Abstract
Natural killer (NK) T cells are innate CD1d-restricted immune cells involved in regulation of immune tolerance, tumor immunity, and immunity to infectious pathogens. Human α-chain variable gene segment 24 (Vα24) NK T cells exist in the periphery as two functionally distinct subsets: one CD4+ and one CD4- subset. However, the developmental pathway of human Vα24 NK T cells is not well understood. Here, we show that Vα24 NK T cells develop in the fetal thymus. The relative number of intrathymic NK T cell precursors decline in a linear manner with gestational age, and they are very rare in the neonatal thymus, indicating that these cells preferentially develop in the early fetal thymus. Their restriction element, CD1d, is expressed by a vast majority of thymocytes. A majority of intrathymic Vα24 NK T cell progenitors are CD4+, whereas a minority are CD4/8+/+. CD4+ Vα24 NK T cell precursors show features of mature NK T cells, such as high levels of their semiinvariant T cell receptor and CD3 and some expression of CD161, whereas the CD4/8+/+ precursors seem less mature. The cytokine IL-7 shows a biphasic effect on Vα24 NK T cell progenitors in fetal thymic organ culture, with high doses driving proliferation of immature CD161-progenitors and low doses supporting survival and maturation. Thus, the data demonstrate that human Vα24 NK T cells of the CD4+, but not the CD4-, subset develop in the early fetal thymus. Furthermore, data suggest an intrathymic pathway of CD4+ Vα24 NK T cell development that is regulated by IL-7.
Human natural killer (NK) T cells are innate immune cells that express the NK cell marker CD161 and possess a semiinvariant T cell receptor (TCR) with uniform use of α-chain variable gene segment 24 (Vα24), paired with β-chain variable gene segment 11 (Vβ11) (1–3). They recognize glycolipids presented by CD1d expressed by antigen presenting cells such as dendritic cells, and they respond by producing cytokines, including IFN-γ, tumor necrosis factor α, and IL-4 (1, 4, 5). The primary role of CD1d-restricted NK T cells is thought to be the regulation of immune responses, and several reports (6–11) support a role for NK T cell dysfunction in the pathogenesis of autoimmune diseases and cancers, as well as in murine models of autoimmunity (12–15). They are lost in HIV-1 infection in humans (16–18) and lymphocytic choriomeningitis virus infection in mice (19), and they can be activated to participate in defense against hepatitis B virus and cytomegalovirus in mice (20, 21).
Human Vα24 NK T cells can be subdivided into CD4+ and CD4- subsets that appear to be both functionally and phenotypically distinct, with differences in homing receptors and cytokine profiles (16, 22–26). The balance between these two subsets is most probably important for the immunoregulatory role of the Vα24 NK T cell compartment. Another interesting feature of NK T cells is their uniform memory T cell-like phenotype with expression of CD45RO and CD28 and frequent expression of CCR5 (27, 28). This phenotype may be linked to their role as innate immune cells, lending them the ability to respond rapidly to CD1d-restricted stimuli.
Classical MHC-restricted human T cells go through a complex process of maturation and selection in the thymus before they can join the peripheral T cell repertoire (reviewed in ref. 29). The earliest thymic precursors express CD34 and lack CD1a, and the transition to a CD34+ CD1a+ stage is strongly associated with T cell commitment (30). Through several discrete stages, these precursors develop into CD4+/+ or CD8+/+ cells, with rearranged TCRα and TCRβ genes, which express the specific TCR and are subject to induction of self-tolerance through positive and negative selection before being allowed to mature into CD4+ or CD8+ cells and leave the thymus (reviewed in refs. 29 and 31). Survival, proliferation, and expansion of thymocytes are supported by IL-7 (32), which is produced by stromal cells in the thymus and appears to play a role at several stages of T cell development (33, 34).
In mice, CD1d-restricted Vα14 NK T cells depend on the thymus for their development (35–38). However, the site and pathway of development and maturation of human Vα24 NK T cells are unknown. Investigation of these pathways and sites is important both for the basic understanding of the biology of these cells and for potential therapeutic interventions to restore or improve Vα24 NK T cell function in diseases in which these cells are lost or impaired. Here, we have found that precursors of the CD4+ Vα24 NK T cell subset can be identified in the early human fetal thymus. Their relative numbers decrease in an inverse linear correlation with gestational age, and they appear to be very rare in the neonatal thymus. Furthermore, we have studied the developmental stages of Vα24 NK T cell in the thymus, the response to IL-7 in fetal thymus organ culture (FTOC), and their maturational stage in relation to NK T cells in cord and peripheral blood. The data indicate that human Vα24 NK T cells of the CD4+, but not the CD4-, subset develop early in the fetal thymus, a finding compatible with early seeding of these cells to peripheral sites during fetal development. Furthermore, data suggest an intrathymic pathway of CD4+ Vα24 NK T cell development that is regulated by IL-7.
