Abstract
Background
Actin is an ancient molecule that shows more than 90% amino acid homology between mammalian and plant actins. The regions of the actin molecule that are involved in F-actin assembly are largely conserved, and it is likely that mammalian actin is able to incorporate into microfilaments in plant cells but there is no experimental evidence until now.
Results
Visualization of microfilaments in onion bulb scale epidermis cells by different techniques revealed that rhodamine-phalloidin stained F-actin besides cytoplasm also in the nuclei whereas GFP-mouse talin hybrid protein did not enter the nuclei. Microinjection of fluorescently labeled actin was applied to study the presence of nuclear microfilaments in plant cells. Ratio imaging of injected fluorescent rabbit skeletal muscle actin and phalloidin staining of the microinjected cells showed that mammalian actin was able to incorporate into plant F-actin. The incorporation occurred preferentially in the nucleus and in the perinuclear region of plant cells whereas part of plant microfilaments, mostly in the periphery of cytoplasm, did not incorporate mammalian actin.
Conclusions
Microinjected mammalian actin is able to enter plant cell's nucleus, whereas incorporation of mammalian actin into plant F-actin occurs preferentially in the nucleus and perinuclear area.
Background
Actin is an ancient molecule and is believed to find its origin at the onset of eukaryotic life 2 billion years ago [1]. Comparison of amino acid sequences has shown more than 90% homology between mammalian and plant actins [2]. The regions of the actin molecule that are involved in actin-actin contacts (F-actin assembly) are largely conserved, and it has been shown that actins from evolutionally distant organisms are able to copolymerize with mammalian skeletal muscle actin [3], [4]. Thus, it is likely that mammalian actin is able to incorporate into microfilaments in plant cells but there is no experimental evidence until now.
Fluorescent analogues of actin and tubulin have been used for studies on cytoskeletal dynamics in animal cells. Mammalian fluorescently labeled neurotubulin has been shown to incorporate into plant microtubules [5], but fluorescent staining of plant microfilaments in living cells has been carried out indirectly, using phalloidin derivates [6], GFP-mouse talin fusion protein [7], and fluorescently labeled fimbrin [8]. The ability of heterologous fluorescent actin to incorporate into endogenous pool of plant microfilaments has been under doubt [9,10] but the reason why mammalian actin did not incorporate into plant microfilaments during few published experiments is still unclear [10,11]. In the current work we applied the commercially available rabbit actin conjugated with Alexa Fluor®, a dye with long fluorescence lifetime as a tool for visualization of microfilaments in living cells.
Actin has been found in cell nucleus of different organisms including plants (reviewed in [12]) but the function of nuclear actin is yet undefined.
Hereby we present experimental evidence about the ability of actin from rabbit skeletal muscle to incorporate into microfilaments in the nucleus of onion bulb scale epidermis cells.
Results
Rhodamine-phalloidin staining of onion bulb scale epidermis cells revealed a dense network of microfilaments in the cytoplasm. In addition, both freeze-shattering and enzyme-mediated permeabilization methods showed diffuse labeling with distinct 1–2 μM spot-like structures in the nuclei (Fig. 1A,1B,1C,1D,1E,1F). Although no obviously filamentous structures were found in the nuclei, the phalloidin staining showed the existence of F-actin in the nuclei of plant cells.
Figure 1.
