Abstract
The merozoite surface protein-9 (MSP-9) has been considered a target for an anti-malarial vaccine since it is one of many proteins involved in the erythrocyte invasion, a critical step in the parasite life cycle. Orthologs encoding this antigen have been found in all known species of Plasmodium parasitic to primates. In order to characterize and investigate the extent and maintenance of msp-9 genetic diversity, we analyzed DNA sequences of the following malaria parasite species: Plasmodium falciparum, P. reichenowi, P. chabaudi, P. yoelii, P. berghei, P. coatneyi, P. gonderi, P. knowlesi, P. inui, P. simiovale, P. fieldi, P. cynomolgi and P. vivax and evaluated the signature of natural selection in all msp-9 orthologs. Our findings suggest that the gene encoding MSP-9 is under purifying selection in Plasmodium vivax and closely related species. We further explored how selection affected different regions of MSP-9 by comparing the polymorphisms in P. vivax and P. falciparum, and found contrasting patterns between these two species that suggest differences in functional constraints. This observation implies that the MSP-9 orthologs in human parasites may interact differently with the host immune response. Thus, studies carried out in one species cannot be directly translated into the other.
Keywords: ABRA, Binding sites, Genetic diversity, Merozoite Surface Proteins, MSP-9, Plasmodium
1. Introduction
Regardless the extraordinary progress achieved in malaria control, this vector-borne disease is still ranking among the most important causes of disease and death worldwide. There are four human malarial parasites; however, two of them, Plasmodium falciparum and P. vivax, are responsible for most malaria morbidity and mortality (WHO 2011). Although the species exhibit great biological differences (Coatney et al., 1971), all human malarial parasites invade erythrocytes in a complex, multi-step process that exhibits important differences among species (Rayner et al., 2005). For instance, P. vivax merozoites invade only reticulocytes, whereas P. falciparum merozoites invade mature erythrocytes as well as reticulocytes. Given that this step is critical in the parasite life cycle, proteins involved in this process are considered to be potential vaccine targets. One of these proteins is the merozoite surface protein-9 (MSP-9) (Vargas-Serrato et al., 2002).
MSP-9 was first identified in P. falciparum as the 101 kDa acidic-basic repetitive antigen (ABRA); then, orthologous genes were identified in other Plasmodium species (Barnwell et al., 1999; Vargas-Serrato et al., 2002; Vargas-Serrato et al., 2003; Lopera-Mesa et al., 2008). It was first described as a hydrophilic protein with a putative 20 amino acid signal peptide, a conserved N-terminal domain with a cluster of four cysteines and a C-terminal region containing species specific blocks of repeated amino acids (Vargas-Serrato et al., 2002; Oliveira-Ferreira et al., 2004). For instance, two repetitive tandems has been reported in the C-terminal domain P. vivax MSP-9 (PvMSP-9) (Vargas-Serrato et al., 2002); whereas only one has been reported in P. falciparum N-terminal (Weber et al., 1988).
The role of MSP-9 in the parasite life cycle is still not clear. It is found on the surface of merozoites as well as in the parasitophorous vacuole within the infected erythrocytes (Weber et al., 1988; Kushwaha et al., 2002; Cowman et al., 2006). Experimental evidence suggests that in P. falciparum, MSP-9 (PfMSP-9) acts as a serine protease and participates in the release of merozoites from infected erythrocytes (Nwagwu et al., 1992). Furthermore, in vitro experiments indicate that PfMSP-9 binds the human erythrocyte surface through its N-terminal cysteine-rich region or by high binding activity peptides (HBAPs) within positions 121-240 (Curtidor et al., 2001). These peptides overlap with the region labelled Δ1a (77-235 aa), one of two segments of PfMSP-9 (the second is Δ2 between 364-528 aa) that intereract with the merozoite surface protein-119 (MSP-119) in a complex that binds the erythrocyte protein band 3 (Kushwaha et al., 2002; Li et al., 2004; Kariuki et al., 2005; Lopera-Mesa et al., 2008). Whereas the P. vivax MSP-9 (PvMSP-9) interaction with PvMSP-119 has not been documented; there is evidence that the N-terminal of PvMSP-9 is immunogenic. Indeed, five T-cell epitopes (pE, pJ, pK, pH and pL) have been identified in the N-terminus that interact with a broad range of HLA class II molecules; these peptides elicited robust cellular responses in individuals naturally exposed to malaria (Lima-Junior et al., 2008).
The role of PfMSP-9 in the erythrocyte invasion, the fact that there are orthologs in several Plasmodium species, and that it is immunogenic as shown in animal models as well as in natural exposed individuals, made MSP-9 a protein of interest for vaccine development (Sharma et al., 1998; Pimtanothai et al., 2000; Kushwaha et al., 2001; Oliveira-Ferreira et al., 2004; Lima-Junior et al., 2010; Lima-Junior et al., 2012). Hence, it is important to further explore its genetic diversity and how such polymorphism is being altered or maintained in Plasmodium. In this study, we explored patterns of genetic polymorphism in P. falciparum and P. vivax and compared those to other Plasmodium species in non-human primates. For instance, we included the chimpanzee parasite P. reichenowi that is closely related to P. falciparum and the species: P. cynomolgi, P. inui, P. simiovale, P. fieldi, P. knowlesi, P. gonderi and P. coatneyi that are a part of a clade that include all these non-human primate malarias and the human parasite P. vivax (Escalante et al., 1998, 2005). In addition, we also included the rodent malarial species, P. chabaudi, P. yoelii and P. berghei, to study the long term evolution of the gene encoding MSP-9. Overall, we found that the genetic polymorphism varies across Plasmodium species. Whereas we detected a pattern consistent with balancing selection in the red blood cell binding region at the N-terminal of PfMSP-9, we found a more complex pattern in P. vivax. PvMSP-9 exhibits evidence consistent with purifying selection in the C terminal- and extensive polymorphism in terms of the number and type of motifs in the low complexity regions. However, the motifs themselves within each low complexity region show an excess of synonymous substitutions, which could be indicative of functional constraints. These contrasting polymorphism patterns between P. falciparum and P. vivax indicate that MSP-9 may be recognized differently across Plasmodium spp.-host interactions.
2. Materials and methods
2.1 Samples
In this study, we processed samples from different geographic locations that included laboratory isolates of P. vivax (Mauritania, Brazil I, Vietnam Palo Alto, Vietnam II, Sumatra, Chesson, and India VII), P. cynomolgi (Mulligan, PT1, Berok-PT2, RO, Ceylonensis, Gombok, and B strain), P. knowlesi (Hackeri, Malayan, Philippine and Nuri), P. fieldi (ABI) and P. simiovale provided by the Centers for Disease control (CDC, see Coatney et al., 1971), and field isolates of P. vivax from Thailand (n=6, hospital based study in Bangkok) and Peru (n=5, Peruvian Amazon region in the Loreto Department). Peru samples were provided by the Naval Medical Research Unit No. 6 in Lima. DNA was purified from 200μL of whole blood samples using the QIAamp DNA Blood Mini kit (Qiagen GmbH, Hilden, Germany).
2.2 PCR amplification, cloning, and sequencing
The gene encoding the MSP-9 for P. vivax and the closely related malarial species found in non-human primates (NHPs) were amplified by polymerase chain reaction (PCR). PvMSP-9 and PcynMSP-9 sequences were amplified using degenerated primers forward AE374 5′-ATG CG(A/C) (C/G)TG A(C/A/G)C (T/A)T CAT C-3′ and reverse AE377 5′-CTA TGG (A/T)GT GAC ATC (G/C)GT G-3′, while the PkMSP-9 sequences were amplified using the primers forward AE374 and reverse AE378 5′-CTA AGG CTC TAC AGT GTT C-3′. P fieldi and P. simiovale sequences were amplified using forward AE444 5′-TGG TGA (A/G)GG G(A/G)C AC(A/G) TAG-3′ and reverse AE377. We also extracted putative MSP9 orthologs from locally assembled 454 (Roche, Applied Science, Basel, Switzerland) reads for P. gonderi (complete CDS) and P. inui (partial CDS). Those sequences are also reported.
