Abstract
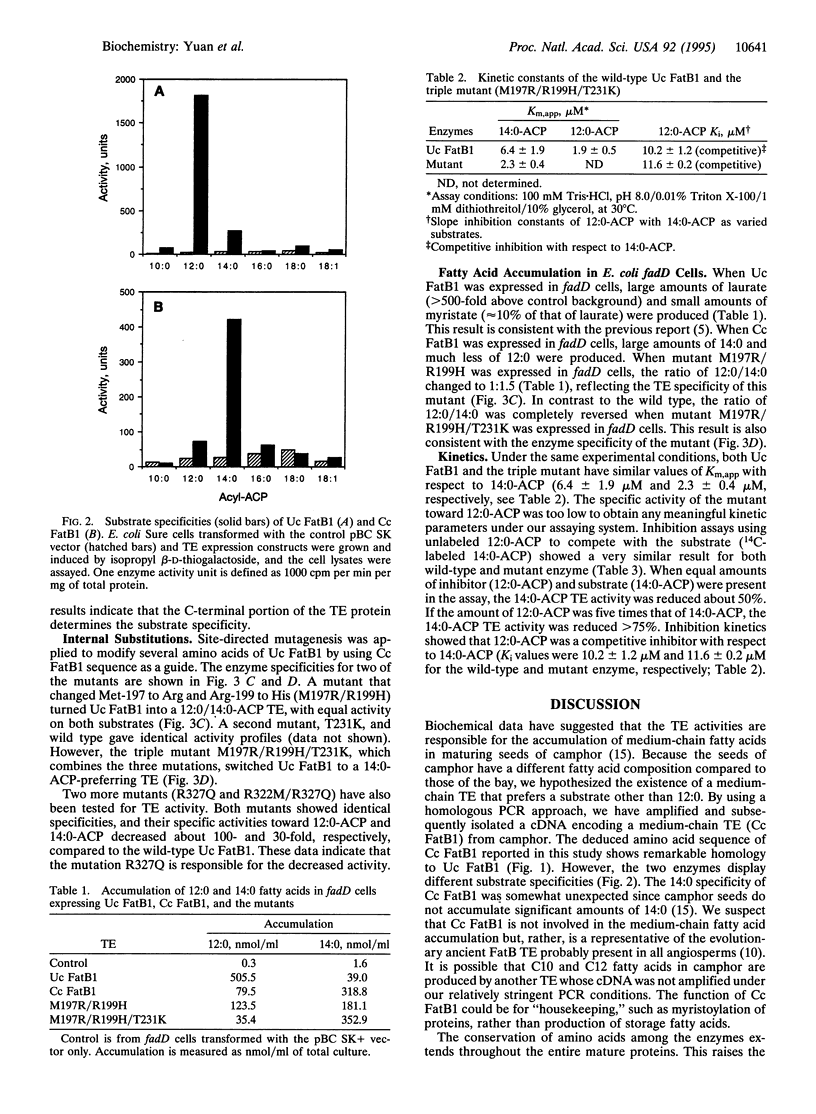
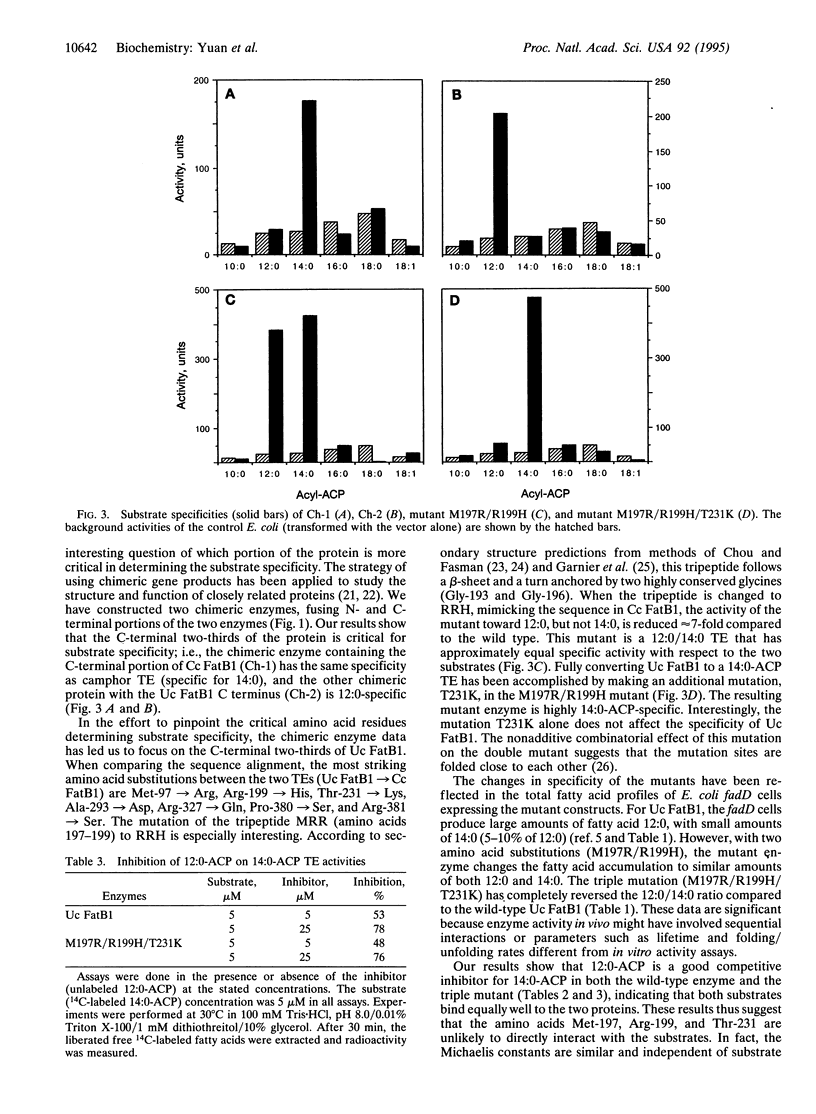
The plant acyl-acyl carrier protein (ACP) thioesterases (TEs) are of biochemical interest because of their roles in fatty acid synthesis and their utilities in the bioengineering of plant seed oils. When the FatB1 cDNA encoding a 12:0-ACP TE (Uc FatB1) from California bay, Umbellularia californica (Uc) was expressed in Escherichia coli and in developing oilseeds of the plants Arabidopsis thaliana and Brassica napus, large amounts of laurate (12:0) and small amounts of myristate (14:0) were accumulated. We have isolated a TE cDNA from camphor (Cinnamomum camphorum) (Cc) seeds that shares 92% amino acid identity with Uc FatB1. This TE, Cc FatB1, mainly hydrolyzes 14:0-ACP as shown by E. coli expression. We have investigated the roles of the N- and C-terminal regions in determining substrate specificity by constructing two chimeric enzymes, in which the N-terminal portion of one protein is fused to the C-terminal portion of the other. Our results show that the C-terminal two-thirds of the protein is critical for the specificity. By site-directed mutagenesis, we have replaced several amino acids in Uc FatB1 by using the Cc FatB1 sequence as a guide. A double mutant, which changes Met-197 to an Arg and Arg-199 to a His (M197R/R199H), turns Uc FatB1 into a 12:0/14:0 TE with equal preference for both substrates. Another mutation, T231K, by itself does not effect the specificity. However, when it is combined with the double mutant to generate a triple mutant (M197R/R199H/T231K), Uc FatB1 is converted to a 14:0-ACP TE. Expression of the double-mutant cDNA in E. coli K27, a strain deficient in fatty acid degradation, results in accumulation of similar amounts of 12:0 and 14:0. Meanwhile the E. coli expressing the triple-mutant cDNA produces predominantly 14:0 with very small amounts of 12:0. Kinetic studies indicate that both wild-type Uc FatB1 and the triple mutant have similar values of Km,app with respect to 14:0-ACP. Inhibitory studies also show that 12:0-ACP is a good competitive inhibitor with respect to 14:0-ACP in both the wild type and the triple mutant. These results imply that both 12:0- and 14:0-ACP can bind to the two proteins equally well, but in the case of the triple mutant, the hydrolysis of 12:0-ACP is severely impaired. The ability to modify TE specificity should allow the production of additional "designer oils" in genetically engineered plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blundell T. L. Problems and solutions in protein engineering--towards rational design. Trends Biotechnol. 