Abstract
The basal nucleus of Meynert (BNM) provides the primary cholinergic inputs to the cerebral cortex. Loss of neurons in the BNM is linked to cognitive deficits in Alzheimer’s disease and other degenerative conditions. Numerous animal studies described cholinergic and non-cholinergic neuronal responses in the BNM; however, work in humans has been hampered by the difficulty of defining the BNM anatomically. Here, on the basis of a previous study that delineated the BNM of post-mortem human brains in a standard stereotaxic space, we sought to examine functional connectivity of the BNM, as compared to the nucleus accumbens (or ventral striatum, VS), in a large resting state functional magnetic resonance imaging data set. The BNM and VS shared but also showed a distinct pattern of cortical and subcortical connectivity. Compared to the VS, the BNM showed stronger positive connectivity with the putamen, pallidum, thalamus, amygdala and midbrain, as well as the anterior cingulate cortex, supplementary motor area and pre-supplementary motor area, a network of brain regions that respond to salient stimuli and orchestrate motor behavior. In contrast, compared to the BNM, the VS showed stronger positive connectivity with the ventral caudate and medial orbitofrontal cortex, areas implicated in reward processing and motivated behavior. Furthermore, the BNM and VS each showed extensive negative connectivity with visual and lateral prefrontal cortices. Together, the distinct cerebral functional connectivities support the role of the BNM in arousal, saliency responses and cognitive motor control and the VS in reward related behavior. Considering the importance of BNM in age-related cognitive decline, we explored the effects of age on BNM and VS connectivities. BNM connectivity to the visual and somatomotor cortices decreases while connectivity to subcortical structures including the midbrain, thalamus, and pallidum increases with age. These findings of age-related changes of cerebral functional connectivity of the BNM may facilitate research of the neural bases of cognitive decline in health and illness.
Keywords: functional connectivity, fMRI, resting state, nucleus of Meynert, ventral striatum, basal forebrain
1. Introduction
A prominent feature of the basal forebrain is the collection of large cortically projecting neurons (basal nucleus of Meynert/BNM or magnocellular basal forebrain cell groups) that serve as the primary source of cholinergic input to the entire cortical mantle (Mufson et al., 2003; Raghanti et al., 2011; Selden et al., 1998; Wenk, 1997; Zaborszky et al. 2012, 2013). In Alzheimer’s and related neurodegenerative diseases, there is a severe loss of cholinergic neurons, and the decrement in cholinergic inputs to the cerebral cortex may underlie cognitive deficits that characterize these age-related conditions (Garibotto et al., 2013). Furthermore, decades of clinical trials showed that medications enhancing cholinergic signals have efficacy in improving cognitive functions in some individuals with Alzheimer’s diseases (Doody et al., 2012; Wallace and Bertrand, 2013). Despite its broad involvement in attention, memory, and other cognitive capacities, how the BNM functionally integrates with the rest of the brain is not well understood. This gap of knowledge arises at least in part from the anatomical complexity of the basal forebrain.
The BNM is interdigitated with several anatomical systems in the basal forebrain, including the ventral striopallidal system (ventral pallidum and nucleus accumbens), and cell groups underneath the globus pallidus that bridge the centromedial amygdala to the bed nucleus of the stria terminalis (so-called ‘extended amygdala’; Heimer et al., 1991). This complexity has hampered precise anatomical delineation of the BNM. Recently, with cytoarchitectonics of postmortem human brains, we presented stereotaxic probabilistic maps of the basal forebrain areas containing the magnocellular cell groups (Zaborszky et al., 2008). On histological sections in ten postmortem brains, the individual compartments of the magnocellular cell groups of the basal forebrain were delineated, 3D reconstructed, and warped to a reference space in Montreal Neurological Institute (MNI) coordinates. The superposition of the cytoarchitectonic maps in the MNI brain shows intersubject variability of the various nuclei and their stereotaxic position relative to other brain structures. Both the right and left BNM showed significantly smaller volumes with increasing age (Zaborszky et al., 2008). This probabilistic map would provide a valuable tool for research of the functions of the BNM in humans that has heretofore been difficult.
Numerous studies have suggested connectivity analysis of resting state fMRI data as a useful alternative to characterizing functional architecture of a brain region. Specifically, low frequency blood oxygenation level dependent (BOLD) signal fluctuations reflect connectivity between functionally related brain regions (Biswal et al., 1995; Fair et al., 2007; Fox and Raichle, 2007). For instance, based on the findings that regions with similar functionality tend to be correlated in their spontaneous BOLD activity, investigators described functional subdivisions of many brain structures including the thalamus (Zhang et al., 2008; Zhang et al., 2010), basal ganglia (Barnes et al., 2010), medial superior frontal cortex (Kim et al., 2010; Zhang et al., 2012), anterior cingulate cortex (Margulies et al., 2007), orbitofrontal cortex (Kahnt et al., 2012), cerebellum (O’Reilly et al., 2010), and precuneus (Margulies et al., 2009; Cauda et al., 2010; Zhang and Li, 2012a).
The current study aimed to employ the probabilistic map (Zaborszky et al., 2008) and characterize whole brain functional connectivity of the BNM, as a step to understanding the systems-level functions of this basal forebrain structure in humans. In particular, we compared the functional connectivities of BNM and nucleus accumbens, a ventral striatal structure that is in proximity of the cholinergic space, in the hope of delineating the distinct roles of these two anatomically adjacent structures. In addition to their spatial proximity, both BNM and VS have been implicated in goal-directed behavior (Da Cunha et al., 2012; Grace et al., 2007; Pauli and O’Reilly, 2008; Sarter et al., 2006). In particular, evidence is accruing that the cholinergic and dopaminergic systems may interact to mediate these motivated behaviors (Lester et al., 2010; Mark et al., 2011; Threlfell and Cragg, 2011). For instance, nicotinic receptor blockade or desensitization alters neuronal bursting and dopamine outflow from the VS (Rice and Cragg, 2004). Depletion of cholinergic signals in the ventral striatum resulted in deficits in sensorimotor gating and other cognitive functions (Laplante et al., 2012). Thus, examining the shared and distinct circuits would also further our understanding of the interacting roles of BNM and VS.
Additional goals were to explore the effects of age and gender on the functional connectivities of the BNM and VS. Because the BNM is implicated in age-related cognitive changes, examining age-dependent patterns of BNM connectivity will facilitate research of the neural bases of mild cognitive impairment, Alzheimer’s disease and other memory disorders.
2. Materials and methods
2.1 Resting state data
Resting-state fMRI scans were pooled from three data sets (Leiden_2180/Leiden_2200, Newark, and Beijing_Zang, n=144), downloadable from the 1000 Functional Connectomes Project (Biswal et al., 2010), and our own data (n=81). In selecting the data, we tried to include as many subjects as possible in order to have more stable findings in the current study, as in our earlier work (Zhang et al., 2012; Zhang and Li, 2014). Thus, we used only datasets acquired under conditions identical to our own datasets (e.g., similar TR, all under 3T, all eye closed). Individual subjects’ images were viewed one by one to ensure that the whole brain was covered. A total of 225 healthy subjects’ resting state data (18–53 years of age; 109 men; one scan per participant; duration: 4.5–10 minutes) were analyzed. Table 1 summarizes these data sets.
Table 1.
Demographic information and imaging parameters of the resting-state functional MRI data obtained from the image repository for the 1000 Functional Connectomes Project and our laboratory.
| Dataset | Subjects | Ages (years) | Time points | TR (s) | Slice acquisition order |
|---|---|---|---|---|---|
| Beijing_Zang | 31 M/66 F | 18 – 26 | 225 | 2 | Interleaved ascending |
| Leiden_2180 | 10 M/0 F | 20 – 27 | 215 | 2.18 | Sequential descending |
| Leiden_2200 | 11 M/8 F | 18 – 28 | 215 | 2.2 | Sequential descending |
| Newark | 9 M/9 F | 21 – 39 | 135 | 2 | Interleaved ascending |
| Our own | 48 M/33 F | 19 – 53 | 295 | 2 | Interleaved ascending |
Note: M, males; F, females; TR: repetition time
2.2 Imaging data preprocessing
Brain imaging data were preprocessed using Statistical Parametric Mapping (SPM 8, Wellcome Department of Imaging Neuroscience, University College London, U.K.). Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. Individual structural image was normalized to an MNI (Montreal Neurological Institute) EPI (echo-planar imaging) template with affine registration followed by nonlinear transformation (Ashburner and Friston, 1999, 2005). The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum.
