Abstract
All of us are familiar with the negative impact of interference on achieving our task goals. We are referring to interference by information, which either impinges on our senses from an external environmental source or is internally generated by our thoughts. Informed by more than a decade of research on the cognitive and neural processing of interference, we have developed a framework for understanding how interference impacts our neural systems and especially how it is regulated and suppressed during efficient on-task performance. Importantly, externally and internally generated interferences have distinct neural signatures, and further, distinct neural processing emerges depending on whether individuals must ignore and suppress the interference, as for distractions, or engage with them in a secondary task, as during multitasking. Here, we elaborate on this cognitive framework and how it changes throughout the human lifespan, focusing mostly on research evidence from younger adults and comparing these findings to data from older adults, children, and cognitively impaired populations. With insights gleaned from our growing understanding, we then describe three novel translational efforts in our lab directed at improving distinct aspects of interference resolution using cognitive training. Critically, these training approaches were specifically developed to target improved interference resolution based on neuroplasticity principles and have shown much success in randomized controlled first version evaluations in healthy aging. Our results show not only on-task training improvements but also robust generalization of benefit to other cognitive control abilities. This research showcases how an in-depth understanding of neural mechanisms can then inform the development of effective deficit-targeted interventions, which can in turn benefit both healthy and cognitively impaired populations.
Keywords: interference, distraction, multitasking, attention, cognitive control, cognitive training, neuroplasticity, aging
1 INTRODUCTION
In our modern-day environment, we are immersed in digital media and related mobile technologies that constantly barrage us with an overload of sensory information. More than ever before, there are constant cognitive demands on our neural systems to selectively attend to sensory inputs that are relevant to immediate goals and critically to ignore or minimize the priority of other interfering sources of information. Those individuals who can successfully identify and prioritize relevant information over interference are able to achieve planned goals and function efficiently in demanding cognitive settings. Understanding cognitive functions that allow us to integrate with our complex world in a goal-directed way is the domain of top-down cognitive control research (Bar, 2003; Corbetta and Shulman, 2002; Frith, 2001).
More than a decade of research in the Gazzaley laboratory has revealed that the two sides of top-down control, enhancement of goal-relevant inputs versus suppression of interference, are both equally important and notably are distinct in their underlying neural mechanisms (Clapp et al., 2010, 2011; Gazzaley et al., 2005a, 2008; Zanto et al., 2010). This emerging research recently inspired a new framework for the characterization of interference (Fig. 1, Clapp and Gazzaley, 2012). Per this framework, interference can be generated from either external or internal information sources. External interference occurs from environmental sensory stimuli and can be further classified into “distractions” and “interruptions.” Distractions are to-be-ignored sensory information, like the background chatter when working at a café. Interruptions are external stimuli that need to be attended but are of secondary priority in our top-down goal sets. Interaction with interruptions while attending to primary goal-relevant stimuli qualifies as multitasking, such as may occur when conversing with a copassenger while driving a car. Indeed, research described in the following sections has shown that distractions and interruptions have distinct neural processing. Importantly, this framework has helped clarify prior research in the field that had generated confusing results when both types of external interference were interchangeably employed in cognitive paradigms.
FIGURE 1.
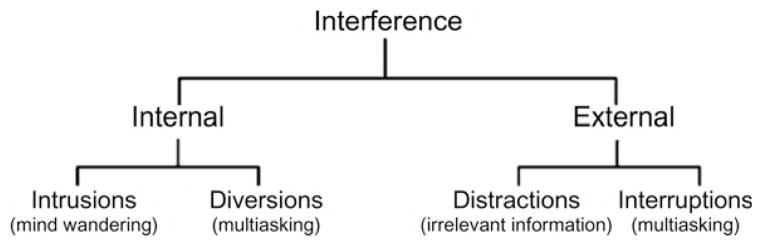
The conceptual framework for classification of different types of interference.
Adapted from Clapp and Gazzaley (2012).
Similar to external interference, internal interference can also be classified either as irrelevant internal distractions termed “intrusions,” as may occur during mind-wandering or daydreaming, or as internal interruptions (i.e., “diversions”) that engage cognitive systems while another primary goal-relevant task is being performed. Planning today’s dinner while reading this chapter is an example of an internal diversion, which like external interruptions involves attempts at multitasking. Note that an understanding of the neural mechanisms that subserve internal interference processing is still a nascent field. While many new studies suggest that internal interference engages a known default mode network (DMN) in the brain (i.e., specific brain regions that are more active during non-task-oriented behaviors; Andrews-Hanna, 2012; Buckner et al., 2008), the exact neural signatures that distinguish internal intrusions versus diversions require sophisticated methodologies such as predictive pattern classification algorithms and are yet to be fully determined.
The following sections serve as an introduction to the basis of the impact of different kinds of interference on human performance. Note that either external or internal interference can occur while one is engaged in either an external or internal goal-relevant primary task. For example, the primary task could be an immediate discrimination task in which individuals must accurately and rapidly respond to relevant stimuli that occur sequentially or simultaneously intermixed with irrelevant inputs, such as taking possession of the ball in a soccer game and making a speedy sensorimotor decision that leads to a goal score in the face of interference from the opponent team. Or the primary task could be a working memory (WM) task that requires maintenance and manipulation of information in mind for short time periods, such as remembering a phone number until the end of a conversation. Alternatively, the primary task could even stretch over longer time scales, drawing on long-term memory (LTM), where stimulus information is maintained over several minutes to hours, while other tasks occur in the interim. Here, we review evidence for interference during each of these multi-time scale cognitive operations: discrimination, WM, and LTM. Note that the research reviewed here is from the standpoint of behavioral and underlying neural performance, that is, neural mechanisms that enable the observed behavioral outcomes. Considering young healthy 20–30-year-old adults as a reference point, we review evidence for modified interference resolution abilities in older age and in children. The second portion of the chapter focuses on novel cognitive training strategies that we have developed as guided by our understanding of interference processing in the brain and the first set of evidence of the effectiveness of these novel approaches.
2 EXTERNAL INTERFERENCE RESOLUTION ACROSS THE LIFESPAN
2.1 Healthy young adults
Almost a decade ago, Gazzaley et al. (2005a,b, 2008) introduced a novel WM paradigm involving delayed recognition, which was used to assess behavioral performance and neural processing of goal-irrelevant distracting images presented in an intermixed sequence with relevant stimuli. An important component of this paradigm was a passive viewing condition, which allowed comparison of neural modulations elicited to attended and ignored stimuli relative to a baseline. These studies showed that healthy young adults enhance sensory neural processing when encoding goal-relevant stimuli relative to passive viewing and further suppress neural activity to irrelevant stimuli. Further, both the magnitude of neural activity and the speed of neural processing were modulated by top-down influences (Gazzaley et al., 2005b). These findings were consistent in fMRI-based BOLD (blood oxygen level-dependent) neural activity in visual cortex and in early sensory ERPs (event-related potentials) and in frontal theta oscillations, which mark top-down engagement with relevant versus irrelevant information.
A more recent study investigated fMRI functional connectivity during a modified version of this experimental paradigm, which used overlapping relevant and irrelevant stimuli presented simultaneously (Chadick and Gazzaley, 2011). This study showed distinct and dynamic cortical connectivity between sensory and prefrontal cortices based on task goals. Relevant stimuli engaged neural connections between sensory and frontoparietal networks, while irrelevant stimuli simultaneously coupled sensory and DMNs. Zanto and Gazzaley (2009) further demonstrated the ecological importance of the neural findings, as optimal WM performance within individuals was related to the extent of neural interference suppression. Finally, Zanto et al. (2011) tightened this neurobehavioral relationship by showing a causal link between prefrontal-mediated modulation of the visual cortex activity during stimulus encoding and WM accuracy, as revealed by a TMS (transcranial magnetic stimulation)-induced perturbation of the inferior frontal junction (IFJ). Note that prior research also found IFJ to be a critical site for cognitive control of interference (Brass and von Cramon, 2004; Brass et al., 2005; Bunge, 2004).
Clapp et al. (2010, 2011) used a different experimental design to probe both types of external interference, distractions and interruptions, introduced during the delay period of a WM task. Again, comparisons to a passive baseline were integral to the study. Performance measures revealed that WM accuracy was significantly reduced in the setting of distraction and even worse when interrupted by another task. Neural data showed suppressed early visual processing of ignored distractions in young adults. In contrast, neural activity to interruptions, which served as stimuli for a secondary discrimination task, was enhanced. Recently, we replicated these findings for intrasensory interference during auditory WM (Mishra et al., 2013). Using fMRI-based functional connectivity analyses, Clapp et al. (2011) further revealed that at the onset of interruptions, connectivity in a visual (visual association cortex, VAC)–prefrontal (middle frontal gyrus, MFG) memory maintenance network was disrupted and dynamically reallocated to the attended interruptor. The memory maintenance network was then reengaged at interruptor offset. Further, the extent of attention-related enhancement to the interruptor directly correlated with reduced WM performance accuracy, revealing how neural network dynamics shape cognitive operations in the face of interference.
