Abstract
Extracellular adenosine 5’ triphosphate (ATP) is a widespread cell-to-cell signaling molecule in the brain, where it activates cell surface P2X and P2Y receptors. P2X receptors define a protein family unlike other neurotransmitter-gated ion channels in terms of sequence, subunit topology, assembly and architecture. Within milliseconds of binding ATP, they catalyze the opening of a cation-selective pore. However, recent data show that P2X receptors often underlie neuromodulatory responses on slower time scales of seconds or longer. Herein, we review these findings at molecular, cellular and systems levels. We propose that, while P2X receptors are fast ligand-gated cation channels, they are most adept at mediating slow neuromodulatory functions that are more widespread and more physiologically utilized than fast ATP synaptic transmission in the CNS.
Keywords: purinergic, glia, synapse, signaling, neuron, secretion, pain, drug, structure, biophysics, astrocyte, channel
The discovery of ATP signaling
The field of ATP signaling has taken nearly a century to evolve since the discovery of ATP in 1929 (Khakh and Burnstock, 2009). The first evidence for the release of ATP from sensory nerves was provided by Pamela Holton in the 1950s (Holton and Holton, 1954; Holton, 1959). In 1972, Geoffrey Burnstock proposed the existence of “purinergic nerves” (Burnstock, 1972), laying the foundations of a new field. Forty years later, there is little doubt that extracellular ATP signaling is widely utilized in cell sensing.
Much of the early data on purinergic nerves derived from physiological experiments on smooth muscle preparations. By the early 1990s, however, ATP was also shown to depolarize neurons (Jahr and Jessell, 1983; Krishtal et al., 1983), to open single ion channels in patches of membrane (Benham and Tsien, 1987; Kolb and Wakelam, 1983) and to mediate fast synaptic transmission at synapses in the peripheral and central nervous systems (Edwards et al., 1992; Evans et al., 1992; Silinsky et al., 1992). By this time, ATP receptors had also been subdivided as belonging to two major families: metabotropic P2Y receptors and ionotropic P2X receptors. With the subsequent cloning of the genes encoding P2X receptors came a new era. In this review, we focus on P2X receptor mediated ATP signaling in the brain, discussing general themes pertinent to mechanisms and neuromodulation at the molecular, cellular, systems and disease levels.
Cell surface receptors for ATP and its metabolites
ATP activates a family of metabotropic P2Y and ionotropic P2X receptors. Collectively, the actions of ATP and its breakdown products produce responses that last from milliseconds to minutes, and even longer time scales through changes in gene regulation via second messengers. Signaling diversity is further increased by the fact that ATP receptors display a very broad range of ATP sensitivities, ranging from nanomolar in the case of P2Y receptors, to hundreds of micromolar for P2X7 receptors (Surprenant et al., 1996). Thus, ATP receptors respond over remarkably broad spatiotemporal scales, and ATP signaling is very dynamic.
The first P2X receptor genes were identified in 1994 (Brake et al., 1994; Valera et al., 1994) and within two years the whole family had been identified (Buell et al., 1996; Chen et al., 1995; Collo et al., 1996; Le et al., 1997; Lewis et al., 1995; Soto et al., 1996; Surprenant et al., 1996). This was an exciting period that culminated with the realization that P2X receptors defined a unique protein family. Each of the homomeric P2X receptors also displays distinct functional properties (Fig. 1, Table 1). The available data on the subunit composition and properties of heteromeric P2X receptors (Coddou et al., 2011) are not considered in depth here.
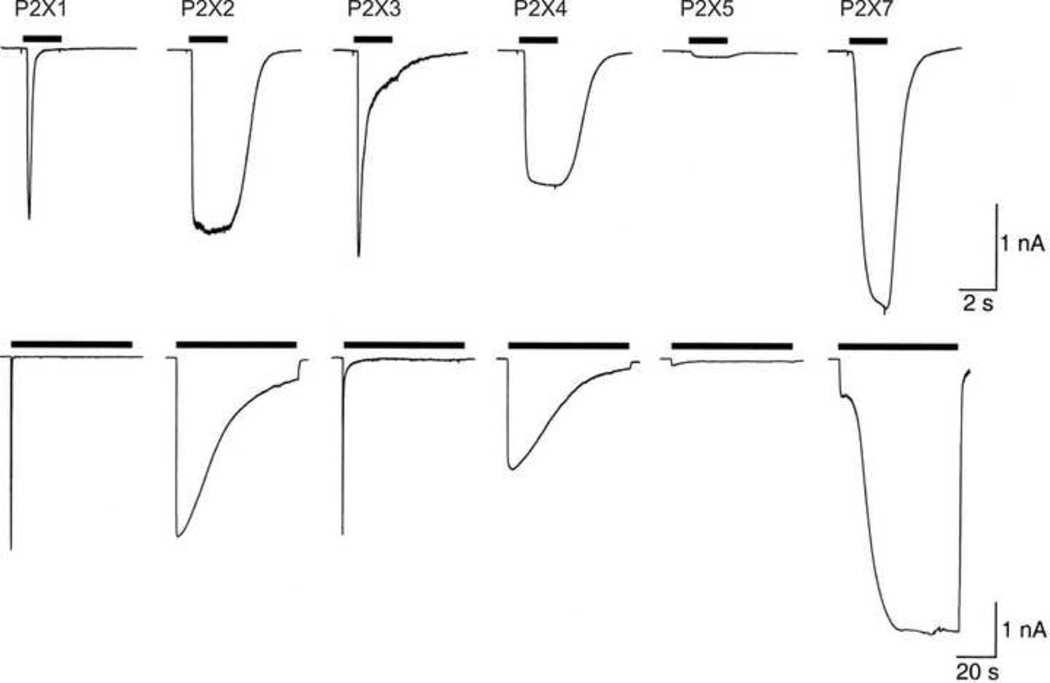
Figure 1. Fast (top) and slow (bottom) desensitization is compared for functional recombinant homomeric rat P2X receptors expressed in HEK-293 cells.
Note the 10-fold difference in time scale between top and bottom panels. Fast desensitization is observed only with P2X1 and P2X3 during brief applications (2 s duration) of ATP (30 µM, except 1 mM for P2X7 owing to its lower sensitivity – see Box 1). Slow desensitization is observed for P2X2 and P2X4 with more prolonged applications (60 s duration) of ATP (30 µM, except 1 mM for P2X7). In all cases except P2X7, the response shown is that seen for the first application of ATP to that cell. For P2X7, one 2 min application of ATP had been made before the application shown. Note that the rising phase of the response recorded for P2X7 receptors is biphasic. This likely reflects pore dilation associated with the second slower phase (discussed in the text; see also Fig 4). The experiments were performed by Dr. A. Surprenant and the figure is reproduced here from North (2002) with the author’s permission.
Table 1.
Key facts and the useful pharmacopeia for exploring P2X receptors.
| P2X1 | P2X2 | P2X3 | P2X4 | P2X5 | P2X6 | P2X7 | |
|---|---|---|---|---|---|---|---|
| Gene name | P2RX1 | P2RX2 | P2RX3 | P2RX4 | P2RX5 | P2RX6 | P2RX7 |
| Desensitization (complete in) | Fast (<1s) | Slow (>20s) | Fast (<1s) | Slow (>20s) | Slow (>20s) | _ | Slow (>20s) |
| Pore “dilation” | No | Yes | No | Yes | _ | _ | Yes |
| PCa/PNa | 4.8 | 2.8 | 1.2 | 4.2 | 1.5 | _ | _ |
| Fractional Ca2+ current (%) | 12.4 | 5.7 | 2.7 | 11 | 4.5 | _ | 4.6 |
| KO made | Yes | Yes | Yes | Yes | _ | _ | Yes |
| Agonist EC50 values (µM) | |||||||
| ATP | 0.07 | 1.2 | 0.5 | 10 | 10 | 12 | 100 |
| 2MeSATP | 0.07 | 1.2 | 0.3 | 10 | 10 | 9 | 100 |
| αβmeATP | 0.3 | > 300 | 0.8 | > 300 | > 300 | > 100 | > 300 |
| BzATP | 0.003 | 0.75 | 0.08 | 7 | > 500 | - | 20 |
| Antagonist IC50 values (µM) | |||||||
| Suramin | 1 | 10 | 3 | >500 | 4 | >100 | 500 |
| PPADS | 1 | 1 | 1 | >500 | 3 | >100 | 50 |
| TNP-ATP | 0.006 | 1 | 0.001 | 15 | - | - | > 30 |
| IP5I | 0.003 | > 300 | 2.8 | Potentiation | - | - | |
| A-317491 | > 10 | > 100 | 0.10 | > 100 | - | > 100 | > 100 |
| RO-3 | > 100 | > 100 | 0.10 | > 100 | > 100 | > 100 | |
| A-740003 | > 100 | > 100 | > 100 | > 100 | - | > 100 | 0.05 |
| AF-353 | > 10 | > 10 | 0.01 | > 10 | > 10 | > 10 | |
| A-438079 | > 100 | > 100 | > 100 | > 100 | - | > 100 | 0.06 |
| A-804598 | > 100 | > 100 | > 100 | > 100 | - | > 100 | 0.01 |
| MRS2179 | 80 | > 100 | > 100 | - | - | > 100 | |
| NF279 | 9 | 30 | 50 | > 100 | - | - | 20 |
| NF449 | 0.7 | > 100 | >100 | > 100 | - | - | > 100 |
| Modulator EC50 values (µM) | |||||||
| Ivermectin | - | > 30 | > 30 | 0.25 | - | - | > 30 |
| Zn2+ | - | Increase EC50 = 7µM | - | Increase 2µM | - | - | - |
| H+ | Decrease pKa 6.3 | Increase pKa 7.3 | Decrease pKa 6.0 | Decrease pKa 6.8 | - | - | Decrease pKa 6.1 |
Notes for table 1: -Indicates that this information is not yet available. The Pf(%) values are for the rat subunits, but the pCa2+/pNa+ values are also shown for human and rat isoforms as appropriate for the available data. In the case of suramin and PPADS IC50 values the data for P2X4 are highly species-dependent.
Abbreviations used in the table: ATP: adenosine 5’-triphosphate, 2meSATP: 2-methythioadenosine 5’-triphosphate, α,βmeATP: alpha beta methylene adenosine 5’-triphosphate, BzATP: 2,3-O-(4-benzoylbenzoyl)-ATP, PPADS: pyridoxal-5’-phosphate-6-azophenyl-2’,4’-disulphonic acid, TNP-ATP: 2’,3’-O-(2,4,6-Trinitrophenyl) adenosine 5’-triphosphate, IP5I: Diinosine pentaphosphate, A-317491: 5-({(3-phenoxybenzyl)[(1S)-1,2,3,4-tetrahydro-1-naphthalenyl]amino}carbonyl)-1,2,4-benzenetricarboxylic acid RO-3: 5-(2-isopropyl-4,5-dimethoxybenzyl)pyrimidine-2,4-diamine, A-740003: N-[1-(N"-cyano-N'-quinolin-5-ylcarbamimidamido)-2,2-dimethylpropyl]-2-(3,4-dimethoxyphenyl)acetamide, AF-353: 5-(5-iodo-2-isopropyl-4-methoxyphenoxy)-pyrimidine-2,4-diamine, A-438079: 3-(5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl)methyl pyridine, A-804598: 2-Cyano- 1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine, MRS2179: 2’-deoxy-N6-methyl adenosine 3’,5’diphosphate, NF279: 8,8’-(carbonylbis(imino-4-1-phenylenecarbonylimino-4,1-phenylenecarbonylimino))bis(naphthalene)-1,3,5-trisulfonic acid, NF449: 4-[({3-[({3,5-bis[(2,4-disulfophenyl)carbamoyl]phenyl}carbamoyl)amino]-5-[(2,4-disulfophenyl)carbamoyl]phenyl}carbonyl)amino]benzene-1,3-disulfonic acid.
P2X receptors are non-selective cation channels with high Ca2+ permeability that carry a depolarizing current under standard physiological conditions. In some cells, P2X channels are also significantly permeable to anions. For example, the full length P2X5 receptor is permeable to Cl− (North, 2002). This remains the exception rather than the rule. Thus at its most fundamental level, P2X receptor mediated neuromodulation starts with chemistry: ATP rapidly gates P2X receptor pores, triggering transmembrane fluxes of selected ions.
The seven mammalian P2X subunits range from 379 (P2X6) to 595 (P2X7) amino acids in length. Each subunit contains two hydrophobic membrane-spanning segments (TM1 and TM2) separated by an ectodomain which contains ten conserved cysteine residues that form disulfide bonds. Representing the simplest known architecture for ligand-gated ion channels, P2X receptors adopt a relatively simple fold, with intracellular N and C termini and most of the molecule forming an extracellular loop. Multiple lines of evidence have established that the channels are trimeric (Barrera et al., 2005; Nicke et al., 1998; Stoop et al., 1999). This contrasts with eukaryotic glutamate-gated cation channels, which form as tetramers, and with the large family of pentameric "Cys-loop" receptors which includes members gated by acetylcholine, glycine, γ-aminobutyric acid, 5-hydroxytryptamine and glutamic acid. TM1 and TM2 both contribute to the function of the P2X pore, but the major pore lining segment is TM2 (Egan et al., 1998; Haines et al., 2001; Jiang et al., 2001; Kracun et al., 2010; Li et al., 2008; Rassendren et al., 1997; Samways et al., 2008).
Molecular Mechanisms
Flukes, fins and beaks
The crystal structure of the zebrafish P2X4 receptor has provided a detailed picture of the atomic anatomy of the receptor (Fig. 3A) (Hattori and Gouaux, 2012; Kawate et al., 2009). In overall shape, a single P2X receptor subunit resembles a dolphin rising from the ocean surface (the cell membrane). The narrow distal part of the dolphin (the fluke) is formed from the two membrane spanning domains as they run through the cell membrane. The body region, with its attached fins and flippers, rises and curves over so that the rostral region corresponding to the head and beak run almost parallel to the membrane surface. The three subunits curl around each other on an axis of symmetry projecting as a perpendicular from the cell membrane, enclosing a central space or cavity.
