Abstract
Calbindin-D28K has been implicated in the regulation of neuronal cell death. Previously, we demonstrated that calbindin-D28K prevents staurosporine (STS)-induced caspase activation and subsequent apoptosis in a neuronal cell line. However, the role of calbindin-D28K in STS-induced activation of calpain and necrotic cell death was not identified. Staurosporine induced the elevation of intracellular calcium after 1 hr of treatment. Overexpression of calbindin-D28K and presence of a calcium chelator, BAPTA, prevented the increase of calcium in STS-treated cells. Cleavage of Bax by calpain was prevented by the overexpressed calbindin-D28K. Permeabilization of the plasma membrane, a factor in necrosis, as well as apoptotic change of the nucleolus induced by STS, was prevented by calbindin-D28K. Thus, our study suggests that calbindin-D28K may exert its protective functions by preventing calpain activation in necrotic cell death, in addition to its effect on the caspase-apoptosis pathway.
Keywords: calbindin-D28k, calcium, calpain, neuronal cell death
INTRODUCTION
Calcium has been implicated in the control of neuronal activity, differentiation, survival, and death [1, 2, 3, 4]. Calcium-binding proteins (CaBPs) are expressed in many types of neurons and maintain calcium homeostasis [5, 6]. Calbindin-D28K belongs to the calbindin family of CaBPs that has a common structural calcium-binding domain designated the EF-hand [7]. Other members of the EF-hand family of CaBPs, such as calmodulin and calpains, have been extensively investigated in neuronal cells [8]. Though the neuroprotection by overexpressed calbindin has been attributed to its calcium-chelating activity, the exact role of calbindin in neuronal cell death remains largely unknown (for review, see [9]). Recently, we reported that calbindin prevents caspase-3 activation and apoptosis in neuronal death induced by staurosporine (STS) [10]. However, the activity of calbindin with respect to calpain, calcium-dependent protease, and necrotic (or late-apoptotic) cell death in STS-induced neuronal death is not clear.
Here, we report that calbindin prevented STS-induced elevation of intracellular levels of free calcium ([Ca2+]i) and calpain activation. We also demonstrated that calbindin expression prevents STS-induced necrotic death, as well as apoptosis.
MATERIALS AND METHODS
Cell culture
The establishment and characterization of MN9D cells that stably overexpress calbindin-D28K (MN9D/Calbindin) or vector control (MN9D/Neo) were described elsewhere [11]. Both MN9D parental cells and MN9D/Neo cells had no detectible levels of endogenous calbindin, as determined by immunoblot analysis [11]. Cells were plated at a density of 2×104 cells/well in 48-well Corning® Costar® plates (coated with 25 µg/ml poly-D-lysine. Cultures were maintained in complete culture medium (CCM), consisting of Dulbecco's Modified Eagle's Medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum and 250 µg G418/ml, for 3 d at 37℃ in an incubator with an atmosphere of 10% CO2. Culture medium was subsequently changed to serum-free N2 medium containing experimental reagents and further incubated for various time periods. Culture reagents were from Life Technologies (Grand Island, NY). Staurosporine was obtained from Sigma-Aldrich (St. Louis, MO, USA) and BAPTA from Calbiochem (La Jolla, CA, USA).
Immunoblot analysis
Cultures of 1×106 MN9D/Neo or MN9D/Calbindin cells in poly-D-lysine-coated Corning® P-100 dishes containing CCM were grown for 3~4 d. Cells were then washed with ice-cold PBS and lysed for 10 min in a buffer containing 50 mM TrisHCl (pH 7.0), 2 mM EDTA, 1% (v/v) Triton X-100, 2 mM PMSF, and 10 µg leupeptin and aprotinin/ml. Lysates were clarified by centrifugation at 13,000 ×g for 30 min at 4℃. Protein content of supernatants was measured using the Bio-Rad protein assay reagent (Bio-Rad; Hercules, CA, USA), and 40 µg from each sample was separated on 12.5% (w/v) PAGE containing SDS. The separated proteins were blotted onto pre-wetted PVDF-nitrocellulose filters (Bio-Rad), and the filters were then further processed for immunoblot analysis with antibodies, e.g., rabbit anti-Bax antibody (1:3000) (Pharmingen, San Jose, CA).
