Transforming growth factor-ß (TGF-ß) is a pleiotropic growth factor with actions that are dependent on circumstances, including dose, target cell type, and context. TGF-ß can elicit both growth-promoting and growth-suppressive activities. In normal tissues, TGF-ß generally acts to restrict growth and maintain differentiation. However, during tumorigenesis, changes in TGF-ß expression and cellular responses can promote tumorigenesis. The present study examines the effects of TGF-ß on the nontumorigenic human prostatic epithelial cell line BPH1 and on three derivative tumorigenic sublines BPH1CAFTD1, BPH1CAFTD3, and BPH1CAFTD5. The data show that TGF-ß has different effects on the nontumorigenic and tumorigenic cells. The nontumorigenic cells are growth inhibited by TGF-ß. In contrast, the tumorigenic sublines are not growth inhibited but instead undergo an epithelial to mesenchymal transformation (EMT) in response to TGF-ß. The tumorigenic lines show constitutively elevated levels of phosphorylated Akt, which modulates their response to TGF-ß by blocking Smad3 and p21 nuclear translocation. On TGF-ß stimulation of the tumorigenic sublines, the activated Akt allows the cell to escape cell cycle arrest. The phosphatidylinositol 3-kinase/Akt pathway is also involved in TGF-ß-induced EMT, defined here by induction of vimentin expression and enhanced cellular motility. In vivo, tumorigenic cells with constitutively active TGF-ß signaling show increased invasion with EMT,which express vimentin, located specifically at the invasive front of the tumor. These data indicate that following malignant transformation TGF-ß can play a direct role in promotingprostatic cancer and further that these responses are context specific in vivo.
Introduction
The transforming growth factor-ß (TGF-ß) belongs to a superfamily of ligands that includes activins and bone morphogenetic proteins (BMP). TGF-ß ligands act through transmembrane type I and type II receptors (TßRI and TßRII) to activate several downstream signal transductionpathways (1). The Smad pathway was the first signaling pathway identified to mediate TGF-ß effects and remains the best characterized. More recently, multiple non-Smad pathways have been implicated in mediating TGF-ß effects downstream of the receptors. Their involvement in the changing responses of cells to TGF-ß are just beginning to be probed (2, 3). Studies in mammary epithelial cells have shown that TGF-ß promotes motility through mechanisms independent of Smad signaling, possibly involving activation of the phosphatidylinositol 3-kinase (PI3K)/Akt and/or mitogen-activated protein kinase (MAPK) pathways (4–6). Additional data suggested that TGF-ß rapidly activates RhoA-dependent signaling pathways to induce stress fiber formation and mesenchymal characteristics (7).
TGF-ßs play complicated roles, including regulating cell proliferation, functional differentiation, extracellular matrix (ECM) production, cell motility, and apoptosis. In tumorigenesis, TGF-ß apparently plays dual roles. In the current paradigm, TGF-ß acts as a growth suppressor in normal tissue, but in tumors changes in TGF-ß expression and cellular responses tip the balance in favor of pro-oncogenic activity (3). In benign prostatic epithelia, by eliciting differentiation, inhibiting proliferation, and inducing apoptosis, TGF-ß provides a mechanism to maintain homeostasis in the prostate (8–10). The ability of TGF-ß to enhance tumorigenicity in vivo is illustrated by its role in many key processes, including stimulating angiogenesis, inhibiting immune surveillance, or promoting the degradation of ECM (10, 11). TGF-ß can also promote local invasion and metastasis through epithelial to mesenchymal transformation (EMT; refs. 7, 12, 13). EMT is a poorly documented feature of human prostate cancer arguably because of a lack of good antibodies against the EMT-related transcription factors.
Specific responses to TGF-ß reflect the complex network of cross-talking signals that constitute the transduction mechanism by which the cell responds to stimulation by the TGF-ß ligand (14). Integration of the TGF-ß pathway with other signaling cascades may modulate TGF-ß responses. The discovery of a physical interaction between Akt and Smad3 has presented a mechanism by which cancer cells may avoid TGF-ß apoptotic or growth-inhibitory responses (15, 16). Akt, also known as protein kinase B, is a serine/threonine kinase. Following stimulation by growth factors, PI3K is recruited and activated resulting in the production of phosphatidylinositol-3,4,5-triphosphate. This attracts Akt to the membrane where it is phosphorylated at T308 and S473 by phosphoinositide-dependent kinase-1. Activated Akt is then released from the membrane and translocates to other subcellular compartments (17, 18). Akt has been reported to be constitutively activated in many cancers, including prostate cancer (18, 19).
The present study investigates the potential of TGF-ß signaling to play a role in mediating the promotion of human prostate cancer. To approach this question, the effects of TGF-ß signaling on the nontumorigenic human prostatic epithelial cell line BPH1 and derivative tumorigenic BPH1CAFTD lines were investigated. These derivative tumorigenic strains were permanently transformed by exposure to human prostatic carcinoma associated fibroblasts (20) and have a series of characteristic genetic changes resulting from their exposure to a cancer stroma environment (21). This communication records the profound differences in the responses of the parental and derivative lines to stimulation by TGF-ß and illustrates mechanisms by which a single ligand can play different roles depending on the cell type and context in which it is acting.
Materials and Methods
Animals. Adult male severe combined immunodeficient (SCID) mice [C.B.17/IcrHsd-scid] were obtained (Harlan, Indianapolis, IN).
Cell lines. BPH1 and the derivative BPH1CAFTD cell lines (20, 22) were from our own stocks and were routinely passaged in RPMI 1640 containing antibiotic-antimycotic mix (Life Technologies,Grand Island, NY) and 5% fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA).
Growth inhibition. Each cell line was seeded at 350,000 cells per well in 24-well plates in serum-free RPMI 1640 (Life Technologies). After growth and attachment overnight, the cells were treated with or without 5 ng/mL TGF-ß1 (R&D Systems, Inc., Minneapolis, MN) in serum-free RPMI 1640 (Invitrogen, Carlsbad, CA). Cell growth was terminated by adding Cell Titer 96 Aqueous One solution (Promega, Madison, WI) at indicated time points. After 2 hours of incubation at 37°C, absorbance at 490 nm wavelength was read using a microplate reader.
Genetic modification of cell lines. Dominant active TßRI (DATßRI; constitutively active TßRI; ref.23), myristylated Akt1 (myrAkt1; ref. 24), and full-length human Smad3 (25) were inserted into a LZRS-EGFP backbone (Nolan Laboratory, Stanford, CA) as described previously (26). Viral particles were generated using the amphotropic PHNXA packaging cells, which were obtained from American Type Culture Collection (Manassas, VA). The viral supernatant from the transfected cells was centrifuged at 3,000 rpm and passed through a 0.45 μm filter. Successiverounds of infection over 5 days were employed. The infected cells were selected based on expression of a bicistronic fluorescent tag. The empty vector was used to generate negative controls. The cells were maintained in RPMI 1640 per the source lines. 3TP-Luc promoter activity was used to confirm that the TGF-ß signaling is constitutively active in dominant active or Smad3 cells (27, 28). Western blotting assay was used to confirm that phosphorylated Akt level is elevated in myrAkt1 cells.