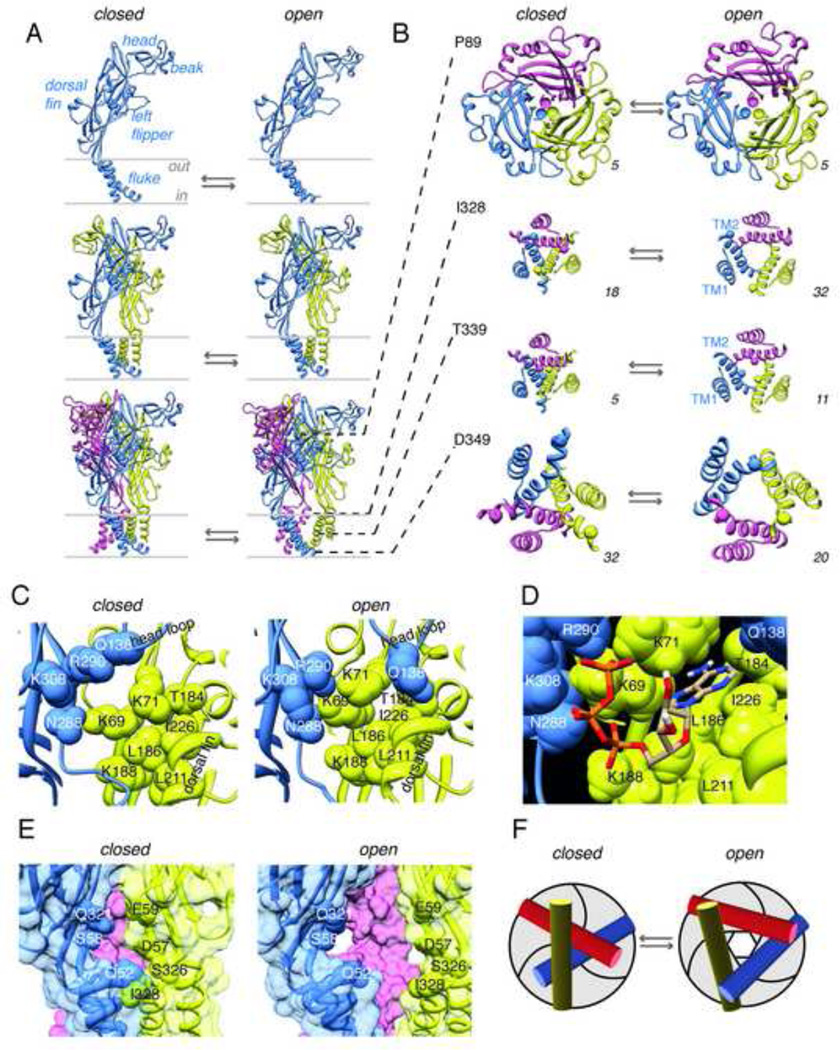
Figure 3. P2X receptor structure, the ATP binding site, and the permeation pathway.
A. Assembly of P2X2 receptors from three subunits, depicted in closed (left) and open (right) states. Upper, middle and lower panels show one, two and three subunits. B. Cross-sections of open and closed rat P2X2 receptors, at the level of the residues indicated, the Cβ atoms of which are shown as spheres. Number in lower right of each panel is the diameter of the circle (in Å), passing through the three Cβ atoms. D349 viewed from below, others viewed from above. C. Key residues in the ATP binding pocket, in open and closed configurations. Two subunits depicted. D. ATP molecule positioned in binding pocket. The U-shape of the triphosphate chain curls around the nitrogen atom of K69. Further explanation in text. E. The lateral portal between two subunits (blue and yellow; pink subunit visible through the portal. Key residues on the edge of the portal are indicated. F. Schematic to illustrate iris-like movement of TM2 domains. Molecular models created in Modeller 9v7 (Sali and Blundell, 1993)using 4DW0 (closed) and 4DW1 (open) as templates, energy minimized using MolProbity (Davis et al., 2007) and displayed in Chimera 1.6 (Pettersen et al., 2004).
The tip of the P2X receptor stands some 70 Å proud of the cell membrane, its turret formed by loops from each subunit surrounding a central aperture that is too narrow for hydrated ions to pass (Fig. 3). This turret is formed by the upper hairpin of a two-stranded beta sheet, the three copies of which form the wall of a slightly widened central cavity as they pass down toward the cell membrane (the upper vestibule).
Extending laterally from the upper body is the head region, a relatively poorly conserved tangle of loops and short β-strands that is stabilized by three disulfide bonds in mammalian receptors. Three prolines cluster at the central axis, corresponding to P89 of the rat P2X2 receptor, which is highly conserved (all numbering refers to rat P2X2). The surrounding region appears to stabilize the upper body as a "brace" with respect to the movements of the lower body that occur during channel opening. Below P89, the central vestibule widens again, its wall formed by three-stranded β-sheets (Fig. 3B). This forms the lower body region, and amino acid side chains projecting into the central vestibule give it a strongly acidic surface. The β-sheets extend down to join directly to the outer ends of the transmembrane domains and, as they do, lateral portals open between them just above the level of the outer membrane surface (Fig. 3A).
Where ATP binds
Facing outwards from the β-sheets that form the wall of the lower body, some 45 Å from the cell surface, one finds the key residues involved in ATP binding. The triphosphate chain of the ATP bends in a U-shape around the ammonium nitrogen atom of K69, and oxygen atoms of the α, β and γ phosphates make several interactions with K69, K70, N288, R290 and K308 (Hattori and Gouaux, 2012) (Fig. 3D). At least one oxygen atom of the γ phosphate is exposed to surrounding solution. The ribose ring of ATP likely makes hydrophobic contact with L211 and I226 (Fig. 3D), leaving its 2' and 3' oxygen atoms exposed to the extracellular solution. The adenine moiety lies deeper in the binding cavity, its side-chain nitrogen hydrogen bonding to backbone carbonyl oxygens of K70 and T184, and its main ring nitrogen atom hydrogen bonding with the side-chain oxygen of T184. These interactions account for the observed selectivity of P2X receptors for ATP, rather than CTP, UTP or GTP (Hattori and Gouaux, 2012).
How ions permeate
The lateral portals between the β-strands of the lower body provide the access route for ions into the central vestibule of the protein (Hattori and Gouaux, 2012; Kawate et al., 2011; Samways et al., 2011). These portals are situated just above the outer membrane surface, ringed by polar side-chains of Q52 and Q321 (A chain), and I328, D57, E59, and K195 (B chain) (Fig. 3E). They are likely partially occluded by lipid at their lower edge. They are sufficiently wide in the closed channel to allow passage of hydrated ions, and they enlarge markedly with channel opening (Fig. 3E). Ions passing through these portals enter the central vestibule, where the excess of negative charges on its inner wall probably serves to concentrate cations. A role in ion selectivity for the portals themselves is indicated by the observation that mutation of some of their negatively charged side-chains changes the relative permeability of calcium (Samways et al., 2012; Samways and Egan, 2007).
The permeation pathway through the cell membrane forms down the central axis of the three tilted TM2 domains. They are widely separated at the outer membrane surface (Fig. 3B), but the extracellular vestibule formed within them narrows as the TM2s approach and cross each other. In the closed structure, the first narrowing occurs at the level of T336, but the narrowest part of the transmembrane pore is formed one helical turn further along TM2 at T339 (Fig. 3A,B). At this point, side-chains occlude the channel when closed, and project into it when open. When T339 is replaced by lysine in one, two or three of the subunits, the unitary conductance is reduced stepwise, and the relative chloride permeability is similarly increased, suggesting a critical interaction with permeating ions (Browne et al., 2011). Thus, the side chains of T339 which occlude the permeation pathway in the closed channel (Fig. 3B) also form the narrowest part of the constriction of the open channel (Hattori and Gouaux, 2012). In the P2X2 receptor, the side chain of the next residue along the helix (S340) also becomes accessible to the permeating ions as the helices separate during channel opening: positively-charged substitutions here markedly increase outward currents (Cao et al., 2009), probably as a result of increasing permeability to chloride ions (Browne et al., 2010).
Passing further into the cell, the splaying TM2 domains enclose a widening intracellular vestibule. A conserved glycine residue situated one helical turn interior to T339 (G342) allows TM2 to kink during channel opening (Fujiwara et al., 2009; Hattori and Gouaux, 2012). The importance of this helix bend is unclear, but it is known that addition of a side chain at this position by mutagenesis does not prevent the response to ATP (Cao et al., 2009; North, 2002). Indeed, in the P2X4 receptor, the identity of such a side-chain profoundly alters kinetic behavior (Khakh et al., 1999a). The side-chain at this position would not protrude towards the permeation pathway of the closed channel, and these effects likely result from stabilizing one of the two open conformations.
The side chain of the V343 is orientated toward the center of the permeation pathway, and this is consistent with the observation that a high affinity metal binding site may be formed here by cysteine substitution (Li et al., 2010), although the cadmium block reversed with washout. Cadmium also has some blocking effect at S345 and D349, but the further splaying of the helices as one passes into the cell makes it unlikely that this results from stable coordination within the central axis of the pore (Kracun et al., 2010). At S345 Cβ radii are 12 Å and 11 Å for open and closed channels and at D349 the radii are 10 Å (open) and 16 Å (closed) (Fig. 3B). The electronegativity at D349 may serve a "cation concentrating" role in the inner vestibule (Cao et al. 2009).
From closed to open
ATP binding initiates several substantial rearrangements (Jiang et al., 2012; Lörinczi et al., 2012; Roberts et al., 2012). The first step may be the disruption of the strong repulsion between the quaternary nitrogen atoms of K308 (on β14 of chain A) and K69 (on β1 of chain B) as the highly negative phosphate tail of ATP is drawn into the binding cleft. These nitrogen atoms are 8 Å apart in the closed structure, and approach to 4 Å in the open structure when they interact with negative oxygen atoms on the phosphate chain. R290 (on β13 of chain A) forms a salt bridge with E167 (projecting down from the head domain of the same subunit) in the closed channel: this is exchanged for a salt bridge with the γ phosphate of ATP (Hausmann et al., 2012) (Fig. 3C). The lateral movement of Q138 allows the head domain to drop down. At the same time, S284 on the left flipper retracts clear of the binding cleft. On the B chain an upward movement of the dorsal fin allows it to make hydrophobic interactions with the ribose moiety and adenine base, principally through L211 and I226 (Fig. 3E). Of interest, the dorsal fin of the P2X7 receptor lacks one helical turn, which includes the first of these two hydrophobic residues: this might contribute to the relative insensitivity to ATP shown by this subtype.
The downward movement of the head domain can be followed by the approach of H120 to H213. Nagaya et al. showed that these residues contributed to an intersubunit zinc binding site (Nagaya et al., 2005) and that, when H120 and H213 are both replaced by cysteine, a new intersubunit disulfide can form which inhibits channel opening. The intimate approximation of the head domain (chain A) with the dorsal fin (chain B) appears to be in itself sufficient to lead to channel opening in a suitably mutated “reporter” receptor (Jiang et al., 2012).
The movements of the left flipper (chain A) and dorsal fin (chain B) exert tension on the β-sheeted wall of the lower body of the same subunit, causing it to flex outwards by a slight separation of its constituent β-sheets and increasing its circumference by a progressively greater amount as it passes down toward the outer surface of the membrane (Roberts et al., 2012). Here, at the outer end of TM2 the "diameter" increases from 18 to 32 Å (Fig. 3C). This widening of the lower body causes the transmembrane helices to separate at their outer ends, and expands the pore like a three-leafed iris (Fig. 3F).
Caveats and limitations
The foregoing interpretation and description gives a compelling account of how a P2X receptor can bind ATP and transform from a closed to an open state. Yet there are caveats and limitations. The first is that the determination of a single open structure does not preclude the existence of other stable forms, and obviously it does not address the movements that occur in the most flexible parts of the protein. Studies using normal mode analysis and molecular dynamic simulations (Du et al., 2012; Jiang et al., 2012) are likely to be most informative in providing insight into conformational dynamics. Additionally, the zebrafish P2X4 receptor used for crystallography lacked intracellular N and C termini, which will likely have an effect on channel properties. Likewise, the membrane proximal regions of these N- and C-terminal domains contain conserved motifs in which minor substitutions can impair receptor function (North, 2002). There is also evidence that these membrane proximal intracellular regions are involved in desensitization (Werner et al., 1996), and the molecular rearrangements underlying desensitization remain unclear. Further, P2X receptors have three ATP binding sites (Bean et al., 1990), and the present structures provide little insight into the molecular basis of the observed cooperativity (Ding and Sachs, 1999; North, 2002). Finally, the stationary snapshots and nanosecond simulations leave us with much to learn about the kinetics of receptor activation, and how these proteins may be better suited to respond to longer-lasting diffusing signals than to the short, sharp pulses of a fast transmitter.
Drugs and allosteric modulators
There has been considerable activity in the drug development world focused on P2X receptors, and this has been well reviewed (Coddou et al., 2011; Ford, 2012). The ATP-bound structure of Hattori and Gouaux (2012) shows clearly how molecules such as TNP-ATP could be accommodated in the ATP binding pocket of the receptor. Wolf and colleagues (2011) have studied NF770, a derivative of suramin, which blocks P2X2 receptors at about 20 nM (Wolf et al., 2011). By homology modelling and docking, they demonstrated a direct hydrogen bond between R290 (critical for ATP binding, see Fig. 3D–F) and the methoxy oxygen atom of NF770, thus accounting for its high affinity as a competitive antagonist.
Ivermectin, strongly potentiates ATP-induced currents at P2X receptors, and this effect is largest for P2X4 receptors (Khakh et al., 1999b). Residues involved in ivermectin binding have been identified on both TMs as lipid-facing (Jelínkova et al., 2008; Silberberg et al., 2007). The ATP-bound structure confirms the outward facing orientation of these residues around the helix crossing point, and substantiates the suggestion by Silberberg et al. (2007) that ivermectin interacts at the protein-lipid interface so as to stabilize an open state.
