Abstract
The predominant distribution of γ δ T cells in the mucosal and epithelial tissues makes these unconventional lymphocytes the “guards” to contact external environment (like allergens) and to contribute to immune surveillance, as well as “vanguards” to participate in initiating mucosal inflammation. Therefore, γ δ T cells have been considered to bridge the innate and adaptive immunity. The role these cells play in allergy seems to be complicated and meaningful, so it makes sense to review the characteristics and role of γ δ T cells in allergic diseases.
1. Introduction
γδ T cells are a minor population of lymphocytes expressing γ and δ T cell receptor (TCR) chains, which are often considered to bridge innate and adaptive immune responses. Recent studies have shown that γδ T cells can comprise up to 50% of the T cells within epithelium or mucosa-rich tissues and less than 10% in peripheral blood [1]. The specific localization and abundance of these cells suggest that they might be markedly implicated in epithelial/mucosal immunity [2, 3]. In contrast to recognition of antigens by αβ T cells, γδ T cells recognize antigens directly without any requirement for antigen processing and presentation or major histocompatibility complex (MHC) molecules [4]. It has been indicated that γδ T cells may play crucial roles in the development and perpetuation of allergic inflammation as effector and immunoregulatory cells, via production of T helper (Th)1-, Th2-, and Th17-associated cytokines [5], which not only induce the synthesis of IgE but also recruit effector cells like eosinophils and basophils into the site of allergic inflammation [6]. Besides, different subsets of γδ T cells can show different functions, depending on what tissue they are found in and which specific TCRs they bear [7]. Even though there is a growing consensus about the importance of these cells in allergic immune responses, the specific mechanisms remain elusive. The present review focuses on the latest knowledge on characteristics and role of γδ T cells in allergic diseases.
2. γ δ T Cells Have Diverse Subsets with Specific Locations and Functions
As research continues, it has been realized that γδ T cells are not a homogeneous population of cells with a single physiological role, and their subset complexity is being characterized, both in mice and humans [7]. TCR Vγ- and Vδ-encoded chain pairs may interact with distinct ligands in different tissues and be expanded on that basis. Defined by the usage of either Vδ1 or Vδ2 TCR (Vδ3 and Vδ5 making up minor populations), human γδ T cells fall into two major subsets: Vδ2 T cells account for the majority (50–95%) of circulating γδ T cells, whereas Vδ1 T cells are rare in the blood but appear at increased frequencies in mucosal tissues and in the skin [7–10].
The Vγ9Vδ2 (also termed Vγ2Vδ2, collectively designated Vδ2) T cells in the peripheral blood can sometimes identify over 50% of leucocytes after certain bacterial or parasitic infections and rapidly get activated; therefore, such TCR-dependent activation of Vγ9Vδ2 T cells enables them to respond to a diverse range of pathogens [11]. According to the surface expression of CD45RA and CD27, markers more commonly used to identify the naive, effector, or memory status of conventional γδ T cells, human Vγ9Vδ2 T cells, are often subdivided into four subsets: “naive” (Tnaive) CD45RA+CD27+ cells; “central memory” (TCM) CD45RA−CD27+ cells; “effector memory” (TEM) CD45RA−CD27− cells; “CD45RA+ effector memory” (TEMRA) CD45RA+CD27− cells [7, 12]. Tnaive and TCM cells express lymph node homing receptors, abound in lymph nodes, and lack immediate effector functions. Conversely, TEM and TEMRA cells, which express receptors for homing to inflamed tissues, are poorly represented in the lymph nodes while abounding in sites of inflammation and display immediate effector functions. It indicates a lineage differentiation pattern for human Vδ2 T cells that generates naive cells circulating in lymph nodes, effector/memory cells patrolling the blood, and terminally differentiated effector cells residing in inflamed tissues [12].
In contrast to Vδ2, the TCR-γ chain usage by the tissue-associated Vδ1 T cells varies at distinct anatomic locations. Vδ1 T cells in the periphery express a naive phenotype and may migrate preferentially to localized sites when they are activated [13]. For instance, Vγ2, Vγ3, Vγ5, Vγ6, and Vγ7 are used predominantly by γδ T cells in peripheral lymphoid organs, skin, small intestine, tongue, and reproductive system, respectively [14, 15]. In contrast, Vγ1 and Vγ4 are preferentially expressed in the respiratory system like nasal mucosa and lung [16–18]. Recent studies have indicated that these tissue-associated Vδ1 T cells may play an important function not only in maintaining immune homeostasis in the local microenvironment [19] but also in wound healing, removing distressed or transformed epithelial cells and subduing excessive inflammation, in both mice and humans [20–22]. Besides, the role of these mucosa predominantly expressed γδ T cells in allergic diseases has also been noticed. Our preliminary studies found that the infiltration of γδ T cells significantly increases in the nasal mucosa of patients with perennial allergic rhinitis (AR) (data not shown). Moreover, Pawankar et al. [23] proved that the increased population of γδ T cells in the perennial AR patients' nasal mucosa mainly comprises of Vγ1Vδ1 subsets. In mice sensitized and challenged with OVA, Cook et al. [24] observed that Vγ1+ T cells spontaneously enhance airway hyperresponsiveness (AHR), whereas Vγ4+ T cells, after being induced by allergen sensitization and challenge, suppress AHR. These data suggest that γδ T cells of distinct phenotypes may play different, sometimes opposed, functions in airway allergic inflammation. However, it is still premature to speculate whether the Vγ4+ subsets of γδ T cells may exert an important role in maintaining immune homeostasis in local microenvironment of healthy humans; on the other hand, the Vγ1+ subsets may take an essential part in the development and perpetuation of allergic inflammation as effectors in atopy patients. Besides, our another recent study showed that different subsets of γδ T cells in peripheral blood of perennial AR patients before and after specific immunotherapy (SIT) appear with distinct expression patterns [25]. But whether γδ T cells in peripheral blood and in mucosa function separately or synergistically remains an unsolved problem.