Methods
Human Subjects and Tissues. Heparinized samples of whole blood from healthy donors and cord blood were obtained after informed consent. Peripheral blood mononuclear cells were isolated by Ficoll/Paque Plus density-gradient centrifugation (Amersham Biosciences). Fetal thymus tissue was obtained from Advanced Bioscience Resources (Alameda, CA). The study was based on protocols approved by the local institutional review board.
Flow Cytometry. The frequency of Vα24 NK T cells in peripheral blood, cord blood, and thymocyte suspensions, as well as the expression of surface markers, were assessed by four-color flow cytometry. The following reagents were used: anti-Vα24 phycoerythrin and anti-Vβ11 FITC and biotin conjugates, and anti-IL-7Rα FITC were obtained from Immunotech (Marseilles, France); streptavidin–allophycocyanin (APC), anti-CD3 peridinin–chlorophyll protein, anti-CD4 APC, anti-CD8 peridinin–chlorophyll protein, anti-CD45RO APC, anti-CD62L APC, anti-CD1d phycoerythrin, anti-CCR5 APC, anti-CD161 FITC, anti-CD161 APC, anti-HLA class 1 APC, and anti-HLA-DR APC were obtained from PharMingen. Single-cell suspensions of thymocytes were stained for 30 min on ice in PBS buffer supplemented with 5% FCS. Samples were analyzed on a FACSCalibur (Becton Dickinson) instrument by using cellquest software. We collected 1 × 106 lymphocyte events per sample.
FTOC. Fetal thymus tissue was dissected into pieces of ≈2 mm3 so that every piece was likely to contain cortex and have similar cellularity. Three pieces of tissue were placed on sterile polycarbonate membrane filters (Millipore) placed on gel foam sponge (Upjohn) rafts in 700 μl of Yssel's medium containing 1% human serum (Gemini Biological Products, Calabasas, CA) in 24-well plates. Graded doses of IL-7 (0–250 ng/ml) (R & D Systems) were added to the culture medium. Medium and cytokines were changed on days 1 and 3. On day 7, thymus fragments were harvested individually with a sterile micropipette tip. A single-cell suspension was made by placing the fragment into a sterile nylon-mesh bag, submerging the bag in PBS with 2% FCS in a 60-mm tissue culture dish, and dispersing the tissue between the nylon layers with forceps. The medium from cultures was collected separately and analyzed for cellular content. Cells that were recovered from tissue and medium were stained for flow cytometry in a 96-well V-bottom plate.
Vα24 NK T Cell Expansion Culture. FTOC was performed as described above, and cells leaving the tissue were collected in the surrounding medium at day 7. Next, we cultured 1 × 106 cells for 8 days in Yssel's medium supplemented with 1% human serum in the presence of 1 × 105 irradiated and α-galactosylceramide (α-GalCer)-pulsed (1 μg/ml for 1 h at 37°C) CD1d-transfected DT407 cells. The culture was analyzed for Vα24 NK T cells by flow cytometry on days 4 and 8.
Statistical Analysis. Data were analyzed by descriptive statistics, paired t test, and linear regression by using sigmastat software (SPSS, Chicago).
Results
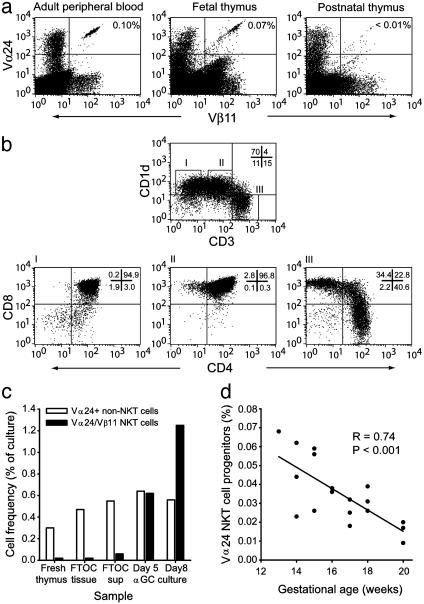
Vα24 NK T Cell Progenitors in the Human Fetal Thymus. Data presented in a recent study (39) indicated that Vα24 NK T cells were not present in the postnatal human thymus. This finding was interpreted to suggest that these cells develop in a thymus-independent manner (39). An alternative explanation is that Vα24 NK T cells are produced at a specific time during development or even late in postnatal life. To investigate these possibilities, we obtained fetal, postnatal, and adolescent thymic tissue and analyzed these tissues for the presence of Vα24+ Vβ11+ NK T cells by flow cytometry. By this assay, we could confirm that Vα24 NK T cells were very rare or absent from the postnatal and adolescent thymi (Fig. 1a). In contrast, Vα24+ Vβ11+ cells were clearly detectable in the fetal thymus at a gestational age of 13 weeks (Fig. 1a). CD1d, the ligand of Vα24 NK T cells, was highly expressed by thymocytes in the CD3 low or negative subset, in particular by CD4/8+/+ cells but also in small numbers of CD4+ CD3-/- cells and CD4/8-/- CD3- cells (Fig. 1b).
