Abstract
Objective
Infection with hepatitis C (HCV) or human immunodeficiency virus (HIV) may be associated with atherosclerosis and vascular disease. Macrophages are a major component of atherosclerotic plaque, and classically activated (M1) macrophages contribute to plaque instability. Our goal was to identify plasma biomarkers that reflect macrophage inflammation and are associated with subclinical atherosclerosis.
Approach and results
We tested whether M1 macrophages produce galectin-3 binding protein (Gal-3BP) in-vitro. Then we measured Gal-3BP and the soluble macrophage biomarkers sCD163 and sCD14 in 264 participants in the Women’s Interagency HIV Study. Women were positive for HIV, HCV, both, or neither (66 in each group, matched for age, race/ethnicity and smoking status). Carotid artery disease was assessed by ultrasound measurement of right distal common carotid artery intima-media thickness (cIMT), distensibility, and presence of atherosclerotic lesions (IMT>1.5 mm). Plasma Gal-3BP was higher in HCV+ than HCV− women (p<0.01), but did not differ by HIV status. The three inflammatory macrophage markers were significantly correlated with each other and negatively correlated with CD4+ counts in HIV-infected women. We defined a macrophage score as 1, 2 or 3 biomarkers elevated above the median. In models adjusted for traditional risk factors, higher macrophage scores were significantly associated with increased atherosclerotic lesions and lower carotid distensibility. Receiver-operator curve analysis of lesions revealed that the markers added predictive value beyond traditional risk factors and C-reactive protein.
Conclusions
The macrophage inflammatory markers Gal-3BP, sCD163 and sCD14 are significantly associated with carotid artery disease in the setting of HIV and HCV infection.
Key word list: atherosclerosis, women, AIDS, immune system, risk factors
INTRODUCTION
Many risk factors, including dyslipidemia, older age, high blood pressure, diabetes and smoking are established predictors of cardiovascular disease (CVD) 1. The chronic inflammatory process in atherosclerosis is increasingly recognized as a contributing factor 2. High sensitivity C-reactive protein (hsCRP) has shown some utility in atherosclerosis risk assessment3 as a global marker of inflammation, but other markers may reflect more specific pathways.
Atherosclerosis is characterized by infiltration of monocytes into the wall of large and medium-sized arteries, where they form atherosclerotic plaque. These monocytes differentiate to macrophages and foam cells, some of which undergo apoptosis and secondary necrosis, forming a necrotic core that makes plaque vulnerable to rupture and clinical events like myocardial infarctions and stroke. Plaque macrophages show several different phenotypes that can be distinguished by surface markers using flow cytometry and immunofluorescence 4. Atherosclerotic lesions contain macrophages with phenotypic characteristics of M1 and M2 polarization. In general, M1 macrophages are thought to be associated with inflamed, vulnerable plaques and M2 macrophages with a thick fibrous cap and less with risk of rupture 5.
In a study of aortas of Ldlr−/− mice, Leitinger et al. found that 40% of CD68+ macrophages showed an M1 phenotype and 25% an M2 phenotype, where M1 was identified by expression of CD86 and M2 by expression of mannose receptor (CD206) 6. In human atherosclerotic lesions, macrophages with both M1 (expressing iNOS, HLA, CD86 and MARCO) and M2 (expressing mannose receptor, CD163 and dectin-1) characteristics have been found, often side-by-side in the same histological section 5. M1 macrophages dominated in the rupture-prone shoulder regions of the plaque, M1 and M2 macrophages were equally represented in the fibrous caps, and M2 macrophages dominated in adventitial tissue. Mice lacking the nuclear receptor Nr4a1 showed enhanced atherosclerotic lesions in spite of decreased numbers of circulating monocytes 7. This was associated with increased expression of the M1 marker IL-12p70 and decreased expression of the M2 marker arginase-1 in peritoneal macrophages and increased mRNA expression for TNF, CD36 and SRA-1 in F4/80+ macrophages sorted from aortae of Apoe−/−Nr4a1−/− mice 7. Tissue macrophage polarization is a multifactorial cascade which may be driven by infective agents or endogenously formed TLR2 and TLR4 ligands, NOD like receptor activation of the NLRP3 inflammasome, and T helper cytokines 8,9. In addition, the hematopoietic growth factor CSF2 (GM-CSF) promotes M1-like macrophages and CSF1 (M-CSF) promotes M2-like macrophages in atherosclerosis 10.