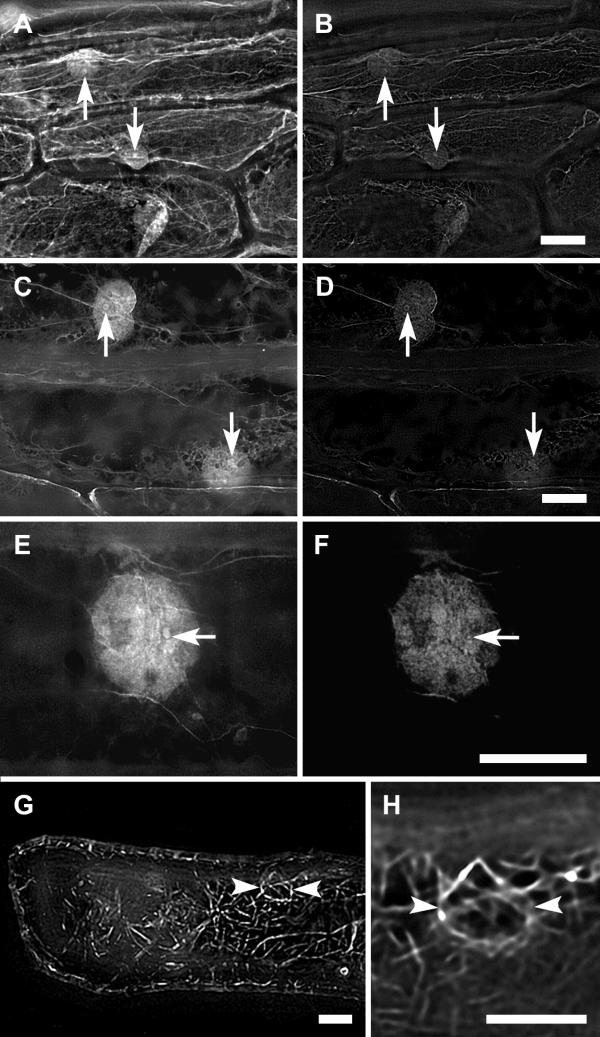
Labeling of microfilaments in onion bulb scale epidermis cells. Labeling of microfilaments in onion bulb scale epidermis cells with rhodamine-phalloidin by freeze shattering (A-B, E-F) and enzyme-mediated permeabilization (C-D), and with GFP-talin (H-G). Sum projections of 90 optical slices (A, C, E, G), and single optical slices through the central region of nuclei (B, D, F, H). Arrows, the spot-like F-actin structures in the nuclei; arrowheads, the nuclear basket. Bars, 20 μm.
GFP-talin decorated microfilaments in the cytoplasm, including "nuclear basket", but no labeling was detected in the nuclei (Fig. 1G,1H).
Fluorescent actin was injected into cytoplasm-rich regions of onion bulb scale epidermis cells: perinuclear area or the corner of the cell (Fig. 2 and 3). The fluorescent label spread rapidly all over the cell's cytoplasm, and stained the nucleus strongly within several minutes after injection (Fig. 2E). The high concentration of fluorescent actin decreased relatively slowly from the injection site whereas virtually no dynamics of fluorescence could be observed in the nuclei (Fig. 2 and 3). The intrusion of actin into nucleus was not dependent on the region of injection; nuclei of cells injected to perinuclear area (Fig. 2) or to corner of the cell (Fig. 3) became fluorescent within a minute after start of the injections.
Figure 2.
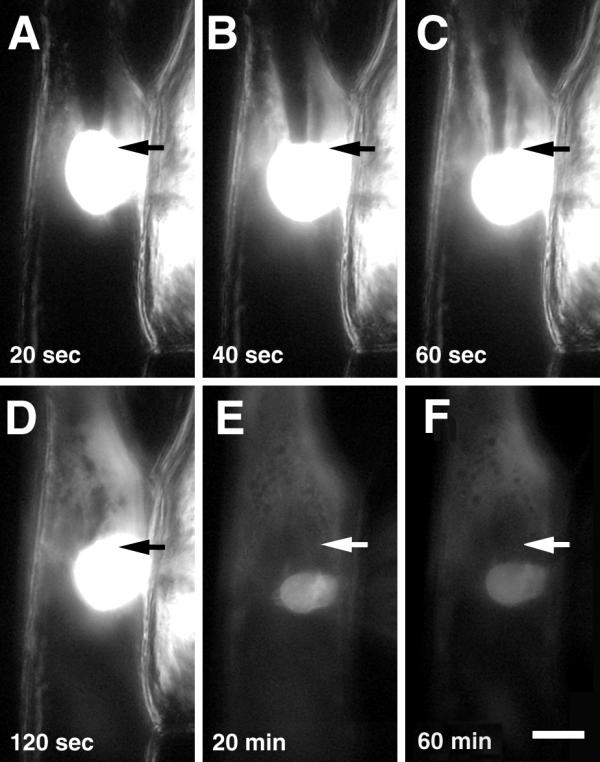
Microinjection of fluorescently labeled rabbit actin into onion bulb scale epidermis cell. A-D, capillary tip is in the cell; E, F, after removal of the capillary. Time: from start of the injection; arrows, the injection site as determined by phase contrast. Bar, 50 μm.