Fig. 1.
Detection of Vα24 NK T cell progenitors responsive to α-GalCer in human fetal thymus depends on gestational age. (a) Flow cytometric assessment of Vα24+ Vβ11+ NK T cells in adult peripheral blood and fetal and postnatal thymus. (b) CD1d expression in fetal thymocytes, with data gated on CD1d-high and CD3- (I), CD1d-high and CD3-low (II), and CD1d- and CD3-high (III). (c)Vα24+ Vβ11+ cells collected in FTOC supernatant after culture expand in response to stimulation with α-GalCer (αGC) over an 8-day culture. (d) The frequency of Vα24+ Vβ11- non-NK T cells is shown as an internal-reference population. Vα24+ Vβ11+ thymocytes were enumerated in thymi by flow cytometry at gestational ages of 13–20 weeks (n = 18). Solid line shows the linear regression of all data (R = 0.74; P < 0.001).
The dual expression of Vα24 and Vβ11 TCR segments accurately identifies CD1d-restricted NK T cells that recognize and respond to the prototypical CD1d-presented antigen α-Gal-Cer in human peripheral blood (24, 40, 41). To demonstrate that Vα24+ Vβ11+ thymocytes in the fetal thymus respond to α-GalCer, we used a FTOC system, in which pieces of tissue were cultured for 7 days, and cells that had exited the tissue were harvested from the surrounding medium. These FTOC tissue “emigrants” were enriched in Vα24 NK T cells as compared with the thymic tissue, and the cells expanded 50-fold in response to α-GalCer over an 8-day culture (Fig. 1c). In contrast, the frequency Vα24+ Vβ11- non-NK T cells in the culture did not change in response to α-GalCer.
Together, these data demonstrate that the Vα24 NK T cell ligand CD1d is expressed in the thymus and that Vα24 NK T cells are generated in the human fetal thymus.
Vα24 NK T Cell Progenitors Appear Early During Development. The striking difference in Vα24 NK T cell progenitor frequency between fetal and postnatal thymus prompted a more careful analysis of these cells in the fetal stage of development. We obtained 18 fetal thymi from gestational ages of 13–20 weeks. Strikingly, Vα24 NK T progenitors were most frequent in the early tissue specimens, and numbers showed an inverse linear correlation with gestational age over the 13- to 20-week span (R = 0.74; P < 0.001) (Fig. 1d). The data indicate that development of Vα24 NK T cells preferentially occurs in an early phase of thymic development. However, these results do not exclude the possibility of Vα24 NK T cell development in the postnatal thymus.
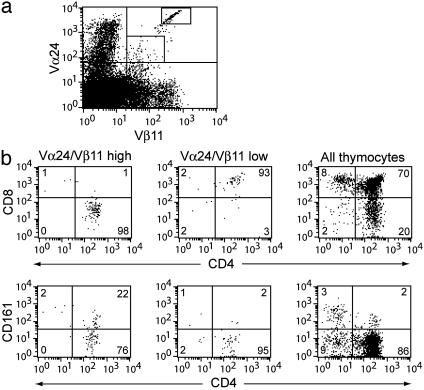
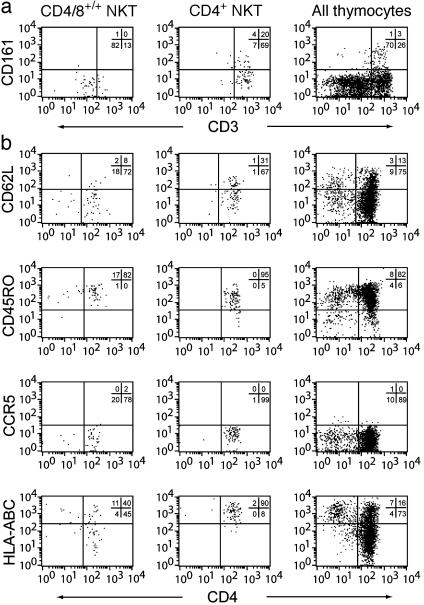
Stages of Vα24 NK T Development and Maturation. These findings prompted us to identify stages of intrathymic Vα24 NK T progenitor maturation. Careful examination of Vα24 and Vβ11 flow cytometry dot plots revealed that cells were either high or low in their expression of the invariant TCR (Fig. 2a). Cells with high expression of the invariant TCR were predominantly CD4+, and low TCR expression was associated with a CD4/8+/+ phenotype (Fig. 2b). These two populations would fit into one immature CD4/8+/+ stage and one more mature CD4+ stage similar to those identified for regular MHC-restricted T cells (29). Of the invariant TCR-high NK T cell progenitors, 20–30% expressed CD161, a marker of mature Vα24 NK T cells in the periphery (Fig. 2b). Expression of CD161 did not show any correlation with gestational age (data not shown). CD161 was not expressed in the CD4/8+/+ stage, again suggesting that these cells are less mature (Fig. 2b). CD4/8+/+ NK T cell progenitors expressed intermediate levels of CD3, whereas the CD4+ cells expressed high levels (Fig. 3a). Expression of CD161 was found only in CD4+ NK T cell progenitors (Fig. 3a). Similarly, HLA class I and to some extent CD62L were up-regulated in the CD4+ stage (Fig. 3b). The RO isoform of CD45 remained high throughout both double-and single-positive stages, and CCR5 expression was undetectable (Fig. 3b). These data support a model of intrathymic maturation from the CD4/8+/+, TCR/CD3-low, and CD161- immature Vα24 NK T cell progenitors to mature CD4+, TCR/CD3-high cells that start to express CD161 and CD62L before they exit into the periphery.
