Abstract
This paper reviews recent work investigating the influence of sleep disturbances on maternal hyperglycemia, particularly gestational diabetes mellitus (GDM). The incidence and prevalence of hyperglycemia are increasing worldwide, which is cause for concern because GDM and even mild hyperglycemia are associated with adverse pregnancy outcomes. A better understanding of sleep-related risk factors for maternal hyperglycemia is an important health matter. Evidence demonstrates associations between sleep disturbances, especially sleep-disordered breathing, and hyperglycemia, but causal effects and the underlying mechanisms linking these conditions have not been fully elucidated. Subjective sleep assessments show associations between sleep disturbances and maternal hyperglycemia. There are, however, few studies using objective measures to support these findings. Large prospective studies are required to examine causal relationships between sleep disturbances and maternal hyperglycemia. There is also a need for smaller mechanistic studies to understand the pathophysiology. Furthermore, interventional studies are required to address whether improvement of sleep parameters can prevent/decrease the risk of developing maternal hyperglycemia. Taken together, the data suggests that sleep disturbances during pregnancy are important to identify and manage in order to minimize maternal hyperglycemia and GDM, and improve maternal and fetal well-being.
Keywords: Sleep disturbance, gestational diabetes mellitus, maternal hyperglycemia, sleep-disordered breathing, obstructive sleep apnea, daytime nap, slow wave sleep, sleep duration, sleep parameters, restless leg syndrome, narcolepsy, adverse pregnancy outcomes, obesity, mechanistic pathways, sleep-disordered breathing symptoms, snoring, excessive daytime sleepiness, Pittsburgh Sleep Quality Index
Introduction
Hyperglycemia occurring during pregnancy refers to a continuum of disease severity ranging from glucose concentrations slightly above normal to gestational diabetes mellitus (GDM) [1-4]. GDM complicates approximately 7% of all pregnancies, affecting 200,000 pregnant women annually in the US [5]. The prevalence rises with increasing rates of maternal obesity and type 2 diabetes mellitus [2 4].
GDM and mild hyperglycemia even in non-diabetic ranges are associated with adverse maternal, fetal and neonatal outcomes [3 4]. Mothers with GDM have an increased risk of pre-eclampsia, cesarean section, preterm labor from polyhydramnios and infection [3 6 7]. GDM is also a risk factor for type 2 diabetes, obesity, cardiovascular disease and metabolic syndrome among both mothers and infants [4 8]. Furthermore, infants of diabetic mothers are at increased risk of macrosomia, related birth injuries, respiratory problems, neonatal hypoglycemia, jaundice [4 6] and impaired intellectual and psychomotor development [9]. GDM increased national medical costs by $636 million in 2007—$596 million for maternal costs and $40 million for neonatal costs in the US [10]. The causes of GDM and its rising incidence remain uncertain, though it is presumed to be related to obesity. Therefore, identifying modifiable risk factors that might help prevent GDM is of urgent public health importance.
Emerging literature shows that sleep disturbances during pregnancy are associated with hyperglycemia. In this article, sleep disturbances refer to internal and external factors that change the duration and/or structure of normal sleep architecture and cause poor sleep quality and daytime sleepiness, including disorders of initiating and maintaining sleep, disorders of the sleep–wake schedule, and dysfunctions associated with sleep, sleep stages, or partial arousals (transitions from deep sleep stages to lighter stages and/or partial wakefulness) in the absence of mental disorders, prescribed medications, substance abuse, or medical conditions [11]. We will review recent data examining associations between GDM and sleep disorders and sleep parameters in pregnant women, focusing especially on sleep quality, sleep duration and sleep-disordered breathing (SDB). We will also examine potential underlying mechanisms for these relationships; identify gaps in scientific knowledge; and propose directions for future research.
Sleep characteristics and disturbances during pregnancy
Sleep disturbances are surprisingly common during pregnancy. The vast majority of pregnant women (over 75%) experience sleep difficulties in the form of multiple nocturnal awakenings [12], likely due to hormonal changes, physical discomfort and anxiety associated with fear of labor or having an unhealthy baby and forthcoming life changes [13 14]. Sleep disturbances can commence with the onset of pregnancy (11 to 12 days), and increase in frequency as pregnancy progresses [15-19]. Some complaints associated with sleep disturbances (Table 1) are specific to one trimester, such as vomiting (morning sickness), but others occur across trimesters, such as urinary frequency and leg cramps [15 16 19-23]. A longitudinal survey study of 325 Finnish women showed that the number of nocturnal awakenings was highest in the third trimester [15]. A recent prospective study of 260 women using the Pittsburgh Sleep Quality Index (PSQI) [24] reported that sleep quality deteriorated from the second to the third trimester. Thirty-six percent of women were poor sleepers in the second trimester and 56% of women in the third. A third study using polysomnography (PSG) also found more nocturnal awakenings and lower sleep efficiency in late pregnancy compared to early pregnancy and the non-pregnant state [25].
Table 1.
The most common etiologies of sleep disturbances during pregnancy
| Sleep Disturbances | 1st Trimester | 2nd Trimester | 3rd Trimester |
|---|---|---|---|
| Nighttime | |||
| General discomfort | + | + | + |
| Psychological Stress | + | + | + |
| Urinary frequency | + | + | + |
| Low back pain/joint pain | + | + | + |
| Leg cramps | + | + | + |
| Heartburn | + | + | |
| Snoring | + | + | |
| Dreams and nightmares | + | + | |
| Fetal movements | + | + | |
| Irregular uterus contractions | + | + | |
| Nausea and vomiting | + | ||
| Daytime | |||
| Fatigue | + | + | |
| Daytime sleepiness | + | + |
Sleepiness and fatigue are the most common first trimester complaints. Sleepiness has been attributed to increased progesterone levels because of its sedative effect and inhibitory effect on muscles, which may result in frequent urination and snoring in late pregnancy [21]. In the first trimester of pregnancy, sleep has a polyphasic character including nocturnal sleep and often one or more daytime naps. Although the majority of women (75%) reported napping during pregnancy, some may not [12]. In the second trimester, sleep again becomes monophasic as in non-pregnant women [26]. By late in the second trimester, total nocturnal sleep time decreases. Total sleep time tends to be longer in the third trimester than pre-pregnancy period due to frequent daytime naps but sleep efficiency and percentage of slow wave sleep (SWS: deep sleep, consisting of stages 3 and 4 of Non-REM, occurring early in the sleep cycle) are lower than in the pre-pregnancy period or in early pregnancy [15 19 23 25]. The physiologic and biochemical changes of pregnancy may place women at risk for developing specific sleep disorders such as obstructive sleep apnea (OSA) and restless legs syndrome (RLS) [16 27-29]. Pregnancy may also exacerbate existing sleep disorders [19 23 30 31]. However, sleep disorders during pregnancy are frequently undiagnosed or overlooked.