3. γ δ T Cells in Different Age and Gender Groups
With age, there comes the change from having fairly diverse pairs of γδ T cells (of which Vδ1+ subsets serve as the majority in cord blood at birth) to increasingly restricted pairings (with Vγ9Vδ2 T cells becoming the major subsets with very limited receptor diversity by adulthood). From birth to about 10 years of age, the absolute number of γδ T cells in the periphery increases, with the Vγ9Vδ2 T cell subsets expanding from a minor population at birth to usually more than 75% of circulating γδ T cells [13, 26]. Vγ9Vδ2 T cells are known to respond to many different phosphoantigens, so it is likely that the exposure to a variety of pathogens results in the selection of these cells in early life [27]. This clonal expansion has been seen as evidence of the vital role these T cells play in responding to environmental challenges in early life. However, it has not been demonstrated whether this phenomenon is in fact primary in response to environmental challenges but not, at least in part, endogenous stimuli, as an extension of the adaptive changes taking place within the newborn [10].
Previous longitudinal cohort studies have shown that most childhood asthma begins in infancy, and between 40% and 75% of children with asthma will have complete resolution of symptoms by adolescence or adulthood [28]. Respiratory syncytial virus (RSV) infection in lower respiratory tract in early childhood is a risk factor for the subsequent development of allergic sensitization such as wheezing up to age of 11 years [29]. Aoyagi et al. [30] reported that compared to age-matched controls, infants affected by RSV-bronchiolitis have lower frequencies of IFN-γ-producing γδ T cells in peripheral blood. Moreover, they noticed normalization of this frequency during the convalescent phase, suggesting that the defective IFN-γ production by these cells may play an important role in the development of asthma. However, it is too early to conclude whether the expansion of Vδ2Vγ9 T cells with age is associated with childhood asthma spontaneous remission by adolescence.
In adulthood, studies have also found the possible great impact of age and gender on the γδ T cell repertoires: in contrast to childhood, the absolute number of γδ T cells decreases, as the result of reduction of Vδ2, but not Vδ1 T cells. Besides, the number of total γδ T cells and Vδ2 T cells are both significantly higher in males than in females [31, 32]. It indicates that age- and gender-matched controls are essential for clinical studies of γδ T cell repertoires in patients.
The term “allergic march” refers to the natural history of atopic manifestations, which is characterized by a typical sequence of IgE antibody responses and clinical symptoms that appear early in life, persist over years or decades, and often remit spontaneously with age. Several studies have shown that the “new” allergy can occur throughout life; generally, allergy prevalence and severity tend to decrease after young adult life [33], and Th2-type responses may weaken with age [34]. Hansen et al. [35] found that immunization dose, sex, and age are highly influential on allergy outcomes in murine models. Nevertheless, further researches are required to make certain whether the change of γδ T cell subsets is associated with the age and gender related allergic march.
4. The Antigen Recognition of γ δ T Cells in Allergy
Selective allergen recognition by TCR that binds specific regions of the antigen molecules is the priming and initiation of antigen-specific T cell immune responses. So far, more than 4000 substances in the environment, the vast majority of which are proteins, mostly enzymes, have been identified as allergens that elicit an IgE-mediated immune response in a genetically predisposed individual. Different from MHC-restricted recognition of bound peptides by αβ TCRs, the antigen specificity of γδ T cells involves the immunoglobulin-like structure of the γδ TCR [36] with the recognition of unprocessed peptides, small organic phosphate molecules, or alkylamines derived from microbes and edible plants [37].
Studies have shown that the epithelial-associated γδ T cells can recognize stress-induced self-antigens, which enables them to monitor multiple insults to the epithelium [38]. However, data from humans and mice seem to indicate the relevance of mucosa-associated γδ T cells in allergen recognition and airway inflammation, perhaps mediated by interaction of foreign antigens with CD1+ dendritic cells (DCs) [39]. The study by Russano et al. [40] showed that CD1+ immature DCs expand in the respiratory mucosa of allergic subjects and are able to process both proteins and lipids, and CD1-restricted phospholipids (PL)-specific γδ T cells represent the key mucosal regulatory subsets for the control of early host reactivity against tree pollens. These CD1-restricted γδ T cells can respond promptly to lipid-antigen recognition by secreting a wide array of cytokines, including high amounts of IL-4, and expand at mucosal allergic inflammation sites [38]. In addition, γδ T cells derived from nasal mucosa in allergic subjects could also recognize pollen derived PE in a CD1d-restricted fashion [40]. Therefore, it may be speculated from above that the early allergic response initiates with primary mucosal recognition of allergen by CD1+ DCs and CD1-restricted γδ T cells, which ensure rapid handling of the foreign inhaled grain.