Specific serum or plasma proteins may act as biomarkers for macrophage activation. CD14, a GPI-linked co-receptor for lipopolysaccharide (LPS) expressed by both monocytes and macrophages, exists in a soluble form that can be detected in human plasma. M1 macrophages, which can be produced in the presence of interferon-γ and bacterial LPS, express CCR7, CD86 and MHCII and secrete IL-12p70 11. M2 macrophages, which can be activated in vitro through several pathways including addition of IL-4 9, express many lectin-like cell surface receptors, among them the hemoglobin-haptoglobin receptor CD163 12. CD163 is shed from the cell surface by a proteolytic mechanism involving ADAM-17 13, and this shed form is soluble and appears in the serum at detectable concentrations.
Since IL-12p70 is usually undetectable in plasma, we sought to find an alternative marker protein for M1 macrophages. Gal-3BP, a secreted 585-amino acid protein and member of the MSR cysteine-rich domain superfamily 14, is easily detected in plasma; it is a well-established plasma marker of viral infection and cancer 15–17; however, its potential role in CVD has not been investigated. Correlating plasma Gal-3BP and CVD can be relevant in light of the pathogen burden hypothesis, which states that cumulative infection burden contributes to atherogenesis 18.
In the present study, we found that in vitro Gal-3BP is produced by M1, but not M2 or M0 (unpolarized) human macrophages. Then, we tested whether plasma markers of macrophage abundance and activation (sCD163, sCD14 and Gal-3BP) correlated with subclinical carotid atherosclerosis, using epidemiologic data from a subset of women participating in the Women’s Interagency HIV Study (WIHS). Previous WIHS studies have established a relationship among known inflammatory markers, HIV infection, antiretroviral therapy, and cardiovascular risk factors with CVD19, 20. We selected four groups of women with HIV and/or HCV infection. Since both HIV and HCV infection have been associated with premature atherosclerosis, we hypothesized they would be also associated with macrophage activation as measured by sCD163, sCD14 and Gal3BP.
METHODS
Link to the online supplement.
RESULTS
In vitro study: Expression of Gal-3BP, CD14 and CD163 in M1 and M2 macrophages
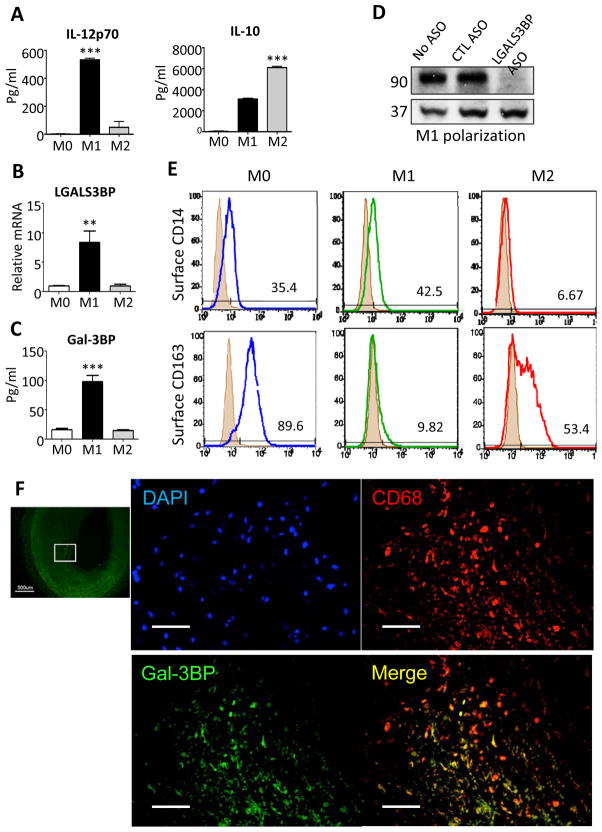
Human monocytes were isolated from blood of healthy volunteers and differentiated to macrophages (M0) in M-CSF using standard methods under serum-free conditions21. These primary macrophages secreted IL-12p70, but not IL-10 into the cell culture supernatant when differentiated to M1 by incubation with LPS and IFN-γ for 24 hours (Figure 1A). M2 macrophages polarized by LPS and IL-4 for 24 hours expressed ample IL-10, but no IL-12 (p70) (Figure 1A). LGALS3BP (human gene encoding Gal-3BP) mRNA (Figure 1B) was expressed in M1, but not M2 macrophages, and Gal-3BP protein was secreted by M1, but not M2 macrophages (Figure 1C). The secreted material migrated at 90 kDa as expected and disappeared after knocking down LGALS3BP by antisense (Figure 1D).
