Significance
Proximity-dependent biotinylation (BioID) is a readily accessible method for identifying protein associations that occur in living cells. Fusion of a promiscuous biotin ligase to a bait protein for expression in live cells enables covalent biotin labeling, and thus identification, of proteins proximate to the bait. Here we used BioID to probe the organization of the nuclear pore complex, a large structure that regulates molecular transport between the nucleus and cytoplasm. These studies enhance our understanding of major subcomplexes within the nuclear pore complex and demonstrate the utility of BioID for studying the organization of large protein assemblies. Additionally, we have measured the labeling radius of BioID, thus enabling the rational application of this method and more meaningful data interpretation.
Abstract
Proximity-dependent biotin identification (BioID) is a method for identifying protein associations that occur in vivo. By fusing a promiscuous biotin ligase to a protein of interest expressed in living cells, BioID permits the labeling of proximate proteins during a defined labeling period. In this study we used BioID to study the human nuclear pore complex (NPC), one of the largest macromolecular assemblies in eukaryotes. Anchored within the nuclear envelope, NPCs mediate the nucleocytoplasmic trafficking of numerous cellular components. We applied BioID to constituents of the Nup107–160 complex and the Nup93 complex, two conserved NPC subcomplexes. A strikingly different set of NPC constituents was detected depending on the position of these BioID-fusion proteins within the NPC. By applying BioID to several constituents located throughout the extremely stable Nup107–160 subcomplex, we refined our understanding of this highly conserved subcomplex, in part by demonstrating a direct interaction of Nup43 with Nup85. Furthermore, by using the extremely stable Nup107–160 structure as a molecular ruler, we defined the practical labeling radius of BioID. These studies further our understanding of human NPC organization and demonstrate that BioID is a valuable tool for exploring the constituency and organization of large protein assemblies in living cells.
The refined characterization of protein assemblies is a prerequisite for understanding functional protein networks. Proximity-dependent biotin identification (BioID) is an approach recently developed to address this problem. BioID is based on expression of a “bait” protein fused to a promiscuous biotin ligase (BirA*) that will generate a history of the bait’s proximity-dependent associations over a period by the biotinylation of interacting or neighboring “prey” proteins (1). BioID biotinylates proteins in situ before their solubilization and subsequent purification and identification. Issues related to bait (and prey) protein solubility and the stability and/or duration of their interaction are thus overcome. BioID has been used successfully to screen for constituents of the relatively insoluble mammalian nuclear lamina (1), the trypanosome bilobe (2), cell junction complexes (3–5), and centrosomes (6, 7). The method also has been used to screen for proteins involved in the Hippo signaling pathway (8).
Biotinylation by BioID is a mark of proximity and not evidence for physical interaction. An outstanding issue concerning this method is the radius of biotinylation. Previous application of BioID to lamin A (LaA) suggested that a majority of the candidates resided within ∼20–30 nm of nuclear envelope (NE)-associated LaA (1). Significantly, distinct subsets of BioID candidates were identified when BirA* was fused to the N versus the C terminus of the cell-junction protein ZO-1 (3). These studies suggested that BioD has a limited nanometer-scale (<20 nm) labeling radius. However, its precise range remained uncertain.
Certain parameters are needed to analyze the range of biotinylation by BioID in live cells more carefully. An ideal test would involve a stable multiprotein structure, preferably with known dimensions that extend beyond 20 nm. Protein stability within this complex is essential to generate accurate measurements. Ideally the complex also should be relatively stable in cells. In nondividing cells the nuclear pore complex (NPC) as been shown to be an extremely stable structure, with many of its constituents exhibiting long residence times (9, 10) and low turnover (11, 12). Anchored within the NE, NPCs mediate the nucleocytoplasmic trafficking of numerous cellular components. NPCs are composed of multiple copies of ∼30 distinct proteins (nucleoporins or “Nups”) arranged with eightfold radial symmetry, leading to an assembly of 500–1,000 proteins with an estimated mass of ∼125 MDa in vertebrates. The mammalian NPC has a core structure composed of two outer membrane-proximal rings (built up by Nup107–160 scaffold complexes) that enclose a central spoke ring containing the Nup93 complex. Interactions of these scaffold Nups with integral membrane proteins contribute to the anchoring of the NPC within the pore membrane. Tethered by this membrane-embedded central framework, peripheral NPC components (notably a subset of Nups containing Phe-Gly repeats, FG-Nups) extend into the central pore channel and into the cytoplasm and the nucleoplasm, where they form cytoplasmic filaments and the nuclear pore basket respectively (reviewed in refs. 13–16) (Fig. 1A).
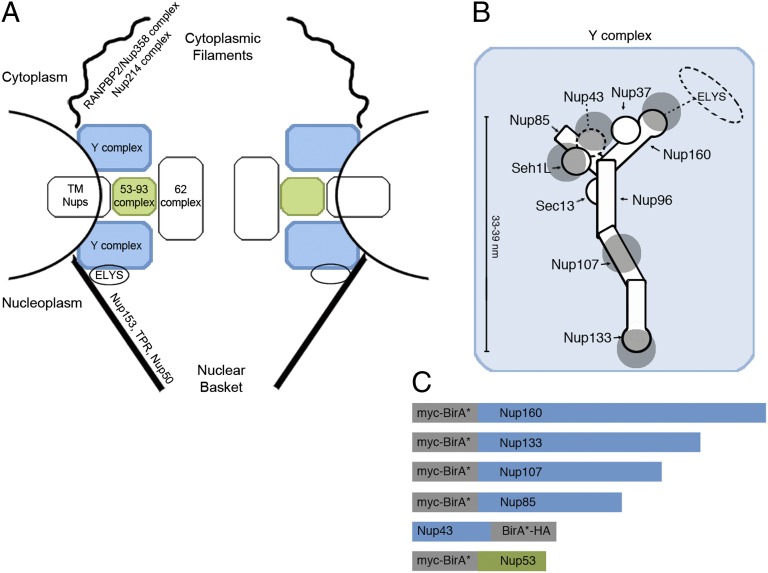
Fig. 1.
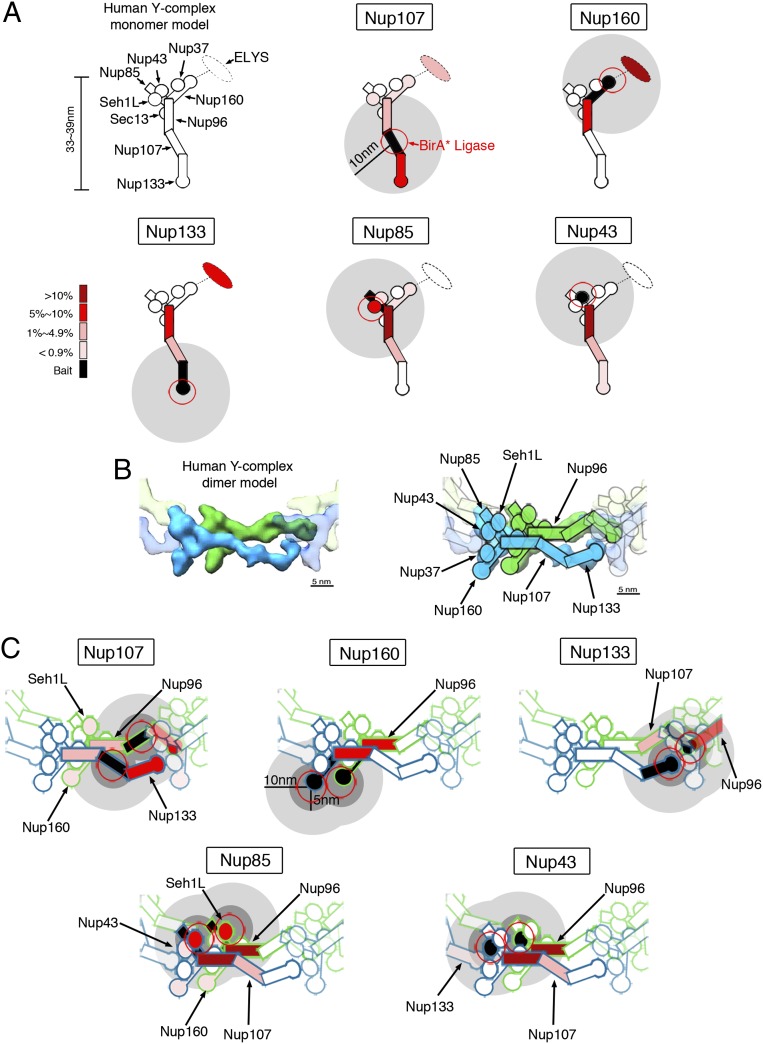
Organization of the mammalian NPC, Y-complex, and BirA*-fusion proteins. (A) Positioning of the Y-complex (blue) and Nup53–93 complex (green) within a simplified model of NPC organization. A full description of the members of each pore subcomplex is shown in Table 1, leftmost column. TM Nups, transmembrane Nups. (B) Model of the human Y-complex. Its many β-propeller domains are schematized by circles or bulges; alpha-solenoid folds are depicted by rectangles. Nup43 is drawn with a dashed line because its localization was unknown at the time these studies were performed. The dotted line and oval indicates possible residence of ELYS near Nup160 and Nup37, extrapolated from studies in yeast (63, 64). Gray disks represent the predicted localization of the BirA*-ligase based on available structural data. (C) Linear model for the NPC proteins fused to BirA* for the BioID studies.