5. The Cytokine Production of γ δ T Cells in Allergy
It is proposed that γδ T cells may act as an extended arm of αβT cells, by providing a rapid but weaker response before the αβ T-cell response has fully developed. Substantial evidence has been accumulated to indicate that γδ T cells have the potential to produce Th17-type cytokines (like IL-17) and Th2-type cytokines (IL-4, -5, and -13) and thus enhance airway allergic inflammation and AHR [41]. In contrast, Th1-type cytokines (IL-12 and IFN-γ) produced by γδ T cells might be induced after special immunotherapy and inhibition of these allergic diseases [42, 43].
The study by Ribot et al. [44] showed that in the spleen and lymph nodes and in the peripheral tissues of mice, the presence or absence of the cytokine CD27 distinguishes two γδ T cell subsets: the CD27+ cells produce IFN-γ, whereas the CD27− cells (50% of Vγ4+ and 11% of Vγ1+) produce primarily IL-17. It suggests the existence of a differential requirement for optimum activation of these distinct γδ T cell subsets in the peripheral immune compartment. In humans, 80% of circulating Vγ9Vδ2 T cells are IFN-γ producers, while less than 1% produce IL-17 [45]. Caccamo et al. [46] observed that IFN-γ + Vγ9Vδ2 T cells have a predominant TEM and at a lower extent TEMRA phenotype, while IL-17+ Vγ9Vδ2 T cells exhibit a TEMRA phenotype. However, IL-17+ Vγ9Vδ2 T cells significantly increase and secrete abundant IL-17 at sites of inflammation (perhaps primarily at epithelial surfaces), which may directly shape the inflammatory infiltrate, for example, by attracting neutrophils during bacterial infection [45]. Zhao et al. [47] also found that the IFN-γ +/IL-17+ ratio in γδ T cells significantly decreases in patients with allergic asthma compared with healthy controls. What is more, several γδ T cells are indicated to be a chief source of IL-17 in peripheral tissues such as lung, which share certain common features with Th17 cells [48–50]. With regard to humans, γδ T cells are divided into two different phenotypes. An IL-4-producing phenotype, which possesses Vγ1/Vδ1 segments, enhances allergic inflammation. Vγ9/Vδ2 segments have an IFN-γ-producing phenotype and might thus have a partial ability to modulate allergen-specific Th2-skewed immunity [43].
In a word, after quick antigen recognition and full activation, diverse subsets of γδ T cells in the circulating blood and various lymphoid compartments subsequently produce an array of different cytokines, performing proinflammation as well as pleiotropic immunoregulatory functions, as it will be further discussed.
6. γ δ T Cells Are Both Effector and Regulatory Cells in Allergic Inflammation
Lately, Kalyan and Kabelitz [10] provided a biographical sketch of γδ T cells: in a continuum, innate natural killer (NK) cells and adaptive αβ T cells respond to the “missing self” and the “dangerous nonself,” respectively, while γδ T cells respond to the “safe nonself” and deal with the inevitable “distressed self.” What is more, NK cells could contribute to responding to the “distressed self,” whereas αβ T cells have some regulatory training to temper the response to the “safe nonself” [10] (Figure 1). This sketch supports the viewpoint that γδ T cells serve as the bridge between innate and adaptive immunity. Allergy is primarily considered as a classic Th2-driven immune response against allergens (safe nonself), with important contributions to pathology by Th2-type cytokines IL-4, -5, and -13, which not only induce the synthesis of IgE but also recruit effector cells like eosinophils and basophils into the site of allergic inflammation [42]. However, inflammatory responses in allergic diseases are more complex than simple overexpression of Th2 cytokines. A recent hypothesis has been put forward to rely on the genetically determined barrier deficiency and disruption by environmental and endogenous proteases in the epithelial barrier (distressed self), which might result in the allergen uptake as a primary defect in the pathogenesis of allergic reactions [51]. It seems that allergy is both an epithelial disease and a disease of the immune system. Using an adoptive cell transfer approach, Jin et al. [52] found that NK and γδ T cells (only Vγ1Vδ5 subsets) are necessary for the acute stages of AHR in mice but not for the later airway eosinophilic inflammation. Another study on AHR demonstrated that NK cells secreted IL-4 and -13 to produce their effector function, but γδ T cells did not have this effect [53]. In addition, γδ T cells, similar to NK cells, express the NKG2D receptor that may contribute to effective stress-responses as well as immune surveillance, which may be relevant in the induction of food allergy [54]. These data suggest that the interaction of innate and adaptive immune cells and the impact of the inflammatory responses on this collaboration seem to be important and worthy of further research.
Figure 1.