Figure 3.
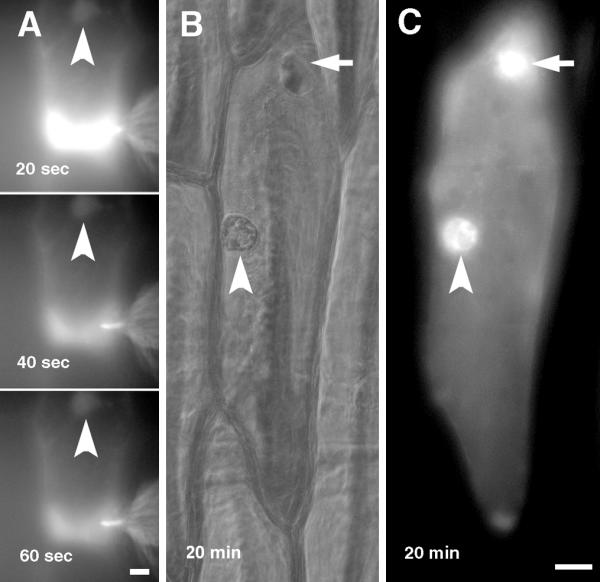
Microinjection of fluorescently labeled rabbit actin into onion bulb scale epidermis cell. A, Microinjection of fluorescently labeled rabbit actin into the corner of onion bulb scale epidermis cell. B, C, An injected cell after removal of injection capillary. B, phase contrast, C, fluorescence. Time: from start of the injection; arrows, the injection site; arrowheads, the nucleus. Bars, 20 μm.
During the incubation of injected cells for 1 hour at room temperature the label of microinjected actin did not show qualitative changes of the distribution (Fig. 2F). Then the cells were fixed and stained with phalloidin to monitor the incorporation of injected actin to microfilaments. After fixation, permeabilization, and washing diffuse fluorescence of microinjected actin conjugate disappeared (Fig. 4), suggesting that G-actin was washed away during the procedures, and actin-Alexa Fluor® 488 remained in the cell as F-actin. The F-actin staining of injected cells was similar to staining of untreated cells (Fig. 1A,1B,1C,1D,1E,1F) suggesting that the injection of fluorescent actin has no effect on the structure of the actin cytoskeleton. Most microfilaments showed both green (injected actin, Fig. 4A) and red (phalloidin staining, Fig. 4B) labeling whereas all injected (green) actin was labeled with phalloidin (Fig. 4C). Some of large F-actin cables, mostly located in the periphery of the cells, were labeled only with phalloidin conjugate (Fig. 4D), indicating that the injected actin had not incorporated into those microfilaments. The nuclei and perinuclear area of injected cells were similarly labeled with green and red fluorochromes, resulting in a yellow colour on overlaid RGB image (Fig. 4E), showing that all microfilaments of this region had incorporated injected actin.
Figure 4.