PCR amplifications were carried out in a 50 μl volume reaction using 20 ng of total genomic DNA, 3 mM MgCl2, 1X PCR buffer, 1.25 mM of each deoxynucleoside triphosphate, 0.4 mM of each primer, and 0.03 U/μl AmpliTaq Gold® DNA polymerase (Applied Biosystems, Roche-USA). Amplification was achieved using the following conditions: an initial denaturation step at 94°C for 3 minutes; 36 cycles of: denaturation at 94°C for 1 minute, annealing at 57°C for 1 minute and extension at 72°C for 2minutes; followed by a final extension at 72°C for 10 min. PCR products were purified from a gel using the QIAquick Gel extraction kit (Qiagen GmbH, Hilden, Germany). In order to rule out PCR errors, at least two independent PCR products were cloned in the pGEM®-T Easy Vector Systems (Promega, Madison, WI, USA) in all cases, and both strands for three or four clones from each sample were sequenced using an Applied Biosystems 3730 capillary sequencer. The identity of the sequences was determined by comparing our sequences with those previously deposited in the GenBank database by using BLAST. The sequences reported in this study were deposited in Genbank under the accession numbers KC935389 to KC935421 and KF516232.
2.2 Genetic diversity and phylogenetic analyses
Nucleotide sequence and protein alignments of the conserved N-terminal were performed using ClustalW as implemented in MEGA v4.1 software (Tamura et al., 2007) with manual editing. We also included sequences available in GenBank and PlasmoDB for P. falciparum (AF213645, XM_001350647, FJ406812, FJ406815, FJ406822, FJ406825, FJ406816, FJ406813, FJ406809, FJ406814, FJ406821, FJ406820, FJ406810, FJ406818, FJ406817, FJ406811, FJ406823, FJ406819 and J03902), P. reichenowi (CDC-1 Oscar, FJ406808), P. vivax (XM_001617324, AF435853), P. cynomolgi (B-strain, PCYB_145430), P. knowlesi (Malaysia H, AF435855), P. coatneyi (AY135361), Plasmodium yoelii yoelii str. 17XNL (XM725739), P. berghei (isolate NK-65, AY302245; isolate ANKA, PBANKA_144330), and P. chabaudi chabaudi (PCHAS_144550).
Intra specific variation in regions that could be aligned (repeats were excluded) was characterized in terms of nucleotide diversity (Pi) and the effect of natural selection was evaluated by estimating the difference of synonymous and non-synonymous substitutions (Ds-Dn) between a pair of sequences using the Nei and Gojobori's method with the Jukes and Cantor correction. The null hypothesis of neutrality was rejected if Dn/Ds ≠ 1. Standard error was calculated using bootstrap with 1000 replications and rates of Ds and Dn were compared by the Z-test of selection using MEGA v4.1 (Tamura et al., 2007). The assumption of neutrality was also tested using the McDonald and Kreitman test or MK test (McDonald and Kreitman, 1991) as implemented in DnaSP v5.0, which compares the intra and inter specific number of synonymous and non-synonymous sites; significance was assessed by using a Fisher's exact test for the 2×2 contingency table as implemented in the program DnaSP v5.0 (Librado and Rozas, 2009). In this analysis using the MK test, we compared the following species: P. vivax with P. cynomolgi and P. falciparum with P. reichenowi.
Phylogenetic relationships of msp-9 orthologs were estimated on a nucleotide sequences alignment of the N-terminal (1294 bp) that lacks low complexity regions in most Plasmodium species but P. falciparum and P. reichenowi; thus, the alignment also excluded the R1 repetitive region found solely in these two species (see Fig 1). A phylogeny was estimated by using the Neighbor-Joining method (Saitou and Nei, 1987) with the Kimura 2-parameters model as implemented in MEGA v5.0 (Tamura et al., 2007). The reliability of the nodes in the NJ trees was assessed by bootstrap method with 1000 pseudo-replications (Nei and Kumar, 2000). In addition, a Bayesian phylogenetic analysis was performed as implemented in MrBayes v3.2.1 (Ronquist and Huelsenbeck, 2003). We used a general time reversible model + gamma (GTR+G); this model had the lower number of parameter that best fit the data as estimated by MEGA v5.0. Bayesian support for the nodes was inferred in MrBayes using 3 ×106 Markov Chain Monte Carlo (MCMC) steps, and after convergence was reached, we discarded the 50% of the sample as a burn-in. Sampling was performed every 100 generations.
Fig. 1.
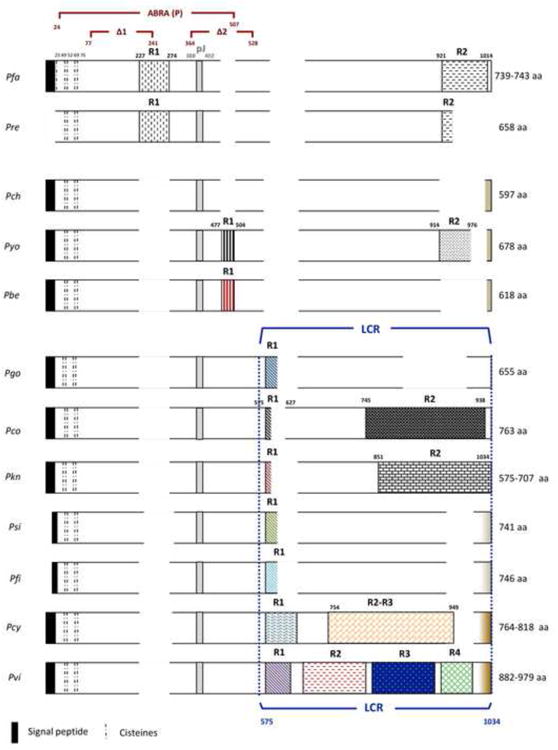
Diagram of the MSP-9 Plasmodium spp protein alignment (partial sequence of P. inui was not included). The length of the protein is indicated for each species. The putative signal peptide, cysteines, RBC binding regions (Kushwaha et al., 2002, Li et al., 2004) and repetitive regions (R, R1, R2, R3 and R4) are shown. Blocks with different patterns represent different repetitive regions. Gaps in the protein alignment are represented by blank areas.
The long term effect of selection on the N-terminal was explored using maximum likelihood approaches as implemented in HyPhy (Kosakovsky Pond et al., 2005). Specifically, the ratio of kA to kS (=ω), in which kA and kS are the number of nonsynonymous and synonymous changes per nonsynonymous and synonymous sites, respectively, was estimated for branches in the phylogenetic trees using the branch model implemented in HyPhy. The ω ratio measures the direction and magnitude of selection on amino acid replacements, with values of ω < 1 (purifying selection), ω = 1 (neutral evolution), and ω > 1 (positive selection). The heterogeneity of ω among branches was incorporated using the free-ratio model where each branch has its independent ω value. HyPhy was run using a codon partition, MG94 substitution model. We performed a likelihood ratio test comparing our null model – the P. vivax branch was constrained to having an omega of 1 – against our alternative model in which the branch was undergoing positive or negative selection. The models were compared using a likelihood ratio test (LRT). Since the tree topology affects the ω values, we explored the robustness of our results by performing a set of tests where our antigen was studied using the phylogeny estimated from mitochondrial genomes (Pacheco et al., 2011). Since selection might be affecting only a subset of lineages (episodic selection), we also used a branch- and branch-site type models, to recognize lineages where selection is episodic (positively selected sites) as implemented in HyPhy (Kosakovsky Pond et al., 2005; 2011). In all analyses we only considered the conserved regions within species and between species from the MSP-9 alignments. Moreover, the repetitive regions were not included in the phylogeny since they are not conserved in all orthologs.
2.3 Protein structure prediction
To predict important aspects of the protein such as its secondary structure (PROFsec) and its solvent accessibility (PROFacc), we used the PredictProtein automatic service (http://www.predictprotein.org) (Rost et al., 2004). In addition, we used SEG to divide amino acid sequences into protein segments of low and high-complexity as implemented in http://mendel.imp.ac.at/METHODS/seg.server.html (Wootton, 1994). The parameters that best determine low complexity regions in MSP-9 were: trigger window length (W): 25, trigger complexity (K1): 3.0 and extension complexity (K2): 3.3. We also used the web-based I-TASSER server, available at http://zhanglab.ccmb.med.umich.edu/I-TASSER/, which is an on-line workbench for high-resolution modeling of protein structure. All predictions from this server are tagged with a confidence score which tells how accurate the predictions are without knowing the experimental data (Roy et al., 2010; 2012; Zhang 2008).
