Abstract
Mutation in TAR DNA binding protein 43 (TDP-43) is a causative factor of amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). Neurodegeneration may not require the presence of pathogenic TDP-43 in all types of relevant cells. Rather, expression of pathogenic TDP-43 in neurons or astrocytes alone is sufficient to cause cell-autonomous or non-cell-autonomous neuron death in transgenic rats. How pathogenic TDP-43 in astrocytes causes non-cell-autonomous neuron death, however, is not clear. Here, we examined the effect of pathogenic TDP-43 on gene expression in astrocytes. Microarray assay revealed that pathogenic TDP-43 in astrocytes preferentially altered expression of the genes encoding secretory proteins. Whereas neurotrophic genes were down-regulated, neurotoxic genes were up-regulated. Representative genes Lcn2 and Chi3L1 were markedly up-regulated in astrocytes from primary culture and intact transgenic rats. Further, synthetic Chi3L1 induced neuron death in a dose-dependent manner. Our results suggest that TDP-43 pathogenesis is associated with the simultaneous induction of multiple neurotoxic genes in astrocytes, which may synergistically produce adverse effects on neuronal survival and contribute to non-cell-autonomous neuron death.
Keywords: astrocytes, TAR DNA binding protein 43, TDP-43, amyotrophic lateral sclerosis, Chi3L1, Lcn2, non-cell-autonomous neuron death
Introduction
Astrocytes, which are the most numerous non-neuronal cell types in the central nervous system, communicate with and protect neurons under physiological conditions. For instance, astrocytes express glutamate transporters that clear the neurotransmitter glutamate from synaptic clefts (Rothstein et al., 1996), preventing glutamate from persistently stimulating post-synaptic neurons. Receptors on astrocytes sense neuronal activity and form gap junctions to propagate signaling (Escartin and Bonvento, 2008). Furthermore, astrocytes synthesize and release cytokines, chemokines, and free radicals that modify neuronal activity (Lee et al., 2007, Escartin and Bonvento, 2008, Landreth et al., 2008, Lee et al., 2009). However, astrocytes are also associated with neurodegeneration in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) (Rothstein et al., 1996, Howland et al., 2002, Nagai et al., 2007, Zhou et al., 2010), and are likely involved in the initiation and propagation of neurodegenerative signaling. Indeed, non-cell-autonomous death of motor neurons is induced by selective expression of ALS-linked genes in astrocytes (Lobsiger and Cleveland, 2007, Nagai et al., 2007, Lepore et al., 2008, Tong et al., 2013). Astrocytes enter a reactive state in response to neuron injury (Lee et al., 2007, Escartin and Bonvento, 2008, Landreth et al., 2008, Lee et al., 2009, Huang et al., 2012a). Although controlled astrocyte activation is beneficial to neurons (Okada et al., 2006), over-activation can be harmful (Custer et al., 2006). For example, overactive astrocytes secrete Lcn2, which stimulates the activation of quiescent astrocytes and microglia and thereby promotes neuronal death (Lee et al., 2007, Lee et al., 2009, Bi et al., 2013). How astrocytes contribute to ALS and FTLD pathogenesis, however, is not fully understood.
Mutation of TAR DNA binding protein 43 (TDP-43) is linked to ALS and FTLD (Neumann et al., 2006). Whereas mutant TDP-43 is a causative factor of familial ALS and FTLD (Kabashi et al., 2008, Rutherford et al., 2008, Sreedharan et al., 2008, Van Deerlin et al., 2008), aggregation of normal TDP-43 is commonly observed in sporadic ALS and FTLD (Neumann et al., 2006, Kwong et al., 2007, Kwong et al., 2008). TDP-43 is a major component of ubiquitin-positive inclusions in several neurodegenerative diseases, including ALS and FTLD. The TPD-43 gene is highly conserved among mammals, flies, and Caenorhabditis elegans (Wang et al., 2004, Ayala et al., 2005). TDP-43 belongs to the family of heterogeneous nuclear ribonucleoproteins, which is characterized by the ability to bind DNA and RNA sequences through RNA recognition motifs (RRMs). TDP-43 contains two RRMs, RRM1 and RRM2, and a glycine-rich region in its C-terminus. RRM1 is required for nucleotide binding, RRM2 is required for correct complex formation (Buratti and Baralle, 2001, Ayala et al., 2005, Ayala et al., 2008), and the C-terminus is required for the formation of ribonucleoprotein complexes (Buratti et al., 2005). Pathogenic mutations in TDP-43 are concentrated in the glycine-rich domain (Pesiridis et al., 2009), but how mutation alters TDP-43 functionality is currently unknown. Studies in rodent models show that over-expression of both wild-type and mutant forms of TDP-43 causes neuron death and disease phenotypes (Tsai et al., 2010, Zhou et al., 2010, Igaz et al., 2011, Swarup et al., 2011, Huang et al., 2012b, Tong et al., 2012), suggesting that a functional gain in TDP-43 mutation is a major contributor to TDP-43 pathogenesis. We previously reported that selective over-expression of mutant TDP-43 in motor neurons or astrocytes alone is sufficient to induce cell-autonomous or non-cell-autonomous motor neuron death in rats (Huang et al., 2012b, Tong et al., 2013). Previous findings indicate that disease induction does not require the presence of pathogenic TDP-43 in all relevant cell types, although the molecular mechanism underlying non-cell-autonomous neuron death is not yet understood.