Why are sleep disturbances serious during pregnancy?
Maternal sleep quality and duration can affect a number of endocrine, metabolic, and neurological functions that are critical to maintain a healthy pregnancy and fetal growth [21]. Thus sleep disturbances may be detrimental to fetal growth and maternal well-being. The collective evidence from epidemiological, experimental and clinical studies in the general population demonstrates associations between sleep disturbances and an array of adverse health conditions such as hyperglycemia, insulin resistance, type 2 diabetes, metabolic syndrome, coronary artery disease and stroke [32-37]. Emerging evidence suggests an analogous association in pregnancy such that women with SDB and short sleep duration (SSD) as assessed by questionnaires, actigraphy or PSG are at increased risk of developing adverse maternal and fetal outcomes such as GDM and pre-eclampsia [18 20• 38-41•••]. Several studies have reported that SSD increased the duration of, and pain perception during, labor, with a higher rate of caesarean delivery and preterm birth [42 43]. Although the results of these studies should be interpreted cautiously due to their small sample sizes, recall bias and other study limitations, this evidence supports the concept that extremes of sleep duration and sleep disorders such as SDB can precipitate or exacerbate a number of pregnancy complications, including GDM.
How obesity, sleep and glucose metabolism are related in pregnancy/postpartum
Obesity has been regularly reported to be associated with increased risk for pregnancy complications, including pre-eclampsia, hypertension, and GDM. The prevalence of obesity has been increasing steadily among reproductive-aged women in many parts of the world, with rates ranging from 10% up to 38% in Western societies [2 44 45]. Existing data shows that obesity is a major risk factor for insulin resistance, GDM and SDB [4 6 40•• 46], a term that refers to a spectrum of abnormal respiratory events during sleep ranging from habitual snoring to OSA, in which recurrent episodes of upper airway collapse occur during sleep [21]. This section will examine the associations between obesity, sleep disturbances (especially SDB) and maternal hyperglycemia, conditions that can potentiate each other, leading to a vicious cycle. Women who are obese prior to or during pregnancy are more likely to suffer from SDB than lean women [40•• 47]. Pregnant women have decreased lung reserve due to pregnancy-related changes [48 49]. The combination of obesity and pregnancy may have adverse effects on the respiratory system, leading to SDB. Conversely, SDB may predispose women to worsening obesity because of sleep fragmentation, daytime sleepiness, and insulin resistance. As reviewed below, SDB may also be associated with changes in leptin and ghrelin levels, increased appetite and caloric intake, again exacerbating obesity, which potentially contributes to hyperglycemia.
Human and animal studies have reported that short or long sleep duration (generally defined as <6 h and >9 h, respectively), sleep restriction and sleep disturbance have metabolic effects that predispose to weight gain and impaired glucose metabolism [32 33 50 51]. Although there is limited evidence regarding whether a relationship exists between sleep duration and weight gain in pregnancy, a number of studies have examined associations between postpartum sleep duration and weight retention. A prospective study of 904 postpartum women found that women reporting short sleep duration (<5 hours/day) at 6 months postpartum were 2.3 times more likely to retain at least 5 kg at 1 year independent of potential confounders including pre-pregnancy body mass index (BMI), gestational weight gain, parity, and postpartum behaviors [52]. Another prospective cohort of 586 women reported that postpartum sleep ≤5 hours/day was associated with higher postpartum weight retention, higher subscapular + triceps skinfold thickness and higher waist circumference at 3 years postpartum, after adjusting for age, pre-pregnancy BMI, and other relevant confounding factors [53].
Although poor sleep quality and sleep loss among pregnant women can occur as a result of hormonal and physical factors [1], voluntary sleep restriction is also common due to work and household responsibilities. Sleep loss or self-imposed SSD during pregnancy may also impair glucose regulation given that women who sleep less have more opportunities to eat. Furthermore, sleepiness and fatigue, which are common conditions in pregnancy, are independently associated with insulin resistance [54•], resulting in increased risk of obesity [2]. Stress and fatigue due to inadequate sleep may also lead to comfort eating, physical inactivity and eventually obesity. Thus, avoiding obesity before and during pregnancy may minimize the risk of developing sleep disorders and hyperglycemia during pregnancy.
Does lack of sleep cause maternal hyperglycemia during pregnancy?
In non-pregnant populations, SSD and fragmented sleep are known as major determinants of metabolic health, and are associated with poor glucose control in patients with diabetes [33 34 37 55-57]. Some prospective studies have also reported that long sleep duration and poor sleep quality and sleep maintenance [32-34 58] are associated with a higher risk of impaired glucose tolerance or type 2 diabetes.
In the pregnant population, data in this area are conflicting. The majority of studies demonstrate an association between sleep duration and increased risk of hyperglycemia [18 50 59•]. In a prospective study of 189 nulliparous women with a singleton gestation, 26% of women reported SSD (<7 hours sleep/night) in the first trimester, 40% in the third trimester and 48% of all women in one or both trimesters. Women who reported SSD were 2.6 (1.3-5.7) times more likely to have 1-hour oral glucose testing values ≥130 and GDM (OR 10.6, 1.3-85.5) after adjusting for age, race, prepregnancy BMI and snoring [18]. In a large prospective cohort study of 1290 women before 20 weeks gestation, self-reported sleep duration was categorized as ≤4, 5-8, 9 (the reference sleep category), or ≥10 hours/night. Regardless of maternal pre-pregnancy weight, both short and long sleep durations were associated with higher frequencies of glucose intolerance (1 hr oral glucose tolerance test (OGTT) ≥140 mg/dl). Women sleeping ≤4 hours/night had a 5.56-fold (1.31-23.69) increased risk of GDM compared with women sleeping 9 hours/night after adjusting for age and race. However, the association was no longer significant after adjusting for pre-pregnancy BMI [50].
