Abstract
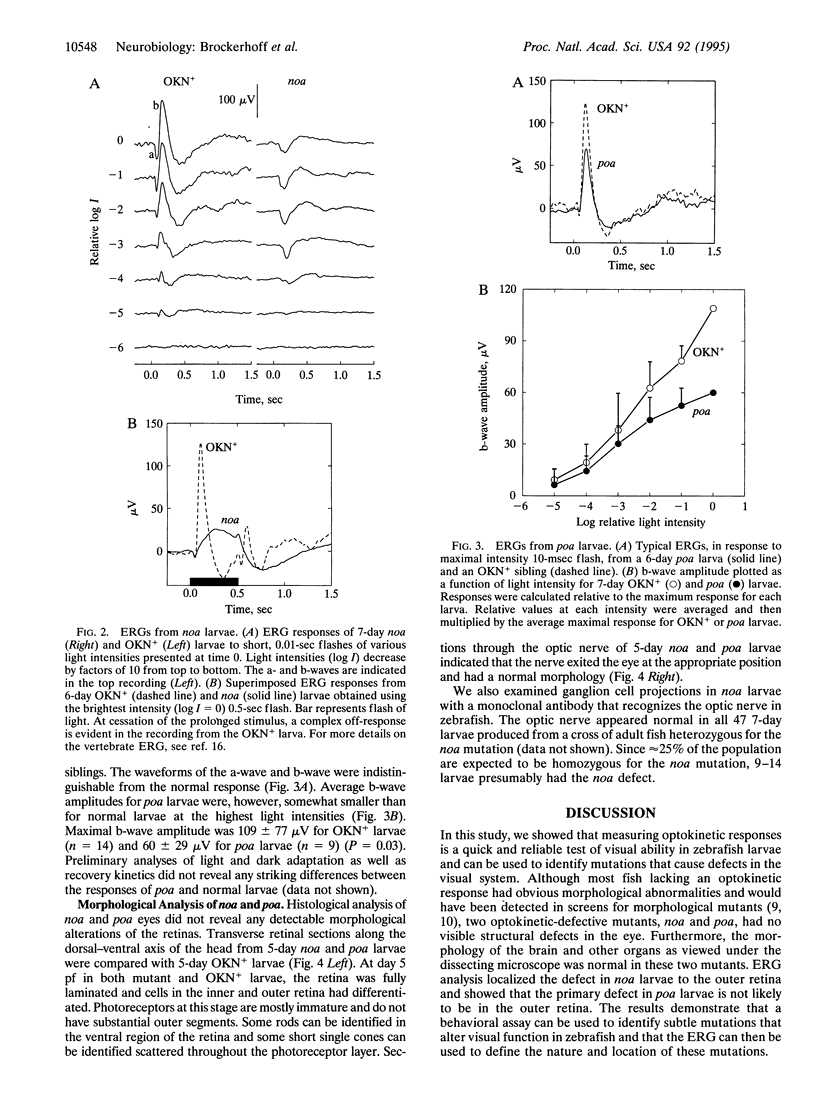
Optokinetic and phototactic behaviors of zebrafish larvae were examined for their usefulness in screening for recessive defects in the visual system. The optokinetic response can be reliably and rapidly detected in 5-day larvae, whereas the phototactic response of larvae is variable and not robust enough to be useful for screening. We therefore measured optokinetic responses of mutagenized larvae as a genetic screen for visual system defects. Third-generation larvae, representing 266 mutagenized genomes, were examined for abnormal optokinetic responses. Eighteen optokinetic-defective mutants were identified and two mutants that did not show obvious morphological defects, no optokinetic response a (noa) and partial optokinetic response a (poa), were studied further. We recorded the electroretinogram (ERG) to determine whether these two mutations affect the retina. The b-wave of noa larvae was grossly abnormal, being delayed in onset and significantly reduced in amplitude. In contrast, the ERG waveform of poa larvae was normal, although the b-wave was reduced in amplitude in bright light. Histologically, the retinas of noa and poa larvae appeared normal. We conclude that noa larvae have a functional defect in the outer retina, whereas the outer retina of poa larvae is likely to be normal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzer S. BEHAVIORAL MUTANTS OF Drosophila ISOLATED BY COUNTERCURRENT DISTRIBUTION. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchek T. The development of photoreceptors in the zebrafish, brachydanio rerio. II. Function. J Comp Neurol. 1984 Mar 20;224(1):116–122. doi: 10.1002/cne.902240110. [DOI] [PubMed] [Google Scholar]
- Driever W., Stemple D., Schier A., Solnica-Krezel L. Zebrafish: genetic tools for studying vertebrate development. Trends Genet. 1994 May;10(5):152–159. doi: 10.1016/0168-9525(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Harris W. A., Stark W. S., Walker J. A. Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J Physiol. 1976 Apr;256(2):415–439. doi: 10.1113/jphysiol.1976.sp011331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Benzer S. Abnormal electroretinograms in visual mutants of Drosophila. Nature. 1969 Apr 26;222(5191):354–356. doi: 10.1038/222354a0. [DOI] [PubMed] [Google Scholar]
- Kljavin I. J. Early development of photoreceptors in the ventral retina of the zebrafish embryo. J Comp Neurol. 1987 Jun 15;260(3):461–471. doi: 10.1002/cne.902600311. [DOI] [PubMed] [Google Scholar]
- Mullins M. C., Hammerschmidt M., Haffter P., Nüsslein-Volhard C. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol. 1994 Mar 1;4(3):189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Mullins M. C., Nüsslein-Volhard C. Mutational approaches to studying embryonic pattern formation in the zebrafish. Curr Opin Genet Dev. 1993 Aug;3(4):648–654. doi: 10.1016/0959-437x(93)90102-u. [DOI] [PubMed] [Google Scholar]
- Postlethwait J. H., Johnson S. L., Midson C. N., Talbot W. S., Gates M., Ballinger E. W., Africa D., Andrews R., Carl T., Eisen J. S. A genetic linkage map for the zebrafish. Science. 1994 Apr 29;264(5159):699–703. doi: 10.1126/science.8171321. [DOI] [PubMed] [Google Scholar]
- Robinson J., Schmitt E. A., Hárosi F. I., Reece R. J., Dowling J. E. Zebrafish ultraviolet visual pigment: absorption spectrum, sequence, and localization. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6009–6012. doi: 10.1073/pnas.90.13.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E. A., Dowling J. E. Early eye morphogenesis in the zebrafish, Brachydanio rerio. J Comp Neurol. 1994 Jun 22;344(4):532–542. doi: 10.1002/cne.903440404. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L., Schier A. F., Driever W. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics. 1994 Apr;136(4):1401–1420. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujii R., Novales R. R. Tetrodotoxin: effects on fish and frog melanophores. Science. 1968 Jun 7;160(3832):1123–1124. doi: 10.1126/science.160.3832.1123. [DOI] [PubMed] [Google Scholar]