Figure 1. Gal-3BP, CD14 and CD163 expression in human macrophages.
Human blood monocytes (all subsets) were incubated with M-CSF (100 ng/ml) for 6 days to produce monocyte-derived macrophages (M0). These macrophages were incubated with interferon-γ for 1 day to produce M1 or with IL-4 to produce M2 macrophages and then challenged with LPS (10 ng/ml). (A) Level of IL-12p70 and IL-10 in the conditioned media were measured by Elisa (B) Gal-3BP mRNA expression was measured by quantitative RT-PCR, expressed relative to H18S. ** P=0.0056 by paired t test, n=3 donors. (C) Gal-3BP protein production was measured using ELISA (P=0.0008). (D) M1 polarized macrophages were treated with cell-permeable antisense (ASO) against LGALS3BP or control sequence (CTL) for 48hr, conditioned media harvested for immunoblotting with anti-Gal-3BP and anti-ERK2 (loading control) antibodies. (E) CD-14 and CD-163 determined by flow cytometry in M0, M1 and M2 polarized macrophages (representative of 3 healthy blood donors). (F) Human postmortem coronary arteries from patients with coronary artery disease were stained with antibodies against Gal-3BP (FITC, green), CD68 (Texas red), and nuclear stain (DAPI, blue) to assess expression of Gal-3BP in plaque macrophages. Lowest panel shows merge of Gal-3BP and CD68; right panels magnify boxed areas. Top: overview (scale bar = 500 μm), other scale bars = 100 μm
As expected, CD14 was expressed on the surface of M0 and M1 macrophages and was reduced upon M2 differentiation (Figure 1E). Conversely, CD163 was expressed by M0 and M2 macrophages and disappeared upon M1 polarization (Figure 1E).
To confirm the relevance of these in vitro findings to human disease, we analyzed human postmortem coronary arteries from patients with coronary artery disease by immunofluorescence. We found expression of Gal-3BP in CD68+ plaque macrophages (Figure 1F).
Population-based study: Study population and inflammatory macrophage markers
We measured sCD14, sCD163 and Gal-3BP in plasma obtained from 264 women in the WIHS. Participants were selected based on their HIV and HCV status and stratified into four groups, each containing 66 women: HIV+/HCV+, HIV+/HCV−, HIV−/HCV+, and HIV−/HCV (Table 1). The median age was 45 (interquartile range: 41, 50); 62% of the study population were black, 26% were Hispanic, and 12% were white or of other race. At the time of assessment, 76% were current smokers, with the remaining 24% either past or never smokers. Mean BMI was 29.3 (SD 7.4), with HIV+ individuals on average having a lower BMI (mean 27.6 vs. 31.0, p=0.02). Among HIV+ women, 77% were on antiretroviral therapy at the time of their visit. Among HCV+ women, only 2% were currently on interferon treatment, with an additional 8% reporting past interferon use.
Table 1.
Characteristics of study population, by HIV/HCV strata.
|
HIV−/HCV−
(N=66) N (%) |
HIV+/HCV−
(N=66) N (%) |
HIV−/HCV+
(N=66) N (%) |
HIV+/HCV+
(N=66) N (%) |
|
|---|---|---|---|---|
| Age (median, IQR) | 44 (40–48) | 45 (41–49) | 47 (43–51) | 47 (43–52) |
| Race/ethnicity | ||||
| Black | 41 (62) | 41 (62) | 41 (62) | 41 (62) |
| Hispanic | 17 (26) | 17 (26) | 17 (26) | 17 (26) |
| Other | 8 (12) | 8 (12) | 8 (12) | 8 (12) |
| Current smoker | 50 (76) | 50 (76) | 50 (76) | 50 (76) |
| Body mass index (mean, SD) | 29.9 (6.5) | 28.4 (6.8) | 32.0 (8.9) | 26.8 (6.0) |
| C-reactive protein level (μg/mL) (median, IQR) | 3.2 (1.1–6.8) | 2.8 (1.2–5.6) | 1.5 (0.4–4.3) | 0.85 (0.3–2.45) |
| CD4+ count (cells/μL) (median, IQR) | N/A | 410 (248–599) | N/A | 392 (215–538) |
| HIV viral load (copies/mL) (median, IQR) | N/A | 885 (80–21000) | N/A | 98.5 (80–2200) |
| ART regimen at time of study | ||||
| Protease inhibitor-based | N/A | 26 (39) | N/A | 33 (50) |
| Non-protease inhibitor-based | N/A | 21 (32) | N/A | 22 (33) |
| Not on treatment | N/A | 19 (29) | N/A | 11 (17) |
| Inflammatory macrophage score (range: 0–3) | ||||
| 0 | 21 (32) | 15 (23) | 8 (12) | 4 (6) |
| 1 | 35 (53) | 24 (36) | 11 (17) | 9 (14) |
| 2 | 8 (12) | 14 (21) | 28 (42) | 21 (32) |
| 3 | 2 (3) | 13 (20) | 19 (29) | 32 (48) |
| Gal-3BP (μg/mL) (mean, SD) (range: 1.36–26.90) | 8.75 (5.61) | 8.43 (4.56) | 12.69 (5.99) | 12.14 (5.98) |
| sCD163 (ng/mL, log2-transformed) (mean, SD) (range: 8.23–13.83) | 10.07 (0.62) | 10.43 (0.79) | 11.13 (0.75) | 11.25 (0.75) |
| sCD14 (ng/mL) (mean, SD) (range: 439–3360) | 1,179 (287) | 1,477 (464) | 1,317 (330) | 1,649 (560) |
HCV = hepatitis C virus, HIV = human immunodeficiency virus, IQR = interquartile range, SD = standard deviation.