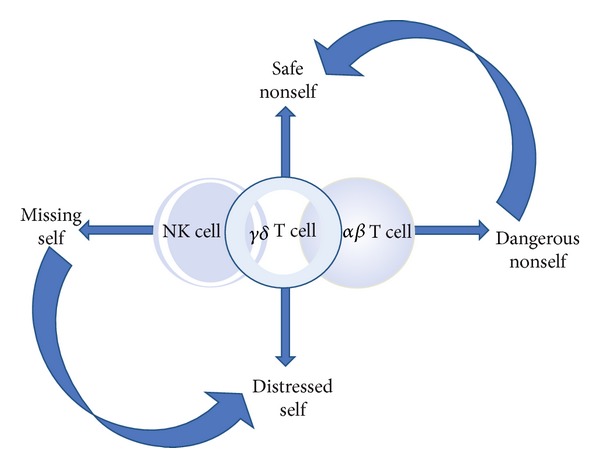
A simplified paradigm illustrating where in the continuum of immune protection and homeostasis γδ T cells fall in relation to innate NK cells and the adaptive αβT cells. Innate NK and adaptive αβ T cells respond to the “missing self” and the “dangerous nonself,” respectively, while, between these two extremes, γδ T cells respond to the “safe nonself” and deal with the inevitable “distressed self.” These different “selves” and the immune response(s) that they trigger exist in a continuum and are modulated by the context in which they are presented. Besides, NK cells could contribute to responding to the “distressed self,” whereas αβ T cells have some regulatory training to temper the response to the “safe nonself” (cited from [10]).
Abundant populations of γδ T cells have been found in the epidermis of rodents [55]. The respiratory mucosa such as nasal mucosa, bronchial mucosa, and lung contain γδ T cells as well [56]. This may be of importance in the remarkable resistance of the airway against environmental stimuli. Substantial evidence has been accumulated to indicate that γδ T cells take part in Th2 immune responses. γδ T cells themselves can not only take the function of follicular Th cells in certain responses but also can support responses that are dependent on classical help provided by αβ T cells. An increase in γδ T cells expressing Th2-type cytokines has been reported in bronchoalveolar lavage (BAL) fluids of allergen challenged asthmatic patients [57]. In addition to proinflammatory function, the γδ T cells also engage as regulators of Th2 immunity [41], in particular regulating the IgE antibodies [3, 38, 40]. Svensson et al. [58] showed that γδ T cell-deficient mice exhibited a diminished allergen specific IgE response compared with wild-type (WT) mice, indicating that γδ T cells contribute to B cell secretion of allergen-specific IgE, either by promoting Ig class switch to IgE or by providing activation signals to differentiated IgE-producing cells. Likewise, Zuany-Amorim et al. [59] reported a low antigen specific IgE and IL-5 release and a decrease in T cell infiltration in the same mouse models. They further found that the response could be restored when IL-4 was administered, suggesting that γδ T cells contribute to type 2-mediated airway inflammation by inducing IL-4 dependent IgE and IgG1 responses. In contrast, Lahn et al. [60] showed that γδ T cells exert a suppressive role in the Th2 response to allergen challenge. Therefore, it is clear that γδ T cells might have various, possibly opposing roles for CD4+ T cells.
Studies have demonstrated that, in the airway, distinct subsets of γδ T cells, defined by their expression of TCR-γ, seem to exhibit differential and sometimes opposed Th-like reactivities in allergen-induced allergic inflammation [7, 47]. In mouse models of allergic diseases, it has been shown that the Vγ1+ subsets can enhance AHR as well as levels of Th2 cytokines in the airways and eosinophilic infiltrates in the lungs [61, 62], and, in contrast, the Vγ4+ subset can be induced to inhibit AHR [63, 64]. Lahn et al. [64] selectively depleted either subset in the lungs (using aerosolized, inhaled anti-TCR Abs) following airway challenge and observed that AHR is altered in the predicted fashion; that is, depletion of Vγ1+ cells decreases and depletion of Vγ4+ cells increases AHR. After transferring few purified Vγ1+ cells into OVA/alum immunized TCR-δ −/− mice, Huang et al. [65] observed the increase of the OVA-specific IgE responses, suggesting that individual enhancer cells are quite potent. However, the relative importance of γδ T cells in human asthma remains to be determined.
It has been shown for some time that murine γδ T cells become functionally competent in the thymus, particularly regarding the production of proinflammatory cytokines IFN-γ and IL-17 [60]. McMenamin et al. [66] found that γδ T cells regulate IgE responsiveness to inhaled antigens by high production of IFN-γ. By using mice immunized with recombinant vaccine virus expressing RSV F protein and challenged with live RSV, Dodd et al. [63] reported that Vγ4+ subsets are recruited into the lungs and produce IFN-γ in a time-dependent manner. These studies suggest that antigen-specific γδ T cells are able to suppress the pathogenic Th2 response in allergic asthma, whereas a recent study by Chen et al. [67] found that the serum levels of IL-4 and IL-13 in peripheral blood of children with AR and asthma markedly decrease while IFN-γ increases after receiving SIT, suggesting that IFN-γ + γδ T cells might exert their Th2 immunosuppression under certain conditions like SIT. Our recent study showed that the serum levels of IL-17 and IL-23 in the AR patients were significantly higher than those in the healthy subjects, and positive correlations exist between the IL-17 and the IL-23 levels, as well as the IL-17 level and γδ T frequencies [68]. Accordingly, we conjecture that the IL-23R+ IL-17+ γδ T cells may promote αβ T cell-mediated traditional Th2 inflammation via producing abundant IL-17. In addition, IL-17-producing γδ T cells could directly promote the development of other IL-17-producing T cells [69], and these innate IL-17-producing T cells are involved in sensing stress, injury, or pathogens and serve an immunoregulatory role at epithelial sites [63].