Cell and tissue immunofluorescence staining. Cells were plated on sialized glass (Superfrost) slides and allowed to attach and grow overnight. After serum starvation for 24 hours, cells were treated with 5 ng/mL TGF-ß1 for 4 hours. After fixation in methanol for 5 minutes at –20°C, samples were washed twice in PBS, blocked for 30 minutes with 5% goat serum (Vector Laboratories, Burlingame, CA), and incubated at room temperature for 1 hour with primary antibodies against vimentin (1:100; Sigma, St. Louis, MO) and wide spectrum keratin (1:100; DAKO, Carpinteria, CA) followed by washing for 30 minutes in PBS. Staining was visualized using fluorescence-conjugated secondary antibodies. Slides were visualized and imaged using a Zeiss upright microscope with an attached Axiocam camera and proprietary software. Smad3 translocation was quantitated by image analysis using NIH ImageJ software (http://rsb.info.nih.gov/ij/). The photons emitted by the fluorescent secondary antibody concentrated in the nucleus (Phn) and those in the whole cell body (Phw) were measured and the nuclear distribution proportion (Phn/Phw) was calculated.
For histologic analysis, 5 μm tissue sections were dewaxed, and the antigen was unmasked by heating samples in unmasking solution (Vector Laboratories). Slides were blocked in 5% goatserum and 2% bovine serum albumin (BSA) in PBS for 30 minutes at room temperature before incubating with mixed primary antibodies (1:100) mouse anti-vimentin (V-6630, IgG1, which recognizes human but not mouse vimentin) and mouse anti-SV40 Tag (IgG2a; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour. After 1-hour washing in PBS buffer, slides were incubated with secondary antibodies (1:200; Alexa Flour 488 anti-mouse IgG1 and Alexa Flour 546 anti-mouse IgG2a; Molecular Probes, Eugene, OR) for 30 minutes at room temperature. Tissue sections were washed for 30 minutes in PBS, mounted, and visualized.
Cross-linking assay. Cells were cultured in six-well plates until 100% confluent and washed thrice over 30 minutes with 500 μL ice-cold 0.1% BSA dissolved in PBS containing Ca2+ and Mg2+. The cells were then affinity labeled with 100 pmol/L [125I]TGF-ß1 with or without a 100-fold molar excess of unlabeled TGF-ß1 using previously described methods (6). All samples were analyzed using 3% to 12% SDS-PAGE and visualized by autoradiography.
Cell fractionation. Cells were seeded in 75 cm2 flasks with RPMI 1640 containing 5% FBS. After overnight starvation in serum-free RPMI 1640, the cells were treated with 5 ng/mL TGF-ß1 for 4 hours. The nuclear and cytoplasmic subcellular fractions were prepared using Pierce Extraction Reagent (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instruction. Theresulted cytoplasmic and nuclear extracts were quantified by the BCA protein assay using protein assay dye reagent (Bio-Rad, Hercules, CA) and kept at –80°C until use.
Cell motility assays. To test cellular motility, wound healing and Transwell migration assays were used. For wound healing, a section of a confluent cell monolayer was wounded with a pipette tip, and the ability of cells to migrate into the cleared section was observed at different time points as specified in Results (29). A Boyden chamber system was used for Transwell migration assay. Polycarbonate inserts with 8.0 μm pore size (Becton Dickinson Labware, Franklin Lakes, NJ) were coated with 500 μL of 250 μg/mL collagen I, air dried at room temperature, and kept sterile at 4°C before use. One hour before an experiment, the inserts were blocked using 1% BSA in PBS at 37°C. A 100 μL suspension containing 100,000 cells was loaded into each insert. FBS (0, 0.5%, and 2.5%) in 500 μL RPMI 1640 was applied to the lower chamber. After incubation for8 hours, cells remaining in the top of the inserts were removed using a cotton swab. The cells that had migrated through the collagen and the filter were fixed with 11% glutaraldehyde (Sigma)for 20 minutes followed by 0.1% crystal violet staining and counted in five random fields. The mean of the number was used to quantitate the migration. The experiments were done in triplicate wells.
Western blot assays. Cells were detached by trypsinization, washed with cold PBS, and lysed with TNN buffer [50 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.5% NP40 (pH 7.4)] containing proteinase inhibitor (Roche, Nutley, NJ) and phosphatase inhibitor (Sigma). Lysates were clarified at 13,000 rpm for 20 minutes at 4°C, and supernatants were quantified by the BCA protein assay using protein assay dye reagent. For TGF-ß receptor Western blots, clarified lysates were deglycosylated by incubation with peptide N-glycosidase F according to the manufacturer's instructions (New England Biolabs, Beverly, MA; ref. 30). Nondeglycosylated and deglycosylated proteins were loaded and electrophoresed through 10% NuPAGE BisTris gel (Invitrogen) and electrophoretically transferred to nitrocellulose membranes. Membranes were blocked for 1 hour in PBS-Tween 20 containing 5% nonfat dry milk and incubated with primary antibody overnight [vimentin, 1:1,000; E-cadherin, 1:3,000 (Transduction Laboratory, Lexington, KY; BD Biosciences PharMingen, San Diego, CA); keratin, 1:2,000; Smad3, 1:500 and p21, 1:300 (Santa Cruz Biotechnology); phosphorylated Akt, 1:500, phosphorylated Smad2, 1:1,000, and phosphorylated GSK3 /ß, 1:1,000 (Cell Signaling, Danvers, MA); ß-actin, 1:5,000 (Sigma); and histone H1, 1:5,000 (Abcam, Inc., Cambridge, MA)], washed 30 minutes in PBS-Tween 20, and incubated with horseradish peroxidase–conjugated anti-mouse secondary antibody (1:1,000; DAKO) for 1 hour. Enhanced chemiluminescence detection reagent (Amersham Biosciences UK Ltd., Little Chalfont, United Kingdom) was used to visualize protein bands.
Cell cycle analysis. BPH1 or BPH1CAFTD1 cells were starved with serum-free RPMI 1640 overnight and treated with or without 5 ng/mL TGF-ß1 for 4 hours until trypsinization. The cells were washed and resuspended in PBS containing 2% FBS at the concentration of 106/mL before fixing with 100% ethanol at 4°C for 1 hour. Washing twice with PBS, the cells wereincubated with 1 mL propidium iodide (PI)/RNase mixture (working dilution: 50 μg/mL PI/0.5 μg/ mL RNase) at 4°C for 3 hours until analysis with cell flow cytometry.
Xenograft and immunohistochemistry. 100k sorted infected BPH1CAFTD1-EV or BPH1CAFTD1-DA epithelial cell lines were suspended in rat tail collagen (50 μL/graft). The resultant gels were incubated overnight in a 5% CO2 humidified incubator at 37°C in complete RPMI 1640 and subsequently placed beneath the renal capsule of male athymic mice (20, 31). Eight to 12 weeks after grafting, the hosts were sacrificed. Harvested grafts were fixed in paraffin and embedded for histologic and immunohistochemical analysis as described above.