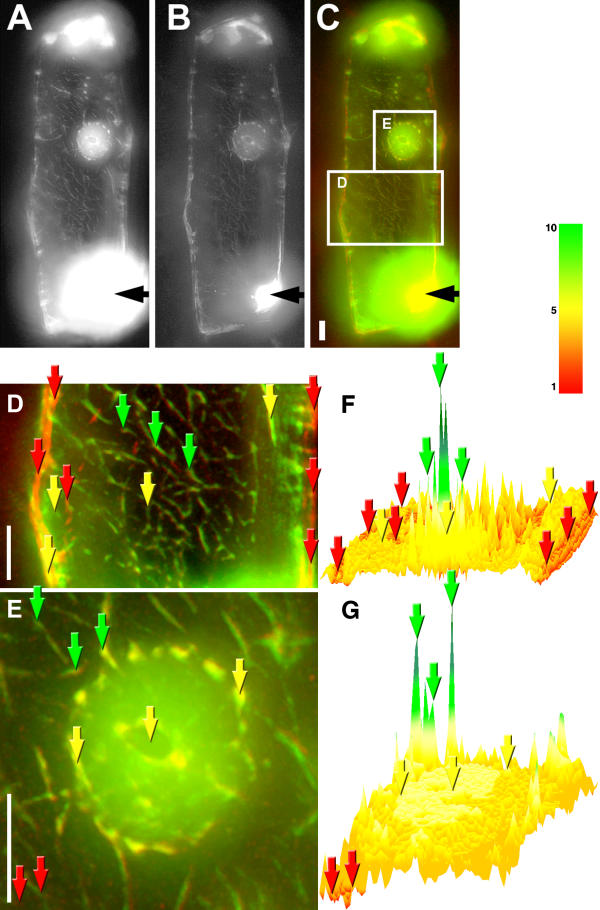
Ratio imaging of microinjected actin-Alexa Fluor® 488 label and rhodamine-phalloidin staining. Microinjected actin-Alexa Fluor® 488 label and phalloidin staining in an onion bulb scale epidermis cell 1 h after injection of actin-Alexa Fluor® 488 conjugate, single optical slice through the central region of nuclei after deconvolution of the images, A, Alexa Fluor® 488 label. B, Rhodamine-phalloidin staining. C, overlay of A (green) and B (red). D, Cytoplasm. E, Nucleus. F and G, ratio images of green/red signals from D and E, respectively. Black arrows, the injection site. Examples of points in the cell with high green/red ratio, green arrows; low green/red ratio, red arrows; medium green/red ratio, yellow arrows. Bars, 10 μm, color bar for F and G, relative value of green/red signals.
Ratio images of green (injected actin) and red (phalloidin staining) showed clearly that although the pictures of injected fluorescent mammalian actin and phalloidin staining were similar at first sight, the injected actin formed large microfilament bundles with relatively low phalloidin signal in the cytoplasm of injected cell (Fig. 4F, green arrows) whereas part of microfilaments showed strong phalloidin staining but contained very little injected actin (Fig. 4F, red arrows). Only few microfilaments in the cytoplasm showed equal green and red signal, resulting a medium value of injected actin/phalloidin signal ratio (Fig. 4F, yellow arrows).
In the nucleus the ratio of green/red signals was almost equally distributed, and had intermediate value (Fig. 4G, yellow arrows) between high and low values around the nucleus. Although the nucleus contained some fibrillar structures, and elements of the "nuclear basket" of microfilaments were visible around the nucleus (Fig. 4E), those structures were not reflected on the ratio image (Fig. 4G).
Discussion
Different methods were applied to visualize plant microfilaments in the current work. The major difference between labeling of microfilaments in living cells with GFP-talin hybrid protein and phalloidin staining of fixed cells was the absence of fluorescence in nuclei of living cells. The authors of GFP-mouse talin fusion protein observed weak, diffuse labeling in the nuclei of tobacco BY-2 cells [7], whereas our results show diffuse but strong phalloidin staining in the nuclei. This difference may be related to binding of GFP-talin to microfilament bundles whereas phalloidin stains all F-actin filaments in fixed cells. Also, the relatively high molecular weight of GFP-talin hybrid protein may affect its nuclear intrusion.