Gonçalves-Sousa et al. [70] reported that murine CD4+CD25+Foxp3+ regulatory T cells (Treg) abolish key effector functions and proliferation of γδ T cells both in vitro and in vivo. They further showed that the suppression is dependent on cellular contact between Treg and γδ T cells and is partially mediated by glucocorticoid-induced TNF receptor-related proteins. It reveals a novel mechanism, by which γδ T-cell function is regulated, and suggests that endogenous Treg may prevent the desired effects of γδ T cell-based immunotherapies. It has also been shown by Hahn et al. [71] that γδ T cells affect the level of IL-10 in the airways and block their function resulting in an increase of Treg in the lung, which suggests that γδ T cells might inhibit Treg function. While these data highlight the importance of understanding how the proinflammatory and immunoregulatory functions of γδ T cells are regulated, the detailed processes remain poorly understood.
7. γ δ T Cells with the Prevention and Treatment of Allergic Diseases
γδ T cells stimulated with bisphosphonate compounds, which are clinically well tolerated and used for γδ T cell expanders in vitro and in vivo, have been considered to be good candidates for cancer immunotherapy, because of their IFN-γ production and cytotoxic effect [72]. Therefore, the adoptive transfer of autologous γδ T cells expanded in vitro might also be an effective strategy for IL-4-mediated allergy.
It has been shown that oral tolerance, which refers to the active state of nonresponsiveness to food and food protein intake, is a unique feature of the (gut-associated) mucosal immune system. And the defects in this process result in allergic sensitization to food proteins [73]. Most intestinal epithelial lymphocytes (IELs) in the mouse consist of γδ T cells, which are localized in the paracellular space between intestinal epithelial cells at the luminal site of the basement membrane [74]. Mengel et al. [75] showed that treating mice with a TCR-δ-specific antibody results in impaired oral tolerance induction and that oral tolerance could be transferred by means of γδ T cells. It suggests that targeting intestinal γδ T cells may provide preventing and therapeutic strategies for food allergy. However, currently IELs are among the least studied cells in the process of allergic sensitization.
Studies have shown that many phosphoantigens and fungal immunomodulators play an important role in γδ T cell-mediated immunotherapy. For example, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate is characterized as a very potent agonist of Vγ9Vδ2 responses and could strengthen the principle of γδ T cell based immunotherapy [76]. In contrast, Gonçalves-Sousa et al. [70] described that Treg could negatively modulate the γδ T cell activities and stressed the importance of combining Treg inhibition with γδ T cell activation for future immunotherapeutic strategies.
In animal models, chronic allergen challenge induces suppression of the Th2 response and reduces AHR and airway inflammation [77, 78]. Reductions of late-phase asthmatic responses to allergen after long-term allergen challenge have also been reported in clinical studies [79, 80]. Lahn et al. [64] indicated that the Vγ4+ subsets appear to mediate such suppressive effect of long-term allergen challenge on AHR. In addition, γδ T cells could also suppress Th2-dependent IgE responses without affecting parallel IgG responses to inhaled antigens [66].
Taken together, γδ T cells may have the potential to help alter the Th2-skewed immunity in patients with allergic diseases. Further accumulated studies to clarify the ability of γδ T cells as an allergic immunotherapy candidate are thus called for.
8. Concluding Remarks
There is ample evidence that γδ T cells are involved in allergy. Recent studies in humans and mice suggest that they can both drive and regulate allergic immune responses through different mechanisms. However, many aspects of the characteristics and role of γδ T cells in allergy remain to be fully elucidated in near future, for instance, the exact effects of various γδ T cell subsets on allergic inflammation; the underlying relations between blood- and mucosa-associated γδ T cells; how age and gender influence the population, distribution, and function of γδ T cells in allergic diseases; the specific regulatory mechanisms of γδ T cells in allergy; how γδ T cells could be applied to prevent and treat allergic diseases, and so on.
Acknowledgments
The study was supported by Grants from National Natural Science Foundation of China (no. 81371072) and Guangdong Natural Science Foundation (no. S2013010016386).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Kabelitz D, Wesch D, Hinz T. γδ T cells, their T cell receptor usage and role in human diseases. Springer Seminars in Immunopathology. 1999;21(1):55–75. [PubMed] [Google Scholar]