The metazoan Nup107–160/yeast Nup84 complex is a conserved and extensively characterized NPC building block (reviewed in ref. 17). In vertebrates, this complex consists of nine subunits: nucleoporin Nup133, nucleoporin Nup107, nucleoporin Nup96, nucleoporin Nup85, nucleoporin Nup160, protein Sec13 homolog Sec13, nucleoporin Seh1L, nucleoporin Nup37, and nucleoporin Nup43 (10, 18, 19), with the nucleoplasmic protein Elys sometimes considered a 10th member of the complex (see Fig. 1B, refs. 20 and 21, and references therein). Biochemical and structural analyses in various species have revealed the precise arrangement of these Nups into Y-shaped complexes (hence the name “Y-complex”) (reviewed in refs. 16 and 21; also see Fig. 1B). Photobleaching studies of mammalian Y-complex Nups (Y-Nups) have revealed their extreme stability within the NPCs, with half-time recoveries exceeding 35 h (22). By applying BioID to proteins that reside at distinct points within the elongated (33–39 nm long) human Y-complex (21), we aimed to define the practical labeling radius of BioID.
To improve our understanding of both BioID specificity and NPC architecture, we also applied BioID to nucleoporin Nup53, a small dimeric membrane-associated protein that belongs to the Nup93 complex, a distinct NPC scaffold complex that links the pore membrane with the Nup62 complex that resides within the central pore channel (refs. 23–27 and references therein; reviewed in ref. 28). Taken together these distinct BioID datasets, encompassing both the Y- and Nup93 complexes, allowed us to define a practical labeling radius and hence the resolution of the BioID technique, at the same time demonstrating the value of the technique in defining both the constituency and organization of large protein complexes.
Results and Discussion
Identification of Proteins Biotinylated in Cells Stably Expressing BioID-Nups.
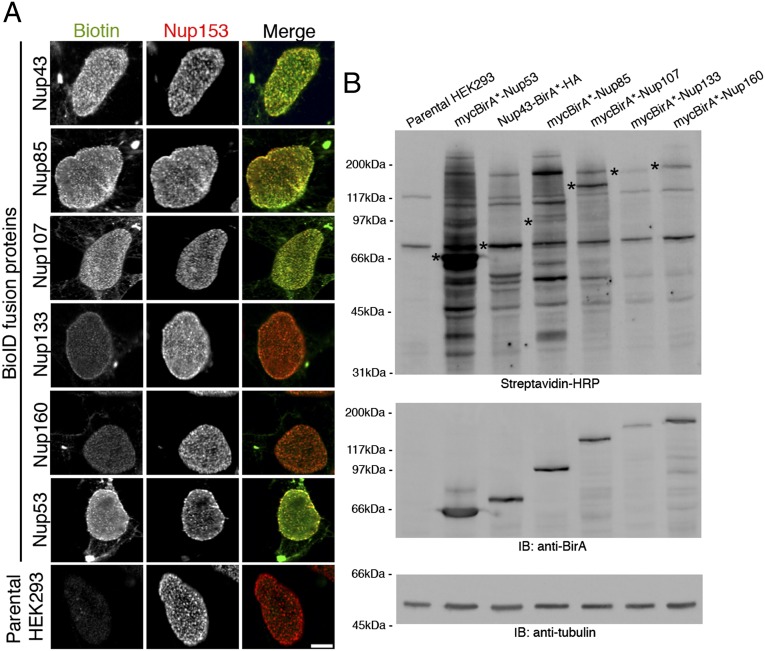
As a prelude to our studies we generated HEK293 cell lines that constitutively express BirA*-tagged members of the Y-complex (Nup160, Nup133, Nup107, Nup85, and Nup43), BirA*-tagged Nup53, or LaA for comparison (Fig. 1C). To minimize artifacts associated with fusion proteins targeting to sites other than NPCs, care was taken to choose subclones of cells that expressed low levels of the fusion protein. Because NPCs disassemble at mitosis, a stage when several Nups associate with other structures [notably at kinetochores in the case of Y-Nups (29, 30)], cell growth was arrested for 72 h in low-serum medium before the induction of biotinylation in all of our experiments,. Immunofluorescence analyses revealed that biotinylation catalyzed by each of the BioID-Nups was largely coincident with the NPCs, as revealed by colocalization with mAb414 (which detects a subset of Nups containing FXFG repeats) (31) and anti-Nup153 (Fig. 2A and Fig. S1B). In addition, the BioID-Nups (but not BioID-LaA) and biotinylated proteins also localized within cytoplasmic structures stained by mAb414 but not by anti-Nup153; these structures likely correspond to populations of endoplasmic reticulum-associated nuclear pores called “annulate lamellae” (9, 31, 32) (Fig. 2A and Fig. S1B). Immunoblot (IB) analysis revealed biotinylation of the BioID-Nups as well as variable levels of unidentified endogenous proteins (Fig. 2B). To identify the proteins biotinylated by each of the BioID-fusion proteins, material isolated from large-scale BioID pull-downs was analyzed by MS (1, 33) (Materials and Methods and Dataset S1). The identities and relative abundance of the candidates (Materials and Methods) associated with the NPC and NE are listed in Table 1.
Fig. 2.
Characterization of cells stably expressing NPC BirA*-fusion proteins. (A) Immunofluorescence analyses of HEK293 cells stably expressing Y-complex members or Nup53 fused to BirA*. The biotin signal generated by the BirA*-fusion proteins is detected with fluorescently labeled streptavidin (green). and NPCs are detected with anti-Nup153 (red). (Scale bar: 7 μm.) (B) Following SDS/PAGE of cell lysates, biotinylated proteins were detected with streptavidin-HRP. Asterisks indicate the location of the BirA*-fusion protein (detected with anti-BirA). Tubulin was used as loading control.
Table 1.
