Abstract
Phthalate exposure during pregnancy has been linked to adverse birth outcomes such as preterm birth, and inflammation and oxidative stress may mediate these relationships. In a prospective cohort study of pregnant women recruited early in gestation in Northern Puerto Rico, we investigated the associations between urinary phthalate metabolites and biomarkers of inflammation, including C-reactive protein, IL-1β, IL-6, IL-10, and TNF-α, and oxidative stress, including 8-hydroxydeoxyguanosine (OHdG) and 8-isoprostane. Inflammation biomarkers were measured in plasma twice during pregnancy (N = 215 measurements, N = 120 subjects), and oxidative stress biomarkers in urine were measured three times (N = 148 measurements, N = 54 subjects) per woman. In adjusted linear mixed models, metabolites of di-2-ethylhexyl phthalate (DEHP) were associated with increased IL-6 and IL-10 but relationships were generally not statistically significant. All phthalates were associated with increases in oxidative stress markers. Relationships with OHdG were significant for DEHP metabolites as well as mono-n-butyl phthalate (MBP) and monoiso-butyl phthalate (MiBP). For 8-isoprostane, associations with nearly all phthalates were statistically significant and the largest effect estimates were observed for MBP and MiBP (49–50% increase in 8-isoprostane with an interquartile range increase in metabolite concentration). These relationships suggest a possible mechanism for phthalate action that may be relevant to a number of adverse health outcomes.
Introduction
Phthalates are used commonly as plasticizers in a number of industrial and consumer products. Some examples include plastic food packaging, shower curtains, vinyl flooring, and cosmetic products such as fragrances and lotions.1 Because phthalates are not chemically bound to plastics, they can leach into food, water, dusts, and air leading to exposure through ingestion and/or inhalation pathways. Additionally, phthalates in personal care products may be absorbed dermally.2 Consequently, phthalate metabolites are ubiquitously detected in urine samples from men and women of all ages in the U.S. and elsewhere, demonstrating widespread human exposure.3
The Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) program is an ongoing prospective cohort study of pregnant women residing in Northern Puerto Rico, where preterm birth rates are the third highest in the world and hazardous waste sites are numerous.4 The aims of the project are to investigate (1) the relationship between exposure to phthalates and other chemical exposures in pregnant women and preterm birth; (2) toxicological mechanisms for these relationships via in vitro studies; (3) groundwater contamination and transfer as a potential route of exposure; and (4) remediation strategies for cleanup. Previous research within this project suggests that pregnant women in Puerto Rico may have higher exposure to some phthalates, such as di-2-ethylhexyl phthalate (DEHP), compared to women of reproductive age from the U.S. general population.5
Phthalates are endocrine disrupting compounds, and have been associated not only with changes in sex and thyroid hormone levels but also with a number of downstream health outcomes in humans.6 An additional mechanism for some of these effects, however, may be through phthalate induced inflammation and oxidative stress. Cellular and animal studies have demonstrated that some phthalate diesters and monoesters, particularly DEHP and one of its metabolites mono-2-ethylhexyl phthalate (MEHP), are capable of inducing proliferation of leukocytes and reactive oxygen species (ROS).7−9 More specifically, the toxicology project under the PROTECT program previously demonstrated that MEHP is capable of inducing oxidative stress responses in placental cells, although precise mechanisms remain unknown.10 Also, some studies in adults suggest that urinary concentrations of various phthalate metabolites may be associated with biomarkers of inflammation and oxidative stress.11−13 Exposure to phthalates during pregnancy has been linked to adverse outcomes including miscarriage, low birth weight, and preterm birth.14−16 The PROTECT program hypothesizes that some of these relationships, particularly preterm birth, may be mediated by phthalate induction of inflammation and oxidative stress.
In the present study, we utilize biomarkers of phthalate metabolites and oxidative stress, measured in urine samples collected from up to three time points per subject across gestation, as well as biomarkers of inflammation, measured in plasma samples collected from up to two time points per subject, to explore the relationship between phthalate exposure and systemic inflammation and oxidative stress in a preliminary subset of pregnant women in the PROTECT study.
Materials and Methods
Study Participants
The present study includes the first 139 participants of the PROTECT program. Recruitment details and inclusion criteria have been described in detail previously.5 Briefly, pregnant women were recruited early in pregnancy (<20 weeks gestation) between 2010 and 2012 in seven prenatal clinics and hospitals in Northern Puerto Rico. Women were excluded if they were carrying more than one fetus, used oral contraceptives less than three months prior to pregnancy, had known obstetric or medical health conditions (e.g., heart conditions or diabetes), or used in vitro fertilization to get pregnant. At the initial study visit (16–20 weeks gestation) they provided urine and blood samples and filled out detailed questionnaires. At visit 2 (20–24 weeks) a second urine sample was collected, and at visit 3 (24–28 weeks) both urine and blood samples were collected again. Women were followed until delivery and detailed birth outcome data was recorded.
Blood samples were processed for collection of plasma and serum components, and both urine and blood components were frozen at −80 °C until batches were shipped overnight on dry ice to the Centers for Disease Control and Prevention (CDC) laboratories or the University of Michigan, where samples were stored at subfreezing temperatures upon arrival until analysis. All study protocols were approved by the ethics and research committees of the University of Michigan School of Public Health, University of Puerto Rico, Northeastern University, and participating hospitals and clinics. The involvement of the CDC laboratory was determined not to constitute engagement in human subjects research.