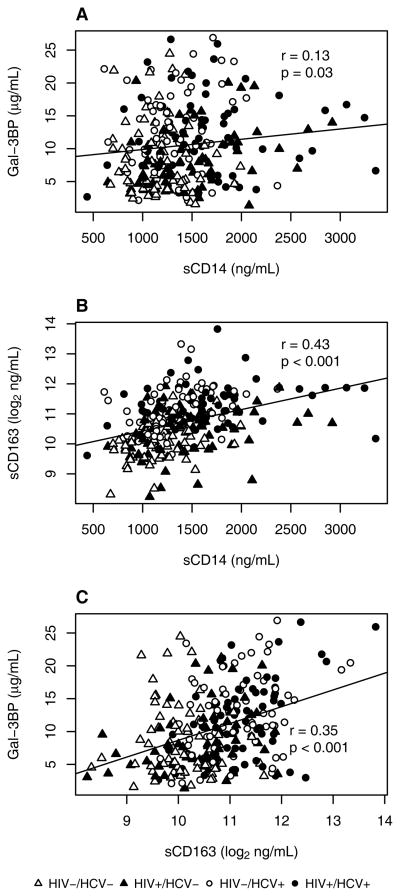
Table 1 shows the mean values of Gal-3BP, sCD163, and sCD14 levels in the study population in each of the four disease strata. Gal-3BP levels were significantly higher among HCV+ women than HCV− women (mean 12.4 μg/mL vs. 8.6 μg/mL, p<0.01), but did not differ based on HIV status (mean 10.3 μg/mL vs. 10.7 μg/mL, p=0.34). Dually-infected individuals had the highest mean levels of sCD163 and sCD14, whereas dually-uninfected individuals had the lowest levels. Women with either HIV infection only or HCV infection only had intermediate levels of sCD163 and sCD14. Correlations among the three macrophage markers were moderate and all positive, suggesting that the biological parameters measured by these biomarkers are partially overlapping (Figure 2). Gal-3BP levels were not significantly associated with traditional CVD risk factors including age, BMI, race/ethnicity or smoking status (data not shown). Regarding other CVD risk factors, both sCD163 and sCD14 levels were higher among older women, and sCD163 levels increased with BMI. Inflammatory macrophage biomarker levels were not highly correlated with levels of hsCRP (Supplemental Figure I). Among HIV+ women, CD4+ count was moderately inversely correlated with inflammatory macrophage biomarkers (Supplemental Figure II).
Figure 2. Spearman correlations comparing (A) Gal-3BP and sCD14, (B) sCD163 and sCD14, and (C) Gal-3BP and sCD163, across four HIV/HCV strata.
White = HIV−/HCV−, red = HIV+/HCV−, blue = HIV−/HCV+, black = HIV+/HCV+.
Line represents regression fit to observed data.
Population-based study: Associations with subclinical carotid artery disease
Subclinical carotid artery disease was common in the study population, with 14% of women having at least one carotid artery lesion. Mean carotid distensibility was 17.1 × 10−6 m2/N (SD 8.5), and the mean cIMT was 0.763 mm (SD 0.135).