Summary of the proteins detected by BioID-Nups, BioID-LaA. and BirA*-only
| Identified candidates | BirA* fusion protein baits |
|||||||
| Nup160 | Nup133 | Nup107 | Nup85 | Nup43 | Nup53 | LaA | BirA* | |
| Nup107 complex (Y-complex + Elys) | ||||||||
| NUP160 | Bait | 0.1 | 0.1 | |||||
| NUP107 | 4.3 | Bait | 1.1 | 4.3 | ||||
| NUP133 | Bait | 7.0 | 0.4 | 0.2 | ||||
| NUP96 | 6.4 | 8.9 | 4.5 | 14.8 | 17.8 | 6.0 | 0.3 | |
| NUP85 | Bait | X | ||||||
| NUP43 | 0.4 | Bait | ||||||
| SEH1L | 0.6 | 7.7 | X | |||||
| ELYS | 14.5 | 6.9 | 1.6 | X | 0.2 | 2.2 | ||
| Percent of total that are Nup107 complex/Elys components | 21 | 20 | 14 | 24 | 23 | 6 | 3 | 0 |
| Nup93 complex | ||||||||
| NUP53 | Bait | |||||||
| NUP93 | 0.1 | |||||||
| NUP155 | 2.7 | 0.1 | ||||||
| NUP205 | 0.3 | |||||||
| NUP188 | X | 5.2 | ||||||
| Nup62 complex | ||||||||
| NUP62* | 5.6 | 17.2 | ||||||
| NUP58/45 | 7.2 | |||||||
| NUP54 | 1.6 | |||||||
| Transmembrane nups | ||||||||
| POM121 | 1.8 | 1.4 | 0.5 | 0.2 | 2.6 | 1.4 | ||
| NDC1 | 1.1 | |||||||
| Percent of total that are expected Nup53 partners | 2 | 1 | 0 | 6 | 0 | 38 | 2 | 0 |
| Cytoplasmic nups | ||||||||
| NUP88 | X | 1.9 | 1.5 | |||||
| NUP214 | 0.9 | 8.7 | 12.5 | <0.1 | ||||
| GLE1 | 0.4 | |||||||
| CG1 | 5.3 | |||||||
| DDX19B/DBP5 | 0.8 | |||||||
| Cytoplasmic filament nups | ||||||||
| RANBP2/Nup358 | 5.1 | 6.5 | 25.1 | 13.5 | 4.0 | 4.7 | 0.8 | |
| RANGAP1 | 2.6 | 2.2 | 0.1 | |||||
| Nuclear pore basket | ||||||||
| NUP153 | 17.7 | 23.1 | 23.3 | 20 | 15.2 | 8.9 | 5.1 | 0.1 |
| NUP50 | 1.2 | 2.8 | 11.7 | 16.5 | 5.4 | 1.3 | 4.4 | |
| TPR | 11.7 | 1.2 | ||||||
| SENP1 | 1.2 | 1.0 | 1.3 | |||||
| SENP2 | 6.7 | 1.3 | 5.2 | |||||
| NUP98 | 0.4 | 0.5 | 6.0 | 0.4 | ||||
| Import/export | ||||||||
| KPNB1 | 0.3 | 0.1 | ||||||
| XPO1 | 0.3 | |||||||
| Percent of total that are NPC-associated | 47 | 61 | 92 | 94 | 54 | 86 | 15 | 2 |
| Nuclear envelope constituents | ||||||||
| TMPO beta | 25.9 | 28.9 | 4.3 | 12.7 | 1.3 | |||
| LEMD3 | 3.5 | 0.6 | 3.7 | <0.1 | ||||
| EMD | 1.5 | 8.9 | ||||||
| SYNE1 | 0.0 | 0.0 | ||||||
| TMEM201 | 0.3 | 0.3 | ||||||
| TMPO alpha | 0.6 | |||||||
| LBR | 0.2 | |||||||
| Percent of total that are NPC/NE-associated | 76 | 90 | 92 | 94 | 54 | 92 | 41 | 4 |
Numbers are the percent of total adjusted peptides (excluding BirA*-fusion protein). Candidates listed as 0.0 are <0.1. Numbers in bold indicate proteins identified by XL-MS on isolated Y complex or intact NPC. X, proteins identified by XL-MS but not BioID. BioID pull-down for LaA was performed with asynchronous cells.
BioID-Nup Analyses Reveal Restricted Specificity.
Immediately obvious in the Nup BioID data of Fig. 2B and Table 1 is the lack of widespread protein biotinylation. Instead, 47–94% of the detected candidates are proteins associated with the NPC. In contrast, Nups represented only 15% of the prey proteins associated with LaA (predominantly nuclear basket) and 2.1% of the prey proteins associated with BirA*-only (Datasets S1–S3). Nup43 was unique in its detection of a prominent non-NPC– or non-NE–associated protein, namely t-complex protein 1 subunit theta (CCT8). CCT8 is a component of the chaperonin complex that mediates folding of proteins containing a WD-repeat, of which Nup43 is a member (reviewed in ref. 34). BirA*-Nup133 was expressed at lower levels than the other baits, and fewer peptides were detected by BioID with Nup133. However, the percentages of those candidates that were constituents of the Y-complex and NPC were similar to the percentages when the other baits were used.
When candidates from the BioID Y-complex and BioID-Nup53 experiments are compared, it is clear that, in large part, they detected spatially distinct populations of proteins (Table 1). Y-complex Nups represented 14–24% of the total BioID candidates that were found when a Y-Nup-BirA* was used as a bait. In contrast, few Y-Nups were found when Nup53 was used as bait (6%, largely Nup96) or when with BirA*-LaA was used (2.7%, largely ELYS). Instead, 38% of the proteins identified by BioID-Nup53 correspond to predicted nearest-neighbor Nups, namely, components of the Nup93 complex, their transmembrane Nup partners nucleoporin Ndc1 and nuclear envelope pore membrane protein Pom121, and the Nup62 complex that is anchored by Nup93 (refs. 35–37 and references therein). These Nups were detected only rarely in Y-Nup–BioID samples. A more global comparison of BioID–Y-Nups versus BioID-Nup53 outcome reveals that, although some candidates overlap, BioID specifically detects different populations of Nup proteins within the larger NPC assembly depending on the residence of the bait (Table 1).
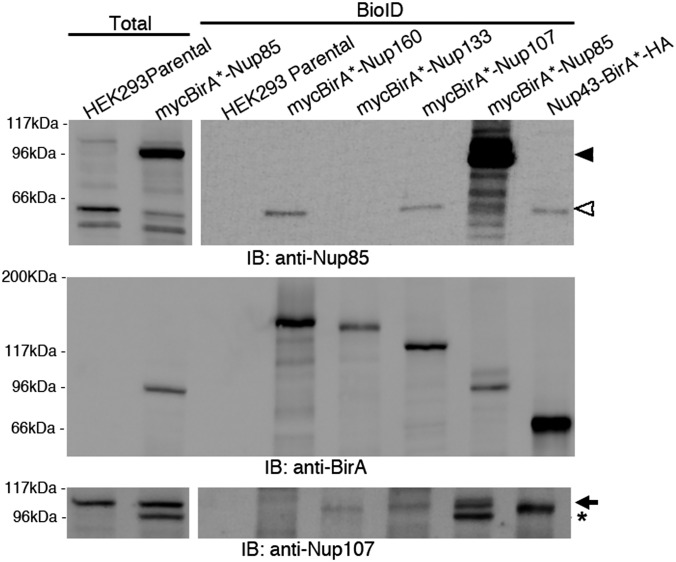
None of the BioID–Y-Nups detected all other members of the Y-complex, although seven of its 10 constituents (including Elys) are biotinylated by at least one of the BioID–Y-Nups. Only Sec13 and Nup37, two small β-propeller Y-Nups (Fig. 1B), were never identified. Endogenous Nup85 was detected only modestly, even though it showed substantial expression and biotinylation of other Y-Nups when fused to biotin ligase (Fig. 2B and Dataset S1). This result suggests that Nup85 may not be able to be efficiently biotinylated. By the more sensitive IB analysis, low levels of endogenous Nup85 were detected in BioID pull-downs for Nup160, Nup107, and Nup43 but not for Nup133 (Fig. 3). These results are not surprising, because BioID-Nup85 detected Nup107 and, to a lesser extent, Nup160 and Nup43, suggesting proximity to these proteins. As a control we reprobed these samples for Nup107 and observed detection of this protein consistent with the MS results. These data reveal one limitation of BioID, namely, that not all proteins are biotinylated with similar efficiency. As in any large-scale experiment, negative results should be treated with caution.