Fig. 2.
Identification of CD4+ and CD4/8+/+ Vα24 NK T cell progenitors in human fetal thymus. (a) Detection of Vα24 NK T cell progenitors in Vα24/Vβ11 TCR low and high stages by flow cytometry. (b) Assessment of CD4, CD8, and CD161 expression in TCR low and TCR high subsets. One representative of 16 experiments is shown.
Fig. 3.
Expression of maturation markers in the developing Vα24 NK T cell progenitors. Flow cytometric assessment of maturation markers and functional receptors CD3 and CD161 (a), as well as CD62L, CD45RO, CCR5, and HLA-A,B,C (b) in CD4/8+/+ and CD4+ stages of NK T cell development, as compared with the overall thymocyte population, are shown. One representative of 10 experiments is shown.
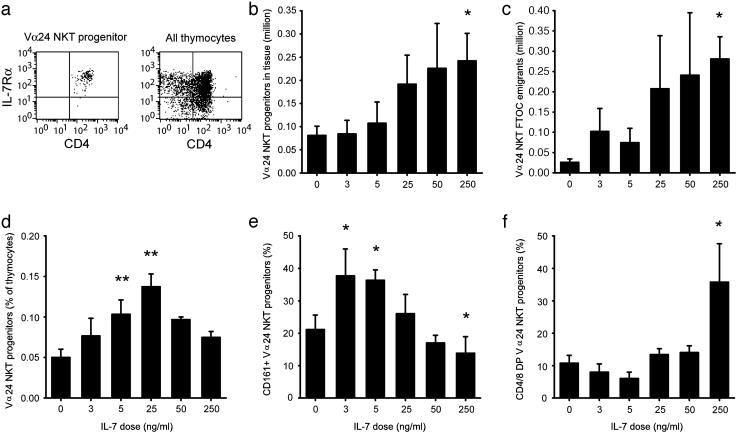
Biphasic Regulation of Vα24 NK T Cell Progenitors by IL-7. IL-7 supports survival, proliferation, and expansion of thymocytes in both humans and mice (32–34). Staining for IL-7Rα clearly showed that intrathymic Vα24 NK T cell progenitors, as well as the vast majority of total thymocytes, expressed IL-7Rα (Fig. 4a). We, therefore, investigated the effects of this cytokine on Vα24 NK T cell development in an FTOC system. Pieces of fetal thymic tissue were cultured for 7 days in the presence of 0–250 ng/ml IL-7, and thymocyte and NK T cell numbers were determined by total cell counts and flow cytometry. Low doses in the 0–5 ng/ml range had moderate effects on the total absolute number of Vα24 NK T cell progenitors retrieved from the tissue cultures, whereas high-dose IL-7 treatment increased the number of Vα24 NK T cell progenitors in the tissue at day 7 (P = 0.03; Fig. 4b) and resulted also in increased Vα24 NK T cell progenitor numbers in the surrounding medium (P = 0.03; Fig. 4c). However, the percentage of Vα24 NK T cell progenitors retrieved from the media fraction of cultures was the highest at 5 and 25 ng/ml IL-7 (P = 0.004 and P = 0.001, respectively), suggesting that these doses preferentially supported survival of these cells over the general thymocyte population (Fig. 4d). We next analyzed expression of the maturation marker CD161 on the developing TCR-high Vα24 NK T cell progenitors in the FTOC. Low doses of IL-7 promoted up-regulation of CD161 (P = 0.03 and P = 0.006), whereas the 250 ng/ml dose resulted in less CD161 expression as compared with untreated control FTOC (P = 0.02) (Fig. 4e). In line with this shift in Vα24 NK T cell progenitor phenotype, high-dose IL-7 treatment shifted the phenotype of these progenitors toward an immature CD4/8+/+ phenotype (P = 0.0; Fig. 4f). Thus, intrathymic Vα24 NK T cell progenitors respond to IL-7 in a biphasic manner: high doses of IL-7 promote proliferation of these cells and maintain them in an immature state, whereas low doses of IL-7 support survival and promote expression of markers associated with mature Vα24 NK T cells.