MTT reduction assay
Cells from each established cell line were plated at 2×104/well in 48-well plates with CCM and grown for 3 d, after which the medium was changed to serum-free N2 medium containing 1 µM STS. These cultures were then further incubated for various time periods in combination with either 1~50 µM BAPTA or vehicle. Following the treatment, cell survival was assessed using the MTT reduction assay as described [12] and determined as percent survival relative to untreated control.
Measurement of cytosolic calcium
Levels of [Ca2+]i were visualized with the cytosolic calcium indicator Fura-2 by confocal laser fluorescence microscopy. Cells were plated onto 35-mm glass-bottom dishes coated with poly-D-lysine and then incubated for treatment with experimental agents. For calcium measurement, the cells were incubated with 5 µM Fura-2/AM for 30 min at room temperature, followed by an additional 30 min in HEPES-buffered isotonic salt solution. Imaging was performed at room temperature on the stage of a confocal laser microscope (Leica TCS NT system) (Leica; Heidelberg, Germany). Images of Fura-2 fluorescence (excitation at 340~380 nm; emission, 510 nm) were acquired with an LSM 510 camera (Carl Zeiss; Jena, Germany). Color coding was set using the LSM 510 image system.
Statistics
Experimental data were expressed as mean values±SEM from a specified number of experiments. Significance of differences between groups was determined by one-way ANOVA and post-hoc Student's t test. Values of p<0.05 were considered to be statistically significant.
RESULTS
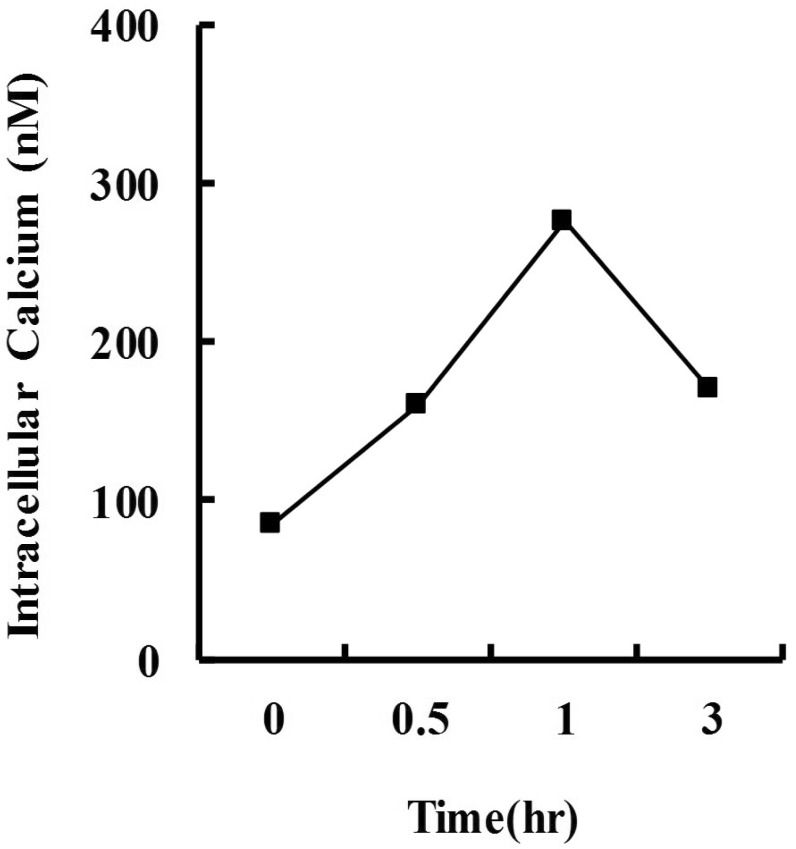