Fig. 3.
Nup85 is a poor substrate for BioID. IB analysis detects low levels of endogenous Nup85 (open arrowhead) in the Nup160, Nup107, and Nup43 BioID pull-down samples and significant levels of the exogenous mycBirA*-Nup85 (arrowheads) in the Nup85 BioID pull-down. (Top) For clarity, total lysates are shown at a lower exposure than the BioID samples. (Middle) For comparison, we detect similar levels of the BirA*-fusion proteins with anti-BirA in these same samples. (Bottom) Reprobing the same membrane with anti-Nup107 reveals levels of endogenous Nup107 (arrow) that correlate with the MS results, thus corroborating those results. Exogenous mycBirA*-Nup85 remains detected below the endogenous Nup107 (asterisk).
Mapping the Position of Nup43 Within the Y-Complex.
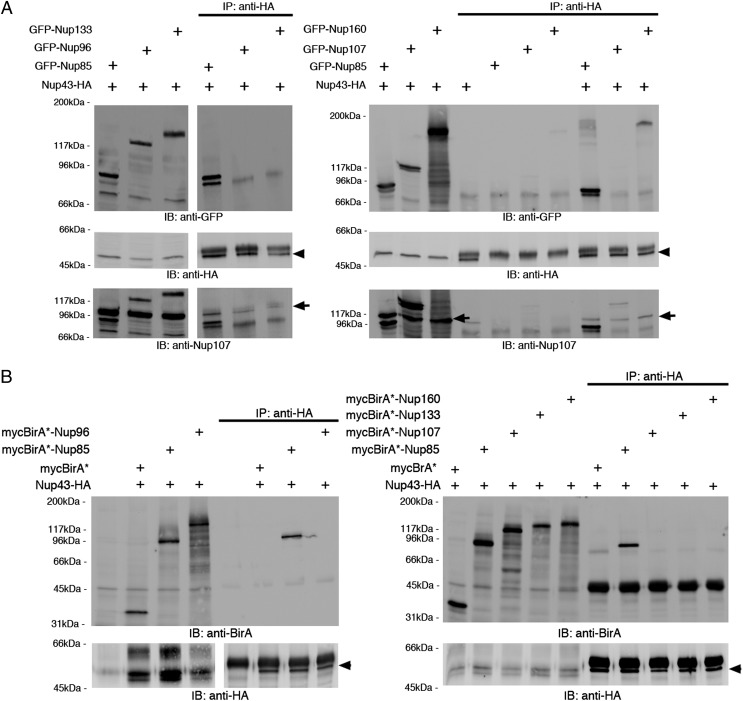
At the time these studies were performed, there was no information as to the location of Nup43 within the Y-complex. Although our Nup43-BioID results suggested that this WD-repeat domain protein is proximate to Nup96, Nup43 was specifically detected by BioID-Nup85. To assess biochemically how Nup43 integrates into the Y-complex, we immunoprecipitated exogenous epitope-tagged complex members and asked if other Nups were coimmunoprecipitated. Assuming that, like the other small β-propeller folded Y-Nups, Nup43 most likely interacts directly with one of the larger proteins, we performed immunoprecipitation (IP) of Nup43-HA and asked if it coimmunoprecipitated with GFP-tagged Nup133, Nup107, Nup96, Nup85, or Nup160. We found that Nup43-HA consistently pulled down GFP-Nup85, and, to a much lesser extent, GFP-Nup160, but inconsistently or never pulled down GFP-Nup107, -Nup133, or -Nup96 (Fig. 4A). As a control, we reprobed these blots with anti-Nup107 and observed that a fraction of endogenous Nup107 was identified in all co-IPs, indicating that our lysis conditions permitted isolation of the endogenous Y-complex (Fig. 4A). However, the relative amount of endogenous Nup107 that was coprecipitated was significantly less substantial than for GFP-Nup85, indicating that the transfected Nup43 and Nup85 likely interact independently of their incorporation into the Y-complex or the NPC.
Fig. 4.
Nup43 interacts with Nup85. (A) Anti-HA co-IP from lysates of HEK293 cells cotransfected with Nup43-HA (Middle, arrowhead) and GFP–Y-Nups (Top) indicates that GFP-Nup85 is pulled down most efficiently by Nup43-HA. (Bottom) Reprobing the samples with anti-Nup107 reveals low levels of endogenous Nup107 (arrow) in all the Nup43-HA pull-down samples. (B) Co-IPs from in vitro transcription/translation reactions in reticulocyte lysates using Nup43-HA (Lower, arrowhead) alone or with mycBirA*-tagged Y-Nups (Upper). Only mycBirA*-Nup85 is detected in the Nup43-HA pull-down fraction.
To validate this result, we turned to an in vitro transcription and translation assay that revealed that Nup43 interacts with Nup85 but with none of the other larger scaffold Y-Nups (Fig. 4B). Together these studies biochemically demonstrate a direct interaction between Nup43 and Nup85. Thus, by demonstrating that Nup43 and Nup85 can associate with each other independently of their integration within the entire NPC, these results strengthen a recent report that identified Nup43 residing in close proximity to Nup85 and Seh1 by XL-MS (21).
Y-Nups as Macromolecular Rulers to Define the Practical Labeling Radius of BioID Accurately.
When analyzed in the context of a monomeric Y-complex, the major BioID Y-Nup candidates appear positioned at 10–20 nm from the baits for Nup160, Nup107, Nup85, and Nup43 (Fig. 5A). In contrast, the strong detection of Nup96 in the case of Nup133 and the weaker identification of Y-Nups distantly positioned from the various baits suggest a much larger radius (Table 1). However, a recent study provides a compelling model for the mammalian NPC in which offset Y-complex dimers are arranged in a head-to-tail staggered parallel fashion to form ring-like structures on the nucleoplasmic and cytoplasmic sides of the pore (21). With this organization it is clear that in some instances we are likely observing intercomplex rather than intracomplex labeling by BioID. Prime examples are the detection of Nup96 by BioID-Nup133 and of Nup107 by BioID-Nup85 or BioID-Nup43 (Table 1 and Fig. 5B). Reevaluating the BioID results from the Y-complex in the context of the whole NPC, we thus can restrict the practical labeling radius of BioID (defined as the ability to detect proteins by MS following BioID pull-down) to ∼10 nm (Fig. 5B). However, because not all Nups within 10 nm are labeled by a BioID-Nup bait, one again must view negative results with caution.
Fig. 5.
Biotinylation of Y-Nups in the context of the whole NPC defines a practical labeling radius. (A) For each BioID-fusion, a model of a single Y-complex subunit is used to depict the relative abundance of Y-Nups detected following BioID pull-down. The red circles depict the approximate position of the BirA* ligase. Gray disks (10-nm radius) provide an approximation of the labeling radius of BioID. (B) Structural model from Bui et al. (21) in which offset Y-complex dimers are arranged in a head-to-tail fashion within the NPC (Left). The approximate positions of Y-Nups are labeled and schematized (Right) on this map. (Modified from ref. 21.) (C) BioID data were applied to the dimer model of Y-complex. The color code in A is used to depict the relative abundance of biotinylated Y-Nups for BioID-fusion proteins. The gray disks (dark: 5-nm radius; light: 10-nm radius) provide an approximation of the labeling radius of BioID.
Refining NPC Organization with BioID.