Fig. 4.
Biphasic regulation of Vα24 NK T cell development by IL-7. (a) Expression of IL-7 receptor α in the thymus. Analysis of NK T cell progenitor numbers and phenotype after 7 days FTOC with 0–250 ng/ml IL-7. The number of Vα24 NK T cell progenitors recovered in tissue is shown in b, and the medium surrounding the tissue (c) was quantified by cell counting and flow cytometric analysis. (d) Percentage of Vα24 NK T cell progenitors in the medium. Percentages of NK T cell precursors expressing a CD161+ (e) and a CD4/8+/+ phenotype (f) after a 7-day FTOC with graded doses of IL-7 are given. Figures represent data from six independent experiments. *, P < 0.05; **, P < 0.005, compared with culture without IL-7, as determined by paired t test.
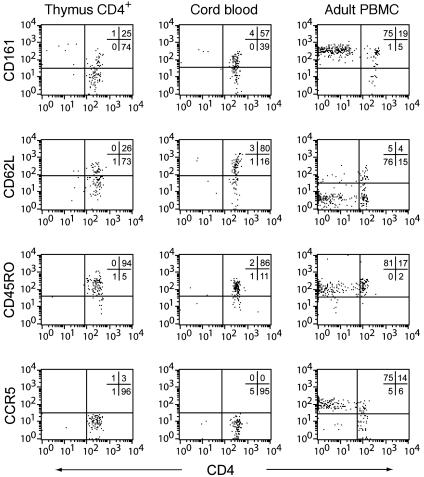
Continued Maturation of Vα24 NK T Cells After Leaving the Thymus. Peripheral blood Vα24 NK T cells in adults contain both CD4+ and CD4- subsets that are functionally distinct (16, 22–26). However, the intrathymic Vα24 NK T progenitors are almost exclusively CD4+ in their CD3/TCR-high stage, and virtually no CD4/8-/- progenitors can be observed (Fig. 2b). This observation prompted a more careful comparison of the intrathymic NK T progenitors, and NK T cells in cord blood and in adult peripheral blood mononuclear cells (Fig. 5). Cord blood Vα24 NK T cells were predominantly CD161+ albeit not to the same extent as in adult peripheral blood. However, the cord blood Vα24 NK T cells were similar to intrathymic progenitors in that they were all CCR5- and CD4+, whereas many peripheral blood NK T cells were CD4- and CCR5+. Vα24 NK T cells of all compartments were CD45RO+, indicative of the memory T cell-like phenotype, but most of the peripheral blood Vα24 NK T cells lacked CD62L and expressed CCR5 at high levels. These data suggest that Vα24 NK T cells differentiate further in the periphery after leaving the thymus, and that the CD4- Vα24 NK T cells frequently found in peripheral blood develop at an alternative site, at another developmental stage, or under particular circumstances.
Fig. 5.
Shifts in Vα24 NK T cell phenotype after leaving the thymus. Expression of CD161, CD62L, CD45RO, and CCR5 in NK T cell progenitors defined by Vα24 and Vβ11 in the TCR high CD4+ intrathymic stage in cord-blood Vα24 NK T cells and adult peripheral blood Vα24 NK T cells, as determined by four-color flow cytometry, are shown.
Discussion
CD1d-restricted Vα24 NK T cells are innate immune cells with the capacity to influence various diseases. Loss or dysfunction of these cells occurs in autoimmune conditions (6–8, 10), cancer (9, 11), and viral infections (16–18, 42). Investigation of the pathways and sites for development and differentiation of human Vα24 NK T cells is, therefore, important both for the basic understanding of the biology of these cells and for potential therapeutic interventions to restore or improve Vα24 NK T cell function in certain diseases. We have shown here that human CD4+ Vα24 NK T cells develop in the thymus. NK T cell progenitors are most frequent in the thymus at an early gestational age, but their relative number declines with age, and they are very rare or absent in postnatal thymus. The size of the thymus, and thus the total cellularity, increase sharply during development of the fetus. The higher percentage of NK T cell progenitors during early fetal age and the subsequent drop in frequency are, therefore, probably a reflection of the timing rather than an absolute measure of NK T cell development, indicating that there is preferential development of Vα24 NK T cells in the early fetal thymus. An early seeding of immunoregulatory Vα24 NK T cells from the thymus to the developing peripheral immune system makes sense from a functional perspective in that their early presence could be involved in establishing immunological tolerance at peripheral sites. In line with this notion, murine Vα14 NK T cells are involved in regulating tolerance to self antigens expressed in the eye (43) and the pancreas (12–15). The NK T cell ligand CD1d is ubiquitously expressed by CD4/8+/+ thymocytes and most likely functions as the selecting ligand for the developing NK T cell progenitors, reminiscent of what is believed to occur in the mouse (37).