Additional preprocessing was applied to reduce spurious BOLD variances that were unlikely to reflect neuronal activity (Rombouts et al., 2003; Fox et al., 2005; Fair et al., 2007; Fox and Raichle, 2007). The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, white matter, and whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular and white matter signals were also included in the regression.
Cordes and colleagues suggested that BOLD fluctuations below a frequency of 0.1Hz contribute to regionally specific BOLD correlations (Cordes et al., 2001). Thus, we applied a temporal band-pass filter (0.009Hz < f < 0.08Hz) to the time course in order to obtain low-frequency fluctuations, as in previous studies (Lowe et al., 1998; Fox et al., 2005; Fair et al., 2007; Fox and Raichle, 2007).
2.3 Head motion
As extensively investigated in Van Dijk et al., 2012, micro head motion (>0.1mm) is an important source of spurious correlations in resting state functional connectivity analysis. Therefore, we applied a “scrubbing” method proposed by Power and colleagues (Power et al., 2012) and successfully applied in previous studies (Smyser et al., 2010; Power et al., 2012; Tomasi and Volkow, 2012) to remove time points affected by head motions. Briefly, for every time point t, we computed the framewise displacement given by FD(t) = |Δdx(t)| + |Δdy(t)| + |Δdz(t)| + r|α(t)| + r|β(t)| + r|gamma;(t)|, where (dx,dy,dz)and (α,β,γ) are the translational and rotational movements, respectively, and r (= 50mm) is a constant that approximates the mean distance between center of MNI space and the cortex and transform rotations into displacements (Power et al., 2012). The second head movement metric was the root mean square variance (DVARS) of the differences in % BOLD intensity I(t) between consecutive time points across brain voxels, computed as follows: , where the brackets indicate the mean across brain voxels. Finally, to compute each subject’s correlation map, we removed every time point that exceeded the head motion limit FD(t)>0.5mm or DVARS(t)>0.5% (Power et al., 2012; Tomasi and Volkow, 2012). On average, 1% of the time points were removed across subjects, and 2 (out of 225) individuals were excluded from further analyses because of excessive head movements.
2.4 Seed regions: basal nucleus of Meynert and ventral striatum
A mask of the basal nucleus of Meynert was created based on a stereotaxic probabilistic map of magnocellular cell groups in the basal forebrain (Zaborszky et al., 2008). Briefly, a T1-weighted MRI scan of 1.17 x 1 x1 mm was obtained of each individual brain (n=10) before histological processing. The outlines of various basal forebrain compartments were traced on 2D images of silver-stained (Merker, 1983) histological sections (20 μm thick, 1.2 mm apart) with a final resolution of 7000 × 6000 pixels. The outlines were processed as contour line for each histological section. As described in Zaborszky et al., 2008, we used a modified version of the Ch1–Ch4 nomenclature of Mesulam (Mesulam et al., 1983) to delineate the magnocellular basal forebrain cell groups. Ch1–Ch2 compartment largely corresponds to the medial septum and the diagonal band of Broca. The Ch3 group corresponds to the horizontal nucleus of the diagonal band of Broca as defined by Hedreen et al (1984). Cell aggregates in the subcommissural-sublenticular region largely correspond to the basal nucleus of Meynert as defined by Vogels et al. (1990) and De Lacalle et al. (1991) and were collectively defined as “Ch4”. Subdivisions of the “Ch4” as suggested by Mesulam et al. (1983) were not delineated.
Magnocellular neurons in the basal forebrain often constitute cell aggregates or clusters that facilitate their recognition. As a rule, borders of delineated areas were drawn where the number of large cells decreased precipitously. Details of reconstruction of these nuclei on a 3D template were described in Zaborszky et al., 2008. Briefly, a 3D volume was reconstructed, with a known algorithm from the digitized histological sections using the MRI scans of the fixed brain as a shape reference with a resolution of 1 mm isotropic (Schormann and Zilles, 1998 and Hoemke, 2006). Each 3D reconstructed histological volume was spatially normalized to the single subject T1-weighted MNI reference brain (Collins et al., 1994 and Holmes et al., 1998) using a combination of affine linear and non-linear, elastic transformations (Henn et al., 1997 and Hoemke, 2006). Based on the 3D whole-brain reconstructions, volume and shape of the magnocellular cell groups were computed. Probability maps were computed separately for each Ch compartment. In this study we used the mask that corresponds to the Ch4 cell groups. These probabilistic maps describe the relative frequency with which the same basal forebrain area (e.g., Ch4) was represented in each voxel of the reference space.
In primates, the nucleus accumbens merges imperceptibly with the rostroventral parts of the caudate nucleus and putamen. We used both cytoarchitectonic and topographical criteria to generate a ventral striatal mask, that largely corresponds to the nucleus accumbens using the following criteria for delineation on enlarged images of the histological sections: there is a higher cell density in the nucleus accumbens than in dorsal striatum; the slightly smaller cells are of round/oval shape; and very few or no fiber bundles pass through the area. Caudally, the lower edge of the ventral pallidum, which appears dark due to its high iron content in silver-stained sections, served as the dorsal border. However, ventral pallidal islands are invaginated into the nucleus accumbens; therefore, caudally the ventral striatal volume inevitably contains some ventral pallidal tissue. In the region of the anterior perforated substance, the nucleus accumbens extends almost to the ventral surface of the brain. Cell groups such as the ‘interface islands’ (Heimer, 2000) were excluded, provided that they are surrounded by thin glial layer.
These two regions of interest are shown in Figure 1. We excluded from each region the overlapping voxels (yellow) to form the BNM and VS seed regions for connectivity analyses.
Figure 1.
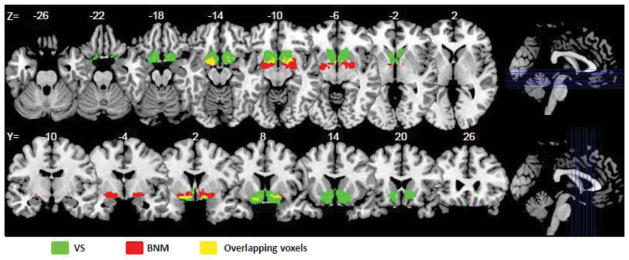
Seed regions: basal nucleus of Meynert (red) and ventral striatum (green), with overlapping voxels in yellow, shown on axial (from z=−26 to +2) and coronal (from y=−10 to +26) sections. Overlapping voxels were removed from each seed region prior to whole brain regressions.
2.5 Orthogonalization
To highlight the distinct functional connectivity patterns between basal nucleus of Meynert (BNM) and the ventral striatum (VS), we constructed BNM and VS seed masks by excluding the overlapping area between them as depicted in Figure 1. Additionally, the Gram-Schmidt method was used to orthogonalize the BNM and VS signals (Margulies et al., 2007; Di Martino et al., 2008; Tomasi and Volkow, 2012). The orthogonalization process highlights the variances unique to BNM and VS signals, by reducing the common confounding fluctuations. However, when querying cerebral functional connectivity of a region of interest (ROI), investigators are primarily interested in connectivities of the ROI without considering whether these results are independent of those of other brain regions. Therefore, we presented the results obtained with the original time series in the main text and those with the orthogonalized time series in the Supplement. In practice, orthogonalization is expected to have little impact when comparing the BNM and VS correlation maps, since common variations are eliminated in the statistical test.
2.6 Seed region-based linear correlation and random effects analysis
The BOLD time courses were averaged spatially over each of the two seeds. For individual subjects, we computed the correlation coefficient between the averaged time course of each seed region and the time courses of all other brain voxels. To assess and compare the resting state “correlograms,” we converted these image maps, which were not normally distributed, to z score maps by Fisher’s z transform (Jenkins and Watts, 1968; Berry and Mielke, 2000): z= 0.5loge [(1 + r)/(1 − r)]. The Z maps were used in group random effect analyses. We performed one-sample t test each on the Z maps of BNM and VS and paired-sample t test comparing the two Z maps.
2.7 Age dependent changes and gender differences in BNM and VS connectivity
We performed a simple regression of the Z maps against age, each for the BNM and VS, to identify age-related changes of functional connectivity in the two structures. To examine gender differences, we compared men and women with age as a covariate in an analysis of variance, each for the BNM and VS. All results were reported for a corrected threshold.