Keeping in mind the estimated labeling radius, we then analyzed the NPC constituents outside the stable Y-complex that were detected by BioID-Nups to get insight into the whole NPC architecture (Fig. 6 and Table 1). Among the identified Nups, a few, most notably FG-Nups associated with the cytoplasmic filaments (nucleoporin Nup358/RANBP2) or the nuclear basket (nucleoporin Nup50 and nucleoporin Nup153), were substantially detected by all Y-Nups. Among them, Nup153 was reported previously to associate with the Y-complex (18), and recent crosslinking-MS (XL-MS) and cryo-electron tomography (cryo-ET) studies recognized the proximity of Nup358 and the Y-complex (21). However, the identification of these large, flexible, or dynamic Nups (22, 38–41) by BioID-Nup53 suggests that these FG-Nups may associate with multiple Nups only transiently as they sample the NPC environment.
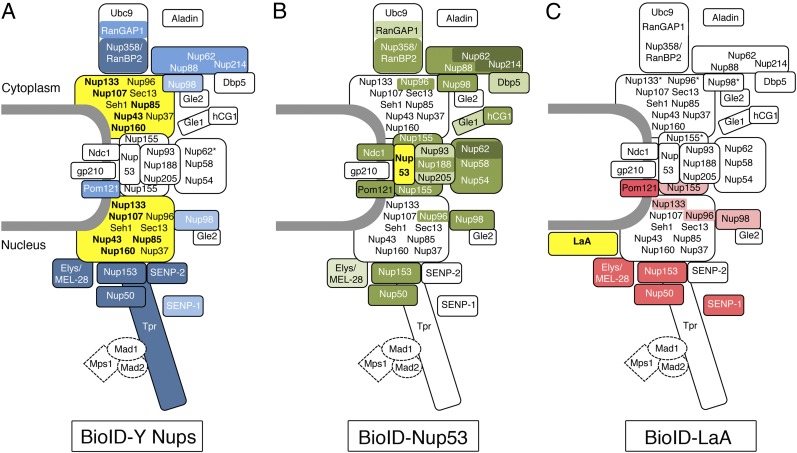
Fig. 6.
Biotinylation of NPC constituents generally correlates with the location of the fusion protein. The candidate Nups identified in this BioID studies are positioned within a simplified model of NPC organization that integrates data from the literature and extrapolations based on previous studies in budding yeast. The baits (bold text) used are shaded yellow. Intensity of (A) blue- (BioID-Y-Nups), (B) green- (BioID-Nup53), or (C) red- (BioID-LaA) shaded candidates correlates with the level of detection of candidates predominantly detected by the different types of BioID-fusion proteins (Table 1). In A, biotinylated Y-Nups are not shaded blue for clarity (Fig. 5). The asterisks next to Nup62 in A and next to Nup133, Nup96, and Nup98 in C represent candidates with multiple locations within the NPC and are unlikely to be biotinylated at that specific place.
Nevertheless, most Nups were identified in a restricted subset of BioID samples, thus validating or refining our actual knowledge of NPC organization. In particular, the more central NPC constituents, such as those in the Nup93 and Nup62 complexes and Ndc1, were all identified when we applied BioID to Nup53 but were largely absent from the BioID–Y-Nups results (Table 1). The identification of this subset of Nups connecting the pore membrane to the more central Nup62–Nup58–Nup54 complex is consistent with the proposed model of the inner pore complex of the NPC (23). Because of its flexibility (42, 43), the Nup62 complex may be further capable of sampling the membrane-proximate region where Nup53 resides. Also well represented in the BioID-Nup53 candidates are proteins reported to localize on the cytoplasmic side of the NPCs, nucleoporin Nup88 and nucleoporin Nup214, which are known to associate with each other (44–48), ATP-dependent RNA helicase Ddx19/Dbp5, which is known to interact with Nup214 (49), and nucleoporin Gle1 and its binding partner nucleoporin hCG1/Npl1 (50, 51) (Fig. 5). The identification of these more distant partners might result from the intrinsic dynamics of Nup53, which has a reported residency half-time of ∼5 h in dividing cells (22), or from the existence of distinct population of the 32 copies of Nup53 within the NPC (52). However, these data also could reflect a more central positioning of these “cytoplasmic” Nups, a feature compatible with the reported interaction between Gle1 and nucleoporin Nup155 (a direct Nup53 binding partner) (53) and with the apparent bending of the Nup214–Nup88 complex toward the central pore channel observed by cryo-ET (21).
Nup133 and Nup160 detected substantially more Pom121 than any of the other Y-Nups that were tested. These two Nups also were unique in detecting TMPO (also known as lamina-associated polypeptide 2, “Lap2”) and LEMD3 (also known as inner nuclear membrane protein, “Man1”), both of which are transmembrane proteins located in the inner nuclear membrane of the NE. Detection of these candidates indicates the proximity of Nup133 and Nup160 to the NPC membrane, a property consistent with previously published data (21, 36, 54, 55). Conversely, Nup85 detected substantially more Nup214 than any of the other tested Y-Nups, a result supported by a recent cryo-ET study (21). Moreover, BioID-Nup85 was unique among the tested Y-Nups in its ability to detect nucleoporin Nup62 and nucleoporin Nup88. Nup62 is a constituent of a subcomplex located in the central channel of the pore, whose other constituents (nucleoporin Nup58/45 and nucleoporin Nup54) are not detected by BioID-Nup85. Although its detection may reveal distinct positioning of Nup62 compared with Nup58/45 and Nup54 (42), Nup62 also was proposed to associate with Nup88 and Nup214 in a distinct complex in Xenopus egg extracts (56–58), as was demonstrated for its ortholog, Nsp1, in budding yeast (59). Our BioID results thus highlight the existence of a Nup62–Nup88–Nup214 complex in human cells and indicate that Nup85 likely projects toward the Nup88–Nup214–Nup62 complex.
Finally, among the identified NPC-associated nuclear basket constituents, the structural nucleoprotein Tpr (39) was detected solely by BioID-Nup107, whereas the SUMO isopeptidase sentrin-specific protease 2 (SENP2), previously reported to associate with the Y-complex (60), was strongly detected by BioID-Nup133 and BioID-Nup43 and to a lesser extent by BioID-Nup107. Our study thus now enables us to position Tpr near Nup107 and SENP2 near the head-to-tail connections between Y-complex dimers (Fig. 5B).
Conclusions
Using a stable protein complex as a molecular ruler, we determined the practical labeling radius of BioID in vivo to be ∼10 nm. We also demonstrated that, when applied to proteins within distinct regions of the NPC, BioID is capable of detecting distinct populations of candidate proteins. The recent studies on the human Y-complex that use XL-MS provide some comparisons with BioID (21) (Table 1). XL-MS permits the identification and precise (amino acid resolution) mapping of extremely close interactions (within a couple of nanometers), whereas BioID has a much larger radius and thus far does not allow the mapping of biotinylated residues within the prey proteins. However, because of sample complexity, the identification by XL-MS of cross-linked peptides from complex assemblies, such as intact NPCs within purified NEs, appears quite challenging. This complexity explains the rather low number of confidently assigned interactions (17 in total, of which 11 involved Y-Nups or Nup53) in this XL-MS study (21). In contrast, BioID does not require prior purification of organelles and is technically far less demanding. Thus BioID is a useful tool for scientists interested in probing the protein constituency and mapping the organization of large structural protein assemblies. In this way it provides a complementary approach to XL-MS. Future studies, including baits from other NPC subcomplexes and evaluation of BioID candidates of the Y-complex during discrete stages of the cell cycle, should provide additional insights into the assembly and function of NPC constituents.
Materials and Methods
Plasmids.