Because Vα24 NK T cells are defined by their TCR, the earliest stage of development at which thymocytes destined to become NK T cells can be identified is in the CD3/TCR low stage. Our data suggest that NK T cell progenitors develop through CD4/8+/+ and CD4+ stages similar to the overall pathway of classical T cells (29). However, single-positive NK T progenitors are clearly different in that they are uniformly CD4+. In addition, they appear to initiate CD161 expression in what may be the final maturation step before leaving the thymus. Unlike classical T cells, Vα24 NK T cells do not switch CD45 isoform expression from RO to RA to obtain the classical naíve CD45RA+ CD62L+ phenotype, but maintain the CD45RO+ CD62L+ central memory T cell-like phenotype in cord blood.
IL-7 plays a key role in the development of T cells in both humans and mice (32–34). We find that IL-7 regulates Vα24 NK T cell progenitors in FTOC in a biphasic manner such that high doses, likely to occur only in the local micro environment, promote proliferation of these cells in an immature CD4/8+/+ state. In contrast, low doses of IL-7 rather support survival and promote expression of the maturation marker CD161. IL-7Rα signals via both the phosphatidylinositol 3-kinase/protein kinase B pathway and a signal transducer and activator of transcription 5-dependent pathway, which regulate thymocyte survival and proliferation, as well as thymocyte differentiation, respectively (44). In addition, IL-7Rα associates with the common γ-chain that is required to initiate expression of the CD161 homologue NK.1.1 in the mouse thymus (45). Thus, the effects of IL-7 on thymic development are complex and involve several signaling pathways that together may account for the dose-dependent effects of IL-7 on Vα24 NK T cell development.
Mature intrathymic progenitors and cord blood Vα24 NK T cells are almost exclusively CD4+. In adult peripheral blood, however, close to 50% of NK T cells are CD4- with no or low expression of CD8. This observation leaves several possibilities: the CD4- subset may be a separate lineage that is extrathymically derived, they may develop in the thymus at a later developmental stage, or the CD4- Vα24 NK T cells may be derived from the CD4+ subset. If the last possibility is correct then the CD4- NK T cell could be a late-stage-activated NK T cell. This model is supported by the effector-like phenotype of CD4- NK T cells, with high levels of CD11a and CCR5 and loss of CD62L, and by the recent observation that human subjects with an expanded Vα24 NK T cell compartment have a preferential expansion of CD4- NK T cells (28). If this model is correct, then CD4 could be viewed as a Vα24 NK T cell differentiation marker that is lost upon maturation into a late-stage effector-type cell. However, other possibilities clearly exist, and further investigations are required to determine the developmental origin of CD4- Vα24 NK T cells.
Development of human Vα24 NK T cells in the thymus displays both similarities and differences to Vα14 NK T cell development in the mouse. One major difference is that intrathymic NK T cell progenitors are frequent in the adult mouse (35–37), whereas in humans, the Vα24 NK T cell progenitors are found in the early fetal thymus and are very hard to find in the postnatal thymus. The two species, thus, appear to be different in the timing of NK T cell development. Another striking difference is that both CD4+ and CD4- NK T cell progenitors are found in the mouse thymus (35, 36), whereas we detect only the CD4+ subset in the human fetal thymus. The site or timing of CD4- NK T cell maturation or development, thus, seems to differ between the two species.
Here, we have shown that human CD4+ Vα24 NK T cells develop in the thymus. We have observed that Vα24 NK T cell progenitors are most frequent in the thymus at an early gestational stage, but their frequency declines with age, and they are rare in the postnatal thymus. Surprisingly, the CD4- Vα24 NK T cells commonly found in adult peripheral blood are not found in the fetal thymus. IL-7 regulates Vα24 NK T cell development in a biphasic manner such that high doses promote proliferation of these cells in an immature CD4/8+/+ state, whereas low doses can support survival and drive expression of the CD161 marker characteristic of mature Vα24 NK T cells. These results shed light on the developmental pathway of CD1d-restricted NK T cells and are important both for the understanding of the basic biology of these cells and for potential therapeutic interventions to restore or improve Vα24 NK T cell function in certain diseases.