- 2.Jaffar Z, Ferrini ME, Herritt LA, Roberts K. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. Journal of Immunology. 2009;182(8):4507–4511. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jameson J, Witherden D, Havran WL. T-cell effector mechanisms: γδ and CD1d-restricted subsets. Current Opinion in Immunology. 2003;15(3):349–353. doi: 10.1016/s0952-7915(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 4.Shin S, El-Diwany R, Schaffert S, et al. Antigen recognition determinants of γδ T cell receptors. Science. 2005;308(5719):252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 5.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. Journal of Allergy and Clinical Immunology. 2009;123(5):986–994. doi: 10.1016/j.jaci.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Pawankar R. γδ T cells in allergic airway diseases. Clinical and Experimental Allergy. 2000;30(3):318–323. doi: 10.1046/j.1365-2222.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- 7.Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of γδ T-cell subsets in mouse and human. Immunology. 2012;136(3):283–290. doi: 10.1111/j.1365-2567.2012.03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holtmeier W, Pfander M, Hennemann A, Zollner TM, Kaufmann R, Caspary WF. The TCR δ repertoire in normal human skin is restricted and distinct from the TCR δ repertoire in the peripheral blood. Journal of Investigative Dermatology. 2001;116(2):275–280. doi: 10.1046/j.1523-1747.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 9.Halary F, Pitard V, Dlubek D, et al. Shared reactivity of Vδ2neg γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. Journal of Experimental Medicine. 2005;201(10):1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalyan S, Kabelitz D. Defining the nature of human γδ T cells: a biographical sketch of the highly empathetic. Cellular and Molecular Immunology. 2013;10(1):21–29. doi: 10.1038/cmi.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunological Reviews. 2007;215(1):59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 12.Dieli F, Poccia F, Lipp M, et al. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. Journal of Experimental Medicine. 2003;198(3):391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T ceel receptor γ/δ repertoire. Journal of Experimental Medicine. 1990;171(5):1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayday AC, Pennington DJ. Key factors in the organized chaos of early T cell development. Nature Immunology. 2007;8(2):137–144. doi: 10.1038/ni1436. [DOI] [PubMed] [Google Scholar]
- 15.Quertermous T, Strauss WM, Van Dongen JJM, Seidman JG. Human T cell γ chain joining regions and T-cell development. Journal of Immunology. 1987;138(8):2687–2690. [PubMed] [Google Scholar]
- 16.Itohara S, Farr AG, Lafaille JJ, et al. Homing of a γδ thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343(6260):754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 17.Kim C-H, Witherden DA, Havran WL. Characterization and TCR variable region gene use of mouse resident nasal γδ T lymphocytes. Journal of Leukocyte Biology. 2008;84(5):1259–1263. doi: 10.1189/jlb.0108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wands JM, Roark CL, Aydintug MK, et al. Distribution and leukocyte contacts of γδ T cells in the lung. Journal of Leukocyte Biology. 2005;78(5):1086–1096. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 19.Ismail AS, Severson KM, Vaishnava S, et al. γδ intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabelitz D, Marischen L, Oberg H-H, Holtmeier W, Wesch D. Epithelial defence by γδ T cells. International Archives of Allergy and Immunology. 2005;137(1):73–81. doi: 10.1159/000085107. [DOI] [PubMed] [Google Scholar]
- 21.Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and γδ intraepithelial lymphocytes during mucosal injury. Journal of Immunology. 2009;182(5):3047–3054. doi: 10.4049/jimmunol.0802705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke NR, Stankovic S, Funda DP, Harrison LC. TCRγδ intraepithelial lymphocytes are required for self-tolerance. Journal of Immunology. 2006;176(11):6553–6559. doi: 10.4049/jimmunol.176.11.6553. [DOI] [PubMed] [Google Scholar]
- 23.Pawankar RU, Okuda M, Suzuki K, Okumura K, Ra C. Phenotypic and molecular characteristics of nasal mucosal γδ T cells in allergic and infectious rhinitis. American Journal of Respiratory and Critical Care Medicine. 1996;153(5):1655–1665. doi: 10.1164/ajrccm.153.5.8630617. [DOI] [PubMed] [Google Scholar]
- 24.Cook L, Miyahara N, Jin N, et al. Evidence that CD8 + dendritic cells enable the development of γδ T cells that modulate airway hyperresponsiveness. Journal of Immunology. 2008;181(1):309–319. doi: 10.4049/jimmunol.181.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng R, Wu X, Huang X, et al. Gene expression pattern of Treg and TCR Vgamma subfamily T cells before and after specific immunotherapy in allergic rhinitis. Journal of Translational Medicine. 2014;12(1):p. 24. doi: 10.1186/1479-5876-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McVay LD, Carding SR. Generation of human γδ T-cell repertoires. Critical Reviews in Immunology. 1999;19(5-6):431–460. [PubMed] [Google Scholar]
- 27.De Rosa SC, Andrus JP, Perfetto SP, et al. Ontogeny of γδ T cells in humans. Journal of Immunology. 2004;172(3):1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 28.Koh MS, Irving LB. The natural history of asthma from childhood to adulthood. International Journal of Clinical Practice. 2007;61(8):1371–1374. doi: 10.1111/j.1742-1241.2007.01426.x. [DOI] [PubMed] [Google Scholar]
- 29.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 30.Aoyagi M, Shimojo N, Sekine K, Nishimuta T, Kohno Y. Respiratory syncytial virus infection suppresses IFN-γ production of γδ T cells. Clinical and Experimental Immunology. 2003;131(2):312–317. doi: 10.1046/j.1365-2249.2003.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michishita Y, Hirokawa M, Guo Y-M, et al. Age-associated alteration of γδ T-cell repertoire and different profiles of activation-induced death of Vδ1 and Vδ2 T cells. International Journal of Hematology. 2011;94(3):230–240. doi: 10.1007/s12185-011-0907-7. [DOI] [PubMed] [Google Scholar]