3. Results
3.1 Description of MSP-9 orthologs in Plasmodium spp
We obtained almost complete msp-9 sequences from 16 different isolates of P. vivax, seven from P. knowlesi, seven from P. cynomolgi, one from P. fieldi, one from P. simiovale, one from P. gonderi, and one partial from P. inui. The MSP-9 analysis was performed using 64 sequences in total, including the ones available in GenBank. The analysis of the MSP-9 sequences showed the signal peptide, four conserved cysteines in positions 49, 52, 69 and 76 (species alignment) and one or more repetitive regions in all species analyzed but in P. chabaudi (Fig 1). Although putative MSP-9 orthologs in P. falciparum and P. vivax are found in different chromosomes (chromosome 12 in P. falciparum and 14 in all the six species with genomic information); the surrounding proteins are also conserved strengthening the hypothesis that these are indeed orthologous genes.
The repeated domains of MSP-9 varied in length, motif composition and number of repetitions among the species studied (Table 1 and Fig. 1). P. falciparum and its relative in African Apes, P. reichenowi, showed two repetitive regions with a similar pattern of motifs. The first repetitive region (R1) in the N terminal was characterized by VNDE(E/D)D and TND(E/D)ED motifs in P. falciparum and by a (V/L)NDEED in P. reichenowi; while the second region in the C terminal had blocks of KE and KEE repeats in both parasites. On the other hand, a repetitive C-terminal end pattern was not maintained in the three rodent parasites since only P. yoelii displayed a repetitive region (R2) of NDKVEDESKPSEST, GDKIEDESETSKST and DDKVEDESKPSEST motifs, but P. berghei and P. chabaudi did not show such a complex pattern; however, P. berghei did have the R1 region similar to the one found in P. yoelii (Table 1 and Fig. 1).
Table 1.
MSP-9 repetitive regions in all species analyzed (only complete sequences are included). The most abundant amino acids are shown in percentage.
| Species (Isolates) | aa composition in protein | LCR position | aa composition in LCR | Repetitive regions | |||||
|---|---|---|---|---|---|---|---|---|---|
| P. falciparum (19) | E | K | N | 209-278 | D | E | N | T | R1: [VNDE(E/D)D]2[TND(E/D)ED]6 |
| 15% | 15% | 13% | 30% | 23% | 23% | 10% | |||
| 637-737 | E | K | R2: [KE/KEE]13-18 | ||||||
| 47% | 21% | ||||||||
|
| |||||||||
| P. yoelii (1) | E | K | S | 404-431 | D | V | Y | R1:[YD]4[DD(I/D)(A/V)]3 | |
| 11% | 10% | 10% | 57% | 11% | 14% | ||||
| 584-676 | D | E | S | R2: [(N/G/D)DK(V/I)EDES(K/E)(P/T)S(E/K)ST]4 | |||||
| 13% | 23% | 18% | |||||||
|
| |||||||||
| P. berghei (2) | I | K | S | 404-431 | A | D | R1: [YD][DD(D/T/V)A]4 | ||
| 11% | 10% | 10% | 18% | 54% | |||||
|
| |||||||||
| P. coatneyi (1) | E | K | T | 519-760 | E | T | Q | R1: [DETD]2 | |
| 13% | 10% | 8% | 19% | 15% | 14% | R2: [TDADQKEVSETE(Q/P)NT]3[AH(V/G)DQQEVSETE(Q/P)NT]8 | |||
|
| |||||||||
| P. gonderi (1) | E | K | 475-653 | E | V | R1: [QTEE(I/L)]3 | |||
| 14% | 12% | 23% | 16% | ||||||
|
| |||||||||
| P. knowlesi (8) | E | K | 466-506 | D | E | R1: [D(L/E)(E/N)D]2 | |||
| 15% | 12% | 24% | 20% | ||||||
| 526-701 | E | Q | T | R2: [VEPE(Q/P)(T/I)(E/Q)E(T/A)Q(N/K)T]1-12 | |||||
| 30% | 15% | 21% | |||||||
|
| |||||||||
| P. simiovale (1) | E | K | V | 450-743 | E | V | R1: DTAE[DTVE]2DTTD | ||
| 13% | 9% | 9% | 19% | 15% | |||||
|
| |||||||||
| P. fieldi (1) | E | K | V | 445-597 | E | V | R1: EDTAGDTV[D(N/D)ATEDT(I/A)]3DDAT | ||
| 13% | 8% | 8% | 21% | 13% | |||||
|
| |||||||||
| P. cynomolgi (8) | A | E | V | 477-818 | A | E | V | R1Bstrain: [DAT(I/S)DA(V/T)N]2 | |
| 9% 1 | 6% | 11% | 17% | 25% | 20% | R1Berok: [EEA(T/A)N(Y/G/D)TAVDTV]2-3[EDATNDTV]0-2 | |||
| R2Bstrain: [VGTEE(V/E)E(A/V)VPEN(A/V/G)E]4-5[V(A/V/E)PEN(T/A)E]5-9 | |||||||||
| R2Berok: [PE(N/H)AEV(A/V/T)]5-10 [PENDEVV]1-5 | |||||||||
|
| |||||||||
| P. vivax (20) | A | E | V | 466-916 | A | E | V | R1: [DET(V/M)]3-6 | |
| 10% | 17% | 14% | 18% | 26% | 21% | R2: [VPENV(E/G)AVTETEEVVE]1-3[(A/V)(A/V)PEN(A/T)EVATEEVVEA(T/P/V)PE(N/D)AEA]1-3 | |||
| R3: [(A/V)VPE(H/N)(T/A)EVATEVVE]2-3[A(V/A)PENA(E/D)]2-7[PVHENA(E/D)]1-6 | |||||||||
| R4: [AVPE(S/N)(V/A/T)E]3-7[AVTEA(V/A)E]2-7 | |||||||||
In other Plasmodia such as P. gonderi, P. simiovale and P. fieldi, only one region of low complexity (repetitive region) was found in their MSP-9 orthologs. Two other non-human primate malarias, P. coatneyi and P. knowlesi, show two repetitive regions in their MSP-9 (R1 and R2) with a pattern of repetitive motifs in a relatively extended region, R2 (Fig. 1). In human Plasmodium species, different patterns of repetitions were observed for P. falciparum and P. vivax (Table 1). Whereas PfMSP-9 only had two low complexity regions, we identified four distinctive repetitive (low complexity) regions in PvMSP-9 that expand in the C terminal (Table 1 and Supplementary Table S1, Fig. 2 and Supplementary Fig. S1). A first repetitive region (R1) displayed a DET(V/M) motif with homologous regions in related species (Supplementary Fig. S1A). The second repetitive region (R2) had the motifs: VPENV(E/G)AVTETEEVVE, AAPENAEVATEEVVEA(T/P)PENAEA or VVPEN(T/A)EVATEEVVEAVPEDAEA and the third repetitive region (R3) had repetitions of (A/V)VPE(H/N)(T/A)EVATEVVE, A(V/A)PENA(E/D) or PVHENA(E/D). Moreover, PvMSP-9 exhibit extensive polymorphism in terms of the number of repeats (Fig. 2). Homologous R2 and R3 regions were only found in P. cynomolgi which is the closest related species to P. vivax (Supplementary Fig. S1B) (Escalante et al., 2005). We also observe that there are two major R2 and R3 patterns within P. cynomolgi that separate the strains into two distinctive clades; however, none of the P. cynomolgi isolate studied has the last repetitive region (R4) that contains mainly heptapeptides (Supplementary Fig. S1B). This R4 forms coiled-coil structures in the protein (Vargas-Serrato et al., 2002). We also found two conserved blocks in PvMSP-9 between R2 - R3 and R3 - R4 of 28 and 30 amino acids, respectively.
Fig. 2.