A semiquantitative study was carried out to analyze the ability of mammalian actin to incorporate into plant microfilaments in living cells. Microinjected fluorescent actin and sequential phalloidin staining showed a similar but not identical picture in the injected cell. Ratio imaging of actin-Alexa Fluor® 488 conjugate and rhodamine-phalloidin signals was applied to reveal the rate of incorporation of exogenous actin into existing microfilaments. Although phallotoxins may bind to mammalian and plant F-actins with different affinity there is no reason to believe that a part of microfilaments remained unstained under current experimental conditions – therefore, the phalloidin staining was considered as labeling of all F-actin filaments in the injected cell. An exception may be short filaments of cortical actin that have proven difficult to preserve and image [13].
The ratio image of an injected cell showed that a part of plant microfilaments did not incorporate exogenous actin or the incorporation rate was not detectable (red filaments), and another part of filaments was newly formed from microinjected actin (green filaments). The green color on the ratio image showed that these filaments were mainly composed of injected actin because the ratio of green/red signals was high compared to the ratio of signals from pre-existing phalloidin-stained filaments (red filaments on the ratio image).
The existence of intermediate values (yellow) of phalloidin/injected actin ratio (mostly in the nuclear region of the cell) indicates that a part of endogenous F-actin had incorporated microinjected fluorescent actin. Phalloidin staining of non-injected cells showed that F-actin was present in the nuclei of onion bulb scale epidermis cells, suggesting that the intermediate green/red ratio in the nuclear region of the injected cell arose because exogenous actin intruded into the nucleus.
There is also a possibility that part of the injected actin did not form F-actin or incorporate into existing microfilaments but attached to existing microfilaments. However, the phalloidin staining confirmed the colocalization of F-actin and injected actin, suggesting that all the actin-Alexa Fluor® 488 conjugate that was visible after fixation and permeabilization of the cell had formed F-actin filaments. The current work that was based on observations using fluorescence microscopy cannot also exclude the possibility that part of newly formed F-actin filaments were colocalized with existing microfilaments through actin filament-crosslinking proteins, e.g. fimbrin [8,14]. However, all known plant actin filament-crosslinking proteins do not decorate microfilaments differentially, and therefore this kind of crosslinking could not explain the different green/red ratio values in the cytoplasm.
We could not observe dynamics and mobility of fluorescent filaments in the microinjected cells. This observation is consistent with the earlier findings that fluorescence analogue cytochemistry with mammalian actin does not work in plant cells [9,10]. Also, it puts under doubt the functionality of the chimeric rabbit-plant microfilaments. However, we did not observe toxic effects of microinjected fluorescent actin. Cytoplasmic streaming was going on until fixation of an injected cell, although it was impossible to determine the origin of the tracks of streaming. The injections did not alter positioning of nuclei. Thus, the microfilament system of the injected cells did not show obvious failures but it remains unclear whether mammalian actin coexisted with plant microfilaments or functioned as a part of these filaments. The lack of dynamics and mobility of visualized microfilaments in living onion cells may be related to differences of nucleotide exchange rate of mammalian and plant actins [15] that may modulate the behavior of microfilaments.
Previous attemps to apply analogue cytochemistry with fluorescently labeled mammalian actin in plant cells have failed because rabbit muscle actin appeared to poison living plant cells [9,10] whereas maize pollen actin have worked perfectly at the same needle concentration. In the current work, actin-Alexa Fluor® 488 conjugate was used because it has significantly better photostability and brighter fluorescence than classical fluorescein or rhodamine derivates. Application of this conjugate made possible to observe the injected actin keeping the needle concentration as low as 5 μM (that is 13 times lower than 65 μM [10]) to avoid potential toxic effects. Also, the injection volume was kept to minimum using manual oil injector. These precautions were most likely crucial for microinjection of mammalian actin into plant cells, although we cannot exclude the possibility that onion bulb scale epidermis is simply better model system for these experiments. The results suggest that the toxic effect of mammalian actin to plant cells may be concentration-dependent, and also could be avoided by selection of model system.