3. Results
3.1 Whole brain functional connectivity of the nucleus of Meynert and ventral striatum
For each seed region, we performed one sample t-test of the Z maps (positively and negatively correlated regions) across the group (n=223). Regions functionally connected with nucleus of Meynert (BNM) and ventral striatum (VS) are presented in Figures 2 and 3, respectively.
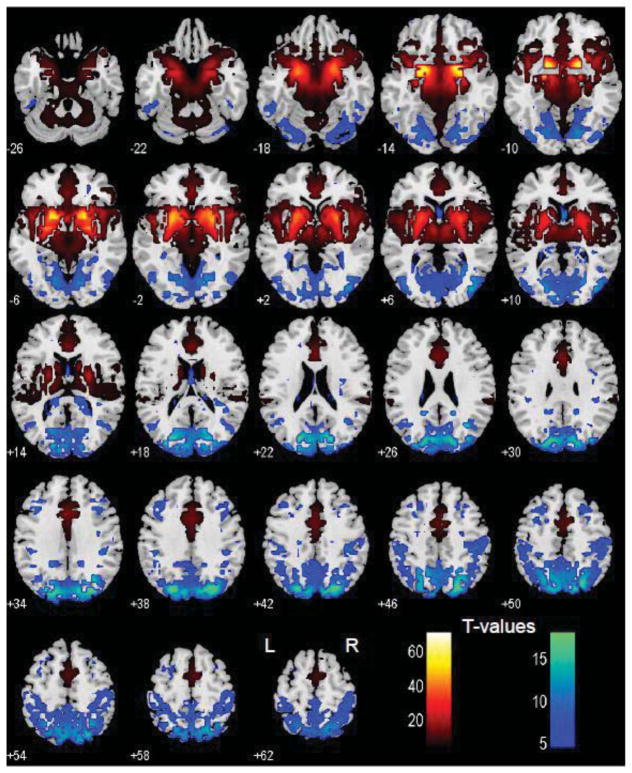
Figure 2.
Brain areas that show positive (warm color) and negative (cool color) functional connectivity to the basal nucleus of Meynert; one-sample t test, p<0.05, corrected for familywise error of multiple comparisons.
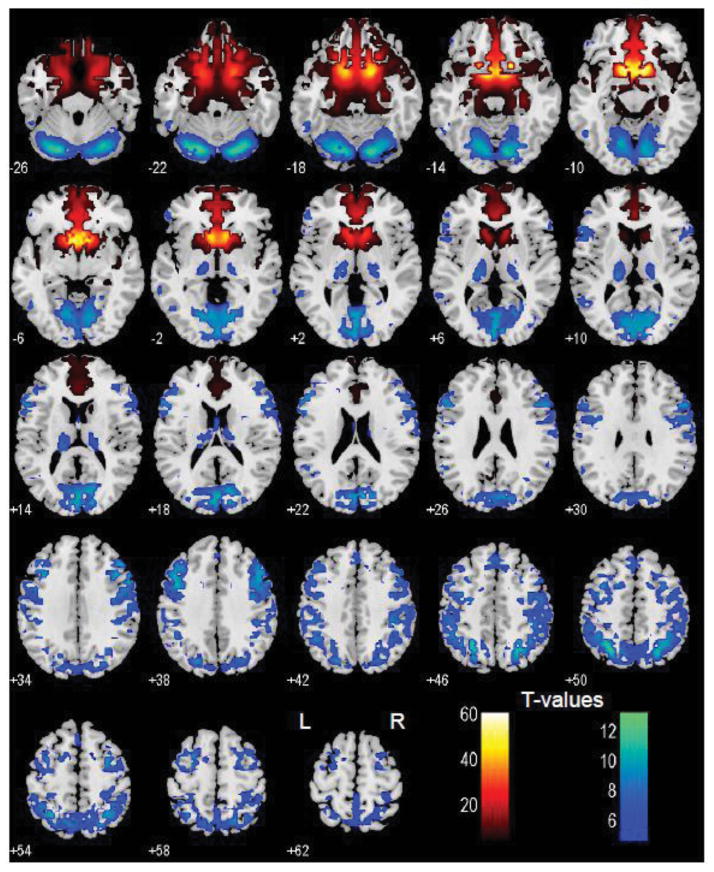
Figure 3.
Brain areas that show positive (warm color) and negative (cool color) functional connectivity to the ventral striatum; one-sample t test, p<0.05, corrected for familywise error of multiple comparisons.
The BNM showed positive connectivity with the medial prefrontal cortex, including dorsal/rostral/perigenual/subgenual anterior cingulate cortex (ACC), supplementary motor area (SMA) as well as pre-SMA, medial orbitofrontal cortex, inferior temporal pole, hippocampus, amygdala, insula, thalamus, midbrain, and basal ganglia. The BNM showed negative connectivity with the cuneus, parahippocampal gyri, precuneus, posterior parietal cortices, middle and lateral frontal cortices, including dorsolateral prefrontal cortex and frontal eye field, and the cerebellum (Figure 2).
The VS showed positive connectivity with the medial orbitofrontal cortex, rectus gyrus, subcallosal gyrus, inferior temporal pole, amygdala, hippocampus, perigenual/subgenual ACC, and ventral part of the caudate head. The VS showed negative connectivity with the visual cortices, posterior parietal cortex, temporoparietal junction, superior temporal gyri, precentral cortex, lateral frontal cortices including the dorsolateral prefrontal cortex, inferior frontal cortices, posterior thalamus/pulvinar, parahippocampal gyrus, and the cerebellum (Figure 3). These results are consistent with previous findings of resting state VS connectivity with the medial orbitofrontal cortex and ventral caudate (Baliki et al., 2013; Cauda et al., 2011; Di Martino et al., 2008; Gopinath et al., 2011), amygdala and midbrain (Cauda et al., 2011), and hippocampus (Cauda et al., 2011; Kahn and Shohamy, 2013).
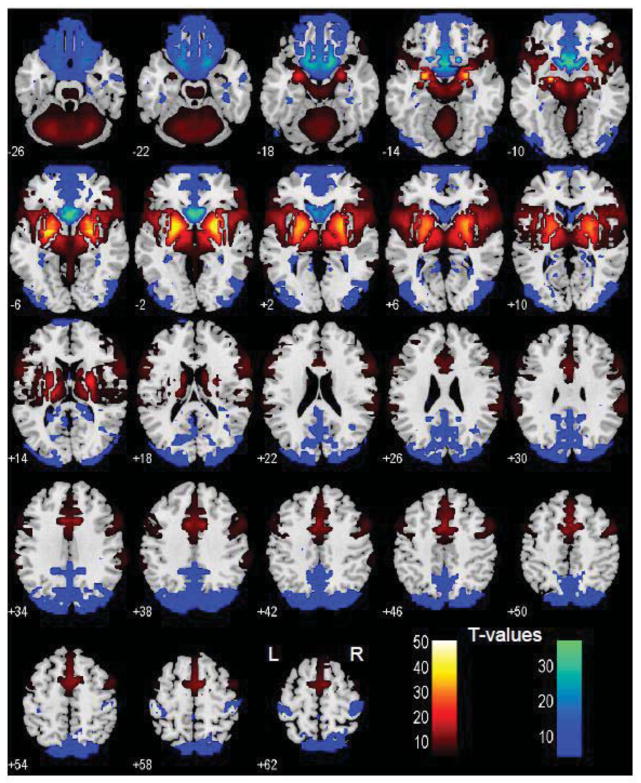
We performed a paired t-test to compare functional connectivity of the BNM and VS (Figure 4). Compared to the VS, the BNM showed greater connectivity with the medial frontal cortex, including rostral/dorsal ACC, SMA, pre-SMA, dorsolateral prefrontal cortex, inferior frontal cortex, lateral orbitofrontal cortex, inferior parietal lobule, temporo-parietal junction, insula, thalamus, dorsal caudate, midbrain, and the cerebellum. Compared to the BNM, VS showed greater connectivity with the medial orbitofrontal cortex, ventral caudate, subcallosal/rectus gyri, olfactory sulcus, cuneus, precuneus, posterior cingulate cortex, parahippocampal gyrus, middle/inferior occipital cortex, and postcentral gyri. We summarize these results in Table 2 and Table 3, where we further distinguish whether a contrast arises from differences in positive or negative connectivity.