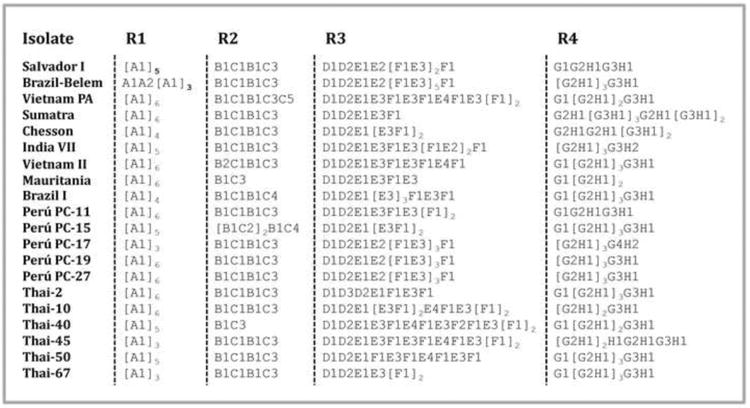
Repetitive regions (R1, R2, R3 and R4) of PvMSP-9. Letters A-H represent repetitive motifs and numbers next to the letters represent motifs with non-synonymous substitutions (see Supplementary Table S1).
3.2 Polymorphism in putative T cell epitopes
One important step for malaria vaccine development is to identify T-cell epitopes that can interact promiscuously with a broad range of HLA class II molecules, as the ones described in PvMSP-9 (Lima-Junior et al., 2008). Epitopes pE, pJ, pK, pH and pL were conserved (100% even at nucleotide level) in all P. vivax isolates analyzed in this study, those have been previously shown to induce the highest cellular response in natural exposed individuals (Lima-Junior et al., 2012). In contrast, we found high nucleotide diversity (n=8, π=0.069, dS-dN=0.1, p>0.05) but with an excess of synonymous over non-synonymous polymorphisms for all putative epitopes (pE, pJ, pH-pK-pL) in P. cynomolgi.
Moreover, when performing the species alignment (Fig. 3), we found high similarity between these peptides in P. vivax and in the closely related species. However, from all five peptides analyzed, the region of the alignment corresponding to peptide pJ had the highest similarity among all the species in the P. vivax clade included in this study (Fig. 3), ranging from 67% (P. gonderi with P. vivax) to 87% between P. cynomolgi and P. vivax. Even further, this peptide was conserved between P. vivax and P. falciparum (73%). Regarding the overlapping peptides pK, pH and pL (Fig. 3), we observed that their highly immunogenic core ASIDSMI (Lima-Junior et al., 2012) was not as conserved as the carboxy-terminal region (DEIDFYEK) of peptide pL, which was found in all non-human primate Plasmodium species (EEIDFYEK) and in P. falciparum and P. reichenowi with two amino acid replacements (DEIDFFEK).
Fig. 3.

Alignment of putative T-cell epitopes of PvMSP-9 (Lima-Junior et al., 2008), including different Plasmodium species. The positions underlined correspond to the carboxy-terminal region of peptide pL.
On the other hand, the epitope ABRA#14 (DSNIMNSINNVMDEIDFFEK), located in positions 487-506 of PfMSP-9 and previously identified as a HLA-DQ-binding peptide that also induced T-cell proliferation and Th1-associated cytokine production in DQ8+ transgenic mice (Pimtanothai et al., 2000), was only found in P. reichenowi but not in the other species included in this study.
3.3 Genetic diversity and phylogenetic analyses
Table 2 describes the genetic variation found in different domains of msp-9 across Plasmodium spp. When we compared the genetic diversity found in PvMSP-9 and PfMSP-9 with the polymorphism observed in non-human primate malarias (P. cynomolgi and P. knowlesi), we found that P. cynomolgi had the highest nucleotide diversity (π=0.069), and P. falciparum the lowest (π=0.001). In addition, the difference between synonymous and non-synonymous substitutions in msp-9 showed an excess of synonymous over non-synonymous polymorphisms in P. vivax, P. cynomolgi and P. knowlesi, a pattern consistent with purifying selection. In these species the evidence of purifying selection found in the overall gene seems to be driven by the C-terminal of the gene. When analyzing samples from Peru (n=5, π=0.005, dS-dN=0.007, p=0.023) and Thailand (n=6, π=0.005, dS-dN=0.011, p=0) we also found an excess of synonymous over non-synonymous substitutions in PvMSP-9. Instead, in P. falciparum, the signature of selection on the gene was positive (consistent with balancing selection) mainly at the polymorphism found on the region previously described as RBC binding (Fig. 1). We also analyzed the patterns of variation on MSP-9 using the McDonald and Kreitman test by comparing P. vivax with P. cynomolgi and also by comparing P. falciparum with P. reichenowi. However, we found no evidence of natural selection acting on the gene by making these comparisons.
Table 2.
Polymorphisms found in the gene encoding MSP-9. Statistical significance is indicated in bold.
| Species | Length (bp) | Π (S.E.) | Ds (S.E.) | Dn (S.E.) | Ds-Dn (S.D.) | p (Z-stat) |
|---|---|---|---|---|---|---|
| P. falciparum (n=18) | ||||||
| Gene+ | 2070 | 0.001(0.001) | 0.001(0) | 0.003(0.001) | -0.002(0.001) | 0.013 (2.525) |
| RBC binding region | 1266 | 0.001(0.001) | 0(0) | 0.002(0.001) | -0.002(0.001) | 0.032 (2.164) |
|
| ||||||
| P. vivax (n=17) | ||||||
| Gene+ | 1821 | 0.005(0.001) | 0.012(0.005) | 0.003(0.001) | 0.008(0.003) | 0.01 (-2.610) |
| RBC binding region* | 1317 | 0.003(0.001) | 0.003(0.002) | 0.003(0.001) | 0.001(0.002) | 0.730 (-0.34) |
|
| ||||||
| P. cynomolgi (n=8) | ||||||
| Gene+ | 2105 | 0.069(0.004) | 0.199(0.018) | 0.055(0.005) | 0.145(0.019) | 0 (-7.805) |
| RBC binding region* | 427 | 0.043(0.003) | 0.162(0.019) | 0.023(0.003) | 0.139(0.021) | 0 (-7.261) |
|
| ||||||
| P. knowlesi (n=8) | ||||||
| Gene+ | 1710 | 0.008(0.001) | 0.021(0.005) | 0.005(0.002) | 0.016(0.005) | 0.002 (-3.122) |
| RBC binding region* | 918 | 0.006(0.001) | 0.013(0.050) | 0.005(0.002) | 0.009(0.005) | 0.1 (-1.66) |
Corresponds to alignable regions (within species) of the gene without repetitions.
Corresponds to the P. falciparum homologous region in the alignment.
The phylogeny of MSP-9 is depicted in Figure 4 using DNA sequences and it is consistent with previous published phylogenies that used house-keeping, mitochondrial, or antigen encoding genes (Escalante et al., 1998, 2004; Pacheco et al., 2007; 2010; 2012). As expected, we observed that P. vivax and related species in nonhuman primates are a monophyletic group with P. cynomolgi and P. vivax; P. knowlesi and P. coatneyi; and P. simiovale and P. fieldi as sister clades. The phylogenetic base methods as implemented in HyPhy gave an overall omega lower than one (ω < 1) that is consistent with purifying selection acting on this gene. Indeed, the branch leading to P. vivax had a value of 0.165 (CI: 0.130-0.199). We also compared this omega value with the one derived from the mitochondrial genes phylogeny (Pacheco et al., 2011) and the results obtained showed a similar omega value of 0.162 (CI: 0.128–0.197). All results indicating negative (purifying) selection were significant as determined by the likelihood ratio test results. In addition, we found no indication of episodic selection on P. vivax.
Fig. 4.