Nup85, Nup107, Nup133, Nup160, Nup53, and Nup96 were amplified by PCR from human cDNA. The PCR products were digested (by XhoI and BamHI for Nup85, Nup107, and Nup160; by XhoI and EcoRI for Nup133; by XhoI and HindIII for Nup53; by XhoI and AflII for Nup96) and inserted into mycBioID pcDNA3.1 (35700; Addgene). Nup43 was amplified and digested with NheI and EcoRI. The digested PCR product was inserted into BioID-HA pcDNA 3.1 (36047; Addgene). Human LaA was inserted into mycBioID pcDNA3.1 following digestion with XhoI and AflII (1). Nup43-HA was PCR-amplified using a reverse primer containing the HA-tag sequence and was inserted into pcDNA 3.1 after NheI and PmeI digestion. GFP-Nup85, GFP-Nup107, GFP-Nup96, GFP-Nup133, and GFP-Nup160 and were used as previously reported (10).
Antibodies.
Mouse monoclonal anti-Nup153 (SA1) was used as previously reported (61). Rabbit anti-Nup107 was used as previously described (19). Rabbit polyclonal anti-HA (ab9110; Abcam), anti-myc (ab9106; Abcam), anti-GFP (ab290; Abcam), anti-Nup85 (A303-977A; Bethyl Laboratories), chicken polyclonal anti-BirA (ab14002; Abcam), mouse monoclonal anti-tubulin (T9026; Sigma) and mAb414 (MMS-120p-500; Eurogentek) were used as primary antibodies.
Cell Lines and Transfection.
Human HEK293 cells were maintained in 5.0% CO2 at 37 °C in DMEM (SH3024301; HyClone) supplemented with 10% (vol/vol) FBS. To generate cells stably expressing BioID fusion proteins, HEK293 cells were transfected via Lipofectamine 2000 (Life Technologies) using the manufacturer’s recommended protocols and subjected to G418 (700 μg/mL) selection. Subclones of cells that expressed low levels of the fusion protein were chosen to minimize potential artifacts associated with spill-over of fusion proteins to sites other than NPCs. Before all the analyses described in this report, cells were growth arrested by incubation in DMEM supplemented with 0.1% FBS for 72 h to arrest cell division, and promiscuous biotinylation by the BioID-Nups was induced by the addition of biotin to this cell-culture medium to a final concentration of 50 μM for 18 h. For the co-IP experiments shown in Fig. 3B, 2.4 × 106 HEK293 cells were cotransfected with equal amounts of HA-Nup43 and GFP-Y-Nups plasmids (1 μg each) 24 h before co-IP.
Immunostaining.
HEK293 cells were fixed in 3% (wt/vol) paraformaldehyde/PBS for 10 min and permeabilized using 0.4% Triton X-100/PBS for 15 min followed by 0.5% SDS/PBS for 10 min. After fixation and permeabilization, cells were labeled with appropriate primary and secondary antibodies for 20 min at 25 °C in 0.4%Triton X-100/PBS. Primary antibodies were detected with Alexa-Fluor 568–conjugated goat anti-mouse (A11031; Life Technologies) or goat anti-rabbit (A11036; Life Technologies) secondary antibodies. Alexa-Fluor 488–conjugated streptavidin (S32354; Life Technologies) was used to detect biotinylated proteins. DNA was detected with Hoechst dye 33258. Coverslips were mounted in 10% (wt/vol) Mowiol 4–88 (17951; Polysciences). Images were obtained using Nikon A1-confocal microscope (60×/1.49 oil APO TIRF Nikon objective) and a CCD camera (CoolSnap HQ; Photometrics) linked to a workstation running NIS-Element software (Nikon).
Immunoblot and Immunoprecipitation.
For immunoblot of total cell lysates, 1.2 × 106 cells were lysed in SDS/PAGE sample buffer, sonicated to shear DNA, and boiled for 5 min. For co-IP analyses transiently transfected HEK293 cells (2.4 × 106) were lysed in 1 mL of IP lysis buffer [50 mM Tris (pH 7.5), 150 mM NaCl, 2.5 mM MgCl2, 1 mM DTT, 1% Triton X-100, and 1× proteinase inhibitor (1861278; Thermo Scientific)]. Lysates were passed through a 21-gauge needle 10 times and centrifuged at 16,500 × g for 10 min at 4 °C. The supernatants were rotated overnight at 4 °C with 20 µL of protein A Sepharose beads (20365; Thermo Scientific) and 2 μg of rabbit anti-HA antibody. Samples were washed thoroughly three times with the IP lysis buffer and twice with wash buffer [50 mM Tris (pH 7.5) and 50 mM NaCl] at 4 °C. Proteins were solubilized in 25 μL SDS/PAGE sample buffer and boiled for 5 min. Proteins were separated on 8% SDS/PAGE and were transferred to nitrocellulose membrane (Bio-Rad), which subsequently was blocked [10% (vol/vol) adult bovine serum, 0.2% Triton X-100, 1× PBS] and incubated with appropriate primary antibodies overnight at 4 °C. After washes with blocking buffer, blots were incubated with HRP-conjugated anti-mouse (F21453; Life Technologies), anti-rabbit (G21234; Life Technologies), or anti-chicken (A9046; Sigma) antibodies to detect proteins following enhanced chemiluminescence. To detect biotinylated proteins, High Sensitivity Streptavidin-HRP (21130; Thermo Scientific) was used as previously described (1, 33). The in vitro transcription and translation was performed by using TnT Quick Coupled Transcription/Translation Systems (Promega) with the manufacturer’s recommended protocol. For 20-μL reactions, 2 μL was reserved for total protein analysis. The remaining volume was added to 0.5 mL of the IP lysis buffer. IP and IB steps were performed as described above.
BioID, On-Bead Protein Digestion, and Identification by 1D LC-MS/MS.
Large-scale (4 × 107 cells) BioID pull-downs for MS analysis were performed as previously described with the exception that pooled lysates were incubated in a 15-mL conical tube overnight at 4 °C before washing. Ninety percent of each sample was used for MS analysis, and 10% was reserved for IB analysis. Sample volume was adjusted to 200 μL with 50 mM ammonium bicarbonate. Then 4 μL of 0.5 M Tris(2-carboxyethyl)phosphine was added to 200 μL of the beads–proteins suspension mix, and proteins were reduced at 40 °C for 30 min. Then 8 μL of 0.5 M Iodoacetamide was added, and proteins were alkylated at room temperature in the dark for 30 min. MS-grade trypsin (Promega) was added (1:20 ratio) for overnight digestion at 37 °C using an Eppendorf Thermomixer at 700 rpm. Digested peptides were separated from magnetic beads by centrifugation and a GE Healthcare MagRack and were transferred to a new tube. Formic acid was added to the peptide solution (to 2%), followed by desalting by Microtrap (catalog no. 77720; Thermo) and then on-line analysis of peptides by high-resolution, high-mass accuracy liquid chromatography tandem MS (LC-MS/MS) consisting of a Michrom HPLC, a 15-cm Michrom Magic C18 column, a low-flow ADVANCED Michrom MS source, and a LTQ-Orbitrap XL (Thermo Fisher Scientific). A 120-min gradient of 10–30% B (0.1% formic acid, 100% acetonitrile) was used to separate the peptides. The total LC time was 140 min. The LTQ-Orbitrap XL was set to scan precursors in the Orbitrap followed by data-dependent MS/MS of the top 10 precursors. Raw LC-MS/MS data were submitted to Sorcerer Enterprise (Sage-N Research Inc.) for protein identification against the ipi.HUMAN.vs.3.73 protein database, which contains semitryptic peptide sequences with the allowance of up to two missed cleavages. Differential search included 16 Da for methionine oxidation, 57 Da for cysteines to account for carboxyamidomethylation, and 226 Da for biotinylation of lysine. Search results were sorted, filtered, statically analyzed, and displayed using PeptideProphet and ProteinProphet (Institute for Systems Biology). The minimum Trans-Proteomic Pipeline (TPP) probability score for proteins was set to 0.95 to assure a TPP error rate lower than 0.01. The relative abundance of each of the identified proteins in different samples was analyzed by QTools, an open-source tool developed in-house for automated differential peptide/protein spectral count analysis (62). Proteins detected in the control sample (cells lacking BirA*) and common BioID background proteins (BirA*-only) (Dataset S2) were subtracted from the results unless their abundance was threefold more than in the BirA*-only. For all datasets, the total spectral counts for each protein then were normalized to account for the total length of the protein in amino acids. The relative abundance of each prey finally was expressed as percentage of the sum of all of the adjusted spectral counts except those of the BioID-fusion protein within a given BioID sample.