Phthalate Metabolite Measurement
Phthalate metabolites were measured in urine (N = 373, N = 139 participants) at the CDC using the same protocols used for the National Health and Nutrition Examination Survey.5 The analytical method entails solid phase extraction with high performance liquid chromatography and tandem mass spectrometry.17 Eleven phthalate metabolites were measured, including metabolites of high molecular weight (HMW) phthalates, namely MEHP, mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), monobenzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), monocarboxyisooctyl phthalate (MCOP), and monocarboxyisononyl phthalate (MCNP), and metabolites of low molecular weight (LMW) phthalates, namely mono-n-butyl phthalate (MBP), monoiso-butyl phthalate (MiBP), and monoethyl phthalate (MEP).18 Concentrations below the limit of detection (LOD) were replaced by the LOD divided by the square root of 2.19 Urinary specific gravity (SG) was measured as an indicator of urine dilution using a digital hand-held refractometer (Atago Co., Ltd., Tokyo, Japan).
Inflammation Biomarker Measurement
For 120 subjects, plasma samples (N = 215 total) from visits 1 (N = 119) and 3 (N = 96) were available for measurement of C-reactive protein (CRP) as well as 4 cytokines as biomarkers of inflammation. These measurements were performed by the University of Michigan Cancer Center Immunological Monitoring Core (Ann Arbor, MI). CRP was measured using a DuoSet enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN), and cytokines, including interleukin (IL)-1β, IL-6, IL-10, and tumor necrosis factor (TNF)-α, were measured using a Milliplex MAP High Sensitivity Human Cytokine Magnetic Bead Panel (EMD Millipore Corp., St. Charles, MO) with a Luminex L200 instrument (Luminex, Austin, TX). Cytokines were analyzed in duplicate and an arithmetic average of the two measurements was created for data analysis. CRP or cytokine levels below the LOD were replaced by the LOD divided by the square root of 2.19
Oxidative Stress Biomarker Measurement
For 58 subjects, urine samples (N = 162 total) from visits 1 (N = 58), 2 (N = 54), and 3 (N = 50) were analyzed for 8-hydroxydeoxyguanosine (OHdG) and 8-isoprostane (isoprostane) as indicators of oxidative stress by Cayman Chemical (Ann Arbor, MI). Both were measured using enzyme immunoassay. Prior to analysis of isoprostane levels urine samples were hydrolyzed and affinity purified. Levels of OHdG or isoprostane below the LOD were replaced by the LOD divided by the square root of 2. For descriptive analyses, oxidative stress biomarkers were standardized to urinary SG using the following formula: OSs = OS[(1.019–1)/(SG-1)], where OSs represents the standardized OS concentration, OS represents the raw concentration, 1.019 was the median specific gravity concentration in all urine samples pooled, and SG is the specific gravity concentration of the specific sample.
Statistical Analysis
Distributions of SG-standardized urinary phthalate metabolite concentrations, plasma inflammation biomarkers, and SG-standardized oxidative stress urinary biomarkers were examined using geometric means and geometric standard deviations as well as selected percentiles. Levels by individual visits were additionally calculated and differences by visit were tested using linear mixed models (LMM) with biomarker regressed on study visit and adjustment for intraindividual correlation using random intercepts for subject ID. To examine the relationship between biomarkers, Spearman correlation coefficients were calculated from all available samples.
To estimate the association between urinary phthalate metabolites and biomarkers of inflammation and oxidative stress, we created LMM with one exposure and one outcome per model. Models were created with random intercepts only to adjust for intraindividual correlation. Crude models were adjusted for urinary SG and visit of sample collection; full models additionally included maternal prepregnancy body mass index (BMI), education, and income level as covariates. For all models observations for one woman who smoked during pregnancy were excluded. For ease of interpretation, results were converted to the percent change in biomarker in association with an interquartile range (IQR) increase in urinary phthalate metabolite. We additionally investigated windows of susceptibility for the effects of phthalate exposure on inflammation and oxidative stress by examining LMM with an interaction term between phthalate metabolite and study visit.
Results
Demographic characteristics of pregnant women in this sample are presented in detail elsewhere.5 Briefly, women were: average 27.5 years of age at study enrollment; well-educated (82.7% had some college education or above); married or cohabitating (71.4%); employed (60.4%); and only one subject reported smoking during pregnancy (0.8%).
Distributions of urinary phthalate metabolites across pregnancy in this group of women have been reported previously.5 Levels in pregnant Puerto Rican women were similar, but for some metabolites (MEHP, MBP, and MEP) higher than levels observed in nonpregnant women ages 18–40 in the general U.S. population. Spearman correlations between different phthalate metabolites were generally moderate (R = 0.23 to 0.45), but strong between metabolites of the same parent compound (R > 0.83 between DEHP metabolites MEHP, MEHHP, MEOHP, and MECPP).5 Repeated measures of metabolites across pregnancy had low to moderate temporal reliability (intraclass correlation coefficients for unadjusted metabolites = 0.09 to 0.43).5
Distributions of inflammation and oxidative stress biomarkers are presented in Table 1. CRP was above the LOD (10 pg/mL) in all samples measured. The LOD was 0.128 pg/mL for all cytokines. IL-1β was below the LOD in 77 (35.8%) samples; IL-6 was below the LOD for 6 (2.8%) samples; IL-10 was below the LOD for 2 (0.9%) samples); and levels of TNF-α were detectable in all samples analyzed. Correlations between inflammation biomarkers were weak to moderate (Spearman R = 0.00 to 0.40) (Table 2). Distributions of inflammation biomarkers by individual visit were examined using boxplots, and differences were tested using LMM with random intercepts only to adjust for intraindividual correlation in measures within subject (Figure 1). No differences by visit were found for IL-1β (p = 0.98), IL-6 (p = 0.97), or TNF-α (p = 0.20), however CRP levels were significantly higher at visit 1 (geometric mean=8.87 μg/mL [geometric standard deviation = 2.28]) compared to those at visit 3 (6.91 μg/mL [2.09]) (p < 0.01). Also, IL-10 levels were significantly higher at visit 1 (8.71 pg/mL [2.77]) compared to visit 3 (7.57 pg/mL [2.74]) (p = 0.01).