Acknowledgments
We thank Kirin Brewery Company, Japan, for the kind gift of the α-GalCer; Karen Beckerman for access to cord blood samples; Jose Rivera for technical assistance; and Alf Grandien and Maire Quigley for careful reading of the manuscript. This work was supported by National Institutes of Health Grants AI52731 and AI54018, the Elizabeth Glaser Pediatric AIDS Foundation, the Swedish Research Council, and the Swedish Foundation for Strategic Research. D.F.N. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: α-GalCer, α-galactosylceramide; APC, allophycocyanin; FTOC, fetal thymus organ culture; NK, natural killer; TCR, T cell receptor; Vα24, α-chain variable gene segment 24.
References
- 1.Godfrey, D. I., Hammond, K. J., Poulton, L. D., Smyth, M. J. & Baxter, A. G. (2000) Immunol. Today 21, 573-583. [DOI] [PubMed] [Google Scholar]
- 2.Porcelli, S., Yockey, C. E., Brenner, M. B. & Balk, S. P. (1993) J. Exp. Med. 178, 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellabona, P., Padovan, E., Casorati, G., Brockhaus, M. & Lanzavecchia, A. (1994) J. Exp. Med. 180, 1171-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Exley, M., Garcia, J., Balk, S. P. & Porcelli, S. (1997) J. Exp. Med. 186, 109-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porcelli, S. A. & Modlin, R. L. (1999) Annu. Rev. Immunol. 17, 297-329. [DOI] [PubMed] [Google Scholar]
- 6.Illes, Z., Kondo, T., Newcombe, J., Oka, N., Tabira, T. & Yamamura, T. (2000) J. Immunol. 164, 4375-4381. [DOI] [PubMed] [Google Scholar]
- 7.Sumida, T., Sakamoto, A., Murata, H., Makino, Y., Takahashi, H., Yoshida, S., Nishioka, K., Iwamoto, I. & Taniguchi, M. (1995) J. Exp. Med. 182, 1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson, S. B., Kent, S. C., Patton, K. T., Orban, T., Jackson, R. A., Exley, M., Porcelli, S., Schatz, D. A., Atkinson, M. A., Balk, S. P., et al. (1998) Nature 391, 177-181. [DOI] [PubMed] [Google Scholar]
- 9.Yanagisawa, K., Seino, K., Ishikawa, Y., Nozue, M., Todoroki, T. & Fukao, K. (2002) J. Immunol. 168, 6494-6499. [DOI] [PubMed] [Google Scholar]
- 10.Kukreja, A., Cost, G., Marker, J., Zhang, C., Sun, Z., Lin-Su, K., Ten, S., Sanz, M., Exley, M., Wilson, B., et al. (2002) J. Clin. Invest. 109, 131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhodapkar, M. V., Geller, M. D., Chang, D. H., Shimizu, K., Fujii, S., Dhodapkar, K. M. & Krasovsky, J. (2003) J. Exp. Med. 197, 1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond, K. J., Poulton, L. D., Palmisano, L. J., Silveira, P. A., Godfrey, D. I. & Baxter, A. G. (1998) J. Exp. Med. 187, 1047-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehuen, A., Lantz, O., Beaudoin, L., Laloux, V., Carnaud, C., Bendelac, A., Bach, J. F. & Monteiro, R. C. (1998) J. Exp. Med. 188, 1831-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharif, S., Arreaza, G. A., Zucker, P., Mi, Q. S., Sondhi, J., Naidenko, O. V., Kronenberg, M., Koezuka, Y., Delovitch, T. L., Gombert, J. M., et al. (2001) Nat. Med. 7, 1057-1062. [DOI] [PubMed] [Google Scholar]
- 15.Hong, S., Wilson, M. T., Serizawa, I., Wu, L., Singh, N., Naidenko, O. V., Miura, T., Haba, T., Scherer, D. C., Wei, J., et al. (2001) Nat. Med. 7, 1052-1056. [DOI] [PubMed] [Google Scholar]
- 16.Sandberg, J. K., Fast, N. M., Palacios, E. H., Fennelly, G., Dobroszycki, J., Palumbo, P., Wiznia, A., Grant, R. M., Bhardwaj, N., Rosenberg, M. G. & Nixon, D. F. (2002) J. Virol. 76, 7528-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Vliet, H. J., von Blomberg, B. M., Hazenberg, M. D., Nishi, N., Otto, S. A., van Benthem, B. H., Prins, M., Claessen, F. A., van den Eertwegh, A. J., Giaccone, G., et al. (2002) J. Immunol. 168, 1490-1495. [DOI] [PubMed] [Google Scholar]