Finally, Wais et al. (2010) demonstrated the negative impact of distractions on LTM. Participants encoded a study list of items and were later probed regarding recognition of these items. Visual distractors during the recall phase significantly reduced LTM accuracy. Neurally, diminished recollection was associated with the disruption of functional connectivity in a network involving the left IFG, hippocampus, and VAC. The authors concluded that bottom-up influences from visual distractions interfere with the top-down selection of episodic details mediated by a capacity-limited frontal control region, resulting in impaired recollection. Subsequently, Wais and Gazzaley (2011) showed a similar impact of auditory distractions on LTM.
Overall, these studies characterize the impact of external interference on cognition in young adults. To put this research in context of everyday function, there is growing concern that the constant presence of media in our daily lives is distracting and diminishing task productivity. Indeed, a recent study investigated cognition in young adults as a factor of their media-multitasking index (MMI; Ophir et al., 2009). The MMI probed how often individuals engaged with more than one form of media simultaneously including print media, television, online videos, music, nonmusical audio, video games, phone calls, instant and text messaging, email, web-surfing, and other computer-based applications. Individuals with high MMIs were significantly more susceptible to interference from irrelevant stimuli in cognitive tasks and could not filter out interference in memory, relative to those with low MMIs. Note that while these findings show that high media multitasking is associated with poor interference resolution as also recently shown by Sanbonmatsu et al. (2013), this evidence is not causal.
In contrast to media multitasking, engagement with a single form of media for extended periods of time and its impact on cognitive control abilities have been extensively studied for action video games (AVGs). Young adults who are AVG experts (i.e., engage in AVGs for >5 h per week) have been consistently shown to have superior attention capacities (Dye et al., 2009; Green and Bavelier, 2003). Interestingly, the neural basis of this superior performance was shown to be enhanced suppression of distracting sensory information compared to neural activity in nongamers (Mishra et al., 2011). Of course, from these findings alone, one must not infer that in order to develop superior interference suppression capacities, one must play AVGs. This is especially the case as commercial AVGs have much violent content that has raised popular concern about the negative impact on social affect. Such concern, however, is not completely based on research. In fact, increasing video-game play over the last few years has been associated with declining crime rates, speculated to be due to availability of a safe alternate avenue to vent real-life frustration in action game play (Puzzanchera et al., 2011). While it was serendipitous that commercial AVGs were found to benefit attention, they are likely unsuitable as therapeutics because game dynamics are developed to maximally immerse the user, but are not targeted to specifically influence or improve neural function. Targeted therapeutics that can selectively address neural, cognitive, and behavioral deficits are an urgent need; we discuss our first interventions that address interference suppression in the second half of this chapter. The next subsection describes alterations in interference processing in healthy aging, which has been a focus area in the Gazzaley laboratory. We then review a few related studies in this field in healthy child populations and briefly describe the status of interference suppression in diverse neuropsychiatric populations.
2.2 Healthy older adults
Several studies from the Gazzaley lab have now supported deficits in interference resolution as a neural processing impairment in healthy aging (Gazzaley, 2013). In fact, poor suppression of irrelevant information is postulated to underlie the diverse cognitive deficits observed in aging spanning multiple functional domains, including perception, attention, WM, LTM, and action (Hasher et al., 1999). For example, older adults do not suppress sensory neural processing of irrelevant information during WM encoding (Gazzaley et al., 2005a, 2008; Zanto et al., 2010) and are susceptible to distractions during WM retention periods (Clapp and Gazzaley, 2012). Clapp et al. (2011) showed that similar to younger adults, older adults also engage active sensory–prefrontal memory maintenance networks that disengage when an interruptor is presented. Notably, however, these networks in older adults fail to reengage post-interruption, with prefrontal control regions remaining functionally connected to the interruptor, even though it is no longer relevant. Cashdollar et al. (2013) behaviorally characterized a prolonged association with distractors to be several hundreds of milliseconds longer in aging, negatively impacting the processing of postinterference-relevant inputs. Finally, our lab has also characterized selective age-related deficits in motor inhibition processes and showed that they seem to be distinct from the sensory inhibition deficits (Anguera and Gazzaley, 2011). A recent placebo-controlled study of a cholinergic enhancer (Donepezil) showed that older adults with mild cognitive impairments regain interference suppression function and neural network connectivity with boosted cholinergic transmission (Pa et al., 2013), suggesting a neurochemical basis of the suppression deficits.
Of note, not all external interference processing seems to be impaired in aging. For example, we did not find a greater impact of distractions and interruptions in the auditory modality in older relative to younger adults (Mishra et al., 2013). Older adults also perform equivalently to younger adults in a multisensory setting, even when the audiovisual stimulus content is incongruent/conflicting across the two sensory domains (Mishra and Gazzaley, 2013). Neural data in the audiovisual paradigm, however, suggest that while older adults on average perform as well as younger adults, only high-performing older adults exhibit preserved neural signatures with aging. An audiovisual WM task version with irrelevant stimuli from the auditory modality interleaved between relevant visual stimuli or vice versa also did not reveal an age-related cross-modal suppression deficit (Guerreiro et al., submitted for publication). In general, evidence from these studies agrees with prior cross-modal research in aging, which consistently demonstrates preserved multisensory performance in aging in the face of unisensory cognitive decline, notably in the visual domain (Hugenschmidt et al., 2009a, 2009b; Laurienti et al., 2006). This body of work suggests that older adults may benefit most when functioning within multisensory than unisensory environments.
We have now started to apply targeted and innovative cognitive training approaches in an effort to remediate deficits in interference resolution observed in older adults. These are reviewed in the second half of this chapter. The next section focuses on our current knowledge of interference processing in children.
2.3 Children
Managing sources of external interference during childhood is an emerging societal concern, especially given the tremendous influx of diverse media technologies in modern times that can present pernicious sources of goal-irrelevant interference (Bavelier et al., 2010; Healy, 1998; Jordan, 2004; Schmidt and Vandewater, 2008). Some research even suggests associations between diagnoses of attention-deficit hyperactivity disorder (ADHD) and media use, although this evidence is far from certain (Acevedo-Polakovich et al., 2006; Chan and Rabinowitz, 2006; Milich and Lorch, 1994). The American Academy of Pediatrics recommends limiting media exposure in children to 2 h per day or less based on findings that elevated media use is associated with poor physical, cognitive, and social development and academic underperformance in children (Johnson et al., 2007; Junco, 2011; Junco and Cotten, 2011, 2012; Ozmert et al., 2002). From a neurodevelopmental standpoint, the cortex does not attain full capacity to manage interference until late adolescence/young adulthood (Giedd, 2012; Hagen and Hale, 1974; Harnishfeger and Bjorklund, 1994; Leon-Carrion et al., 2004; Spronk and Jonkman, 2012), which makes neural systems all the more prone to the negative impacts of heightened exposure to interference. Indeed, a recent study showed that teenagers intentionally engage with sources of interference, such as texting and Facebook, even in an observed study environment, which ultimately was associated with poor outcomes on their academic learning (Rosen et al., 2013).
The interference framework that was recently proposed in adults (Clapp et al., 2010) still remains to be neurophysiologically investigated in children. In a first replication of the WM paradigm introduced by Gazzaley et al. (2005a), Wendelken et al. (2011) showed enhanced fMRI-based BOLD activations for task-relevant stimuli in stimulus-selective visual cortex and in dorsolateral pre-frontal cortex (DLPFC). The strength of these activations increased with age from 8 to 14 years, as did improvements in WM accuracy. In contrast to the young and older adult studies, however, this study did not show modulations in irrelevant-stimulus processing, suggesting this critical cognitive operation may be underdeveloped in children. This is not too surprising as top-down control regions, such as the DLPFC, demonstrate particularly delayed maturation in terms of cortical thickness (Gogtay et al., 2004). Further myelination processes, white matter tract development, and increases in tract coherence progressively advance throughout middle childhood and young adulthood (Barnea-Goraly et al., 2005; Giedd, 2004). Putting these significant developmental structural changes in perspective, much future work is needed to understand interference processing and suppression abilities in developing brains. Finally, we note that future research on neural interference suppression during development is even more imperative from the standpoint of crucial deficits in this ability in various young special needs populations such as ADHD (Minear and Shah, 2006) and autism (Adams and Jarrold, 2012; Allen and Courchesne, 2001; Marco et al., 2011). An understanding of the underlying neural correlates and processing deficits in these neural systems would become important targets for future therapeutic remediation.