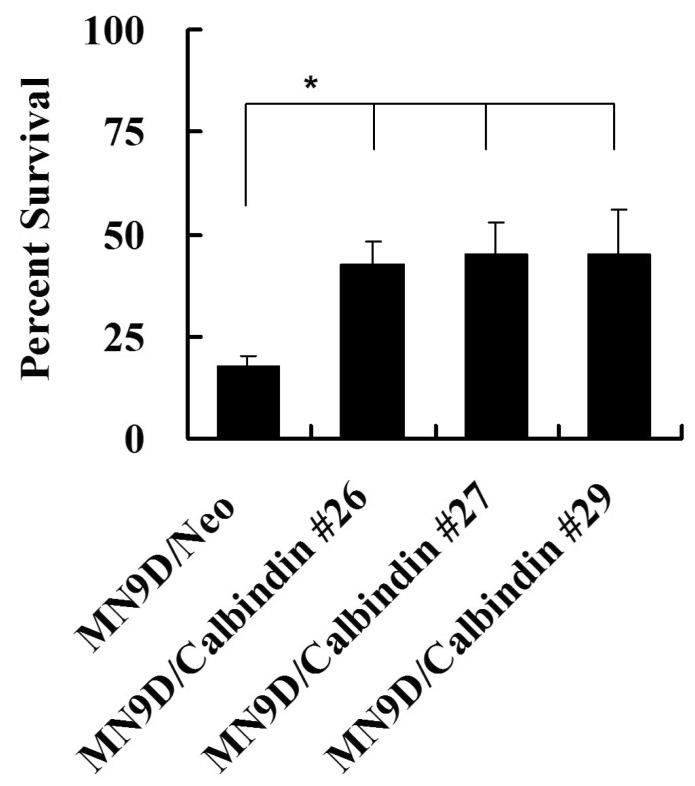
Previously, we demonstrated that STS induced the activation of caspase and calpain in dopaminergic neuron death [10, 13]. Overexpression of calbindin prevented caspase activation and apoptosis in STS-induced cell death [10]. In this study, we investigated the role of calbindin in calpain activation and necrosis in STS-induced cell death. After 30 min of STS treatment, [Ca2+]i was elevated and peaked at 1 hr (Fig. 1). Cotreatment with BAPTA prevented STS-induced [Ca2+]i surge (Fig. 2A and B). Overexpression of calbindin prevented [Ca2+]i elevation after treatment with STS (Fig. 2B). Three individual clones of stable cell lines expressing calbindin were similarly resistant to STS-induced cell death (Fig. 3).
Fig. 1.
Staurosporine (STS) induces the elevation of cytosolic calcium in MN9D cells. Cells were treated with or without 1 µM STS for various times, stained with the calcium-sensitive Fura-2 fluorogenic dye and analyzed by confocal laser microscopy. The intracellular calcium concentration ([Ca2+]i) was quantitated. Values were representitive from three independent experiments.
Fig. 2.
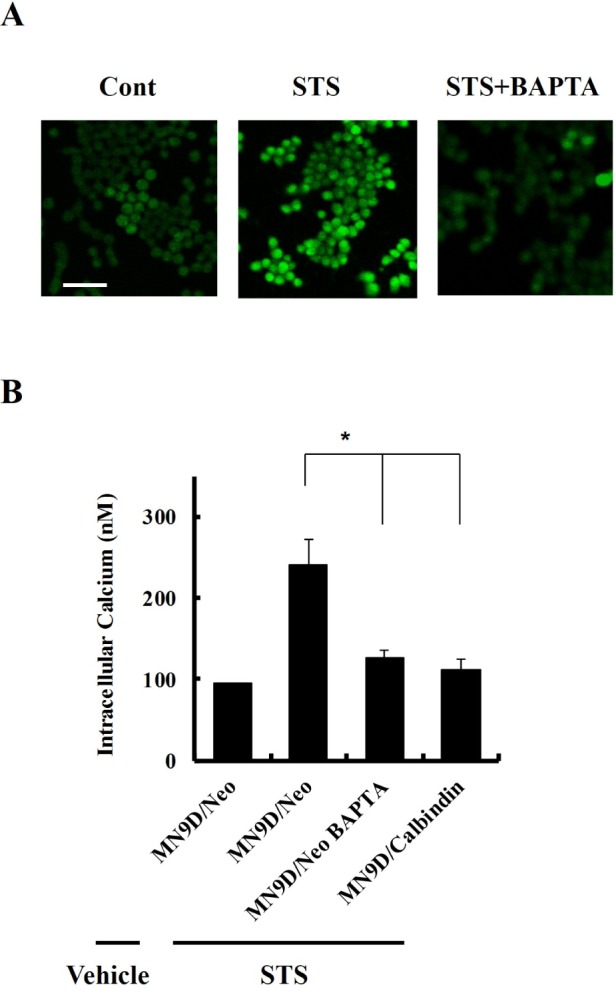
Overexpression of calbindin prevents STS-induced intracellular calcium ([Ca2+]i) surge. (A) Fura-2 staining of MN9D/Neo cells treated with vehicle alone (Cont), 1 µM STS or STS in the presence of 40 µM of the calcium chelator BAPTA. Scale bar, 100 µm. (B) Quantitation of [Ca2+]i in MN9D/Neo cells treated with vehicle, STS, or STS in combination with BAPTA. NM9D/Calbindin cells were treated with STS and [Ca2+]i was compared. *p<0.05.
