Abstract
Head direction (HD) cells have been identified in a number of limbic system structures. These cells encode the animal’s perceived directional heading in the horizontal plane and are dependent on an intact vestibular system. Previous studies have reported that the responses of vestibular neuron within the vestibular nuclei are markedly attenuated when an animal makes a volitional head turn compared to passive rotation. This finding presents a conundrum in that if vestibular responses are suppressed during an active head turn how is a vestibular signal propagated forward to drive and update the HD signal? This review identifies and discusses four possible mechanisms that could resolve this problem. These mechanisms are: 1) the ascending vestibular signal is generated by more than just vestibular-only neurons, 2) not all vestibular-only neurons contributing to the HD pathway have firing rates that are attenuated by active head turns, 3) the ascending pathway may be spared from the affects of the attenuation in that the HD system receives information from other vestibular brainstem sites that do not include vestibular-only cells, 4) the ascending signal is affected by the inhibited vestibular signal during an active head turn, but the HD circuit compensates and uses the altered signal to accurately update the current head direction. Future studies will be needed to decipher which of these possibilities is correct.
Keywords: head direction, navigation, passive movement, self-motion, spatial orientation, vestibular, restraint
Accurate navigation requires knowledge of both location and directional heading
Neurophysiological studies have identified three major cell types representing this kind of information. Place cells, found throughout the three major areas of the hippocampus, discharge in relation to the animal’s specific location within the environment (O’Keefe and Dostrovsky, 1971). Different place cells represent different locations, such that the summed activity of place cells collectively defines the animal’s perceived location. Grid cells fire at multiple locations in a regular, repeating hexagonal grid pattern throughout the environment and are found primarily in the entorhinal cortex, but have also been observed in the pre- and parasubiculum (Hafting et al., 2005; Boccara et al., 2010). The third cell type, head direction cells, are found primarily in the classic Papez circuit, and discharge as a function of the animal’s head direction in the horizontal plane, independent of the animal’s location and on-going behavior (Taube et al., 1990a). Each HD cell is tuned to a particular orientation with the environment and is referred to as the cell’s preferred firing direction. Other spatial cells, which combine various aspects of these three basic spatial cell types, have also been reported in hippocampal-related structures, including place x HD cells (Cacucci et al., 2004) border cells (Lever et al., 2009), which fire along a portion of a border in an enclosed environment, and conjunctive grid x HD cells (Sargolini et al., 2007). The spatial correlates of all these cells respond to landmark cues, such that rotation of the landmark in a cue controlled environment leads to a corresponding shift of the spatial cell’s firing pattern. Thus, a place cell’s place field, a HD cell’s preferred firing direction, and the orientation of the grid cell firing pattern all shift a similar amount when a prominent landmark cue is rotated relative to the environment. These cell types also receive and are affected by information about the animal’s self-motion through the environment. These self-motion (idiothetic) cues include information from vestibular, visual, proprioceptive, and motor efference systems. Both landmark and self-motion information are normally in register with one another as an animal moves through its environment. Motor and proprioceptive information have in particular been shown to be important for updating the HD signal as an animal moves from a familiar environment to a novel one that contains unfamiliar landmarks (Stackman et al., 2003; Yoder et al., 2011). In contrast to updating the orientation of the HD signal’s preferred firing direction relative to landmarks, vestibular information is critical for generating the HD signal, as disruption of vestibular system inputs to the HD network completely disrupts the HD signal in both cortical and subcortical areas. Further, vestibular information allows the HD system to update the perceived orientation without constantly assessing the external environment as is necessary for processing landmark information. Although previous studies have indicated that passive rotation of an animal lowers and in some cases attenuates HD cell responses, more recent work that has recorded from HD cells in rats with their heads and bodies immobile, has shown that passive back-and-forth yaw rotation of the animal’s head in the horizontal plane does not reduce cell firing at the cell’s preferred direction (Shinder and Taube, 2011a).
Studies have also shown that signals conveying information about the animal’s angular head velocity (AHV) are an important component in generating the HD signal. AHV information is an ideal signal for updating the HD response to reflect the change in the animal’s current perceived orientation following any head movement. Cells within the dorsal tegmental nucleus (DTN), which receive inputs from the vestibular system and project to the HD cell network, predominantly contain neural correlates related to the animal’s AHV, although some HD cells have been identified there (Bassett and Taube, 2001; Sharp et al., 2001b). Moreover, lesions of the DTN disrupt the HD signal throughout the HD cell network (Bassett et al., 2007). The origins of the AHV signal could come from a combination of vestibular and motor efference inputs, although it is generally believed that vestibular inputs play the major role because 1) HD responses are not impaired in the absence of head movements, which occurs as a result of full head and body restraint, and 2) the presence of an anatomical circuit between the vestibular system and the nuclei involved in generating the HD signal.
During the past decade, several studies have recorded from vestibular nucleus neurons in head-free behaving monkeys. These studies have shown an important property – namely that responses in some types of vestibular neurons are attenuated or substantially depressed when the monkey makes an active head turn. In one type of vestibular nuclei neuron, passive rotation of the head elicits classic firing rate responses that are correlated primarily with AHV, and are therefore likely candidates as the source of the AHV signal to the HD system. These cells are known as vestibular-only neurons. But responses from these same neurons are substantially diminished when the monkey makes an active head turn (McCrea et al., 1999; Roy and Cullen, 2001, 2004). If the firing of these vestibular-only neurons is attenuated during actively generated head turns, then these vestibular responses would be ‘turned off’ when the animal is in a freely-moving condition and making active head movements. Taken in conjunction with the fact that a vestibular signal is necessary for generating the HD signal, these findings result in a conundrum (Cullen and Roy, 2004). If the vestibular nuclei cells become quiescent during an active head turn, then how does the vestibular system and the associated ascending AHV signal enable the HD network to generate the HD signal during an active head turn? This review discusses this issue and tries to resolve the conundrum. We present four possible mechanisms that may resolve this issue, each of which are not mutually exclusive. First, it is possible that the ascending vestibular signal is created by more than just vestibular-only neurons. Second, it is possible that not all vestibular-only neurons contributing to the HD pathway have firing rates that are equally and severely attenuated by active head turns. Third, the ascending pathway may be partially spared from the effects of the attenuation, in that the HD system might receive information from vestibular brainstem sites that do not include the vestibular-only cells. Finally, we consider the possibility that the ascending signal is affected by the inhibited (or altered) vestibular signal during an active head turn, but the HD circuit compensates and uses the altered signal to accurately update the current head direction.
The HD cell network and signal generation
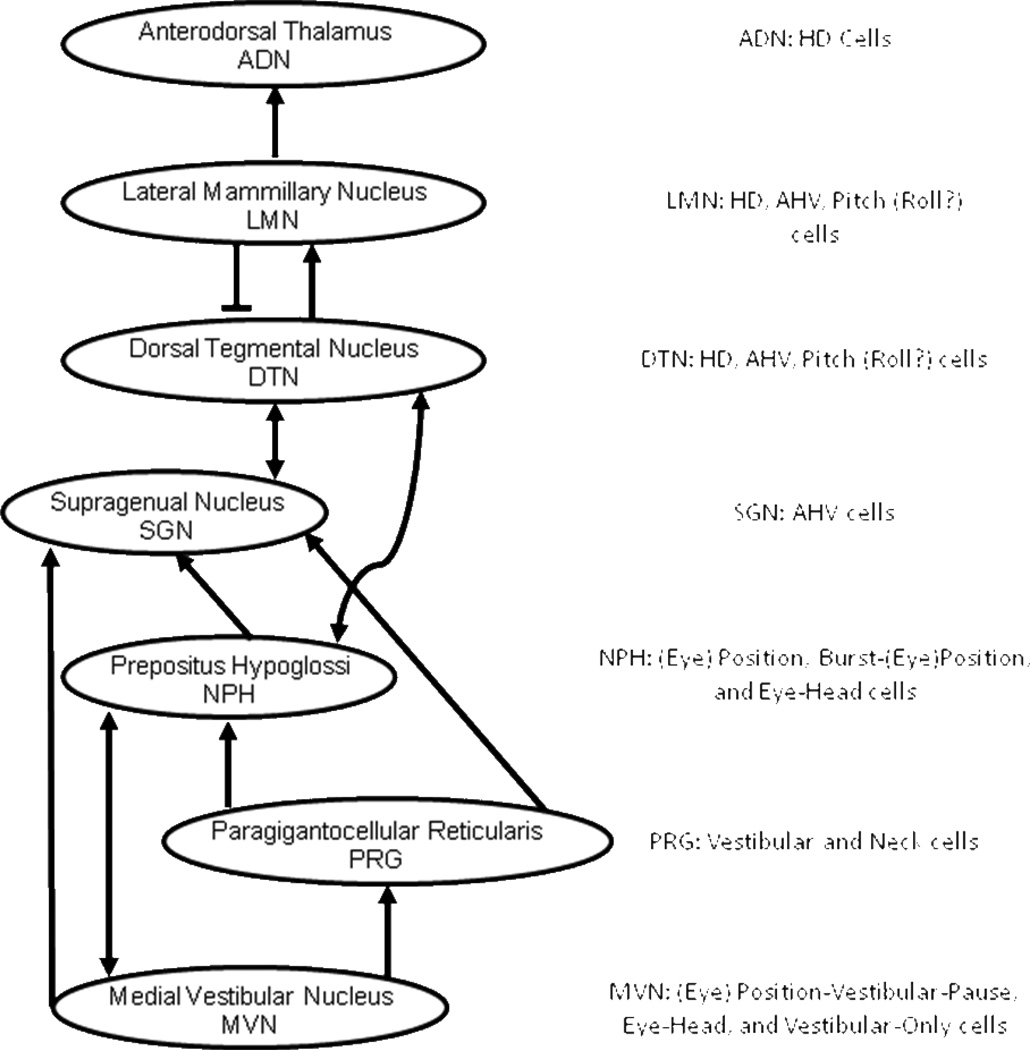
The HD signal has been identified in a number of interconnected areas that form a major part of the Papez circuit, including the DTN, lateral mammillary nuclei, anterodorsal thalamus, postsubiculum, and entorhinal cortex (Sharp et al., 2001a; Taube, 2007). Although HD cells have been found in other brain areas (e.g., dorsal striatum, retrosplenial cortex, medial precentral cortex), the HD signal in these other areas is thought to be dependent on the pathway through the Papez circuit. Previous work has established that the HD cell signal is most likely generated subcortically – either within the DTN or in its connections with the lateral mammillary nuclei. The vestibular system and its associated nuclei play a critical role in the generation of the HD signal. Permanent neurotoxic lesions of the vestibular labyrinth (Stackman and Taube, 1997), inactivation of the vestibular hair cells (Stackman et al., 2002), and occlusion of the semicircular canals (Muir et al., 2009) have all effectively disrupted direction-specific firing in HD cells. Therefore, the ascending pathway from the vestibular nuclei, which contains sensory information about the animal’s AHV and translational head acceleration, is critical for the formation of the HD signal. Vestibular inputs that generate the HD signal can be traced back to the contributions of individual vestibular sensory end organs. Recent experiments in transgenic mice have shown that the horizontal canals, which are mostly sensitive to angular acceleration in the yaw (horizontal) plane when the animal’s head is held in the upright position, are essential for generating the directional signal (Valerio and Taube, 2012). Yoder and Taube (2009) found that the otolith organs, which are sensitive to translational acceleration of the animal, are also known to play some role in the generation of the HD signal. This finding suggests that at some point in the ascending pathway to the HD system, there may be convergence of rotational and translational head movement information. The vestibular nuclei project to a number of different areas, but its projections to the nucleus prepositus (NPH) and to the supragenual nucleus (SGN)(Biazoli et al., 2006) are thought to convey vestibular information to the ascending HD pathway. This view is supported by findings that lesions of either of these nuclei disrupts the normal functioning of the HD signal (NPH: Butler and Taube, 2012; SGN: Clark et al., 2012). Both the SGN and NPH in turn project to the DTN, which contain both HD and AHV sensitive cells. The DTN then project to the lateral mammillary nuclei, which also contain both HD and AHV sensitive cells. From here, the HD signal is conveyed rostrally to brain regions that contain HD cells, but generally have limited AHV sensitivity: the anterodorsal thalamus → postsubiculum → medial entorhinal cortex → hippocampus (for review see Taube, 2007). Within the entorhinal cortex/hippocampal network, the HD signal is thought to be integrated with place and grid cell information to yield a complete representation of the animal’s spatial orientation in its environment. The HD cell network and their pathways are summarized in Figure 1. This overall pathway represents the most direct possible route for vestibular information to ascend to the HD system, and is based upon anatomical connectivity. As will be discussed below, there are possible extended side pathways that include other regions (e.g., the cerebellum), which may play a significant role in the processing of vestibular information for the ascending pathway to the HD system.