Figure 4.
Brain areas that show differences in functional connectivity to the basal nucleus of Meynert (BNM) versus ventral striatum (VS): BNM > VS (warm color); VS > BNM (cool color); paired-sample t test, p<0.05, corrected for familywise error of multiple comparisons.
Table 2.
Regions showing greater connectivity with basal nucleus of Meynert (BNM) as compared to ventral striatum (VS); paired t-test, n=223.
| volume (mm3) | peak voxel (Z) | MNI coordinate | side | identified brain region | connectivity | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | BNM | VS | ||||
| 365472 | inf | 24 | −1 | −8 | R | Putamen/Pallidum/Thalamus/Midbrain/Cerebellum/Amygdala/Insula/TP/lOFC | ++ | +/~/− |
| inf | −24 | −4 | −8 | L | Putamen/Pallidum/Thalamus/Midbrain/Cerebellum/Amygdala/Insula/TP/lOFC | ++ | +/~/− | |
| inf | 9 | 11 | 34 | R | ACC/SMA/pre-SMA | + | ~/− | |
| inf | −9 | 14 | 37 | L | ACC/SMA/pre-SMA | + | ~/− | |
|
| ||||||||
| 4563 | 7.48 | 63 | −31 | 28 | R | Temporo-parietal junction/Supramarginal G | +/~ | −/-- |
| 7.33 | −66 | −28 | 25 | L | Temporo-parietal junction/Supramarginal G | +/~ | −/-- | |
|
| ||||||||
| 1026 | 6.60 | 63 | −46 | 1 | R | Middle temporal G/Inferior frontal G/DLPFC | + | − |
|
| ||||||||
| 729 | 6.05 | −60 | −55 | 1 | L | Middle temporal G/Inferior frontal G/DLPFC | + | − |
Note: inf: infinity; R: right; L: left; TP: temporal pole; lOFC: lateral orbitofrontal cortex; G: gyrus; ACC: anterior cingulate cortex; SMA: supplementary motor area; DLPFC: dorsolateral prefrontal cortex; +/− indicates positive and negative connectivity (with ++/-- for stronger connectivity), and ~ indicates no significant connectivity at one-sample t test, p<0.05, FWE corrected.
Table 3.
Regions showing greater connectivity with ventral striatum (VS) as compared to the basal nucleus of Meynert (BNM); paired t-test, n=223.
| volume mm3 | peak voxel Z | MNI coordinate | side | identified brain region | connectivity | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | BNM | VS | ||||
| 326160 | inf | 12 | 17 | −11 | R | ventral Caudate/mOFC/Subcallosal G/Rectus G/Olfactory S | ~/− | ++ |
| inf | −9 | 14 | −14 | L | ventral Caudate/mOFC/Subcallosal G/Rectus G/Olfactory S | ~/− | ++ | |
| inf | −18 | −49 | 22 | L | pCG/Cuneus/Precuneus/Parahippocampal G | – – | ~/− | |
| inf | 18 | −46 | 25 | R | pCG/Cuneus/Precuneus/Parahippocampal G | – – | ~/− | |
|
| ||||||||
| 1512 | 6.47 | 42 | −28 | 64 | R | Postcentral G | − | ~ |
|
| ||||||||
| 864 | 5.91 | −39 | −31 | 67 | L | Postcentral G | − | ~ |
Note: inf: infinity; R: right; L: left; mOFC: medial orbitofrontal cortex; G: gyrus; S: sulcus; pCG: posterior cingulate gyrus; +/− indicates positive and negative connectivity (with ++/-- indicating stronger connectivity), and ~ indicates no significant connectivity at one-sample t test, p<0.05 FWE corrected.
3.2 The effects of age and gender on BNM and VS connectivity
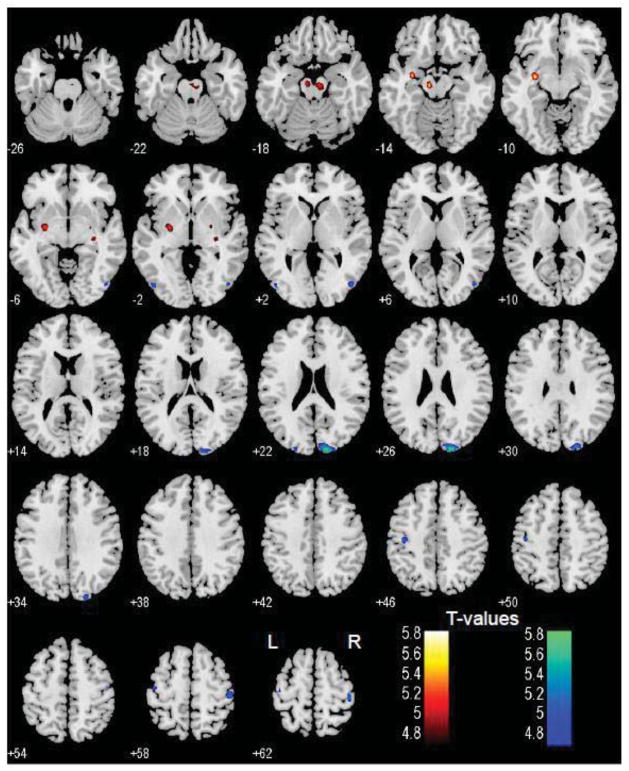
In a simple regression, BNM connectivity to the midbrain in the area of the ventral tegmental area and substantia nigra (MNI coordinates [−9 −16 −14], Z=5.41; and [6 −19, −20], Z=5.08, 1215 mm3), left pallidum and parahippocampal gyrus ([−30 −7 −11], Z=5.62, 1026 mm3), and right pallidum ([27 −7 −5], Z=5.04, 189 mm3) showed a positive correlation with age (Figure 5). In contrast, BNM connectivity to the bilateral motor cortices ([−33 −37 70], Z=5.63; and [−45 −16 61], Z=4.85, 2187 mm3; and [45 −25 64], Z=5.44, 1161 mm3) and right visual cortex ([15 −94 25], Z=5.59, 2133 mm3) showed a negative correlation with age. At the same, corrected threshold, VS connectivities did not show any age dependence.
Figure 5.
Brain areas with functional connectivity to the basal nucleus of Meynert (BNM) that correlates positively (warm color) and negatively (cool color) with age. p<0.05, corrected for familywise error of multiple comparisons.
In a covariance analysis with age as a covariate, we examined gender differences. Men and women did not differ in BNM or VS connectivity at the same, corrected threshold.
4. Discussion
4.1 Functional connectivity of the nucleus of Meynert and ventral striatum
Compared to the VS, the BNM showed stronger positive connectivity with the putamen, pallidum, thalamus, amygdala and midbrain, cerebellum, as well as the anterior cingulate cortex, supplementary motor area and pre-supplementary motor area, a network of brain regions that respond to salient stimuli and orchestrate goal-directed behavior. In contrast, the VS showed stronger positive connectivity with the ventral caudate and medial orbitofrontal cortex, areas implicated in reward processing, compared to the VS. Together, the distinct cerebral functional connectivities support the role of the BNM in arousal and cognitive motor control and the VS in reward related behavior.
The BNM shows connectivity with a selective set of cortical and subcortical structures, consistent with anatomical studies in rodents and non-human primates (Chandler and Waterhouse, 2012; Mesulam et al., 1984; Zaborszky et al., 2013). In particular, studies in monkeys showed that the density of cholinergic fibers and choline acetyltransferase activities are particularly rich in agranular areas of the insula, caudal orbitofrontal cortex (OFC), temporal pole, and parahippocampal region (Mesulam et al., 1984; 1986), a pattern mirrored by the current findings of regions positively connected to the BNM (Figure 2). The VS receives extensive projections from the OFC (Haber et al., 2006), amygdala, and the dysgranular part of the insula that process taste, olfaction and other visceral information (Fudge et al., 2005; Nakano et al., 1999), in accord with VS responses to primary reinforcers.