In a third study of 169 women who completed several sleep questionnaires, including Epworth sleepiness scale (ESS) and PSQI. There was a significant inverse correlation between sleep duration and 1-h glucose values following 50-g OGTT such that one hour of shorter sleep was associated with a 4% increase in glucose levels. Furthermore, short sleep and measures of poor sleep quality were also correlated with preterm delivery and greater rate of admission to neonatal intensive care units in both GDM and non-GDM women. However, in this study age, pre-pregnancy BMI, a family history of diabetes and previous GDM were not controlled [59•]. A prospective study of 260 pregnant women using the PSQI reported that sleep quality worsened during pregnancy, but sleep parameters were not significantly different between pregnancies with and without adverse outcomes including GDM [24].
Studies with relatively small sample sizes (n=52-104) have tended not to find associations between sleep duration and glucose intolerance or GDM [20• 60•]. In a secondary analysis of a prospective study of 104 pregnant women, neither objective nor subjective sleep duration was related to hyperglycemia after controlling for known risk factors for GDM [20•]. However, self-reported nap duration was associated with high glucose challenge test (GCT) results in adjusted models. Similar results were reported by a recent case-control study (n=52) that found no significant differences in either objective or subjective measures of sleep duration between women with and without GDM [60•].
Findings showing an association between longer self-reported nap duration in early pregnancy and high GCT [20•] are compatible with results from studies of older adults, among whom daytime napping has been associated with a higher risk of diabetes [61 62]. Results from Bourjeily [54•] et al. also have demonstrated that severe daytime sleepiness increases the risk of GDM in nonsnoring pregnant women, independent of confounders. These studies suggest that sleep has modulatory influences on glucose regulation regardless of time of day. Hypothetically, disturbed sleep/wake cycles due to napping at unusual times may disturb the sympathetic-parasympathetic balance and cause excessive hormone release that regulate glucose metabolism [20•]. Daytime naps also reduce the amount of SWS, which is associated with glucose homeostasis as detailed below [63]. To elucidate whether napping has clinical implications for the treatment and prevention of GDM, further studies are required.
Is OSA an independent risk factor for glucose dysregulation?
The prevalence of OSA, diagnosed by PSG, in pregnant women is not known because large population-based epidemiological studies using objective sleep measures are lacking. However, self-reported snoring and daytime sleepiness, two symptoms of OSA, are very common. The prevalence of habitual snoring among pregnant women has been estimated to be between 14% and 46% [16-18 28 39• 46 47 50 59• 64]. Longitudinal studies have shown that frequent snoring (≥3 nights/week) increases as pregnancy progress [17-19 39•]. The combination of weight gain and pregnancy-induced physiological, anatomical and hormonal changes in the respiratory system (upper airway, lung mechanics, and control of breathing) make women more prone to snoring and increase the risk of developing OSA and nocturnal desaturation. SDB may be particularly severe during the third trimester [16 28 39•] when oxygen stores in the lung are reduced due to lung compression from the enlarging uterus.
In the pregnant population, most observational studies have demonstrated an association between SDB and hyperglycemia independent of confounders including race and BMI. A prospective cohort study of 1290 pregnant women reported that 1-hour OGT values were higher among women with habitual snoring after adjusting for age, race/ethnicity and BMI (p=0.04). Overweight or obese women who snored had a significantly increased risk of GDM compared to lean nonsnorers (RR 6.91, 95% CI 2.87-16.6). However, the odds of GDM in all snorers were not significantly increased compared to nonsnorers (RR 1.86, CI 0.88-3.94) [50]. Similarly, a large cross-sectional survey of 1000 pregnant women found an association between symptoms of SDB and GDM after adjusting for multiple factors including BMI at delivery (2.1, 95% CI 1.3–3.4) [64]. In a prospective study, 189 healthy nulliparous women completed a sleep survey both at study enrollment and in the third trimester. This study showed that women who reported frequent snoring at both time points were 4.9 times (95% CI, 1.3–18.1) more likely to develop GDM after controlling for BMI compared to nonsnorers [18]. Similarly, in a prospective study of 169 women, a significantly higher risk of developing GDM was observed among pregnant women at increased risk for SDB (after adjustment for BMI) or who endorsed a combination of increased SDB risk and short sleep duration [59•]. Balserak et al also found that self-reported symptoms of SDB in early gestation were independently associated with high GCT values (≥135) in a secondary analysis of a prospective study [20•].
In contrast to these studies, a large, prospective study of 1719 women in the 3rd trimester in which a sleep survey was administered to a clinic-based population demonstrated that neither pregnancy-onset nor chronic snoring was associated with GDM after adjusting for confounders [39•]. Although self-reported SDB symptoms have been linked with hyperglycemia in most of these studies, one must be cautious when interpreting these results because SDB questionnaires have low sensitivity and specificity for predicting OSA in pregnant women or have not been validated in pregnancy.
Studies objectively assessing the impact of SDB on the risk of hyperglycemia in pregnant women are limited and the results of these studies are contradictory. Chen et al. [41•••] convincingly reported that the risk of GDM increased almost two-fold (OR 1.6, 95% CI 1.07–2.8) among women with PSG-confirmed SDB in a large retrospective cohort study involving 759 pregnant women with OSA and 3955 matched controls. A recent retrospective cohort study of 143 women with confirmed moderate to severe OSA (AHI≥15) also demonstrated an increased risk of composite adverse pregnancy outcomes (GDM and pregnancy related hypertension), after adjusted for timing of the sleep study performed before, during or after pregnancy. However, the effect of obesity was not fully controlled because BMI used in the statistical analysis was ascertained at the time of the sleep study [65•]. Negative associations between objectively measured AHI and hyperglycemia have reported in studies with smaller sample sizes (n=52-104) [20• 60•]. For instance, in a cohort study of 175 obese pregnant women, OSA was associated with increased prevalence of pre-eclampsia, neonatal intensive care unit admissions, and cesarean delivery, but the prevalence of GDM was not increased [40••]. This may be due to the study's relatively small sample size and because all subjects were obese.