Divalent and trivalent cations have been used extensively to probe the ectodomain function of P2X receptors (Coddou et al., 2011). There are two salient areas. The first is that all P2X receptors are exquisitely sensitive to the concentrations of normal extracellular ions: all the receptors respond to lower ATP concentrations when the concentrations of extracellular calcium and magnesium are reduced, effects generally more marked for P2X7 receptors (Surprenant et al., 1996). Reduction of extracellular magnesium will increase the fraction of ATP that is in the uncoordinated (ATP4−) state, which is now known to be the active binding form, but this effect should be equal for all receptors. The importance of the divalent ions is particularly seen with P2X7 receptors, where the reduction in calcium and magnesium concentrations strongly promotes "dilatation" and divalent ions appear to serve as "co-agonists" (Jiang et al., 2005; Shinozaki et al., 2009; Surprenant et al., 1996). A similar behavior underlies the astonishing property of P2X3 receptors to "remember" for many minutes a brief exposure to a higher than normal concentration of calcium ions (Cook et al., 1998). This action was ascribed to an acceleration of recovery from a very long-lived desensitized state.
Gadolinium was present in the solution used to grow the first crystals of the zebrafish P2X4 receptor, and gadolinium (100 µM) completely inhibits ATP currents at those receptors (Kawate et al., 2009). X-rays showed it unequivocally to be present in the upper part of the central vestibule, coordinated by E98 from each of the three subunits, but also on the external surface of the body domain, one ion per subunit. In this latter position, the gadolinium is coordinated by carboxylates from D184 and N187. Any general significance of this finding seems unlikely: zfP2X4 D184 is not well conserved among P2X receptors, whereas the Asp at position 187 is conserved but glycosylated (Lenertz et al., 2010; Newbolt et al., 1998; Roberts and Evans, 2006).
Pore dilation
Although all functional P2X receptors undergo conformational changes that result in the opening of a cationic pore within milliseconds of ATP binding, some P2X receptors (notably P2X2, P2X4 and P2X7) also undergo additional slower conformational changes (Fig 4). Pore dilation follows several seconds of ATP activation and is characterized by increases in permeability to organic cations and several dyes (Khakh et al., 1999a; Khakh and Lester, 1999; North, 2002; Virginio et al., 1999). In other P2X receptors, notably P2X1 and P2X3, extended activation by ATP results in channel closure through desensitization. Pore dilation is of interest because it occurs over seconds, endowing P2X receptors with slow signaling capabilities and potentially providing the ability to release intracellular constituents such as ATP itself.
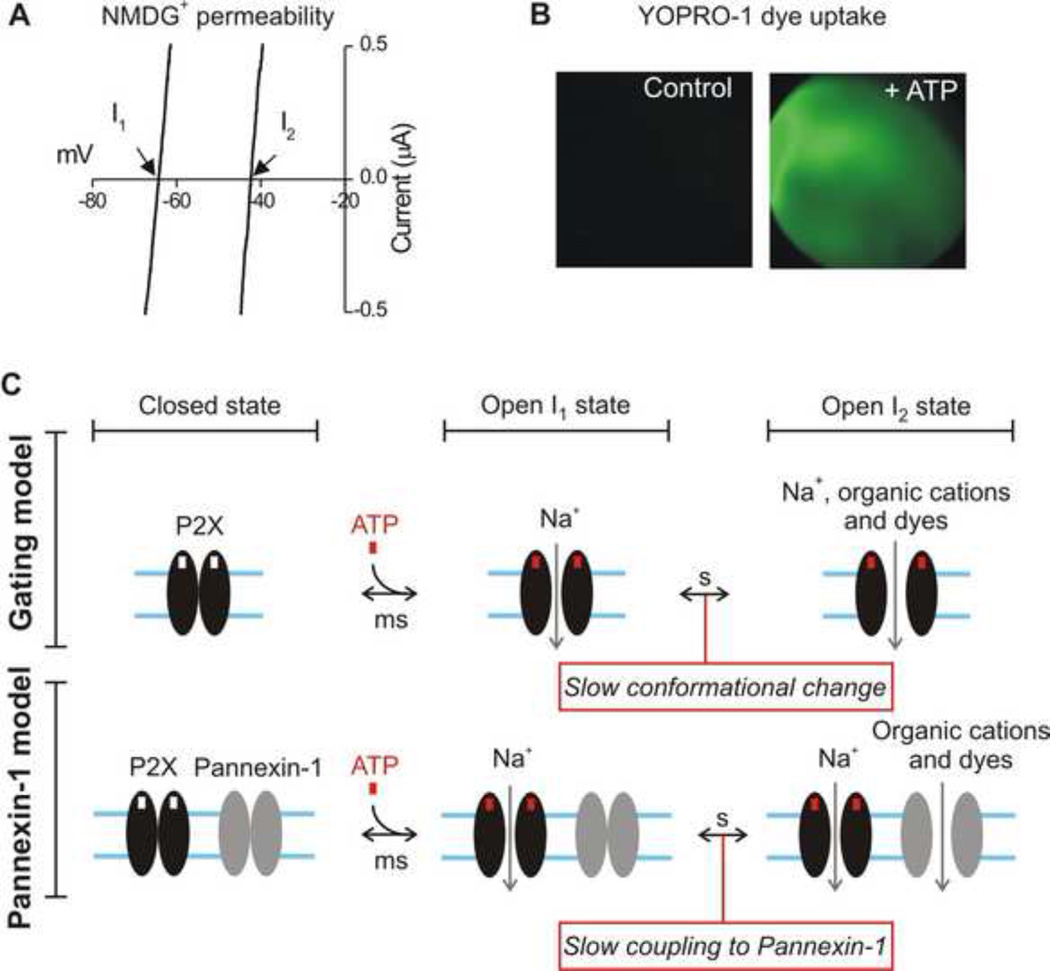
Figure 4. Examples and models of slow conformational changes in P2X receptors displaying pore dilation.
A. Current-voltage plots for example recordings of the I1 and I2 states for P2X2 receptors expressed in Xenopus oocytes are shown. The I1 state has low permeability to the organic cation NMDG+ (hence a negative reversal potential). With time the permeability to NMDG+ increases, indicated as a shift in reversal potential by ~ +16 mV. B. Representative images of a Xenopus oocyte bathed in YOPRO-1 before and during 100 µM ATP (for 30s). The oocyte becomes fluorescent as YOPRO-1 enters the cell, presumably via the I2 state. The data shown in A and B are from Supplementary information accompanying (Chaumont and Khakh, 2008). C. Diagrams illustrating the differences between the Panx-1 and gating models for the I2 state. In the gating model, small cations, organic cations and dyes enter due to slow conformational changes in the P2X pore. In the Panx-1 model, organic cations and dyes enter via an accessory ion channel protein called Panx-1. On balance the field is converging on the gating model, but there is also evidence for the Panx-1 model in the case of P2X7 receptors that are natively expressed (as discussed in the main text). In both models, the cytosolic domains of P2X receptors are important.
Two mechanisms have been proposed for pore dilation (Fig 4). For P2X2, P2X4 and P2X7 receptors, pore dilation appears to involve an intrinsic conformational change in the protein itself (Chaumont and Khakh, 2008; Khadra et al., 2012; Yan et al., 2010; Yan et al., 2011; Yan et al., 2008). However, for natively expressed P2X7 channels, an accessory protein may also be required, and pannexin-1 channels may be involved in receptor pore dilation (Jiang et al., 2005; Pelegrin and Surprenant, 2006; Pelegrin and Surprenant, 2007; Surprenant et al., 1996) in a manner that varies with the particular splice variant being studied (Xu et al., 2012). In all cases the dilated pore state is regulated by cellular processes and mechanisms that involve the C terminal tail. In the case of P2X4 receptors, fast scanning atomic force microscopy has been used to image a slow conformational change that may underlie the phenomenon within single protein molecules (Shinozaki et al., 2009).
Pore dilation may allow P2X receptors to function as intrinsic frequency detectors, by switching to the larger pore state with altered signaling upon repeated ATP activation (Khakh et al., 1999a). Recent data suggest that this particular state of P2X7 receptors may be involved in susceptibility to chronic pain in rodents and humans (Sorge et al., 2012), raising the possibility that pore dilation of other P2X receptors in the brain may also mediate important slow responses. Further structural as well as physiological studies are needed to evaluate precisely how pore dilation and dynamic selectivity filters occur and what their functions are in vivo.
Functional interactions of P2X receptors with other ion channels
P2X and nicotinic receptors undergo functional interactions (Barajas-Lopez et al., 1998; Nakazawa, 1994; Nakazawa et al., 1991; Searl et al., 1998; Searl and Silinsky, 1998; Zhou and Galligan, 1998). Co-expression of P2X2 and α3β4 nicotinic receptors in Xenopus oocytes provided evidence for functional interactions resulting in cross inhibition: the activation of one channel type affected distinct kinetic and conductance states of the other, and co-activation resulted in non-additive responses owing to inhibition of both channel types (Khakh et al., 2000). This study also showed that the functional interactions occurred in synaptically coupled myenteric neurons where nicotinic fast excitatory postsynaptic currents were occluded during activation of endogenously co-expressed P2X channels. Similar experiments have now been repeated with several ion channel combinations showing that cross inhibition between P2X receptors and members of the nicotinic receptor-like family are common (Barajas-Lopez et al., 1998; Barajas-Lopez et al., 2002; Boue-Grabot et al., 2003; Boue-Grabot et al., 2004a; Boue-Grabot et al., 2004b). Most recently, functional interactions have been reported for P2X receptors and acid sensing ion channels (ASIC) (Birdsong et al., 2010) as well as between P2X3 receptors and TRPV1 channels (Stanchev et al., 2009). We comment here on general themes that emerge.
Overall, the data suggest P2X receptors form molecular scale partnerships with distinct ion channels. Fluorescence resonance energy transfer (FRET) experiments show close interactions between P2X2 and α4β2 nicotinic, P2X5 and ASIC, as well as P2X2 and GABAA receptors, which provides a basis for functional interactions within the plasma membrane (Birdsong et al., 2010; Khakh et al., 2005; Shrivastava et al., 2011). Cross-inhibition between P2X receptors and nicotinic channels can occur in the absence of ion flow through P2X2 during a closed-desensitized state, and is likely due to conformational coupling (Khakh et al., 2000). Similarly, the interaction between P2X5 and ASIC channels is independent of ion flow through P2X5 receptors (Birdsong et al., 2010). In the case of spinal neurons, activation of P2X2 receptors increases the lateral mobility of GABAA receptors, adding a previously unknown facet to the interactions between these receptor types (Shrivastava et al., 2011). An important question for future exploration is to determine if cross inhibition has behavioral consequences, either physiologically or during disease states, and it will also be important to nail down the molecular basis for the interactions.
Activation-dependent regulation of P2X receptors
P2X receptors are regulated in a use-dependent manner and it is likely these mechanisms contribute in important ways to their neuromodulatory responses in the brain. To date, two mechanisms have emerged: regulation of trafficking and regulation by Ca2+ sensors.
P2X4 receptors display several types of dynamic trafficking including endocytosis, lysosomal secretion and lateral mobility. A robust observation has been the role of trafficking of P2X4 receptors through dynamin-dependent endocytosis, and P2X4 receptors undergo constitutive and regulated endocytosis mediated by a novel non-canonical endocytic motif (YXXGL) (Bobanovic et al., 2002; Royle et al., 2002; Royle et al., 2005). Additionally, owing to the their extensive glycosylation, P2X4 receptors are resistant to lysosomal degradation and can be secreted from lysosomes to deliver a pool of functional ion channels to the cell surface (Qureshi et al., 2007). Most recently, these insights have been extended to studies of P2X4 receptors within microglia, and it has been shown that both lysosomal secretion and plasma membrane lateral mobility of P2X4 receptors are increased by activation of the microglia (Toulme et al., 2010; Toulme and Khakh, 2012).
A second form of activity-dependent regulation has been demonstrated for P2X2 and P2X7 receptors and is mediated by the Ca2+ sensor proteins VILIP1 and calmodulin, respectively (Chaumont et al., 2008; Richler et al., 2011; Roger et al., 2008). In both cases, Ca2+ fluxes mediated by these P2X receptors result in the recruitment of the cognate Ca2+ sensor to the Cterminal domain of the channel to regulate functional responses. The consequences are subtle for P2X2 receptors, but result in profound facilitation of P2X7 receptor responses (Roger et al., 2008). The interaction with VILIP1, which occurs during endogenous ATP release, requires slow conformational changes resulting in exposure of a VILIP1 binding site in the cytosolic C terminal tail of P2X2 receptors (Chaumont et al., 2008; Chaumont and Khakh, 2008). Singlemolecule experiments reveal that the interaction between P2X2 receptors and VILIP1 regulates plasma membrane lateral mobility of P2X receptors in neuronal dendrites (Richler et al., 2011), perhaps serving to affect recovery from desensitization by controlling the supply of receptors. Determining the full repertoire of proteins that interact with P2X2, P2X4 and P2X7 will help illuminate how these receptors are tuned to perform their tasks in vivo.
Single-molecule imaging experiments now provide accurate and consistent values for P2X2, P2X4 and P2X7 receptor diffusion coefficients in the plasma membrane (0.027, 0.023 and 0.021 µm2/s, respectively) (Arizono et al., 2012; Richler et al., 2011; Toulme and Khakh, 2012). In the case of P2X2 and P2X4 receptors, activation by ATP causes the receptors to diffuse twice as fast in a cell- and subunit specific manner (Richler et al., 2011; Toulme and Khakh, 2012). Accurate P2X receptor diffusion coefficients will be invaluable in modeling receptor movement and plausible roles for lateral mobility in recovery from desensitization during physiological activation such as would occur during point source-like ATP release in vivo.
Cells and Mechanisms
Some general points
P2X receptors are often expressed at low levels, generally in specific compartments such as the edges of spines and within nerve terminals (Le et al., 1998; Rubio and Soto, 2001; Vulchanova et al., 1996), and are activated by quite high amounts of ATP. It seems that sufficient ATP to activate extrasynaptic P2X2 receptors is only released during bursts of action potentials (Richler et al., 2008), suggesting that P2X receptors underlie neuromodulatory responses. Also, we are aware of no example in the brain or spinal cord where endogenous ATP release stimulates postsynaptic P2X receptors to trigger action potential firing (i.e. is a primary fast synaptic transmitter). In this respect, ATP shares features in common with other neuromodulators, such as ACh and serotonin, which also to appear to mediate fast synaptic transmission only rarely in the central nervous system. In these settings, P2X receptor responses may require downstream signaling or protein interactions and not necessarily depolarization of the membrane. Indeed, P2X receptors carry significant Ca2+ fluxes at resting membrane potentials (Egan and Khakh, 2004).