- 18.Motsinger, A., Haas, D. W., Stanic, A. K., Van Kaer, L., Joyce, S. & Unutmaz, D. (2002) J. Exp. Med. 195, 869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobbs, J. A., Cho, S., Roberts, T. J., Sriram, V., Zhang, J., Xu, M. & Brutkiewicz, R. R. (2001) J. Virol. 75, 10746-10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakimi, K., Guidotti, L. G., Koezuka, Y. & Chisari, F. V. (2000) J. Exp. Med. 192, 921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dommelen, S. L., Tabarias, H. A., Smyth, M. J. & Degli-Esposti, M. A. (2003) J. Virol. 77, 1877-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davodeau, F., Peyrat, M. A., Necker, A., Dominici, R., Blanchard, F., Leget, C., Gaschet, J., Costa, P., Jacques, Y., Godard, A., et al. (1997) J. Immunol. 158, 5603-5611. [PubMed] [Google Scholar]
- 23.Takahashi, T., Nieda, M., Koezuka, Y., Nicol, A., Porcelli, S. A., Ishikawa, Y., Tadokoro, K., Hirai, H. & Juji, T. (2000) J. Immunol. 164, 4458-4464. [DOI] [PubMed] [Google Scholar]
- 24.Lee, P. T., Benlagha, K., Teyton, L. & Bendelac, A. (2002) J. Exp. Med. 195, 637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumperz, J. E., Miyake, S., Yamamura, T. & Brenner, M. B. (2002) J. Exp. Med. 195, 625-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, C. H., Johnston, B. & Butcher, E. C. (2002) Blood 100, 11-16. [DOI] [PubMed] [Google Scholar]
- 27.van Der Vliet, H. J., Nishi, N., de Gruijl, T. D., von Blomberg, B. M., van den Eertwegh, A. J., Pinedo, H. M., Giaccone, G. & Scheper, R. J. (2000) Blood 95, 2440-2442. [PubMed] [Google Scholar]
- 28.Sandberg, J. K., Bhardwaj, N. & Nixon, D. F. (2003) Eur. J. Immunol. 33, 588-596. [DOI] [PubMed] [Google Scholar]
- 29.Spits, H. (2002) Nat. Rev. Immunol. 2, 760-772. [DOI] [PubMed] [Google Scholar]
- 30.Galy, A., Verma, S., Barcena, A. & Spits, H. (1993) J. Exp. Med. 178, 391-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderton, S. M. & Wraith, D. C. (2002) Nat. Rev. Immunol. 2, 487-498. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto, Y., Douek, D. C., McFarland, R. D. & Koup, R. A. (2002) Blood 99, 2851-2858. [DOI] [PubMed] [Google Scholar]
- 33.Peschon, J. J., Morrissey, P. J., Grabstein, K. H., Ramsdell, F. J., Maraskovsky, E., Gliniak, B. C., Park, L. S., Ziegler, S. F., Williams, D. E., Ware, C. B., et al. (1994) J. Exp. Med. 180, 1955-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Freeden-Jeffry, U., Vieira, P., Lucian, L. A., McNeil, T., Burdach, S. E. & Murray, R. (1995) J. Exp. Med. 181, 1519-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellicci, D. G., Hammond, K. J., Uldrich, A. P., Baxter, A. G., Smyth, M. J. & Godfrey, D. I. (2002) J. Exp. Med. 195, 835-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benlagha, K., Kyin, T., Beavis, A., Teyton, L. & Bendelac, A. (2002) Science 296, 553-555. [DOI] [PubMed] [Google Scholar]
- 37.Gapin, L., Matsuda, J. L., Surh, C. D. & Kronenberg, M. (2001) Nat. Immunol. 2, 971-978. [DOI] [PubMed] [Google Scholar]
- 38.Tilloy, F., Di Santo, J. P., Bendelac, A. & Lantz, O. (1999) Eur. J. Immunol. 29, 3313-3318. [DOI] [PubMed] [Google Scholar]
- 39.Gurney, K. B., Yang, O. O., Wilson, S. B. & Uittenbogaart, C. H. (2002) J. Immunol. 169, 5338-5346. [DOI] [PubMed] [Google Scholar]
- 40.Benlagha, K., Weiss, A., Beavis, A., Teyton, L. & Bendelac, A. (2000) J. Exp. Med. 191, 1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karadimitris, A., Gadola, S., Altamirano, M., Brown, D., Woolfson, A., Klenerman, P., Chen, J. L., Koezuka, Y., Roberts, I. A., Price, D. A., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 3294-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas, M., Gadola, S., Meier, U., Young, N. T., Harcourt, G., Karadimitris, A., Coumi, N., Brown, D., Dusheiko, G., Cerundolo, V. & Klenerman, P. (2003) J. Virol. 77, 2251-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonoda, K. H., Exley, M., Snapper, S., Balk, S. P. & Stein-Streilein, J. (1999) J. Exp. Med. 190, 1215-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pallard, C., Stegmann, A. P., van Kleffens, T., Smart, F., Venkitaraman, A. & Spits, H. (1999) Immunity 10, 525-535. [DOI] [PubMed] [Google Scholar]
- 45.Lantz, O., Sharara, L. I., Tilloy, F., Andersson, A. & DiSanto, J. P. (1997) J. Exp. Med. 185, 1395-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]