In the Sleep Heart Health Study, individuals with diabetes had an increased risk of poor sleep and some form of SDB (up to 58%), which may impair glucose control [55]. These findings suggest that women with pre-pregnancy diabetes should be screened for OSA [21]. Intervention studies have shown that treatment for OSA with CPAP improves glucose control in non-gravid populations [34 66]. However, whether OSA intervention improves outcomes in pregnancy and whether it may prevent pregnancy complications associated with GDM in the long term is not known. Because of the unique nature of pregnancy, treatment efforts for OSA need to address the distinct attributes of intervening in pregnant women (e.g. frequent nocturnal urination), including issues of CPAP initiation and adherence.
Restless legs syndrome and glucose metabolism
Restless leg syndrome (RLS) is characterized by an urge to move the extremities in response to uncomfortable sensations, a circadian pattern in which symptoms worsen at night, and are relieved by movement. RLS leads to difficulties in initiating and maintaining sleep and affects approximately 25% of women during pregnancy [21 22 29]. RLS has been shown to have a significant association with type 2 diabetes in the non-pregnant population [67 68]. In the pregnant population, little data exists, with only a recent preliminary study showing that women with GDM are significantly more likely to have RLS than age and BMI matched women [60•].
Narcolepsy and glucose metabolism
Narcolepsy is characterized by excessive daytime sleepiness, various combinations of irresistible onset of sleep, REM-related sleep symptoms (e.g. sleep paralysis and hypnagogic or hypnopompic hallucinations), and cataplexy. Cataplexy however is not present in all narcoleptics [69]. Honda et al found that narcolepsy was associated with increased frequency of type 2 diabetes [70]. However, subsequent studies have failed to find evidence that narcolepsy increases the risk of insulin resistance or glucose intolerance (and consequently of type 2 diabetes) independently of BMI [71 72].
In a retrospective cohort study (n=54), pregnant women whose narcolepsy symptoms started before or during pregnancy tended to have greater glucose intolerance or more severe type 2 diabetes mellitus compared to the asymptomatic group. The factors associated with glucose metabolism such as weight gain during pregnancy did not differ between these groups [69]. A recent retrospective cohort study undertaken in European countries also reported that the symptomatic patients with narcolepsy-cataplexy had a significantly higher prevalence of impaired glucose metabolism than the asymptomatic patients [73]. However, these studies had no control groups without narcolepsy.
What are the potential mechanisms underlying the link between sleep disturbances and hyperglycemia?
Mechanisms linking sleep disturbances and pregnancy complications including hyperglycemia are likely multifactorial and are not completely understood. Both Type 2 diabetes and GDM are associated with insulin resistance and impaired insulin secretion, and share the same risk factors and genetic susceptibility [74]. However, normal pregnant women are mildly insulin resistant due to hormonal changes [2 4 74]. Thus, even small changes in sleep parameters could potentially make pregnant women more vulnerable to developing maternal hyperglycemia.
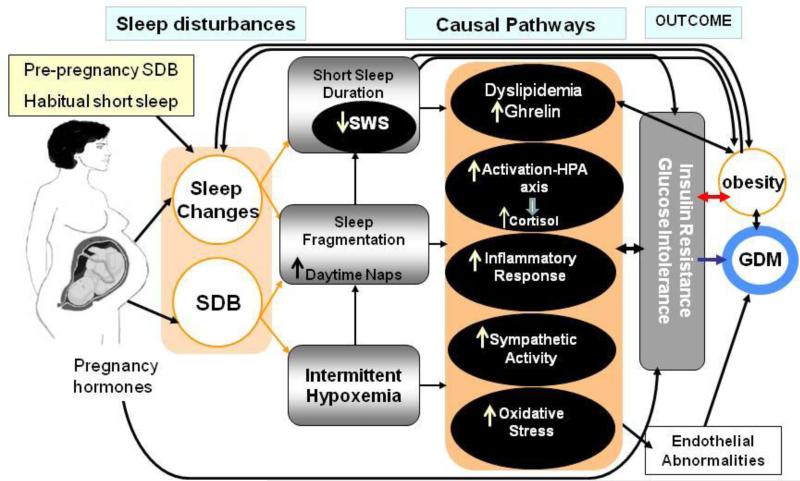
Figure 1 provides guidance for our proposed model explaining potential pathways in pregnancy. Our model suggests that habitual poor sleep quality or SSD, specifically insufficient SWS, can lead to impaired glucose tolerance. As has been described in sleep deprivation studies, SSD and decreased SWS may augment the inflammatory response by increasing circulating concentrations of interleukin-6 (IL-6), tumor necrosis factor alpha (TNFa) and C-reactive protein (CRP) [75-80], which are involved in the pathogenesis of insulin resistance and type 2 diabetes [1]. Decreased PSG sleep duration was also associated with increased TNFa levels in the Cleveland Family Study (n=614); this study reported that increases, but not decreases, in habitual sleep duration were linked to elevated CRP and IL-6 levels [81]. Similarly, in mid and late pregnancy, SSD and poor sleep quality have been found to be associated with higher levels of IL-6 [82]. Naps during pregnancy could also activate pro-inflammatory pathways, leading to hyperglycemia. Increased levels of cytokines can decrease insulin sensitivity and downstream insulin signaling, adversely impacting glucose regulation [2 83].
Figure 1.
Schematic illustration of potential causal pathways that may link sleep / sleep disturbances during pregnancy and hyperglycemia.
SWS may have more significant implications for glucose homeostasis than sleep duration alone due to its important role in transitory metabolic, hormonal, and neurophysiologic changes. These include decreasing brain glucose utilization and sympathetic activity, stimulating growth hormone release, inhibiting the activation of the HPA axis and cortisol secretion [35 84 85], all of which likely affect glucose regulation. Experimental studies in the non-pregnant population have observed that reduction in SWS is associated with increased risk of impaired glucose tolerance and insulin sensitivity [34 35 84]. The duration of SWS decreases in most pregnant women by the 3rd trimester [19 23 25]; we believe that a greater decline in SWS is likely to occur among those who develop SDB.