To extend the findings of our previous studies (Tong et al., 2013), we examined the gene expression profiles of astrocytes isolated from transgenic rats that selectively over-express mutant TDP-43 in astrocytes and develop an ALS-like phenotype. We found that genes encoding secretory proteins were preferentially altered by expression of pathogenic TDP-43 in astrocytes. In particular, the secretory protein Chi3L1 was markedly up-regulated in astrocytes, and synthetic Chi3L1 caused dose-dependent neuron death. These findings suggest that pathogenic TDP-43 alters the expression of secretory proteins and thereby contributes to non-cell-autonomous neuron death.
Materials and Methods
Study approval
Animal use was in accord with NIH guidelines and the animal use protocol was approved by the Institutional Animal Care and Use Committees at Thomas Jefferson University. Both male and female rats were used in this study.
Astrocyte culture and microarray assay
Primary cells were taken from GFAP-tTA/TRE-TDP43M337V double-transgenic rats, which were previously described (Huang et al., 2012b, Tong et al., 2013). Mixed glial cells were isolated from the cortex of 3-day-old postnatal rats, and astrocytes were separated from other glial cells by shaking. Purified astrocytes were deprived of doxycycline (Dox) to allow TDP-43M337V transgene expression. Total RNA was isolated from induced astrocytes for microarray assay, which was performed at SABiosciences-QIAGEN in Frederick, Maryland. Microarray data were deposited to the National Center for Biotechnology Information (NCBI) database with an assigned accession number (GSE42091). Microarray data were analyzed with DAVID 6.7 software, and genes with altered expression were clustered based on functionality. Selected genes were further validated by quantitative polymerase chain reaction (PCR) as previously described (Huang et al., 2012b).
Primary neuron culture and cytotoxicity assay
Cortical neurons were isolated from wild-type rat embryos (embryonic day 19) using published protocols (Liu et al., 2003). Cortical neurons were initially cultured in complete neuron medium for 10 days and then treated with recombinant Chi3L1 protein (Sino Biological Incorporation, China) at varying concentrations for 4 days before viability assay. Cell viability was quantified with a Live/Dead assay kit for mammalian cells (Invitrogen, L3224) as previously described (Bi et al., 2013).
Immunofluorescence staining
Co-localization of human TDP-43 with related proteins (GFAP, Iba-1, APC, and Chi3L1) was examined by double-labeling immunofluorescence staining. Immunofluorescent staining was performed on cultured primary glia or cross-sections of the rat spinal cord. The following primary antibodies were used: mouse monoclonal anti-APC (Calbiochem), mouse monoclonal anti-human TDP-43 (Abnova, clone 2E2-D3), rabbit anti-GFAP (DAKO North America), and rabbit anti-Iba-1 (Wako Chemicals USA). Immunostained tissue sections were examined using a Nikon microscope for immunohistochemistry or a confocal microscope (Imaging Facility of Kimmel Cancer Center at Jefferson). Single-layer images were scanned using a Zeiss LSM510 META confocal system, and Z-stacks of confocal images (at 1-μm intervals) were projected to reconstruct astrocyte structure.