Table 1. Distributions of Biomarkers of Inflammation in Plasma and Oxidative Stress in Urinea.
| percentiles |
|||||||
|---|---|---|---|---|---|---|---|
| biomarker | N | geometric mean (geometric SD) | 25th | 50th | 75th | 95th | Max. |
| Inflammation | |||||||
| CRP (ug/mL) | 215 | 7.93 (2.22) | 4.80 | 7.25 | 14.7 | 29.1 | 80.2 |
| IL-1 β (pg/mL) | 215 | 0.21 (2.47) | 0.09 | 0.20 | 0.41 | 0.80 | 7.74 |
| IL-6 (pg/mL) | 215 | 1.86 (2.84) | 1.07 | 1.94 | 3.35 | 8.88 | 67.1 |
| IL-10 (pg/mL) | 215 | 8.18 (2.76) | 4.85 | 8.33 | 12.8 | 45.6 | 263 |
| TNF-α (pg/mL) | 215 | 3.34 (1.97) | 2.23 | 3.38 | 4.81 | 8.25 | 208 |
| Oxidative Stress | |||||||
| OHdG (ng/mL) | 148 | 125 (1.67) | 91.6 | 122 | 160 | 287 | 842 |
| Isoprostane (pg/mL) | 148 | 258 (1.85) | 177 | 258 | 381 | 623 | 2025 |
Oxidative stress biomarker concentrations standardized to urinary specific gravity.
Table 2. Spearman Correlation Coefficients (P-Values) Of Inflammation and Oxidative Stressa Biomarkers.
| IL-1 β | IL-6 | IL-10 | TNF-α | OHdG | Isoprostane | |
|---|---|---|---|---|---|---|
| CRP | 0.00 (0.99) | 0.24 (<0.01) | 0.12 (0.09) | –0.01 (0.87) | –0.08 (0.46) | –0.02 (0.86) |
| IL-1β | 0.40 (<0.01) | 0.38 (<0.01) | 0.10 (0.13) | –0.13 (0.19) | –0.01 (0.95) | |
| IL-6 | 0.40 (<0.01) | 0.32 (<0.01) | –0.03 (0.81) | 0.05 (0.64) | ||
| IL-10 | 0.39 (<0.01) | –0.10 (0.34) | –0.01 (0.92) | |||
| TNF-α | 0.12 (0.25) | 0.22 (0.03) | ||||
| OHdG | 0.43 (<0.01) |
Oxidative stress biomarker concentrations standardized to urinary specific gravity.
Figure 1.
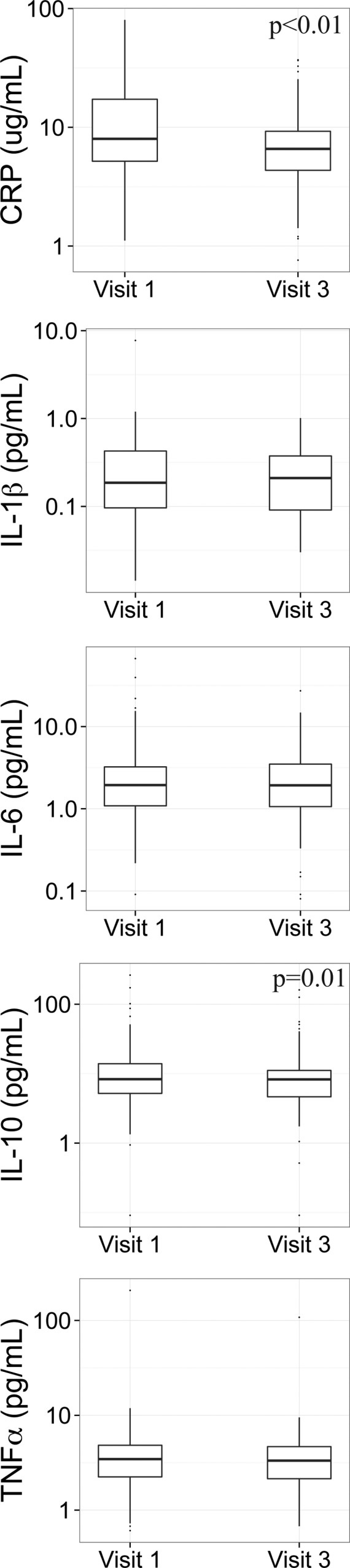
Boxplots comparing inflammation biomarker distributions by study visit.
OHdG and isoprostane were detected in all urine samples analyzed. Information on urinary SG was missing for 14 samples analyzed, so those measures were excluded from analysis (final N = 148 samples). OHdG and isoprostane were moderately but significantly correlated with each other (Spearman R = 0.43), but were not significantly correlated with inflammation biomarkers with the exception of a low correlation between isoprostane and TNF-α. Levels of OHdG or isoprostane at visits 2 or 3 were not significantly different from levels at visit 1 (SI Figure S1).