Our model also posits that recurrent partial or complete airway obstruction results in intermittent hypoxia and frequent arousals from sleep in pregnant women. In the general population, arousals and hypoxia decrease total sleep time in general and SWS in particular [86], and also increase sympathetic activity [1 34 84 87] during sleep, which carries over into wakefulness [35]. Furthermore, intermittent hypoxia results in activation of the hypothalamic-pituitary axis (HPA) and alterations in inflammatory pathways [87 88]. Analogous biological pathways could be involved in maternal hyperglycemia.
Intermittent hypoxia-reoxygenation can initiate oxidative stress, creating a vicious cycle that activates inflammatory pathways [87 89]. Alternately, hyperglycemia has been found to acutely increase circulating cytokine concentrations through an oxidative stress mechanism [87 90]. Both oxidative stress and inflammation are linked to endothelial dysfunction [91], which is present in individuals with GDM [83] and in both obese and non-obese women with a history of GDM, even when they have normal glucose tolerance [92]. These findings suggest that endothelial dysfunction may play a role in the onset of GDM.
Sleep fragmentation, proinflammatory cytokines and intermittent hypoxia can also lead to hyperactivation of the HPA, which in turn leads to increased release of the glucocorticoid cortisol [84 88 93 94]. Cortisol regulates several basal processes including fat and glucose metabolism, blood pressure, inflammatory and immune responses. Long-term secretion of the glucocorticoid cortisol increases susceptibility to insulin resistance and impaired glucose tolerance, and restrains further development of the inflammatory process [84 93 94]. Mean 24-hour plasma cortisol levels have been found to be significantly higher in short sleepers than long sleepers [88]. Disruptions in sleep as a result of emotional stress associated with pregnancy could also increase cortisol levels due to activation of the HPA axis [95 96]. Other causal routes, however, may exist. For instance, stressed women may smoke or eat more which contribute to SDB; stress due to poor or inadequate sleep can also affect immune function and vasculature [96]. Further research is needed to better understand the pathophysiology of maternal hyperglycemia regarding the functional relationships between sleep, stress and HPA axis activity.
Recent evidence also suggests that sleep loss, especially loss of SWS, may affect hormones that regulate appetite and satiety, specifically by reducing leptin sensitivity and increasing levels of ghrelin [33 85]. Among police officers, a U-shaped association was observed. Leptin levels were higher at both short and long extremes of sleep duration. These associations were stronger among women [97]. Leptin levels also significantly correlated with insulin resistance in patients with moderate-to-severe OSA. After 8 weeks of CPAP treatment, insulin secretion capacity improved and leptin levels fell [66].
In pregnancy, either long sleep duration (e.g. due to daytime naps) or sleep loss caused by sleep restriction or sleep problems could contribute to the pathophysiology of GDM [18 20• 41••• 50 59• 64 98], by prompting food intake, weight gain and increased insulin resistance [99 100]. However, data on leptin in GDM are controversial. While some studies have reported that maternal leptin concentrations increased in GDM [4, 98, 99, 101] and hyperleptinaemia in early pregnancy was a predictor of developing GDM in later pregnancy, other studies have found that leptin concentrations were unchanged or decreased among women with GDM [99]. Ghrelin mRNA expression in placental tissue has also been found to be higher in individuals with GDM than in controls, whereas there was no association between circulating ghrelin and GDM [98]. In the pregnant population, all the potential pathways linking sleep and maternal hyperglycemia mentioned above are, due to our lack of knowledge about them, important topics of research that warrant further investigation.
Conclusion
The majority of pregnant women experience sleep disturbances due to pregnancy-related changes that can result in poor sleep quality, insufficient sleep and daytime sleepiness. A burgeoning collection of data demonstrates that extremes of sleep duration, poor sleep quality, severe daytime sleepiness and sleep disorders including SDB and symptomatic narcolepsy during pregnancy are associated with maternal hyperglycemia and GDM. Obesity is a major risk factor for these conditions. Therefore, studies investigating sleep disturbances, especially SDB and GDM, in pregnancy must consider obesity as a significant confounder. The physiological stress of sleep disturbances appears to trigger a cascade of pathophysiological events including oxidative stress, sympathetic over-activity, inflammation and endothelial dysfunction that increase the predisposition for insulin resistance, glucose intolerance and GDM. Maintaining a proper sleep pattern and avoiding obesity are critical for glucose regulation and healthy metabolic function in pregnancy. Factors that would improve future investigations in this area include the use of interventions, objective as well as subjective sleep measurements, and utilization of a large longitudinal study design.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Bilgay Izci-Balserak and Grace W. Pien declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Izci-Balserak B, Pien GW. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. Curr Opin Pulm Med. 2010;16(6):574–82. doi: 10.1097/MCP.0b013e32833f0d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140(3):365–71. doi: 10.1530/REP-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group HSCR. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. NEJM. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 4.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes care. 2007;30(Suppl 2):S251–60. doi: 10.2337/dc07-s225. (Journal Article) [DOI] [PubMed] [Google Scholar]
- 5.Association AD. Standards of medical care in diabetes. Diabetes care. 2004;27(Suppl 1):S15–35. doi: 10.2337/diacare.27.2007.s15. [DOI] [PubMed] [Google Scholar]