To reflect the overall levels of macrophage-associated inflammation within an individual, we created a macrophage score which was defined as the number of biomarkers that were found to have levels above the population-wide median (range 0–3). In statistical models accounting for age, race/ethnicity, current smoking status, BMI, hsCRP, HIV, and HCV, higher macrophage score modeled as a continuous variable was significantly associated with both increased odds of lesion (OR 1.58 per elevated marker, 95% CI 1.15 to 2.18) and lower distensibility (β −1.44 units per marker, 95% CI −2.59 to −0.29) (Table 2). Having at least one elevated marker was associated with greater subclinical carotid artery disease, with the maximum number (i.e., 3) associated with the highest levels of disease.
Table 2.
Association between inflammatory macrophage markers and measures of subclinical carotid artery disease, Women’s Interagency HIV Study.
| Any carotid artery lesion | Distensibility (10−6 × N−1 × m2) | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | β | 95% CI | P | |
| Inflammatory macrophage score, per each elevated marker (range: 0–3) | ||||||
| Among entire study population (N=256) | ||||||
| Modeled as continuous | 1.58 | 1.15, 2.18 | 0.005 | −1.44 | −2.59, −0.29 | 0.01 |
| Modeled as categorical | ||||||
| 0 (reference group) | 1.00 | 0.00 | ||||
| 1 | 4.18 | 0.84, 20.70 | 0.08 | −1.57 | −4.13, 1.00 | 0.23 |
| 2 | 4.97 | 0.78, 31.64 | 0.09 | −1.47 | −4.66, 1.72 | 0.37 |
| 3 | 6.87 | 1.40, 33.71 | 0.02 | −4.65 | −8.06, −1.25 | 0.01 |
| Stratified analyses | ||||||
| Modeled as continuous | ||||||
| In HIV−/HCV− (N=65) | 1.18 | 0.35, 3.98 | 0.78 | 0.59 | −2.14, 3.33 | 0.67 |
| In HIV−/HCV+ (N=63) | 1.24 | 0.48, 3.21 | 0.66 | −2.03 | −3.87, −0.18 | 0.03 |
| In HIV+/HCV− (N=64) | 1.58 | 0.77, 3.24 | 0.21 | −2.36 | −4.27, −0.45 | 0.02 |
| In HIV+/HCV+ (N=64) | 2.81 | 0.96, 8.23 | 0.06 | −1.43 | −3.07, 0.21 | 0.09 |
| Individual inflammatory macrophage markers, per each standard deviation | ||||||
| Among entire study population (N=256) | ||||||
| Gal-3BP | 1.48 | 1.01, 2.15 | 0.04 | −1.44 | −2.28, −0.61 | <0.001 |
| sCD163 | 1.85 | 1.21, 2.83 | 0.005 | −0.05 | −1.26, 1.15 | 0.93 |
| sCD14 | 1.49 | 1.04, 2.12 | 0.03 | −1.11 | −2.10, −0.12 | 0.03 |
CI = confidence interval, HCV = hepatitis C virus, HIV = human immunodeficiency virus, m = meters, N = Newtons.
All analyses adjusted for age, race/ethnicity, smoking status, body mass index, high-sensitivity C-reactive protein, and except for stratified analyses, HIV and HCV infection. Bold indicates significant at p<0.05.
Additional analyses examined individual markers for associations with carotid outcomes. Gal-3BP, sCD163, and sCD14 levels were each significantly associated with an increase in the odds of a carotid artery lesion (Table 2). We found that models that contained the three markers individually each increased the prediction of having a carotid artery lesion (C-statistic 0.735–0.743), compared with a model without these markers (C-statistic 0.714). Including all three markers simultaneously, either using the macrophage score or as individual covariates, further increased the predictive value (C-statistic 0.746 and 0.762, respectively).
Higher levels of the three markers were correlated with reduced distensibility in bivariate analyses (Supplemental Figure III), but only Gal3BP and sCD14 were significantly associated with distensibility after covariate adjustment (Table 2).
In stratified analyses by HIV and HCV infection status, we found an association between increasing macrophage score and the presence of carotid artery lesions among HIV- and HCV-infected participants, with the effect most pronounced and approaching statistical significance among dually-infected individuals (OR 2.81, 95% CI 0.96–8.23) (Table 2). In analyses of individual markers, none of the associations with carotid artery measurements differed significantly across subgroups defined by HIV and HCV after covariate adjustment, despite suggestions of bivariate correlations (e.g., Supplemental Figures IV and V). We observed a trend suggesting stronger associations of subclinical carotid artery disease with sCD163 in HIV-infected as compared with HIV-uninfected groups (pinteraction=0.09, Supplemental Table I). Findings did not differ appreciably after taking into account CD4+ count or current antiretroviral therapy use among HIV-infected women, or interferon therapy use among HCV-infected women (data not shown). In general, neither the macrophage biomarker score nor the three individual macrophage biomarkers were associated with levels of cIMT, either overall or within subgroups defined by HIV and HCV status (Supplemental Tables I and II).