4.2 Functional implications for the basal nucleus of Meynert and ventral striatum
The BNM, along with pulvinar and amygdala, responds to salient stimuli (Morris et al., 1997). Manipulations of cholinergic signaling alter neural responses to salient stimuli in a variety of behavioral paradigms, including odd-ball tasks (Klinkenberg et al., 2013). For instance, during auditory conditioning in humans, augmenting cholinergic signaling with physostigmine (an inhibitor of acetylcholinesterase) enhances processing of behaviorally irrelevant stimuli and attenuates differential conditioning-related cortical activations (Thiel et al., 2002). Scopolamine, an antagonist of muscarinic acetylcholine receptors, impairs categorization of new but not learnt visual stimuli in monkeys (Aggelopoulos et al., 2011). Donepezil, a cholinesterase inhibitor, facilitates reaction time to targets following a cue that instructs voluntary shift of attention (Rokem et al., 2010). In rodents, increasing cholinergic activity in gustatory cortex enhances the salience of a familiar, conditioned stimulus in taste aversion learning (Clark and Bernstein, 2009). Cholinergic deafferentation lowers the signal-to-noise ratio of target evoked responses in posterior parietal neurons (Broussard et al., 2009). Infusion of a cholinergic immunotoxin in the basal forebrain compromises cued target detection (Bushnell et al., 1998). A study using microdialysis demonstrated that acetylcholine release in the frontal cortex and hippocampus is elicited by behavioral conditioning to salient stimuli (Acquas et al., 1996). Taken together, numerous studies support a role of the BNM in processing salient and novel stimuli for learning and cognitive motor control (Wenk, 1997).
Although earlier studies largely focused on the role of the ventral striatum (VS) in reward processing, a growing body of work has suggested that the VS responds not only to rewarding but more generally to stimuli that are just salient (Jensen et al., 2003; 2007; Litt et al., 2011; Pohlack et al., 2012; Veldhuizen et al., 2011; Zink et al., 2003; 2006; see also Brooks and Berns, 2013 for a review). For instance, the VS responds to painful stimulation as well as anticipation of such aversive stimuli (Jensen et al., 2003; Zink et al., 2006). A critical issue then is to distinguish functional roles of the BNM and VS in saliency processing. The current findings on the differences in functional connectivity may help address this question. Compared to the VS, the BNM shows stronger positive connectivity with the medial frontal and fronto-parietal cortices, as well as the thalamus, insula, basal ganglia, and midbrain – areas that form a task-related network (Robinson et al., 2009; Zhang et al., 2008), as well as stronger negative connectivity with the precuneus, a core area of the default mode network (Raichle et al., 2001; Raichle and Snyder, 2007; Zhang and Li, 2012a). These differences suggest that, compared to the VS, the BNM is more directly and readily engaged in task related processing.
This difference in functional connectivity is consistent with a general role of the BNM in task-related behavior such as perception and perceptual motor learning and memory (Chen et al., 2012; Connor et al., 2010; Froemke et al., 2013; Goard and Dan, 2009; Leach et al., 2013; Miasnikov and Weinberger, 2012). In particular, the BNM may mediate learning and memory even when such processes are motivationally neutral. In behavioral conditioning of rats, specific associative memory can be induced by direct activation of the BNM without detectable motivational effects as in seeking reward or avoiding punishment (Miasnikov et al., 2008). Indeed, neurons in the BNM did not respond to primary reinforcers, positive (juice) or negative (salt water), in monkeys (Wilson and Rolls, 1990). While the BNM responds to visual or acoustic stimuli that signal reinforcers, it does not respond to the reinforcers themselves (Wilson and Ma, 2004). Other work suggests that the BNM neurons encode saliency irrespective of valence of the stimuli (Lin and Nicolelis, 2008). These results are concordant with a learning-related or memory-promoting role for the BNM that places it “downstream” of motivational systems (Miasnikov et al., 2008), consistent with the anatomical finding that, the cholinergic neurons in basal forebrain receive input from the nucleus accumbens (Zaborszky and Cullinan, 1992). Through its extensive cortical projections, the BNM may mediate attentional effort in a circuitry that integrates brain systems involved in modulation of input functions, incentive processing and performance monitoring (Sarter et al., 2006).
On the other hand, one is cautioned not to over-interpret the differences of BNM and VS in saliency- and reward- related processing. For instance, reward is intrinsically salient, and cerebral structures such as the medial orbitofrontal cortex, with positive connectivity to both the BNM and VS, is involved in processing reward and emotionally salient stimuli (Hardin et al., 2009; Kühn and Gallinat, 2012; Liu et al., 2011; Rothkirch et al., 2012). Likewise, as discussed earlier, although VS is predominantly implicated in reward processing, there is accumulating evidence that VS responds to salient, non-rewarding stimuli (Esslinger et al., 2013; Jensen et al., 2007; Pohlack et al., 2012; Spicer et al., 2007; Zink et al., 2003; 2006). Future research may take advantage of the current findings in further delineating the respective roles of BNM and VS in motivated behavior.
4.3 Age-related connectivity of the nucleus of Meynert
Previous imaging studies suggest that aging is related in a complicated way to cerebral functional connectivities, with different brain areas increasing and decreasing with age in connectivity to regions of interest (Bernard et al., 2013; Campbell et al., 2013; Hafkemeijer et al., 2013; Hoffstaedter et al., 2014; Liu et al., 2012; Roski et al., 2013; Taniwaki et al., 2007; Ystad et al., 2010; see also Ferreira and Busatto, 2013 for a review). Here, we observed that age is associated with increased BNM connectivity to subcortical structures including the midbrain and pallidum and decreased connectivity to somatomotor and visual cortices. Although statistically highly significant, these results are not straightforward to interpret without a functional concomitant. Given that the current BOLD time series were obtained during resting state, one is tempted to speculate that these changes are not related to specific contextual or task conditions. Future studies are needed to examine the relationship between this contrasting pattern of increased and decreased connectivity to subcortical and cortical structures and cognitive motor performance (Hu et al., 2012; 2013) and evaluate whether and to what extent these changes may reflect a compensatory process.
In over 1,000 adolescents and young adults, Tomasi and Volkow characterized age-related increases in the functional connectivity of the ventral tegmental area (VTA) with limbic regions and with the default mode network and decreases in the connectivity of the substantia nigra (SN) with motor and medial temporal cortices (Tomasi and Volkow, 2012). In terms of mean strength and statistical significance of connectivity, the VTA demonstrated bilateral positive connectivity with the nucleus accumbens, hippocampus, parahippocampus, globus pallidus, caudate, cerebellar vermis, and anterior insula, as well as the angular gyrus, inferior frontal cortex, and anterior cingulate cortex, and negative connectivity with the inferior occipital cortex. The SN demonstrated bilateral positive connectivity with globus pallidus, subthalamic nucleus, thalamus, and cerebellar vermis, as well as the Broca’s and Wernickes’s areas, anterior cingulate cortex, and supplementary motor area, and negative connectivity with orbitofrontal, temporal, and occipital cortices, ventral precuneus, and angular gyrus. Thus, there is much overlap between subcortical and cortical connectivities between the VTA/SN and BNM/VS, suggesting the need in future studies to investigate early developmental changes in functional connectivity of the BNM/VS and their relevance to clinical conditions that manifest early during development.
4.4 Potential clinical implications
In addition to implications for our understanding of the cognitive deficits in Alzheimer’s disease and other forms of age-related cognitive decline, the current findings may also facilitate research of the cholinergic mechanisms of drug and alcohol addiction (Adinoff et al., 2010; Kurosawa et al., 2013; Miki et al., 2013; Paolone et al., 2013; Williams and Adinoff, 2008). Altered cholinergic neurotransmission is known to play a critical role in nicotine addiction (Dani and Balfour, 2011). More broadly, nicotinic acetylcholine receptors are heavily expressed in limbic dopamine circuitry and the medial habenula-interpeduncular nucleus complex, which are critical mediators of reinforced and addictive behavior (Leslie et al., 2013; Mark et al., 2011). For instance, behavioral performance that requires sustained attention is associated with extracellular levels of cortical acetylcholine. Rats that are prone to attribute incentive salience to reward cues (“sign trackers”), relative to “goal-trackers”, are vulnerable to developing obesity and addiction (Paolone et al., 2013). Because of its interaction of the limbic reward and prefrontal cognitive control circuits, cholinergic neurotransmission has been a target for pharmacotherapy development for stimulant addiction (Sofuoglu and Mooney, 2009). Thus, the current findings of the systems level functional connectivity of the BNM will help future studies to pursue the etiology and treatment of addiction on these related fronts.