Results
TGF-ß1 effects on BPH1CAFTD cells. TGF-ß1 at a concentration of 5 ng/mL inhibited the growth of the nontumorigenic BPH1 cells over a 5-day period, consistent with previously publishedobservations (22). In contrast, none of the tumorigenic BPH1CAFTD cell lines were growth inhibited, with treated and untreated cells growing at statistically identical rates (Fig. 1A ).Parental BPH1 cells were phenotypically unchanged by TGF-ß treatment, exhibiting a classic epithelial cobblestone morphology in culture with continued expression of keratin and E-cadherin. In contrast, the tumorigenic BPH1CAFTD lines responded to TGF-ß by subtle morphologic changes, suggesting cellular elongation. Vimentin expression in these cells was robustly induced, whereas keratin expression was repressed (Fig. 1B). Western blotting assays confirmed the immunofluorescence data showing induction of vimentin and suppression of E-cadherin and keratin (Fig. 1C).
Figure 1.
Nontumorigenic and derivative tumorigenic cells show different responses to TGF-ß1. A, growth response of BPH1 and BPH1CAFTD1 (CAFTD1), BPH1CAFTD3 (CAFTD3), and BPH1CAFTD5 (CAFTD5) cells to 5 ng/mL TGF-ß1. The nontumorigenic parental BPH1 cells were growth inhibited by TGF-ß. In contrast, the tumorigenic sublines showed no significant change in their proliferation in response to TGF-ß. B, phenotypic response of BPH1 and BPH1CAFTD1, BPH1CAFTD3, and BPH1CAFTD5 cells to 72 hours of treatment with 5 ng/mL TGF-ß1. Phase-contrast micrographs show no change of BPH1 cell shape in response to TGF-ß. In contrast, the three CAFTD lines exhibited variable degrees of elongation. Immunofluorescence staining reveals changes in protein expression. Wide-spectrum cytokeratin was visualized in red and vimentin in green. Nuclear DNA was stained in blue using 4',6-diamidino-2-phenylindole. In serum-free medium, all four cell lines express cytokeratins with little detectable vimentin. In response to treatment with TGF-ß1, the tumorigenic cells, but not the nontumorigenic parental cells, initiate expression of vimentin and lose keratin expression. C, Western blotting confirmed the immunofluorescence data. In response to 5 ng/mL TGF-ß1 treatment, vimentin expression was induced by 72 hours post-treatment in the tumorigenic BPH1CAFTD1, BPH1CAFTD3, and BPH1CAFTD5 cells, whereas E-cadherin and keratin expression was suppressed in these tumorigenic cells. No equivalent change in vimentin or keratin expression was seen in the nontumorigenic parental BPH1 cells.
Cellular response to TGF-ß. To determine whether the difference between the parental and tumorigenic cell response to TGF-ß resulted from a loss of TGF-ß signaling components or the alteration of downstream signal transduction pathways resulting from changes in expression of other proteins that modulate signaling (2, 32), we used a cross-linking assay to check the binding of iodinated TGF-ß to receptors and also examined the phosphorylation of Smad2 in response to TGF-ß stimulation. The cross-linking assay showed that TßR1, TßRII, and the class three TGF-ß receptors were all expressed in both the BPH1 cells and all of the BPH1CAFTD cell lines (Supplementary Fig. SA). The presence of TßR1 and TßRII was confirmed by Westernblotting. To confirm receptor function, Western blotting assays were done, which showed that, downstream of the receptor, Smad2 phosphorylation in response to TGF-ß stimulation wassimilar in parental and tumorigenic cells (Supplementary Fig. SB). The basal phosphorylated Smad2 seen in the tumorigenic cell lines likely reflects autocrine TGF-ß activity, which is commonly seen to be elevated in tumor cells (reviewed in ref. 3). In contrast to the expression of Smad2, the levels of phosphorylated Akt are very different between the two cell types, with the tumorigenic BPH1CAFTD lines having much higher levels of expression than the non tumorigenic BPH1 cells (Supplementary Fig. SB).
Elevated phosphorylated Akt blocks Smad3 translocation and reduces nuclear p21 to release the cell cycle arrest. To test the potential of elevated phosphorylated Akt to modulate the response to TGF-ß, we checked the R-Smad (Smad2 and Smad3) localization on TGF-ßtreatment in parental BPH1 cells and BPH1CAFTD1 cells. Both Western blotting and immunofluorescence staining show that before TGF-ß treatment, Smad3 is predominantly seen in the cytoplasm. Within 4 hours of TGF-ß treatment, Smad3 was translocated into the nucleus in BPH1 cells. However, in BPH1CAFTD1 cells, this process was apparently only partial with a substantial amount of Smad3 maintained in the cytoplasm. To confirm that this phenomenon is caused by the endogenous levels of phosphorylated Akt, we did two complementary experiments. To examine a gain of function, a constitutively active myrAkt1 was expressed in the BPH1 cells. To show loss of function, the BPH1CAFTD1 cells were pretreated with the PI3K/Akt inhibitors wortmannin (Sigma) or LY294002 (Calbiochem, San Diego, CA; ref. 4) before TGF-ß administration. These experiments showed that myrAkt1 overexpression inhibits nuclear translocation of Smad3 in response to TGF-ß, with a portion of the Smad3 remaining in the cytoplasm of the nontumorigenic BPH1 cells, whereas blocking PI3K/Akt enhanced Smad3 translocation into the nucleus of the BPH1CAFTD cells (Fig. 2 ). The phosphorylation and distribution of Smad2 was apparently the same in BPH1, BPH1CAFTD1, and BPH1-Akt cells: most of total Smad2 translocated into the nucleus in response to TGF-ß treatment; almost all the phosphorylated Smad2 localized in the nucleus. These observations suggest that although Smad2 and Smad3 are similar in structure and physically and functionally interact to mediate TGF-ß signaling, they are under distinct regulatory control, which probably causes their different biological functions. Our data show that activated Akt blocks Smad3 but not Smad2 translocation. These results are consistent with previous studies in prostatic epithelial cells (30, 33).
Figure 2.