Statistical analysis
Cortical neurons isolated from a same animal were cultured in paired wells and were treated with or without Chi3L1. Cell viability between Chi3L1-treated and untreated cells were analyzed using paired t-test. Statistical significance was set at p < 0.05.
Results
Expression of mutant TDP-43 in primary astrocytes
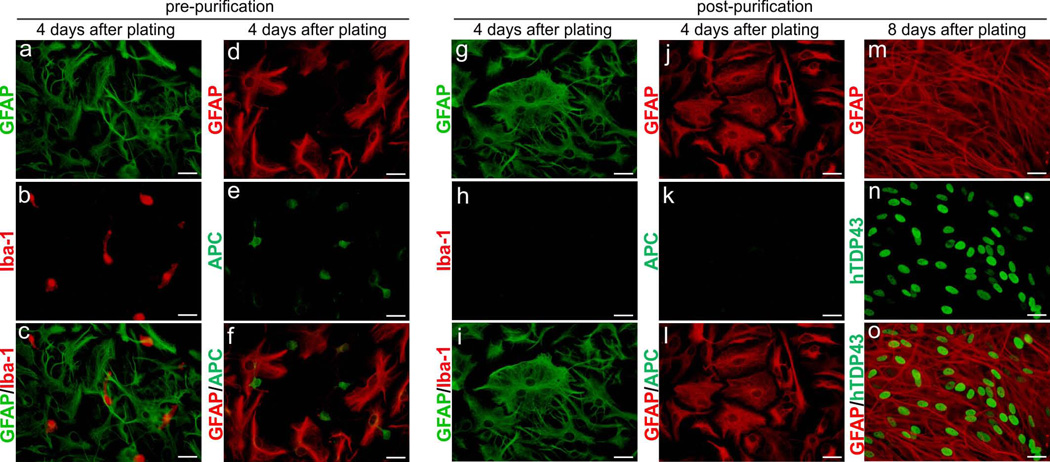
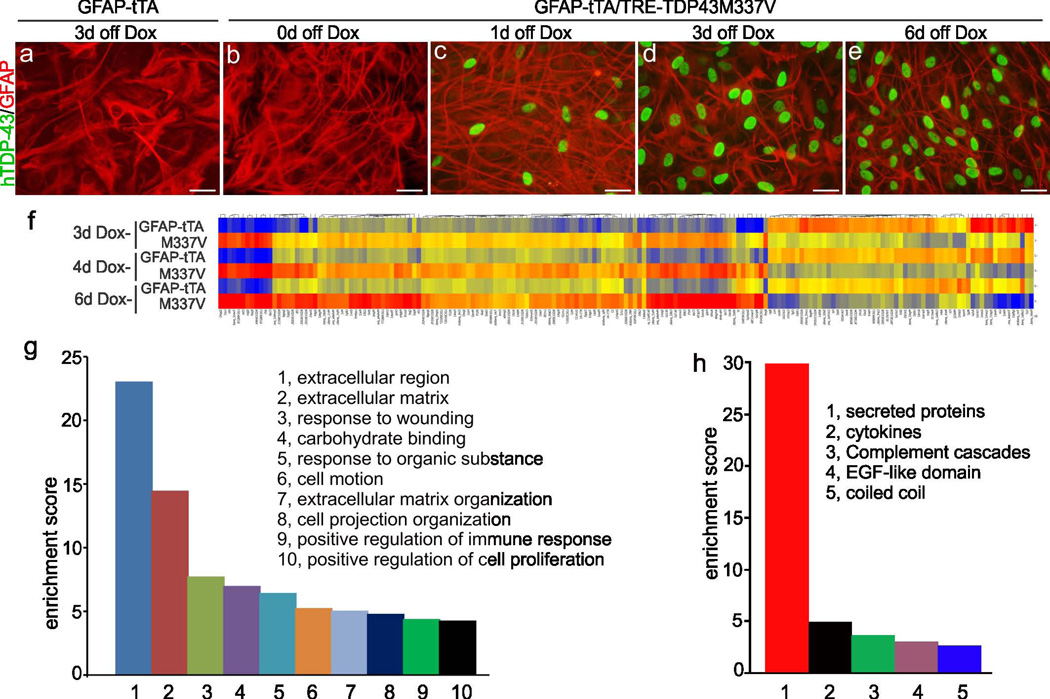
To dissect the molecular mechanisms of TDP-43-induced non-cell-autonomous neuron death, we isolated and purified astrocytes from GFAP-tTA/TRE-TDP43M337V transgenic rats that selectively express pathogenic TDP-43 in astrocytes and develop an ALS-like phenotype (Tong et al., 2013). These astrocytes express a sufficient quantity of pathogenic TDP-43 to induce non-cell-autonomous neuron death and therefore are the ideal model for mechanistic studies. Mixed glial cells were taken from postnatal rats and cultured in glia-preferred medium to diminish the presence of neurons. Flasks containing mixed glial cultures were aggressively shaken overnight to detach microglia and oligodendrocytes from the flask surface. The astrocyte purity of retained cells was examined by immunostaining with an antibody recognizing the astrocyte marker glial fibrillary acidic protein (GFAP). Additional shaking time was required when the astrocyte-enriched culture showed staining for the microglia marker Iba-1 (Figure 1: A-C) or the oligodendrocyte marker APC (Figure 1: D-F). After at least two rounds of purification, Iba-1- (Figure 1: G-I) and APC-positive (Figure 1: J-L) cells were eliminated from the culture. The high purity of the astrocyte culture enabled us to determine gene expression profiles in astrocytes from non-transgenic and TDP-43M337V transgenic cells and hence identify genes whose expression is altered by pathogenic TDP-43. The mutant TDP-43 transgene is expressed from a tetracycline-regulated promoter and thus is subject to regulation by Dox. Pathogenic TDP-43 was initially not expressed in cultured astrocytes in the presence of Dox (Figure 2: A-B) but was rapidly expressed after withdrawal of Dox from the culture (Figure 2: C-E). This inducible, transient expression of mutant TDP-43 enabled us to identify genes highly related to TDP-43 pathogenesis.
Figure 1.
Primary astrocytes were purified and induced to express mutant human TDP-43. (a-f) Double-labeling fluorescent staining revealed that unpurified glial culture contained astrocytes (GFAP), microglia (Iba-1), and oligodendrocytes (APC). Primary glia culture was isolated from the cortex of GFAP-tTA#2/TRE-TDP43M337V transgenic rats at 3 days of age. (g–l) Immunostaining showed that microglia and oligodendrocytes were eliminated from primary astrocyte culture after three cycles of shaking. (m–o) Immunofluorescent staining revealed that mutant human TDP-43 (hTDP43) was robustly expressed in purified astrocytes. Purified astrocytes were plated on 6-well plates and deprived of Dox on day 2 after plating. All scale bars: 30 μm (a-o).
Figure 2.