3 INTERNAL INTERFERENCE RESOLUTION ACROSS THE LIFESPAN
3.1 Healthy young adults
Just as goal-directed activities can be derailed by interference from irrelevant stimuli in the external environment, interference can also arise from the internal milieu in the form of intrusive thoughts, emotions, and urges (Beauregard et al., 2001; Christoff et al., 2009; Dolcos and McCarthy, 2006; Kane et al., 2007; Mason et al., 2007). Following our framework for categorizing different types of external interference, internal interference can also be categorized in a similar manner as either intrusions that parallel external distractions or diversions that parallel external interruptions. Again, the fundamental distinction between intrusions and diversions is the degree of intentionality: intrusions occur spontaneously, often without awareness, while diversions represent volitional thoughts that we entertain while also attempting to complete another task or behavior.
The vast majority of research on internal interference has focused on what we refer to as internal distractions or intrusions. The terminology that appears in the literature, however, is far from standardized. Intrusions have been conceptualized as “mind-wandering” (Mason et al., 2007; Smallwood and Schooler, 2006), “stimulus-independent thought” (Teasdale et al., 1995), “self-generated thought” (Callard et al., 2012), “task-unrelated thought” (Fransson, 2006), “spontaneous cognition” (Andrews-Hanna et al., 2010), “self-focused attention” (Gentili et al., 2009), “introspectively oriented thought” (Fransson, 2005), and more indirectly as “spontaneous fluctuations of attention” (Cohen and Maunsell, 2011). Semantic differences aside, the fundamental characteristic of internal interference is that attention is derailed from an original task or goal and instead becomes focused on internal thoughts. This shift in attentional focus may be either intentional (i.e., diversions) or unintentional (i.e., intrusions) and can occur with or without awareness.
Studies of the frequency of “mind-wandering” show that between 30% and 50% of our waking thoughts are “stimulus-independent” or not related to the primary task or goal at hand (Killingsworth and Gilbert, 2010; McVay et al., 2009; Smilek et al., 2010). These off-task thoughts occur during almost every type of behavior and task that has been monitored (Killingsworth and Gilbert, 2010; McVay et al., 2009), and they result in demonstrable costs in task performance (Smallwood et al., 2007). Internal distractions tend to be overwhelmingly negative with respect to emotional content (Killingsworth and Gilbert, 2010) and the frequency of mind-wandering is inversely associated to telomere length, a molecular marker of severe stress and biological aging (Epel et al., 2013).
There is evidence that “mind-wandering” reflects both state-dependent changes in cognitive status, varying inversely with both task difficulty and arousal (Braboszcz and Delorme, 2011; Smallwood and Schooler, 2006), and trait-level individual differences in executive function (McVay and Kane, 2010). In general, the frequency of reporting internal distractions is inversely correlated with executive processes. Mind-wandering increases as tasks become well practiced (Cunningham et al., 2000; Smallwood et al., 2004); it does not affect performance on easy, mundane tasks, but negatively impacts tasks that involve cognitive control such as WM (Teasdale et al., 1995). Further, the frequency of internal distractions correlates negatively with WM capacity (Kane et al., 2007; Mason et al., 2007; McVay and Kane, 2009). Finally, individuals with ADHD report more internal distractions under a variety of conditions (Shaw and Giambra, 1993).
Neuroimaging research has begun to demonstrate some of the neural correlates of internal distractions, with correlations between mind-wandering and activity in the DMN network as a primary focus. DMN activity is increased during episodes of mind-wandering (Andrews-Hanna et al., 2010; Christoff et al., 2009; Preminger et al., 2011) and the general predilection of participants to mind-wander correlates with increased DMN activity during cognitive tasks (Christoff et al., 2009; Mason et al., 2007). A recent study further showed that DMN activity was high during episodes of mind-wandering when the participants were unaware of the internal distraction but dropped off once the mind-wandering event entered awareness, at which time activity in frontal cognitive control circuits increased (Christoff et al., 2009). A subsequent study of functional connectivity showed positive correlations between DMN activity and cognitive control regions, but a negative correlation with primary sensory and motor areas (Christoff, 2012). This pattern suggests a decoupling of sensory and cognitive control cortices during internal distractions—a finding that is also supported by converging encephalography (EEG) evidence showing attenuated sensory ERPs during episodes of mind-wandering (Kam et al., 2011).
As internal interference is detrimental to on-task performance, it is an imperative target for regulation and suppression. In fact, failure to adequately regulate the impact of internal interference can lead to significant impairments in cognition, social conduct, and affect regulation (Beauregard et al., 2001; Dolcos and McCarthy, 2006; Killingsworth and Gilbert, 2010). Pathological failure to regulate this interference likely plays an important role in a range of mental illnesses (Broyd et al., 2009; Buckner et al., 2008), including ADHD (both inattentive and hyperactive behaviors, Fassbender et al., 2009), posttraumatic stress disorder (intrusive recollections triggered by external cues, Pitman et al., 2012; Yehuda and LeDoux, 2007), major depressive disorder (ruminations and impairments in cognition and attention), traumatic brain injury (executive function deficits), obsessive compulsive disorder (uncontrollable anxieties/obsessions and compulsive behaviors), and substance dependence disorders (uncontrollable cravings and contextual triggers for relapse; Sayette et al., 2010). Because of this vulnerability to disease or trauma, there is much future research needed to understand the capacity and plasticity of internal interference regulation systems, especially for developing targeted interventions that remediate deficits in these regulation processes.
3.2 Healthy older adults
Healthy older adults represent one nonclinical population that has been somewhat better characterized in terms of their susceptibility to internal distractions (Giambra, 1989; Jackson and Balota, 2012; McVay et al., 2013). It is well documented that healthy older adults show decreased WM capacity and deficits in executive control (Braver and Barch, 2002; Gazzaley and D’Esposito, 2007; Salthouse, 1994; Ziegler et al., 2010) and in external distractor suppression (Gazzaley, 2013). Thus, if mind-wandering is inversely related to executive function, we might predict an increased susceptibility of older adults to internal distractions. Surprisingly, several studies have shown that older adults report significantly fewer internal distractions on a variety of tasks (Giambra, 1989; Jackson and Balota, 2012; McVay et al., 2013). One interpretation of these findings is that older adults have insufficient resources to maintain both task-relevant and task-irrelevant thoughts; this notion is consistent with the view that mind-wandering results from failures of cognitive control.
3.3 Children
Very few studies have explored the frequency and nature of internal interference in children. A major hurdle to studying this phenomenon is the potential lack of meta-cognitive insight in children about the target of their attentional focus, although children do appear to have some intuition about the concept of mind-wandering as early as 5½ years of age (Flavell and Flavell, 2004). Early studies that used the Imaginal Processes Inventory to assess mind-wandering and daydreaming found that high school students reported more daydreaming (i.e., intentional diversions as per our framework) but less uncontrolled mind-wandering, when compared to older college students (Taylor et al., 1978). Further, emergence of “constructive daydreaming” and a more positive attitude toward daydreaming occurs between the ages of 5 and 10 (Gold and Henderson, 1990; Henderson and Gold, 1983). More recent studies have sought to assess internal interference by examining relative activity within the DMN during development (Immordino-Yang et al., 2012). In children between 8 and 14 years of age, DMN activity decreased as task demands increased; further, children with ADHD failed to show this deactivation (Fassbender et al., 2009). Children with ADHD showed significantly greater levels of behavioral variability, and this variability was inversely correlated with the degree of DMN deactivation. Converging evidence comes from a study that found that children with ADHD had reduced resting state functional connectivity between DMN regions (Fair et al., 2010). These studies demonstrate the critical importance of the DMN for the regulation of internal interference and again point to a potential neural target for the remediation of deficient interference control in clinical populations such as ADHD.
Having described the recently developed cognitive framework for interference resolution and the impact of interference across the lifespan, we now transition to reviewing novel intervention strategies in this domain. We recently developed these interventions in an attempt to remediate deficits in interference resolution in a targeted manner.
4 NEUROPLASTICITY-TARGETED INTERVENTIONS FOR THE RECOVERY OF DEFICITS IN INTERFERENCE RESOLUTION
It is evident from the research reviewed in the prior section that interference control is a vital component of cognition at every level of cognitive engagement. Also as noted, poor interference control is a common deficit in many neuropsychiatrically impaired populations. Further, the negative impacts of interference seem to be increasing in healthy individuals living in modern environments that are burgeoning with media and mobile technology. Currently, no therapeutics exist that selectively and effectively address the problem of poor interference resolution abilities. Development and scientific evaluation of such interventions, especially in the form of computerized cognitive training that is accessible to many people, is a major research emphasis at the Gazzaley laboratory.