Phosphorylated Akt blocks Smad3 translocating to the nucleus in human prostatic epithelial cells. A, cells were incubated with 5 ng/mL TGF-ß1 for 4 hours and processed to give cytoplasmic (C) and nuclear (N) extracts. Specificity was confirmed using histone H1 and ß-actin as nuclear and cytoplasmic markers and loading controls. Analysis of Smad3 localization showed that in nontumorigenic BPH1 cells Smad3 was predominantly localized to the nucleus after TGF-ß1 treatment. In contrast, in BPH1CAFTD1 cells, Smad3 was seen at similar levels in both nuclear and cytoplasmic fractions. Overexpression of myrAkt1 in BPH1 cells (BPH1-Akt) inhibited Smad3 translocation to the nucleus, whereas treatment using the PI3K inhibitors 10 μmol/L Ly294002 (ly) or 100 nmol/L wortmannin (WM) enabled Smad3 to translocate into the nucleus of BPH1CAFTD1. Total and phosphorylated Smad2 localization did not change markedly in response to Akt activation. B, immunofluorescence staining illustrates Smad3 localization in cells with or without TGF-ß1 treatment. Smad3 is visualized in green, whereas nuclei are stained using PI and are shown in red. Without treatment, Smad3 was localized in the cytoplasm in both BPH1 and BPH1CAFTD1 cells. After TGF-ß treatment, almost all of the Smad3 was translocated into the nucleus in BPH1 cells; however, in BPH1CAFTD1 cells, both nuclear and cytoplasmic localization were seen. Pretreatment with wortmannin enhanced nuclear localization of Smad3 in BPH1CAFTD1 cells. C, Smad3 translocation was quantitated using NIH ImageJ software. The average photons locating in the nucleus or in the whole-cell body were measured on a cell-by-cell basis in five random fields. The proportion of the nuclear staining was plotted. BPH1 cells showed much more nuclear Smad3 on TGF-ß1 treatment (25% for pretreatment versus 71% for post-treatment; P < 0.01), but BPH1CAFTD1 did not show significant Smad3 relocalization in response to TGF-ß1 (the nuclear staining proportion was 21% and 24%, respectively; P > 0.05). Wortmannin pretreatment increased the Smad3 nuclear distribution proportion on TGF-ß1 to 61% (P < 0.01).
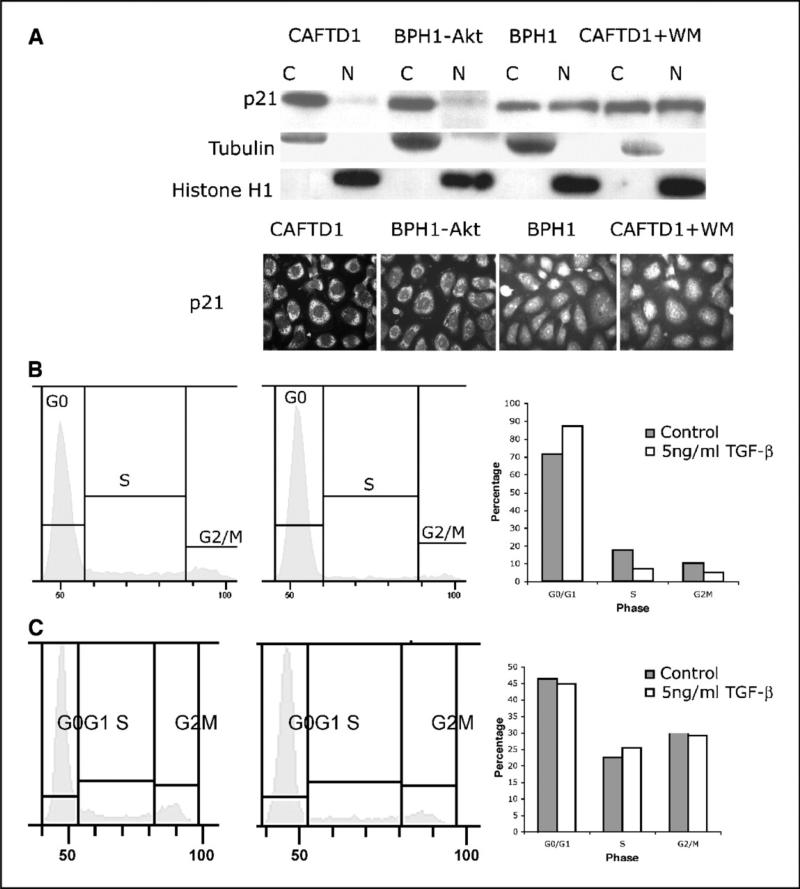
To further assess the influence of TGF-ß on genes downstream of the Smad pathway, we examined the subcellular localization of the growth inhibitor p21CIP. Figure 3A shows that, in tumorigenetic BPH1CAFTD1 cells, TGF-ß treatment results in almost all p21 being localized in the cytoplasm. This is in marked contrast to the nontumorigenic BPH1 cells where p21 is evenly distributed between the cytoplasm and the nucleus. This differential distribution pattern involves phosphorylated Akt because, in BPH1 cells overexpressing myrAkt1, all p21 was seen to be localized in the cytoplasm. Pretreatment of BPH1CAFTD cells with wortmannin resulted in cytoplasmic and nuclear distribution of p21. The distribution of p21 was confirmed by immunofluorescence staining (Fig. 3A). Because the inhibitory function of p21CIP occurs in the nucleus, its exclusion from the nucleus allows the cells to go through G1hase, which could allow BPH1CAFTD1 cells to skip cell cycle arrest on TGF-ß treatment. Cell cycle analysis (Fig. 3B and C) is consistent with this concept.
Figure 3.
Phosphorylated Akt can reduce nuclear p21 and cause the release of G1-S arrest on TGF-ß. A BPH1 and BPH1CAFTD1 cells were treated with TGF-ß1 for 4 hours. Cytoplasmic and nuclear fractions were extracted. Western blotting results show that, in BPH1 cells, p21 locates in both cytosolic and nuclear fractions. In BPH1CAFTD1 cells, only cytoplasmic p21 is seen. Overexpression of myrAkt1 in BPH1 cells profoundly reduces p21 levels in the nuclear fraction, whereas treatment with wortmannin maintains nuclear p21 levels in BPH1CAFTD1 cells. The p21 distribution pattern was confirmed by immunofluorescence staining. B and C, flow cytometric analysis shows that, after 4 hours of TGF-ß1 treatment, BPH1 cells are arrested at G1-S phase (B), whereas BPH1CAFTD1 cells (C) skipped the cell cycle check.
PI3K/Akt signaling is involved in vimentin induction by TGF-ß. To further study the cellular response to TGF-ß, we used cell lines expressing a DATßRI, myrAkt1, or full-length Smad3. The empty vector was infected into the cells as a negative control. In line with observations on the effect of TGF-ß1 (Fig. 1) in nontumorigenic BPH1 cells, the overexpression of dominant active receptor, myrAkt1, or Smad3 did not result in obvious changes in either cell morphology or protein expression profile. In contrast, in tumorigenic BPH1CAFTD cells expressing either the dominant active receptor or myrAkt1, both Western blotting and immunofluorescence staining showed that the mesenchymal marker vimentin was robustly induced, whereas the epithelialmarkers cytokeratin and E-cadherin were concurrently suppressed (Fig. 4A and B ). At the same time, the morphology changed from a cobblestone to a more elongated phenotype. There wereno phenotypic changes induced in these cells by overexpression of Smad3. These results showed that in the tumorigenic cell line BPH1CAFTD1 the activation of TßRI or expression of myrAkt1 led to an expression profile suggestive of EMT, which is characterized by morphologic changes, induction of vimentin, and suppression of cytokeratin and E-cadherin. The results above showed that overexpression of myrAkt1 elicited similar effects on BPH1CAFTD1 cells to treatment with TGF-ß or to overexpression of DATßRI. To further investigate whether PI3K/Akt is necessary for TGF-ß to elicit these effects, we infected BPH1CAFTD1 cells with an adenovirus containing a dominant-negative Akt (DN-Akt; K179M). As an additional separate test, we pretreated cells with the PI3K inhibitor wortmannin before exposure to TGF-ß. The Western blotting results (Fig. 4C) showed that the induction of vimentin by TGF-ß was impaired in BPH1CAFTD1 cells when the PI3K/Akt pathway was blocked by wortmannin or DN-Akt, indicating that the PI3K/Akt pathway is involved in the EMT response to TGF-ß. As a downstream target gene of PI3K/Akt, GSK3ß can be inhibited by Akt-mediated phosphorylation. GSK3ß failed to phosphorylate in this assay, supporting the idea that the DN-Akt construct was active in these cells.