Figure 1.
Diagram of the anatomical connections of the ascending vestibular pathway leading to the HD circuit. The physiological cell types found in each region depicted in the circuit are labeled to the right of each region.
Passive versus Active Properties of HD cells
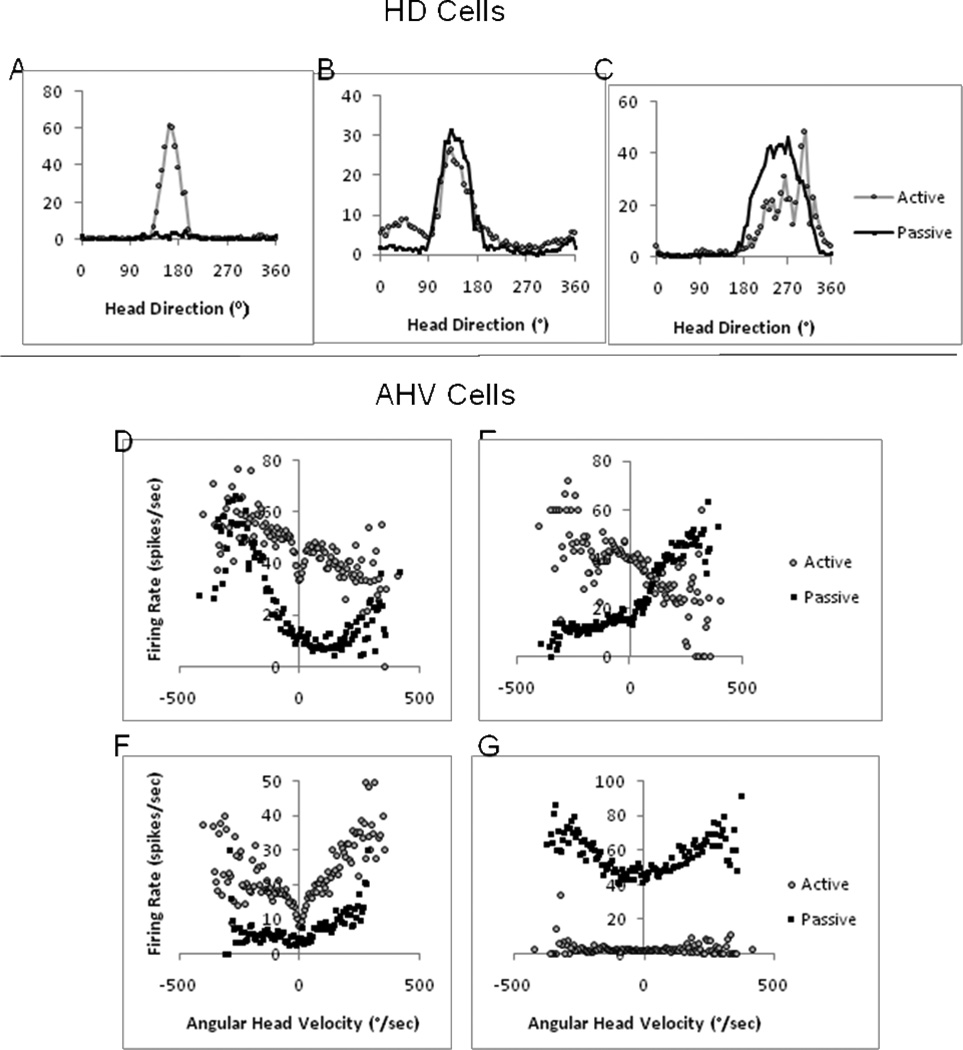
In the initial study on HD cells in the postsubiculum, passive rotation of the animals resulted in reductions in the cells’ peak firing rates compared to the peak firing rates seen during an active, freely-moving session (Taube, 1990b). Passive rotation was conducted by loosely wrapping the animal in a towel, and then rotating the animal in the experimenter’s hands. Firing rates were reduced on average ∼50% in seven of nine cells. This effect of reduced firing rates with passive rotation was found to occur in other brain areas that contained HD cells – most notably in the anterodorsal thalamus (Fig. 2A) and retrosplenial cortex (Taube, 1995, Knierim et al., 1995, Chen et al., 1994, Golob et al., 1998). The reduced firing rates during passive rotation sessions also extended to some, but not all, AHV cells in the DTN using a similar hand-held approach (Sharp et al., 2001b). Further, a trend emerged, which showed that milder reductions in firing rates occurred when loose restraint was used during the passive rotation (Zugaro et al., 2001, Bassett et al., 2005) compared to studies that used tighter restraint (Taube, 1995). The interpretation of this trend was that it reflected possible influences of motor and proprioceptive signals in the HD signal network.
Figure 2.
Top: Head direction (HD) cells recorded during active and passive rotation in the anterodorsal thalamus (ADN) (A,B) and dorsal tegmental nucleus (DTN) (C). The cell in A displayed attenuated directional responses when the animal was passively restrained and rotated back-and-forth in the yaw plane in the experimenter’s hands around the cell’s PFD. The HD cells in B and C were recorded using similar methods except the rat’s’ head was bolted to a restraint device. Under these conditions, HD cell activity was not attenuated. (D–G) Angular head velocity (AHV) cells recorded from the DTN during active and passive rotation. D and E are examples of asymmetric DTN AHV cells, and F and G are examples of symmetric AHV cells. The most common response during passive rotation was a decrease in the firing rate at all AHVs (D, F), but an increase in firing rate during passive rotation was observed occasionally (G). The cell depicted in E had an unusual response during passive rotation as it responded in a reversed asymmetric manner compared to the active session - firing rate increased for CW head turns in the active session, but increased for CCW head turns in the passive session. The firing rate response for each cell during active movement is denoted by gray circles, and the response during passive rotation is denoted by black squares throughout the figure. CW and CCW head turns are denoted by negative and positive values, respectively.
Recently, Shinder and Taube (2011a) used a more rigorous method to restrain the animal’s head. The animal’s head was attached to a device via an implanted head bolt and its body was tightly wrapped in a towel and placed in a fixed plexiglass tube that prohibited head, neck, and body movement. The device was then attached to a turntable and the rat was passively rotated back-and-forth clockwise (CW) and counterclockwise (CCW) in the horizontal plane, using similar movements as were used during the hand-held passive sessions conducted in previous studies. Under these conditions, passive rotation did not lead to a reduction in HD cell firing rates in the anterodorsal thalamus compared to an active, freely-moving session (Fig. 2B). In preliminary work, these findings extended to HD cells recorded in the DTN (Fig. 2C). Thus, reductions in motor and proprioceptive inputs to the HD system, by themselves, could not account for the previously seen reductions in firing rate. HD cell firing rates are known to be modulated a little by the animal’s AHV, particularly for HD cells in the anterodorsal thalamus and lateral mammillary nuclei (Taube, 1995; Stackman and Taube, 1998). Thus, it was possible that the amount or frequency distribution of AHV experienced by the animal differed between the hand-held and head-fixed passive sessions, and in turn, this different velocity experience may have led to differences in the firing rates in the two passive sessions. However, Shinder and Taube (2011a) reported that passive restraint in the head-fixed animals did not change cell sensitivity to AHV, suggesting that any differences in AHV between the different passive session types were unlikely to explain the firing rate differences.
Another important factor to consider is whether stress might have impacted cell firing rates during passive rotation. Restraining an animal by wrapping it in a towel can certainly be stressful, and there are pathways involved in stress, which are connected to the vestibular nuclei (Bruchey and Gonzalez-Lima, 2006). However, stress is unlikely to account for the differences because animals in both studies were acclimated to the passive testing conditions before conducting the first cell recordings. In addition, it is noteworthy that Shinder and Taube found no differences in the passive sessions between animals that were naïve to the test conditions and animals that were acclimated to the experimental conditions for several days. In sum, it is clear that passive rotation of a head-fixed rat did not lead to significant changes in peak firing rates when the animal faced the cell’s preferred firing direction, or in the background firing rate when the animal faced away from the cell’s preferred firing direction. Precisely why firing rates are sometimes reduced in restrained hand-held animals remains unclear.