Brain plasticity research has shown that it is possible to develop cognitive training approaches that powerfully drive plasticity of specific neural circuits and can be used to achieve behavioral and neurological remediation (Anguera et al., 2013; Merzenich and deCharms, 1996; Merzenich et al., 1991). Such neuroplasticity-targeted training is built on two important principles: (1) continuous performance feedback and (2) performance-adaptive modulations of task challenge. Punctuated rewarding feedback is provided to the trainee throughout training at multiple time scales: on every behavioral trial that lasts a few seconds, summary feedback at end of every training session block that occurs every few minutes, daily summary feedback provided at end of each day of training, and finally feedback related to training progress over multiple days. This elaborate scheme of performance feedback has been empirically found to be vital for engaging the trainee and providing them insight into their specific cognitive impairments, which can then be recovered through performance-adaptive training. Per the second principle, the training is continuously adaptive to each trainee’s performance capacities. Adaptive mechanics scale up task challenge when performance is accurate and at reasonably fast response times, while task challenge is reduced when poor performance is encountered. Importantly, the adaptive staircase algorithms are set such that on average, the training generates 75–85% correct performance; at these performance levels, it has been observed that the trainee is optimally engaged, enjoys training, and has high compliance. Thus, continuous performance feedback and adaptive task challenge uniquely customize the training to the cognitive status of each individual. These training principles have been previously applied in successful language learning programs for dyslexic children in over 3.5 million children worldwide by Scientific Learning® (Hayes et al., 2003; Tallal et al., 1996; Temple et al., 2003), in more than 200,000 elderly adults to ameliorate declining cognitive function in old age by Posit Science® (Anderson et al., 2013; Ball et al., 2010; Mahncke et al., 2006; Smith et al., 2009; Wolinsky et al., 2013), and in WM training for children with attention deficits developed by CogMed® (Klingberg, 2010; Rutledge et al., 2012), to name a few. Importantly, however, cognitive training for interference regulation and suppression has not been directly targeted in past efforts. Notably, there is a need for training that is specifically targeted to the interference regulation deficit given that nonspecific brain training programs show little or no transfer of benefit to these critical cognitive functions, especially in the setting of daily life cognitive tasks (Owen et al., 2010; Zelinski, 2009).
4.1 Adaptive training to remediate external distractibility in older adults
In a recently completed study, we developed a novel cognitive training approach to specifically address the issue of heightened distractibility—an impactful cognitive deficit in aging and in many neuropsychiatric populations (Mishra et al., submitted for publication). A need for a targeted intervention was evident, since despite previous efforts, no training approach had resulted in reduced distractibility in aging (Berry et al., 2010; Buitenweg et al., 2012; van Muijden et al., 2012; Wilkinson and Yang, 2012) or in child populations (Stevens et al., 2008; Thorell et al., 2009). Of note, Berry et al. (2010) did demonstrate successful cognitive and neurophysiological improvements as a result of visual perceptual training in aging. Ten hours of training to discriminate simple visual Gabor patches, which expanded or contracted within short 50–200 ms display durations, not only improved on-task perception but also benefitted delayed-recognition WM of untrained dot motion kinematogram stimuli. Moreover, this transfer of benefit was not just confined to WM behavioral accuracy, neural evidence showed functional plasticity of early sensory processing of the encoded WM stimuli within the visual N1 ERP component, which correlated with the performance gains. Thus, this study was unique in showing robust transfer of benefit in both cognitive performance and underlying neurophysiology. However, an assay of WM in the presence of distractions conducted in the study did not yield any transfer effects.
We recently approached the problem of heightened distractibility in aging from a neural perspective with the goal of training participants to suppress the neural processing of distracting stimuli via engagement in an environment of progressively increasing distractor challenge. The way to succeed in such training is to discriminate relevant informative targets amidst irrelevant distractor nontargets, which resemble the target to a greater and greater extent as performance improves. The degree of distractor challenge is thus adaptively determined by the discrimination performance of the trainee on each trial. This adaptive distractor training was conceptualized in collaboration with Merzenich and colleagues, who had recently shown that aging rats also exhibit a distractor suppression deficit (de Villers-Sidani et al., 2010). Intriguingly, this rat study revealed that when aging rats are subjected to adaptive target training (i.e., progressively increased target challenge amidst a fixed background of distractors), many age-related cortical processing deficits were recovered, but with the exception of the distraction suppression deficit, again stressing the need for a targeted intervention approach.
We thus embarked on adaptive distractor training in parallel experiments on older rats and older humans. Training was implemented in the auditory modality, given its greater feasibility in rats. Thirty-six training sessions were undertaken in both species over a 1-month training period: 1 h per session in rats and 10 min per session in humans. In each training session block, the rat or human discriminated a new target tone frequency chosen pseudorandomly in the 0.4–2 kHz frequency range amidst distractor tones that spanned a 0.2–4 kHz range. A triplet tone sequence was presented on each trial and the trainees discriminated whether the target tone was present within that sequence. Success was rewarded by a game score increase in humans and a food reward in rats. Critically, each trial’s performance led to adaptive modification of the distractor frequency range on the next trial, which was progressively moved within a ±2 to ±0.1 octave range relative to the target.
At the end of the adaptive distractor training, we observed a 43% and 50% improvement in target discrimination amidst distractors in rats and human, respectively. This translated to improvements in octave resolution of large effect sizes (Cohen’s d) of 1.06 in rats and 1.48 in humans. A hits versus false alarms analysis showed that training-related discrimination improvements were specifically driven by diminished distractor-related false-positive errors, which reduced by 53% and 36% from onset of training in rats and humans, respectively, while target hit rate remained constant through training.
The neural plasticity underlying these behavioral effects was analyzed in single-and multiunit neuronal recordings in anesthetized rats and high-density EEG recordings in awake humans. Neurons in the rat auditory cortex consistently showed suppression of distractor responses in the trained but not untrained animals, while target responses measured to “oddball” deviant stimuli were unaffected. Concomitantly, the tonotopic organization of the rat auditory cortex was improved in its spectral and spatial response sensitivity as revealed by sharper neuronal tuning bandwidth measures and reduced spatial receptive field overlap, respectively. Based on prior literature, it is most likely that the improvements in the organization of the aging auditory tonotopic map are a direct outcome of the improved distractor response inhibition at the single neuron level (Zheng and Knudsen, 1999).
In parallel, the neural population recordings in awake humans analyzed using ERPs showed that early sensory auditory processing of distractors was selectively attenuated in trained, but not untrained individuals. Moreover, this sensory modulation (i.e., reduced distractor processing) directly correlated with improved on-task behavioral performance. In humans, we had the opportunity to probe top-down engagement with distracting information using frontal theta oscillations. Early 50–150 ms frontal theta power was indiscriminately enhanced in repeat assessments in untrained individuals suggesting increased overall, but non-stimulus-selective engagement in the second versus first assessment. In contrast, trained individuals showed selective enhancement of target-linked theta while distractor-related theta remained near pretraining levels. These data suggested selectively restrained top-down engagement with distractors. In fact, individuals with reduced post- versus pretraining distractor-related frontal theta also showed the most improved sensory signal-to-noise contrasts in post- versus pretraining ERPs. This early frontal theta localized to known cognitive control sites that have been shown to be involved in interference regulation in the region of the IFJ (Brass et al., 2005; Zanto et al., 2011). Finally, a close link between the frontal and sensory regions was revealed by theta phase coherence modulations between frontal and temporal (auditory sensory) regions at peak activity electrode sites. This phase coherence was exclusively diminished for distractors posttraining and selectively in trained older humans. This reduced frontosensory phase coherence for trained distractors was interpreted as reduced distractor encoding in a functional network that represents task-relevant targets. These coherence effects are in line with recent research from the lab showing that sensory cortices encoding task-relevant versus distracting information preferentially connect with different cognitive control networks, the frontoparietal network and the DMN, respectively (Chadick and Gazzaley, 2011).
Overall, the neural data demonstrated converging and multiple scales of neural plasticity exhibited in both rats and humans as a result of adaptive distractor training. The investigation in anesthetized rats provided insight into pure bottom-up sensory plasticity in the absence of top-down cognitive control influences. This can be rarely achieved in humans. The neural findings in humans complemented the results in rat data, showing evidence for improved sensory distractor suppression posttraining. Human neurophysiology further highlighted that distractor suppression is a dynamic network process, such that top-down frontal regulation is intimately modulated along with the observed sensory changes. Finally, in this study, we evaluated transfer of benefit of the tone-based distractor training in humans on standard measures of cognitive control, which assayed WM span, delayed WM recognition accuracy in the presence of interference and sustained attention. WM span was exclusively benefitted in the training group at an effect size of 0.94; it was notable that these gains were observed for sequence span of letter and number stimuli despite the fact that the training used elementary tone stimuli. While the other two cognitive tests did not show group-level improvements, individual-level benefits were observed such that individuals who improved most in adaptive distractor training also showed the most gains in sustained attention and in WM recognition in the presence of interference, thus offering some evidence of improved interference regulation with training. Overall, this training study showed how adaptive training can be used to selectively tune deficient neural circuits by focusing the adaptive task challenge on the deficient neurobehavioral process, here distractor suppression. This critical insight paves the way for the effective development of future cognitive training and neurotherapeutic approaches that are selectively targeted to specific neural dysfunctions.