Figure 4.
PI3K/Akt is involved in vimentin induction by TGF-ß signaling. A, immunofluorescence staining of BPH1 and BPH1CAFTD1 cells expressing empty vector (EV), DATßRI (DA), myrAkt1 (Akt), and Smad3 (S3) retroviral constructs. Cells are stained to visualize expression of vimentin, keratin, and E-cadherin. In the nontumorigenic BPH1 cells, a cobblestone epithelial phenotype was maintained under all conditions, with expression of E-cadherin and cytokeratins being maintained and little to no immunoreactivity to a vimentin antibody. In contrast, the tumorigenic BPH1CAFTD1 cells show vimentin induction and suppression of E-cadherin when either the dominant active receptor or myrAkt1 is expressed. Of note, stimulation of the Smad pathway in these tumorigenic cells does not elicit phenotypic changes consistent with EMT. B Western blot analysis confirms the immunocytochemical observations with no vimentin expression seen in BPH1 cells under any circumstance, whereas BPH1CAFTD1 cells show marked increases in vimentin expression when either the dominant active receptor or myrAkt1 was expressed. Normal human prostate fibroblasts (NPF) were used as a positive control C, BPH1CAFTD1 cells were serum starved overnight and treated with TGF-ß1 (5 ng/mL) for 60 hours in the presence or absence of PI3K inhibitor wortmannin (100 nmol/L). The cell lysates were probed with vimentin antibody. After pretreatment with wortmannin, vimentin induction by TGF-ß was impaired. In a parallel study, BPH1CAFTD1 cells were infected by DN-Akt adenovirus 24 hours before treatment with TGF-ß1. The result indicates that, after DN-Akt infection, TGF-ß does not induce vimentin expression in these cells. Phosphorylated GSK3ß was used to confirm kinase inactivation of Akt. ß-Actin was used as loading control.
Activation of TßRI or Akt signaling elicits enhancement of motility. BPH1CAFTD1 cells show enhanced motile ability in wound closure and Transwell migration assays. In a wound-healingassay, the motile ability was checked by quantifying the rate of wound closure (Fig. 5A). The results showed that the BPH1CAFTD1 cells overexpressing either DATßRI or myrAkt1 closedwounds significantly faster (Student's t test, P < 0.01) than empty vector control cells. Smad3-overexpressing cells showed no significant difference from empty vector cells. In a Boyden chamber assay as shown in Fig. 5B, the ability of cells to invade collagen and migrate to the underside of the inserts is determined by a 12-hour response to conditioned medium containingdifferent concentrations of FBS in the lower chamber. The data showed that significantly more DATßRI- and myrAkt1-expressing cells migrated to the underside of the filter compared with the empty vector control cells (Student's t test, P < 0.01). Overexpression of full-length Smad3 showed a trend toward slower migration, which did not reach significant difference from emptyvector controls. These two assays showed that tumorigenic BPH1CAFTD1 cells acquired enhanced motility when either DATßRI or myrAkt1 but not when Smad3 is forcibly activated. The same experiments were done on parental BPH1 cells where no significant changes in wound closure or motility were found.
Figure 5.
Activation of TGF-ß or Akt signaling enhances cell motility. A, in a wound-healing assay, a measure of the speed of BPH1CAFTD1 cells closing the wound showed that both DATßRI and myrAkt1 enhanced cell mobility compared with empty vector, whereas Smad3 expression had no effect. All the experiments were done in triplicate. Columns, mean; bars, SD. P < 0.01 (Student's t test). B, a Transwell migration assay showed similar effects to the wound-healing assay, with both DATßRI and myrAkt1 significantly enhancing invasion in the presence of serum, whereas Smad3 overexpression had a nonsignificant inhibitory effect. All the experiments were done in triplicate. Columns, mean; bars, SD. P < 0.01 (Student's t test).
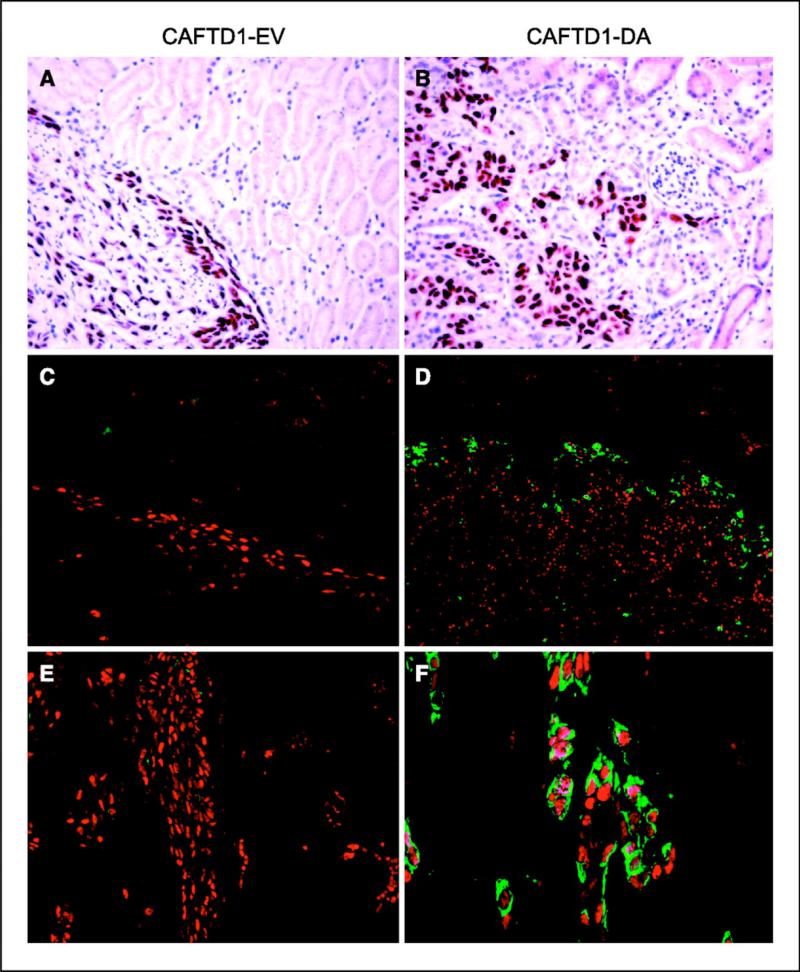
EMT cells lead invasive front in vivo. To confirm that the results observed in vitro corresponded to in vivo phenomena and to test whether EMT is associated with invasion, we suspended BPH1CAFTD1 cells expressing either empty vector (control) or DATßRI in collagen gel and xenografted beneath the kidney capsule of male athymic hosts. Two months later, the grafts were harvested for analysis. At a histologic level, the empty vector control cells grow within a very limited area, which has a sharply delineated border with the host kidney. This is consistent with previous descriptions of the growth of BPH1CAFTD1 tumors that exhibit very little invasive activity (20). In contrast, the DATßRI-overexpressing cells developed large tumors and showed obvious invasion into the adjacent kidney. When staining against SV40 T antigen was used to identify the grafted epithelial cells (Fig. 6), the transgenic DATßRI cells were clearly seen to exhibit an aggressive behavior, invading the kidney parenchyma and surrounding kidney tubules, which was not seen in the empty vector control cells. Double staining with antibodies against SV40T antigen and vimentin shows that control grafts did not express vimentin at the tumor margin. In stark contrast, the invasive DATßR1 cells expressed vimentin along the invasive front but interestingly not in the body of the tumor. Cells coexpressing SV40T antigenand vimentin are clearly visible along this front, confirming their EMT nature. This finding shows not only that EMT can occur in tumorigenic human prostatic epithelium but also that theexpression of this phenotype, even in the face of constitutive signaling, is context dependent.