- 6.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes care. 2012;35(4):780–6. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowe LP, Metzger BE, Lowe WL, Jr., Dyer AR, McDade TW, McIntyre HD, et al. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95(12):5427–34. doi: 10.1210/jc.2010-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–13. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Lieshout RJ, Voruganti LP. Diabetes mellitus during pregnancy and increased risk of schizophrenia in offspring: a review of the evidence and putative mechanisms. J Psychiatry Neurosci. 2008;33(5):395–404. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YZ, Quick WW, Yang WY, Zhang YD, Baldwin A, Moran J, et al. Cost of Gestational Diabetes Mellitus in the United States in 2007. Popul Health Manag. 200912(3):165–74. doi: 10.1089/pop.2009.12303. [DOI] [PubMed] [Google Scholar]
- 11.Cormier R. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Butterworths; Boston: 1990. Sleep Disturbances. [PubMed] [Google Scholar]
- 12.National Sleep F . Sleep in America poll 2007. Washington (DC): 2007. [Google Scholar]
- 13.Hall WA, Hauck YL, Carty EM, Hutton EK, Fenwick J, Stoll K. Childbirth fear, anxiety, fatigue, and sleep deprivation in pregnant women. J Obstet Gynecol Neonatal Nurs. 2009;38(5):567–76. doi: 10.1111/j.1552-6909.2009.01054.x. [DOI] [PubMed] [Google Scholar]
- 14.Beebe KR, Lee KA, Carrieri-Kohlman V, Humphreys J. The effects of childbirth self-efficacy and anxiety during pregnancy on prehospitalization Labor. Jognn. 2007;36(5):410–18. doi: 10.1111/j.1552-6909.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- 15.Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV. Effects of pregnancy on mothers' sleep. Sleep Med. 2002;3(1):37–42. doi: 10.1016/s1389-9457(01)00130-7. [DOI] [PubMed] [Google Scholar]
- 16.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28(10):1299–305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 17.Guilleminault C, Querra-Salva M, Chowdhuri S, Poyares D. Normal pregnancy, daytime sleeping, snoring and blood pressure. Sleep Med. 2000;1(4):289–97. doi: 10.1016/s1389-9457(00)00046-0. [DOI] [PubMed] [Google Scholar]
- 18.Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203(2):142, e1–5. doi: 10.1016/j.ajog.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pien GW. A longitudinal cohort study of SDB in pregnancy, Presented at Sleep Medicine Division Grand Rounds. Philadelphia, PA- USA: Oct 20, 2011. [Google Scholar]
- 20•.Izci Balserak B, Jackson N, Ratcliffe SA, Pack AI, Pien GW. Sleep-disordered breathing and daytime napping are associated with maternal hyperglycemia. Sleep Breath. 2013;17(3):1093–102. doi: 10.1007/s11325-013-0809-4. [This secondary analysis of a prospective study of sleep in pregnant women using both objective and subjective sleep measures is the first to find an association between nap duration and maternal hyperglycemia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izci-Balserak B, Lee KA. Sleep Disturbances and Sleep-Related Disorders in Pregnancy. In: Kryger M, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine. Saunders; Philadelphia: 2010. pp. 1572–86. [Google Scholar]
- 22.Lee KA, Zaffke ME, Baratte-Beebe K. Restless legs syndrome and sleep disturbance during pregnancy: the role of folate and iron. J Womens Health Gend Based Med. 2001;10(4):335–41. doi: 10.1089/152460901750269652. [DOI] [PubMed] [Google Scholar]
- 23.Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95(1):14–8. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- 24.Naud K, Ouellet A, Brown C, Pasquier JC, Moutquin JM. Is sleep disturbed in pregnancy? J Obstet Gynaecol Can. 2010;32(1):28–34. doi: 10.1016/S1701-2163(16)34400-0. [DOI] [PubMed] [Google Scholar]
- 25.Wilson DL, Barnes M, Ellett L, Permezel M, Jackson M, Crowe SF. Decreased sleep efficiency, increased wake after sleep onset and increased cortical arousals in late pregnancy. Aust N Z J Obstet Gynaecol. 2011;51(1):38–46. doi: 10.1111/j.1479-828X.2010.01252.x. [DOI] [PubMed] [Google Scholar]
- 26.Karacan I, Wayne H, Harman AW, Williams R, Webb W, Ross JJ. Characteristics of sleep patterns during late pregnancy and the postpartum periods. Am J Obstet Gynecol. 1968;101:579–86. Journal Article. [Google Scholar]
- 27.Reid J, Skomro R, Cotton D, Ward H, Olatunbosun F, Gjevre J, et al. Pregnant Women with Gestational Hypertension May Have a High Frequency of Sleep Disordered Breathing. Sleep. 2011;34(8):1033–38. doi: 10.5665/SLEEP.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izci B, Martin SE, Dundas KC, Liston WA, Calder AA, Douglas NJ. Sleep complaints: snoring and daytime sleepiness in pregnant and pre-eclamptic women. Sleep Med. 2005;6(2):163–9. doi: 10.1016/j.sleep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Manconi M, Govoni V, De Vito A, Economou NT, Cesnik E, Casetta I, et al. Restless legs syndrome and pregnancy. Neurology. 2004;63(6):1065–69. doi: 10.1212/01.wnl.0000138427.83574.a6. [DOI] [PubMed] [Google Scholar]
- 30.Guilleminault C, Kreutzer M, Chang JL. Pregnancy, sleep disordered breathing and treatment with nasal continuous positive airway pressure. Sleep Med. 2004;5(1):43–51. doi: 10.1016/j.sleep.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Edwards N, Blyton DM, Hennessy A, Sullivan CE. Severity of sleep-disordered breathing improves following parturition. Sleep. 2005;28(6):737–41. doi: 10.1093/sleep/28.6.737. [DOI] [PubMed] [Google Scholar]
- 32.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knutson KL. Does inadequate sleep play a role in vulnerability to obesity? Am J Hum Biol. 2012;24(3):361–71. doi: 10.1002/ajhb.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Punjabi NM. Do sleep disorders and associated treatments impact glucose metabolism? Drugs. 2009;69(Suppl 2):13–27. doi: 10.2165/11531150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes care. 2009;32(6):1017–19. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–13. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izci B, Riha RL, Martin SE, Vennelle M, Liston WA, Dundas KC, et al. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med. 2003;167(2):137–40. doi: 10.1164/rccm.200206-590OC. [DOI] [PubMed] [Google Scholar]