Due to the similarity between crude and adjusted analyses only fully adjusted associations between inflammation biomarkers and urinary phthalate metabolite concentrations are presented in Table 3. Few statistically significant associations were detected. Effect estimates from models of HMW phthalate metabolites and IL-6 were generally positive, and for MEHHP (percent change in outcome with IQR increase [%Δ] = 15.8, 95% confidence interval [CI]=-2.23, 37.2) MEOHP (%Δ = 16.9, 95% CI = −1.57, 38.9), MECPP (%Δ = 18.8, 95% CI = −0.59, 42.1), and MCNP (%Δ = 16.8, 95% CI =2.69, 32.9) the relationships were marginally (p < 0.10) or statistically significant (p < 0.05). No associations were detected with metabolites of LMW phthalates. For IL-10, increases were observed in association with most metabolites of HMW phthalates (significant for MECPP), although the association with MBzP was inverse and marginally significant. For metabolites of LMW phthalates, inverse associations were observed but confidence intervals were wide. No significant associations were detected for the other biomarkers of inflammation, except for marginally significant increases in CRP in association with IQR increases in MCPP (%Δ = 13.0, 95% CI = −1.23, 29.4) and MCNP (%Δ = 10.1, 95% CI = −0.86, 22.2). Models with interaction terms between study visit and phthalate metabolites did not suggest differences in susceptibility by study visit (data not shown).
Table 3. Percent Change (95% Confidence Interval)a in Plasma Inflammation Biomarker in Association with Interquartile Range Increase in Urinary Phthalate Metabolite Concentration.
| CRP |
IL-1β |
IL-6 |
||||
|---|---|---|---|---|---|---|
| %Δ (95% CI) | p | %Δ (95% CI) | p | %Δ (95% CI) | p | |
| MEHP | 8.55 (−7.27, 27.1) | 0.31 | 5.41 (−12.0, 26.3) | 0.57 | 15.9 (−4.58, 40.8) | 0.14 |
| MEHHP | 4.13 (−9.33, 19.6) | 0.57 | 5.31 (−9.93, 23.1) | 0.52 | 15.8 (−2.23, 37.2) | 0.09 |
| MEOHP | 4.14 (−9.55, 19.9) | 0.57 | 5.91 (−9.68, 24.2) | 0.48 | 16.9 (−1.57, 38.9) | 0.08 |
| MECPP | 4.25 (−9.95, 20.7) | 0.58 | 8.06 (−8.37, 27.4) | 0.36 | 18.8 (−0.59, 42.1) | 0.06 |
| MCPP | 13.0 (−1.23, 29.4) | 0.08 | –4.50 (−18.1, 11.3) | 0.56 | 2.87 (−13.0, 21.7) | 0.74 |
| MCOP | 4.21 (−9.48, 20.0) | 0.57 | 6.12 (−9.78, 24.8) | 0.48 | 2.61 (−13.9, 22.3) | 0.77 |
| MCNP | 10.1 (−0.86, 22.2) | 0.08 | 10.4 (−1.93, 24.3) | 0.11 | 16.8 (2.69, 32.9) | 0.02 |
| MBzP | –1.67 (−16.7, 16.1) | 0.84 | –11.6 (−26.7, 6.55) | 0.20 | –1.50 (−19.8, 21.0) | 0.89 |
| MBP | 9.68 (−5.18, 26.9) | 0.22 | 0.97 (−14.4, 19.1) | 0.91 | –0.73 (−17.1, 18.8) | 0.94 |
| MiBP | 1.79 (−11.6, 17.3) | 0.81 | –0.04 (−14.9, 17.3) | 1.00 | –1.15 (−17.0, 17.7) | 0.90 |
| MEP | –2.22 (−18.4, 17.1) | 0.81 | –3.69 (−21.9, 18.7) | 0.73 | 7.70 (−14.0, 34.9) | 0.52 |
| IL-10 |
TNF-α |
|||
|---|---|---|---|---|
| %Δ (95%CI) | p | %Δ (95%CI) | p | |
| MEHP | 17.2 (−2.36, 40.7) | 0.09 | 4.51 (−4.34, 14.2) | 0.33 |
| MEHHP | 15.8 (−1.10, 35.7) | 0.07 | 4.13 (−3.38, 12.2) | 0.29 |
| MEOHP | 17.6 (0.18, 38.1) | 0.05 | 4.48 (−3.16, 12.7) | 0.26 |
| MECPP | 24.5 (5.53, 46.9) | 0.01 | 4.90 (−3.00, 13.5) | 0.24 |
| MCPP | 2.63 (−12.3, 20.1) | 0.75 | –0.13 (−7.32, 7.62) | 0.97 |
| MCOP | 18.0 (0.02, 39.2) | 0.05 | 1.42 (−6.53, 10.0) | 0.74 |
| MCNP | 7.73 (−4.54, 21.6) | 0.23 | 0.26 (−5.21, 6.03) | 0.93 |
| MBzP | –17.8 (−31.9, −0.75) | 0.05 | 1.31 (−7.70, 11.2) | 0.78 |
| MBP | –8.28 (−22.4, 8.33) | 0.31 | 3.54 (−4.35, 12.1) | 0.39 |
| MiBP | –5.02 (−19.3, 11.8) | 0.54 | 2.54 (−5.10, 10.8) | 0.53 |
| MEP | –7.50 (−25.3, 14.5) | 0.48 | 1.91 (−8.35, 13.3) | 0.73 |
Estimates created from linear mixed models with random slopes for subject ID and adjustment for urinary specific gravity, visit of sample collection, and maternal prepregnancy BMI, education, and income level. N = 87 subjects; N = 157 observations.