The microinjected fluorescent actin was rapidly (within seconds) transported into the nucleus, and the incorporation of injected G-actin into plant F-actin filaments occurred preferentially in this region. Although the images of injected fluorescent actin (that showed the location of injected actin), and phalloidin staining (that showed the location of both endogenous and newly formed F-actin filaments) were qualitatively very similar, ratio imaging revealed significant differences in the incorporation of injected actin between the nucleus and the cytoplasm of the injected cell.
The presence of actin in plant cell nucleus has been shown immunocytochemically and by phalloidin staining [16,17]. Collings with co-workers [17] showed also the localization of F-actin in nuclear grooves and invaginations, suggesting its possible role in nuclear transport. Although transport of actin through nuclear pores has not been directly shown in plant cells, the intrusion of microinjected actin into the nucleus was not an unexpected result, considering the high concentration and relatively low molecular weight (around 43 kD) of actin-Alexa Fluor® 488 conjugate. However, the appearance of relatively bright fluorescence in the nucleus compared to the fluorescence in cytoplasm in a short time after the start of the injections suggests that the intrusion of actin into nucleus is not simply a concentration-dependent diffusion through nuclear pores. Although the current study did not reveal the dynamics of actin incorporation into plant F-actin in the nuclei, the sequential phalloidin staining and ratio imaging showed that injected mammalian actin had incorporated preferentially in the nucleus. The incorporation into F-actin may be the reason why the nucleus of the injected cell stained rapidly and permanently with the label of injected actin, although amino acid sequence of actin from rabbit skeletal muscle [18] contains nuclear export signal identical to the signal that has been found from plants [19]. The dynamics of incorporation of G-actin into F-actin is related to the structure of actin isoforms. Differential expression of actin isoforms in plant tissues has been studied [20] but nothing is known about their subcellular localization. Therefore, the data that are currently available do not allow even speculations about the molecular basis of preferential incorporation of mammalian actin in plant cell's nucleus.
The current work shows that mammalian actin is able to incorporate into microfilaments in living plant cells, particularly in the nucleus. The functions of nuclear microfilaments are poorly understood but they may have fundamental importance in the light of a work that identified nuclear myosin interacting with RNA polymerase II [21]. However, the reasons and mechanisms of the differential incorporation of mammalian actin into plant microfilaments remain to be elucidated before the application of fluorescently labeled actin as a tool in studies on the plant cytoskeleton.
Conclusions
Microinjected mammalian actin is able to enter plant cell's nucleus, whereas incorporation of mammalian actin into plant F-actin occurs preferentially in the nucleus and perinuclear area.
Methods
F-actin staining with rhodamine-phalloidin (Molecular Probes, USA) of onion bulb scale epidermis cells by freeze shattering and enzyme-mediated permeabilization were performed as described [22].
For GFP-talin expression, the epidermal cell layers of onion bulbs were placed inside up on a layer of 2% agarose. Cells were transformed with GFP-mouse talin fusion cDNA [7] biolistically using PDS – 100/He Biolistic® device (BioRad, USA), 1,0 μm gold particles and 1300 psi rupture discs. After bombardment the tissues were incubated in the dark for 24–48 h at room temperature.