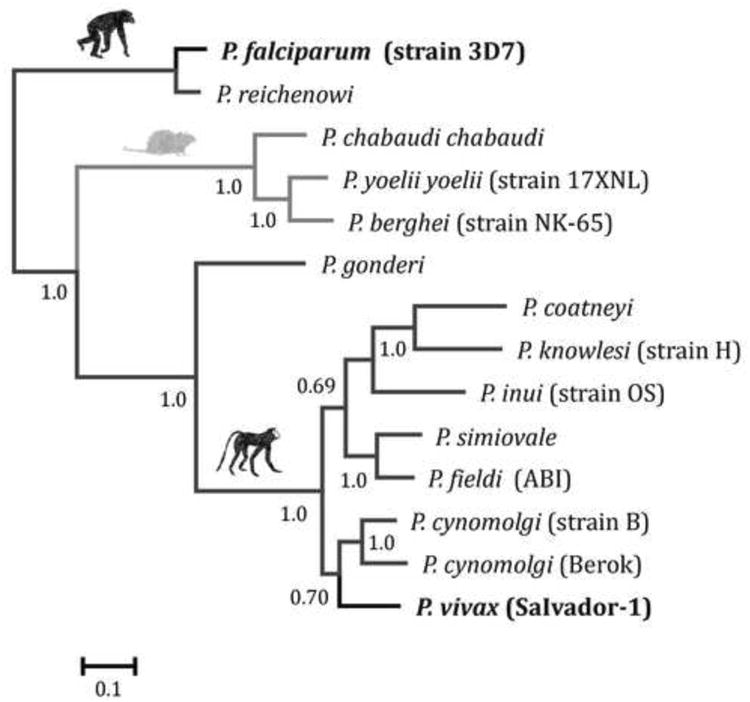
Bayesian phylogenetic tree of the gene encoding MSP-9 using MrBayes. The phylogeny was estimated using solely alignable sites on the N-terminal (1,294 bp). The numbers on nodes of the tree are posterior probabilities represented as percentage based on 3×106 Markov Chain Monte Carlo (MCMC) steps; the first 1,000,000 (50%) were discarded as a burn-in. Sampling was performed every 100 generations.
3.4 Protein characteristics and predicted structure
According to the secondary structure predicted results, there are changes in the percentage of coil elements (loops) found in the protein across the phylogeny (Table 3). Whereas P. falciparum has a greater percentage of helices, P. vivax and related species have an increased amount of loops (Table 3). Regarding the solvent accessibility prediction, the percentage of exposed residues in MSP-9 ranged from 72-84% in different Plasmodium species with a predominance of glutamic acid, lysine, and valine in the amino acid composition.
Table 3.
Results of MSP-9 secondary protein structure (H: helix, E: extended sheet, L: loop) and solvent accessibility prediction (e: exposed residues, b: buried residues). In bold are labeled the most common amino acids.
| Species | Secondary structure (% in protein) | Solvent accessibility (% in protein) | aa composition (% of residues) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | E | L | b | E | |||||||||||
| P. falciparum | 81.73 | 1.49 | 16.78 | 24.63 | 75.37 | A: 2.8 | C: 0.8 | D: 6.9 | E: 14.6 | F: 2.6 | G: 3.0 | H: 1.1 | I: 5.5 | K: 14.5 | L: 8.7 |
| M: 3.0 | N: 12.9 | P: 2.4 | Q: 4.1 | R: 0.5 | S: 3.9 | T: 4.2 | V: 5.4 | W: 0.1 | Y: 3.0 | ||||||
|
| |||||||||||||||
| P. yoelii | 31.27 | 6.64 | 62.09 | 28.17 | 71.83 | A: 2.8 | C: 0.9 | D: 8.8 | E: 10.8 | F: 1.5 | G: 4.6 | H: 0.7 | I: 9.4 | K: 9.9 | L: 9.0 |
| M: 1.5 | N: 8.7 | P: 3.4 | Q: 2.2 | R: 1.9 | S: 9.9 | T: 5.6 | V: 5.2 | W: 0.0 | Y: 3.2 | ||||||
|
| |||||||||||||||
| P. coatneyi | 20.29 | 2.36 | 77.36 | 15.71 | 84.29 | A: 4.2 | C: 0.5 | D: 6.8 | E: 12.7 | R: 0.8 | G: 5.5 | H: 2.9 | I: 6.4 | K: 9.9 | L: 7.3 |
| M: 2.5 | N: 6.2 | P: 2.1 | Q: 6.9 | S:5.1 | T: 8.4 | V: 8.0 | W: 0.0 | Y: 1.8 | |||||||
|
| |||||||||||||||
| P. knowlesi | 14.12 | 3.42 | 82.45 | 18.54 | 81.46 | A: 2.4 | C: 0.7 | D: 5.3 | E: 15.1 | F: 2.3 | G: 6.0 | H: 2.1 | I: 6.7 | K: 12.3 | L: 8.0 |
| M: 3.0 | N: 6.1 | P: 3.0 | Q: 5.6 | R: 0.4 | S: 3.3 | T: 8.4 | V: 7.1 | W: 0.0 | Y: 2.1 | ||||||
|
| |||||||||||||||
| P. cynomolgi | 14.71 | 2.7 | 82.6 | 15.44 | 84.56 | A: 9.1 | C: 0.5 | D: 7.0 | E: 15.9 | F: 2.5 | G: 5.0 | H: 2.0 | I: 5.4 | K: 8.3 | L: 6.1 |
| M: 2.8 | N: 7.0 | P: 3.7 | Q: 2.6 | R: 0.9 | S: 3.2 | T: 5.5 | V: 11 | W: 0.0 | Y: 1.6 | ||||||
|
| |||||||||||||||
| P. vivax | 23.14 | 5.57 | 71.29 | 22.6 | 77.4 | A: 10.4 | C: 0.4 | D: 6.1 | E: 17.1 | F: 2.1 | G: 4.3 | H: 2.6 | I: 4.2 | K: 7.4 | L: 5.6 |
| M: 2.2 | N: 5.8 | P: 3.9 | Q: 2.3 | R: 0.6 | S: 3.1 | T: 6.9 | V: 13.5 | W: 0.0 | Y: 1.6 | ||||||
We also obtained the predicted tertiary structure of PfMSP-9 (C-score=-2.53 and TM-score=0.42±0.14) and PvMSP-9 (C-score=-0.51 and TM-score=0.65±0.13) (Supplementary Fig. S2). The protein is depicted in rainbow coloring scheme Blue (N-terminus) to Red (C-terminus). In P. falciparum, we observed that the greatest percentage of alpha helices and beta strands were found at the N-terminal of the protein; while a long region of coil elements, indicating an unstructured or disordered region was found at the C-terminal, corresponding to the repetitive region of the protein (LCR). In addition, when analyzing the predicted solvent accessibility results, we also found that the N-terminal part was the one containing most of the buried residues (Supplementary Fig. S3) which delineates the core region of the protein, whereas regions with solvent exposed and hydrophilic residues such as the ones found at the LCR are potential hydration/functional sites. To further explore protein function, we analyze the predicted ligand binding sites of PvMSP-9 reported by I-TASSER. The highest confidence of the binding site score (0.69) was observed when the template 1w36b was compared with the query protein. When the BS-score is greater than 0.5 and the predicted binding site residues are clustered together, then the confidence level of the prediction is usually high. Based on this score, we found four predicted binding site residues in P. vivax: 449D, 454Y, 455E and 456K; which correspond to the carboxy-terminal region (DEIDFYEK, Fig. 3) of the immunogenic peptide pL (Lima-Junior et al., 2008). These putative binding site residues were also found in P. cynomolgi, P. inui, P fieldi, P. simiovale, P. knowlesi, P. gonderi and P. coatneyi with only one substitution in position 449 (E/D); it is worth noting that both amino acids are acid.
4. Discussion
Merozoite surface protein-9 is a promising vaccine candidate antigen found in rodents, non-human primates and human Plasmodium species. Like previous studies (Vargas- Serrato et al., 2002), the common features founded in all the species were that glutamic acid (E) and lysine (K) were the most predominant amino acid in this protein (Table 1), four cysteines were highly conserved at the N-terminus, and the C-terminus exhibited great polymorphism, with repetitive regions clearly defined in most species (Fig. 1). Neither the four conserved cysteines nor the tandem repeat region, appear to participate directly in the PfMSP-9-5ABC interaction (Li et al., 2004); however, they might confer specific structural characteristics to the protein. It has been suggested that the N-terminus interacts with red blood cells by binding the band 3 protein in association with PfMSP-1 (Kushwaha et al., 2002). Moreover, when we compared the five erythrocyte high activity binding peptides (HABPs) found on positions 121-240 in P. falciparum (Curtidor et al., 2001) against P. reichenowi, which are part of ABRA(P) and Δ1 peptides (Li et al., 2004; Kushwaha et al., 2002), they were highly conserved between both species (121LQSHKKLIK(A/G)LKKNIESIQN140, 141KK(H/V)L(I/V)YKNKSYNPLLL(S/T)(C/F)VK160, 161KMNMLKENVDYIQKNQNLFK180, 201LKSqGHKKETSqNQNEN(n/K)DN220 and 221QKYQEVNDE(D/E)(D/E)VNDEE(D/E)(T/V)ND240) but not in the species conformed by P. vivax and other non-human primate malaria; this finding suggests that P. falciparum and P. vivax may have a different way of interacting with reticulocytes and that other peptides might be involved.