Fig. 3.
Overexpression of calbindin prevents drug-induced cell death. Following 24 hr of 1 µM STS exposure, MTT reduction assay of MN9D/Neo and MN9D/Calbindin cells was performed. Values were expressed as a percentage relative to the untreated control (100%). *p<0.05.
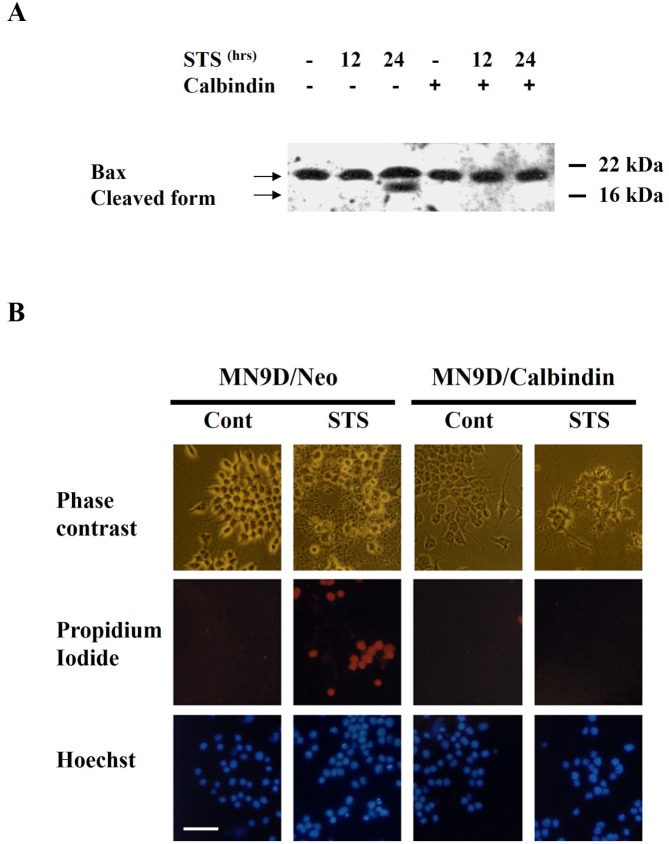
To further define the protective mechanism of calbindin, we tested the effect of calbindin on the cleavage of Bax, which is a substrate of calpain. As we previously reported, STS treatment induced calpain activation in MN9D/Neo cells, as indicated by calpain-mediated cleavage of an 18 kDa Bax fragment, at the late phase of cell death (Fig. 4A; 24-hr time point). Calbindin overexpression prevented Bax cleavage in STS-induced neuron death, suggesting that calbindin inhibited calpain activation (Fig. 4A).
Fig. 4.
Calbindin-D28K prevents calpain activation and permeabilization of the cell membrane during cell death induced by STS. (A) Immunoblot analysis of calpain-mediated Bax cleavage in MN9D/Neo and MN9D/Calbindin cells. (B) MN9D/Neo and MN9D/Calbindin cells were exposed to 1 µM STS for 20 hr. Nuclei of STS-treated cells were stained with propidium iodide or Hoechst dye. In MN9D/Neo cells, many nuclei were stained in red as the cell membrane was permeabilized to propidium iodide entry, indicating necrotic cell death. This necrotic change was significantly attenuated in MN9D/Calbindin cells. Scale bar, 100 µm.