Furthermore, the VS and its cerebral connectivity has been implicated in various neuropsychiatric disorders such as autism (Delmonte et al., 2013; Di Martino et al., 2011) or psychological processes of clinical interest such as consciousness (Cauda et al., 2009a) and pain (Baliki et al., 2013; Cauda et al., 2009b; 2010). For instance, with differential functional and structural connectivity, the shell and core regions of the VS may each mediate prediction of monetary reward and cessation of pain (Baliki et al., 2013). Functional connectivity of the VS and hippocampus may allow integration of motivational significance and memory to guide behavior (Kahn and Shohamy, 2013), a process of relevance to depression and psychomotor retardation. Notably, animal studies have also implicated the BNM in pain (Zhang et al., 2010) and anesthesia (Laalou et al., 2008); thus, the current findings may shed light on future studies to directly contrast the roles of VS and BNM in these neural processes of clinical significance.
4.5 A methodological consideration
Negative functional connectivity has been observed and reported since the very beginning of the resting state fMRI study (Biswal et al., 1995). Negative functional connectivity, also called anti-correlation, represents negative cross-correlation in spontaneous BOLD signal between two brain regions. It was suggested that the global signal regression, a common step of data preprocessing in seed region based functional connectivity analyses, is a likely cause of anti-correlated functional networks (Murphy et al., 2009; Weissenbacher et al., 2009). However, recent investigations demonstrated that the negative correlations are not an artifact but have biological origins (Fox et al., 2009; Chen et al., 2011; Chai et al., 2012). For instance, negative functional connectivity is associated predominantly with long range connections and correlates with the shortest path length in the human brain network (Scholvinck et al., 2010; Chen et al., 2011; Schwarz and McGonigle, 2011). Indeed, the negative correlations between brain regions with presumably opposing functional roles have been consistently observed in different studies (Greicius et al., 2003; Fox et al., 2005; Fransson, 2005; Kelly et al., 2008; Uddin et al., 2009; Chen et al., 2011), including those using independent component analysis, which does not involve global signal regression (Cole et al., 2010; Zuo et al., 2010; Zhang and Li, 2012b). Furthermore, the existence of the negative functional connectivity was also suggested by computational simulations of cerebral network activities in both monkeys and humans (Honey et al., 2007; Izhikevich and Edelman, 2008; Deco et al., 2009) and supported by simultaneous recording of unit activity and local field potential from task-positive and task-negative (default mode) networks in cats (Popa et al., 2009). Together, these earlier studies suggest functional significance of negative functional connectivity.
Supplementary Material
Acknowledgments
This study was supported by NIH grants R01DA023248, K02DA026990, R21AA018004, R01AA021449 to C-S.R.L. and NINDS grant 23945 to LZ. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. J.S.I. was supported by Young Researcher Award (Sao Paulo Research Foundation FAPESP 2011/08573-4). We thank investigators of the 1000 Functional Connectomes Project and those who shared the data set for making this study possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acquas E, Wilson C, Fibiger HC. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation, and fear. J Neurosci. 1996 May 1;16(9):3089–96. doi: 10.1523/JNEUROSCI.16-09-03089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Devous MD, Sr, Williams MJ, Best SE, Harris TS, Minhajuddin A, Zielinski T, Cullum M. Altered neural cholinergic receptor systems in cocaine-addicted subjects. Neuropsychopharmacology. 2010 Jun;35(7):1485–99. doi: 10.1038/npp.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggelopoulos NC, Liebe S, Logothetis NK, Rainer G. Cholinergic control of visual categorization in macaques. Front Behav Neurosci. 2011 Nov 15;5:73. doi: 10.3389/fnbeh.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J Neurosci. 2013 Oct 9;33(41):16383–93. doi: 10.1523/JNEUROSCI.1731-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Peltier SJ, Wiggins JL, Jaeggi SM, Buschkuehl M, Fling BW, Kwak Y, Jonides J, Monk CS, Seidler RD. Disrupted cortico-cerebellar connectivity in older adults. Neuroimage. 2013 Dec;83:103–19. doi: 10.1016/j.neuroimage.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KA, Cohen AL, Power JD, Nelson SM, Dosenbach YB, Miezin FM, Petersen SE, Schlaggar BL. Identifying Basal Ganglia divisions in individuals using resting-state functional connectivity MRI. Front Syst Neurosci. 2010;4:18. doi: 10.3389/fnsys.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry KJ, Mielke PW., Jr A Monte Carlo investigation of the Fisher Z transformation for normal and nonnormal distributions. Psychol Rep. 2000;87:1101–1114. doi: 10.2466/pr0.2000.87.3f.1101. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke P, Brown S, Ngan E, Liotti M. Functional brain organization of preparatory attentional control in visual search. Brain Res. 2013 Sep 12;1530:32–43. doi: 10.1016/j.brainres.2013.07.032. [DOI] [PubMed] [Google Scholar]
- Brooks AM, Berns GS. Aversive stimuli and loss in the mesocorticolimbic dopamine system. Trends Cogn Sci. 2013 Jun;17(6):281–6. doi: 10.1016/j.tics.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Broussard JI, Karelina K, Sarter M, Givens B. Cholinergic optimization of cue-evoked parietal activity during challenged attentional performance. Eur J Neurosci. 2009 Apr;29(8):1711–22. doi: 10.1111/j.1460-9568.2009.06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ, Chiba AA, Oshiro WM. Effects of unilateral removal of basal forebrain cholinergic neurons on cued target detection in rats. Behav Brain Res. 1998 Jan;90(1):57–71. doi: 10.1016/s0166-4328(97)00082-x. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Grigg O, Saverino C, Churchill N, Grady CL. Age differences in the intrinsic functional connectivity of default network subsystems. Front Aging Neurosci. 2013 Nov 14;5:73. doi: 10.3389/fnagi.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Cocito D, Paolasso I, Isoardo G, Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 2010 Jul;81(7):806–11. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- Cauda F, Geminiani G, D’Agata F, Sacco K, Duca S, Bagshaw AP, Cavanna AE. Functional connectivity of the posteromedial cortex. PLoS One. 2010 Sep 30;5(9) doi: 10.1371/journal.pone.0013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Cavanna AE, D’agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011 Oct;23(10):2864–77. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Cauda F, Micon BM, Sacco K, Duca S, D’Agata F, Geminiani G, Canavero S. Disrupted intrinsic functional connectivity in the vegetative state. J Neurol Neurosurg Psychiatry. 2009a Apr;80(4):429–31. doi: 10.1136/jnnp.2007.142349. [DOI] [PubMed] [Google Scholar]
- Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009b;4(2):e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D, Waterhouse BD. Evidence for broad versus segregated projections from cholinergic and noradrenergic nuclei to functionally and anatomically discrete subregions of prefrontal cortex. Front Behav Neurosci. 2012 May 21;6:20. doi: 10.3389/fnbeh.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chen G, Xie C, Li SJ. Negative functional connectivity and its dependence on the shortest path length of positive network in the resting-state human brain. Brain Connect. 2011;1:195–206. doi: 10.1089/brain.2011.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, Sur M. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):E2832–41. doi: 10.1073/pnas.1206557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EW, Bernstein IL. Boosting cholinergic activity in gustatory cortex enhances the salience of a familiar conditioned stimulus in taste aversion learning. Behav Neurosci. 2009 Aug;123(4):764–71. doi: 10.1037/a0016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Conner JM, Kulczycki M, Tuszynski MH. Unique contributions of distinct cholinergic projections to motor cortical plasticity and learning. Cereb Cortex. 2010 Nov;20(11):2739–48. doi: 10.1093/cercor/bhq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Da Cunha C, Gomez-A A, Blaha CD. The role of the basal ganglia in motivated behavior. Rev Neurosci. 2012;23(5–6):747–67. doi: 10.1515/revneuro-2012-0063. [DOI] [PubMed] [Google Scholar]
- Dani JA, Balfour DJ. Historical and current perspective on tobacco use and nicotine addiction. Trends Neurosci. 2011 Jul;34(7):383–92. doi: 10.1016/j.tins.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lacalle S, Iraizoz J, Ma Gonzalo L. Differential changes in cell size and number in topographic subdivisions of human basal nucleus in normal aging. Neuroscience. 1991;43:445–456. doi: 10.1016/0306-4522(91)90307-a. [DOI] [PubMed] [Google Scholar]