1994 May;12(5):145–148. doi: 10.1016/0167-7799(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Davies H. M., Anderson L., Fan C., Hawkins D. J. Developmental induction, purification, and further characterization of 12:0-ACP thioesterase from immature cotyledons of Umbellularia californica. Arch Biochem Biophys. 1991 Oct;290(1):37–45. doi: 10.1016/0003-9861(91)90588-a. [DOI] [PubMed] [Google Scholar]
- De Renobales M., Rogers L., Kolattukudy P. E. Involvement of a thioesterase in the production of short-chain fatty acids in the uropygial glands of mallard ducks (Anas platyrhynchos). Arch Biochem Biophys. 1980 Dec;205(2):464–477. doi: 10.1016/0003-9861(80)90129-0. [DOI] [PubMed] [Google Scholar]
- Dörmann P., Kridl J. C., Ohlrogge J. B. Cloning and expression in Escherichia coli of a cDNA coding for the oleoyl-acyl carrier protein thioesterase from coriander (Coriandrum sativum L.). Biochim Biophys Acta. 1994 Apr 14;1212(1):134–136. doi: 10.1016/0005-2760(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Dörmann P., Voelker T. A., Ohlrogge J. B. Cloning and expression in Escherichia coli of a novel thioesterase from Arabidopsis thaliana specific for long-chain acyl-acyl carrier proteins. Arch Biochem Biophys. 1995 Jan 10;316(1):612–618. doi: 10.1006/abbi.1995.1081. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hardy L. W., Nalivaika E. Asn177 in Escherichia coli thymidylate synthase is a major determinant of pyrimidine specificity. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9725–9729. doi: 10.1073/pnas.89.20.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstad R. H., Morash S. C., McMaster C. R., Bell R. M. Chimeric enzymes. Structure-function analysis of segments of sn-1,2-diacylglycerol choline- and ethanolaminephosphotransferases. J Biol Chem. 1994 Aug 19;269(33):20995–21002. [PubMed] [Google Scholar]
- Jones A., Davies H. M., Voelker T. A. Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutionary origin of plant acyl-ACP thioesterases. Plant Cell. 1995 Mar;7(3):359–371. doi: 10.1105/tpc.7.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. G., Chandrasegaran S. Chimeric restriction endonuclease. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):883–887. doi: 10.1073/pnas.91.