Angular head velocity within the HD system
In the HD system, AHV signals have been found throughout the ascending pathway from the vestibular nuclei to the anterodorsal thalamus portion of the HD circuit (Fig. 1). About 44% of the cells encountered in the lateral mammillary nuclei are primarily sensitive to AHV (Stackman and Taube, 1998) and ∼75% of the cells in the DTN are responsive to AHV (Sharp et al., 2001b; Bassett and Taube, 2001). Both regions contain HD cells and AHV cells within the same region, although HD cells appear to make up a higher percentage of the population in the lateral mammillary nuclei. Anatomically, it appears that the AHV signal in the DTN is derived predominantly from signals that originate from the NPH and the SGN (Biazoli et al., 2006) and both of these brainstem regions receive input from the medial vestibular nucleus (for review see Taube, 2007). The SGN receives input from the NPH, as well as from the vestibular nucleus, and lesions of the SGN disrupt the HD signal in the anterodorsal thalamus (Clark et al., 2012). Partial sparing of the SGN leads to a reduction in the number of HD cells and to poor stability in the preferred firing direction of these cells. In contrast, the NPH is reciprocally connected with the medial vestibular nuclei and preliminary results suggest that lesions of the NPH also lead to a reduction in the number of HD cells identified in the anterodorsal thalamus, and the ones that were found had preferred firing directions that were unstable in the dark (Butler and Taube, 2012). To date, however, beyond encoding AHV, we do not know the exact nature of the vestibular signal that is projected forward into the NPH and SGN, nor is there information about the specific functional processing that occurs within each of the regions as the signal ascends towards the DTN. Understanding these issues is particularly important in light of the conundrum described above. If the input originating from the medial vestibular nuclei is the origin of the AHV signal, and medial vestibular nuclei activity is suppressed during active head movements, then how does the HD signal continue to function during active head movements while remaining dependent upon vestibular signal integrity?
AHV signals and the lateral mammillary nuclei
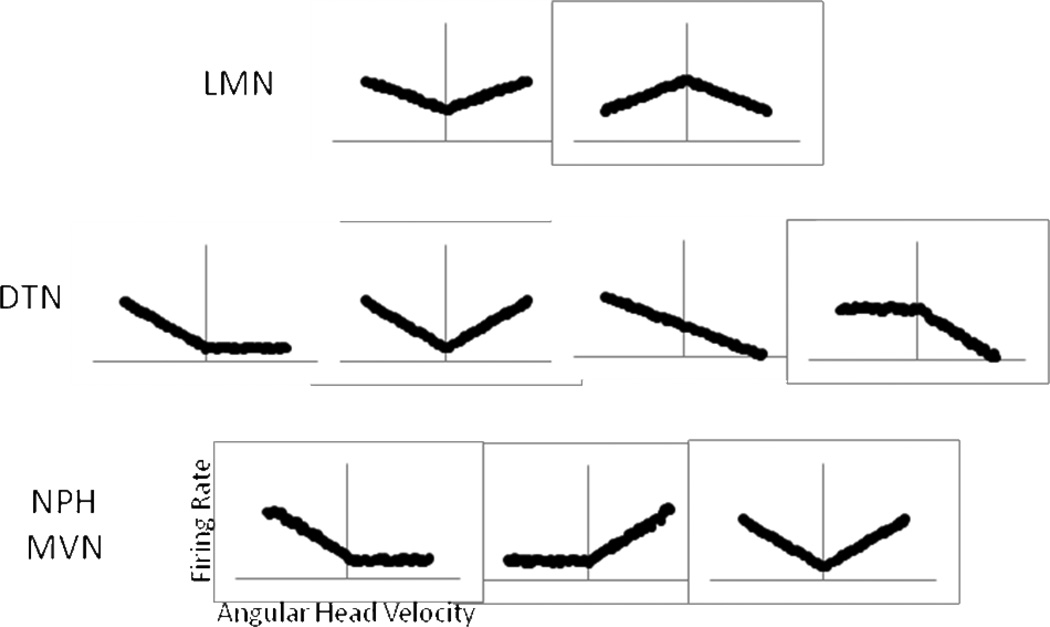
Since we do not yet know precisely the physiological signals that generate the AHV signal in the lateral mammillary nuclei and DTN, we can compare the AHV signal across these regions as it ascends the brainstem to the Papez circuit. This assessment can then be used as a predictor for the type of signal processing that might occur or be required to produce such AHV signals. To begin, the lateral mammillary nuclei are part of the core HD circuit, and are synaptically the furthest HD cell-related region from the medial vestibular nuclei, in which an AHV signal can be seen. Within the lateral mammillary nuclei, there are three types of spatial cells present (Stackman and Taube, 1998). First, there are HD cells, which encode the direction of the animal’s head. Second, there are head pitch cells, which encode the orientation of the head in the vertical plane relative to the gravity axis, independent of the animal’s directional heading in the horizontal plane. With 0° defined as earth horizontal, all lateral mammillary nuclei head pitch cells have their maximum firing rate tuned to straight up −90° vertical, and their firing rate decreases linearly as the pitch angle is reduced to 0°. Third, there are AHV cells. Unlike the other cell types, AHV cells display a continuum of responses from weak to strong and do not have a singular preferred AHV to which they respond. Lateral mammillary nuclei AHV cells display firing rates that are proportional to the animal’s AHV in the horizontal plane (Fig. 3). It remains unknown whether the AHV sensitivity is independent of the animal’s head orientation relative to gravity and the extent to which the lateral mammillary AHV sensitivity resembles horizontal canal-sensitive neurons in the vestibular nuclei. However, one of the distinctive features of these mammillary AHV cells is that they are insensitive to the direction of head rotation (i.e., CW vs. CCW) – they consistently display increasing or decreasing firing rates for increasing head velocities in either direction. This finding points out another distinctive feature of these mammillary AHV cells. Half of them are positively correlated with AHV, where the firing rate increases proportionately to angular velocity, and the other half are negatively correlated to AHV such that firing rates decrease proportionately with increasing AHV.
Figure 3.
Diagram of neural response types in the ascending vestibular pathway. Simplified firing rate responses are created based upon the known angular head velocity sensitivities seen in the medial vestibular nucleus, NPH, DTN and LMN. Positive AHV values correspond to leftward (CCW) head turns, and negative AHV values correspond to rightward (CW) head movements (graph scheme is similar to Figure 2D–G and depicted in the lower left plot).
AHV signals and the DTN
The symmetric positive and negative lateral mammillary nuclei AHV signals stand in contrast to the AHV signal seen in the DTN (Fig. 3), just one synapse back in the ascending HD pathway. In the DTN, symmetric AHV cells, like those found in the lateral mammillary nuclei, were reported in one study (Bassett and Taube, 2001), but not in another study (Sharp et al., 2001b). The symmetric AHV cells were all positively correlated with AHV, such that firing rates to both CW and CCW head turns always increased with increasing AHV (Fig. 2F). Along with an absence of negatively correlated symmetric AHV sensitivity (i.e., decreased firing rates with increasing AHV to both CW and CCW head turns), around 36% of all AHV cells encountered were asymmetric with a firing rate response to AHV that differed depending upon which direction the head was turning (Fig. 2D–E). It should be noted that in the study that failed to find symmetric AHV sensitivity, all of the AHV cells were found to be asymmetric. Thus, further evaluation will be required to resolve this difference. The asymmetric responses found in both studies included two types of asymmetric response. One type of asymmetry is characterized by firing rate responses that are relatively unaffected during head turns in one direction, but have increasing or decreasing firing rates for increasing angular head velocities in the other direction. The other type of asymmetric response involves responding to head turns in both directions, but with positively correlated firing rates in one direction and negatively correlated firing rates in the other direction (Fig. 2D–E). Further, in addition to AHV cells, there are also signals in the DTN that resemble those mentioned above in the lateral mammillary nuclei (Fig. 3), including head pitch and HD. Moreover, unlike the lateral mammillary nuclei, there is more multimodal convergence in DTN with many cells being sensitive to more than one stimulus type. For example, AHV cells in the DTN can also display pitch responses, be sensitive to translational movements, or can be modulated by the animal’s directional heading – characteristics not reported in lateral mammillary cells (Bassett and Taube, 2001; Sharp et al., 2001b). In contrast, AHV sensitivity in the lateral mammillary nuclei is more unimodal, in the sense that there is little convergence of pitch or HD information onto AHV cells (Stackman and Taube, 1998).
An important consideration in understanding the contributions of the vestibular signal to the HD pathway is that the studies on DTN and LMN neurons were performed with freely moving rats – thereby using naturalistic stimulation of the vestibular system. In contrast, neurons in the vestibular brainstem (vestibular nuclei and nucleus prepositus) have been studied primarily with passive sinusoidal rotation. The complex, natural movement of a freely moving rat contains a mixture of rotational amplitudes and frequencies, which are difficult to evaluate with individual, independent motion parameters – with the head freely moving, the speed, duration, and frequency of the head rotations are all changing at the same time. The differences between these procedures could lead to a misrepresentation of the predicted firing rate response during behavior because active movements contain a mixture of head movement velocities and frequencies. Individual head movements tend to correlate the frequency of the head rotation (number of changes in direction over time) with the amplitude of the head rotation (the speed of the head rotation). For example, low velocity head movements tend to be performed at low frequencies in order to minimize the work (torque) that is required by the muscles to make the movement (at 10 deg/sec, a 0.1 Hz sinusoidal head rotation produces 6.3 deg/sec2 of acceleration, while a 2 Hz sinusoidal head rotation produces 125.7 deg/sec2 of acceleration). Thus, the increased firing rates produced by increases in rotational frequency are confounded with increasing angular head velocities. Further, the mixed frequencies and amplitudes of natural head movements are not summed linearly by the vestibular system (Massot et al., 2012). Therefore, comparisons of active head movements in the DTN (Bassett and Taube, 2001; Sharp et al., 2001b) with head movement sensitivities in the MVN and NPH must account for the differences in the types of head movements experienced during the neural response.
Only one of the two DTN recording studies evaluated AHV cells for active versus passive rotational sensitivity (Sharp et al., 2001b). However, this study used hand-held restraint methodologies similar to those used in the previous studies of active-passive responding for HD cells in which significant reductions in firing rate were found (Taube, 1990b, Taube, 1995, Knierim et al, 1995, Chen et al., 1994, Golob et al., 1998). Although this method of restraint and the findings reported by Sharp et al. (2001b) may not provide as clear and unambiguous picture as one would like (see above), they are still worth considering and relevant to our conundrum. If firing of vestibular neurons is altered during active motion, then these vestibular signals would either fail to represent angular head velocity or would represent only a subset of head movements (head on neck rotations) when the animal is in the freely-moving condition, and one might expect less influence from these vestibular neurons on HD cell responses as compared to other types of inputs, such as motor or visual cues, during active motion. However, Sharp et al. reported that the most common response they found was the exact opposite effect in the DTN – namely that during passive restraint, there was less firing related to AHV than during active head movements. These results, demonstrate attenuated responses during passive restraint similar to those seen in HD cells with hand-held restraint, but contrary to what would be predicted from the vestibular signal, which should encode angular velocity more robustly under passive restraint. Specifically, Sharp et al. found that passive rotations for 12 asymmetric AHV cells produced mixed results, with 8 cells losing their AHV sensitivity during the passive rotations, while the other 4 cells maintained their AHV responses. The authors noted that the animals frequently struggled during restraint and this behavior would compromise the ability to monitor a true passive response because various motor and proprioceptive pathways would be activated during the struggling period. Of the 4 cells that maintained an AHV response during passive restraint, 2 cells maintained a response that was similar to that recorded during active rotation and the other 2 cells either changed the direction of motion that the cell responded to or lost their AHV sensitivity later in the session. No cells were found that increased their firing rate responses during passive restraint. Preliminary recordings of DTN AHV cells from our laboratory using full head and body restraint have replicated the most common responses reported by Sharp et al. (2001b). However, we observed other types of responses to passive restraint that encompassed a broader range of response profiles; for example, some cells had minimal AHV sensitivity during active foraging, but displayed robust sensitivity to AHV when tested with passive rotation as predicted by the improved AHV encoding during passive rotation (Fig. 2G). How all of these disparate findings are to be resolved remains unclear, but the conundrum focuses attention on the AHV signal coming from the ascending pathway. It is possible that the AHV signals in DTN represent integrated motor and/or proprioceptive information rather than processed vestibular signals. However, this explanation does not account for the vestibular dependency of HD signals during active head turns. Therefore, it is important to examine the ascending vestibular signal from the SGN and NPH.