4.2 A multitasking video game to enhance cognitive control in older adults
In our interference framework, external interruptions are induced by multitasking behavior that demands the performance of a secondary task concurrently with a primary task. In a recent study of 174 individuals using a custom-designed, targeted 3D video game, “NeuroRacer,” we showed a rapid decline in multitasking abilities from ages 20 to 79 (Anguera et al., 2013). “NeuroRacer,” developed in the lab in collaboration with professional game designers, assessed perceptual discrimination abilities (“Sign” task) with and without concurrent visuomotor tracking (“Drive” task). The “Sign” task required discrimination of a specific colored shape target (e.g., a green circle) amidst a rapid sequential stream of eight other colored shapes that had either one or no common features with the target shape. During “Sign and Drive,” the “Sign” task had to be performed concurrently with the “Drive” task to maintain a car in the center of a winding road using a joystick (Fig. 2). Concurrent “Sign and Drive” performance was compared to “Sign Only” performance to generate a multitasking cost index. Multitasking performance was observed to steadily deteriorate in a linear fashion across the lifespan, with an average cost of −26% in the second decade of life declining to −65% in the seventh decade.
FIGURE 2.
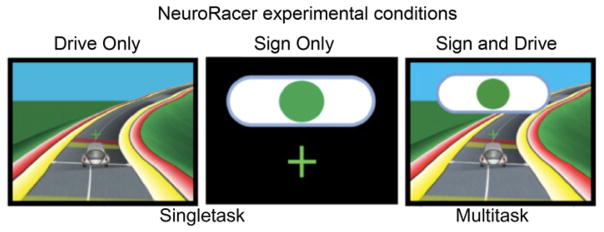
“NeuroRacer” experimental conditions shown as screenshot captures from the training task.
Adapted from Anguera et al. (2013).
We then used “NeuroRacer” to investigate if multitasking abilities on the game can be improved through training. In a randomized controlled trial design, 46 older adults (60–85 years) were assigned to one of three groups: multitasking training (MTT; n =16), single-task training (STT; n =15) as an active control, or no-contact control (NCC; n =15). Training involved playing an assigned version of “Neuro-Racer” on a laptop at home for 1 h a day, three times a week for 4 weeks (12 total hours of training), with all groups returning for 1 month posttraining and 6-month follow-up assessments. The MTT group exclusively engaged in the “Sign and Drive” condition during the training period, while the STT group divided their time between a “Sign Only” and a “Drive Only” condition trained separately on each training day. Thus, MTT and STT groups were matched for all factors except for the presence of simultaneous interference that only occurred for the MTT group. Training results showed that multitasking performance costs on the game were significantly reduced exclusively in the MTT group (−64.2% to −16.2% pre- vs. posttraining cost); interestingly, these improvements reached levels that were superior to performance of a 20-year-old cohort that performed a single session of “NeuroRacer” (−36.7% cost, p <0.001). Of note, training-driven multitasking performance improvements on the game remained stable at a follow-up assessment 6-month after the completion of training (−21.2% cost at follow-up).
Following the training period, we assessed for generalized improvements in cognitive control abilities that are known to be impaired in aging on untrained tasks of sustained attention, divided attention, and WM. We found improvements were exclusively present for the MTT group for both WM (Clapp et al., 2011) and sustained attention (TOVA; Greenberg, 1996). These important transfer of benefits from “NeuroRacer” to untrained cognitive control tasks suggested that a common, underlying mechanism of cognitive control was challenged and enhanced in the MTT group. Of note, these transfer of benefits brought about by multitasking in an engaging and immersive 3D video game were of larger scale than observed for prior dual-task training approaches, which have been implemented in more sparse environments (Erickson et al., 2007; Lussier et al., 2012).
To assess the neural basis of the performance improvements, we quantified event-related spectral perturbations (ERSPs) and long-range phase coherence time-locked to the occurrence of “Signs” in an assessment version of “NeuroRacer” at pre- and posttraining. We specifically assessed ERSP and frontal–occipital coherence measures in midline frontal theta (4–7 Hz) oscillations that are a known marker of top-down engagement as shown in previous studies of WM (Onton et al., 2005) and sustained attention (Sauseng et al., 2007). Separate analyses for theta band power and coherence each revealed significant 3-way interactions of condition (“Sign and Drive” and “Sign Only”), X session (pre and post), and X group (MTT, STT, and NCC), with follow-up analyses indicating that only the MTT group demonstrated a significant increase from pre- to posttraining in both neural measures and exclusively for the “Sign and Drive” condition. Notably, midline frontal theta power, which was deficient relative to theta observed in a younger cohort (20–29 years), was enhanced to levels comparable to younger adults selectively in the MTT group. These data clearly demonstrated that selective neuroplastic changes stemmed from the cognitively demanding interference between the “Sign” and “Drive” tasks when participants were motivated to engage in them simultaneously. Coupled with previous findings of increased midline frontal theta on a variety of cognitive control tasks (Mitchell et al., 2008), our results support a common neural basis for cognitive control processes, which can be enhanced by immersion in an adaptive, high-interference environment. This interpretation is bolstered by evidence indicating that MTT-induced increases in midline frontal theta power during “Sign and Drive” were positively correlated with improvements in the sustained attention transfer task (Fig. 3). Thus, MTT-induced enhancement of midline frontal theta power was associated with generalized benefits on an untrained cognitive control task, reflecting its utility as a neural signature of plasticity in cognitive control processes.
FIGURE 3.
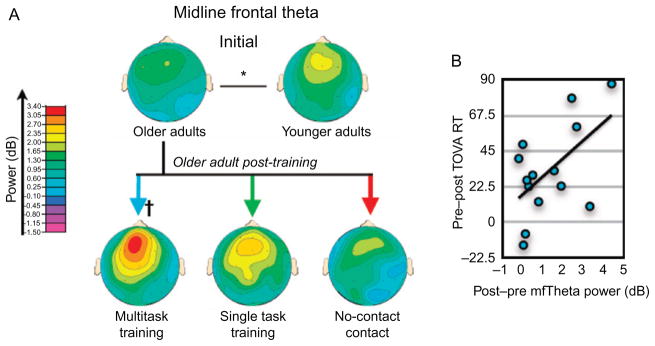
Midline frontal theta activity modulation in “NeuroRacer,” adapted from Anguera et al. (2013). (A) Theta power 1-month after training improved significantly only for the multitask training group; and increased power was observed for younger versus older adults. (B) Correlation in the multitask training group between the change in midline frontal theta power and behavioral improvement on the TOVA (a test of sustained attention). †p <0.05 within group improvement from pre to post, *p <0.05 between groups.
Parallel research has shown that midline frontal theta power is inversely correlated with activity in medial prefrontal cortex related to the DMN (Scheeringa et al., 2008). Thus, it can be hypothesized that “NeuroRacer” may benefit cognitive control by improving the ability of older adults to suppress the DMN during task engagement, a process known to be compromised in aging (Damoiseaux et al., 2008). Future neurophysiological and neurochemical studies are imperative to clarify the causal relation between medial prefrontal activity and the robust performance gains observed here. Overall, the large and sustained reduction in multitasking costs and the generalization of benefits to untrained cognitive control abilities evidenced in our immersive, interference-laden video-game training emphasize the importance of deficit- and circuit-targeted neurotherapeutic approaches to treat clinical populations with deficient cognitive control (e.g., ADHD, depression, and dementia).
4.3 Enhancing self-regulation of internal distractions
Very few studies have examined the potential for training humans to improve their ability to self-regulate against the negative impact of internal interference. A recent study examined the efficacy of a 2-week mindfulness training course in improving on-task performance and reducing episodes of mind-wandering (Mrazek et al., 2013). Compared to a control group that completed a nutrition education program, participants in the training group showed a significant decline in the number of task-unrelated thoughts measured using both self-report and thought probe methods. This decrease in mind-wandering was associated with an increase in reading comprehension scores and WM capacity, a finding consistent with an earlier study that showed a negative association between scores on a mindfulness inventory and the frequency of mind-wandering and attentional lapses on a sustained attention task (Mrazek et al., 2012). Interestingly, a study of long-term meditators found a dynamic relationship between time spent practicing concentration meditation and DMN activity: meditators with a moderate amount of training showed greater DMN activity, compared to novice controls, while expert meditators showed a decrease in DMN activity (Brefczynski-Lewis et al., 2007). These findings suggest common processes may underlie self-regulation of internal distractions and some aspects of meditation or mindfulness and support the notion that such practices may influence plasticity of internal distractor suppression networks. A more detailed understanding of the neural underpinnings of internal interference is bound to inform future training approaches, allowing them to target and drive remediative plasticity within the specific neural networks that generate aberrant and unregulated levels of internal interference.