- 39•.O'Brien LM, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Chames MC, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487, e1–9. doi: 10.1016/j.ajog.2012.08.034. [This large, prospective study administered a sleep survey to a clinic-based population and demonstrated that neither pregnancy-onset nor chronic snoring was associated with GDM after adjusting for confounders.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Louis J, Auckley D, Miladinovic B, Shepherd A, Mencin P, Kumar D, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120(5):1085–92. doi: 10.1097/AOG.0b013e31826eb9d8. [This prospective cohort study of obese pregnant women using home PSG reported that OSA was associated with increased prevalence of pre-eclampsia, neonatal intensive care unit admissions, and cesarean delivery, but not the prevalence of GDM. This finding emphasizes that obesity is a significant confounding variable when studying hyperglycemia in pregnancy among women with OSA.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•••.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136, e1–5. doi: 10.1016/j.ajog.2011.09.006. [This is the largest retrospective cohort study of pregnant women with PSG-confirmed SDB showing that the risk of GDM increased almost two-fold (OR 1.6, 95% CI 1.07–2.8) among women known to have antenatal SDB after controlling a number of maternal characteristics such as age, education level and marital status.] [DOI] [PubMed] [Google Scholar]
- 42.Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol. 2004;191(6):2041–46. doi: 10.1016/j.ajog.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 43.Okun ML, Luther JF, Wisniewski SR, Sit D, Prairie BA, Wisner KL. Disturbed sleep, a novel risk factor for preterm birth? Journal of women's health. 2012;21(1):54–60. doi: 10.1089/jwh.2010.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 45.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993-2003. Obesity. 2007;15(4):986–93. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 46.Kapsimalis F, Kryger M. Sleep breathing disorders in the U.S. female population. Journal of women's health. 2009;18(8):1211–9. doi: 10.1089/jwh.2008.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maasilta P, Bachour A, Teramo K, Polo O, Laitinen LA. Sleep-related disordered breathing during pregnancy in obese women. Chest. 2001;120(5):1448–54. doi: 10.1378/chest.120.5.1448. [DOI] [PubMed] [Google Scholar]
- 48.Costa D, Barbalho MC, Miguel GPS, Forti EMP. Azevedo JLMC. The Impact of Obesity on Pulmonary Function in Adult Women. Clinics. 2008;63(6):719–24. doi: 10.1590/S1807-59322008000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirnle L, Lysenko L, Gerber H, Lesnik P, Baranowska A, Rachwalik M, et al. Respiratory function in pregnant women. Adv Exp Med Biol. 2013;788:153–60. doi: 10.1007/978-94-007-6627-3_23. [DOI] [PubMed] [Google Scholar]
- 50.Qiu C, Enquobahrie D, Frederick IO, Abetew D, Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health. 2010;10:17. doi: 10.1186/1472-6874-10-17. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coomans CP, van den Berg SA, Houben T, van Klinken JB, van den Berg R, Pronk AC, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27(4):1721–32. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 52.Gunderson EP, Rifas-Shiman SL, Oken E, Rich-Edwards JW, Kleinman KP, Taveras EM, et al. Association of fewer hours of sleep at 6 months postpartum with substantial weight retention at 1 year postpartum. Am J Epidemiol. 2008;167(2):178–87. doi: 10.1093/aje/kwm298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taveras EM, Rifas-Shiman SL, Rich-Edwards JW, Gunderson EP, Stuebe AM, Mantzoros CS. Association of maternal short sleep duration with adiposity and cardiometabolic status at 3 years postpartum. Obesity. 2011;19(1):171–8. doi: 10.1038/oby.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Bourjeily G, El Sabbagh R, Sawan P, Raker C, Wang C, Hott B, et al. Epworth sleepiness scale scores and adverse pregnancy outcomes. Sleep Breath. 2013;17(4):1179–86. doi: 10.1007/s11325-013-0820-9. [This survey study is the first study to find that severe daytime sleepiness increases the risk of GDM in nonsnoring pregnant women.] [DOI] [PubMed] [Google Scholar]
- 55.Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes care. 2003;26(3):702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 56.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165(8):863–68. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 57.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen K, et al. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care. 2010;33(7):1573–7. doi: 10.2337/dc09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kita T, Yoshioka E, Satoh H, Saijo Y, Kawaharada M, Okada E, et al. Short sleep duration and poor sleep quality increase the risk of diabetes in Japanese workers with no family history of diabetes. Diabetes Care. 2012;35(2):313–8. doi: 10.2337/dc11-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Reutrakul S, Zaidi N, Wroblewski K, Kay HH, Ismail M, Ehrmann DA, et al. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes care. 2011;34(11):2454–7. doi: 10.2337/dc11-0780. [This questionnaire based study found a significant inverse correlation between sleep duration and 1-h glucose values following 50-g OGTT such that one hour of shorter sleep was associated with a 4% increase in glucose levels. Furthermore, it noted that increased SDB risk and a combination of increased SDB risk and short sleep duration were associated with a significantly higher risk of developing GDM.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Marc I, Series F, Giguere Y, Kimoff RJ, Meltzer S, Pamidi S, et al. Sleep Disturbances In Gestational Diabetes: A Case -Control Study. Am J Respir Crit Care Med. 2013;187:A5940. [This preliminary case-control study found no significant differences in either objective or subjective measures of sleep duration between women with and without GDM. This is the first study investigating differences of RDIs between women with GDM and controls.] [Google Scholar]
- 61.Lam KH, Jiang CQ, Thomas GN, Lao XQ, McGhee SM, Zhang WS, et al. Napping Is Associated with Increased Risk of Type 2 Diabetes: The Guangzhou Biobank Cohort Study. Sleep. 2010;33(3):402–07. doi: 10.1093/sleep/33.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes care. 2010;33(1):78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borbely AA. From slow waves to sleep homeostasis: new perspectives. Arch Ital Biol. 2001;139(1-2):53–61. [PubMed] [Google Scholar]
- 64.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep- disordered breathing. Eur Respir J. 2010;36(4):849–55. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 65•.Facco FL, Liu CS, Cabello AA, Kick A, Grobman WA, Zee PC. Sleep-disordered breathing: a risk factor for adverse pregnancy outcomes? Am J Perinatol. 2012;29(4):277–82. doi: 10.1055/s-0031-1295658. [This retrospective cohort study demonstrated an increased risk of composite adverse pregnancy outcomes (GDM and pregnancy related hypertension) among women with confirmed moderate to severe OSA.] [DOI] [PubMed] [Google Scholar]