Integration of the vestibular AHV signal
As discussed above, there are a number of different behaviors that can modulate AHV responses in the DTN, including HD, head pitch, and translational movement. In contrast, lateral mammillary nuclei cells tend to respond primarily to only one stimulus each. As the DTN signals ascend to the lateral mammillary nuclei, there is also an increase in symmetry – both in turn direction and in the balance of positive and negative correlations with angular velocity (Fig. 3). We can now extrapolate this progression backward into the vestibular brainstem to the SGN and NPH. What might the role of these two nuclei be in transmitting the vestibular signal from the vestibular nuclei to the DTN? It should be noted that AHV responses in the lateral mammillary nuclei and DTN were studied primarily for head movements in the horizontal plane and it has not been determined whether the AHV sensitivity is referenced only to this plane or if it holds for other orientations of the animal’s head outside this plane. It is possible that AHV cell firing may remain referenced to the horizontal plane of the head, rather than the horizontal plane of the environment. In which case, during locomotion in the vertical plane, the HD response would simply shift with the animal to represent the animal’s directional orientation in the plane of locomotion, which is tilted 90°. On the other hand, if the mammillary HD signal is referenced to rotational head movement in the horizontal plane (in reference to gravity) and not specifically to yaw rotation of the head, then the reference frame of the ascending vestibular signal would have to be transformed from a head-based coordinate system to a gravity-centric system. This transformation may occur at or prior to the development of the AHV signal in the lateral mammillary or DTN. It is possible that the AHV signal becomes referenced to gravity within the ascending pathway, since the other signals available in the lateral mammillary and DTN contain the necessary information for determining the orientation of the head relative to the horizontal plane including vertical pitch position and possibly roll orientation. Therefore, the processing of the vestibular canal and otolith signals to compute pitch and roll position, in addition to changes in yaw, most likely occurs early in the ascending pathway. Combining rotational and pitch/roll acceleration signals may occur within the medial vestibular nuclei, where there are cells that display vestibular sensitivity that are referenced to gravity (Yakusheva et al., 2007; Angelaki and Yakusheva, 2009). There are projections to the medial vestibular nuclei from the cerebellar nodulus and uvula, which are strongly gravity-referenced (Carleton and Carpenter, 1983, Horowitz et al., 2005). These motion signals must be integrated to reflect orientation, rather than velocity or acceleration, and this integration may occur in the NPH or SGN (Anastasio and Robinson, 1991; Kaneko, 1997).
Vestibular system
While the exact nature of the vestibular signals that contribute to the HD system remain unknown, some insight about the vestibular system’s contribution can be provided based on the anatomical projections to the HD system and the functional properties of these regions. The DTN receives ascending input from the vestibular pathway including the NPH and SGN (Hayakawa and Zyo, 1985; Liu et al., 1984; Biazoli et al., 2006; Groenewegen and Van Dijk, 1984; McCrea and Baker, 1985). Moreover, the medial vestibular nuclei are interconnected with the NPH (McCrea and Horn, 2006; Horowitz et al., 2005) and also project to the SGN (Biazoli et al., 2006). Thus, the medial vestibular nuclei can be viewed as the initial stage for processing the vestibular signal and contributing a spatial signal to the ascending HD pathway (Brown et al., 2005).
Types of vestibular neurons
There are primarily three principal classes of neurons that have been described within the medial vestibular nuclei: position-vestibular-pause (referred to as PVP) neurons, vestibular-only (referred to as VO) neurons, and eye-head (referred to as EH) neurons (Scudder and Fuchs, 1992; reviewed in Cullen and Roy, 2004). Vestibular sensitivity in these neurons is modulated by AHV during passive rotations in the dark. In animals that visually track objects, both position-vestibular-pause and eye-head neurons show suppression of vestibular modulation during directed active head movements or gaze redirection (i.e., combined head and eye movement) that ends once the eye-in-space position is stable (McCrea and Luan, 2003; Roy and Cullen, 2002). During active head turns, these neurons therefore show AHV modulation only during the later portion of the active head rotation, when the head is being brought into line with the eyes, once the eyes have acquired the target. The third type of neuron, the vestibular-only neuron, suppresses vestibular responses throughout the entire head movement, regardless of the eye movements involved (Roy and Cullen, 2001). Furthermore, the suppression of modulation during active head movements only occurs when the monkey turns its head relative to its body, activating neck-related motor neurons. This active movement suppression does not occur when the monkey actively "steers" the chair in which they are sitting, by using its hands to turn its head and body together without rotating its neck. The authors concluded that an efference copy of the neck motor command, rather than higher-order motor commands involved in planning head-in-space movements, was responsible for influencing vestibular firing patterns. The influence of neck proprioception during active head movement is also influential in the mouse, but instead of suppressing the encoding of angular head velocity altogether, angular head velocity is encoded by non-vestibular inputs. During active head on neck rotations in the mouse, vestibular-only neurons reflect neck proprioception, but do not display the robust vestibular sensitivity seen during passive head movement, and encode head on neck instead of head in space rotation (Medrea and Cullen, 2013). These findings demonstrate the important contribution that motor and proprioceptive signals play in driving vestibular nuclei neurons. The fact that vestibular response changes during active movement depends upon neck proprioception stands in contrast to HD cell activity, which encodes the animal’s current heading independent of any neck rotation (Taube et al., 1990a). However, as discussed above, neck muscle reafference (or neck motor efference) could supplement the attenuated response of the vestibular signal in the HD pathway, or selectively gate which vestibular cells contribute to the ascending pathway.
In one of the initial primate studies that showed attenuated firing in vestibular-only neurons during active head movements, the authors reported that the suppression was near complete in about 75% of the cells (McCrea et al., 1999). Importantly, however, the amount of attenuation varied between 20–75% in the remaining cells. This finding indicates that not all vestibular-only neurons are markedly suppressed during active head turns, and it is quite possible that these mildly suppressed neurons are responsible for contributing to the ascending AHV signal in the HD cell pathway.
Interestingly, in the mouse, the response of vestibular-only neurons to active and passive head rotation is different. During passive rotation the neurons encode head rotation, but during active rotation these neurons respond to neck proprioception (Medrea and Cullen, 2013). While these neurons encode head rotation relative to the body during active movement, they do not encode head rotation relative to the environment as is required for the HD signal. Thus, vestibular-only neurons have not been found to provide the same angular head velocity information during active and passive rotation across different species. In addition, in the mouse the population of bimodal vestibular-neck cells represented 70% of the vestibular-only cells (Medrea and Cullen 2013). Of these cells, neck sensitivity did not change from active to passive rotation, while vestibular sensitivity displayed a significant reduction in sensitivity. There may have been a few cells where there was some evidence that the vestibular change in sensitivity was limited, but this affect could only be noted in neurons with low vestibular sensitivity and the change in vestibular sensitivity cannot be distinguished from the variance in the responses across the population of neurons. Thus, while it is possible that the ascending vestibular signal to the HD pathway is derived from a minority of vestibular-only cells that consistently encode vestibular-related head movements during both active and passive rotation, it remains to be determined if such a sub-population of cells can and does drive the ascending AHV signal.
NPH neurons
In contrast to the medial vestibular nuclei, McFarland and Fuchs (1992) classified monkey NPH neurons into three somewhat different types. Two types of NPH neurons responded only during eye movements and were not sensitive to vestibular stimulation (position and burst-position neurons), and a eye-head type that was sensitive to both eye and head movement in one direction only. The firing rates of these NPH eye-head cells were usually modulated more by eye velocity than by head velocity, but NPH eye-head cells can also contain visual motion or neck proprioceptive sensitivity (Gresty and Baker, 1976). However, McFarland and Fuchs found that just under half of the eye-head neurons were insensitive to eye position and might be better classified as NPH vestibular-only neurons. In the rodent, Kaufman et al. (2000) reported that 30% of NPH cells were eye-head cells, and that head movement sensitivity was stronger in cells classified as eye-head as opposed to vestibular-only. It is possible that the NPH vestibular-only cell could be comparable to asymmetric AHV cells found in the DTN. Interestingly, the NPH and medial vestibular nuclei, along with regions in the HD pathway including the DTN and lateral mammillary nuclei, were labeled by transneuronal retrograde anatomical tracing that started with an injection into the medial rectus eye muscle (Graf et al., 2002; also see Brown et al., 2005). Thus, the HD pathway appears to share some circuitry in common with the oculomotor-related vestibular circuits. Therefore, it is possible that the ascending HD pathway receives input from eye-head neurons as well. The contribution of eye-head neurons to the ascending HD pathway is not inconsistent with the ocular behavior of the rodent species. While saccadic eye movements are present in motionless, head-restrained rats (Chelazzi et al., 1989; Hikosaka and Sakamoto, 1987), eye movements are normally associated with head movements in freely-moving rats (Meier and Dieringer, 1993; Tempia et al., 1992; Wallace et al., 2013). Thus, the eye movement contribution, if there is one, would complement the head movement signal on eye-head cells. Current evidence indicates that an ocular contribution may be unlikely, because HD cell activity continues to reflect a constant head direction despite possible changes in the direction of the animal’s gaze when the animal is motionless (during which occasional eye movements would be expected to occur) (Shinder and Taube, 2011b).