We have recently developed an original meditation-inspired, plasticity-based cognitive training program for improving self-regulation of attention, metacognitive awareness, and suppression of internal distractions. Our training paradigm consists of a portable application deployed on an iPod, which is designed to integrate aspects of meditation training, specifically, attention to breath and monitoring quality of attention. In addition to incorporating meditation-based practices, our training application integrates plasticity-based cognitive training methods, including quantifiable goals, performance feedback, and performance-driven adaptivity of task challenge. Our approach to studying internal distraction regulation training is not intended to replace the many physiological benefits that meditation engenders, including stress reduction, body awareness, and compassion. Instead, by focusing on a constrained aspect of meditation (i.e., focused attention and awareness of internal distractions), we reduce the variability involved in standard meditation practice, thus allowing us to study the effects of directed training on self-regulation of internal distractions.
We are currently assessing the efficacy of our new internal distraction training paradigm (Ziegler et al., 2013). In a pilot study, participants completed a 1-week, daily, at-home training study in which they used our novel interactive iPod application designed to adaptively increase their ability to sustain attention to their breath while minimizing internal distractions. Before and after training, healthy younger adults performed a mental rotation task with and without auditory noise delivered through headphones. After each trial, participants reported if they were distracted by internal thoughts or by some external factor. Analyses of baseline behavioral data revealed an interesting pattern whereby internal distractions were more frequent than external distractions in the no-noise condition, while presence of external auditory noise led to an increase in external distractions and a decrease in internal distractions. Focused training was associated with significant increases in the total distraction-free time over the course of the 1-week training. Following training, we found a significant decrease in reports of internal distractions during the noise-absent mental rotation task, while reports of external distractions did not differ significantly between pre- and posttraining sessions (Fig. 4). These results suggest that internal and external distractions interact dynamically depending on the stimulus environment. These pilot data provide additional evidence supporting the idea that regulation of internal distractions can be modified by a training application that integrates meditation principles with plasticity-based cognitive training methods. A randomized controlled evaluation of this novel training paradigm is currently underway.
FIGURE 4.
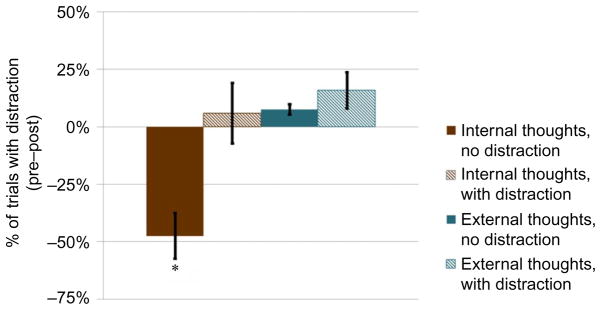
Difference in reports of internal and external thoughts between pre- and posttraining cognitive sessions during performance of a mental rotation task either with auditory background noise (distraction) or without background noise (no distraction). Errors bars are standard errors of mean, *p <0.02, one sample t-test.
5 CONCLUSIONS
In this chapter, we describe our recently developed framework for interference in cognitive processing. Much research from our lab laid the groundwork for the development of the interference framework, which divides cognitive interference as either externally or internally generated. Each of these divisions can then be further subdivided into two levels based on the extent of required top-down engagement with the external/internal interference. External and internal distractions imply irrelevant information that must be ignored, while external and internal interruptions set up multitasking scenarios where the individual must engage in two simultaneous tasks. Notably, this multilevel classification has helped resolve much debate in the literature regarding interference processing, as research from our lab has shown that distractions versus interruptions have distinct underlying neural mechanisms and neural network dynamics. Critically, we have further elucidated that older adults exhibit aberrant neural dynamics of both types of interference processing relative to young adults, which impacts their performance on a variety of cognitive tasks. We are now in the process of characterizing interference processing in the developing child brain, which notably has much real-world relevance in our media and technology-heavy, interference-laden modern environments.
Finally, we describe three novel cognitive training approaches that we recently developed to selectively target and improve processing of external distractions, external interruptions/multitasking, and internal distractions. The need for distinct training approaches for each of these forms of interference follows logically from neuroscientific evidence for distinct underlying neural networks subserving these interference processes. We principally demonstrate that neuroplasticity-based training strategies, which provide continuous performance feedback and adaptively modify interference challenge during training, are highly successful in the remediation of interference resolution abilities. Thus, we envisage that our approaches can be potentially applied to benefit clinical populations that have significant deficits in interference resolution and thus inform the future of targeted neurotherapeutic interventions.
Acknowledgments
This work was supported by the National Institutes of Health grants 5R01AG030395 (AG), 5RO1AG0403333 (AG), 5R21AG041071 (AG), and 5R24TW007988-05 subaward VUMC38412 (JM); the UCSF Institutional Research and Career Development Award (JAA); the Robert Wood Johnson Foundation; and the Posit Science Corporation.
References
- Acevedo-Polakovich ID, Lorch EP, Milich R, Ashby RD. Disentangling the relation between television viewing and cognitive processes in children with attention-deficit/hyperactivity disorder and comparison children. Arch Pediatr Adolesc Med. 2006;160:354–360. doi: 10.1001/archpedi.160.4.354. [DOI] [PubMed] [Google Scholar]
- Adams NC, Jarrold C. Inhibition in autism: children with autism have difficulty inhibiting irrelevant distractors but not prepotent responses. J Autism Dev Disord. 2012;42:1052–1063. doi: 10.1007/s10803-011-1345-3. [DOI] [PubMed] [Google Scholar]
- Allen G, Courchesne E. Attention function and dysfunction in autism. Front Biosci. 2001;6:D105–D119. doi: 10.2741/allen. [DOI] [PubMed] [Google Scholar]
- Anderson S, White-Schwoch T, Parbery-Clark A, Kraus N. Reversal of age-related neural timing delays with training. Proc Natl Acad Sci U S A. 2013;110:4357–4362. doi: 10.1073/pnas.1213555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The Brain’s default network and its adaptive role in internal mentation. Neurosci: Rev J Bringing Neurobiol Neurol Psychiatr. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera JA, Gazzaley A. Dissociation of motor and sensory inhibition processes in normal aging. Clin Neurophysiol. 2011;123:730–740. doi: 10.1016/j.clinph.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, Kong E, Larraburo Y, Rolle C, Johnston E, Gazzaley A. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Edwards JD, Ross LA, McGwin G., Jr Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58:2107–2113. doi: 10.1111/j.1532-5415.2010.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J Cogn Neurosci. 2003;15:600–609. doi: 10.1162/089892903321662976. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Green CS, Dye MW. Children, wired: for better and for worse. Neuron. 2010;67:692–701. doi: 10.1016/j.neuron.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Lévesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, Gazzaley A. The influence of perceptual training on working memory in older adults. PLoS One. 2010;5:e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braboszcz C, Delorme A. Lost in thoughts: neural markers of low alertness during mind wandering. Neuroimage. 2011;54:3040–3047. doi: 10.1016/j.neuroimage.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Selection for cognitive control: a functional magnetic resonance imaging study on the selection of task-relevant information. J Neurosci. 2004;24:8847–8852. doi: 10.1523/JNEUROSCI.2513-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuro-modulation. Neurosci Biobehav Rev. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Bio-behav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buitenweg JIV, Murre JMJ, Ridderinkhof KR. Brain training in progress: a review of trainability in healthy seniors. Front Hum Neurosci. 2012;6:183. doi: 10.3389/fnhum.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Callard F, Smallwood J, Margulies DS. Default positions: how neuroscience’s historical legacy has hampered investigation of the resting mind. Front Psychol. 2012;3:321. doi: 10.3389/fpsyg.2012.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar N, Fukuda K, Bocklage A, Aurtenetxe S, Vogel EK, Gazzaley A. Prolonged disengagement from attentional capture in normal aging. Psychol Aging. 2013;28:77–86. doi: 10.1037/a0029899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadick JZ, Gazzaley A. Differential coupling of visual cortex with default or frontal–parietal network based on goals. Nat Neurosci. 2011;14:830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PA, Rabinowitz T. A cross-sectional analysis of video games and attention deficit hyperactivity disorder symptoms in adolescents. Ann Gen Psychiatr. 