Multiple P2X receptor knockout mice have been generated, all of which survive to adulthood (Chessell et al., 2005; Cockayne et al., 2005; Cockayne et al., 2000; Mulryan et al., 2000; Souslova et al., 2000; Ulmann et al., 2008). Few immediately obvious CNS phenotypes have been reported, yet these same mouse models show that P2X channels are strongly involved in a whole host of pathologies. Thus, it appears that endogenously released ATP does not generally affect the immediate integrative properties of neuronal circuits, but pathological alterations in signaling can have profound effects.
Fast ATP P2X receptor-mediated synaptic transmission in the CNS: small and underwhelming
The first evidence for fast ATP synaptic responses in the brain was provided in the medial habenula (Edwards et al., 1992; Edwards et al., 1997). Since then evidence for ATP as a synaptic transmitter has been provided in the locus coeruleus (Nieber et al., 1997; Silinsky et al., 1992), the hippocampus (Mori et al., 2001; Pankratov et al., 1998; Pankratov et al., 2002), in spinal neurons (Bardoni et al., 1997; Jo and Schlichter, 1999), hypothalamic neurons (Jo et al., 2011; Jo and Role, 2002) and cortex (Lalo et al., 2007; Pankratov et al., 2003; Pankratov et al., 2007). While these studies found evidence for ATP synaptic transmission, in all cases the interpretation that P2X receptors are involved is based on the use of P2X antagonists and agonists that are known to be imperfect in their selectivity (Khakh et al., 2001; North, 2002). Also, none of these studies employed P2X receptor subunit knock-out mice or provided a detailed pharmacological/biophysical characterization of the underlying P2X receptors (Table 1 shows the emerging useful pharmacopeia of P2X receptors). Additionally, ATP-mediated EPSCs detected in this manner tend to be small (about 10% of the size of EPSCs mediated by glutamate), infrequent, only observed in subpopulations of neurons within a given brain nucleus, and they generally require strong electrical stimulation to evoke. There is little evidence that the small EPSCs are physiologically effective in the neurons from which they were detected. Thus, the evidence in favor of ATP as an important synaptic neurotransmitter mediating fast synaptic potentials in the brain remains weak.
The evidence for ATP as a fast synaptic neurotransmitter with important functional roles is much stronger in the periphery, for example at neuro-effector junctions (Mulryan et al., 2000; Sneddon and Burnstock, 1984; Sneddon et al., 1982), neuro-neuronal synapses (Evans et al., 1992) and in the gastrointestinal system (Bian et al., 2003; Galligan and Bertrand, 1994). An important feature of these papers is that key experiments have been repeated in P2X1 and P2X2 subunit knock-out mice, and the underlying receptors have been characterized in detail.
Neuromodulatory influences of postsynaptic P2X receptors: a diversity of mechanisms
Three noteworthy studies suggest that ATP signaling via postsynaptic P2X receptors plays a neuromodulatory role at brain synapses. The first concerns the role of P2X receptors in long-term synaptic potentiation (LTP) of glutamate synapses onto CA1 pyramidal neurons (Pankratov et al., 2002). In this case, Ca2+ flux through P2X receptors dampens NMDA receptor-dependent LTP at low frequencies of action potential firing in Schaffer collateral axons (Pankratov et al., 2002). Consequently, when P2X receptors are blocked, NMDA receptor dependent LTP occurs at lower action potential frequencies. Second, recent studies have utilized P2X4 receptor knock-out mice to analyze synaptic transmission and plasticity in CA1 pyramidal neurons, and no evidence for a role of P2X4 receptors in excitatory synaptic transmission was found (Baxter et al., 2011). Moreover, although the P2X4 deletion mice display subtly reduced LTP (Sim et al., 2006), ATP fast synaptic transmission does not seem to be the cause, and the data suggest that Ca2+ entry through P2X4 receptors may regulate NMDA receptor incorporation into fast synapses (Baxter et al., 2011). A third set of experiments suggest roles for P2X4 receptors in inhibitory synaptic transmission onto a specific population of steroidogenic factor 1 (SF-1) positive neurons in the ventromedial nucleus of the hypothalamus (Jo et al., 2011). Blocking P2X4 receptor endocytosis increases responses evoked by exogenous ATP in SF-1 neurons, but no evidence was found for fast ATP synaptic transmission. However, blocking P2X4 endocytosis reduced inhibitory IPSCs, which appears to be due to increased cross inhibition between P2X4 receptors and synaptic GABAA receptors.
Demonstrating synaptic consequences for the interaction between P2X and other receptor classes is relevant to future efforts to explore the physiological roles of P2X receptors in the brain. From this perspective, the interactions between P2X5 and ASIC channels are particularly noteworthy (Birdsong et al., 2010), as they demonstrate strong functional interplay between the two ion channels in a manner that utilizes a P2X receptor subunit that is only weakly functional as a homomer (Collo et al., 1996). In this case, the interaction is independent of ion flow through the P2X receptor and dependent on a molecular interaction between the cognate subunits reminiscent of interactions between P2X and nicotinic receptors (Khakh et al., 2005). One should consider, therefore, the realistic possibility that seemingly silent P2X receptors in brain neurons may nonetheless be exerting important modulatory influences on other ion channels, particularly in cases such as ischemia when ATP is known to be released in high amounts (Birdsong et al., 2010).
Overall, recent studies that employ knock-out mice or other more specific molecular interventions suggest that P2X receptors modulate fast synaptic responses mediated by other more established fast neurotransmitters. Consistent with this suggestion, electron microscopy shows that P2X2 and P2X4 receptors are generally not located in synapses at sites directly opposite neurotransmitter release from the presynaptic terminal (Fig 5), residing instead at the edges of the postsynaptic density (Rubio and Soto, 2001). Recent single molecule imaging and tracking experiments in cell culture support these findings and show that P2X2 receptors do not enter fast glutamatergic synapses even when heterologously over expressed and strongly activated by ATP (Richler et al., 2011). These data offer an explanation for the rarity of fast ATP neurotransmission in the brain and also raise important cell biological questions about why P2X receptors are excluded from synapses.
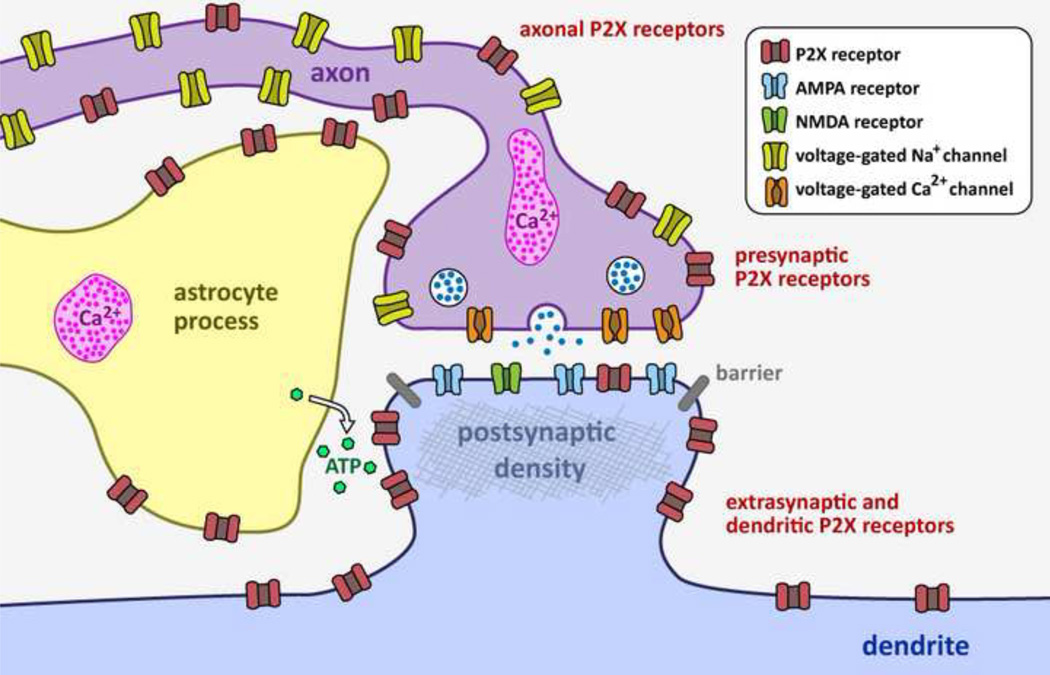
Figure 5. Summary showing locations of P2X receptors in synapses.
Note: this is a cartoon summarizing twenty years of work on various synapses. As such we are not aware of any synapse that expresses P2X receptors in all the locations shown in this cartoon. ATP is released from astrocytes from lysosomes, vesicles and through hemichannels, as discussed in the text. All three of these release mechanisms need to be considered on a case-by-case basis, because it is likely that they operate in different settings and in different brain nuclei. Electron microscopy and single molecule imaging shows that P2X2 and P2X4 receptors do not enter synapses (Masin et al., 2006; Richler et al., 2011; Rubio and Soto, 2001), implying the existence of a restrictive barrier which is shown here as a gray bar at the edge of the postsynaptic density.
Presynaptic P2X receptors and the control of release probability
In general, activation of presynaptic P2X receptors increases neurotransmitter release probability due to influx of calcium (Fig 5), although depression of action potential evoked neurotransmitter release can also occur as a result of action potential failure and/or shunting in axons (Engelman and MacDermott, 2004; Khakh and Henderson, 2000). Presynaptic P2X receptors may be activated by endogenous ATP release in some synapses. Although, presynaptic P2X responses have now been described in many parts of the brain, we lack a satisfactory understanding of the precise physiological function of this form of presynaptic facilitation.
A presynaptic action of ATP forms a key aspect of its overall effect in the hippocampus, within the feed-forward circuit formed between CA3 pyramidal neurons, GABAergic interneurons and output CA1 pyramidal neurons. Progress from several groups is beginning to converge and suggest ways in which ATP signaling may be involved in the integrative actions of this circuit. Presynaptic P2X2 receptors increase glutamate release onto interneurons but not pyramidal neurons (Khakh et al., 2003), whereas postsynaptic P2Y1 receptors depolarize interneurons (Bowser and Khakh, 2004; Kawamura et al., 2004). However, most likely because there are few pre- or post-synaptic ATP receptors that depolarize CA1 pyramidal neurons directly (Baxter et al., 2011; Khakh et al., 2003), the net effect on output neurons is dominated by heightened GABAergic synaptic inhibition. Thus, in the stratum radiatum region of the hippocampus, the cellular effects of ATP are excitatory, but the overall result on the network is dominated by increased inhibition, implying that ATP acts as a ‘physiological brake’ to excitation within this feed-forward circuit (Bowser and Khakh, 2004). Recent studies show that this type of inhibition by ATP is indeed engaged via ATP release from astrocytes through connexin hemichannels, triggered by activity-dependent decreases in extracellular Ca2+ levels within the hippocampal circuit and propagated by slow astrocyte Ca2+ signaling mediated by ATP (Torres et al., 2012).
Multiplicative scaling of synaptic efficacy by P2X receptors in magnocellular neurons of the hypothalamus
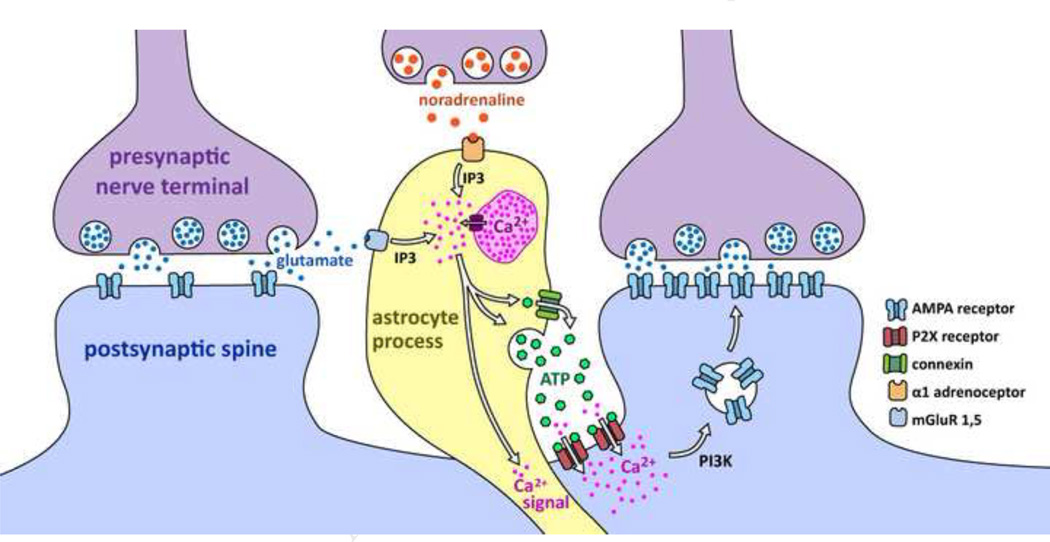
One of the best examples of neuromodulation by ATP acting at P2X receptors is in magnocellular neurons (MCNs) of the hypothalamus (Fig 6). Endogenous ATP acting at extrasynaptic P2X receptors plays a crucial role in modulating excitatory synaptic transmission in MCNs (Gordon et al., 2005; Gordon et al., 2009). Early work suggested that ATP mediates part of the excitatory drive to MCNs following activation of catecholamine containing cells (Day et al., 1993), and electrophysiological studies showed that MCNs expressed functional P2X receptors that mediated membrane potential depolarization and increases in membrane conductance (Hiruma and Bourque, 1995). Later studies found ample evidence for the presence of P2X receptor mRNAs and proteins that mediated ATP-evoked inward currents and strong elevations in intracellular Ca2+ levels in MCNs. It seems MCNs express a mixture of P2X receptors containing P2X2, P2X4 and possibly P2X7 subunits (Vavra et al., 2011).
Figure 6. Cartoon summary of synaptic scaling by ATP acting on P2X receptors in magnocellular neurons (MCNs) of the hypothalamus.