Figure 6.
TGF-ß stimulates cell invasion in vivo. Grafts of BPH1CAFTD1 cells carrying empty vector control (A, C, and E) or DATßRI (B, D, and F). Cells were grafted to the renal capsule of SCID mice and harvested after 2 months. Sections were stained for expression of SV40T antigen (A and B) or double stained for SV40T antigen (red) and vimentin (green) using immunofluorescence (C-F). BPH1CAFTD1 cells were chosen for this assay as they are minimally invasive, an observation confirmed in the empty vector controls (A, C, and E). These tumors grow on the surface of the kidney with limited invasion and no expression of vimentin. In contrast, cells expressing DATßRI invaded the host kidney infiltrating between, and surrounding, kidney tubules (B, D, and F). Vimentin expression by the epithelial cells was located specifically at the invading front and not in the tumor body (D and F). Coexpression of vimentin and SV40T (shown in detail in F) confirms that the cells that expressed vimentin were derived from the BPH1CAFTD1 line.
Discussion
Many studies have shown that TGF-ß plays a complex role in carcinogenesis, being growth inhibitory for normal epithelial cells but tumor promoting for cancer cells (34). The local concentration of extracellular TGF-ß1 is significantly elevated in prostate cancer compared with normal or benign prostate tissues (35). Cancer cells can acquire resistance to growth inhibitionby TGF-ß through mutation or deletion of either components of the receptors or the signaling cascade or, more often, through the regulation of the downstream signaling molecules (2, 3, 13, 36). Because such an elevated level of a normally growth-suppressive factor would act to inhibit progression, it is clear that a loss in the ability of tumor cells to perceive TGF-ß as a growth inhibitor would provide a significant growth advantage and would thus be expected to be an early change seen in tumor evolution. This elevated level TGF-ß1 is suggested to be indirectly involved in the development of prostate cancer by stimulating angiogenesis and inhibiting immune responses directed against tumor cells (37). Elevated TGF-ß levels can also affect the cancer cells directly, because loss of sensitivity to inhibition of tumor growth by TGF-ß is not synonymous with complete loss of TGF-ß signaling (32, 38).
In the present study, the response to TGF-ß signaling was examined in a related group of nontumorigenic parental and tumorigenic derivative human prostatic epithelial cell lines. During malignant transformation by their stromal environment, the tumorigenic cell lines lost the growth-suppressive response to TGF-ß and acquired a constitutively activated Akt pathway. Elevation of Akt is commonly a response to the loss of the phosphatase and tensin homologue (PTEN) tumor suppressor (39), which occurs frequently in human prostate cancer (40, 41). Therefore, this molecular characteristic of these cells is consistent with differences between normal and malignant cells in many prostate cancer patients. The receptor signaling response to TGF-ß is apparently intact in the tumorigenic BPH1CAFTD1 cells as measured by their ability to phosphorylate Smad3 in response to TGF-ß stimulation. However, we observed that in the tumorigenic cells (in contrast to the nontumorigenic cells) the nuclear translocation of the Smad complex in response to TGF-ß signaling was impaired. We were able to show that the elevated Akt level in the tumorigenic cells was sufficient for this response, because nuclear translocation was restored by blocking the PI3K/Akt pathway. To confirm this, we expressed a myrAkt1 in the benign cells and saw the same inhibition of Smad nuclear translocation as in the tumorigenic cells.
The subcellular localization of the growth inhibitor p21CIP was found to be influenced by elevated levels of activated Akt in the tumorigenic cell lines. p21 is a mediator of TGF-ß signaling, which is active in the nucleus where it acts to inhibit progression through the cell cycle (42, 43). We observed that in the tumorigenic cell lines p21 was excluded from the nucleus; this was not seen in the nontumorigenic cells. This nuclear exclusion could be blocked by wortmannin, which inhibits the Akt/PI3K pathway. When myrAkt1 was expressed in nontumorigenic cells, p21 failed to localize to the nucleus from the cytoplasm following exposure to TGF-ß. This suggests an additional mechanism by which tumorigenic cells with elevated levels of activated Akt might skip growth arrest in response to TGF-ß signaling.
The induction of EMT is another way for TGF-ß to promote carcinogenesis (7, 12). Normal development of the mesodermal germ layer is a result of EMT occurring in the ectodermal epithelium. This fundamental process is required for the existence of animals with three germ layers (44). In both normal tissue differentiation and carcinogenesis, select populations of epithelial cells may undergo EMT, lose epithelial polarity, form actin stress fibers, and differentiate into a mesenchymal, fibroblast phenotype (45). Additionally, delocalization of cell adhesion markers, including E-cadherin, ZO-1, and integrin ß1, allows cells to digest and to migrate through the ECM (46). EMT has been associated with tumor metastasis due to the need for cells to be motile and invasive.
The role of EMT in human cancers is still controversial and their presence or absence in specific tumor types is under investigation. A major problem is the ability of pathologists to positivelyidentify such cells given that their appearance and location would be consistent with mesenchymal cells. A firm resolution of the situation in clinical tumors will await the developmentand application of good antibodies to transcription factors, such as snail and twist, which are involved in EMT. In the meantime, model systems with built-in tracers provide a useful means ofinvestigating the mechanisms underlying the process.
The use of MEK inhibitors and DN-TßRII helped show that TGF-ß is an inducer of EMT (12, 45, 47). Cells containing constructs coexpressing DNRhoA and DNp160ROCK also inhibited EMT (48). Either MAPK/extracellular signal-regulated kinase, PI3K, or both are important in TGF-ß-mediated EMT and/or motility in transformed and metastatic mammary epithelial cells (49). To our knowledge, no data have been reported concerning the role of TGF-ß in the induction of EMT in human prostatic epithelium.