For microinjection and subsequent rhodamine staining, rounded piece of onion bulb scale epidermis was placed into Attofluor® cell chamber (Molecular Probes, USA) on a glass coverslip, leaving a layer of 2% agarose between the coverslip and the tissue but not on top of the epidermis. The tissue was attached to the cell chamber using a slice of silicone tubing. Actin from rabbit muscle, Alexa Fluor® 488 conjugate (Molecular Probes, USA) was diluted in G buffer (5 mM Tris, pH 8.1, 0.2 mM CaCl2, 0.2 mM dithiothreitol, 0.2 mM ATP) according to manufacturer's instruction to concentration of 0.2 mg/ml (5 μM needle concentration), centrifuged 14,000 rpm at room temperature, and injected into 10 onion bulb scale epidermis cells cytoplasm with Eppendorf (Germany) Cell Tram Oil 5176. The injection capillary was held in the cell for at least 20 minutes, and viability of injected cells was controlled by monitoring cytoplasmic streaming during the microinjections. Alexa Fluor® 488 was visualized with an Olympus IMT-2 inverted microscope with reflected light fluorescence attachment using XF-100 filter set (Omega Optical, Inc., USA) and 40 × long working distance objective during the injection, and 40 × oil objective, NA 1.3 after phalloidin staining. Recording was performed by digital CCD camera (CF 8/1 DX, KAPPA, Germany) and KAPPA ImageBase Time software that was driving electronic shutters (Applied Scientific Instruments, USA) during the injections, or Z-stepper during the imaging of fixed and stained cells.
The course of the movements of fluorescent actin in the injected cells was monitored for 1 hour, then the epidermis was stained in the cell chamber with rhodamine-phalloidin 6 units/ml in 2 mM Tris pH 8.0, 0.1% saponin, 2% paraformaldehyde (EM grade, Electron Microscopy Sciences, USA) for 1 hour, and washed in 2 mM Tris pH 8.0 for 30 minutes. Phalloidin staining was visualized with standard longpass filter for rhodamine. AutoDeblur 9.1 (AutoQuant Imaging, Inc., USA) 3D deconvolution was used for 90–120 optical slices (0.15 μM Z-step) of each cell.
Ratio images of green/red signals were constructed from 10-pixel-wide (0.3 μm and 0.2 μm for cytoplasmic and nuclear regions, respectively) stripes of images using ImageQuaNT software (Molecular Dynamics, USA). Numeric values of 10-pixels parts along the stripes were averaged before calculation of ratio images to eliminate the camera noise.
Authors' contributions
HP carried out the microinjection, microscopy, and image analysis. ET conceived of the study, and participated in its design and coordination. Both authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The work was supported by Estonian Science Foundation Grants 4220 and 4932.
Contributor Information
Heiti Paves, Email: heiti@kbfi.ee.
Erkki Truve, Email: erkki@kbfi.ee.
References
- Doolittle RF. Reconstructing history with amino acid sequences. Protein Sci. 1992;1:191–200. doi: 10.1002/pro.5560010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Vandekerckhove J, Ampe C. Structural modules in actin-binding proteins: towards a new classification. Biochim Biophys Acta. 1999;1448:323–348. doi: 10.1016/S0167-4889(98)00152-9. [DOI] [PubMed] [Google Scholar]
- Mortara RA. Studies on trypanosomatid actin. I. Immunochemical and biochemical identification. J Protozool. 1989;36:8–13. doi: 10.1111/j.1550-7408.1989.tb02666.x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J, Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978;126:783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- Zhang D, Wadsworth P, Hepler PK. Microtubule dynamics in living dividing plant cells: confocal imaging of microinjected fluorescent brain tubulin. Proc Natl Acad Sci U S A. 1990;87:8820–8824. doi: 10.1073/pnas.87.22.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl U, Zhang D, Hepler PK. Actin microfilaments are associated with the migrating nucleus and the cell cortex in the green alga Micrasterias. Studies on living cells. J Cell Sci. 1994;107:1929–1934. doi: 10.1242/jcs.107.7.1929. [DOI] [PubMed] [Google Scholar]