2006;5:16. doi: 10.1186/1744-859X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K. Undirected thought: neural determinants and correlates. Brain Res. 2012;1428:51–59. doi: 10.1016/j.brainres.2011.09.060. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Gazzaley A. Distinct mechanisms for the impact of distraction and interruption on working memory in aging. Neurobiol Aging. 2012;33:134–148. doi: 10.1016/j.neurobiolaging.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Gazzaley A. Mechanisms of working memory disruption by external interference. Cereb Cortex. 2010;20:859–872. doi: 10.1093/cercor/bhp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc Natl Acad Sci U S A. 2011;108:7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. When attention wanders: how uncontrolled fluctuations in attention affect performance. J Neurosci. 2011;31:15802–15806. doi: 10.1523/JNEUROSCI.3063-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cunningham S, Scerbo MW, Freeman FG. The electrocortical correlates of daydreaming during vigilance tasks. J Ment Imag. 2000;24:61–72. [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SARB. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- De Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RCS, Merzenich MM. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci U S A. 2010;107:13900–13905. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye MW, Green CS, Bavelier D. The development of attention skills in action video game players. Neuropsychologia. 2009;47:1780–1789. doi: 10.1016/j.neuropsychologia.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Puterman E, Lin J, Blackburn E, Lazaro A, Mendes WB. Wandering minds and aging cells. Clin Psychol Sci. 2013;1:75–83. [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kim JS, Alvarado M, Kramer AF. Training-induced plasticity in older adults: effects of training on hemispheric asymmetry. Neurobiol Aging. 2007;28:272–283. doi: 10.1016/j.neurobiolaging.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TGC, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatr. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell JH, Flavell ER. Development of children’s intuitions about thought–action relations. J Cogn Dev. 2004;5:451–460. [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Frith C. A framework for studying the neural basis of attention. Neuropsychologia. 2001;39:1367–1371. doi: 10.1016/s0028-3932(01)00124-5. [DOI] [PubMed] [Google Scholar]
- Gazzaley A. Top-down modulation deficit in the aging brain: an emerging theory of cognitive aging. In: Knight RT, Stuss DT, editors. Principles of Frontal Lobe Function. Oxford University Press; USA: 2013. pp. 593–608. [Google Scholar]
- Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005a;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005b;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D’Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, Guazzelli M. Beyond amygdala: default Mode Network activity differs between patients with social phobia and healthy controls. Brain Res Bull. 2009;79:409–413. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Giambra LM. Task-unrelated-thought frequency as a function of age: a laboratory study. Psychol Aging. 1989;4:136–143. doi: 10.1037/0882-7974.4.2.136. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN. The digital revolution and adolescent brain evolution. J Adolesc Health. 2012;51:101–105. doi: 10.1016/j.jadohealth.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SR, Henderson BB. Daydreaming and curiosity: stability and change in gifted children and adolescents. Adolescence. 1990;25:701–708. [PubMed] [Google Scholar]
- Green CS, Bavelier D. Action video game modifies visual selective attention. Nature. 2003;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- Greenberg LM. TOVA continuous performance test manual. Los Alamitos, CA: Universal Attention Disorders; 1996. [Google Scholar]
- Guerreiro M, Anguera J, Mishra J, Van Gerven P, Gazzaley A. Age-equivalent top-down modulation during cross-modal selective attention. submitted for publication. [DOI] [PubMed] [Google Scholar]
- Hagen JW, Hale GA. The Development of Attention in Children. Educational Testing Service; Princeton, NJ: 1974. [Google Scholar]
- Harnishfeger KK, Bjorklund DF. The development of inhibition mechanisms and their relation to individual differences in children’s cognitions. Learn Individ Differ. 1994;6:331–335. [Google Scholar]
- Hasher L, Zacks RT, Rahhal TA. Timing, instructions, and inhibitory control: some missing factors in the age and memory debate. Gerontology. 1999;45:355–357. doi: 10.1159/000022121. [DOI] [PubMed] [Google Scholar]
- Hayes EA, Warrier CM, Nicol TG, Zecker SG, Kraus N. Neural plasticity following auditory training in children with learning problems. Clin Neurophysiol. 2003;114:673–684. doi: 10.1016/s1388-2457(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Healy JM. Failure to Connect: How Computers Affect Our Children’s Minds—and What We Can Do About It. SImon and Schuster Paperbacks; New York: 1998. [Google Scholar]
- Henderson BB, Gold SR. Intellectual styles: a comparison of factor structures in gifted and average children and adolescents. J Pers Soc Psychol. 1983;45:624–632. [Google Scholar]
- Hugenschmidt CE, Mozolic JL, Laurienti PJ. Suppression of multisensory integration by modality-specific attention in aging. Neuroreport. 2009a;20:349–353. doi: 10.1097/WNR.0b013e328323ab07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, McCoy TP, Hayasaka S, Laurienti PJ. Preservation of crossmodal selective attention in healthy aging. Exp Brain Res. 2009b;198:273–285. doi: 10.1007/s00221-009-1816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino-Yang MH, Christodoulou JA, Singh V. Rest is not idleness: implications of the brain’s default mode for human development and education. Perspect Psychol Sci. 2012;7:352–364. doi: 10.1177/1745691612447308. [DOI] [PubMed] [Google Scholar]
- Jackson JD, Balota DA. Mind-wandering in younger and older adults: converging evidence from the sustained attention to response task and reading for comprehension. Psychol Aging. 2012;27:106–119. doi: 10.1037/a0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Brook JS. Extensive television viewing and the development of attention and learning difficulties during adolescence. Arch Pediatr Adolesc Med. 2007;161:480–486. doi: 10.1001/archpedi.161.5.480. [DOI] [PubMed] [Google Scholar]
- Jordan A. The role of media in children’s development: an ecological perspective. J Dev Behav Pediatr. 2004;25:196–206. doi: 10.1097/00004703-200406000-00009. [DOI] [PubMed] [Google Scholar]
- Junco R. Too much face and not enough books: the relationship between multiple indices of Facebook use and academic performance. Comput Hum Behav. 2011;28:187–198. [Google Scholar]
- Junco R, Cotten SR. Perceived academic effects of instant message use. Comput Educ. 2011;56:370–378. [Google Scholar]
- Junco R, Cotten SR. No A 4 u: the relationship between multitasking and academic performance. Comput Educ. 2012;59:505–514. [Google Scholar]
- Kam JWY, Dao E, Farley J, Fitzpatrick K, Smallwood J, Schooler JW, Handy TC. Slow fluctuations in attentional control of sensory cortex. J Cogn Neurosci. 2011;23:460–470. doi: 10.1162/jocn.2010.21443. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: an experience-sampling study of working memory and executive control in daily life. Psychol Sci. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Killingsworth Ma, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends Cogn Sci. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Burdette JH, Maldjian JA, Wallace MT. Enhanced multisensory integration in older adults. Neurobiol Aging. 2006;27:1155–1163. doi: 10.1016/j.neurobiolaging.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Leon-Carrion J, Garcia-Orza J, Perez-Santamaria FJ. Development of the inhibitory component of the executive functions in children and adolescents. Int J Neurosci. 2004;114:1291–1311. doi: 10.1080/00207450490476066. [DOI] [PubMed] [Google Scholar]
- Lussier M, Gagnon C, Bherer L. An investigation of response and stimulus modality transfer effects after dual-task training in younger and older. Front Hum Neurosci. 2012;6:129. doi: 10.3389/fnhum.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Schooler JW, Reichle ED. Out for a smoke: the impact of cigarette craving on zoning out during reading. Psychol Sci. 2010;21:26–30. doi: 10.1177/0956797609354059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, Joyce NM, Boniske T, Atkins SM, Merzenich MM. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A. 2006;103:12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 2011;69:48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Conducting the train of thought: working memory capacity, goal neglect, and mind wandering in an executive-control task. J Exp Psychol Learn Mem Cogn. 2009;35:196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008) Psychol Bull. 2010;136:188–197. doi: 10.1037/a0018298. discussion 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ, Kwapil TR. Tracking the train of thought from the laboratory into everyday life: an experience-sampling study of mind wandering across controlled and ecological contexts. Psychon Bull Rev. 2009;16:857–863. doi: 10.3758/PBR.16.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Meier ME, Touron DR, Kane MJ. Aging ebbs the flow of thought: adult age differences in mind wandering, executive control, and self-evaluation. Acta Psychol. 2013;142:136–147. doi: 10.1016/j.actpsy.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, DeCharms R. Neural representations, experience, and change. In: Llinas R, Churchland P, editors. The Mind-Brain Continuum. The MIT Press; Boston: 1996. pp. 61–81. [Google Scholar]