In this study, we saw that TGF-ß induced EMT (characterized as mesenchymal marker induction, epithelial marker suppression, and associated enhanced cell motility) in tumorigenic but not parental nontumorigenic cells. Induction of an EMT phenotype in these cells was impaired by blocking the PI3K/Akt pathway, suggesting that this non-Smad pathway is required for mediating this response in these tumorigenic cells. These data suggest that although the elevated levels of Akt seen in the tumorigenic cells are sufficient to inhibit nuclear translocation of the Smad complex they are insufficient per se to elicit an EMT. Multiple reports suggest that no single component of the TGF-ß signaling pathway can stimulate EMT (5, 6, 8), suggesting that Akt activation is necessary but not sufficient for EMT. Inhibition of Smad nuclear localization, which is seen to occur in the tumorigenic cells, apparently does not inhibit the activation of other signaling pathways.
Stimulation of the tumorigenic cell lines by exogenous TGF-ß or by the introduction of DATßRI (situations in which phosphorylated Akt can cooperate with other TGF-ß pathways) is sufficient to elicit an EMT. However, expression of myrAkt1 also elicits an EMT in these cells. An explanation for this is provided in Supplementary Fig. SB where the tumorigenic lines can be seen to exhibit a basal level of Smad2 phosphorylation presumably due to autocrine activity. This suggests that TGF-ß signaling is active in these cells at some level even in the absence of exogenous ligand, thus providing a mechanism by which other TGF-ß signaling pathways, which can cooperate with myrAkt1, might be stimulated. The observation that the cells do not spontaneously show an EMT phenotype is likely due to a failure of their basal levels of phosphorylated Akt and the weak signaling provided by autocrine TGF-ß to reach a critical signaling threshold necessary for such a transformation.
In vivo expression of DATßRII in BPH1CAFTD1 cells results in the formation of tumors that are much more invasive than the empty vector controls. EMT cells lead the aggressive invasive front but are apparently not present in the noninvading body of the tumor. These observations reflect the importance of cellular context on the responses to specific pathways and support the concept of cells as signaling integrators. Such diverse and cell type–specific effects have been reported by Dr. Roy-Burman's group, who showed that BMP7 arrested growth at G1 phase of BPH1 while inducing EMT in PC-3 cells (50). One of the advantages of the BPH1-BPH1CAFTD cells is the fact that these are nontumorigenic parental and tumorigenic derivative sublines originally from a common clone. Few such interrelated human prostate cells exist. Therefore, comparisons with other cell lines are more generic. There are limited nontumorigenic human prostatic epithelial cell lines available; however, we tested 957E/hTERT immortalized but nontumorigenic prostate epithelial cells (generously supplied by Dr. John Isaacs; refs. 51, 52),which showed growth inhibition but not vimentin induction in response to TGF-ß treatment. In contrast, the tumorigenic RWPE2 cell line (53) showed vimentin induction 72 hours after TGF-ß treatment. Published observations also show that DU145 cells undergo EMT in response to TGF-ß (54).
This study supports a view that TGF-ß has different roles in this model of human prostatic carcinogenesis. In the benign cells, TGF-ß acts as a tumor suppressor by inhibiting cell growth and likely supporting differentiation. However, this growth-inhibitory function can be lost early intumorigenesis as a result of apparently commonly occurring changes, such as loss of PTEN activity resulting in enhanced activation of Akt providing a mechanism to escape growth arrest (41). TGF-ß is expressed at high levels in both epithelial and stromal cells of human prostate tumors, with expression levels negatively correlated with patient prognosis (55). The ability of TGF-ß to stimulate invasion via the induction of EMT may represent an important contribution to the carcinogenic process in the prostate.
Supplementary Material
Acknowledgments
Grant support: NIH grants CA96403 and DK067049 (S.W. Hayward) and DOD-PCRP predoctoral fellowship W81XWH-05-1-0583 (M. Ao). The Vanderbilt University Medical Center Institutional Flow Cytometry Core was supported by the Vanderbilt Ingram Cancer Center (grant P30CA68485).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C.Section 1734 solely to indicate this fact.
We thank Dr. Rik Derynck (University of California at San Francisco) for supplying the DATßRI (T204D) construct, Dr. William R Sellers (Dana-Farber Cancer Institute) for the myrAkt1 construct, Dr. Carlos L. Arteaga (Vanderbilt University Medical Center) for the DN-Akt adenovirus, Evangeline Easterly for help and advice with the iodinated TGF-ß cross-linking assay, and Dr. James N. Higginbotham for his help in the fluorescence-activated cell sorting and cell cycle analysis.
References
- 1.Massague J. TGF-ß signal transduction. Annu Rev Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753. [CrossRef] [Medline] [DOI] [PubMed] [Google Scholar]
- 2.Akhurst RJ, Derynck R. TGF-ß signaling in cancer—a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [Medline] [DOI] [PubMed] [Google Scholar]
- 3.Wakefield LM, Roberts AB. TGF-ß signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–9. doi: 10.1016/s0959-437x(01)00259-3. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 4.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor ß-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–10. doi: 10.1074/jbc.M005912200. [Abstract/ Free Full Text] [DOI] [PubMed] [Google Scholar]
- 5.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFß-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–206. doi: 10.1242/jcs.115.15.3193. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 6.Dumont N, Arteaga CL. Targeting the TGFß signaling network in human neoplasia. Cancer Cell. 2003;3:531–6. doi: 10.1016/s1535-6108(03)00135-1. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 7.Bhowmick NA, Ghiassi M, Bakin A, et al. Transforming growth factor-ß1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyprianou N. Activation of TGF-ß signalling in human prostate cancer cells suppresses tumorigenicity via deregulation of cell cycle progression and induction of caspase-1 mediated apoptosis: significance in prostate tumorigenesis. Prostate Cancer Prostatic Dis. 1999;2:S18. doi: 10.1038/sj.pcan.4500344. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Kyprianou N. Restoration of transforming growth factor ß signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res. 1999;59:1366–71. [Abstract/Free Full Text] [PubMed] [Google Scholar]
- 10.Lee C, Sintich SM, Mathews EP, et al. Transforming growth factor-ß in benign and malignant prostate. Prostate. 1999;39:285–90. doi: 10.1002/(sici)1097-0045(19990601)39:4<285::aid-pros9>3.0.co;2-7. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 11.Barrack ER. TGFß in prostate cancer: a growth inhibitor that can enhance tumorigenicity. Prostate. 1997;31:61–70. doi: 10.1002/(sici)1097-0045(19970401)31:1<61::aid-pros10>3.0.co;2-m. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 12.Ellenrieder V, Hendler SF, Boeck W, et al. Transforming growth factor ß1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001;61:4222–8. [Abstract/ Free Full Text] [PubMed] [Google Scholar]