Over-expression of mutant TDP-43 in astrocytes altered gene expression profile. (a-e) Immunostaining revealed that the TDP43M337V transgene (hTDP43) was rapidly induced in astrocytes purified from GFAP-tTA#2/TRE-TDP43M337V double-transgenic rats (M337V: b-e) but not in control cells purified from GFAP-tTA#2 single-transgenic rats (a). (f) Hierarchical cluster analysis revealed differential gene expression between tTA and M337V transgenic astrocytes at varying times after induction (Dox withdrawal: Dox-). Columns on the heat map correspond to individual probe sets, with red indicating higher expression, yellow indicating intermediate expression, and blue indicating lower expression. Heat map shows selected 449 genes with an absolute value of fold change > 2 at any of the three induction times (3, 4, or 6 days after Dox withdrawal). (g) Biological pathway analysis revealed many enriched pathways altered by TDP43M337V expression. Higher enrichment score indicates greater enrichment. (h) Further analysis revealed that most genes involved in the most highly enriched pathway (extracellular region of panel g) encode secretory proteins. Scale bars: 30 μm (a-e).
Mutant TDP-43 in astrocytes preferentially affects secretory protein-encoding genes
For the transient expression of pathogenic TDP-43, glial cells were initially cultured in Dox-containing medium, which repressed the mutant TDP-43 transgene (Figure 2: A-B). Purified astrocytes were then deprived of Dox, which rapidly induced mutant TDP-43 transgene expression in astrocytes (Figure 1: M-O; Figure 2: A-E). By day 3 of Dox withdrawal, TDP-43 transgene was expressed in the majority of astrocytes. Gene expression profiles in cultured astrocytes were determined by microarray assay on day 3, 4, or 6 after Dox withdrawal (Figure 2: F). Genes with progressive change in expression were considered relevant to TDP-43 pathogenesis and were further analyzed for functionality. Intriguingly, clusters of genes encoding secretory proteins displayed the highest enrichment scores (Figure 2: G-H). Representative genes were validated by quantitative PCR, and their progressive changes in expression were verified in cultured astrocytes across all time points examined (Table 1). Changes in the expression of selected genes were confirmed in the spinal cord of transgenic rats that selectively expressed pathogenic TDP-43 in astrocytes (Table 1). Collectively, these results confirm the authenticity of altered gene expression profiles.
Table 1.
Quantitative PCR Validates Gene Expression Changes Revealed by Microarray
| Entrez Gene ID |
gene Symbol |
microarray assay | quantitative PCR | |||
|---|---|---|---|---|---|---|
| folds changed in astrocytes | folds changed in astrocyte culture |
folds changed in rat spinal cord |
||||
| 3 days | 4 days | 6 days | ||||
| 114592 | Aurkb | 4.8 | 6.1 | 9.7 | 6 | 4 |
| 24825 | Tf | 3.6 | 2.5 | 2.0 | 4 | 3 |
| 312707 | Lrrc23 | 3.1 | 3.0 | 2.4 | 3 | 2 |
| 266803 | Sostdc1 | 2.2 | 2.0 | 1.2 | 4 | 3 |
| 24231 | C2 | 2.1 | 2.3 | 2.5 | 3 | 8 |
| 25742 | S100b | 2.1 | 1.5 | 1.6 | 2 | 5 |
| 170496 | Lcn2 | 2.1 | 3.0 | 2.1 | 5 | 12 |
| 245920 | Cxcl10 | 1.8 | 2.3 | 1.9 | 2 | 6 |
| 89824 | Chi3l1 | 1.8 | 1.5 | 1.2 | 2 | 9 |
| 282583 | Fbln2 | 2.4 | 3.0 | 3.3 | 3 | 4 |
| 59107 | Ltbp1 | 2.5 | 2.2 | 3.7 | 3 | 3 |
| 25118 | Itga1 | 4.2 | 3.2 | 3.6 | 4 | 3 |
| 24484 | Igfbp3 | 5.7 | 4.0 | 4.8 | 5 | 6 |
Notes: For quantitative PCR, mRNA was extracted from astrocyte culture or rat spinal cord and reverse-transcribed into cDNA pools. Genes except Chi3L1 were selected for PCR verification based on their fold change > 2 at any of the three induction times (3, 4, or 6 days after Dox withdrawal). Chi3L1 is reported to be upregulated in neurodegenerative diseases and thus its expression in rat tissue was further analyzed by PCR. Quantitative PCR amplified cDNA of interested genes and also amplified the cDNA of L17. The levels of each mRNA in TDP-43M337V expressing cells or tissues were normalized to those in GFAP-tTA single transgenic cells or tissues and were expressed as the folds changed. Numbers in red indicate the increase in the folds of gene expression and numbers in blue indicate the decrease in the folds of gene expression.