Results for models of oxidative stress biomarkers in relation to urinary phthalate metabolites are presented in Table 4. For both biomarkers, increases were observed in association with all urinary phthalate metabolites. For OHdG, the highest percent increases were in association with IQR increase in MBP (%Δ = 23.9, 95% CI = 9.37, 40.4) and MiBP (%Δ = 19.9, 95% CI = 5.52, 36.2), although associations for DEHP metabolites were also statistically significant. For isoprostane, effect estimates were greater compared to those for OHdG, and all were statistically significant except for the association with MEP. As with OHdG, the strongest associations were for MBP (%Δ = 49.7, 95%CI = 32.0, 69.8) and MiBP (%Δ = 48.8, 95% CI = 30.4, 69.8).
Table 4. Percent Change (95% Confidence Interval)a in Urinary Oxidative Stress Biomarker in Association with Interquartile Range Increase in Urinary Phthalate Metabolite Concentration.
| OHdG |
Isoprostane |
|||
|---|---|---|---|---|
| %Δ (95% CI) | p | %Δ (95% CI) | p | |
| MEHP | 17.7 (2.35, 35.3) | 0.03 | 34.9 (15.9, 57.1) | <0.01 |
| MEHHP | 14.5 (0.73, 30.2) | 0.04 | 30.2 (13.5, 49.3) | <0.01 |
| MEOHP | 17.9 (3.00, 34.9) | 0.02 | 34.3 (16.4, 55.0) | <0.01 |
| MECPP | 15.1 (0.34, 32.1) | 0.05 | 28.4 (10.9, 48.7) | <0.01 |
| MCPP | 2.31 (−9.31, 15.4) | 0.71 | 23.7 (9.04, 40.4) | <0.01 |
| MCOP | 3.18 (−8.22, 16.0) | 0.60 | 24.2 (8.94, 41.6) | <0.01 |
| MCNP | 7.72 (−2.62, 19.2) | 0.15 | 18.9 (6.94, 32.2) | <0.01 |
| MBzP | 13.3 (−3.35, 32.8) | 0.13 | 35.3 (14.5, 59.9) | <0.01 |
| MBP | 23.9 (9.37, 40.4) | <0.01 | 49.7 (32.0, 69.8) | <0.01 |
| MiBP | 19.9 (5.52, 36.2) | 0.01 | 48.8 (30.4, 69.8) | <0.01 |
| MEP | 11.4 (−5.50, 31.3) | 0.20 | 19.4 (−1.56, 44.8) | 0.08 |
Estimates created from linear mixed models with random slopes for subject ID and adjustment for urinary specific gravity, visit of sample collection, and maternal prepregnancy BMI, education, and income level. N = 46 subjects; N = 125 observations.
Interaction terms between study visit and individual phthalate metabolites were not statistically significant for models of OHdG (data not shown). However, for isoprostane, we observed inverse interaction terms that were marginally significant between visit 3 and DEHP metabolites, including MEHHP (β = −0.53, p = 0.09), MEOHP (β = −0.54, p = 0.07), and MECPP (β = −0.90, p = 0.05). These results suggest that pregnant women in this study population may have a weaker isoprostane response to DEHP exposures later in pregnancy compared to early pregnancy, although additional evidence with larger sample sizes at each visit would be necessary for confirmation.
Discussion
The present study explored the association between urinary phthalate metabolites and biomarkers of inflammation and oxidative stress, all measured at multiple time points during pregnancy. We observed that metabolites of DEHP were associated with slight increases in IL-6 and IL-10 cytokines. We also observed that a majority of phthalate metabolites were associated with increases in urinary biomarkers of oxidative stress, including OHdG and isoprostane, and that these associations were strongest for MBP and MiBP. These results may represent important mechanisms for adverse birth outcomes and other health effects.
Inflammation biomarker levels in this population were mostly similar to those measured in a nested case-control study of preterm birth in Boston, in which plasma (N = 1585 samples, N = 481 subjects) from up to 4 time points across pregnancy (median gestational ages 10, 18, 26, and 35 weeks) was analyzed by the same methods and laboratory as in the present study.20 CRP levels were somewhat higher in this population of Puerto Rican women (geometric mean [geometric standard deviation] = 7.93 μg/mL [2.22]) compared to those from women in Boston (5.80 μg/mL [2.89]). For cytokines measured, IL-1β, IL-6, and TNF-α plasma levels were very similar in both populations, but IL-10 plasma levels were slightly lower in the present Puerto Rican population (8.18 pg/mL [2.76]) compared to the Boston population (13.9 pg/mL [2.58]).20
For inflammation biomarkers, associations were generally null; we observed few statistically significant associations or clear patterns across the data. Most HMW phthalate metabolites were associated with increases in IL-6 and IL-10, but relationships were only statistically significant (p < 0.05) between MCNP and IL-6 and between MECPP and IL-10. As this analysis was exploratory with a relatively small sample size, we also noted some suggestive associations (p < 0.10) between MEHHP, MEOHP, and MECPP and increased IL-6, as well as between MEHP, MEHHP, MEOHP, and MCOP and increased IL-10. Nevertheless, these findings may have been spurious. Additional research with a larger sample size and/or additional time points is necessary before firm conclusions can be drawn.