- 13.Muraoka RS, Dumont N, Ritter CA, et al. Blockade of TGF-ß inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–9. doi: 10.1172/JCI15234. [CrossRef] [Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massague J. How cells read TGF-ß signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 15.Remy I, Montmarquette A, Michnick SW. PKB/Akt modulates TGF-ß signalling through a direct interaction with Smad3. Nat Cell Biol. 2004;6:358–65. doi: 10.1038/ncb1113. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 16.Conery AR, Cao Y, Thompson EA, et al. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-ß induced apoptosis. Nat Cell Biol. 2004;6:366–72. doi: 10.1038/ncb1117. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 17.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561–76. [CrossRef][Medline] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 19.Danielpour D. Functions and regulation of transforming growth factor-ß (TGF-ß) in the prostate. Eur J Cancer. 2005;41:846–57. doi: 10.1016/j.ejca.2004.12.027. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 20.Hayward SW, Wang Y, Cao M, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–42. [Abstract/Free Full Text] [PubMed] [Google Scholar]
- 21.Phillips JL, Hayward SW, Wang Y, et al. The consequences of chromosomal aneuploidy on gene expression profiles in a cell line model for prostate carcinogenesis. Cancer Res. 2001;61:8143–9. [Abstract/Free Full Text] [PubMed] [Google Scholar]
- 22.Hayward SW, Dahiya R, Cunha GR, et al. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31:14–24. doi: 10.1007/BF02631333. [Medline] [DOI] [PubMed] [Google Scholar]
- 23.Wieser R, Wrana JL, Massague J. GS domain mutations that constitutively activate TßRI, the downstream signaling component in the TGF-ß receptor complex. EMBO J. 1995;14:2199–208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barthel A, Kohn AD, Luo Y, Roth RA. A constitutively active version of the Ser/Thr kinase Akt induces production of the ob gene product, leptin, in 3T3-1 adipocytes. Endocrinology. 1997;138:3559–62. doi: 10.1210/endo.138.8.5263. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 25.Bernard DJ. Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone ß subunit in mouse gonadotrope cells. Mol Endocrinol. 2004;18:606–23. doi: 10.1210/me.2003-0264. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 26.Williams K, Fernandez S, Stien X, et al. Unopposed c-MYC expression in benign prostatic epithelium causes a cancer phenotype. Prostate. 2005;63:369–84. doi: 10.1002/pros.20200. [CrossRef] [Medline] [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Yue J, Frey RS, Zhu Q, Mulder KM. Transforming growth factor ß signaling through Smad1 in human breast cancer cells. Cancer Res. 1998;58:4752–7. [Abstract/ Free Full Text] [PubMed] [Google Scholar]
- 28.Nagarajan RP, Chen F, Li W, et al. Repression of transforming-growth-factor-ß-mediated transcription by nuclear factor B. Biochem J. 2000;348(Pt 3):591–6. [CrossRef][Medline] [PMC free article] [PubMed] [Google Scholar]
- 29.Mochizuki H, Matsubara A, Teishima J, et al. Interaction of ligand-receptor system between stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human prostate cancer: a possible predictor of metastasis. Biochem Biophys Res Commun. 2004;320:656–63. doi: 10.1016/j.bbrc.2004.06.013. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 30.Song K, Cornelius SC, Reiss M, Danielpour D. Insulin-like growth factor-I inhibits transcriptional responses of transforming growth factor-ß by phosphatidylinositol 3-kinase/Akt-dependent suppression of the activation of Smad3 but not Smad2. J Biol Chem. 2003;278:38342–51. doi: 10.1074/jbc.M304583200. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 31.Olumi AF, Grossfeld GD, Hayward SW, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11. doi: 10.1186/bcr138. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piek E, Roberts AB. Suppressor and oncogenic roles of transforming growth factor-ß and its signaling pathways in tumorigenesis. Adv Cancer Res. 2001;83:1–54. doi: 10.1016/s0065-230x(01)83001-3. [Medline] [DOI] [PubMed] [Google Scholar]
- 33.Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-ß/ALK5-mediated Smad3 activation. EMBO J. 2006;25:58–69. doi: 10.1038/sj.emboj.7600917. [CrossRef][Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakefield LM, Piek E, Bottinger EP. TGF-ß signaling in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2001;6:67–82. doi: 10.1023/a:1009568532177. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 35.Bello-DeOcampo D, Tindall DJ. TGF-ß1/Smad signaling in prostate cancer. Curr Drug Targets. 2003;4:197–207. doi: 10.2174/1389450033491118. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 36.Derynck R, Akhurst RJ, Balmain A. TGF-ß signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 37.Wikstrom P, Damber J, Bergh A. Role of transforming growth factor-ß1 in prostate cancer. Microsc Res Tech. 2001;52:411–9. doi: 10.1002/1097-0029(20010215)52:4<411::AID-JEMT1026>3.0.CO;2-8. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 38.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-ß family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 39.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–5. doi: 10.1073/pnas.96.8.4240. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies MA, Koul D, Dhesi H, et al. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551–6. [Abstract/Free Full Text] [PubMed] [Google Scholar]
- 41.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 42.Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-ß. Genes Dev. 1995;9:1831–45. doi: 10.1101/gad.9.15.1831. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 43.Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-ß signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91. doi: 10.1016/s0165-2478(02)00023-8. [CrossRef] [Medline] [DOI] [PubMed] [Google Scholar]
- 44.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [Medline] [DOI] [PubMed] [Google Scholar]
- 45.Guo Y, Kyprianou N. Overexpression of transforming growth factor (TGF) ß1 type II receptor restores TGF-ß1 sensitivity and signaling in human prostate cancer cells. Cell Growth Differ. 1998;9:185–93. [Abstract] [PubMed] [Google Scholar]
- 46.Yue J, Mulder KM. Activation of the mitogen-activated protein kinase pathway by transforming growth factor-ß. Methods Mol Biol. 2000;142:125–31. doi: 10.1385/1-59259-053-5:125. [Medline] [DOI] [PubMed] [Google Scholar]
- 47.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-ß induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–36. doi: 10.1083/jcb.127.6.2021. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhowmick NA, Chytil A, Plieth D, et al. TGF-ß signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 49.Janda E, Lehmann K, Killisch I, et al. Ras and TGFß cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang S, Zhong C, Frenkel B, Reddi AH, Roy-Burman P. Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res. 2005;65:5769–77. doi: 10.1158/0008-5472.CAN-05-0289. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 51.Yasunaga Y, Nakamura K, Ko D, et al. A novel human cancer culture model for the study of prostate cancer. Oncogene. 2001;20:8036–41. doi: 10.1038/sj.onc.1205002. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 52.Dalrymple S, Antony L, Xu Y, et al. Role of notch-1 and E-cadherin in the differential response to calcium in culturing normal versus malignant prostate cells. Cancer Res. 2005;65:9269–79. doi: 10.1158/0008-5472.CAN-04-3989. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 53.Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis. 1997;18:1215–23. doi: 10.1093/carcin/18.6.1215. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 54.Brown KA, Aakre ME, Gorska AE, et al. Induction by transforming growth factor-ß1 of epithelial to mesenchymal transition is a rare event in vitro. Breast Cancer Res. 2004;6:R215–31. doi: 10.1186/bcr778. [CrossRef][Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wikstrom P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A. Transforming growth factor ß1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate. 1998;37:19–29. doi: 10.1002/(sici)1097-0045(19980915)37:1<19::aid-pros4>3.0.co;2-3. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.