Mutant TDP-43 in astrocytes leads to down-regulation of neurotrophic genes but up-regulation of neurotoxic genes
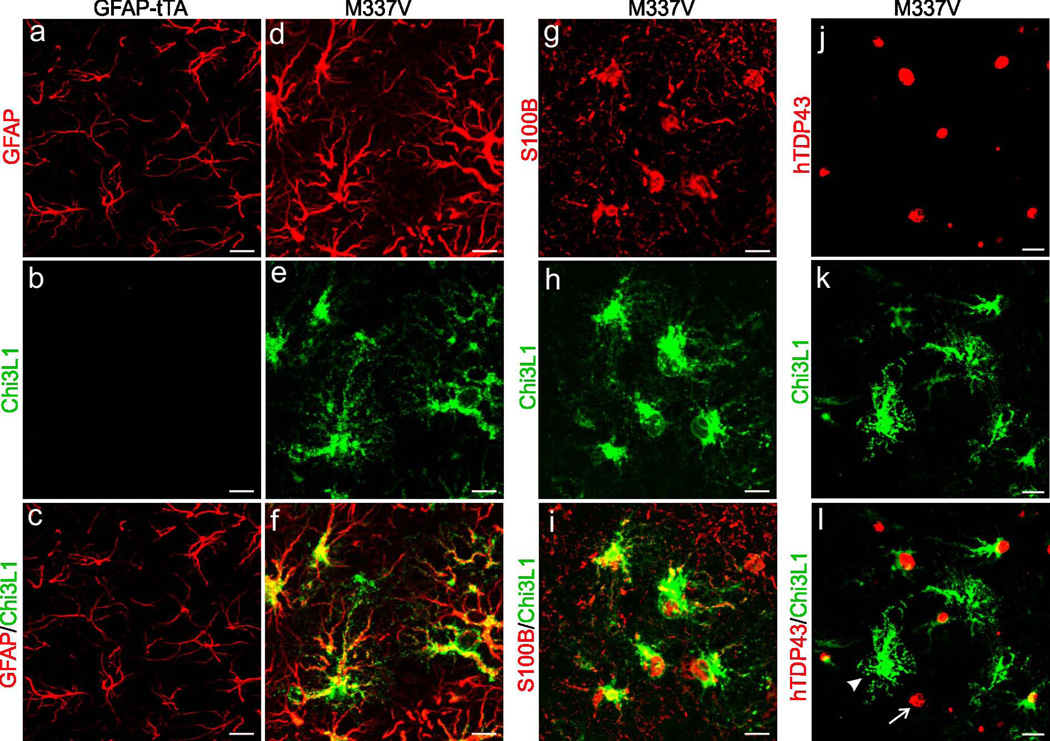
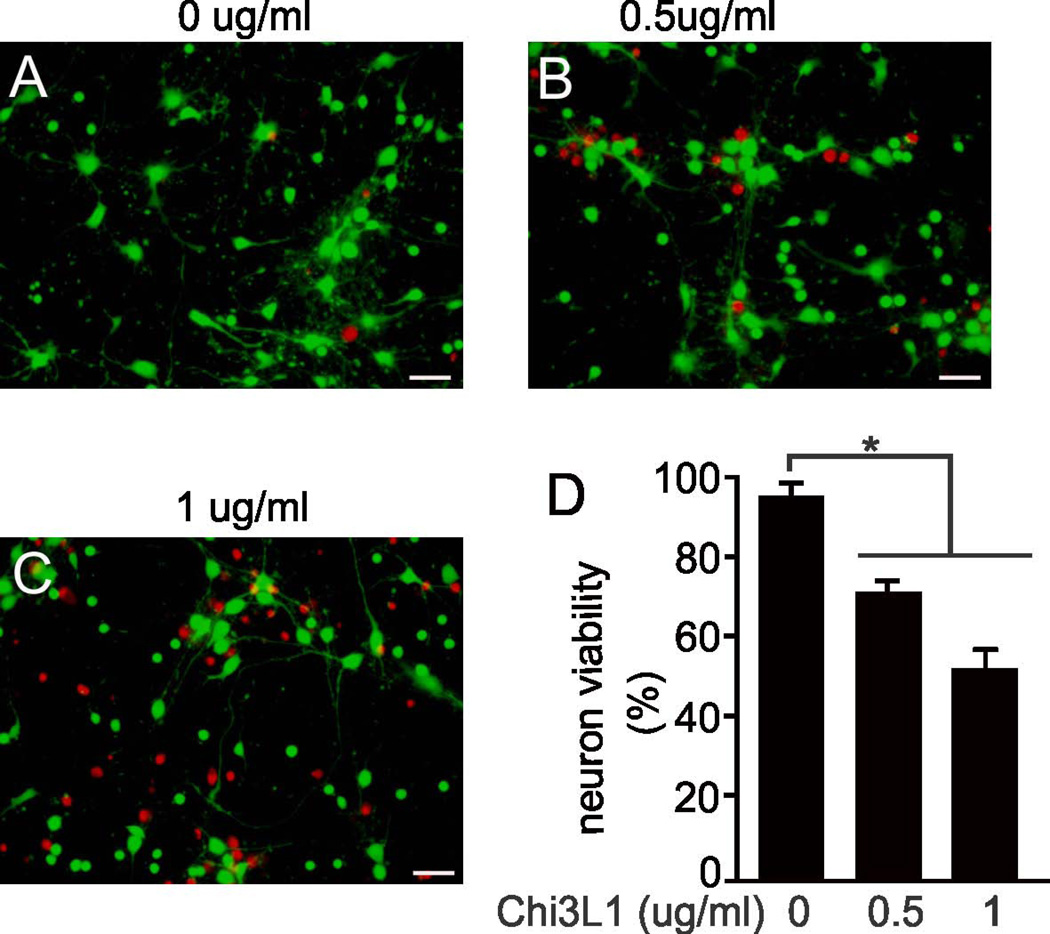
In-depth analysis of gene expression in astrocytes revealed that neurotrophic genes were down-regulated whereas potentially neurotoxic genes were up-regulated (Table 1). In response to pathogenic TDP-43 expression, some genes (i.e., Lcn2, CXCL10, and Chi3L1) were up-regulated and others (i.e., LTBP1, Fbln2, and ITGA1) were down-regulated in astrocytes from primary culture and transgenic rats (Table 1). Among these, Lcn2 belongs to the lipocalin family and its up-regulation is associated with neurotoxicity (Bi et al., 2013). Lcn2 stimulates the activation of quiescent astrocytes and microglia (Lee et al., 2007, Lee et al., 2009), thereby amplifying glia-mediated neuron damage. CXCL10 is up-regulated in astrocytes in response to excitatory neurotoxicity, and its deficiency diminishes NMDA-induced neuron death (van Weering et al., 2011). LTBP1 targets complexes of transforming growth factor beta to the extracellular matrix (Dobolyi and Palkovits, 2008), and its deficiency may lead to a loss of neurotrophic effects. Chi3L1 was up-regulated in the astrocytes of transgenic rats that selectively express mutant TDP-43 in astrocytes (Figure 3), but its induction appeared to be independent of mutant TDP-43 presence (Figure 3: I-L). We further examined the effect of Chi3L1 on neuron survival and found that synthetic Chi3L1 killed cortical neurons in a dose-dependent manner (Figure 4). Therefore, pathogenic TDP-43 in astrocytes induced up-regulation of genes such as Lcn2 and Chi3L1, whose protein products are cytotoxic to neurons.