Based on work by the Bains lab (Gordon et al., 2005; Gordon et al., 2009). Glutamate and noradrenaline cause release of intracellular Ca2+ within astrocyte processes, leading to ATP release in to the extracellular milieu. This activates extrasynaptic P2X receptors, which through a Ca2+- and PI3K-dependent mechanism, leads to synaptic scaling by insertion of AMPA receptors into dendritic spines of MCNs. The astrocyte Ca2+ signals may spread within astrocytes leading to similar effects at other synapses. The effects of glutamate and noradrenaline in this context are both reduced when astrocyte processes withdraw from MCNs during lactation and dehydration (see text for details). In this context, the astrocyte process is not actively contributing to synaptic transmission, but rather via ATP it is exerting a strong activation-dependent neuromodulation. Similar responses at other brain synapses with astrocyte processes nearby ought to be explored.
It has been shown that P2X receptor signaling mediates the effect of noradrenaline (NA) on AMPA receptor-mediated mEPSCs arriving onto MCNs of the rat paraventricular nucleus (Gordon et al., 2005). Brief applications of NA increased mEPSC amplitudes for long periods of time, perhaps permanently, by increasing AMPA receptor insertion into synapses (Fig. 6). NA did not act postsynaptically via adrenoceptors in MCNs; instead, NA acted on astrocytes to release ATP. This paper provided strong evidence that ATP acting via P2X receptors resulted in modulation of glutamatergic synaptic efficacy, and astrocytes were directly implicated as the source of endogenous ATP (Gordon et al., 2005). Bains and colleagues have established that the ATP signaling mechanism is engaged during afferent activity, thus demonstrating that the effects of endogenous astrocyte derived ATP are likely to be utilized in vivo during physiological action potential firing impinging on MCNs (Gordon et al., 2009).
The work on MCNs also provides several interesting points that may be relevant more broadly to the study of brain P2X receptor-mediated signaling. First, the authors failed to find fast ATP synaptic transmission. Second, they found that the sources of endogenous ATP were astrocytes rather than neurons, and that bursts of action potentials were needed to engage astrocyte signaling. Third, activation of P2X receptors resulted in multiplicative scaling of all synapses in MCNs. Multiplicative scaling is a form of homeostatic plasticity, whose underpinnings are only beginning to be explored. Perhaps P2X receptors are also involved in other areas of the brain in this form of plasticity? Fourth, the effect of P2X receptor activation on synaptic efficacy in MCNs took many minutes to develop, even though P2X receptor activation occurs in seconds once ATP is bound (Vavra et al., 2011). Fifth, recent data suggest that presynaptic P2X receptors also exist in the supraoptic nucleus, where their activation facilitates glutamate and GABA release (Vavra et al., 2011). The neurosecretory cells of the hypothalamus thus emerge as the best characterized model system to explore the dynamic neuromodulatory influences of pre- and postsynaptic P2X receptors (Fig 6).
Astrocyte P2X receptors and the regulation of cortical UP states by astrocytic ATP
Astrocytes are increasingly recognized as important cellular elements within neuronal circuits not only for providing metabolic and structural support to neurons, but also for their ability to regulate neuronal function through a variety of mechanisms (Attwell et al., 2010; Halassa and Haydon, 2010). Cortical astrocytes express functional P2X7 and P2X1/5 receptors (Lalo et al., 2008; Oliveira et al., 2011) in distinct populations of astrocytes in the somatosensory and prefrontal cortices, respectively, although genome-wide analysis of astrocyte mRNA expression did not reveal any P2X receptor as being particularly enriched within astrocytes (Cahoy et al., 2008). P2X1/5 receptors on cortical astrocytes may be activated by endogenous ATP release from neurons and mediate Ca2+ fluxes (Palygin et al., 2010).
A recent study demonstrated that astrocytes utilize ATP signaling to regulate cortical UP states, which are network driven membrane depolarizations recorded from cortical neurons (Poskanzer and Yuste, 2011). During an UP state, the membrane potential is depolarized for hundreds of milliseconds and individual neurons fire bursts of action potentials. The available data do not allow one to conclude whether the key signals/events are mediated by astrocytic or neuronal P2X receptors; however, given that cortical astrocytes and neurons both express P2X receptors, this study provides strong evidence for how astrocytes function as the source of ATP to regulate network phenomena that occur on a time scale of hundreds of milliseconds. In future studies, it will be interesting to explore the contributions of specific P2X receptors to cortical UP states using knock-out mice and the emerging pharmacology of P2X receptors and thus attempt to correlate altered UP state dynamics with possible behavioral deficits. Finally, one wonders if neuronal P2X receptor signaling scales synaptic efficacy within principal neurons or interneurons of the cortex and regulates the output of the cortical neurons, as seen in MCNs in the hypothalamus (Gordon et al., 2005; Gordon et al., 2009).
Central chemoreception and the control of respiratory drive
An important step in peripheral sensation is activation of P2X and P2Y receptors on primary afferent terminals. Such responses are fundamental to nociception (North, 2004) and in ventilatory responses to hypoxia mediated by the carotid body (Rong et al., 2003). Recent data suggest that ATP serves similar roles in the CNS and contributes to the regulation of respiratory drive. Hypercapnia (an increase in blood CO2; pCO2) increases breathing, and specific areas of the medulla function as central chemoreceptors (Feldman et al., 2003). Gourine and colleagues demonstrated ATP release in micromolar amounts from the ventral surface of the medulla during hypercapnia (Gourine et al., 2005). Using miniaturized ATP biosensors, these authors mapped the sites of ATP release to the retrotrapezoid nucleus (RTN), an area known to contain pH sensitive neurons (Mulkey et al., 2004). To explore roles for endogenous ATP in contributing to respiratory drive during hypercapnia, the authors blocked P2X and P2Y receptors and observed reduced sensitivity and gain of the respiratory response to increasing CO2 levels (Gourine et al., 2005). Exogenous applications of ATP to chemosensitive areas of the medulla mimicked the effects of CO2 on breathing, and a P2Y-preferring agonist produced qualitatively different effects, implying important roles for P2X receptors. Furthermore, studies in brain slices show that ATP-mediated signaling can affect the firing properties of RTN neurons, but that chemosensitivity of these neurons does not derive from ATP (the neurons responded to changes in pH even when P2X and P2Y receptors were blocked (Mulkey et al., 2006)). Taken together, these data suggest that RTN neurons respond directly to pH changes (Mulkey et al., 2006; Mulkey et al., 2004) and that another process releases ATP in response to pH changes to influence the firing properties of RTN neurons (Gourine et al., 2005).
Recent data suggest that the cellular sources of ATP mediating the purinergic component of the central chemosensory response to hypercapnia are astrocytes located within the ventral surface of the medulla (i.e. near the RTN) and that the astrocytes within this area are particularly pH sensitive (Gourine et al., 2010). Hence, “excited” astrocytes propagate a Ca2+ signal among them due to intercellular ATP release acting on P2X and P2Y receptors. Additionally, the authors found that the acid pH-evoked depolarization of RTN neurons was abolished when ATP signaling was blocked, implying that the neuronal response was secondary to ATP release rather than due to intrinsic chemosensitivity of the RTN neurons themselves. Moreover, the authors found that expression and illumination of channelrhodopsin within astrocytes led to light-evoked ATP release and depolarization of RTN neurons via ATP. The use of channelrhodopsin within an in vivo preparation showed that light-evoked astrocyte Ca2+ elevations lead to respiratory activity that was blocked by a mixed P2X1 and P2Y1 receptor antagonist. Taken together, this study suggests that a key step in central chemoreception involves ATP release from astrocytes located on the ventral surface of the medulla, that this signal is further propagated by ATP release acting on P2X and P2Y receptors, ultimately arriving at RTN neurons to depolarize them via ATP receptors that are likely of the P2X1 or P2Y1 class (Gourine et al., 2010).
Subsequent studies have confirmed that astrocytes release ATP in response to elevations in pCO2, but in a manner that is independent of pH changes and by a mechanism involving connexin 26 hemichannels (Huckstepp et al., 2010). Astrocytes in other parts of the brain may also release ATP in response to hypercapnia and so the mechanism may not be specific for the respiratory centers (Dulla et al., 2005). Moreover, recent work has shown that the purinergic component of the respiratory drive is at most 30%, a fraction that relies on hemichannel mediated ATP release, and that the actions of endogenous ATP on RTN neurons were not mediated by P2Y1 receptors, leaving open the intriguing possibility P2X receptors play a critical role (Wenker et al., 2012). Furthermore, in newborn rats, RTN chemoreception depends only on the intrinsic chemosensitivity of neurons (Onimaru et al., 2012). In summary, the available data indicate that a component of central chemoreception is mediated by astrocyte ATP that likely acts on P2X receptors in the RTN, demonstrating how ATP neuromodulation has profound effects on RTN neuron function with clear behavioral outcomes in the form of respiratory responses to hypercapnia.
Systems and Mechanisms
Afferent sensations and signaling in the peripheral nervous system
Afferent nerves that carry information into the central nervous system can be excited at their peripheral ends either by direct mechanical distortion or by transmitters released from specialized sensory cells. Considerable evidence now supports the view that the transmitter released at some such sensory 'first synapses' is ATP, and that it activates P2X receptors on the primary afferent nerve endings.
Taste and chemosensation
In response to gustatory stimulation, Type II taste buds release ATP that acts on P2X2/3 heteromeric receptors to excite primary afferent nerves that run to the CNS in the facial or glossopharyngeal nerves (Finger et al., 2005). Indeed, the release of ATP is itself driven in part by positive feedback through P2X2 receptors on the taste buds themselves (Huang et al., 2011). A similar situation pertains with respect to sensory cells of the carotid body. In this case the glomus cells sense arterial oxygen levels. In response to hypoxia they release ATP, and this acts on P2X receptors to initiate impulses in the carotid sinus nerve (Rong et al., 2003).
Cough
Pulmonary vagal afferents can be directly excited by activation of P2X2/3 receptors (Kwong et al., 2008), and P2X3 receptor antagonists reduce cough in a commonly used guinea pig model (Kamei et al., 2005). ATP inhalation in humans induces cough and dyspnea, likely by direct activation of P2X receptors (Basoglu et al., 2005). There are now available highly selective antagonists for receptors containing P2X3 subunits (Gever et al., 2010) that may prove to have clinical utility.
Urinary bladder and intestine
As the bladder becomes distended, the stretch in its wall can lead to ATP release from urothelial cells (Ferguson et al., 1997). This excites the terminals of afferent fibers expressing heteromeric P2X2/3 receptors (Zhong et al., 2003), and mice with a disrupted P2X3 receptor gene exhibit bladder hyporeflexia (Cockayne et al., 2000). The central ends of these primary afferent fibers in the dorsal horn of the spinal cord also express P2X2/3 receptors, evidenced by the observation that intrathecal administration of a selective P2X2/3 receptor antagonist also strongly inhibits the micturition reflex (Kaan et al., 2010). A similar situation pertains in the intestine, where ATP and αβmeATP directly excite mesenteric afferents running from the wall of the small intestine in the rat (Kirkup et al., 1999). Action potential discharge in pelvic nerve afferent fibers elicited by colon distension are much attenuated in P2X3 knock-out mice (Shinoda et al., 2009; Wynn et al., 2003). These observations all point to a role for ATP acting directly to excite primary afferent nerves in physiological and probably pathological circumstances.
Pain
Interest in P2X receptors and pain dates from early observations that ATP itself evokes pain when applied to blisters (Bleehen et al., 1976). This is likely mediated by activation of P2X3 subunits, which are restricted in their distribution to a subset of primary afferent neurons (Chen et al., 1995; Lewis et al., 1995) that also express receptors for capsaicin (TRPV1) and isolectin B4 (Guo et al., 1999; Vulchanova et al., 1998). The relevance of this observation to any role in chronic inflammatory or neuropathic pain remains less clear, however. P2X3 knockout mice do have an afferent phenotype, most notably reduced mechanical allodynia (Cockayne et al., 2000), and antagonists selective for receptors containing P2X3 subunits (Jarvis et al., 2002) reduce chronic neuropathic and inflammatory pain in the rat. Local administration of the P2X2/3 antagonist A-317491, or prior depletion of P2X3 receptor expression by intrathecal antisense oligonucleotides, also reduce the mechanical hyperalgesia evoked by carageenan (Oliveira et al., 2009) or complete Freund's adjuvant (Ballini et al., 2011). This is consistent with a role for ATP acting on peripherally situated P2X2/3 receptors, as a component of a local "inflammatory soup".
Selective P2X3 receptor antagonists can prevent the mechanical allodynia seen in neuropathic pain models, whether applied locally or intrathecally (Gum et al., 2012; McGaraughty et al., 2003). Locally, this is associated with sensitization (an increased in expression of membrane P2X3 receptors) rather than increased ATP release (Chen et al., 2005).
Cochlea
Several cells types in the cochlea express P2X receptors, and their functional roles have been comprehensively reviewed (Housley et al., 2009). The most compelling is that part played by ATP released from Köllicker’s organ. This organ (also known as the greater epithelial ridge) consists of columnar supporting cells, and it is prominent in the neonatal cochlea before the onset of hearing. At this developmental stage, ATP is released from the supporting cells in bursts and depolarizes the adjacent inner hair cells, causing them to fire bursts of action potentials which, in turn, excites auditory nerves through release of glutamate (Tritsch and Bergles, 2010; Tritsch et al., 2007). The sporadic, spontaneous ATP release begins a few days after birth, and ceases with the onset of hearing. It provides the primary excitatory stimulus to the auditory nerve during this period, and this is responsible for the downstream survival and maturation of auditory neurons.
Retina
An extensive literature pertains to P2X receptor expression in the retina, both in neurons and in supporting cells (Housley et al., 2009). The speculation around their roles in normal function or disease currently lacks insight at the cellular level, but this is clearly an area worth exploring.
Disease and Mechanisms in the CNS
Pain
Recently, it has been proposed that central P2X4 receptors are involved in neuropathic pain (Trang and Salter, 2012). The key evidence here is that removal of P2X4 receptors strikingly prevents the development of mechanical allodynia following peripheral nerve injury (Tsuda et al., 2009; Tsuda et al., 2003; Ulmann et al., 2008). Peripheral nerve injury is followed by activation of spinal microglia. It is suggested that ATP acting on P2X4 receptors drives the release of BDNF from spinal microglia, and that this in turn is critical for the re-wiring that underlies the perception of mild tactile stimuli as noxious. The neuronal subtypes and specific microcircuitry involved remain to be elucidated.