DISCUSSION
In our study, we found plasma markers of macrophage abundance, polarization and activation (Gal-3BP, sCD163 and sCD14) to be associated with two measures of subclinical carotid artery disease. Specifically, we found that higher macrophage scores, defined by elevations in multiple macrophage biomarkers, were significantly associated with both increased odds of carotid artery lesions and reduced carotid artery distensibility (inverse of stiffness). Additional analyses suggested that associations of the macrophage markers with carotid artery parameters may have been augmented in HIV− and/or HCV-infected women, as compared with women free of HIV and HCV. These associations remained after taking into account CD4+ count or adjusting for hsCRP, a known inflammation marker of CVD. We did not observe correlations between macrophage biomarkers and hsCRP levels. Thus, our data suggest that circulating macrophage biomarkers may reflect the inflammatory process contributing to atherosclerosis, above and beyond hsCRP, and therefore may provide additional predictive and/or diagnostic ability in the future with respect to subclinical CVD. We also found each of the macrophage markers to be more elevated in HIV-infected women with lower CD4+ counts, particularly sCD163 and sCD14, which is consistent with ongoing inflammation and immune activation in these women.
We identified Gal-3BP as a measurable and soluble M1 macrophage marker in our in vitro study, and then found that Gal-3BP was consistently up-regulated and positively correlated with subclinical carotid artery disease markers in a clinical study after adjustment for potential confounders. Accumulating data suggest that some plaque macrophages express M1 markers and others M2 markers 6; although contribution to plaque instability and inflammation is mostly attributed to M1 markers 5. Recent studies have given rise to interest in galectins as potential biomarkers of CVD 22. In prior work, higher plasma levels of galectin-3 have been associated with increased heart failure risk 23 and higher mortality in those with heart failure 24, 25. Human Gal-3BP binds specifically to human galectin-3 14, which is interestingly an M2 marker26. To our knowledge, ours is the first study to examine associations between Gal-3BP and measures of subclinical CVD. Plasma Gal-3BP levels were increased in HCV-positive individuals, but did not differ by HIV infection status. The finding relating to HCV is consistent with prior data 15. The lack of association between HIV and Gal-3BP levels differs from other studies 16, 17. Underlying differences in study populations, calendar time effects, or availability of newer drugs in our study could have contributed to differences between studies.
Our study also provides further support of a role for sCD163 in CVD, as has been suggested by several recent studies 27–30. While our study focused on women, a cross-sectional analysis of 102 HIV-infected men with undetectable viremia and 41 HIV-uninfected men without a history of CAD found sCD163 to be significantly associated with the presence of non-calcified atherosclerotic plaque, even after adjusting for HIV, traditional CVD risk factors, and age and race 28. Another recent cross-sectional study among 81 HIV-infected individuals using positron emission tomography found sCD163 to be significantly associated with arterial inflammation in the aorta, after adjusting for traditional CV risk factors 30. However, other small cross-sectional or case-control studies in individuals with HIV have not found associations between sCD163 and plaque or myocardial infarction 31, 32, and therefore larger, prospective studies may help to further clarify the role of these markers in CVD pathogenesis. Although CD163 is expressed on M2 macrophages, sCD163 may not reflect this, because ADAM-17, the enzyme cleaving CD163 to become soluble, is expressed by M1 macrophages 13.
Higher sCD14 levels have been associated with all-cause mortality in studies of both the general population 33, as well as in those with HIV 34. Here, we found that sCD14 level was associated with increased carotid artery lesions and decreased carotid artery distensibility after adjusting for HIV and HCV infection, corroborating other findings in the general population.41, 42 Another HIV study showed an association between sCD14 levels and yearly rate of change in cIMT 35. At least one other HIV study failed to find an association between sCD14 and cIMT or lesions, although the authors of that study acknowledged limited power (N=60) 32.
