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Schoenbrunn A, Frentsch M, Kohler S, et al. A converse 4-1BB and CD40 ligand expression pattern delineates activated regulatory T cells (Treg) and conventional T cells enabling direct isolation of alloantigen-reactive natural Foxp3+ Treg. J Immunol. 2012;189(12):5985–5994. doi: 10.4049/jimmunol.1201090. [DOI] [PubMed] [Google Scholar]
- 18.Cooley S, Foley B, Verneris MR, et al. Haploidentical natural killer (NK) cells expanding in vivo after adoptive transfer exhibit hyperfunction that partially overcomes self tolerance and leads to clearance of refractory leukemia. Blood. 2011;118(21):355. [abstract] [Google Scholar]
- 19.Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor Rev. 2002;13(6):429–439. doi: 10.1016/s1359-6101(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 20.Gasteiger G, Hemmers S, Firth MA, et al. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med. 2013;210(6):1167–1178. doi: 10.1084/jem.20122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sitrin J, Ring A, Garcia KC, Benoist C, Mathis D. Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL-2. J Exp Med. 2013;210(6):1153–1165. doi: 10.1084/jem.20122248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung DJ, Rossi M, Romano E, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114(3):555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodumudi KN, Weber A, Sarnaik AA, Pilon-Thomas S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol. 2012;189(11):5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, Green WR. Immunotherapy of murine retrovirus-induced acquired immunodeficiency by CD4 T regulatory cell depletion and PD-1 blockade. J Virol. 2011;85(24):13342–13353. doi: 10.1128/JVI.00120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charbonnier LM, Vokaer B, Lemaitre PH, Field KA, Leo O, Le Moine A. CTLA4-Ig restores rejection of MHC class-II mismatched allografts by disabling IL-2-expanded regulatory T cells. Am J Transplantation. 2012;12(9):2313-2321. [DOI] [PubMed]
- 26.Ghiringhelli F, Ménard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202(8):1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuoka K, Koreth J, Kim HT, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5(179):179ra43. doi:10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed]
- 28.Saito W, Kitamura H, Hijikata A, et al. Identification of therapeutic targets on quiscent chemotherapy-resistant human leukemia stem cells. Sci Transl Med. 2010;2(17):17ra9. doi:10.1126/scitranslmed.3000349. [DOI] [PMC free article] [PubMed]
- 29.Gerber JM, Gucwa JL, Esopi D, et al. Genome-wide comparison of the transcriptomes of highly enriched normal and chronic myeloid leukemia stem and progenitor cell populations. Oncotarget. 2013;4(5):715–728. doi: 10.18632/oncotarget.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerny J, Yu H, Ramanathan M, et al. Expression of CD25 independently predicts early treatment failure of acute myeloid leukaemia (AML). Br J Haematol. 2013;160(2):262–266. doi: 10.1111/bjh.12109. [DOI] [PubMed] [Google Scholar]
- 31.Tolar J, Curtsinger J, McElmurry R, et al. Optimal xenogeneic adoptive transfer of human NK cells: Fresh NK cells and IL-15 administration are superior to frozen NK cells and IL-2. Blood. 2012;120(21) [Google Scholar]
- 32.Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE. 2012;7(1):e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lapteva N, Durett AG, Sun J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012;14(9):1131–1143. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooley S, Verneris MR, Curtsinger J, et al. Recombinant human IL-15 promotes in vivo expansion of adoptively transferred NK cells in a first-in-human phase I dose escalation study in patients with AML. Blood. 2012;120:894. [abstract] [Google Scholar]