Paragigantocellular reticularis nucleus
The possibility that eye-head cells in the NPH contribute to the ascending HD pathway may depend upon the contributions of the SGN and another vestibular-related region, the dorsal part of the paragigantocellular reticular nucleus (PGRNd). Anatomically, the PGRNd is a part of the ascending vestibular pathway to the HD system (Biazoli et al., 2006; Brown et al., 2005), and may also play a role in the oculomotor pathway described above (Graf et al., 2002). Interestingly, in the cat PGRNd neurons respond to head, eye, and neck rotation (Kitama et al., 1995; Grantyn et al., 1987; Vidal et al., 1983). Kitama et al. classified 37% of PGRNd neurons as orienting-related eye-neck neurons, such that they responded during active head movements, which involved coordination of eye and head movements during gaze shifts. These PGRNd eye-neck neurons did not appear to have notable vestibular sensitivity. In 14.7% of the remaining neurons that were unrelated to orienting movements, there was a noticeable vestibular sensitivity when tested with passive rotation. Thus, information about both active and passive head rotation is encoded in PGRNd neurons. Preliminary work in our laboratory has found comparable findings in freely-moving rats, where PGRNd neurons were identified that were sensitive to AHV and classified as the asymmetric cell type (S.S. Winter and J.S. Taube, unpublished observations). Because the PGRNd projects to the NPH (McCrea and Baker, 1985; Biazoli et al., 2006), these signals could contribute to the active head movement suppression seen in the NPH (Dale and Cullen, 2013). Further, given the PGRNd projection to the SGN (Biazoli et al., 2006), these signals, in theory, could cancel such active head movement related suppression in the SGN. Finally, the vestibular-only PGRNd neuron sub-type could also represent another possible source for the horizontal AHV signal. It is noteworthy that the PGRNd is not the only region that projects to both the NPH and SGN that could supply such complimentary information, as the frontal eye fields in the frontal cortex also contain information about active head movements and project to both brainstem nuclei (Stanton et al., 1988). Thus, this area could also provide both motor and sensory input to the ascending HD pathway through its descending projections to the brainstem.
Ascending AHV information
Taken together, the SGN and NPH anatomically represent the principle second stage in vestibular signal processing in the ascending pathway to the HD system. Both receive input from the medial vestibular nuclei and both project to the DTN; further, the NPH also projects to the SGN (see Taube, 2007 for review). Lesions of either region disrupt direction-specific firing in anterodorsal thalamus HD cells (Clark et al., 2012; Butler and Taube, 2012), with both regions forming parallel pathways to the DTN (Fig. 1). Yet, the function and nature of the pathways through the SGN and NPH, which generate the different types of responses in the DTN remains unclear. For instance, it is unclear whether the SGN was labeled as part of the HD-oculomotor pathway in the transneuronal viral study described above (Graf et al., 2002). Although portions of the vestibulo-ocular pathway that included the NPH and medial vestibular nuclei, as well as the DTN, were labeled, Graf et al. do not report any SGN labeling, and in their figure 4A there is no indication of labeling dorsomedial to the genu of the seventh nerve at the level of the abducens (although it is unclear if this particular section might be just caudal to the SGN). Further, while the NPH and SGN also share anatomical connectivity with the cerebellar flocculus and paraflocculus, these two regions also display divergent connectivity, as the NPH, but not the SGN, is connected with the cerebellar nodulus and uvula (Kaufman et al., 1996). In addition, it must be remembered that the SGN and NPH are involved in the vestibulo-trigeminal pathway (Giaconi et al., 2006), indicating that the SGN may not be exclusively involved in processing HD-related AHV signals and that the vestibular pathway through the SGN may not necessarily be oculomotor in nature. Thus, while it is possible that there is only one signal processing pathway through the vestibular brainstem that generates the AHV signal for the HD system, it is possible that there are multiple sources of vestibular information contributing to the ascending vestibular pathway to the HD system.
Velocity storage
The possibility that there may be two perceptual vestibular circuits, one overlapping and the other independent of oculomotor circuits is consistent with experiments that have manipulated velocity storage and monitored the vestibulo-ocular reflex and perceptual output (Shaikh et al., 2013). Velocity storage is the mechanism by which a short, fast response in the vestibular nerve becomes extended in time in the vestibular brainstem (Raphan et al., 1979; Buettner et al., 1978). However, most natural movements would not require such extensive processing. Current efforts to understand the vestibular circuitry underlying velocity storage find that what we have defined as velocity storage is a byproduct of the vestibular system’s attempt to resolve ambiguous sensory information using previous information about the animal’s current orientation and movement (Laurens and Angelaki, 2011).
The primary reference to align vestibular sensory information is an internal model of gravity, which can be used to resolve head movements into changes in orientation and motion in the planes of the environment. Interestingly, controlling and referencing the velocity storage response to gravity is also related to the vestibular pathway that connects to the nodulus and uvula (Laurens et al., 2013; Yakusheva et al., 2007; Solomon and Cohen, 1994; Wearne et al., 1998; Cohen et al., 2002). As discussed above, these parts of the caudal cerebellum may provide a similar reference control for the ascending angular velocity signal in the NPH (Carleton and Carpenter, 1983, Horowitz et al., 2005), and would reference the AHV signal sent to the HD circuit by aligning the head rotation with gravity (Karmali and Merfeld, 2012). The importance of referencing the vestibular sensory information externally (to gravity) as opposed to internally (to the head), may not be clearly evident when the animal is moving with their head upright. However, as the animal moves in three dimensions, the difference in how sensory information is referenced defines whether the animal is able to continuously maintain orientation with its environment (Taube and Shinder, 2013). This view, that the cerebellum uses gravity to realign vestibular canal and otolith input in order to extract movement in the horizontal and vertical planes, has not been tested. However, the vertical position sensitivity seen in the DTN and LMN implies that whether the computation is cerebellar or intrinsic to the ascending pathway, it is important to be able to convert vestibular self-motion information from being aligned with the head to being aligned with the horizontal plane of the environment. In this way, the cerebellum may play an important role in sensory processing, not only for spatial orientation in the HD system, but also in referencing translational movements to the planes of the environment for other limbic spatial representations (Rochefort et al. 2013). Consistent with this view is recent evidence demonstrating that hippocampal place cell properties are disrupted in transgenic mice that have a specific deficit in protein kinase C-dependent plasticity at parallel fiber-Purkinje synapses (Rochefort et al. 2011). In particular, place cell properties were impaired in these mice when they had to rely on self-motion cues during a path integration task. In sum, there may be more than one kind of vestibular signal transformation that occurs in the pathway from the medial vestibular nuclei through the NPH and SGN, which are related to the AHV signals used in the HD system.
As noted above, the phenomena of velocity storage may play a role in linking the perception of self-motion with oculomotor responses (Bronstein et al., 2008; Bertolini et al., 2012; Shaikh et al., 2013; Okada et al., 1999). Whether velocity storage plays a role in generating the HD signal was recently tested by severing the commissural projections between the vestibular nuclei, which disrupts velocity storage (Katz et al., 1991; Tham et al., 1989). Under these conditions, it was predicted that if the animal’s AHV was under-signaled, then a larger head movement would be required to create a specified shift in network activity (Taube and Bassett, 2005). The authors reported that HD cell preferred firing directions appeared to drift constantly in the direction of movement, and that the onset of the drifts was abrupt and usually preceded by low frequency, long duration head turns, suggesting that the HD cell network receives a vestibular signal that is prolonged by the velocity storage integrator. Given that the NPH and medial vestibular nuclei are interconnected, and that the cerebellar nodulus and uvula provide inputs into these two areas, the pathways are present for the control and referencing of velocity storage (Waespe et al., 1985; Solomon and Cohen, 1994; Bertolini et al., 2012). Interestingly, however, only a small percentage of NPH neurons (6.6%) display evidence of velocity storage (Lannou et al., 1984), and the NPH is usually not directly linked with the production of the velocity storage itself (Godaux et al., 1993). In sum, these findings suggest that the velocity storage signal is first processed in the medial vestibular nuclei prior to its input to the NPH and SGN.
Velocity sensitivity
The firing rates of NPH neurons have complex response profiles to rotational stimuli and might provide some insight into the ascending signal to the HD circuit. For example, the firing rates of NPH neurons do not consistently represent all head rotations at a given velocity equivalently. For natural, continuous head movements, the head moves at a particular velocity only for short time periods. The head speeds up, slows down, and changes direction during normal, ongoing behavior. The rate at which changes are made in the direction and velocity can be denoted by the frequency of the head movement. The firing rate sensitivity of NPH neurons at a constant head velocity generally decreases as the frequency, or number of changes in the direction of the head rotation, increases during smooth or low frequency head movements. However, the NPH neurons display the opposite - increased firing rates with increasing rotational frequency when the head movements contain more perturbations, or are in a range of higher frequencies, which would be seen during locomotion (Kaufman et al., 2000; Lannou et al., 1984). There is currently little information about NPH neuron activity in relation to changes in rotational velocity for head movements of similar frequency (smooth vs. interrupted). However, Lannou et al. (1984) noted decreasing firing rates in the NPH for increasing optokinetic visual stimulation velocities. Further, Kaufman et al. (2000) tested 10 cells across multiple velocities of head rotation with the frequency of the head rotation (number of head turns per second) held constant. Of these 10 cells, the firing rate sensitivity to head velocity for 7 cells was negatively correlated to the velocity of the head, 1 cell had a near zero slope, and 2 cells had firing that was positively correlated with increases in the animal’s AHV. Thus, because these NPH neurons were not sensitive to the static position of the head, the changes in firing rate sensitivity were attributed to the changes in peak head velocity. Therefore, there is some indication that there is a similar negative relationship between firing rate and AHV in the NPH as is seen in the medial vestibular nuclei (Newlands et al., 2009). It is unclear which of these medial vestibular or NPH cells are contributing to the ascending pathway. The DTN is the first area of the ascending pathway that contains AHV sensitive cells where neural responses could give an indication of the types of vestibular signals that may provide input to the ascending pathway. However, the previous studies of AHV sensitivity in DTN cells did not evaluate rotational frequency, but as discussed above Bassett and Taube (2001) found a mixed population of AHV cell types in the DTN – with tuning curves that were categorized as either symmetric or asymmetric. While Basset & Taube reported that the majority of DTN cells were of the symmetric type, Sharp et al. (2001b) found that all 35 of their DTN cells were of the asymmetric AHV cell type. While some ambiguity in the known responses of DTN AHV cells remains, there is clearly a difference between the strong asymmetric AHV cell type in the medial vestibular nuclei and NPH, and the mixed symmetric and asymmetric cell types in the DTN (Fig. 3). This difference might be accounted for by inhibitory output from the NPH to the DTN. Whether the pathway from NPH to the DTN is excitatory or inhibitory is not known, inhibitory output from the NPH is known to exist, as the NPH → abducens nucleus projection stems from eye-head neurons that respond to head rotation in only one direction (Escudero et al., 1992). If the eye-head neurons are contributing to the ascending pathway, then the asymmetric and inhibitory output of these cells to the SGN and DTN could provide the basis for switching the negative velocity-to-firing rate relationship to a positive relationship in the DTN, which results in the AHV symmetric cell type.