After 20 hr of treatment with STS, both apoptotic (chromatin condensation and fragmentation) and necrotic (propidium-iodide staining) events occurred in MN9D/Neo cells; whereas, calbindin overexpression significantly prevented both types of events (Fig. 4B). Few MN9D/Calbindin cells showed chromatin condensation or fragmentation, and virtually no cells were stained with propidium iodide (Fig. 4B). These data suggest that calbindin may exert its protective functions by preventing calpain activation and necrotic cell death, as well as by effects on the caspase-mediated apoptotic pathway [10].
DISCUSSION
Previously, we reported that calbindin prevents STS-induced caspase activation [10]. However, the role of calbindin on calcium elevation and calcium-dependent calpain activation has not been investigated in STS-induced dopaminergic neuron death. In this study, we demonstrated that calbindin overexpression prevented elevation of [Ca2+]i, calpain activation, and cleavage of Bax in STS-induced dopaminergic neuron death. Our previous results demonstrated that MPP+-induced Bax cleavage was prevented by the expression of calbindin. Taken together, our data suggest that the prevention of [Ca2+]i elevation, calpain activation, and Bax cleavage might be a common mechanism underlying the protective role of calbindin in dopaminergic neuron death. In support of this hypothesis, calpain activation has been reported in post-mortem midbrain of patients with Parkinson's disease (PD). Furthermore, calpain regulates other molecules in dopaminergic neuron death [14]. Therefore, the calpain pathway may likely be a good research area for discovery of new PD treatments.
Interestingly, the protection rate by calbindin in STS-induced cell death is much higher than in MPP+-induced death (approximately 40% vs. 25%, respectively). This implies that the protective role of calbindin is accomplished through inhibition of both the calcium/calpain and caspase pathways in parallel in STS-induced dopaminergic cell death. Calbindin's protective effect is relatively smaller in MPP+-induced cell death, in which only the calcium-calpain pathway is inhibited [10].
Permeabilization of the plasma membrane is a typical characteristic of necrotic cell death [15]. In this study, we observed that a large portion of STS-treated MN9D cells lost the integrity of their cell membrane, although the morphology of the nucleoli was not apoptotic, suggesting that STS-induced cell death is necrotic in at least a part of the cell population. Our data suggested that calbindin prevents both the necrotic and apoptotic components of cell death in STS-induced dopaminergic cell death. Indeed, the cell death mechanisms are rather complicated in PD. In brain of PD patients, both caspase and calpain activation have been reported [16, 17, 18]. Therefore, a multifunctional molecule, e.g., calbindin, that can prevent involvement of both caspase and calpain pathways, might be needed to protect dopaminergic neurons in the brain.
Interestingly, it has been demonstrated that calbindin is located in the midbrain dopaminergic neurons, which are relatively invulnerable to degeneration as seen in animal PD models [19, 20]. In PD patients, dopaminergic neurons of the substantia nigra pars compacta that express calbindin appear to be relatively more resistant to cell death than those not expressing calbindin [21]. Therefore, this protein might be part of endogenous protective machinery, and further research may reveal a potential treatment based on it to prevent dopaminergic neuron death in PD.
ACKNOWLEDGEMENTS
We thank Dr. A. Heller for allowing us to use the MN9D cell line. This work was financially supported by Chonnam National University, 2012.