Despite the lack of associations observed in the present study, previous research has demonstrated that some phthalate metabolites, particularly MEHP, are capable of inducing inflammatory and anti-inflammatory responses in various cell types.9,21,22 Systemic inflammatory responses in relation to phthalate exposure have also been observed in epidemiologic studies using CRP23 as well as alkaline phosphatase and absolute neutrophil count measured in serum.11 To our knowledge this is the first study to examine circulating plasma cytokines and urinary concentrations of phthalate metabolites in human subjects, although one experimental study also observed that subjects nasally exposed to DEHP-contaminated house dusts had higher IL-6, IL-5, and granulocyte-colony-stimulating factor concentrations in mucosal secretions.24
IL-6 is a proinflammatory cytokine released from macrophages in response to pathogenic stimuli as part of the acute phase response.25 IL-6 has been used as a biomarker in previous studies investigating the inflammatory effect of other environmental pollutants, such as air contaminants26 and lead.27 Circulating levels have also been linked to a number of adverse birth outcomes, including preeclampsia and preterm birth.28 In addition to its usefulness as a biomarker of inflammation, IL-6 may also play a critical role in the pathway to spontaneous preterm birth by initiating an inflammatory cascade that leads to the release of other signaling molecules, cervical ripening and other physical changes, and finally preterm parturition.29 IL-6 levels at the maternal-fetal interface may be most relevant to this pathway. We examined the relationship between urinary phthalate metabolites and IL-6 in peripheral plasma samples, as opposed to amniotic fluid, placental tissue, or umbilical cord blood, which may explain the lack of observed associations.
Our null findings for an association between phthalate metabolites and CRP were somewhat unexpected, as this marker is used commonly to indicate systemic inflammation30 and increases have been observed in association with MiBP and MBzP in a previous study in human adults.23 One explanation is that CRP, an acute phase protein formed in hepatocytes in response to IL-6 stimulation, is more representative of the balance between inflammatory and anti-inflammatory responses.25 While we observed increases in IL-6, we additionally observed increases in IL-10, which has anti-inflammatory properties.25 These parallel positive associations for both pro and anti-inflammatory cytokines are not unexpected, nor is the moderately strong and positive correlation between the two markers (Spearman R = 0.40). In the human system, the magnitude of an inflammatory response is tempered by a simultaneous anti-inflammatory response to avoid cellular damage.31 If these changes are observed in unison, as they were in this analysis, the cumulative inflammatory response may be minimal. Phthalate metabolites were also not associated with and IL-1β and TNF-α in this analysis although IL-1β and TNF-α, like IL-6, are pro-inflammatory acute phase proteins.25 However, as with CRP, these cytokines showed only modest correlations with IL-6 indicating that levels in plasma may be representative of different cellular responses. These null associations may also have been due to our relatively small sample size and limited ability to detect more subtle associations with these markers.
The stronger findings in our study were between the urinary concentrations of phthalate metabolites and biomarkers of oxidative stress. The distribution of OHdG was similar in the population of pregnant women from Boston, where both markers were measured in urine samples from up to 4 time points during gestation (N = 1678 samples, N = 482 subjects) using the same methods and laboratory as in the present study. However, isoprostane levels were higher in this population (258 pg/mL [1.85]) compared to the women in the Boston study (189 pg/mL [2.57]).32 This could be due to higher exposure to phthalates and other chemicals that cause oxidative stress in Puerto Rico compared to the US,5 though alternative explanations cannot be ruled out.
Previous studies in various cell types have demonstrated that phthalate metabolites, especially MEHP, are capable of producing reactive oxygen species and consequently oxidative stress.8,10 These effects may result from the ability of MEHP and other metabolites to bind to peroxisome proliferator activated receptors (PPARs).33 Several studies in humans have observed associations between phthalate metabolites and biomarkers of oxidative stress, including gammaglutamyl transferase (GGT),23 OHdG,12 and malondialdehyde (MDA; like isoprostane a marker of lipid peroxidation) in the elderly13 and in children.34 In our recent study of pregnant women in Boston, we also observed significant positive associations between nearly all urinary phthalate metabolites measured and OHdG and isoprostane.32 In that study, the percent change in oxidative stress marker was also highest in association with the LMW metabolites MBP and MiBP and associations for isoprostane were higher compared to OHdG.32 The results from this study of Puerto Rican pregnant women are similar; effect estimates were generally higher in association with metabolites of LMW phthalates, particularly MBP and MiBP, and across all phthalate metabolites effect estimates were higher for isoprostane compared to OHdG. This replication of the associations we observed in pregnant women in Boston supports a broader relationship between environmental phthalate exposure and measures of oxidative stress during pregnancy.
These associations may be particularly important in the quest to identify causes and pathways leading to preterm birth. In Puerto Rico, rates of preterm birth have grown rapidly over the past two decades and are the third highest in the world, following Malawi and Congo.35,36 Despite the prevalence and severity of this problem in Puerto Rico and elsewhere, preterm birth is poorly understood and effective preventions are limited.37 Environmental contaminants may be important contributing factors.38 While phthalates have been linked to preterm birth in epidemiologic studies, causality has not been clearly demonstrated. This suggestive evidence for an association between phthalate metabolites and biomarkers of inflammation and oxidative stress, both plausible intermediates in the pathway between phthalate exposure and preterm birth, support this hypothesis and in the future could provide a target for intervention strategies.