Supragenual nucleus
The role that the SGN serves in processing the AHV signal from the medial vestibular and NPH nuclei to the ascending pathway is not known, but it is clear that it plays an important role because lesions of it disrupt the HD cell signal (Clark et al. 2012). This uncertainty, in part, stems from the fact that there have been no published reports on response characteristics of SGN cells. Moreover, the results from the DTN studies, which could guide expectations for the cell types to be observed in the SGN, are again ambiguous due to the aforementioned conflicting reports. One possibility is that the SGN acts in concert with the NPH to balance what might otherwise be an inhibitory, asymmetric AHV type response to head rotations contralateral to the side of the head the NPH neuron is located in (Kaufman et al. 2000). The NPH output is believed to be inhibitory (Escudero et al. 1992), and modeling the NPH contribution to oculomotor pathways suggests that NPH output comes from vestibular neurons that primarily respond to head movement in one direction (Kaufman et al. 2000). Thus, if the output of NPH neurons is jointly projected to oculomotor pathways and the DTN (Graf et al., 2002), then the SGN output to the DTN could balance the NPH output and provide information to the DTN about head movements in both directions. In the Bassett and Taube (2001) study, there was a notable amount of symmetric AHV sensitivity, and when asymmetric responses were found, there was a trend for DTN AHV cells to increase their firing to head rotations in the direction contralateral to the side of the recorded cell (9 out of 11 asymmetric cells favored contralateral rotation). In contrast, in the Sharp et al. (2001b) study, there was a trend for cells to respond to head rotations toward the side that was ipsilateral to the recording site (Sharp et al., 2001b). In both cases there was at least a partial mix of cells that responded to ipsilateral and contralateral head turns. The mixture of these cell types could originate from projections of AHV information from inhibitory NPH cells that project contralaterally (the inhibitory eye-head outputs mentioned above) and converge with excitatory inputs from SGN cells that have a bias for ipsilateral rotation. Preliminary work in our laboratory indicates that many SGN cells can be classified as asymmetric AHV cells (S.S. Winter and J.S. Taube, unpublished observations). Therefore, the SGN may be complementary to the NPH in providing AHV signals to the DTN. If the SGN receives inhibitory NPH output with a contralateral rotational bias, the peak response would be to ipsilateral rotation. Because the SGN projects to the contralateral DTN (Biazoli et al., 2006), the input to the DTN would be biased to contralateral rotation by the SGN and attenuated to ipsilateral rotation because of the inhibitory input from uncrossed NPH projections. However, it is not yet known whether the NPH eye-head neurons that have a combined vestibular and oculomotor sensitivity are the same neurons that project to both the oculomotor and HD pathways. As noted above, the mix in ipsilateral and contralateral rotational biases found in the DTN is consistent with contributions from NPH eye-head cells from both sides of the brain converging in each DTN. However, it is also possible that the PGRNd supplies an AHV signal through the SGN and that the NPH integrates vertical and roll information. Such possibilities require experimental confirmation.
AHV and oculomotor signals
One issue to consider is the involvement of oculomotor signals on the processing of AHV information as it ascends towards the HD cell network. Many of the vestibular signals stemming from the vestibular nuclei are mixed in with eye position or eye velocity information. This information is not relevant to directional heading and must be removed from any signal being conveyed to the HD circuitry. One solution would be to counter the ocular-only portion of the eye-head signal to produce a signal with only head-related AHV information. This requirement suggests one possible role for the SGN might be to remove the eye-related information from the AHV signal. However, this possibility does not account for the direct projection from the NPH to the DTN. If the NPH eye-head cells project to the DTN directly, then any conversion of the eye-head signal must occur within the DTN. Eye-head cells, which are sensitive to eye and head velocity, could encode head movement consistently across both active and passive conditions (McCrea and Gdowski, 2003; Roy and Cullen, 2003). However, because of their ocular sensitivity, eye-head neurons display different firing rate responses during head rotations involved in cancellation of the vestibulo-ocular reflex and similar head rotations in the dark when the vestibulo-ocular reflex is active (Roy and Cullen, 2003; McFarland and Fuchs, 1992). Thus, even the potential projection of eye-head cell information to the HD system presents a problem – not necessarily because active head movements might be encoded as a mixture of eye and head rotation, as opposed to exclusively head rotation, but because not all head movements are treated the same when ocular responses are being volitionally modulated. The problem is ameliorated if other signals can be used to segregate out the vestibular-only portion of the ascending signal, but this process would yield the same signal type seen in vestibular-only neurons. Vestibular-only neurons, which differ in their active and passive responses, may be used in conjunction with eye-head and eye-related cells from the NPH or eye-neck cells from the PGRNd, such that for any given head movement, there is no single neuron type that consistently encodes the animal’s AHV, but rather, there is a collective vestibular signal from all cell types that provides information to the HD system.
Possible solutions to the conundrum
We now return to the conundrum: how is AHV information projected onto the HD system when it appears that many vestibular related neurons are quiescent during active head turns? In the introduction we proposed four possible nonexclusive mechanisms that might reconcile the conundrum.
The first possibility is that, as discussed above, the ascending AHV signal is the integrated output of multiple cell types (eye-head, eye-neck, and vestibular-only cells) across multiple regions (PGRNd, NPH and SGN nuclei), each of which responds differently to various conditions of head motion. Given these various cell types and the number of brainstem areas that provide inputs into the HD cell network, it is likely that the AHV signal is derived from a number of different sources, not all of which will be derived from the vestibular system and attenuated during an active head turn. Because proprioceptive and efference copy information can be used to determine head rotation in space during active head movement when some vestibular signals are known to be quiescent, the combination of vestibular and non-vestibular self-motion cues could encode total head rotation by the sum of the movement components that each system encodes. Thus, both proprioceptive feedback and motor efference copy information could play important roles and the DTN could serve as an important area where this integration takes place. It remains to be determined whether the signals ascending to the DTN are gated or controlled by the behavioral condition, or whether there is a defined mechanism of signal summation that generates a consistent AHV signal.
A second possibility is that there is a subset of vestibular-only neurons in the SGN, PGRNd or NPH that accurately encode all head movements and contribute to the ascending HD pathway. It was noted above that there are more oculomotor related neurons in the primate NPH than in rodent species, and that the differences in how vestibular-only cells respond to active and passive head movements differs depending upon the species. The increased vestibular related ocular circuitry in primates may be built on top of, rather than as a replacement for the evolutionary older vestibular-only related pathways through the NPH. While the reduced vestibular sensitivity during active head movements has been found in the majority of vestibular related NPH and medial vestibular nuclei cells in primates, the attenuation falls along a range of values. In most cases, there is a small percentage of cells found alongside the strongly suppressed cells that display minimal attenuation during active head movement (McCrea et al., 1996, 1999; Phillips et al., 1996; Khalsa et al., 1987). These cells have not been classified as a separate neural type, and have not been evaluated for possible contributions to the ascending pathway to the HD system. This possible explanation for a source of vestibular input to the ascending AHV signal suggests that the vestibular-only cells in the rodent continue to display head in space rotational sensitivity during active head movements, which is not the case (Medrea and Cullen, 2013). However, as the ascending vestibular pathway remains to be mapped in either the rat or a primate, it should be remembered that there could be a subpopulation of vestibular sensitive neurons in both species that may be the simple and direct source of vestibular input to the HD system.
The third possibility is that while vestibular-only neurons in the vestibular nuclei may contribute to the ascending pathway, and have reduced AHV sensitivity during active head turns, another AHV signal from a different brainstem area, like the PGRNd or cerebellum, may be used to drive the ascending HD circuit. In this case, the ascending AHV signal may not be the result of a distributed network, but rather a gated network where vestibular-only neurons only contribute during passive head movements and another signal from the SGN, PGRNd or NPH is used during active head movements. In such an arrangement, efference signals during active head movements would not directly encode motion, but play a critical role in gating vestibular information during active head turns.
A fourth possibility is that the AHV signal in the HD circuit is more complex than has been previously suspected. When an animal moves to face into the recorded cell’s preferred firing direction, there is no significant difference in the cell’s firing rate between when the animal actively moves to face that direction or when a fully-restrained, head-fixed animal is passively rotated in the dark to face that direction (Shinder and Taube, 2011a). Because vestibular lesions impair HD function during active head movement (Stackman and Taube, 1997), these findings taken together suggest that vestibular information likely plays the primary role in generating the HD signal, since motor efference and proprioceptive cues would be absent under passive conditions and could not replace vestibular signals during active conditions. However, the AHV signal in the rodent DTN has only been tested under passive conditions in one study with mixed results, where some cells had differential responses to active vs. passive rotation of the head (Sharp et al., 2001b). Thus, it is possible that the HD circuit adjusts for these changes by using visual, motor, and proprioceptive information such that the altered AHV input is interpreted differently under each condition, and a consistent HD signal is maintained across conditions. It is possible that such mechanisms exist to remove differences created by different behavioral conditions, such that the AHV information that contributes to generation of the HD signal consistently represents the same head motion the same way under all conditions. Similar mechanisms have been proposed for the mechanisms of how the HD system addresses other variations in vestibular sensory sensitivity for different velocities and frequencies of head movements that produce identical changes in direction (van der Meer et al., 2007; Kim et al., 2011).
Even during active movement, HD cell function depends upon vestibular input, and the sensitivity of many vestibular cells processing head movement information is suppressed during those same active head turns. The conundrum is created in which we are left to determine what possible mechanisms are available to provide vestibular information to the HD circuit while the head is actively turned. Given what we do know about the ascending vestibular pathway to the HD circuit, we can surmise several possibilities. The overlap of the HD and oculomotor pathways and the progression of AHV information along the ascending HD pathway provide indications of the types of processing required for the generation of the AHV signal for the HD circuit. While it remains possible that a simple circuit is driving the ascending pathway, the number of factors that need to be processed, such as reference frame, active vs. passive movement, and three planes of possible motion, most likely implies that there is more than a simple relay of horizontal AHV. This possibility appears even more likely if there is a reference change in the vestibular signal requiring involvement of cerebellar and commissural circuitry. Therefore, the conundrum of how the ascending pathway to the HD circuit is derived from a vestibular signal that is suppressed during active head movements, may in fact be one small part of a larger problem faced by the brain as it manipulates vestibular information derived from similar head movements under different behavioral conditions in order to yield accurate perceptions of directional heading (Dokka et al. 2013; Frissen et al. 2011; Zaidel et al. 2013).
In order to further understand and resolve this conundrum, future experiments will need to better understand the vestibular contribution to the HD system, and define active and passive movement encoding in the ascending vestibular pathway. Importantly, much remains unresolved about the AHV processing in the DTN. Currently, the available data contains many conflicting findings and do not provide definitive conclusions about the AHV sensitivity of the DTN during active and passive movement. Similarly, there is no information about the types of signals being processed in the SGN, and limited information about how medial vestibular nucleus and NPH neurons encode movements driven by active locomotion. All of these issues will require resolution in order to better understand whether the changes in the vestibular signal seen during active and passive head movements translate into differences in AHV signals ascending to the HD system.