References
- 1.Orrenius S, McCabe MJ, Jr, Nicotera P. Ca(2+)-dependent mechanisms of cytotoxicity and programmed cell death. Toxicol Lett. 1992:357–364. doi: 10.1016/0378-4274(92)90208-2. 64-65 Spec No. [DOI] [PubMed] [Google Scholar]
- 2.Finkbeiner S, Greenberg ME. Ca2+ channel-regulated neuronal gene expression. J Neurobiol. 1998;37:171–189. [PubMed] [Google Scholar]
- 3.Farnsworth CL, Freshney NW, Rosen LB, Ghosh A, Greenberg ME, Feig LA. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 4.Chan CS, Gertler TS, Surmeier DJ. Calcium homeostasis, selective vulnerability and Parkinson's disease. Trends Neurosci. 2009;32:249–256. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller RJ. The control of neuronal Ca2+ homeostasis. Prog Neurobiol. 1991;37:255–285. doi: 10.1016/0301-0082(91)90028-y. [DOI] [PubMed] [Google Scholar]
- 6.Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- 7.Persechini A, Moncrief ND, Kretsinger RH. The EF-hand family of calcium-modulated proteins. Trends Neurosci. 1989;12:462–467. doi: 10.1016/0166-2236(89)90097-0. [DOI] [PubMed] [Google Scholar]
- 8.Fanò G, Biocca S, Fulle S, Mariggiò MA, Belia S, Calissano P. The S-100: a protein family in search of a function. Prog Neurobiol. 1995;46:71–82. doi: 10.1016/0301-0082(94)00062-m. [DOI] [PubMed] [Google Scholar]
- 9.Christakos S, Prince R. Estrogen, vitamin D, and calcium transport. J Bone Miner Res. 2003;18:1737–1739. doi: 10.1359/jbmr.2003.18.10.1737. [DOI] [PubMed] [Google Scholar]
- 10.Choi WS, Lee E, Lim J, Oh YJ. Calbindin-D28K prevents drug-induced dopaminergic neuronal death by inhibiting caspase and calpain activity. Biochem Biophys Res Commun. 2008;371:127–131. doi: 10.1016/j.bbrc.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Choi WS, Chun SY, Markelonis GJ, Oh TH, Oh YJ. Overexpression of calbindin-D28K induces neurite outgrowth in dopaminergic neuronal cells via activation of p38 MAPK. Biochem Biophys Res Commun. 2001;287:656–661. doi: 10.1006/bbrc.2001.5649. [DOI] [PubMed] [Google Scholar]
- 12.Choi WS, Yoon SY, Oh TH, Choi EJ, O'Malley KL, Oh YJ. Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic neuronal cell death: role of caspases, ROS, and JNK. J Neurosci Res. 1999;57:86–94. doi: 10.1002/(SICI)1097-4547(19990701)57:1<86::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 13.Kim JE, Oh JH, Choi WS, Chang II, Sohn S, Krajewski S, Reed JC, O'Malley KL, Oh YJ. Sequential cleavage of poly(ADP-ribose)polymerase and appearance of a small Bax-immunoreactive protein are blocked by Bcl-X(L) and caspase inhibitors during staurosporine-induced dopaminergic neuronal apoptosis. J Neurochem. 1999;72:2456–2463. doi: 10.1046/j.1471-4159.1999.0722456.x. [DOI] [PubMed] [Google Scholar]
- 14.Smith PD, Mount MP, Shree R, Callaghan S, Slack RS, Anisman H, Vincent I, Wang X, Mao Z, Park DS. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson EB. Special topic: apoptosis. Annu Rev Physiol. 1998;60:525–532. doi: 10.1146/annurev.physiol.60.1.525. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci U S A. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilsland J, Roy S, Xanthoudakis S, Nicholson DW, Han Y, Grimm E, Hefti F, Harper SJ. Caspase inhibitors attenuate 1-methyl-4-phenylpyridinium toxicity in primary cultures of mesencephalic dopaminergic neurons. J Neurosci. 2002;22:2637–2649. doi: 10.1523/JNEUROSCI.22-07-02637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viswanath V, Wu Y, Boonplueang R, Chen S, Stevenson FF, Yantiri F, Yang L, Beal MF, Andersen JK. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J Neurosci. 2001;21:9519–9528. doi: 10.1523/JNEUROSCI.21-24-09519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang CL, Sinton CM, German DC. Midbrain dopaminergic neurons in the mouse: co-localization with Calbindin-D28K and calretinin. Neuroscience. 1996;75:523–533. doi: 10.1016/0306-4522(96)00228-x. [DOI] [PubMed] [Google Scholar]
- 20.Parent A, Lavoie B. The heterogeneity of the mesostriatal dopaminergic system as revealed in normal and parkinsonian monkeys. Adv Neurol. 1993;60:25–33. [PubMed] [Google Scholar]
- 21.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain. 1999;122(Pt 8):1421–1436. doi: 10.1093/brain/122.8.1421. [DOI] [PubMed] [Google Scholar]