P2X7 receptors can also fashion the behavioral responses to painful stimuli and, in sharp contrast to the situation with P2X4 receptors, there is now a wealth of pharmacological antagonists to be used as experimental tools, some of which are in clinical trials (Gum et al., 2012; Jarvis, 2010). The predominant expression of P2X7 receptors in the nervous system is on microglia, astrocytes and oligodendrocytes. However, some re-interpretation of experiments which used knock-out mice may be required. The mice produced by Pfizer (Masin et al., 2012) continue to express two shortened, alternatively spliced, forms of the P2X7 receptor (P2X7 13B and P2X7 13C). The mice produced by Glaxo have a functional P2X7 splice variant (P2X7(k)) that continues to be expressed in these mice: the corresponding protein is widely expressed, but it has a different N-terminus and TM1 (Nicke et al., 2009). It forms receptors which are more sensitive to ATP and which undergo a more rapid increase in permeability to organic cations (a measure of pore dilation).
The development of mechanical hypersensitivity in models of neuropathic pain is absent in the Glaxo P2X7 deletion mouse (Chessell et al., 2005), and a similar phenoptye is observed in mice lacking both isoforms of IL-1β (Honore et al., 2006b). Mechanical hypersensitivity is also prevented by intrathecal P2X7 antagonist A-438079 (Kobayashi et al., 2011) and Brilliant Blue G (He et al., 2012) or systemic administration of P2X7 receptor antagonists (Honore et al., 2006a). These effects in pain models appear to require release of IL-1β, given that A-438079 blocks not only the ATP evoked release of IL-1β but also the release evoked by LPS (Clark et al., 2010). Indeed, LPS-induced release of IL-1β is absent in lumbar spinal cord slices from P2X7 deletion mice. These results imply that the rewiring of microcircuitry in the spinal cord that occurs during prolonged inflammation or nerve injury involves ATP release, the activation of P2X7 receptors on spinal microglia, and the release of IL-1β. Our understanding of the source of ATP has advanced little since Pamela Holton showed that sensory nerves release ATP when stimulated electrically (Holton, 1959). As for the target action of the released IL-1β, one suggestion is that it leads to increased phosphorylation of the NR1 and NR2B subunits of the NMDA receptor and perhaps an LTP-like phenomenon (Zhang et al., 2008), which has been associated with behavioral hyperalgesia (Brenner et al., 2004).
Some independent support for considering P2X7 receptors as potential targets in pain therapies is also provided by genetic associations. Six of seven inbred mouse strains that showed less than average allodynia also expressed P2X7 receptors with a single nucleotide polymorphism resulting in the P451L mutation (Sorge et al., 2012). Mouse P2X7[P451L] receptors have impaired function as measured by ATP-evoked uptake of YO-PRO-1 (a commonly used optical measure of pore dilation) but not when measured as ATP-induced ionic current (Adriouch et al., 2002; Young et al., 2006). This polymorphism, as well as two others known to result in impaired P2X7 receptor function (H155Y, R270H) were found more often in subjects reporting a higher than average pain level following mastectomy, or in osteoarthirits (Sorge et al., 2012).
Demyelinating disease
Inflammation is a component of the degeneration that occurs in several nervous system disorders, and the involvement of P2X receptors has been investigated in certain animal models of disease. In mice, experimental autoimmune encephalitis (EAE) can be induced by immunization with myelin oligodendrocyte glycoprotein. Studies on P2X7 deletion mice appear to be contradictory. In the Pfizer mice, the clinical and pathological features of EAE are more marked in “knock-outs”, whereas in the Glaxo mice the deficiency of the P2X7 receptor suppressed the development of EAE (Sharp et al., 2008).
Spinal cord injury
Considerable interest was created by the report that blocking P2X7 receptors improves functional recovery after spinal cord injury in rats (Wang et al., 2004). The receptor antagonists used (oxoATP and PPADS) are non-selective, and their actions cannot be reliably attributed to P2X7 receptor blockade. The results were later extended by using Brilliant Blue G (Cotrina and Nedergaard, 2009; Peng et al., 2009), which is a more selective blocker of mouse and rat P2X7 receptors (Jiang et al., 2000). However, a recent study failed to replicate these results (Marcillo et al., 2012). Additional systematic approaches are clearly required, using the highly selective P2X7 receptor antagonists that are now available. A role for P2X4 receptors has also been suggested. In mice with deleted P2X4 receptors, there was a decrease in caspase-1 cleavage and IL-1β production at the injury site, which is correlated both with diminished neutrophil and macrophage infiltration at the injury site, and improved functional outcome (de Rivero Vaccari et al., 2012).
Stroke
Large amounts of ATP are released from damaged cells as a result of ischemia, which may activate P2X7 receptors. This provides the logic for considering blockade of P2X7 receptors as a possible therapeutic regimen. Pretreatment with PPADS improves recovery from experimental stroke in rats with permanent middle cerebral artery occlusion (Lämmer et al., 2011). Treatment with brilliant Blue G beginning 30 minutes after middle cerebral artery occlusion caused a 60% reduction in brain damage measured three days later (Arbeloa et al., 2012). These studies need to be extended to determine if P2X7 receptors are valid targets in stroke and whether the relevant receptors are located on astrocytes or neurons.
Summary and future opportunities
In this review, we have highlighted some of the recent literature that sheds new light on how P2X receptors work and how they mediate neuromodulation in diverse systems. Representing a novel structural class of ion channels with several unique functional properties, and mediating fascinating slow responses, the physiology of ATP P2X receptors has challenged our precepts of how a fast neurotransmitter-gated cation channel should look and behave. New biophysics and biology has been discovered, and many early biophysical and physiological insights have been supported with high resolution crystal structures, optical approaches and molecular genetics.
Based on the aforementioned latest breakthroughs, we propose that P2X receptors have evolved to fulfill unique biological functions and occupy signaling niches that are not readily met by other fast neurotransmitter systems. Given that glia constitute about half the cells in the brain, express multiple ATP receptors and release ATP through a variety of mechanisms, we suggest that a major facet of ATP and P2X receptor biology is related to glia and their slow neuromodulatory functions in the nervous system. Viewed from this neuromodulatory capacity and largely unexplored potential, ATP acting via P2X receptors is a physiologically important signal, particularly for linking slow glial communications with fast neural microcircuit computations.
For continued progress, it will be vital that we explore P2X receptor functions with the best available tools. Luckily, many P2X receptor knockout mice are now available and selective antagonists are being discovered (Table 1). With the publication of the P2X crystal structures, several classic biophysical questions have been answered and there can be little doubt the field has moved into an exciting new era. We now await the structure of a full length P2X receptor with its cytosolic domains, which will allow us to relate the findings to the wealth of studies on receptor function and gating. One would also like to know if the ATP bound state represents the only open state or an intermediate conformation of the permeation pathway. It will be important to continue to use simple systems to explore ion channel biophysics, but more nuanced cell biological issues such as trafficking, protein-protein interactions and receptor regulation should be performed in intact systems such as native cell lines, primary cultures and in vivo to render the findings meaningful in a physiological manner. Additionally, the generation of a battery of P2X reporter mice will allow for the study of live tissue specimens, thus ending our reliance on staining with antibodies of limited selectivity and on dead tissue. Use of such reporter mice is perhaps the only immediately obvious way to address the issue of heterogeneous responses, because it would allow researchers to record from genetically specified cell populations. It also seems necessary to generate mouse lines expressing tamoxifen inducible Cre recombinase under the P2X4 and P2X7 receptor promoters because of their roles in pain, allowing mechanistic experiments in vivo to commence, thus ending correlative interpretations and aiding exploration of causation in the spinal pain microcircuitry. Our improved knowledge of ATP binding and gating in P2X receptors also presents itself as an opportunity to design/reengineer P2X receptors to perform specific tasks, such as chaperoning other proteins to the edges of dendritic spines or to nerve terminals in the treatment of synaptic diseases, or to provide known and desired fluxes in specific domains of neurons that need them. It seems necessary to evaluate if cross inhibition between P2X receptors and other ion channels has behavioral consequences, either physiologically or during disease states, and thus explore this neuromodulatory regulation. Similarly, we need to determine the proteome of key P2X receptors to understand both physiology and biophysics of these proteins: P2X4 and P2X7 receptors seem to be a good place to start given their roles in pain.
The field of P2X receptor signaling holds great promise to treat human diseases, which is an important remit and mandate of publicly funded biological research. Recent exciting breakthroughs offer ample chances for national research agencies to invest in an area with largely untapped clinical potential and thus deliver on their promise to advance human therapeutics. From this perspective, the entire field eagerly awaits the results of ongoing clinical trials on the first generation of drugs targeting P2X receptors to treat pain states, bladder dysfunction and cough (Ford, 2012; Gum et al., 2012). There is huge unexplored potential to exploit ATP signaling for treating CNS disease. We need to explore these possibilities from a grass roots level and up.
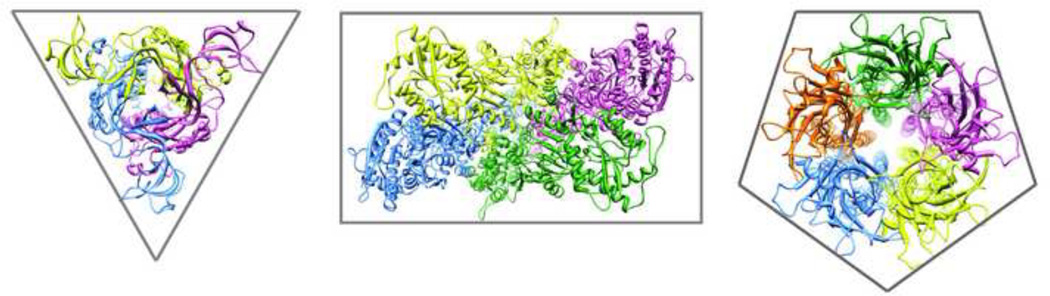
Figure 2. P2X receptors define a unique family of ligand-gated ion channels.
Trimeric, tetrameric and pentameric ligand-gated ion channels, viewed from the outer surface. Left, P2X receptor family. ATP-gated P2X4 cation channel from zebrafish (Danio rerio) in an open state. Modified from 4DW1. Middle, AMPA receptor family. Glutamate-gated GluA2 cation channel from rat, in closed state (Rattus norvegicus). Modified from 3KG2. Right, Nicotinic acetylcholine receptor family. Glutamate-gated chloride channel from the nematode worm (Caenorrhabditis elegans) in open state. Modified from 3RHW.