- Delmonte S, Gallagher L, O’Hanlon E, McGrath J, Balsters JH. Functional and structural connectivity of frontostriatal circuitry in Autism Spectrum Disorder. Front Hum Neurosci. 2013 Aug 6;7:430. doi: 10.3389/fnhum.2013.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa V, McIntosh AR, Sporns O, Kotter R. Key role of coupling, delay, and noise in resting brain fluctuations. Proc Natl Acad Sci U S A. 2009;106:10302–10307. doi: 10.1073/pnas.0901831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008 Dec;18(12):2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, Lord C, Castellanos FX, Milham MP. Aberrant striatal functional connectivity in children with Autism. Biological Psychiatry. 2011 May;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Cummings JL, Farlow MR. Reviewing the role of donepezil in the treatment of Alzheimer’s disease. Curr Alzheimer Res. 2012 Sep;9(7):773–81. doi: 10.2174/156720512802455412. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Braun U, Schirmbeck F, Santos A, Meyer-Lindenberg A, Zink M, Kirsch P. Activation of midbrain and ventral striatal regions implicates salience processing during a modified beads task. PLoS One. 2013;8(3):e58536. doi: 10.1371/journal.pone.0058536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 2013 Mar;37(3):384–400. doi: 10.1016/j.neubiorev.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, Martins AR, Zaika N, Bernstein H, Wachs M, Levis PA, Polley DB, Merzenich MM, Schreiner CE. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci. 2013 Jan;16(1):79–88. doi: 10.1038/nn.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005 Sep 19;490(2):101–18. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibotto V, Tettamanti M, Marcone A, Florea I, Panzacchi A, Moresco R, Virta JR, Rinne J, Cappa SF, Perani D. Cholinergic activity correlates with reserve proxies in Alzheimer’s disease. Neurobiol Aging. 2013 Nov;34(11):2694.e13–8. doi: 10.1016/j.neurobiolaging.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009 Nov;12(11):1444–9. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath K1, Ringe W, Goyal A, Carter K, Dinse HR, Haley R, Briggs R. Striatal functional connectivity networks are modulated by fMRI resting state conditions. Neuroimage. 2011 Jan 1;54(1):380–8. doi: 10.1016/j.neuroimage.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007 May;30(5):220–7. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006 Aug 9;26(32):8368–76. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedreen JC, Struble RG, Whitehouse PJ, Price DL. Topography of the magnocellular basal forebrain system in human brain. J Neuropath Exp Neurol. 1984;43:1–21. doi: 10.1097/00005072-198401000-00001. [DOI] [PubMed] [Google Scholar]
- Hafkemeijer A, Altmann-Schneider I, Oleksik AM, van de Wiel L, Middelkoop HA, van Buchem MA, van der Grond J, Rombouts SA. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. 2013;3(4):353–62. doi: 10.1089/brain.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Pine DS, Ernst M. The influence of context valence in the neural coding of monetary outcomes. Neuroimage. 2009 Oct 15;48(1):249–57. doi: 10.1016/j.neuroimage.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L. Basal forebrain in the context of schizophrenia. Brain Res Rev. 2000;31:205–235. doi: 10.1016/s0165-0173(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Heimer L, de Olmos J, Alheid GF, Zaborszky L. “Perestroika” in the basal forebrain; Opening the border between Neurology and Psychiatry. Progr Brain Res. 1991;87:109–165. doi: 10.1016/s0079-6123(08)63050-2. [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Roski C, Caspers S, Zilles K, Eickhoff SB. Age-related decrease of functional connectivity additional to gray matter atrophy in a network for movement initiation. Brain Struct Funct. 2014 Jan 8; doi: 10.1007/s00429-013-0696-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci U S A. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chao HH, Zhang S, Ide JS, Li CS. Changes in cerebral morphometry and amplitude of low-frequency fluctuations of BOLD signals during healthy aging: correlation with inhibitory control. Brain Struct Funct. 2013 Apr 4; doi: 10.1007/s00429-013-0548-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chao HH, Winkler AD, Li CS. The effects of age on cerebral activations: internally versus externally driven processes. Front Aging Neurosci. 2012 Apr 24;4:4. doi: 10.3389/fnagi.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM, Edelman GM. Large-scale model of mammalian thalamocortical systems. Proc Natl Acad Sci U S A. 2008;105:3593–3598. doi: 10.1073/pnas.0712231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG. Spectral Analysis and Its Applications. San Francisco: Holden-Day; 1968. [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003 Dec 18;40(6):1251–7. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S. Separate brain regions code for salience vs. valence during reward prediction in humans. Hum Brain Mapp. 2007 Apr;28(4):294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Shohamy D. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus. 2013 Mar;23(3):187–92. doi: 10.1002/hipo.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Chang LJ, Park SQ, Heinzle J, Haynes JD. Connectivity-based parcellation of the human orbitofrontal cortex. J Neurosci. 2012;32:6240–6250. doi: 10.1523/JNEUROSCI.0257-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JM, Jo HJ, Kim SH, Lee JH, Kim ST, Seo SW, Cox RW, Na DL, Kim SI, Saad ZS. Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method. Neuroimage. 2010;49:2375–2386. doi: 10.1016/j.neuroimage.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A, Riedel WJ, Sambeth A. Cholinergic modulation of auditory processing, sensory gating and novelty detection in human participants. Psychopharmacology (Berl) 2013 Feb;225(4):903–21. doi: 10.1007/s00213-012-2872-0. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. The neural correlates of subjective pleasantness. Neuroimage. 2012 May 15;61(1):289–94. doi: 10.1016/j.neuroimage.2012.02.065. [DOI] [PubMed] [Google Scholar]
- Kurosawa R, Taoka N, Shinohara F, Minami M, Kaneda K. Cocaine exposure enhances excitatory synaptic drive to cholinergic neurons in the laterodorsal tegmental nucleus. Eur J Neurosci. 2013 Jul 3; doi: 10.1111/ejn.12296. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Laalou FZ, de Vasconcelos AP, Oberling P, Jeltsch H, Cassel JC, Pain L. Involvement of the basal cholinergic forebrain in the mediation of general (propofol) anesthesia. Anesthesiology. 2008 May;108(5):888–96. doi: 10.1097/ALN.0b013e31816d919b. [DOI] [PubMed] [Google Scholar]
- Laplante F, Zhang ZW, Huppé-Gourgues F, Dufresne MM, Vaucher E, Sullivan RM. Cholinergic depletion in nucleus accumbens impairs mesocortical dopamine activation and cognitive function in rats. Neuropharmacology. 2012 Nov;63(6):1075–84. doi: 10.1016/j.neuropharm.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Leach ND, Nodal FR, Cordery PM, King AJ, Bajo VM. Cortical cholinergic input is required for normal auditory perception and experience-dependent plasticity in adult ferrets. J Neurosci. 2013 Apr 10;33(15):6659–71. doi: 10.1523/JNEUROSCI.5039-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie FM, Mojica CY, Reynaga DD. Nicotinic receptors in addiction pathways. Mol Pharmacol. 2013 Apr;83(4):753–8. doi: 10.1124/mol.112.083659. [DOI] [PubMed] [Google Scholar]
- Lester DB, Rogers TD, Blaha CD. Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010 Jun;16(3):137–62. doi: 10.1111/j.1755-5949.2010.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Nicolelis MA. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron. 2008 Jul 10;59(1):138–49. doi: 10.1016/j.neuron.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cereb Cortex. 2011 Jan;21(1):95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- Liu Z, Bai L, Dai R, Zhong C, Wang H, You Y, Wei W, Tian J. Exploring the effective connectivity of resting state networks in mild cognitive impairment: an fMRI study combining ICA and multivariate Granger causality analysis. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:5454–7. doi: 10.1109/EMBC.2012.6347228. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011 Apr;35(5):1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark GP, Shabani S, Dobbs LK, Hansen ST. Cholinergic modulation of mesolimbic dopamine function and reward. Physiol Behav. 2011 Jul 25;104(1):76–81. doi: 10.1016/j.physbeh.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker B. Silver staining of cell bodies by means of physical development. J Neurosci Meth. 1983;9:235–241. doi: 10.1016/0165-0270(83)90086-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Rosen AD, Mufson EJ. Regional variations in cortical cholinergic innervation: chemoarchitectonics of acetylcholinesterase-containing fibers in the macaque brain. Brain Res. 1984 Oct 8;311(2):245–58. doi: 10.1016/0006-8993(84)90087-8. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Volicer L, Marquis JK, Mufson EJ, Green CR. Systematic regional differences in the cholinergic innervation of the primate cerebral cortex: distribution of enzyme activities and some behavioral implications. Ann Neurol. 