Supplementary Material
Acknowledgments
We thank Brian Burke and Benoit Palancade for helpful discussions and advice. These studies were supported by Grants RO1GM102203, RO1GM102486, and RO1EB014869 (to K.J.R.) from the National Institutes of Health; Sanford Research startup funds (K.J.R.); French National Research Agency Grant ANR-12-BSV2-0008-01 (to V.D.); and Fondation ARC pourla Recherche sur le Cancer (V.D.). This project used the Imaging Core and Protein Biochemistry Core at Sanford Research, which are supported by Institutional Development Awards from the National Institute of General Medical Sciences and the National Institutes of Health under Grants P20GM103548 and P20GM103620.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406459111/-/DCSupplemental.
References
- 1.Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196(6):801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morriswood B, et al. Novel bilobe components in Trypanosoma brucei identified using proximity-dependent biotinylation. Eukaryot Cell. 2013;12(2):356–367. doi: 10.1128/EC.00326-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Itallie CM, et al. The N and C termini of ZO-1 are surrounded by distinct proteins and functional protein networks. J Biol Chem. 2013;288(19):13775–13788. doi: 10.1074/jbc.M113.466193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Itallie CM, et al. Biotin ligase tagging identifies proteins proximal to E-cadherin, including lipoma preferred partner, a regulator of epithelial cell-cell and cell-substrate adhesion. J Cell Sci. 2014;127(Pt 4):885–895. doi: 10.1242/jcs.140475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steed E, et al. MarvelD3 couples tight junctions to the MEKK1-JNK pathway to regulate cell behavior and survival. J Cell Biol. 2014;204(5):821–838. doi: 10.1083/jcb.201304115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comartin D, et al. CEP120 and SPICE1 cooperate with CPAP in centriole elongation. Curr Biol. 2013;23(14):1360–1366. doi: 10.1016/j.cub.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Firat-Karalar EN, Rauniyar N, Yates JR, 3rd, Stearns T. Proximity interactions among centrosome components identify regulators of centriole duplication. Curr Biol. 2014;24(6):664–670. doi: 10.1016/j.cub.2014.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couzens AL, et al. Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci Signal. 2013;6(302):rs15. doi: 10.1126/scisignal.2004712. [DOI] [PubMed] [Google Scholar]
- 9.Daigle N, et al. Nuclear pore complexes form immobile networks and have a very low turnover in live mammalian cells. J Cell Biol. 2001;154(1):71–84. doi: 10.1083/jcb.200101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loïodice I, et al. The entire Nup107-160 complex, including three new members, is targeted as one entity to kinetochores in mitosis. Mol Biol Cell. 2004;15(7):3333–3344. doi: 10.1091/mbc.E03-12-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136(2):284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savas JN, Toyama BH, Xu T, Yates JR, 3rd, Hetzer MW. Extremely long-lived nuclear pore proteins in the rat brain. Science. 2012;335(6071):942. doi: 10.1126/science.1217421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floch A, Palancade B, Doye V. Fifty years of nuclear pores and nucleocytoplasmic transport studies: Multiple tools revealing complex rules. Methods Cell Biol. 2014;122:1–40. doi: 10.1016/B978-0-12-417160-2.00001-1. [DOI] [PubMed] [Google Scholar]
- 14.Grossman E, Medalia O, Zwerger M. Functional architecture of the nuclear pore complex. Annu Rev Biophys. 2012;41:557–584. doi: 10.1146/annurev-biophys-050511-102328. [DOI] [PubMed] [Google Scholar]
- 15.Raices M, D’Angelo MA. Nuclear pore complex composition: A new regulator of tissue-specific and developmental functions. Nat Rev Mol Cell Biol. 2012;13(11):687–699. doi: 10.1038/nrm3461. [DOI] [PubMed] [Google Scholar]
- 16.Hoelz A, Debler EW, Blobel G. The structure of the nuclear pore complex. Annu Rev Biochem. 2011;80:613–643. doi: 10.1146/annurev-biochem-060109-151030. [DOI] [PubMed] [Google Scholar]
- 17.González-Aguilera C, Askjaer P. Dissecting the NUP107 complex: Multiple components and even more functions. Nucleus. 2012;3(4):340–348. doi: 10.4161/nucl.21135. [DOI] [PubMed] [Google Scholar]
- 18.Vasu S, et al. Novel vertebrate nucleoporins Nup133 and Nup160 play a role in mRNA export. J Cell Biol. 2001;155(3):339–354. doi: 10.1083/jcb.200108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belgareh N, et al. An evolutionarily conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J Cell Biol. 2001;154(6):1147–1160. doi: 10.1083/jcb.200101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wozniak R, Burke B, Doye V. Nuclear transport and the mitotic apparatus: An evolving relationship. Cell Mol Life Sci. 2010;67(13):2215–2230. doi: 10.1007/s00018-010-0325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bui KH, et al. Integrated structural analysis of the human nuclear pore complex scaffold. Cell. 2013;155(6):1233–1243. doi: 10.1016/j.cell.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 22.Rabut G, Doye V, Ellenberg J. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat Cell Biol. 2004;6(11):1114–1121. doi: 10.1038/ncb1184. [DOI] [PubMed] [Google Scholar]
- 23.Amlacher S, et al. Insight into structure and assembly of the nuclear pore complex by utilizing the genome of a eukaryotic thermophile. Cell. 2011;146(2):277–289. doi: 10.1016/j.cell.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhardt N, Redolfi J, Antonin W. Interaction of Nup53 with Ndc1 and Nup155 is required for nuclear pore complex assembly. J Cell Sci. 2014;127(Pt 4):908–921. doi: 10.1242/jcs.141739. [DOI] [PubMed] [Google Scholar]
- 25.Hawryluk-Gara LA, Platani M, Santarella R, Wozniak RW, Mattaj IW. Nup53 is required for nuclear envelope and nuclear pore complex assembly. Mol Biol Cell. 2008;19(4):1753–1762. doi: 10.1091/mbc.E07-08-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansfeld J, et al. The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol Cell. 2006;22(1):93–103. doi: 10.1016/j.molcel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Sachdev R, Sieverding C, Flötenmeyer M, Antonin W. The C-terminal domain of Nup93 is essential for assembly of the structural backbone of nuclear pore complexes. Mol Biol Cell. 2012;23(4):740–749. doi: 10.1091/mbc.E11-09-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vollmer B, Antonin W. The diverse roles of the Nup93/Nic96 complex proteins - structural scaffolds of the nuclear pore complex with additional cellular functions. Biol Chem. 2014;395(5):515–528. doi: 10.1515/hsz-2013-0285. [DOI] [PubMed] [Google Scholar]
- 29.Chatel G, Fahrenkrog B. Nucleoporins: Leaving the nuclear pore complex for a successful mitosis. Cell Signal. 2011;23(10):1555–1562. doi: 10.1016/j.cellsig.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Zuccolo M, et al. The human Nup107-160 nuclear pore subcomplex contributes to proper kinetochore functions. EMBO J. 2007;26(7):1853–1864. doi: 10.1038/sj.emboj.7601642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis LI, Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- 32.Cordes VC, Reidenbach S, Franke WW. Cytoplasmic annulate lamellae in cultured cells: Composition, distribution, and mitotic behavior. Cell Tissue Res. 1996;284(2):177–191. doi: 10.1007/s004410050578. [DOI] [PubMed] [Google Scholar]