Figure 3.
Over-expression of TDP43M337V in rats induced expression of the neurotoxic factor Chi3L1 in astrocytes. (a-f) Double-labeling fluorescent staining revealed that Chi3L1 was induced in astrocytes in GFAP-tTA#2/TRE-TDP43M337V double-transgenic rats (M337V: d-f) but not in GFAP-tTA#2 single-transgenic rats (a-c). (Slifer et al.) Fluorescent staining revealed that Chi3L1 precisely co-localized with S100B but with human TDP-43 (hTDP43). Arrowhead points to an astrocyte immunostained for Chi3L1 but not hTDP43. Arrow points to an astrocyte immunostained for hTDP43 but not Chi3L1. Images show immunostaining of lumbar cords taken from M337V rats at paralysis stages or GFAP-tTA rats at matched ages (a-l).
Figure 4.
The astrocyte factor Chi3L1 was neurotoxic. (a-c) Micrographs show a dose-dependent response of cortical neurons to Chi3L1 toxicity. Cortical neurons isolated from wild-type SD rats were treated with varying doses of Chi3L1 for 4 days. Cell viability was examined using a cell Live/Dead assay which stained live cells in green and dead cells in red. Scale bars: 40 μm. (d) Cell counts show cortical neuron viability after Chi3L1 treatment. Data are shown as mean ± SEM (n = 7), *p < 0.05.
Discussion
TDP-43, a multifunctional ribonucleoprotein, binds both DNA and RNA (Buratti, 2001). In neurons, TDP-43 binds thousands of mRNA molecules as detected by sequencing TDP-43-bound RNA (Sephton et al., 2011, Sephton et al., 2012). As we aimed to examine the molecular mechanisms of non-cell-autonomous neuron death related to TDP-43 pathogenesis, we used microarray assay instead of RNA sequencing to detect genes affected by pathogenic TDP-43 expression in astrocytes. We found that the expression of many genes in astrocytes was altered upon pathogenic TDP-43 expression. The selected genes are not necessarily the binding targets of TPD-43 but should reflect the functional status of astrocytes expressing pathogenic TDP-43. Our findings thus suggest that TDP-43 has numerous targets in astrocytes. Futher functionality analysis revealed that secretory protein-encoding genes were preferentially affected by pathogenic TDP-43 in astrocytes. Whereas neurotrophic factors were down-regulated, expression of genes with potential neurotoxicity were markedly up-regulated. Therefore, pathogenic TDP-43 in astrocytes differentially affects genes with neurotoxic or neurotrophic effects.
Secretory proteins Lcn2 and Chi3L1 were up-regulated in cultured astrocytes and transgenic rats that selectivley express pathogenic TDP-43 in astrocytes (Tong et al., 2013). Our previous study contains multiple lines of evidence that reactive astrocyte-secreated Lcn2 is highly and selectively toxic to neurons (Bi et al., 2013): 1) Lcn2 is induced in astrocytes of transgenic rats that express mutant TDP-43 in neurons and develop progressive neurodegeneration; 2) abundant Lcn2 is secreted into the medium of cultured rat brain slices that expresses pathogenic TDP-43 in neurons; 3) the conditioned medium of cultured rat brain slices is toxic to neurons, and this neurotoxicity is sigificantly reduced by depletion of Lcn2 from the conditioned medium; and 4) synthetic Lcn2 is highly toxic to neurons but is innocuous to glial cells (i.e., astrocytes, microglia, and oligodendrocytes). Up-regulated Lcn2 therefore adversely and appreciably affects neuron survival. Similar to Lcn2 (Bi et al., 2013), Chi3L1 adversely affected neuron survival in a dose-dependent manner as revealed in our current study. Chi3L1 is reportedly induced in reactive astrocytes (Bonneh-Barkay et al., 2010), and its levels are elevated in the cerebrospinal fluid of Alzheimer’s disease patients (Craig-Schapiro et al., 2010). Although Lcn2 and Chi3L1 may not be the binding targets of TDP-43, their induction in astrocytes likely reflects aberrant functionality. Thus, secretory proteins such as Lcn2 and Chi3L1 could be targeted for treatment of neurodegenerative disorders, and their levels could be used to monitor disease progression and therapeutic outcome.