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Knutzon D. S., Bleibaum J. L., Nelsen J., Kridl J. C., Thompson G. A. Isolation and characterization of two safflower oleoyl-acyl carrier protein thioesterase cDNA clones. Plant Physiol. 1992 Dec;100(4):1751–1758. doi: 10.1104/pp.100.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. M., Derewenda U., Serre L., Ferri S., Szittner R., Wei Y., Meighen E. A., Derewenda Z. S. Structure of a myristoyl-ACP-specific thioesterase from Vibrio harveyi. Biochemistry. 1994 Aug 16;33(32):9382–9388. doi: 10.1021/bi00198a003. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B. Design of New Plant Products: Engineering of Fatty Acid Metabolism. Plant Physiol. 1994 Mar;104(3):821–826. doi: 10.1104/pp.104.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Pollard M. R., Anderson L., Fan C., Hawkins D. J., Davies H. M. A specific acyl-ACP thioesterase implicated in medium-chain fatty acid production in immature cotyledons of Umbellularia californica. Arch Biochem Biophys. 1991 Feb 1;284(2):306–312. doi: 10.1016/0003-9861(91)90300-8. [DOI] [PubMed] [Google Scholar]
- Sandberg W. S., Terwilliger T. C. Engineering multiple properties of a protein by combinatorial mutagenesis. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8367–8371. doi: 10.1073/pnas.90.18.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. Mechanism of chain length determination in biosynthesis of milk fatty acids. J Dairy Sci. 1980 Feb;63(2):337–352. doi: 10.3168/jds.S0022-0302(80)82935-3. [DOI] [PubMed] [Google Scholar]
- Voelker T. A., Davies H. M. Alteration of the specificity and regulation of fatty acid synthesis of Escherichia coli by expression of a plant medium-chain acyl-acyl carrier protein thioesterase. J Bacteriol. 1994 Dec;176(23):7320–7327. doi: 10.1128/jb.176.23.7320-7327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker T. A., Worrell A. C., Anderson L., Bleibaum J., Fan C., Hawkins D. J., Radke S. E., Davies H. M. Fatty acid biosynthesis redirected to medium chains in transgenic oilseed plants. Science. 1992 Jul 3;257(5066):72–74. doi: 10.1126/science.1621095. [DOI] [PubMed] [Google Scholar]
- el Hawrani A. S., Moreton K. M., Sessions R. B., Clarke A. R., Holbrook J. J. Engineering surface loops of proteins--a preferred strategy for obtaining new enzyme function. Trends Biotechnol. 1994 May;12(5):207–211. doi: 10.1016/0167-7799(94)90084-1. [DOI] [PubMed] [Google Scholar]