According to the protein predicted results, residues (454Y, 455E and 456K) found in P. vivax might be important ligand-binding sites. These residues were conserved not only in all P. vivax isolates but also in P. cynomolgi, P. inui, P. fieldi, P. simiovale, P. knowlesi, P. gonderi and P. coatneyi. Interestingly, these residues are part of the immunogenic peptide DEIDFYEK (Fig. 3). In addition, its predicted structure is mainly composed of coil elements and exposed residues; these characteristics make it a peptide to be considered in studies aiming to understand the immunogenicity of PvMSP-9 in naturally exposed populations. Although the peptide pJ is highly conserved in all malarial species (Fig. 1 and 3), including P. falciparum, its predictive solvent accessibility suggests that it is mostly “buried” and appear to be part of the core domain of the protein (Lima-Junior et al., 2008).
It is worth noting that MSP-9, in P. vivax and related species, has repetitive motifs or low complexity regions at the C-terminal of the gene, these regions are relatively large when compared with other Plasmodium including P. falciparum. The only other species with similar low complexity regions is P. cynomolgi, its closest known related species (Escalante et al., 2005), indicating that these regions originated before P. vivax became a human parasite. Like other repetitive proteins in Plasmodium spp. (Rodriguez et al., 2008), PvMSP-9 has extensive polymorphism in the number of motifs; however, when the repetitive motifs are aligned among them (see Rich et al., 1997) the motifs themselves accumulate more synonymous than non-synonymous substitutions (Table 4). These repeated structures in proteins often occur in linear arrays or as a superhelix (Andrade et al., 2001) such as in MSP-9. The coil-coiled structures at the C-terminus end of PfMSP-9 and PvMSP-9 exhibit an extensive solvent-accessible surface that could be well suited to binding large substrates (Andrade et al., 2001). In malaria, it has been proposed that repeat regions may function as ligands for host proteins, or serve to suppress the development of immunity through a strategy of serological cross reactivity (Nussenzweig V and Nussenzweig R.S., 1989). Furthermore, certain domains might provide an activation signal to B cells, as the first step of a strategy to force them down an inappropriate (T-cell-independent) differentiation pathway (Schofield, 1991). Even though there is no direct evidence of this phenomenon in MSP-9, there are studies indicating that this protein is immunogenic.
Table 4.
Polymorphisms found in the low complexity region of the gene encoding PvMSP-9. Descriptions of the repetitive regions are in Table 1 (R1, R2, R3 and R4). Statistical significance is indicated in bold.
| Repetitive regions | Π (S.E.) | Ds-Dn (S.D.) | p (Z-stat) |
|---|---|---|---|
| RLC1 | 0.037±0.034 | 0.219±0.168 | 0.189 (-1.321) |
| RLC2 | 0.085±0.018 | 0.322±0.174 | 0.001 (-3.308) |
| RLC3A* | 0.085±0.031 | 0.214±0.194 | 0.054 (-1.949) |
| RLC3B* | 0.074±0.034 | 0.189±0.204 | 0.259 (-1.134) |
| RLC3C* | 0.001±0.001 | 0.002±0.002 | 0.270 (1.108) |
| RLC4 | 0.056±0.020 | 0.138±0.115 | 0.096 (-1.675) |
| RLC1+RCL2+RCL3+RLC4 | 0.015±0.002 | 0.038±0.010 | 0.000 (-4.053) |
A, B and C correspond to each repetitive motif of R4 (Table 1)
For instance, experiments in rabbits found that peptides based on PfMSP-9 residues covering the most hydrophilic regions and a peptide representing its repetitive sequence, elicited antibody responses and exerted a strong inhibitory effect on the merozoite invasion of erythrocytes (Sharma et al., 1998). Moreover, immunogenicity studies with recombinant PfMSP-9 proteins in rabbits and mice indicated that T-cell epitopes were localized in the middle portion of the protein (Pimtanothai et al., 2000; Kushwaha et al., 2001). Similar studies in P. vivax using the MSP-9 N-terminal and the second block of repeats (PvMSP-9-RII) generated an immunological response with high titers of specific antibodies that recognized the parasite's protein (Oliveira-Ferreira et al., 2004; Lima-Junior et al., 2012). The actual motifs of this LCR accumulate more synonymous than non-synonymous substitutions indicating some functional constrain. On the other hand, the N-terminal region of PvMSP-9 induced IFN-γ and IL-4 cytokine responses, confirming the presence of T-cell epitopes in this region of the protein (Lima-Junior et al., 2010). The presence of these T cell epitopes (Lima-Junior et al., 2010) indicates that the response induced by the LCRs could be low grade and non-neutralizing (Schofield 1991). Overall, there is an elicited immune response which association with protection in P. falciparum and/or P. vivax remains to be determined.
Generally, there is relatively high genetic diversity on individual functional proteins expressed on the surface of the merozoite and that are involved in the invasion of the red blood cell (Escalante et al., 1998, 2004; VanBuskirk et al., 2004); this is not the case for MSP-9 (Table 2) where there was limited variation outside the low complexity regions. However, the fact that this protein could also be involved in the protein complex for red blood cell invasion may select against the accumulation of polymorphism in those non-repetitive regions. Studying the role that natural selection may play in the absence of polymorphism is difficult simply because it could be the result of demographic processes such as population expansions after a bottleneck. This situation is not unique to MSP-9, other merozoite proteins such as merozoite surface protein-8 (MSP-8) and merozoite surface protein-10 (MSP-10) show very limited genetic polymorphism (Pacheco et al., 2012). Establishing whether such conserved parts of the protein are indeed under purifying selection is important since they may offer valuable targets for antimalarial vaccines if protective immune responses could be elicited against them (Baum et al., 2003; Cole-Tobian and King, 2003; Martinez et al., 2004; Pacheco et al., 2010; 2012). Based on our knowledge of MSP-9 from P. falciparum, we could speculate that in PvMSP-9 the putative binding sites are conserved by purifying selection in order to interact with MSP-119 kDa fragment in a complex that binds the red blood cell. The analysis of genetic diversity of MSP-119 and MSP-133 regions in P. vivax and P. falciparum confirmed that MSP-119 is less polymorphic than MSP-133 in both human malarial parasites (Pacheco et al., 2007). Moreover, in P. vivax and related species, there were more synonymous over non-synonymous substitutions in MSP-9, which contrasts to what has been previously reported in other antigenic proteins (Escalante et al., 1998; Tetteh et al., 2009). This suggests that the repetitive parts of this protein are under strong functional constraints and implies a much more complex interaction between merozoite surface proteins that could result in a different host's immunological response.
5. Conclusion
We found evidence consistent with purifying selection in the gene encoding PvMSP-9. Our findings could indicate functional constraint for maintaining the protein's structure in P. vivax and related species due to a role in the invasion process. We also observed a different pattern in P. falciparum, where the observed polymorphism (particularly in the N-terminal) could be explained by balancing selection. Thus, the genetic polymorphism found in the MSP9 orthologs of the two major human parasites may be the result of different mechanisms of interaction with the host. It is worth noting that it has been hypothesized that natural selection is less efficient when acting at the blood stage of P. vivax than P. falciparum (Schneider and Escalante 2013). Such predictions, together with the contrasting patterns observed in orthologous merozoite proteins such as MSP-1, MSP-10 (Pacheco et al 2007; 2012) and the one studied here; further reinforce the notion that the differences between P. falciparum and P. vivax need to be considered so findings in one species are not simply translated to the other.