There were several limitations to this analysis. As this was a preliminary subset of an ongoing cohort study, we had a relatively small sample size. However, we had the power of multiple measurements of exposure and outcomes across pregnancy, which enabled us to detect associations with some precision. Despite having repeated measures, there is still considerable variability in subject-specific phthalate levels over pregnancy,5 and some variability in inflammation and oxidative stress biomarker levels. A greater number of measures may have improved our detection of associations, particularly for the inflammation marker analysis where we only had markers at two time points available instead of the three which were available for oxidative stress markers. Our study was also limited by lack of information on intrauterine infection, for example, chorioamnionitis, which would be strongly linked to inflammation and potentially oxidative stress markers and could have to some extent obscured real effects. However, such conditions during pregnancy are rare (1–4% in normal pregnancy), and as we have no reason to expect that they would be associated with phthalate exposure their presence would only bias effect estimates toward the null.39
Despite these limitations our study had numerous strengths. We measured exposure and outcome biomarkers at multiple time points during pregnancy, which enabled us to utilize mixed effect models to more powerfully detect associations with repeated measures since individuals can serve as their own reference over time. The usefulness of this modeling technique is clear when our results are compared to other studies with single measures per subject. We also measured multiple biomarkers of both inflammation and oxidative stress, as different markers may result from different mechanisms and may lead to different downstream health effects. Finally, markers were measured at disparate time points during pregnancy which allowed us to look at changes in levels and windows of vulnerability across gestation. These preliminary results provide evidence for a relationship between maternal phthalate exposure during pregnancy and systemic oxidative stress, as well as suggestive evidence for a relationship with systemic inflammation, which may be important mechanisms for the range of adverse health effects that have been linked to phthalates.
Acknowledgments
We thank Manori Silva, Ella Samandar, Jim Preau, and Tao Jia of the CDC for measuring the urinary phthalate metabolites; Joel Whitfield of the Cancer Center Immunology Core, University of Michigan, Ann Arbor, Michigan, for his analysis of biomarkers of inflammation; and Elizabeth Hurst and colleagues of Cayman Chemical in Ann Arbor, Michigan, for oxidative stress biomarker analysis. This work was supported by the National Institute of Environmental Health Sciences, National Institutes of Health (Grants P42ES017198, R01ES018872, and P30ES017885). The content is solely the responsibility of the authors and does not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supporting Information Available
Additional information as noted in the text is available online. This material is available free of charge via the Internet at http://pubs.acs.org/.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Schettler T. Human exposure to phthalates via consumer products. Int. J. Androl 2006, 291134–9discussion 181-5. [DOI] [PubMed] [Google Scholar]
- Janjua N. R.; Frederiksen H.; Skakkebaek N. E.; Wulf H. C.; Andersson A. M. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int. J. Androl 2008, 312118–30. [DOI] [PubMed] [Google Scholar]
- Meeker J. D.; Sathyanarayana S.; Swan S. H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc., B 2009, 36415262097–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D.; Cantonwine D. E.; Rivera-Gonzalez L. O.; Ferguson K. K.; Mukherjee B.; Calafat A. M.; Ye X.; Anzalota Del Toro L. V.; Crespo-Hernandez N.; Jimenez-Velez B.; Alshawabkeh A. N.; Cordero J. F. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ. Sci. Technol. 2013, 4773439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine D. E.; Cordero J. F.; Rivera-Gonzalez L. O.; Anzalota Del Toro L. V.; Ferguson K. K.; Mukherjee B.; Calafat A. M.; Crespo N.; Jimenez-Velez B.; Padilla I. Y.; Alshawabkeh A. N.; Meeker J. D. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: Distribution, temporal variability, and predictors. Environ. Int. 2014, 62, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A.Phthalates: human exposure and related health effects. Dioxins and Health: Including Other Persistent Organic Pollutants and Endocrine Disruptors, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, 2012; 415−443. [Google Scholar]
- Chen X.; Wang J.; Qin Q.; Jiang Y.; Yang G.; Rao K.; Wang Q.; Xiong W.; Yuan J. Mono-2-ethylhexyl phthalate induced loss of mitochondrial membrane potential and activation of Caspase3 in HepG2 cells. Environ. Toxicol Pharmacol 2012, 333421–30. [DOI] [PubMed] [Google Scholar]
- Erkekoglu P.; Rachidi W.; Yuzugullu O. G.; Giray B.; Favier A.; Ozturk M.; Hincal F. Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium. Toxicol. Appl. Pharmacol. 2010, 248152–62. [DOI] [PubMed] [Google Scholar]
- Jepsen K. F.; Abildtrup A.; Larsen S. T. Monophthalates promote IL-6 and IL-8 production in the human epithelial cell line A549. Toxicol In Vitro 2004, 183265–9. [DOI] [PubMed] [Google Scholar]