- 32.Caccamo N, Dieli F, Wesch D, Jomaa H, Eberl M. Sex-specific phenotypical and functional differences in peripheral human Vγ9/Vδ2 T cells. Journal of Leukocyte Biology. 2006;79(4):663–666. doi: 10.1189/jlb.1105640. [DOI] [PubMed] [Google Scholar]
- 33.Dahl R, Andersen PS, Chivato T, Valovirta E, de Monchy J. National prevalence of respiratory allergic disorders. Respiratory Medicine. 2004;98(5):398–403. doi: 10.1016/j.rmed.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Smith P, Dunne DW, Fallon PG. Defective in vivo induction of functional type 2 cytokine responses in aged mice. European Journal of Immunology. 2001;31(5):1495–1502. doi: 10.1002/1521-4141(200105)31:5<1495::AID-IMMU1495>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Hansen JS, Alberg T, Rasmussen H, Lovik M, Nygaard UC. Determinants of experimental allergic responses: interactions between allergen dose, sex and age. Scandinavian Journal of Immunology. 2011;73(6):554–567. doi: 10.1111/j.1365-3083.2011.02529.x. [DOI] [PubMed] [Google Scholar]
- 36.Xu B, Pizarro JC, Holmes MA, et al. Crystal structure of a αδ T-cell receptor specific for the human MHC class I homolog MICA. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(6):2414–2419. doi: 10.1073/pnas.1015433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bukowski JF, Morita CT, Brenner MB. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11(1):57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 38.Ferrick DA, King DP, Jackson KA, et al. Intraepithelial γδ T lymphocytes: sentinel cells at mucosal barriers. Springer Seminars in Immunopathology. 2000;22(3):283–296. doi: 10.1007/s002810000047. [DOI] [PubMed] [Google Scholar]
- 39.De Libero G, Mori L. Recognition of lipid antigens by T cells. Nature Reviews Immunology. 2005;5(6):485–496. doi: 10.1038/nri1631. [DOI] [PubMed] [Google Scholar]
- 40.Russano AM, Agea E, Corazzi L, et al. Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d-restricted γδ T cells. Journal of Allergy and Clinical Immunology. 2006;117(5):1178–1184. doi: 10.1016/j.jaci.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Born WK, Yafei H, Jin N, Hua H, O’Brien RL. Balanced approach of γδ T cells to type 2 immunity. Immunology and Cell Biology. 2010;88(3):269–274. doi: 10.1038/icb.2009.105. [DOI] [PubMed] [Google Scholar]
- 42.Pawankar R, Mori S, Ozu C, Kimura S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011;1(3):157–167. doi: 10.5415/apallergy.2011.1.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korematsu S, Tanaka Y, Nagakura T, Minato N, Izumi T. Human γδ T cells modulate the mite allergen-specific T-helper type 2-skewed immunity. Clinical and Experimental Allergy. 2007;37(11):1681–1687. doi: 10.1111/j.1365-2222.2007.02826.x. [DOI] [PubMed] [Google Scholar]
- 44.Ribot JC, deBarros A, Pang DJ, et al. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nature Immunology. 2009;10(4):427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vγ2Vδ2 T cells. Journal of Immunology. 2010;184(12):7268–7280. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caccamo N, La Mendola C, Orlando V, et al. Differentiation, phenotype, and function of interleukin-17-producing human vγ9vδ2 T cells. Blood. 2011;118(1):129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Yang J, Gao Y-D. Altered expressions of helper T cell (Th)1, Th2, and Th17 cytokines in CD8+ and γδ T cells in patients with allergic asthma. Journal of Asthma. 2011;48(5):429–436. doi: 10.3109/02770903.2011.570403. [DOI] [PubMed] [Google Scholar]
- 48.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. Journal of Immunology. 2007;179(8):5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petermann F, Rothhammer V, Claussen MC, et al. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33(3):351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. Journal of Immunology. 2007;178(7):4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 51.Holgate ST. Has the time come to rethink the pathogenesis of asthma? Current Opinion in Allergy and Clinical Immunology. 2010;10(1):48–53. doi: 10.1097/ACI.0b013e3283347be5. [DOI] [PubMed] [Google Scholar]
- 52.Jin N, Miyahara N, Roark CL, et al. Airway hyperresponsiveness through synergy of gammadelta T cells and NKT cells. Journal of Immunology. 2007;179(5):2961–2968. doi: 10.4049/jimmunol.179.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin N, Roark CL, Miyahara N, et al. Allergic airway hyperresponsiveness-enhancing γδ T cells develop in normal untreated mice and fail to produce IL-4/13, unlike Th2 and NKT cells. Journal of Immunology. 2009;182(4):2002–2010. doi: 10.4049/jimmunol.0803280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31(2):184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Stingl G, Gunter KC, Tschachler E, et al. Thy-1+ dendritic epidermal cells belong to the T-cell lineage. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(8):2430–2434. doi: 10.1073/pnas.84.8.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Augustin A, Kubo RT, Sim G-K. Resident pulmonary lymphocytes expressing the γ/δ T-cell receptor. Nature. 1989;340(6230):239–241. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- 57.Spinozzi F, Agea E, Bistoni O, et al. Increased allergen-specific, steroid-sensitive γδ T cells in bronchoalveolar lavage fluid from patients with asthma. Annals of Internal Medicine. 1996;124(2):223–227. doi: 10.7326/0003-4819-124-2-199601150-00005. [DOI] [PubMed] [Google Scholar]