Supplementary Material
Supplementary Fig. S1. A) Alignment of repetitive region 1 (R1) in P. vivax and related species. B) Alignment of repetitive regions R2-R4 of P. vivax and P. cynomolgi.
Supplementary Fig. S2. Predicted results of MSP-9 tertiary structure representing putative protein domains (A and C) and β-strands (yellow), α-helices and coil-coiled structures (pink) (B and D) in P. falciparum and P. vivax.
Supplementary Fig. S3. Protein prediction results in A) P. falciparum and B) P. vivax. PROF_sec: secondary structure prediction with a reliability index greater than 5 (H: helix, E: extended sheet, L: loop). PROF_acc: accessibility prediction with a reliability index greater than 5 (e: exposed residues, i: intermediate and b: all other residues).
Supplementary Table S1. Amino acid motifs in repetitive regions R1, R2, R3 and R4 of PvMSP-9. Substitutions in the nucleotide sequence are in parentheses; non-synonymous substitutions are in bold.
Highlights.
We investigated the gene encoding the merozoite surface protein (MSP-9) in several Plasmodium spp.
Repeated domains in malarial parasites species varied in length, motif composition and number of repetitions.
Natural selection is operating differently in P. falciparum and P. vivax.
We found evidence of purifying selection on P. vivax and related species.
Acknowledgments
This work was supported by the US National Institutes of Health (R01 GM080586 to AAE). We thank the DNA laboratory at the School of Life Sciences for their technical support. We also thank Jane Carlton for providing access to 454 preliminary data from P. inui and P. gonderi. The content is solely the responsibility of the authors and does not represent the official views of the NIH, the Centers for Disease Control and Prevention, Department of the Navy, Department of Defense, or the US Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stella M. Chenet, Email: schenet@asu.edu.
M. Andreína Pacheco, Email: Maria.Pacheco@asu.edu.
David J. Bacon, Email: david.bacon@nrl.navy.mil.
William E. Collins, Email: wec1@cdc.gov.
John W. Barnwell, Email: wzb3@cdc.gov.
References
- Andrade MA, Perez-Iratxeta C, Ponting CP. Protein repeats: structures, functions, and evolution. J Struct Biol. 2001;134:117–131. doi: 10.1006/jsbi.2001.4392. [DOI] [PubMed] [Google Scholar]
- Barnwell JW, Galinski MR, DeSimone SG, Perler F, Ingravallo P. Plasmodium vivax, P. cynomolgi, and P. knowlesi: identification of homologue proteins associated with the surface of merozoites. Exp Parasitol. 1999;91:238–249. doi: 10.1006/expr.1998.4372. [DOI] [PubMed] [Google Scholar]
- Baum J, Thomas AW, Conway DJ. Evidence for diversifying selection on erythrocyte-binding antigens of Plasmodium falciparum and P. vivax. Genetics. 2003;163:1327–1336. doi: 10.1093/genetics/163.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coatney RG, Collins WE, Warren M, Contacos PG. The Primate Malaria. US Government Printing Office; Washington DC: 1971. [Google Scholar]
- Cole-Tobian J, King CL. Diversity and natural selection in Plasmodium vivax Duffy binding protein gene. Mol Biochem Parasitol. 2003;127:121–132. doi: 10.1016/s0166-6851(02)00327-4. [DOI] [PubMed] [Google Scholar]
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Curtidor H, Urquiza M, Suarez JE, Rodriguez LE, Ocampo M, Puentes A, Garcia JE, Vera R, Lopez R, Ramirez LE, Pinzon M, Patarroyo ME. Plasmodium falciparum acid basic repeat antigen (ABRA) peptides: erythrocyte binding and biological activity. Vaccine. 2001;19:4496–4504. doi: 10.1016/s0264-410x(01)00202-x. [DOI] [PubMed] [Google Scholar]
- Escalante AA, Lal AA, Ayala FJ. Genetic polymorphism and natural selection in the malaria parasite Plasmodium falciparum. Genetics. 1998;149:189–202. doi: 10.1093/genetics/149.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante AA, Cornejo OE, Rojas A, Udhayakumar V, Lal AA. Assessing the effect of natural selection in malaria parasites. Trends Parasitol. 2004;20:388–395. doi: 10.1016/j.pt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Escalante AA, Cornejo OE, Freeland DE, Poe AC, Durrego E, Collins WE, Lal AA. A monkey's tale: the origin of Plasmodium vivax as a human malaria parasite. Proc Natl Acad Sci U S A. 2005;102:1980–1985. doi: 10.1073/pnas.0409652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki MM, Li X, Yamodo I, Chishti AH, Oh SS. Two Plasmodium falciparum merozoite proteins binding to erythrocyte band 3 form a direct complex. Biochem Biophys Res Commun. 2005;338:1690–1695. doi: 10.1016/j.bbrc.2005.10.154. [DOI] [PubMed] [Google Scholar]
- Kosakovsky Pond SL, Frost SD, Muse SV. HyPhy: hypothesis testing using phylogenies. Bioinformatics. 2005;21:676–679. doi: 10.1093/bioinformatics/bti079. [DOI] [PubMed] [Google Scholar]
- Kosakovsky Pond SL, Murrell B, Fourment M, Frost SD, Delport W, Scheffler K. A random effects branch-site model for detecting episodic diversifying selection. Molecular Biology and Evolution. 2011 doi: 10.1093/molbev/msr125. first published online June 13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha A, Rao PP, Suresh RP, Chauhan VS. Immunogenicity of recombinant fragments of Plasmodium falciparum acidic basic repeat antigen produced in Escherichia coli. Parasite Immunol. 2001;23:435–44. doi: 10.1046/j.1365-3024.2001.00390.x. [DOI] [PubMed] [Google Scholar]
- Kushwaha A, Perween A, Mukund S, Majumdar S, Bhardwaj D, Chowdhury NR, Chauhan VS. Amino terminus of Plasmodium falciparum acidic basic repeat antigen interacts with the erythrocyte membrane through band 3 protein. Mol Biochem Parasitol. 2002;122:45–54. doi: 10.1016/s0166-6851(02)00077-4. [DOI] [PubMed] [Google Scholar]
- Li X, Chen H, Oo TH, Daly TM, Bergman LW, Liu SC, Chishti AH, Oh SS. A co-ligand complex anchors Plasmodium falciparum merozoites to the erythrocyte invasion receptor band 3. J Biol Chem. 2004;13:5765–5771. doi: 10.1074/jbc.M308716200. [DOI] [PubMed] [Google Scholar]
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Lima-Junior JC, Tran TM, Meyer EV, Singh B, De-Simone SG, Santos F, Daniel-Ribeiro CT, Moreno A, Barnwell JW, Galinski MR, Oliveira-Ferreira J. Naturally acquired humoral and cellular immune responses to Plasmodium vivax merozoite surface protein 9 in Northwestern Amazon individuals. Vaccine. 2008;26:6645–6654. doi: 10.1016/j.vaccine.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Junior JC, Banic DM, Tran TM, Meyer VS, De-Simone SG, Santos F, Porto LC, Marques MT, Moreno A, Barnwell JW, Galinski MR, Oliveira-Ferreira J. Promiscuous T-cell epitopes of Plasmodium merozoite surface protein 9 (PvMSP-9) induces IFN-gamma and IL-4 responses in individuals naturally exposed to malaria in the Brazilian Amazon. Vaccine. 2010;28:3185–3191. doi: 10.1016/j.vaccine.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Junior JC, Rodrigues-da-Silva RN, Banic DM, Jiang J, Singh B, Fabrício-Silva GM, Porto LC, Meyer EV, Moreno A, Rodrigues MM, Barnwell JW, Galinski MR, de Oliveira-Ferreira J. Influence of HLA-DRB1 and HLA-DQB1 alleles on IgG antibody response to the P. vivax MSP-1, MSP-3a and MSP-9 in individuals from Brazilian endemic area. PLoS One. 2012;7:e36419. doi: 10.1371/journal.pone.0036419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopera-Mesa TM, Kushwaha A, Mohmmed A, Chauhan VS. Plasmodium berghei merozoite surface protein-9: immunogenicity and protective efficacy using a homologous challenge model. Vaccine. 2008;26:1335–1343. doi: 10.1016/j.vaccine.2007.12.042. [DOI] [PubMed] [Google Scholar]