- Tetz L. M.; Cheng A. A.; Korte C. S.; Giese R. W.; Wang P.; Harris C.; Meeker J. D.; Loch-Caruso R. Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro. Toxicol. Appl. Pharmacol. 2013, 268147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K. K.; Loch-Caruso R.; Meeker J. D. Exploration of oxidative stress and inflammatory markers in relation to urinary phthalate metabolites: NHANES 1999–2006. Environ. Sci. Technol. 2012, 461477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y. C.; Park E. Y.; Park M. S.; Ko J. A.; Oh S. Y.; Kim H.; Lee K. H.; Leem J. H.; Ha E. H. Community level exposure to chemicals and oxidative stress in adult population. Toxicol. Lett. 2009, 1842139–44. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Park H. Y.; Bae S.; Lim Y. H.; Hong Y. C. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: A panel study. PLoS One 2013, 88e71392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K. K.; McElrath T. F.; Meeker J. D. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2013, 168, 61–67 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft G.; Jonsson B. A.; Lindh C. H.; Jensen T. K.; Hjollund N. H.; Vested A.; Bonde J. P. Association between pregnancy loss and urinary phthalate levels around the time of conception. Environ. Health Perspect 2012, 1203458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Lin L.; Cao Y.; Chen B.; Zheng L.; Ge R. S. Phthalate levels and low birth weight: A nested case-control study of Chinese newborns. J. Pediatr. 2009, 1554500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. J.; Samandar E.; Preau J. L. Jr.; Reidy J. A.; Needham L. L.; Calafat A. M. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2007, 8601106–12. [DOI] [PubMed] [Google Scholar]
- Zota A. R.; Calafat A. M.; Woodruff T. J. Temporal trends in phthalate exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect 2014, 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung R. W.; Reed L. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar]
- Ferguson K. K.; McElrath T. F.; Chen Y. H..; Mukherjee B.; Meeker J. D.. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. 2014, DOI: 10.1111/aji.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rael L. T.; Bar-Or R.; Ambruso D. R.; Mains C. W.; Slone D. S.; Craun M. L.; Bar-Or D. Phthalate esters used as plasticizers in packed red blood cell storage bags may lead to progressive toxin exposure and the release of pro-inflammatory cytokines. Oxid. Med. Cell. Longevity 2009, 23166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakkestad K. E.; Holme J. A.; Paulsen R. E.; Schwarze P. E.; Becher R. Mono(2-ethylhexyl) phthalate induces both pro- and anti-inflammatory responses in rat alveolar macrophages through crosstalk between p38, the lipoxygenase pathway and PPARalpha. Inhal.Toxicol. 2010, 222140–50. [DOI] [PubMed] [Google Scholar]
- Ferguson K. K.; Loch-Caruso R.; Meeker J. D. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999–2006. Environ. Res. 2011, 1115718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschle T.; Reiter R.; Butte W.; Heinzow B.; Keck T.; Riechelmann H. A controlled challenge study on di(2-ethylhexyl) phthalate (DEHP) in house dust and the immune response in human nasal mucosa of allergic subjects. Environ. Health Perspect 2008, 116111487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.; Travers P.; Walport M.. Janeway’s Immunobiology, 7th ed.; Garland Science, Taylor & Francis Group, LLC: New York, NY, 2008. [Google Scholar]
- Delfino R. J.; Staimer N.; Tjoa T.; Gillen D. L.; Polidori A.; Arhami M.; Kleinman M. T.; Vaziri N. D.; Longhurst J.; Sioutas C. Air pollution exposures and circulating biomarkers of effect in a susceptible population: Clues to potential causal component mixtures and mechanisms. Environ. Health Perspect 2009, 11781232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. L.; Kubzansky L. D.; Ikeda A.; Fang S. C.; Sparrow D.; Weisskopf M. G.; Wright R. O.; Vokonas P.; Hu H.; Schwartz J. Lead concentrations in relation to multiple biomarkers of cardiovascular disease: The Normative Aging Study. Environ. Health Perspect 2012, 1203361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins J. R.; Gomez-Lopez N.; Robertson S. A. Interleukin-6 in pregnancy and gestational disorders. J. Reprod. Immunol. 2012, 951–21–14. [DOI] [PubMed] [Google Scholar]
- Challis J. R.; Lockwood C. J.; Myatt L.; Norman J. E.; Strauss J. F. 3rd; Petraglia F. Inflammation and pregnancy. Reprod. Sci. 2009, 162206–15. [DOI] [PubMed] [Google Scholar]
- Marnell L.; Mold C.; Du Clos T. W. C-reactive protein: Ligands, receptors and role in inflammation. Clin. Immunol. 2005, 1172104–11. [DOI] [PubMed] [Google Scholar]
- Saraiva M.; O’Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol 2010, 103170–81. [DOI] [PubMed] [Google Scholar]
- Ferguson K. K.; McElrath T. F.; Chen Y. H.; Mukherjee B.; Meeker J. D., Urinary phthalate metabolites are associated with increased oxidative stress biomarkers in pregnant women. 2014, submitted. [Google Scholar]
- Hurst C. H.; Waxman D. J. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol. Sci. 2003, 742297–308. [DOI] [PubMed] [Google Scholar]
- Kim S.; Kang S.; Lee G.; Lee S.; Jo A.; Kwak K.; Kim D.; Koh D.; Kho Y. L.; Kim S.; Choi K. Urinary phthalate metabolites among elementary school children of Korea: Sources, risks, and their association with oxidative stress marker. Sci. Total Environ. 2013, 472C, 49–55. [DOI] [PubMed] [Google Scholar]
- Blencowe H.; Cousens S.; Oestergaard M. Z.; Chou D.; M?ller A. B.; Narwal R.; Adler A.; Vera Garcia C.; Rohde S.; Say L.; Lawn J. E. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 37998322162–72. [DOI] [PubMed] [Google Scholar]
- Martin J. A.; Hamilton B. E.; Ventura S. J.; Osterman M. J.; Kirmeyer S.; Mathews T. J.; Wilson E. C. Births: Final data for 2009. National Vital Statistics Reports 2011, 6011–70. [PubMed] [Google Scholar]
- Behrman R. E.; Butler A. S.. Preterm Birth: Causes, Consequences, And Prevention; The National Academies Press: Washington, DC, 2007. [PubMed] [Google Scholar]
- Ferguson K. K.; O’Neill M. S.; Meeker J. D. Environmental contaminant exposures and preterm birth: A comprehensive review. J. Toxicol. Environ. Health, Part B 2013, 16269–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. S.; Duff P. Progress in pathogenesis and management of clinical intraamniotic infection. Am. J. Obstet. Gynecol. 1991, 1645 Pt 11317–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