- 66.Cuhadaroglu C, Utkusavas A, Ozturk L, Salman S, Ece T. Effects of nasal CPAP treatment on insulin resistance, lipid profile, and plasma leptin in sleep apnea. Lung. 2009;187(2):75–81. doi: 10.1007/s00408-008-9131-5. [DOI] [PubMed] [Google Scholar]
- 67.Cuellar NG, Ratcliffe SJ. A comparison of glycemic control, sleep, fatigue, and depression in type 2 diabetes with and without restless legs syndrome. JCSM. 2008;4(1):50–56. [PMC free article] [PubMed] [Google Scholar]
- 68.Merlino G, Fratticci L, Valente M, Del Giudice A, Noacco C, Dolso P, et al. Association of restless legs syndrome in type 2 diabetes: a case-control study. Sleep. 2007;30(7):866–71. doi: 10.1093/sleep/30.7.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maurovich-Horvat E, Tormasiova M, Slonkova J, Kemlink D, Maurovich-Horvat L, Nevsimalova S, et al. Assessment of pregnancy outcomes in Czech and Slovak women with narcolepsy. Med Sci Monit. 2010;16(12):SR35–40. [PubMed] [Google Scholar]
- 70.Honda Y, Doi Y, Ninomiya R, Ninomiya C. Increased frequency of non-insulin-dependent diabetes mellitus among narcoleptic patients. Sleep. 1986;9(1 Pt 2):254–9. doi: 10.1093/sleep/9.1.254. [DOI] [PubMed] [Google Scholar]
- 71.Beitinger PA, Fulda S, Dalal MA, Wehrle R, Keckeis M, Wetter TC, et al. Glucose tolerance in patients with narcolepsy. Sleep. 2012;35(2):231–6. doi: 10.5665/sleep.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engel A, Helfrich J, Manderscheid N, Musholt PB, Forst T, Pfutzner A, et al. Investigation of insulin resistance in narcoleptic patients: dependent or independent of body mass index? Neuropsychiatr Dis Treat. 2011;7:351–6. doi: 10.2147/NDT.S18455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maurovich-Horvat E, Kemlink D, Hogl B, Frauscher B, Ehrmann L, Geisler P, et al. Narcolepsy and pregnancy: a retrospective European evaluation of 249 pregnancies. J Sleep Res. 2013;22(5):496–512. doi: 10.1111/jsr.12047. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med. 2004;21(2):103–13. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 75.Kaushal N, Ramesh V, Gozal D. TNF-alpha and temporal changes in sleep architecture in mice exposed to sleep fragmentation. PLoS One. 2012;7(9):e45610. doi: 10.1371/journal.pone.0045610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosa Neto JC, Lira FS, Venancio DP, Cunha CA, Oyama LM, Pimentel GD, et al. Sleep deprivation affects inflammatory marker expression in adipose tissue. Lipids Health Dis. 2010;9:125. doi: 10.1186/1476-511X-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24(1):54–7. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas KS, Motivala S, Olmstead R, Irwin MR. Sleep depth and fatigue: role of cellular inflammatory activation. Brain Behav Immun. 2011;25(1):53–8. doi: 10.1016/j.bbi.2010.07.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 80.Altman NG, Izci-Balserak B, Schopfer E, Jackson N, Rattanaumpawan P, Gehrman PR, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med. 2012;13(10):1261–70. doi: 10.1016/j.sleep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okun ML, Hall M, Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007;14(6):560–7. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- 83.Mordwinkin NM, Ouzounian JG, Yedigarova L, Montoro MN, Louie SG, Rodgers KE. Alteration of endothelial function markers in women with gestational diabetes and their fetuses. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013;26(5):507–12. doi: 10.3109/14767058.2012.736564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 86.Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, et al. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med. 2006;7(1):7–16. doi: 10.1016/j.sleep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 87.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32(4):447–70. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steiger A. Sleep and the hypothalamo-pituitary-adrenocortical system. Sleep Med Rev. 2002;6(2):125–38. doi: 10.1053/smrv.2001.0159. [DOI] [PubMed] [Google Scholar]
- 89.Lavie L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front Biosci. 2012;4:1391–403. doi: 10.2741/469. [DOI] [PubMed] [Google Scholar]
- 90.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 91.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117(17):2270–8. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anastasiou E, Lekakis JP, Alevizaki M, Papamichael CM, Megas J, Souvatzoglou A, et al. Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care. 1998;21(12):2111–5. doi: 10.2337/diacare.21.12.2111. [DOI] [PubMed] [Google Scholar]
- 93.Szelenyi J, Vizi ES. The catecholamine cytokine balance: interaction between the brain and the immune system. Ann N Y Acad Sci. 2007;1113:311–24. doi: 10.1196/annals.1391.026. [DOI] [PubMed] [Google Scholar]
- 94.Venihaki M, Dikkes P, Carrigan A, Karalis KP. Corticotropin-releasing hormone regulates IL-6 expression during inflammation. J Clin Invest. 2001;108(8):1159–66. doi: 10.1172/JCI12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.LeWinn KZ, Stroud LR, Molnar BE, Ware JH, Koenen KC, Buka SL. Elevated maternal cortisol levels during pregnancy are associated with reduced childhood IQ. Int J Epidemiol. 2009;38(6):1700–10. doi: 10.1093/ije/dyp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schetter DC GL. Stress in pregnancy: empirical evidence and theoretical issues to guide interdisciplinary research. In: Contrada R, Baum A, editors. Handbook of Stress Science. Springer; New York: 2011. pp. 321–43. [Google Scholar]
- 97.Charles LE, Gu JK, Andrew ME, Violanti JM, Fekedulegn D, Burchfiel CM. Sleep duration and biomarkers of metabolic function among police officers. J Occup Environ Med. 2011;53(8):831–7. doi: 10.1097/JOM.0b013e31821f5ece. [DOI] [PubMed] [Google Scholar]
- 98.Telejko B, Kuzmicki M, Zonenberg A, Modzelewska A, Niedziolko-Bagniuk K, Ponurkiewicz A, et al. Ghrelin in gestational diabetes: serum level and mRNA expression in fat and placental tissue. Exp Clin Endocrinol Diabetes. 2010;118(2):87–92. doi: 10.1055/s-0029-1238313. [DOI] [PubMed] [Google Scholar]
- 99.Miehle K, Stepan H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol. 2012;76(1):2–11. doi: 10.1111/j.1365-2265.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- 100.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. NEJM. 2009;361(14):1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen D, Xia G, Xu P, Dong M. Peripartum serum leptin and soluble leptin receptor levels in women with gestational diabetes. Acta Obstet Gynecol Scand. 2010;89(12):1595–9. doi: 10.3109/00016349.2010.514040. [DOI] [PubMed] [Google Scholar]