- 58.Svensson L, Lilliehöök B, Larsson R, Bucht A. γδ T cells contribute to the systemic immunoglobulin E response and local B-cell reactivity in allergic eosinophilic airway inflammation. Immunology. 2003;108(1):98–108. doi: 10.1046/j.1365-2567.2003.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuany-Amorim C, Ruffié C, Hailé S, Vargaftig BB, Pereira P, Pretolani M. Requirements for Γδ T cells in allergic airway inflammation. Science. 1998;280(5367):1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 60.Lahn M, Kanehiro A, Takeda K, et al. Negative regulation of airway responsiveness that is dependent on gammadelta T cells and independent of alphabeta T cells. Nature Medicine. 1999;5(10):1150–1156. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 61.Hahn Y-S, Taube C, Jin N, et al. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ Cells, but not Vγ4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. Journal of Immunology. 2004;172(5):2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 62.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of Vγ1-expressing γ/δ T lymphocytes in normal mice. Journal of Experimental Medicine. 1995;182(6):1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dodd J, Riffault S, Kodituwakku JS, Hayday AC, Openshaw PJM. Pulmonary Vγ4+ γδ T cells have proinflammatory and antiviral effects in viral lung disease. Journal of Immunology. 2009;182(2):1174–1181. doi: 10.4049/jimmunol.182.2.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lahn M, Kanehiro A, Takeda K, et al. MHC class I-dependent Vγ4+ pulmonary T cells regulate αβ T cell-independent airway responsiveness. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8850–8855. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Y, Jin N, Roark CL, et al. The influence of IgE-enhancing and IgE-suppressive γδ T cells changes with exposure to inhaled ovalbumin. Journal of Immunology. 2009;183(2):849–855. doi: 10.4049/jimmunol.0804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265(5180):1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z-G, Li M, Chen Y-F, et al. Effects of dermatophagoides pteronyssinus allergen-specific immunotherapy on the serum interleukin-13 and pulmonary functions in asthmatic children. Chinese Medical Journal. 2009;122(10):1157–1161. [PubMed] [Google Scholar]
- 68.Huang X, Yang Q, Chen Y, Li P, Zhang G, Li Y. Expressions of IL-17, IL-21 and IL-23 in the serum of allergic rhinitis patients. Journal of Medical Biochemistry. 2011;30(4):323–327. [Google Scholar]
- 69.Cui Y, Shao H, Lan C, et al. Major role of γδ T cells in the generation of IL-17+ uveitogenic T cells. Journal of Immunology. 2009;183(1):560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonçalves-Sousa N, Ribot JC, DeBarros A, Correia DV, Caramalho Í, Silva-Santos B. Inhibition of murine γδ lymphocyte expansion and effector function by regulatory αβ T cells is cell-contactdependent and sensitive to GITR modulation. European Journal of Immunology. 2010;40(1):61–70. doi: 10.1002/eji.200939715. [DOI] [PubMed] [Google Scholar]
- 71.Hahn Y-S, Ji XY, Woo S-I, et al. Vγ1+ γδ T cells reduce IL-10-producing CD4+CD25+ T cells in the lung of ovalbumin-sensitized and challenged mice. Immunology Letters. 2008;121(2):87–92. doi: 10.1016/j.imlet.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human γδ T cells by nonpeptide antigens. Journal of Immunology. 2001;167(9):5092–5098. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- 73.Mousallem T, Burks AW. Immunology in the clinic review series; focus on allergies: immunotherapy for food allergy. Clinical and Experimental Immunology. 2012;167(1):26–31. doi: 10.1111/j.1365-2249.2011.04499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishikawa H, Naito T, Iwanaga T, et al. Curriculum vitae of intestinal intraepithelial T cells: their developmental and behavioral characteristics. Immunological Reviews. 2007;215(1):154–165. doi: 10.1111/j.1600-065X.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 75.Mengel J, Cardillo F, Aroeira LS, Williams O, Russo M, Vaz NM. Anti-γδ T cell antibody blocks the induction and maintenance of oral tolerance to ovalbumin in mice. Immunology Letters. 1995;48(2):97–102. doi: 10.1016/0165-2478(95)02451-4. [DOI] [PubMed] [Google Scholar]
- 76.Ali Z, Shao L, Halliday L, et al. Prolonged (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-driven antimicrobial and cytotoxic responses of pulmonary and systemic Vγ2Vδ2 T cells in macaques. Journal of Immunology. 2007;179(12):8287–8296. doi: 10.4049/jimmunol.179.12.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakai K, Yokoyama A, Kohno N, Hamada H, Hiwada K. Prolonged antigen exposure ameliorates airway inflammation but not remodeling in a mouse model of bronchial asthma. International Archives of Allergy and Immunology. 2001;126(2):126–134. doi: 10.1159/000049503. [DOI] [PubMed] [Google Scholar]
- 78.Jungsuwadee P, Dekan G, Stingl G, Epstein MM. Recurrent aerosol antigen exposure induces distinct patterns of experimental allergic asthma in mice. Clinical Immunology. 2002;102(2):145–153. doi: 10.1006/clim.2001.5157. [DOI] [PubMed] [Google Scholar]
- 79.Cui Z-H, Joetham A, Aydintug MK, Hahn Y-S, Born WK, Gelfand EW. Reversal of allergic airway hyperreactivity after long-term allergen challenge depends on γδ T cells. American Journal of Respiratory and Critical Care Medicine. 2003;168(11):1324–1332. doi: 10.1164/rccm.200305-634OC. [DOI] [PubMed] [Google Scholar]
- 80.Palmqvist M, Cui Z-H, Sjöstrand M, Lindén A, Lötvall J. Reduced late asthmatic response by repeated low-dose allergen exposure. European Respiratory Journal. 2001;17(5):872–880. doi: 10.1183/09031936.01.17508720. [DOI] [PubMed] [Google Scholar]