In contrast to neurotoxic factors, neurotrophic factors were largely down-regulated in astrocytes upon expression of pathogenic TDP-43. Intriguingly, glutamate transporters EAAT1 and EAAT2 are markedly down-regulated in astrocytes of transgenic rats that selectively express pathogenic TDP-43 in astrocytes (Tong et al., 2013). However, EAAT1 and EAAT2 expression was unaltered in cultured astrocytes in the current study. These differing results obtained from in vitro and in vivo studies are not surprising, as primary cells may change their properties under culturing conditions. Whereas selective expression of mutant TDP-43 in astrocytes of intact rats clearly causes non-cell-autonomous motor neuron death and an ALS-like phenotype (Tong et al., 2013), expression of mutant TDP-43 in cultured astrocytes does not adversely affect neuron survival (Serio et al., 2013). That is, findings from in vitro studies do not always translate to live animals. Taken together, our results suggest that drastic alteration in the expression of secretory protein-encoding genes is a major contributor to non-cell-autonomous neuron death caused by expression of pathogenic TDP-43 in astrocytes.
Acknowledgement
This work is supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NS073829 to H. Z. and NS072113 and NS084089 to X.G.X). The content is the author’s responsibility and does not necessarily represent the official view of the NIH institutes.
Abbreviation used
- Chi3L1
Chitinase-3-like protein 1
- TDP-43
TAR DNA binding protein 43
- ALS
amyotrophic lateral sclerosis
- FTLD
frontotemporal lobar degeneration
Footnotes
Conflict of interest: The authors declare that no conflict of interest exists.
References
- Ayala YM, Pantano S, D'Ambrogio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE. Human, Drosophila, and C.elegans TDP43: nucleic acid binding properties and splicing regulatory function. J Mol Biol. 2005;348:575–588. doi: 10.1016/j.jmb.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Ayala YM, Zago P, D'Ambrogio A, Xu YF, Petrucelli L, Buratti E, Baralle FE. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. doi: 10.1242/jcs.038950. [DOI] [PubMed] [Google Scholar]
- Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, Li F, Xu Z, Bowser R, Xia XG, Zhou H. Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci U S A. 2013;110:4069–4074. doi: 10.1073/pnas.1218497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. Journal of Neuroinflammation. 2010;7:34. doi: 10.1186/1742-2094-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratti E. Characterization and Functional Implications of the RNA Binding Properties of Nuclear Factor TDP-43, a Novel Splicing Regulator of CFTR Exon 9. Journal of Biological Chemistry. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- Buratti E, Brindisi A, Giombi M, Tisminetzky S, Ayala YM, Baralle FE. TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: an important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J Biol Chem. 2005;280:37572–37584. doi: 10.1074/jbc.M505557200. [DOI] [PubMed] [Google Scholar]
- Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D'Angelo G, Malone JP, Townsend RR, Morris JC, Fagan AM, Holtzman DM. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer SK, Garden GA, Gill N, Rueb U, Libby RT, Schultz C, Guyenet SJ, Deller T, Westrum LE, Sopher BL, La Spada AR. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nature Neuroscience. 2006;9:1302–1311. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M. Expression of latent transforming growth factor beta binding proteins in the rat brain. J Comp Neurol. 2008;507:1393–1408. doi: 10.1002/cne.21621. [DOI] [PubMed] [Google Scholar]
- Escartin C, Bonvento G. Targeted Activation of Astrocytes: A Potential Neuroprotective Strategy. Molecular Neurobiology. 2008;38:231–241. doi: 10.1007/s12035-008-8043-y. [DOI] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tong J, Bi F, Wu Q, Huang B, Zhou H, Xia XG. Entorhinal cortical neurons are the primary targets of FUS mislocalization and ubiquitin aggregation in FUS transgenic rats. Hum Mol Genet. 2012a;21:4602–4614. doi: 10.1093/hmg/dds299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tong J, Bi F, Zhou H, Xia XG. Mutant TDP-43 in motor neurons promotes the onset and progression of ALS in rats. J Clin Invest. 2012b;122:107–118. doi: 10.1172/JCI59130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Lee EB, Chen-Plotkin A, Swanson E, Unger T, Malunda J, Xu Y, Winton MJ, Trojanowski JQ, Lee VM. Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J Clin Invest. 2011;121:726–738. doi: 10.1172/JCI44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, Pradat PF, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Neumann M, Sampathu DM, Lee VM, Trojanowski JQ. TDP-43 proteinopathy: the neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol. 2007;114:63–70. doi: 10.1007/s00401-007-0226-5. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Uryu K, Trojanowski JQ, Lee VM. TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis. Neurosignals. 2008;16:41–51. doi: 10.1159/000109758. [DOI] [PubMed] [Google Scholar]