Conclusions
In summary, many neurons in the vestibular nuclei, which respond to a passive head turn, respond differently to an active head turn. HD cells discharge when the head is turned passively or actively. Because the generation of the HD signal is dependent on an intact vestibular system, a conundrum arises to account for how an active head turn, which results in non-active vestibular responses, leads to updating of the HD signal. The HD signal is believed to be generated from the reciprocal connections between the DTN and lateral mammillary nuclei. The principal cell type found within the DTN are cells that fire based on the animal’s AHV. But AHV signals, which are also the primary correlate of vestibular neurons, are found throughout all the brainstem nuclei that are involved in the circuitry from the vestibular nuclei to the DTN – namely the NPH, SGN, and PGRNd. With the possible exception of NPH, very little is known about how the AHV signal is processed in these areas and how it might differ from the AHV signals seen in the vestibular nuclei. We have presented several possible explanations that might explain the conundrum. Foremost among these are the observations that not all vestibular cells shut down during an active head turn. In addition, the proprioceptive and motor inputs into the intervening nuclei between the vestibular nuclei and the DTN allow for integration of various head movement signals and can provide a further explanation out of the conundrum. Undoubtedly, as we learn more about the nature of the AHV signals in these intervening nuclei – in particular how they respond to active vs. passive head turns, we should achieve not only a more definitive answer to the conundrum, but also a better understanding of how the HD signal is generated. The demonstration that normal HD cell responses are maintained following head-fixed restraint provides an excellent opportunity for future experiments to take advantage of the possibility that recordings can be conducted intracellularly in HD cells. This approach makes it possible to record from HD cells and relate the cell’s level of depolarization to the animal’s behavior, as was recently demonstrated for hippocampal place cells (Harvey et al. 2009). Further, because hippocampal place cell activity appears to be maintained in virtual reality environments, albeit at a reduced level sometimes (Guifen et al., 2013; Ravassard et al., 2013), the combining of intracellular recording with passive restraint and virtual reality techniques offers opportunities for understanding how the HD signal is processed that did not exist before.
Highlights.
Head direction cells in the limbic system encode the animal’s perceived directional heading.
Lesions of the vestibular system abolish the head direction signal.
Responses of vestibular neurons are markedly attenuated during volitional head turns.
A conundrum arises as to how a vestibular signal is fed forward to update the HD signal.
This review discusses four possible mechanisms that could resolve this problem.
Acknowledgements
This work was supported by the National Institutes of Health Grants NS053907, DC009318.i
Glossary
- AHV
angular head velocity
- DTN
dorsal tegmental nucleus
- HD
head direction
- NPH
nucleus prepositus
- PGRNd
paragigantocellular reticular nucleus, dorsal part
- SGN
supragenual nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anastasio TJ, Robinson DA. Failure of the oculomotor neural integrator from a discrete midline lesion between the abducens nuclei in the monkey. Neurosci Lett. 1991;127:82–86. doi: 10.1016/0304-3940(91)90900-e. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Yakusheva TA. How vestibular neurons solve the tilt/translation ambiguity. Comparison of brainstem, cerebellum, and thalamus. Ann N Y Acad Sci. 2009;1164:19–28. doi: 10.1111/j.1749-6632.2009.03939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JP, Taube JS. Neural correlates for angular head velocity in the rat dorsal tegmental nucleus. J Neurosci. 2001;21:5740–5751. doi: 10.1523/JNEUROSCI.21-15-05740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JP, Tullman ML, Taube JS. Lesions of the tegmento-mammillary circuit in the head direction system disrupts the head direction signal in the anterior thalamus. J Neurosci. 2007;27:7564–7577. doi: 10.1523/JNEUROSCI.0268-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JP, Zugaro MB, Muir GM, Golob EJ, Muller RU, Taube JS. Passive movements of the head do not abolish anticipatory firing properties of head direction cells. J Neurophysiol. 2005;93:1304–1316. doi: 10.1152/jn.00490.2004. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Ramat S, Bockisch CJ, Marti S, Straumann D, Palla A. Is vestibular self-motion perception controlled by the velocity storage? Insights from patients with chronic degeneration of the vestibulo-cerebellum. PLoS One. 2012;7:e36763. doi: 10.1371/journal.pone.0036763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biazoli CE, Jr, Goto M, Campos AM, Canteras NS. The supragenual nucleus: a putative relay station for ascending vestibular signs to head direction cells. Brain Res. 2006;1094:138–148. doi: 10.1016/j.brainres.2006.03.101. [DOI] [PubMed] [Google Scholar]
- Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser M-B. Grid cells in pre- and parasubiculum. Nat Neurosci. 2010;13:987–994. doi: 10.1038/nn.2602. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Grunfeld EA, Faldon M, Okada T. Reduced self-motion perception in patients with midline cerebellar lesions. Neuroreport. 2008;19:691–693. doi: 10.1097/WNR.0b013e3282fbf9f6. [DOI] [PubMed] [Google Scholar]
- Brown JE, Card JP, Yates BJ. Polysynaptic pathways from the vestibular nuclei to the lateral mammillary nucleus of the rat: substrates for vestibular input to head direction cells. Exp Brain Res. 2005;161:47–61. doi: 10.1007/s00221-004-2045-4. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Gonzalez-Lima F. Brain activity associated with fear renewal. Eur J Neurosci. 2006;24:3567–3577. doi: 10.1111/j.1460-9568.2006.05229.x. [DOI] [PubMed] [Google Scholar]
- Buettner UW, Büttner U, Henn V. Transfer characteristics of neurons in vestibular nuclei of the alert monkey. J Neurophysiol. 1978;41:1614–1628. doi: 10.1152/jn.1978.41.6.1614. [DOI] [PubMed] [Google Scholar]
- Butler WN, Taube JS. Abstract Viewer/Itinerary Planner. Washington DC: Society for Neuroscience; 2012. The nucleus prepositus contributes to head direction cell stability in rats. Program No. 920.13. 2012 Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacucci F, Lever C, Wills TH, Buregess N, O’Keefe J. Theta-modulated place-by-direction cells in the hippocampal formation in the rat. J Neurosci. 2004;24:8265–8277. doi: 10.1523/JNEUROSCI.2635-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton SC, Carpenter MB. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res. 1983;278:29–51. doi: 10.1016/0006-8993(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Rossi F, Tempia F, Ghirardi M, Strata P. Saccadic eye movements and gaze holding in the head-restrained pigmented rat. Eur J Neurosci. 1989;1:639–646. doi: 10.1111/j.1460-9568.1989.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. II. Contributions of visual and ideothetic information to the directional firing. Exp Brain Res. 1994;101:24–34. doi: 10.1007/BF00243213. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Brown JE, Taube JS. Head direction cell activity in the anterodorsal thalamus requires intact supragenual nuclei. J Neurophysiol. 2012;108:2767–2784. doi: 10.1152/jn.00295.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, John P, Yakushin SB, Buettner-Ennever J, Raphan T. The nodulus and uvula: source of cerebellar control of spatial orientation of the angular vestibulo-ocular reflex. Ann N Y Acad Sci. 2002;978:28–45. doi: 10.1111/j.1749-6632.2002.tb07553.x. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol. 2004;91:1919–1933. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- Dale A, Cullen KE. The nucleus prepositus predominantly outputs eye movement-related information during passive and active self-motion. J Neurophysiol. 2013;109:1900–1911. doi: 10.1152/jn.00788.2012. [DOI] [PubMed] [Google Scholar]
- Dokka K, Macneilage PR, Deangelis GC, Angelaki DE. Multisensory self-motion compensation during object trajectory judgments. Cereb Cortex. 2013 doi: 10.1093/cercor/bht247. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero M, de la Cruz RR, Delgado-García JM. A physiological study of vestibular and prepositus hypoglossi neurones projecting to the abducens nucleus in the alert cat. J Physiol. 1992;458:539–560. doi: 10.1113/jphysiol.1992.sp019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A, Derdikman D, Foerster J, Las L, Ulanovsky N. Abstract Viewer/Itinerary Planner. Washington DC: Society for Neuroscience; 2012. 3-D head-direction cells in the bat presubiculum. Program No. 203.19 2012 Online. [Google Scholar]
- Frissen I, Campos JL, Souman JL, Ernst MO. Integration of vestibular and proprioceptive signals for spatial updating. Exp Brain Res. 2011;212:163–176. doi: 10.1007/s00221-011-2717-9. [DOI] [PubMed] [Google Scholar]
- Giaconi E, Deriu F, Tolu E, Cuccurazzu B, Yates BJ, Billig I. Transneuronal tracing of vestibulo-trigeminal pathways innervating the masseter muscle in the rat. Exp Brain Res. 2006;171:330–339. doi: 10.1007/s00221-005-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godaux E, Mettens P, Cheron G. Differential effect of injections of kainic acid into the prepositus and the vestibular nuclei of the cat. J Physiol. 1993;472:459–482. doi: 10.1113/jphysiol.1993.sp019956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golob EJ, Wolk DA, Taube JS. Recordings of postsubicular head direction cells following lesions of the lateral dorsal thalamic nucleus. Brain Research. 1998;780:9–19. doi: 10.1016/s0006-8993(97)01076-7. [DOI] [PubMed] [Google Scholar]
- Graf W, Gerrits N, Yatim-Dhiba N, Ugolini G. Mapping the oculomotor system: the power of transneuronal labelling with rabies virus. Eur J Neurosci. 2002;15:1557–1562. doi: 10.1046/j.1460-9568.2002.01994.x. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Ong-Meang Jacques V, Berthoz A. Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. II. Morphological properties as revealed by intra-axonal injections of horseradish peroxidase. Exp Brain Res. 1987;66:355–377. doi: 10.1007/BF00243310. [DOI] [PubMed] [Google Scholar]
- Gresty M, Baker R. Neurons with visual receptive field, eye movement and neck displacement sensitivity within and around the nucleus prepositus hypoglossi in the alert cat. Exp Brain Res. 1976;24:429–433. doi: 10.1007/BF00235008. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Van Dijk CA. Efferent connections of the dorsal tegmental region in the rat, studied by means of anterograde transport of the lectin Phaseolus vulgaris-leucoagglutinin (PHA-L) Brain Res. 1984;304:367–371. doi: 10.1016/0006-8993(84)90341-x. [DOI] [PubMed] [Google Scholar]
- Guifen Chena G, King JA, Burgess N, O’Keefe J. How vision and movement combine in the hippocampal place code. Proc Natl Acad Sci USA. 2013;110:378–383. doi: 10.1073/pnas.1215834110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461:941–946. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Zyo K. Afferent connections of Gudden's tegmental nuclei in the rabbit. J Comp Neurol. 1985;235:169–181. doi: 10.1002/cne.902350203. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M. Dynamic characteristics of saccadic eye movements in the albino rat. Neurosci Res. 1987;4:304–308. doi: 10.1016/0168-0102(87)90046-0. [DOI] [PubMed] [Google Scholar]