Acknowledgements
We regret that space limitations prevented us from citing many important original papers and of course we could not cover the entire field. The authors thank Drs. JS Bains and DK Mulkey for discussion and comments. We thank past and current laboratory members for energizing and critical discussions. The Khakh lab is supported by the CHDI Foundation and the NIH NINDS (NS060677, NS063186, NS073980). The North lab is supported by the Wellcome Trust (093140) and the Medical Research Council. Thanks to Dr. Liam Browne for help with molecular modeling, and drawing Figure 3F, and to Janet Iwasa (http://www.onemicron.com/) for drawing Figures 5 and 6.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- Arbeloa J, Pérez-Samartín A, Gottlieb M, Matute C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis. 2012;45:954–961. doi: 10.1016/j.nbd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Arizono M, Bannai H, Nakamura K, Niwa F, Enomoto M, Matsu-Ura T, Miyamoto A, Sherwood MW, Nakamura T, Mikoshiba K. Receptor-selective diffusion barrier enhances sensitivity of astrocytic processes to metabotropic glutamate receptor stimulation. Sci Signal. 2012 Apr 3;5(218):ra27. doi: 10.1126/scisignal.2002498. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballini E, Virginio C, Medhurst SJ, Summerfield SG, Aldegheri L, Buson A, Carignani C, Chen YH, Giacometti A, Lago I, et al. Characterization of three diaminopyrimidines as potent and selective antagonists of P2X3 and P2X2/3 receptors with in vivo efficacy in a pain model. Br J Pharmacol. 2011;163:1315–1325. doi: 10.1111/j.1476-5381.2011.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-Lopez C, Espinosa-Luna R, Zhu Y. Functional interactions between nicotinic and P2X channels in short-term cultures of guinea-pig submucosal neurons. J Physiol (Lond) 1998;513:671–683. doi: 10.1111/j.1469-7793.1998.671ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-Lopez C, Montano LM, Espinosa-Luna R. Inhibitory interactions between 5-HT3 and P2X channels in submucosal neurons. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1238–G1248. doi: 10.1152/ajpgi.00054.2002. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J Neurosci. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera NP, Ormond SJ, Henderson RM, Murrell-Lagnado RD, Edwardson JM. Atomic Force Microscopy Imaging Demonstrates that P2X2 Receptors Are Trimers but That P2X6 Receptor Subunits Do Not Oligomerize. J Biol Chem. 2005;280:10759–10765. doi: 10.1074/jbc.M412265200. [DOI] [PubMed] [Google Scholar]
- Basoglu OK, Pelleg A, Essilfie-Quaye S, Brindicci C, Barnes PJ, Kharitonov SA. Effects of aerosolized adenosine 5'-triphosphate vs adenosine 5'-monophosphate on dyspnea and airway caliber in healthy nonsmokers and patients with asthma. Chest. 2005;128:1905–1909. doi: 10.1378/chest.128.4.1905. [DOI] [PubMed] [Google Scholar]
- Baxter AW, Choi SJ, Sim JA, North RA. Role of P2X4 receptors in synaptic strengthening in mouse CA1 hippocampal neurons. Eur J Neurosci. 2011;34:213–230. doi: 10.1111/j.1460-9568.2011.07763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP, Williams CA, Ceelen PW. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990;10:11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bian X, Ren J, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan J. Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol (Lond) 2003;551:309–322. doi: 10.1113/jphysiol.2003.044172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, et al. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleehen T, Hobbiger F, Keele CA. Identification of algogenic substances in human erythrocytes. J Physiol. 1976;262:131–149. doi: 10.1113/jphysiol.1976.sp011589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobanovic LK, Royle SJ, Murrell-Lagnado RD. P2X receptor trafficking in neurons is subunit specific. J Neurosci. 2002;22:4814–4824. doi: 10.1523/JNEUROSCI.22-12-04814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boue-Grabot E, Barajas-Lopez C, Chakfe Y, Blais D, Belanger D, Emerit MB, Seguela P. Intracellular cross talk and physical interaction between two classes of neurotransmitter-gated channels. J Neurosci. 2003;23:1246–1253. doi: 10.1523/JNEUROSCI.23-04-01246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boue-Grabot E, Emerit MB, Toulme E, Seguela P, Garret M. Cross-talk and co-trafficking between rho1/GABA receptors and ATP-gated channels. J Biol Chem. 2004a;279:6967–6975. doi: 10.1074/jbc.M307772200. [DOI] [PubMed] [Google Scholar]
- Boue-Grabot E, Toulme E, Emerit MB, Garret M. Subunit specific coupling between GABA type A and P2X2 receptor channels. J Biol Chem. 2004b;279:52517–52525. doi: 10.1074/jbc.M410223200. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. ATP excites interneurons and astrocytes to increase synaptic inhibtion in neuronal networks. J Neurosci. 2004;24:8606–8620. doi: 10.1523/JNEUROSCI.2660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- Browne LE, Cao L, Broomhead HE, Bragg L, Wilkinson WJ, North RA. P2X receptor channels show threefold symmetry in ionic charge selectivity and unitary conductance. Nat Neurosci. 2011;14:17–18. doi: 10.1038/nn.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne LE, Jiang LH, North RA. New structure enlivens interest in P2X receptors. Trends Pharmacol Sci. 2010;31:229–237. doi: 10.1016/j.tips.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. Embo J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Broomhead HE, Young MT, North RA. Polar residues in the second transmembrane domain of the rat P2X2 receptor that affect spontaneous gating, unitary conductance, and rectification. J Neurosci. 2009;29:14257–14264. doi: 10.1523/JNEUROSCI.4403-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont S, Compan V, Toulme E, Richler E, Housley GD, Rassendren F, Khakh BS. Regulation of P2X2 receptors by the neuronal calcium sensor VILIP1. Sci Signal. 2008;1(41) doi: 10.1126/scisignal.1162329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont S, Khakh BS. Patch-clamp coordinated spectroscopy shows P2X2 receptor permeability dynamics require cytosolic domain rearrangements, but not Panx-1 channels. Proc Natl Acad Sci U S A. 2008;105:12063–12068. doi: 10.1073/pnas.0803008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li GW, Wang C, Gu Y, Huang LY. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain. 2005;119:38–48. doi: 10.1016/j.pain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Chessell I, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci. 2010;30:573–582. doi: 10.1523/JNEUROSCI.3295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, et al. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, Rodland KD, McCleskey EW. A memory for extracellular Ca2+ by speeding recovery of P2X receptors from desensitization. J Neurosci. 1998;18:9238–9244. doi: 10.1523/JNEUROSCI.18-22-09238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signal. 2009;5:223–232. doi: 10.1007/s11302-009-9138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WBr, Snoeyink J, Richardson JS, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007 Jul;35(Web Server issue):W375–W383. doi: 10.1093/nar/gkm216. Epub 2007 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA, Sibbald JR, Khanna S. ATP mediates an excitatory noradrenergic neuron input to supraoptic vasopressin cells. Brain Res. 1993;607:341–344. doi: 10.1016/0006-8993(93)91528-z. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, Keane RW, Lacroix S. P2X4 receptors influence inflammasome activation after spinal cord injury. J Neurosci. 2012;32:3058–3066. doi: 10.1523/JNEUROSCI.4930-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Sachs F. Single channel properties of P2X2 purinoceptors. J Gen Physiol. 1999;113:695–720. doi: 10.1085/jgp.113.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Dong H, Zhou HX. Gating mechanism of a P2X4 receptor developed from normal mode analysis and molecular dynamics simulations. Proc Natl Acad Sci U S A. 2012;13:4140–4145. doi: 10.1073/pnas.1119546109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;48:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Robertson SJ, Gibb AJ. Properties of ATP receptor-mediated synaptic transmission in the rat medial habenula. Neuropharmacology. 1997;36:1253–1268. doi: 10.1016/s0028-3908(97)00127-5. [DOI] [PubMed] [Google Scholar]
- Egan TM, Haines WR, Voigt MM. A domain contributing to the ion channel of ATP-gated P2X2 receptors identified by the substituted cysteine accessibility method. J Neurosci. 1998;18:2350–2359. doi: 10.1523/JNEUROSCI.18-07-02350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TM, Khakh BS. Contribution of calcium ions to P2X channel responses. J Neurosci. 2004;24:3413–3420. doi: 10.1523/JNEUROSCI.5429-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes--a possible sensory mechanism? J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012;8(Suppl 1):3–26. doi: 10.1007/s11302-011-9271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Keceli B, Nakajo K, Kubo Y. Voltage- and [ATP]-dependent gating of the P2X(2) ATP receptor channel. J Gen Physiol. 2009;133:93–109. doi: 10.1085/jgp.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. J Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gever JR, Soto R, Henningsen RA, Martin RS, Hackos DH, Panicker S, Rubas W, Oglesby IB, Dillon MP, Milla ME, et al. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160:1387–1398. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64:391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gum RJ, Wakefield B, Jarvis MF. P2X receptor antagonists for pain management: examination of binding and physicochemical properties. Purinergic Signal. 2012 Feb;8(Suppl 1):41–56. doi: 10.1007/s11302-011-9272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Haines WR, Voigt MM, Migita K, Torres GE, Egan TM. On the contribution of the first transmembrane domain to whole-cell current through an ATP-gated ionotropic P2X receptor. J Neurosci. 2001;21:5885–5892. doi: 10.1523/JNEUROSCI.21-16-05885.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R, Gunther J, Kless A, Kuhlmann D, Kassack MU, Bahrenberg G, Markwardt F, Schmalzing G. Salt bridge switching from Arg290/Glu167 to Arg290/ATP promotes the closed-to-open transition of the P2X2 receptor. Mol Pharm. 2012 doi: 10.1124/mol.112.081489. in press. [DOI] [PubMed] [Google Scholar]
- He WJ, Cui J, Du L, Zhao YD, Burnstock G, Zhou HD, Ruan HZ. Spinal P2X(7) receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model. Behav Brain Res. 2012;226:163–170. doi: 10.1016/j.bbr.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Hiruma H, Bourque CW. P2 purinoceptor-mediated depolarization of rat supraoptic neurosecretory cells in vitro. J Physiol. 1995;489:805–811. doi: 10.1113/jphysiol.1995.sp021093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton FA, Holton P. The capillary dilator substances in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. J Physiol. 1954;126:124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J Physiol (Lond) 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006a;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- Honore P, Wade CL, Zhong C, Harris RR, Wu C, Ghayur T, Iwakura Y, Decker MW, Faltynek C, Sullivan J, et al. Interleukin-1alphabeta gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not postoperative pain. Behav Brain Res. 2006b;167:355–364. doi: 10.1016/j.bbr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci. 2009;32:128–141. doi: 10.1016/j.tins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Huang YA, Stone LM, Pereira E, Yang R, Kinnamon JC, Dvoryanchikov G, Chaudhari N, Finger TE, Kinnamon SC, Roper SD. Knocking out P2X receptors reduces transmitter secretion in taste buds. J Neurosci. 2011;31:13654–13661. doi: 10.1523/JNEUROSCI.3356-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Jessell TM. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983;304:730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- Jarvis MF. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33:48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelínkova I, Vávra V, Jindrichova M, Obsil T, Zemkova HW, Zemkova H, Stojilkovic SS. Identification of P2X(4) receptor transmembrane residues contributing to channel gating and interaction with ivermectin. Pflugers Arch. 2008;456:939–950. doi: 10.1007/s00424-008-0450-4. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Mackenzie A, Zhang YH, Surprenant A, North RA. N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X(7) receptors. Am J Physiol Cell Physiol. 2005;289:C1295–C1302. doi: 10.1152/ajpcell.00253.2005. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Spelta V, Surprenant A, North RA. Amino acid residues involved in gating identified in the first membrane-spanning domain of the rat P2X(2) receptor. J Biol Chem. 2001;276:14902–14908. doi: 10.1074/jbc.M011327200. [DOI] [PubMed] [Google Scholar]
- Jiang R, Taly A, Lemoine D, Martz A, Cunrath O, Grutter T. Tightening of the ATP-binding sites induces the opening of P2X receptor channels. EMBO J. 2012;31:2134–2143. doi: 10.1038/emboj.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y-H, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nature Neuroscience. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Jo YH, Donier E, Martinez A, Garret M, Toulmé E, Boué-Grabot E. Crosstalk between P2X4 and gamma-aminobutyric acid, type A receptors determines synaptic efficacy at a central synapse. J Biol Chem. 2011;286:19993–20004. doi: 10.1074/jbc.M111.231324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Role LW. Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J Neurosci. 2002;22:4794–4804. doi: 10.1523/JNEUROSCI.22-12-04794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaan TK, Yip PK, Grist J, Cefalu JS, Nunn PA, Ford AP, Zhong Y, McMahon SB. Endogenous purinergic control of bladder activity via presynaptic P2X3 and P2X2/3 receptors in the spinal cord. J Neurosci. 2010;30:4503–4507. doi: 10.1523/JNEUROSCI.6132-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Takahashi Y, Yoshikawa Y, Saitoh A. Involvement of P2X receptor subtypes in ATP-induced enhancement of the cough reflex sensitivity. Eur J Pharmacol. 2005;528:158–161. doi: 10.1016/j.ejphar.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Gachet C, Inoue K, Kato F. Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J Neurosci. 2004;24:10835–10845. doi: 10.1523/JNEUROSCI.3028-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Robertson JL, Li M, Silberberg SD, Swartz KJ. Ion access pathway to the transmembrane pore in P2X receptor channels. J Gen Physiol. 2011;137:579–590. doi: 10.1085/jgp.201010593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadra A, Yan Z, Coddou C, Tomic M, Sherman A, Stojilkovic SS. Gating properties of the P2X2a and P2X2b receptor channels: Experiments and mathematical modeling. J Gen Physiol. 2012;139:333–348. doi: 10.1085/jgp.201110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Bao X, Labarca C, Lester HA. Neuronal P2X receptor-transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci. 1999a;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G. The double life of ATP. Sci Am. 2009;301:84–92. doi: 10.1038/scientificamerican1209-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PPA. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- Khakh BS, Fisher JA, Nashmi R, Bowser DN, Lester HA. An angstrom scale interaction between plasma membrane ATP-gated P2X2 and a4b2 nicotinic channels measured with FRET and TIRF microscopy. J Neurosci. 2005;20:6911–6920. doi: 10.1523/JNEUROSCI.0561-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Gittermann D, Cockayne DA, Jones A. ATP Modulation of Excitatory Synapses onto Interneurons. J Neurosci. 2003;23:7426–7437. doi: 10.1523/JNEUROSCI.23-19-07426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Henderson G. Modulation of fast synaptic transmission by presynaptic ligand-gated cation channels. J Auton Nerv Syst. 2000;81:110–121. doi: 10.1016/s0165-1838(00)00111-9. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Lester HA. Dynamic Selectivity Filters in Ion Channels. Neuron. 1999;23:653–658. doi: 10.1016/s0896-6273(01)80025-8. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric Control of Gating and Kinetics at P2X4 Receptor Channels. J Neurosci. 1999b;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Zhou X, Sydes J, Galligan JJ, Lester HA. State-dependent cross-inhibition between transmitter-gated cation channels. Nature. 2000;406:405–410. doi: 10.1038/35019066. [DOI] [PubMed] [Google Scholar]
- Kirkup AJ, Booth CE, Chessell IP, Humphrey PP, Grundy D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. J Physiol. 1999;520:551–563. doi: 10.1111/j.1469-7793.1999.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K. Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett. 2011;504:57–61. doi: 10.1016/j.neulet.2011.08.058. [DOI] [PubMed] [Google Scholar]
- Kolb HA, Wakelam MJ. Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature. 1983;303:621–623. doi: 10.1038/303621a0. [DOI] [PubMed] [Google Scholar]