Our study is a first step in understanding the pathophysiology and potential role as risk predictors of these promising new biomarkers in our HIV/HCV population. However, it has limitations. Because the current study is cross-sectional, both macrophage markers and carotid artery outcomes were assessed at the same visit. Therefore, we cannot infer temporality or causality in our study, and thus longitudinal analyses will be important to further elucidate the roles of these markers in predicting the development of CVD, both with and without the presence of chronic viral infection. Indeed, before these markers can be considered for use as risk predictors in clinical practice, they will need to be validated in other populations as well as in the prediction of major adverse cardiac events. Second, this study was limited to women. Since it is well-recognized that the ability to accurately predict CVD risk differs in men compared with women 36, it would be important to replicate our findings to assess the role of these markers in men. Finally, we were limited by a relatively small sample size. However, our study is in fact larger than several other studies examining similar subclinical CVD outcomes in the HIV population. Furthermore, due to the excellent characterization of WIHS participants since 1993, we were able to include a substantial number of exceptionally well-matched women with documented HIV and HCV infection as well as those without infection, to be able to explore differences based on these factors and assess potential effect modification.
In conclusion, we demonstrated that three inflammatory biomarkers of macrophages that are measurable in human plasma are significantly associated with subclinical carotid artery lesions and distensibility in a subset of well-characterized women both with and without chronic viral infection, suggesting their potential for predictive or diagnostic value with respect to atherosclerosis. The association of plasma macrophage biomarkers with CVD may provide new insights pointing to possible new strategies for prevention or treatment of CVD in patients with and without HIV and HCV.
Supplementary Material
Significance.
Macrophages are principal contributors to vascular inflammation and atherosclerosis pathology; they can adopt distinctive phenotypes, from inflammatory (M1) to wound healing (M2) phenotypes. While both present in the atherosclerotic plaque, M1 macrophages are more likely to contribute to inflammation and plaque rapture. There are limited tools to evaluate the inflammatory status of macrophages that populate the plaque. Virus infection can contribute to both CVD pathogenesis and macrophages polarization. Herein we measure plasma protein markers secreted from macrophages, in the blood collected from women positive for HIV, HCV, both or neither and correlate their levels to carotid artery disease, assessed by ultrasound measurement of right distal common carotid artery intima-media thickness (cIMT), distensibility, and presence of atherosclerotic lesions. We conclude that the macrophage activation status markers Gal-3BP (M1), sCD163 (M2) and sCD14 (M0) are significantly associated with carotid artery disease in the setting of HIV and HCV infection.
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). We thank Dr. Maria-Beatriz Lopes, University of Virginia, Charlottesville, for providing post mortem coronary artery specimens.
Funding Sources. WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen), U01-AI-034993; Metropolitan Washington WIHS (Mary Young), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Alexandra Levine and Marek Nowicki), U01-HD-032632 (WIHS I - WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA). Additional funding was provided by R01-HL-083760 and R01-HL-095140. In addition, I.S. is supported by American Heart Association postdoctoral fellowship.
Abbreviations
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- cIMT
carotid artery intima-media thickness
- Gal-3BP
galectin-3 binding protein
- sCD163
soluble cluster of differentiation 163
- sCD14
soluble cluster of differentiation 14
Footnotes
Disclosures: Authors have no conflicts of interest to disclose.
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
References
- 1.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. doi: 10.1016/S0140-6736(13)61752-3. ePub: September 29, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004;116(Suppl 6A):9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E, de Winther MP. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:1120–1126. doi: 10.1161/ATVBAHA.112.300173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 10.Wolfs IM, Donners MM, de Winther MP. Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb Haemost. 2011;106:763–771. doi: 10.1160/TH11-05-0320. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 13.Etzerodt A, Maniecki MB, Moller K, Moller HJ, Moestrup SK. Tumor necrosis factor alpha-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J Leukoc Biol. 2010;88:1201–1205. doi: 10.1189/jlb.0410235. [DOI] [PubMed] [Google Scholar]