- Merzenich MM, Recanzone GH, Jenkins W. How the brain functionally rewires itself. In: Arbib M, Robinson JA, editors. Natural and Artificial Parallel Computations. MIT Press; New York: 1991. [Google Scholar]
- Milich R, Lorch EP. Advances in Clinical Child Psychology. Plenum; New York: 1994. Television viewing methodology to understand cognitive processing of ADHD children. [Google Scholar]
- Minear M, Shah P. Working Memory and Education. Academic Press; Burlington, MA: 2006. Sources of working memory deficits in children and possibilities for remediation. [Google Scholar]
- Mishra J, Gazzaley A. Preserved discrimination performance and neural processing during crossmodal attention in aging. PLOS One. 2013 doi: 10.1371/journal.pone.0081894. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Zinni M, Bavelier D, Hillyard SA. Neural basis of superior performance of action videogame players in an attention-demanding task. J Neurosci. 2011;31:992–998. doi: 10.1523/JNEUROSCI.4834-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, de Villers-Sidani E, Merzenich M, Gazzaley A. Adaptive Training Diminishes Distractibility in Aging across Species. doi: 10.1016/j.neuron.2014.10.034. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Zanto T, Nilakantan A, Gazzaley A. Comparable mechanisms of working memory interference by auditory and visual motion in youth and aging. Neuropsychologia. 2013;51:1896–1906. doi: 10.1016/j.neuropsychologia.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta” Prog. Neurobiol. 2008;86:156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Mrazek MD, Smallwood J, Schooler JW. Mindfulness and mind-wandering: finding convergence through opposing constructs. Emotion. 2012;12:442–448. doi: 10.1037/a0026678. [DOI] [PubMed] [Google Scholar]
- Mrazek MD, Franklin MS, Phillips DT, Baird B, Schooler JW. Mindfulness training improves working memory capacity and GRE performance while reducing mind wandering. Psychol Sci. 2013;24:776–781. doi: 10.1177/0956797612459659. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Ophir E, Nass C, Wagner AD. Cognitive control in media multitaskers. Proc Natl Acad Sci U S A. 2009;106:15583–15587. doi: 10.1073/pnas.0903620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, Howard RJ, Ballard CG. Putting brain training to the test. Nature. 2010;465:775–778. doi: 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozmert E, Toyran M, Yurdakok K. Behavioral correlates of television viewing in primary school children evaluated by the child behavior checklist. Arch Pediatr Adolesc Med. 2002;156:910–914. doi: 10.1001/archpedi.156.9.910. [DOI] [PubMed] [Google Scholar]
- Pa J, Berry AS, Compagnone M, Boccanfuso J, Greenhouse I, Rubens MT, Johnson JK, Gazzaley A. Cholinergic enhancement of functional networks in older adults with mild cognitive impairment. Ann Neurol. 2013;73:762–773. doi: 10.1002/ana.23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preminger S, Harmelech T, Malach R. Stimulus-free thoughts induce differential activation in the human default network. Neuroimage. 2011;54:1692–1702. doi: 10.1016/j.neuroimage.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Puzzanchera C, Adams B, Sickmund M. Juvenile Court Statistics 2008. National Center for Juvenile Justice; Pittsburgh, PA: 2011. [Google Scholar]
- Rosen LD, Carrier LM, Cheever N. Facebook and texting made me do it: Media-induced task-switching while studying. Comput Hum Behav. 2013;29:948–958. [Google Scholar]
- Rutledge KJ, van den Bos W, McClure SM, Schweitzer JB. Training cognition in ADHD: current findings, borrowed concepts, and future directions. Neurother J Am Soc Exp NeuroTher. 2012;9:542–558. doi: 10.1007/s13311-012-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. The aging of working memory. Neuropsychology. 1994;8:535–543. [Google Scholar]
- Sanbonmatsu DM, Strayer DL, Medeiros-Ward N, Watson JM. Who multi-tasks and why? Multi-tasking ability, perceived multi-tasking ability, impulsivity, and sensation seeking. PLoS One. 2013;8:e54402. doi: 10.1371/journal.pone.0054402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Hoppe J, Klimesch W, Gerloff C, Hummel FC. Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. Eur J Neurosci. 2007;25:587–593. doi: 10.1111/j.1460-9568.2006.05286.x. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MCM, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int J Psychophysiol. 2008;67:242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Schmidt ME, Vandewater EA. Media and attention, cognition, and school achievement. Future Child. 2008;18:63–85. doi: 10.1353/foc.0.0004. [DOI] [PubMed] [Google Scholar]
- Shaw GA, Giambra LM. Task unrelated thoughts of college students diagnosed as hyperactive in childhood. Dev Neuropsychol. 1993;9:17–30. [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Davies JB, Heim D, Finnigan F, Sudberry M, O’Connor R, Obonsawin M. Subjective experience and the attentional lapse: task engagement and disengagement during sustained attention. Conscious Cogn. 2004;13:657–690. doi: 10.1016/j.concog.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Fishman DJ, Schooler JW. Counting the cost of an absent mind: mind wandering as an underrecognized influence on educational performance. Psychon Bull Rev. 2007;14:230–236. doi: 10.3758/bf03194057. [DOI] [PubMed] [Google Scholar]
- Smilek D, Carriere JS, Cheyne JA. Failures of sustained attention in life, lab, and brain: ecological validity of the SART. Neuropsychologia. 2010;48:2564–2570. doi: 10.1016/j.neuropsychologia.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM. A cognitive training program based on principles of brain plasticity: results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. J Am Geriatr Soc. 2009;57:594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk M, Jonkman LM. Electrophysiological evidence for different effects of working memory load on interference control in adolescents than adults. Int J Psychophysiol. 2012;83:24–35. doi: 10.1016/j.ijpsycho.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Stevens C, Fanning J, Coch D, Sanders L, Neville H. Neural mechanisms of selective auditory attention are enhanced by computerized training: electrophysiological evidence from language-impaired and typically developing children. Brain Res. 2008;1205:55–69. doi: 10.1016/j.brainres.2007.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallal P, Miller SL, Bedi G, Byma G, Wang X, Nagarajan SS, Schreiner C, Jenkins WM, Merzenich MM. Language comprehension in language-learning impaired children improved with acoustically modified speech. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- Taylor P, Fulcomer M, Taylor F. Daydreaming in the adolescent years: instrument development, factor analysis, and sex differences. Adolescence. 1978;13:735–750. [PubMed] [Google Scholar]
- Teasdale JD, Dritschel BH, Taylor MJ, Proctor L, Lloyd CA, Nimmo-Smith I, Baddeley AD. Stimulus-independent thought depends on central executive resources. Mem Cogn. 1995;23:551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JDE. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci U S A. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell LB, Lindqvist S, Bergman Nutley S, Bohlin G, Klingberg T. Training and transfer effects of executive functions in preschool children. Dev Sci. 2009;12:106–113. doi: 10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Van Muijden J, Band GPH, Hommel B. Online games training aging brains: limited transfer to cognitive control functions. Front Hum Neurosci. 2012;6:221. doi: 10.3389/fnhum.2012.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais PE, Gazzaley A. The impact of auditory distraction on retrieval of visual memories. Psychon Bull Rev. 2011;18:1090–1097. doi: 10.3758/s13423-011-0169-7. [DOI] [PubMed] [Google Scholar]
- Wais PE, Rubens MT, Boccanfuso J, Gazzaley A. Neural mechanismsunderlying the impact of visual distraction on retrieval of long-term memory. J Neurosci. 2010;30:8541–8550. doi: 10.1523/JNEUROSCI.1478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Baym CL, Gazzaley A, Bunge SA. Neural indices of improved attentional modulation over middle childhood. Dev Cogn Neurosci. 2011;1:175–186. doi: 10.1016/j.dcn.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson AJ, Yang L. Plasticity of inhibition in older adults: retest practice and transfer effects. Psychol Aging. 2012;27:606–615. doi: 10.1037/a0025926. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One. 2013;8:e61624. doi: 10.1371/journal.pone.0061624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. J Neurosci. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Bollinger J, Gazzaley A. Top-down modulation of visual feature processing: the role of the inferior frontal junction. Neuroimage. 2010;53:736–745. doi: 10.1016/j.neuroimage.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EM. Far transfer in cognitive training of older adults. Restor Neurol Neurosci. 2009;27:455–471. doi: 10.3233/RNN-2009-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Knudsen EI. Functional selection of adaptive auditory space map by GABAA-mediated inhibition. Science. 1999;284:962–965. doi: 10.1126/science.284.5416.962. [DOI] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 2010;31:1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Finnegan CB, Gazzaley A. Meditation-inspired cognitive training promotes self-regulation of internal distractions. Entertainment Software & Cognitive Neurotherapeutics Society (ESCoNS) Conference.2013. [Google Scholar]