- Kracun S, Chaptal V, Abramson J, Khakh BS. Gated access to the pore of a P2X receptor: structural implications for closed-open transitions. J Biol Chem. 2010;285:10110–10121. doi: 10.1074/jbc.M109.089185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Marchenko SM, Pidoplichko VI. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett. 1983;31:41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol. 2008;295:L858–L865. doi: 10.1152/ajplung.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, Verkhratsky A. P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci. 2008;28:5473–5480. doi: 10.1523/JNEUROSCI.1149-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Verkhratsky A, Pankratov Y. Ivermectin potentiates ATP-induced ion currents in cortical neurones: evidence for functional expression of P2X4 receptors? Neurosci Lett. 2007;421:158–162. doi: 10.1016/j.neulet.2007.03.078. [DOI] [PubMed] [Google Scholar]
- Lämmer AB, Beck A, Grummich B, Förschler A, Krügel T, Kahn T, Schneider D, Illes P, Franke H, Krügel U. The P2 receptor antagonist PPADS supports recovery from experimental stroke in vivo. PLoS One. 2011:e19983. doi: 10.1371/journal.pone.0019983. Epub 2011 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le KT, Paquet M, Nouel D, Babinski K, Seguela P. Primary structure and expression of a naturally truncated human P2X ATP receptor subunit from brain and immune system. FEBS Lett. 1997;418:195–199. doi: 10.1016/s0014-5793(97)01380-x. [DOI] [PubMed] [Google Scholar]
- Le KT, Villeneuve P, Ramjaun AR, McPherson PS, Beaudet A, Seguela P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998;83:177–190. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- Lenertz LY, Wang Z, Guadarrama A, Hill LM, Gavala ML, Bertics PJ. Mutation of putative N-linked glycosylation sites on the human nucleotide receptor P2X7 reveals a key residue important for receptor function. Biochemistry. 2010;49:4611–4619. doi: 10.1021/bi902083n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP- gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Li M, Chang TH, Silberberg SD, Swartz KJ. Gating the pore of P2X receptor channels. Nat Neurosci. 2008;11:883–887. doi: 10.1038/nn.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Kawate T, Silberberg SD, Swartz KJ. Pore-opening mechanism in trimeric P2X receptor channels. Nat Commun. 2010 Jul 27;1:44. doi: 10.1038/ncomms1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörinczi E, Bhargava Y, Marino SF, Taly A, Kaczmarek-Hájek K, Barrantes-Freer A, Dutertre S, Grutter T, Rettinger J, Nicke A. Involvement of the cysteine-rich head domain in activation and desensitization of the P2X1 receptor. Proc Natl Acad Sci U S A. 2012;109:11396–11401. doi: 10.1073/pnas.1118759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcillo A, Frydel B, Bramlett HM, Dietrich WD. A reassessment of P2X7 receptor inhibition as a neuroprotective strategy in rat models of contusion injury. Exp Neurol. 2012;233:687–692. doi: 10.1016/j.expneurol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masin M, Kerschensteiner D, Dumke K, Rubio ME, Soto F. FE65 interacts with P2X2 subunits at excitatory synapses and modulates receptor function. J Biol Chem. 2006;281:4100–4108. doi: 10.1074/jbc.M507735200. [DOI] [PubMed] [Google Scholar]
- Masin M, Young C, Lim K, Barnes SJ, Xu XJ, Marschall V, Brutkowski W, Mooney ER, Gorecki DC, Murrell-Lagnado R. Expression, assembly and function of novel C-terminal truncated variants of the mouse P2X7 receptor: re-evaluation of P2X7 knockouts. Br J Pharmacol. 2012;165:978–993. doi: 10.1111/j.1476-5381.2011.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaraughty S, Wismer CT, Zhu CZ, Mikusa J, Honore P, Chu KL, Lee CH, Faltynek CR, Jarvis MF. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol. 2003;140:1381–1388. doi: 10.1038/sj.bjp.0705574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Heuss C, Gahwiler BH, Gerber U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J Physiol (Lond) 2001;535:115–123. doi: 10.1111/j.1469-7793.2001.t01-1-00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1368. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Tittle RK, Saar N, Dellal SS, Hume RI. An intersubunit zinc binding site in rat P2X2 receptors. J Biol Chem. 2005;280:25982–25993. doi: 10.1074/jbc.M504545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J Neurosci. 1994;14:740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Fujimori K, Takanaka A, Inoue K. Comparison of adenosine triphosphate- and nicotine-activated inward currents in rat phaeochromocytoma cells. J Physiol. 1991;434:647–660. doi: 10.1113/jphysiol.1991.sp018491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbolt A, Stoop R, Virginio C, Surprenant A, North RA, Buell G, Rassendren F. Membrane topology of an ATP-gated ion channel (P2X receptor) J Biol Chem. 1998;273:15177–15182. doi: 10.1074/jbc.273.24.15177. [DOI] [PubMed] [Google Scholar]
- Nicke A, Baumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A, Kuan YH, Masin M, Rettinger J, Marquez-Klaka B, Bender O, Górecki DC, Murrell-Lagnado RD, Soto F. A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. J Biol Chem. 2009;284:25913–25922. doi: 10.1074/jbc.M109.033134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieber K, Poelchen W, Illes P. Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurones of the rat. Br J Pharmacol. 1997;122:423–430. doi: 10.1038/sj.bjp.0701386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol. 2004;554:301–308. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JF, Riedel T, Leichsenring A, Heine C, Franke H, Krügel U, Nörenberg W, Illes P. Rodent cortical astroglia express in situ functional P2X7 receptors sensing pathologically high ATP concentrations. Cereb Cortex. 2011;21:806–820. doi: 10.1093/cercor/bhq154. [DOI] [PubMed] [Google Scholar]
- Oliveira MC, Pelegrini-da-Silva A, Tambeli CH, Parada CA. Peripheral mechanisms underlying the essential role of P2X3,2/3 receptors in the development of inflammatory hyperalgesia. Pain. 2009;141:127–134. doi: 10.1016/j.pain.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Postsynaptic mechanisms of CO(2) responses in parafacial respiratory neurons of newborn rats. J Physiol. 2012;590:1615–1624. doi: 10.1113/jphysiol.2011.222687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palygin O, Lalo U, Verkhratsky A, Pankratov Y. Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium. 2010;48:225–231. doi: 10.1016/j.ceca.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur J Neurosci. 1998;10:3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal O, Verkhratsky A. P2X receptor-mediated excitatory synaptic currents in somatosensory cortex. Mol Cell Neurosci. 2003;24:842–849. doi: 10.1016/s1044-7431(03)00233-1. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Verkhratsky A, North RA. Quantal release of ATP in mouse cortex. J Gen Physiol. 2007;129:257–265. doi: 10.1085/jgp.200609693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov YV, Lalo UV, Krishtal OA. Role for P2X receptors in long-term potentiation. J Neurosci. 2002;22:8363–8369. doi: 10.1523/JNEUROSCI.22-19-08363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Poskanzer KE, Yuste R. Astrocytic regulation of cortical UP states. Proc Natl Acad Sci U S A. 2011;45:18453–18458. doi: 10.1073/pnas.1112378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado R. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci. 2007;120:3838–3849. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- Rassendren F, Buell G, Newbolt A, North RA, Surprenant A. Identification of amino acid residues contributing to the pore of a P2X receptor. Embo J. 1997;16:3446–3454. doi: 10.1093/emboj/16.12.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler E, Chaumont S, Shigetomi E, Sagasti A, Khakh BS. An approach to image activation of transmitter-gated P2X receptors in vitro and in vivo. Nat Methods. 2008;5:87–93. doi: 10.1038/nmeth1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler E, Shigetomi E, Khakh BS. Neuronal P2X2 receptors are mobile ATP sensors that explore the plasma membrane when activated. J Neurosci. 2011;31:16716–16730. doi: 10.1523/JNEUROSCI.3362-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Allsopp RC, El Ajouz S, Vial C, Schmid R, Young MT, Evans RJ. Agonist binding evokes extensive conformational changes in the extracellular domain of the ATP-gated human P2X1 receptor ion channel. Proc Natl Acad Sci U S A. 2012;109:4663–4667. doi: 10.1073/pnas.1201872109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Evans RJ. Contribution of conserved polar glutamine, asparagine and threonine residues and glycosylation to agonist action at human P2X1 receptors for ATP. J Neurochem. 2006;96:843–852. doi: 10.1111/j.1471-4159.2005.03593.x. [DOI] [PubMed] [Google Scholar]
- Roger S, Pelegrin P, Surprenant A. Facilitation of P2X7 receptor currents and membrane blebbing via constitutive and dynamic calmodulin binding. J Neurosci. 2008;28:6393–6401. doi: 10.1523/JNEUROSCI.0696-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle SJ, Bobanovic LK, Murrell-Lagnado RD. Identification of a non-canonical tyrosine-based endocytic motif in an ionotropic receptor. J Biol Chem. 2002;277:35378–35385. doi: 10.1074/jbc.M204844200. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Qureshi OS, Bobanovic LK, Evans PR, Owen DJ, Murrell-Lagnado RD. Non-canonical YXXG{Phi} endocytic motifs: recognition by AP2 and preferential utilization in P2X4 receptors. J Cell Sci. 2005;118:3073–3080. doi: 10.1242/jcs.02451. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Soto F. Distinct localisation of P2X receptors at excitatory postsynaptic specializations. J Neurosci. 2001;21:641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Samways DS, Khakh BS, Dutertre S, Egan TM. Preferential use of unobstructed lateral portals as the access route to the pore of human ATP-gated ion channels (P2X receptors) Proc Natl Acad Sci U S A. 2011;108:13800–13805. doi: 10.1073/pnas.1017550108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samways DS, Khakh BS, Egan TM. Allosteric modulation of Ca2+ flux in ligand-gated cation channel (P2X4) by actions on lateral portals. J Biol Chem. 2012;287:7594–7602. doi: 10.1074/jbc.M111.322461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samways DS, Migita K, Li Z, Egan TM. On the role of the first transmembrane domain in cation permeability and flux of the ATP-gated P2X2 receptor. J Biol Chem. 2008;283:5110–5117. doi: 10.1074/jbc.M708713200. [DOI] [PubMed] [Google Scholar]
- Samways DSK, Egan TM. Acidic amino acids impart enhanced Ca2+ permeability and flux in two members of the ATP-gated P2X receptor family. J Gen Physiol. 2007;129:245–256. doi: 10.1085/jgp.200609677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searl TJ, Redman RS, Silinsky EM. Mutual occlusion of P2X ATP receptors and nicotinic receptors on sympathetic neurons of the guinea-pig. J Physiol (Lond) 1998;510:783–791. doi: 10.1111/j.1469-7793.1998.783bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searl TJ, Silinsky EM. Cross-talk between apparently independent receptors. J Physiol (Lond) 1998;513:629–630. doi: 10.1111/j.1469-7793.1998.629ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AJ, Polak PE, Simonini V, Lin SX, Richardson JC, Bongarzone ER, Feinstein DL. P2x7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J Neuroinflammation. 2008;5 doi: 10.1186/1742-2094-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137:2096–2104. doi: 10.1053/j.gastro.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki Y, Sumitomo K, Tsuda M, Koizumi S, Inoue K, Torimitsu K. Direct Observation of ATP-Induced Conformational Changes in Single P2X4 Receptors. PLoS Biol. 2009;7(5) doi: 10.1371/journal.pbio.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava AN, Triller A, Sieghart W, Sarto-Jackson I. Regulation of GABA(A) receptor dynamics by interaction with purinergic P2X(2) receptors. J Biol Chem. 2011;286:14455–14468. doi: 10.1074/jbc.M110.165282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg SD, Li M, Swartz KJ. Ivermectin Interaction with transmembrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron. 2007;54:263–274. doi: 10.1016/j.neuron.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Silinsky EM, Gerzanich V, Vanner SM. ATP mediates excitatory synaptic transmission in mammalian neurones. Br J Pharmacol. 1992;106:762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P, Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984;100:85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P, Westfall DP, Fedan JS. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982;218:693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, Austin JS, Zaykin DV, Meulen HV, Costigan M, et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med. 2012;18:595–599. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci U S A. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- Stanchev D, Blosa M, Milius D, Gerevich Z, Rubini P, Schmalzing G, Eschrich K, Schaefer M, Wirkner K, Illes P. Cross-inhibition between native and recombinant TRPV1 and P2X(3) receptors. Pain. 2009;143:26–36. doi: 10.1016/j.pain.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Stoop R, Thomas S, Rassendren F, Kawashima E, Buell G, Surprenant A, North RA. Contribution of individual subunits to the multimeric P2X(2) receptor: estimates based on methanethiosulfonate block at T336C. Mol Pharmacol. 1999;56:973–981. doi: 10.1124/mol.56.5.973. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Torres A, Wang F, Xu Q, Fujita T, Dobrowolski R, Willecke K, Takano T, Nedergaard M. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci Signal. 2012;5(208):ra8. doi: 10.1126/scisignal.2002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulme E, Garcia A, Samways D, Egan TM, Carson MJ, Khakh BS. P2X4 receptors in activated C8-B4 cells of cerebellar microglial origin. J Gen Physiol. 2010;135:333–353. doi: 10.1085/jgp.200910336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulme E, Khakh BS. Imaging P2X4 Receptor Lateral Mobility in Microglia: REGULATION BY CALCIUM AND p38 MAPK. J Biol Chem. 2012;287:14734–14748. doi: 10.1074/jbc.M111.329334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Salter MW. P2X4 purinoceptor signaling in chronic pain. Purinergic Signal. 2012;8:621–628. doi: 10.1007/s11302-012-9306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the Mammalian cochlea. J Neurosci. 2010;30:1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Vavra V, Bhattacharya A, Zemkova H. Facilitation of glutamate and GABA release by P2X receptor activation in supraoptic neurons from freshly isolated rat brain slices. Neuroscience. 2011;188:1–12. doi: 10.1016/j.neuroscience.2011.04.067. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci U S A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS, Mulkey DK. Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signalling. J Physiol. 2012;590:2137–2150. doi: 10.1113/jphysiol.2012.229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner P, Seward EP, Buell GN, North RA. Domains of P2X receptors involved in desensitization. Proc Natl Acad Sci U S A. 1996;93:15485–15490. doi: 10.1073/pnas.93.26.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C, Rosefort C, Fallah G, Kassack MU, Hamacher A, Bodnar M, Wang H, Illes P, Kless A, Bahrenberg G, et al. Molecular determinants of potent P2X2 antagonism identified by functional analysis, mutagenesis, and homology docking. Mol Pharmacol. 2011;79:649–661. doi: 10.1124/mol.110.068700. [DOI] [PubMed] [Google Scholar]
- Wynn G, Rong W, Xiang Z, Burnstock G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology. 2003;125:1398–1409. doi: 10.1016/j.gastro.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Boumechache M, Robinson LE, Marschall V, Gorecki DC, Masin M, Murrell-Lagnado R. Splice-variants of the P2X7 receptor reveal differential agonist-dependence and functional coupling with pannexin-1. J Cell Sci. 2012 May 2; doi: 10.1242/jcs.099374. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Yan Z, Khadra A, Li S, Tomic M, Sherman A, Stojilkovic SS. Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci. 2010;30:14213–14224. doi: 10.1523/JNEUROSCI.2390-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Khadra A, Sherman A, Stojilkovic SS. Calcium-dependent block of P2X7 receptor channel function is allosteric. J Gen Physiol. 2011;138:437–452. doi: 10.1085/jgp.201110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Li S, Liang Z, Tomić M, Stojilkovic SS. The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol. 2008;132:563–573. doi: 10.1085/jgp.200810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MT, Pelegrin P, Surprenant A. Identification of Thr283 as a key determinant of P2X7 receptor function. Br J Pharmacol. 2006;149:261–268. doi: 10.1038/sj.bjp.0706880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Banning AS, Cockayne DA, Ford AP, Burnstock G, Mcmahon SB. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–675. doi: 10.1016/s0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]
- Zhou X, Galligan JJ. Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. J Physiol (Lond) 1998;513:685–697. doi: 10.1111/j.1469-7793.1998.685ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]