- 14.Koths K, Taylor E, Halenbeck R, Casipit C, Wang A. Cloning and characterization of a human Mac-2-binding protein, a new member of the superfamily defined by the macrophage scavenger receptor cysteine-rich domain. J Biol Chem. 1993;268:14245–14249. [PubMed] [Google Scholar]
- 15.Dorrucci M, Ciccozzi M, Iacobelli S, Rezza G. Temporal trend in the level of 90K glycoprotein after HIV seroconversion among persons coinfected with hepatitis C virus. Infection. 2005;33:101–102. doi: 10.1007/s15010-005-4076-6. [DOI] [PubMed] [Google Scholar]
- 16.Groschel B, Braner JJ, Funk M, Linde R, Doerr HW, Cinatl J, Jr, Iacobelli S. Elevated plasma levels of 90K (Mac-2 BP) immunostimulatory glycoprotein in HIV-1-infected children. J Clin Immunol. 2000;20:117–122. doi: 10.1023/a:1006634530672. [DOI] [PubMed] [Google Scholar]
- 17.Natoli C, Ortona L, Tamburrini E, Tinari N, Di Stefano P, D’Egidio M, Ghinelli F, Sighinolfi L, D’Ostilio N, Piazza M. Elevated serum levels of a 90,000 daltons tumor-associated antigen in cancer and in infection by human immunodeficiency virus (HIV) Anticancer Res. 1994;14:1457–1460. [PubMed] [Google Scholar]
- 18.Epstein SE, Zhu J, Najafi AH, Burnett MS. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation. 2009;119:3133–3141. doi: 10.1161/CIRCULATIONAHA.109.849455. [DOI] [PubMed] [Google Scholar]
- 19.Tien PC, Schneider MF, Cole SR, Cohen MH, Glesby MJ, Lazar J, Young M, Mack W, Hodis HN, Kaplan RC. Association of hepatitis C virus and HIV infection with subclinical atherosclerosis in the women’s interagency HIV study. AIDS. 2009;23:1781–1784. doi: 10.1097/QAD.0b013e32832d7aa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, Anastos K, Tien PC, Xue X, Lazar J, Parrinello CM, Benning L, Tracy RP. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr. 2012;60:359–368. doi: 10.1097/QAI.0b013e31825b03be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho HJ, Shashkin P, Gleissner CA, Dunson D, Jain N, Lee JK, Miller Y, Ley K. Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genomics. 2007;29:149–160. doi: 10.1152/physiolgenomics.00051.2006. [DOI] [PubMed] [Google Scholar]
- 22.Gruson D, Ko G. Galectins testing: new promises for the diagnosis and risk stratification of chronic diseases? Clin Biochem. 2012;45:719–726. doi: 10.1016/j.clinbiochem.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010;99:323–328. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Velde AR, Gullestad L, Ueland T, Aukrust P, Guo Y, Adourian A, Muntendam P, van Veldhuisen DJ, de Boer RA. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail. 2013;6:219–226. doi: 10.1161/CIRCHEARTFAILURE.112.000129. [DOI] [PubMed] [Google Scholar]
- 26.Novak R, Dabelic S, Dumic J. Galectin-1 and galectin-3 expression profiles in classically and alternatively activated human macrophages. Biochim Biophys Acta. 2012;1820:1383–1390. doi: 10.1016/j.bbagen.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno JA, Dejouvencel T, Labreuche J, Smadja DM, Dussiot M, Martin-Ventura JL, Egido J, Gaussem P, Emmerich J, Michel JB, Blanco-Colio LM, Meilhac O. Peripheral artery disease is associated with a high CD163/TWEAK plasma ratio. Arterioscler Thromb Vasc Biol. 2010;30:1253–1262. doi: 10.1161/ATVBAHA.110.203364. [DOI] [PubMed] [Google Scholar]
- 29.Urbonaviciene G, Martin-Ventura JL, Lindholt JS, Urbonavicius S, Moreno JA, Egido J, Blanco-Colio LM. Impact of soluble TWEAK and CD163/TWEAK ratio on long-term cardiovascular mortality in patients with peripheral arterial disease. Atherosclerosis. 2011;219:892–899. doi: 10.1016/j.atherosclerosis.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK. Arterial inflammation in patients with HIV. Jama. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knudsen A, Moller HJ, Katzenstein TL, Gerstoft J, Obel N, Kronborg G, Benfield T, Kjaer A, Lebech AM. Soluble CD163 does not predict first-time myocardial infarction in patients infected with human immunodeficiency virus: a nested case-control study. BMC Infect Dis. 2013;13:230. doi: 10.1186/1471-2334-13-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, Lederman MM, McComsey GA. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14:385–390. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiner AP, Lange EM, Jenny NS, Chaves PH, Ellis J, Li J, Walston J, Lange LA, Cushman M, Tracy RP. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol. 2013;33:158–164. doi: 10.1161/ATVBAHA.112.300421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206:1558–1567. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paynter NP, Everett BM, Cook NR. Cardiovascular Disease Risk Prediction in Women: Is There a Role for Novel Biomarkers? Clin Chem. 2013;60:88–97. doi: 10.1373/clinchem.2013.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.