- Landreth G, Jiang Q, Mandrekar S, Heneka M. PPARgamma agonists as therapeutics for the treatment of Alzheimer's disease. Neurotherapeutics. 2008;5:481–489. doi: 10.1016/j.nurt.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee J, Kim S, Park JY, Lee WH, Mori K, Kim SH, Kim IK, Suk K. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol. 2007;179:3231–3241. doi: 10.4049/jimmunol.179.5.3231. [DOI] [PubMed] [Google Scholar]
- Lee S, Park JY, Lee WH, Kim H, Park HC, Mori K, Suk K. Lipocalin-2 Is an Autocrine Mediator of Reactive Astrocytosis. Journal of Neuroscience. 2009;29:234–249. doi: 10.1523/JNEUROSCI.5273-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation–based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nature Neuroscience. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Zhuang Z, Hoffman PW, Bai G. Functional analysis of the rat N-methyl-D-aspartate receptor 2A promoter: multiple transcription starts points, positive regulation by Sp factors, and translational regulation. J Biol Chem. 2003;278:26423–26434. doi: 10.1074/jbc.M211165200. [DOI] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nature Neuroscience. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nature Neuroscience. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nature Medicine. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Pesiridis GS, Lee VM, Trojanowski JQ. Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis. Hum Mol Genet. 2009;18:R156. doi: 10.1093/hmg/ddp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rutherford NJ, Zhang YJ, Baker M, Gass JM, Finch NA, Xu YF, Stewart H, Kelley BJ, Kuntz K, Crook RJ, Sreedharan J, Vance C, Sorenson E, Lippa C, Bigio EH, Geschwind DH, Knopman DS, Mitsumoto H, Petersen RC, Cashman NR, Hutton M, Shaw CE, Boylan KB, Boeve B, Graff-Radford NR, Wszolek ZK, Caselli RJ, Dickson DW, Mackenzie IR, Petrucelli L, Rademakers R. Novel Mutations in TARDBP (TDP-43) in Patients with Familial Amyotrophic Lateral Sclerosis. PLoS Genet. 2008;4:e1000193. doi: 10.1371/journal.pgen.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton CF, Cenik B, Cenik BK, Herz J, Yu G. TDP-43 in central nervous system development and function: clues to TDP-43-associated neurodegeneration. Biol Chem. 2012;393:589–594. doi: 10.1515/hsz-2012-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton CF, Cenik C, Kucukural A, Dammer EB, Cenik B, Han Y, Dewey CM, Roth FP, Herz J, Peng J, Moore MJ, Yu G. Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. J Biol Chem. 2011;286:1204–1215. doi: 10.1074/jbc.M110.190884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio A, Bilican B, Barmada SJ, Ando DM, Zhao C, Siller R, Burr K, Haghi G, Story D, Nishimura AL, Carrasco MA, Phatnani HP, Shum C, Wilmut I, Maniatis T, Shaw CE, Finkbeiner S, Chandran S. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc Natl Acad Sci U S A. 2013;110:4697–4702. doi: 10.1073/pnas.1300398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifer MA, Martin ER, Bronson PG, Browning-Large C, Doraiswamy PM, Welsh-Bohmer KA, Gilbert JR, Haines JL, Pericak-Vance MA. Lack of association between UBQLN1 and Alzheimer disease. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:208–213. doi: 10.1002/ajmg.b.30298. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup V, Phaneuf D, Dupre N, Petri S, Strong M, Kriz J, Julien JP. Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor kappaB-mediated pathogenic pathways. J Exp Med. 2011;208:2429–2447. doi: 10.1084/jem.20111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Huang C, Bi F, Wu Q, Huang B, Liu X, Li F, Zhou H, Xia XG. Expression of ALS-linked TDP-43 mutant in astrocytes causes non-cell-autonomous motor neuron death in rats. EMBO J. 2013;32:1917–1926. doi: 10.1038/emboj.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Huang C, Bi F, Wu Q, Huang B, Zhou H. XBP1 depletion precedes ubiquitin aggregation and Golgi fragmentation in TDP-43 transgenic rats. J Neurochem. 2012;123:406–416. doi: 10.1111/jnc.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KJ, Yang CH, Fang YH, Cho KH, Chien WL, Wang WT, Wu TW, Lin CP, Fu WM, Shen CK. Elevated expression of TDP-43 in the forebrain of mice is sufficient to cause neurological and pathological phenotypes mimicking FTLD-U. J Exp Med. 2010;207:1661–1673. doi: 10.1084/jem.20092164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, Steinbart E, McCluskey L, Grossman M, Neumann M, Wu IL, Yang WS, Kalb R, Galasko DR, Montine TJ, Trojanowski JQ, Lee VM, Schellenberg GD, Yu CE. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering HR, Boddeke HW, Vinet J, Brouwer N, de Haas AH, van Rooijen N, Thomsen AR, Biber KP. CXCL10/CXCR3 signaling in glia cells differentially affects NMDA-induced cell death in CA and DG neurons of the mouse hippocampus. Hippocampus. 2011;21:220–232. doi: 10.1002/hipo.20742. [DOI] [PubMed] [Google Scholar]
- Wang HY, Wang IF, Bose J, Shen CK. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004;83:130–139. doi: 10.1016/s0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- Zhou H, Huang C, Chen H, Wang D, Landel CP, Xia PY, Bowser R, Liu YJ, Xia XG. transgenic rat model of neurodegeneration caused by mutation in the TDP gene. PLOS Genetics. 2010;6:e1000887. doi: 10.1371/journal.pgen.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]