- Martinez P, Suarez CF, Cardenas PP, Patarroyo MA. Plasmodium vivax Duffy binding protein: a modular evolutionary proposal. Parasitology. 2004;128:353–366. doi: 10.1017/s0031182003004773. [DOI] [PubMed] [Google Scholar]
- McDonald J, Kreitman M. Adaptive protein evolution at the Adh locus in Drosophila. Nature. 1991;351:652–654. doi: 10.1038/351652a0. [DOI] [PubMed] [Google Scholar]
- Nei M, Kumar S. Molecular Evolution and Phylogenetics. Oxford University Press; New York: 2000. [Google Scholar]
- Nussenzweig V, Nussenzweig RS. Rationale for the development of an engineered sporozoite malaria vaccine. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- Nwagwu M, Haynes JD, Orlandi PA, Chulay JD. Plasmodium falciparum: chymotryptic-like proteolysis associated with a 101-kDa acidic-basic repeat antigen. Exp Parasitol. 1992;75:399–414. doi: 10.1016/0014-4894(92)90253-7. [DOI] [PubMed] [Google Scholar]
- Oliveira-Ferreira J, Vargas-Serrato E, Barnwell JW, Moreno A, Galinski MR. Immunogenicity of Plasmodium vivax merozoite surface protein-9 recombinant proteins expressed in E. coli. Vaccine. 2004;22:2023–2030. doi: 10.1016/j.vaccine.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Pacheco MA, Poe AC, Collins WE, Lal AA, Tanabe K, Kariuki SK, Udhayakumar V, Escalante AA. A comparative study of the genetic diversity of the 42kDa fragment of the merozoite surface protein 1 in Plasmodium falciparum and P. vivax. Infect Genet Evol. 2007;7:180–187. doi: 10.1016/j.meegid.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco MA, Ryan EM, Poe AC, Basco L, Udhayakumar V, Collins WE, Escalante AA. Evidence for negative selection on the gene encoding rhoptry-associated protein 1 (RAP-1) in Plasmodium spp. Infect Genet Evol. 2010;10:655–661. doi: 10.1016/j.meegid.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco MA, Battistuzzi FU, Junge RE, Cornejo OE, Williams CV, Landau I, Rabetafika L, Snounou G, Jones-Engel L, Escalante AA. Timing the origin of human malarias: the lemur puzzle. BMC Evol Biol. 2011;11:299. doi: 10.1186/1471-2148-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco MA, Elango AP, Rahman AA, Fisher D, Collins WE, Barnwell JW, Escalante AA. Evidence of purifying selection on merozoite surface protein 8 (MSP8) and 10 (MSP10) in Plasmodium spp. Infect Genet Evol. 2012;12:978–86. doi: 10.1016/j.meegid.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimtanothai N, Parra M, Johnson AH, David CS, Katovich Hurley C. Assessing the binding of four Plasmodium falciparum T helper cell epitopes to HLA-DQ and induction of T-cell responses in HLA-DQ transgenic mice. Infect Immun. 2000;68:1366–73. doi: 10.1128/iai.68.3.1366-1373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner JC, Tran TM, Corredor V, Huber CS, Barnwell JW, Galinski MR. Dramatic difference in diversity between Plasmodium falciparum and Plasmodium vivax reticulocyte binding-like genes. Am J Trop Med Hyg. 2005;72:666–74. [PubMed] [Google Scholar]
- Rich SM, Hudson RR, Ayala FJ. Plasmodium falciparum antigenic diversity: evidence of clonal population structure. Proc Natl Acad Sci USA. 1997;94:13040–45. doi: 10.1073/pnas.94.24.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez LE, Curtidor H, Urquiza M, Cifuentes G, Reyes C, Patarroyo ME. Intimate molecular interactions of P. falciparum merozoite proteins involved in invasion of red blood cells and their implications for vaccine design. Chem Rev. 2008;108:3656–705. doi: 10.1021/cr068407v. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rost B, Yachdav G, Jinfeng L. The PredictProtein server. Nucleic Acids Res. 2004;32:W321–W326. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nature Protocols. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Yang J, Yang Zhang Y. COFACTOR: an accurate comparative algorithm for structure-based protein function annotation. Nucleic Acids Research. 2012;40:W471–W477. doi: 10.1093/nar/gks372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schofield L. On the function of repetitive domains in protein antigens of Plasmodium and other eukaryotic parasites. Parasitol Today. 1991;5:99–105. doi: 10.1016/0169-4758(91)90166-l. [DOI] [PubMed] [Google Scholar]
- Sharma P, Kumar A, Singh B, Bharadwaj A, Sailaja VN, Adak T, Kushwaha A, Malhotra P, Chauhan VS. Characterization of protective epitopes in a highly conserved Plasmodium falciparum antigenic protein containing repeats of acidic and basic residues. Infect Immun. 1998;6:2895–2904. doi: 10.1128/iai.66.6.2895-2904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KA, Escalante AA. Fitness components and natural selection: why are there different patterns on the emergence of drug resistance in Plasmodium falciparum and Plasmodium vivax? Malar J. 2013;11:12–15. doi: 10.1186/1475-2875-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tetteh KK, Stewart LB, Ochola LI, Amambua-Ngwa A, Thomas AW, Marsh K, Weedall GD, Conway DJ. Prospective identification of malaria parasite genes under balancing selection. PLoS One. 2009;4:e5568. doi: 10.1371/journal.pone.0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBuskirk KM, Cole-Tobian JL, Baisor M, Sevova ES, Bockarie M, King CL, Adams JH. Antigenic drift in the ligand domain of Plasmodium vivax duffy binding protein confers resistance to inhibitory antibodies. J Infect Dis. 2004;190:1556–1562. doi: 10.1086/424852. [DOI] [PubMed] [Google Scholar]
- Vargas-Serrato E, Barnwell JW, Ingravallo P, Perler FB, Galinski MR. Merozoite surface protein-9 of Plasmodium vivax and related simian malaria parasites is orthologous to p101/ABRA of P. falciparum. Mol Biochem Parasitol. 2002;120:41–52. doi: 10.1016/s0166-6851(01)00433-9. [DOI] [PubMed] [Google Scholar]
- Vargas-Serrato E, Corredor V, Galinski MR. Phylogenetic analysis of CSP and MSP-9 gene sequences demonstrates the close relationship of Plasmodium coatneyi to Plasmodium knowlesi. Infect Genet Evol. 2003;3:67–73. doi: 10.1016/s1567-1348(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Weber JL, Lyon JA, Wolff RH, Hall T, Lowell GH, Chulay JD. Primary structure of a Plasmodium falciparum malaria antigen located at the merozoite surface and within the parasitophorous vacuole. J Biol Chem. 1988;263:11421–11425. [PubMed] [Google Scholar]
- World Health Organization (WHO) World malaria report 2011. [accessed July 24, 2012];2011 http://www.who.int/malaria/world_malaria_report_2011/en.
- Wootton JC. Non-globular domains in protein sequences: automated segmentation using complexity measures. Comput Chem. 1994;18:269–285. doi: 10.1016/0097-8485(94)85023-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. A) Alignment of repetitive region 1 (R1) in P. vivax and related species. B) Alignment of repetitive regions R2-R4 of P. vivax and P. cynomolgi.
Supplementary Fig. S2. Predicted results of MSP-9 tertiary structure representing putative protein domains (A and C) and β-strands (yellow), α-helices and coil-coiled structures (pink) (B and D) in P. falciparum and P. vivax.
Supplementary Fig. S3. Protein prediction results in A) P. falciparum and B) P. vivax. PROF_sec: secondary structure prediction with a reliability index greater than 5 (H: helix, E: extended sheet, L: loop). PROF_acc: accessibility prediction with a reliability index greater than 5 (e: exposed residues, i: intermediate and b: all other residues).
Supplementary Table S1. Amino acid motifs in repetitive regions R1, R2, R3 and R4 of PvMSP-9. Substitutions in the nucleotide sequence are in parentheses; non-synonymous substitutions are in bold.