- Kost B, Spielhofer P, Chua NH. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 1998;16:393–401. doi: 10.1046/j.1365-313x.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- Kovar DR, Gibbon BC, McCurdy DW, Staiger CJ. Fluorescently-labeled fimbrin decorates a dynamic actin filament network in live plant cells. Planta. 2001;213:390–395. doi: 10.1007/s004250000494. [DOI] [PubMed] [Google Scholar]
- Hepler PK, Cleary AL, Gunning BES, Wadsworth P, Wasteneys GO, Zhang DH. Cytoskeletal dynamics in living plant cells. Cell Biol Int. 1993;17:127–142. doi: 10.1006/cbir.1993.1050. [DOI] [Google Scholar]
- Ren H, Gibbon BC, Ashworth SL, Sherman DM, Yuan M, Staiger CJ. Actin Purified from Maize Pollen Functions in Living Plant Cells. Plant Cell. 1997;9:1445–1457. doi: 10.1105/tpc.9.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger CJ, Hussey PJ. Actin and actin-modulating proteins. In: Hussey PJ, editor. Annual Plant Reviews. Vol. 10. 2004. pp. 32–80. [Google Scholar]
- de Lanerolle P, Cole AB. Cytoskeletal proteins and gene regulation: form, function, and signal transduction in the nucleus. Sci STKE. 2002;139:PE30. doi: 10.1126/stke.2002.139.pe30. [DOI] [PubMed] [Google Scholar]
- Traas JA, Doonan JH, Rawlins DJ, Shaw PJ, Watts J, Lloyd CW. An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J Cell Biol. 1987;105:387–395. doi: 10.1083/jcb.105.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Drobak BK, Staiger CJ. Maize profilin isoforms are functionally distinct. Plant Cell. 2000;12:583–598. doi: 10.1105/tpc.12.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar David R., Yang Pinfen, Sale Winfield S., Drobak Bjorn K., Staiger Christopher J. Chlamydomonas reinhardtii produces a profilin with unusual biochemical properties. J Cell Sci. 2001;114:4293–4305. doi: 10.1242/jcs.114.23.4293. [DOI] [PubMed] [Google Scholar]
- Skubatz H, Orellana MV, Yablonka-Reuveni Z. Cytochemical evidence for the presence of actin in the nucleus of the voodoo lily appendix. Histochem J. 2000;32:467–474. doi: 10.1023/A:1004140215519. [DOI] [PubMed] [Google Scholar]
- Collings DA, Carter CN, Rink JC, Scott AC, Wyatt SE, Allen NS. Plant nuclei can contain extensive grooves and invaginations. Plant Cell. 2000;12:2425–2440. doi: 10.1105/tpc.12.12.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JH, Elzinga M. The primary structure of actin from rabbit skeletal muscle. Completion and analysis of the amino acid sequence. J Biol Chem. 1975;250:5915–5920. [PubMed] [Google Scholar]
- Haasen D, Kohler C, Neuhaus G, Merkle T. Nuclear export of proteins in plants: AtXPO1 is the export receptor for leucine-rich nuclear export signals in Arabidopsis thaliana. Plant J. 1999;20:695–705. doi: 10.1046/j.1365-313X.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- Meagher RB, McKinney EC, Kandasamy MK. Isovariant dynamics expand and buffer the responses of complex systems: the diverse plant actin gene family. Plant Cell. 1999;11:995–1006. doi: 10.1105/tpc.11.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestic-Dragovich L, Stojiljkovic L, Philimonenko AA, Nowak G, Ke Y, Settlage RE, Shabanowitz J, Hunt DF, Hozak P, de Lanerolle P. A myosin I isoform in the nucleus. Science. 2000;290:337–341. doi: 10.1126/science.290.5490.337. [DOI] [PubMed] [Google Scholar]
- Wasteneys GO, Willingale-Theune J, Menzel D. Freeze shattering: a simple and effective method for permeabilizing higher plant cell walls. J Microsc. 1997;188 ( Pt 1):51–61. doi: 10.1046/j.1365-2818.1977.2390796.x. [DOI] [PubMed] [Google Scholar]