1986;19:144–151. doi: 10.1002/ana.410190206. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Gross N, Poytress BS, Weinberger NM. Motivationally neutral stimulation of the nucleus basalis induces specific behavioral memory. Neurobiol Learn Mem. 2008 Jul;90(1):125–37. doi: 10.1016/j.nlm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Weinberger NM. Detection of an inhibitory cortical gradient underlying peak shift in learning: a neural basis for a false memory. Neurobiol Learn Mem. 2012 Nov;98(4):368–79. doi: 10.1016/j.nlm.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Kusaka T, Yokoyama T, Ohta KI, Suzuki S, Warita K, Jamal M, Wang ZY, Ueki M, Liu JQ, Yakura T, Tamai M, Sumitani K, Hosomi N, Takeuchi Y. Short-term ethanol exposure causes imbalanced neurotrophic factor allocation in the basal forebrain cholinergic system: a novel insight into understanding the initial processes of alcohol addiction. J Neural Transm. 2013 Sep 6; doi: 10.1007/s00702-013-1085-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Dolan RJ. Neural responses to salient visual stimuli. Proc Biol Sci. 1997 May 22;264(1382):769–75. doi: 10.1098/rspb.1997.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26(2003):233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Chiba T. Afferent connections to the ventral striatum from the medial prefrontal cortex (area 25) and the thalamic nuclei in the macaque monkey. Ann N Y Acad Sci. 1999 Jun 29;877:667–70. doi: 10.1111/j.1749-6632.1999.tb09297.x. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013 May 8;33(19):8321–35. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli WM, O’Reilly RC. Attentional control of associative learning--a possible role of the central cholinergic system. Brain Res. 2008 Apr 2;1202:43–53. doi: 10.1016/j.brainres.2007.06.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Ruttorf M, Schad LR, Flor H. Activation of the ventral striatum during aversive contextual conditioning in humans. Biol Psychol. 2012 Sep;91(1):74–80. doi: 10.1016/j.biopsycho.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Popa D, Popescu AT, Pare D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. J Neurosci. 2009;29:1191–1201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Simic G, Watson S, Stimpson CD, Hof PR, Sherwood CC. Comparative analysis of the nucleus basalis of Meynert among primates. Neuroscience. 2011 Jun 16;184:1–15. doi: 10.1016/j.neuroscience.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007 Oct 1;37(4):1083–90. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Robinson S, Basso G, Soldati N, Sailer U, Jovicich J, Bruzzone L, Kryspin-Exner I, Bauer H, Moser E. A resting state network in the motor control circuit of the basal ganglia. BMC neuroscience. 2009;10:137. doi: 10.1186/1471-2202-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokem A, Landau AN, Garg D, Prinzmetal W, Silver MA. Cholinergic enhancement increases the effects of voluntary attention but does not affect involuntary attention. Neuropsychopharmacology. 2010 Dec;35(13):2538–44. doi: 10.1038/npp.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. Neuroimage. 2003;20:1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- Roski C, Caspers S, Langner R, Laird AR, Fox PT, Zilles K, Amunts K, Eickhoff SB. Adult age-dependent differences in resting-state connectivity within and between visual-attention and sensorimotor networks. Front Aging Neurosci. 2013 Oct 29;5:67. doi: 10.3389/fnagi.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkirch M, Schmack K, Schlagenhauf F, Sterzer P. Implicit motivational value and salience are processed in distinct areas of orbitofrontal cortex. Neuroimage. 2012 Sep;62(3):1717–25. doi: 10.1016/j.neuroimage.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006 Aug;51(2):145–60. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz AJ, McGonigle J. Negative edges and soft thresholding in complex network analysis of resting state functional connectivity data. Neuroimage. 2011;55:1132–1146. doi: 10.1016/j.neuroimage.2010.12.047. [DOI] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121(Pt 12):2249–2257. doi: 10.1093/brain/121.12.2249. 1998. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M. Cholinergic functioning in stimulant addiction: implications for medications development. CNS Drugs. 2009 Nov;23(11):939–52. doi: 10.2165/11310920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer J, Galvan A, Hare TA, Voss H, Glover G, Casey B. Sensitivity of the nucleus accumbens to violations in expectation of reward. Neuroimage. 2007 Jan 1;34(1):455–61. doi: 10.1016/j.neuroimage.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniwaki T, Okayama A, Yoshiura T, Togao O, Nakamura Y, Yamasaki T, Ogata K, Shigeto H, Ohyagi Y, Kira J, Tobimatsu S. Age-related alterations of the functional interactions within the basal ganglia and cerebellar motor loops in vivo. Neuroimage. 2007 Jul 15;36(4):126. doi: 10.1016/j.neuroimage.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional Connectivity of Substantia Nigra and Ventral Tegmental Area: Maturation During Adolescence and Effects of ADHD. Cereb Cortex. 2012 Dec 12; doi: 10.1093/cercor/bhs382. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M, Eichele T, Lundervold AJ, Lundervold A. Subcortical functional connectivity and verbal episodic memory in healthy elderly--a resting state fMRI study. Neuroimage. Aug 1;52(1):379–88. doi: 10.1016/j.neuroimage.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Bentley P, Dolan RJ. Effects of cholinergic enhancement on conditioning-related responses in human auditory cortex. Eur J Neurosci. 2002 Dec;16(11):2199–206. doi: 10.1046/j.1460-9568.2002.02272.x. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Cragg SJ. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Syst Neurosci. 2011 Mar 3;5:11. doi: 10.3389/fnsys.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional Connectivity of Substantia Nigra and Ventral Tegmental Area: Maturation During Adolescence and Effects of ADHD. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Douglas D, Aschenbrenner K, Gitelman DR, Small DM. The anterior insular cortex represents breaches of taste identity expectation. J Neurosci. 2011 Oct 12;31(41):14735–44. doi: 10.1523/JNEUROSCI.1502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels OJM, Broere CAJ, Ter Laak HJ, Ten Donkelaar HJ, Nieuwenhuys R, Schulte BPM. Cell loss and shrinkage in the nucleus basalis Meynert complex in Alzheimer’s disease. Neurobiol Aging. 1990;11:3–13. doi: 10.1016/0197-4580(90)90056-6. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Bertrand D. Importance of the nicotinic acetylcholine receptor system in the prefrontal cortex. Biochem Pharmacol. 2013 Jun 15;85(12):1713–20. doi: 10.1016/j.bcp.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem. 1997 Mar;67(2):85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008 Jul;33(8):1779–97. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA, Ma YY. Reinforcement-related neurons in the primate basal forebrain respond to the learned significance of task events rather than to the hedonic attributes of reward. Cognitive Brain Research. 2004;19(1):74–81. doi: 10.1016/j.cogbrainres.2003.11.003. 2004. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Rolls ET. Neuronal responses related to reinforcement in the primate basal forebrain. Brain Research. 1990;509(2):213–231. doi: 10.1016/0006-8993(90)90546-n. 1990. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE. Projections from the nucleus accumbens to cholinergic neurons of the ventral pallidum: A correlated light and electron microscopic double immunolabeling study in rat. Brain Res. 1992;570:92–101. doi: 10.1016/0006-8993(92)90568-t. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: An experimental study based on retrograde tracing and 3D reconstruction. Cereb Cortex. 2013 Aug 19; doi: 10.1093/cercor/bht210. In press. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Van den Pol A, Gyengesi E. The basal forebrain cholinergic projection system in mice. In: Watson C, Paxinos G, Puelles L, editors. The Mouse Nervous System. 1. Amsterdam: Elsevier; 2012. pp. 684–718. [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. Journal of neurophysiology. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li CS. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex. 2012;22:99–111. doi: 10.1093/cercor/bhr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012a;59:3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional networks for cognitive control in a stop signal task: independent component analysis. Hum Brain Mapp. 2012b;33:89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Mei J, Lü SG, Zhao ZQ. Age-related alterations in responses of nucleus basalis magnocellularis neurons to peripheral nociceptive stimuli. Brain Res. 2002 Sep 6;948(1–2):47–55. doi: 10.1016/s0006-8993(02)02947-5. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006 Feb 1;29(3):977–83. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003 Sep 3;23(22):8092–7. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.