- 33.Roux KJ, Kim DI, Burke B. BioID: A screen for protein-protein interactions. Curr Protoc Protein Sci. 2013;74:19.23.1–19.23.14. doi: 10.1002/0471140864.ps1923s74. [DOI] [PubMed] [Google Scholar]
- 34.Craig EA. Eukaryotic chaperonins: Lubricating the folding of WD-repeat proteins. Curr Biol. 2003;13(23):R904–R905. doi: 10.1016/j.cub.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Eisenhardt N, Redolfi J, Antonin W. Interaction of Nup53 with Ndc1 and Nup155 is required for nuclear pore complex assembly. J Cell Sci. 2014;127(Pt 4):908–921. doi: 10.1242/jcs.141739. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell JM, Mansfeld J, Capitanio J, Kutay U, Wozniak RW. Pom121 links two essential subcomplexes of the nuclear pore complex core to the membrane. J Cell Biol. 2010;191(3):505–521. doi: 10.1083/jcb.201007098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandi P, et al. Nup93, a vertebrate homologue of yeast Nic96p, forms a complex with a novel 205-kDa protein and is required for correct nuclear pore assembly. Mol Biol Cell. 1997;8(10):2017–2038. doi: 10.1091/mbc.8.10.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fahrenkrog B, et al. Domain-specific antibodies reveal multiple-site topology of Nup153 within the nuclear pore complex. J Struct Biol. 2002;140(1-3):254–267. doi: 10.1016/s1047-8477(02)00524-5. [DOI] [PubMed] [Google Scholar]
- 39.Krull S, Thyberg J, Björkroth B, Rackwitz HR, Cordes VC. Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket. Mol Biol Cell. 2004;15(9):4261–4277. doi: 10.1091/mbc.E04-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulillo SM, et al. Nucleoporin domain topology is linked to the transport status of the nuclear pore complex. J Mol Biol. 2005;351(4):784–798. doi: 10.1016/j.jmb.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 41.Denning DP, Patel SS, Uversky V, Fink AL, Rexach M. Disorder in the nuclear pore complex: The FG repeat regions of nucleoporins are natively unfolded. Proc Natl Acad Sci USA. 2003;100(5):2450–2455. doi: 10.1073/pnas.0437902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solmaz SR, Chauhan R, Blobel G, Melčák I. Molecular architecture of the transport channel of the nuclear pore complex. Cell. 2011;147(3):590–602. doi: 10.1016/j.cell.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz-Herion K, Maco B, Sauder U, Fahrenkrog B. Domain topology of the p62 complex within the 3-D architecture of the nuclear pore complex. J Mol Biol. 2007;370(4):796–806. doi: 10.1016/j.jmb.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 44.Bernad R, Engelsma D, Sanderson H, Pickersgill H, Fornerod M. Nup214-Nup88 nucleoporin subcomplex is required for CRM1-mediated 60 S preribosomal nuclear export. J Biol Chem. 2006;281(28):19378–19386. doi: 10.1074/jbc.M512585200. [DOI] [PubMed] [Google Scholar]
- 45.Bernad R, van der Velde H, Fornerod M, Pickersgill H. Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export. Mol Cell Biol. 2004;24(6):2373–2384. doi: 10.1128/MCB.24.6.2373-2384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fornerod M, et al. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 1997;16(4):807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roth P, et al. The Drosophila nucleoporin DNup88 localizes DNup214 and CRM1 on the nuclear envelope and attenuates NES-mediated nuclear export. J Cell Biol. 2003;163(4):701–706. doi: 10.1083/jcb.200304046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stochaj U, Bański P, Kodiha M, Matusiewicz N. The N-terminal domain of the mammalian nucleoporin p62 interacts with other nucleoporins of the FXFG family during interphase. Exp Cell Res. 2006;312(13):2490–2499. doi: 10.1016/j.yexcr.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Schmitt C, et al. Dbp5, a DEAD-box protein required for mRNA export, is recruited to the cytoplasmic fibrils of nuclear pore complex via a conserved interaction with CAN/Nup159p. EMBO J. 1999;18(15):4332–4347. doi: 10.1093/emboj/18.15.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Folkmann AW, et al. Gle1 functions during mRNA export in an oligomeric complex that is altered in human disease. Cell. 2013;155(3):582–593. doi: 10.1016/j.cell.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kendirgi F, Rexer DJ, Alcázar-Román AR, Onishko HM, Wente SR. Interaction between the shuttling mRNA export factor Gle1 and the nucleoporin hCG1: A conserved mechanism in the export of Hsp70 mRNA. Mol Biol Cell. 2005;16(9):4304–4315. doi: 10.1091/mbc.E04-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ori A, et al. Cell type-specific nuclear pores: A case in point for context-dependent stoichiometry of molecular machines. Mol Syst Biol. 2013;9:648. doi: 10.1038/msb.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rayala HJ, Kendirgi F, Barry DM, Majerus PW, Wente SR. The mRNA export factor human Gle1 interacts with the nuclear pore complex protein Nup155. Mol Cell Proteomics. 2004;3(2):145–155. doi: 10.1074/mcp.M300106-MCP200. [DOI] [PubMed] [Google Scholar]
- 54.Drin G, et al. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14(2):138–146. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- 55.Szymborska A, et al. Nuclear pore scaffold structure analyzed by super-resolution microscopy and particle averaging. Science. 2013;341(6146):655–658. doi: 10.1126/science.1240672. [DOI] [PubMed] [Google Scholar]
- 56.Hülsmann BB, Labokha AA, Görlich D. The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell. 2012;150(4):738–751. doi: 10.1016/j.cell.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 57.Macaulay C, Meier E, Forbes DJ. Differential mitotic phosphorylation of proteins of the nuclear pore complex. J Biol Chem. 1995;270(1):254–262. doi: 10.1074/jbc.270.1.254. [DOI] [PubMed] [Google Scholar]
- 58.Finlay DR, Meier E, Bradley P, Horecka J, Forbes DJ. A complex of nuclear pore proteins required for pore function. J Cell Biol. 1991;114(1):169–183. doi: 10.1083/jcb.114.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belgareh N, et al. Functional characterization of a Nup159p-containing nuclear pore subcomplex. Mol Biol Cell. 1998;9(12):3475–3492. doi: 10.1091/mbc.9.12.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goeres J, et al. The SUMO-specific isopeptidase SENP2 associates dynamically with nuclear pore complexes through interactions with karyopherins and the Nup107-160 nucleoporin subcomplex. Mol Biol Cell. 2011;22(24):4868–4882. doi: 10.1091/mbc.E10-12-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Q, et al. Functional association of Sun1 with nuclear pore complexes. J Cell Biol. 2007;178(5):785–798. doi: 10.1083/jcb.200704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brill LM, Motamedchaboki K, Wu S, Wolf DA. Comprehensive proteomic analysis of Schizosaccharomyces pombe by two-dimensional HPLC-tandem mass spectrometry. Methods. 2009;48(3):311–319. doi: 10.1016/j.ymeth.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bilokapic S, Schwartz TU. Structural and functional studies of the 252 kDa nucleoporin ELYS reveal distinct roles for its three tethered domains. Structure. 2013;21(4):572–580. doi: 10.1016/j.str.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thierbach K, et al. Protein interfaces of the conserved Nup84 complex from Chaetomium thermophilum shown by crosslinking mass spectrometry and electron microscopy. Structure. 2013;21(9):1672–1682. doi: 10.1016/j.str.2013.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.