- Horowitz SS, Blanchard J, Morin LP. Medial vestibular connections with the hypocretin (orexin) system. J Comp Neurol. 2005;487:127–146. doi: 10.1002/cne.20521. [DOI] [PubMed] [Google Scholar]
- Kaneko CR. Eye movement deficits after ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys. I. Saccades and fixation. J Neurophysiol. 1997;78:1753–1768. doi: 10.1152/jn.1997.78.4.1753. [DOI] [PubMed] [Google Scholar]
- Karmali F, Merfeld DM. A distributed dynamic, parallel computational model: the role of noise in velocity storage. J Neurophysiol. 2012;108:390–405. doi: 10.1152/jn.00883.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman GD, Mustari MJ, Miselis RR, Perachio AA. Transneuronal pathways to the vestibulocerebellum. J Comp Neurol. 1996;370:501–523. doi: 10.1002/(SICI)1096-9861(19960708)370:4<501::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kaufman GD, Shinder ME, Perachio AA. Convergent properties of vestibular-related brain stem neurons in the gerbil. J Neurophysiol. 2000;83:1958–1971. doi: 10.1152/jn.2000.83.4.1958. [DOI] [PubMed] [Google Scholar]
- Katz E, Vianney de Jong JM, Buettner-Ennever J, Cohen B. Effects of midline medullary lesions on velocity storage and the vestibulo-ocular reflex. Exp Brain Res. 1991;87:505–520. doi: 10.1007/BF00227076. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Tomlinson RD, Schwarz DW, Landolt JP. Vestibular nuclear neuron activity during active and passive head movement in the alert rhesus monkey. J Neurophysiol. 1987;57:1484–1497. doi: 10.1152/jn.1987.57.5.1484. [DOI] [PubMed] [Google Scholar]
- Kim B, Dickman JD, Taube JS, Angelaki DE. Abstract Viewer/Itinerary Planner. New Orleans: Society for Neuroscience; 2011. Head directional responses in the primate anterodorsal nucleus of the anterior thalamus during a passive rotation task. Program No. 199.01. 2011 Online. [Google Scholar]
- Kitama T, Grantyn A, Berthoz A. Orienting-related eye-neck neurons of the medial ponto-bulbar reticular formation do not participate in horizontal canal-dependent vestibular reflexes of alert cats. Brain Res Bull. 1995;38:337–347. doi: 10.1016/0361-9230(95)00106-o. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. J Neurosci. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannou J, Cazin L, Precht W, Le Taillanter M. Responses of prepositus hypoglossi neurons to optokinetic and vestibular stimulations in the rat. Brain Res. 1984;301:39–45. doi: 10.1016/0006-8993(84)90400-1. [DOI] [PubMed] [Google Scholar]
- Laurens J, Angelaki DE. The functional significance of velocity storage and its dependence on gravity. Exp Brain Res. 2011;210:407–422. doi: 10.1007/s00221-011-2568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens J, Meng H, Angelaki DE. Computation of linear acceleration through an internal model in the macaque cerebellum. Nat Neurosci. 2013;16:1701–1708. doi: 10.1038/nn.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O’Keefe J, Burgess N. Boundary vector cells in the subiculum of the hippocampal formation. J Neurosci. 2009;29:9771–9777. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Chang L, Wickern G. The dorsal tegmental nucleus: an axoplasmic transport study. Brain Res. 1984;310:123–132. doi: 10.1016/0006-8993(84)90015-5. [DOI] [PubMed] [Google Scholar]
- Massot C, Schneider AD, Chacron MJ, Cullen KE. The vestibular system implements a linear-nonlinear transformation in order to encode self-motion. PLoS Biol. 2012;10:e1001365. doi: 10.1371/journal.pbio.1001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Baker R. Anatomical connections of the nucleus prepositus of the cat. J Comp Neurol. 1985;237:377–407. doi: 10.1002/cne.902370308. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Chen-Huang C, Belton T, Gdowski GT. Behavior contingent processing of vestibular sensory signals in the vestibular nuclei. Ann N Y Acad Sci. 1996;781:292–303. doi: 10.1111/j.1749-6632.1996.tb15707.x. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Gdowski GT. Firing behaviour of squirrel monkey eye movement-related vestibular nucleus neurons during gaze saccades. J Physiol. 2003;546:207–224. doi: 10.1113/jphysiol.2002.027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibule-spinal and other non-eye-movement related neurons. J Neurophysiol. 1999;82:416–428. doi: 10.1152/jn.1999.82.1.416. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Horn AK. Nucleus prepositus. Prog Brain Res. 2006;151:205–230. doi: 10.1016/S0079-6123(05)51007-0. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Luan H. Signal processing of semicircular canal and otolith signals in the vestibular nuclei during passive and active head movements. Ann N Y Acad Sci. 2003;1004:169–182. doi: 10.1196/annals.1303.015. [DOI] [PubMed] [Google Scholar]
- McFarland JL, Fuchs AF. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. J Neurophysiol. 1992;68:319–332. doi: 10.1152/jn.1992.68.1.319. [DOI] [PubMed] [Google Scholar]
- Medrea I, Cullen KE. Multisensory integration in early vestibular processing in mice: The encoding of passive versus active motion. J Neurophysiol. 2013;110:2704–2717. doi: 10.1152/jn.01037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier RK, Dieringer N. The role of compensatory eye and head movements in the rat for image stabilization and gaze orientation. Exp Brain Res. 1993;96:54–64. doi: 10.1007/BF00230438. [DOI] [PubMed] [Google Scholar]
- Muir GM, Brown JE, Carey JP, Hirvonen TP, Della Santina CC, Minor LB, Taube JS. Disruption of the head direction cell signal after occlusion of the semicircular canals in the freely moving chinchilla. J Neurosci. 2009;29:14521–14533. doi: 10.1523/JNEUROSCI.3450-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlands SD, Lin N, Wei M. Response linearity of alert monkey non-eye movement vestibular nucleus neurons during sinusoidal yaw rotation. J Neurophysiol. 2009;102:1388–1397. doi: 10.1152/jn.90914.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Grunfeld E, Shallo-Hoffmann J, Bronstein AM. Vestibular perception of angular velocity in normal subjects and in patients with congenital nystagmus. Brain. 1999;122:1293–1303. doi: 10.1093/brain/122.7.1293. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Phillips JO, Ling I, Siebold C, Fuchs AF. Behavior of primate vestibulo-ocular reflex neurons and vestibular neurons during head-free gaze shifts. Ann N Y Acad Sci. 1996;781:276–291. doi: 10.1111/j.1749-6632.1996.tb15706.x. [DOI] [PubMed] [Google Scholar]
- Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR) Exp Brain Res. 1979;35:229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- Ravassard P, Kees A, Willers B, Ho D, Aharoni D, Cushman J, Aghajan ZM, Mehta MR. Multisensory control of hippocampal spatiotemporal selectivity. Science. 2013;340:1342–1346. doi: 10.1126/science.1232655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Arabo A, André M, Poucet B, Save E, Rondi-Reig L. Cerebellum shapes hippocampal spatial code. Science. 2011;334:385–389. doi: 10.1126/science.1207403. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Lefort JM, Rondi-Reig L. The cerebellum: a new key structure in the navigation system. Front Neural Circuits. 2013;7:35. doi: 10.3389/fncir.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci. 2001;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol. 2002;87:2337–2357. doi: 10.1152/jn.2002.87.5.2337. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Brain stem pursuit pathways: dissociating visual, vestibular, and proprioceptive inputs during combined eye-head gaze tracking. J Neurophysiol. 2003;90:271–290. doi: 10.1152/jn.01074.2002. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Woitter MP, Moser MB, Moser EI. Conjunction representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol. 1992;68:244–264. doi: 10.1152/jn.1992.68.1.244. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Palla A, Marti S, Olasagasti I, Optican LM, Zee DS, Straumann D. Role of cerebellum in motion perception and vestibulo-ocular reflex-similarities and disparities. Cerebellum. 2013;12:97–107. doi: 10.1007/s12311-012-0401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PE, Blair HT, Cho J. The anatomical and computational basis of the rat head-direction cell signal. Trends Neurosci. 2001a;24:289–294. doi: 10.1016/s0166-2236(00)01797-5. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Tinkelman A, Cho J. Angular velocity and head direction signals recorded from the dorsal tegmental nucleus of gudden in the rat: implications for path integration in the head direction cell circuit. Behav Neurosci. 2001b;115:571–588. [PubMed] [Google Scholar]
- Shinder ME, Taube JS. Active and passive movement are encoded equally by head direction cells in the anterodorsal thalamus. J Neurophysiol. 2011a;106:788–800. doi: 10.1152/jn.01098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinder ME, Taube JS. Abstract Viewer/Itinerary Planner. New Orleans: Society for Neuroscience; 2011b. Firing rates of head direction cells are different between when moving and when motionless. Program No. 491.10. 2011 Online. [Google Scholar]
- Solomon D, Cohen B. Stimulation of the nodulus and uvula discharges velocity storage in the vestibulo-ocular reflex. Exp Brain Res. 1994;102:57–68. doi: 10.1007/BF00232438. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Golob EJ, Bassett JP, Taube JS. Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol. 2003;90:2862–2874. doi: 10.1152/jn.00346.2003. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002;12:291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing properties of head direction cells in rat anterior thalamic neurons: Dependence upon vestibular input. J Neurosci. 1997;17:4349–4358. doi: 10.1523/JNEUROSCI.17-11-04349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing properties of rat lateral mammillary single units: head direction, head pitch, and angular head velocity. J Neurosci. 1998;18:9020–9037. doi: 10.1523/JNEUROSCI.18-21-09020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. J Comp Neurol. 1988;271:493–506. doi: 10.1002/cne.902710403. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Ann Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Bassett JP. Abstract Viewer/Itineary Planner. Washington DC: Society for Neuroscience; 2005. The velocity storage integrator contributes to stable head direction representations. Program No. 392.1. 2005 Online. [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990a;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990b;10:436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Shinder ME. On the nature of three-dimensional encoding in the cognitive map: Commentary on Hayman, Verriotis, Jovalekic, Fenton, and Jeffery. Hippocampus. 2013;23:14–21. doi: 10.1002/hipo.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempia F, Ghirardi M, Dotta M, Strata P. Spontaneous gaze shifts in intact head-free rats and following inferior olive and cerebellar lesions. Eur J Neurosci. 1992;4:1239–1248. doi: 10.1111/j.1460-9568.1992.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Tham R, Larsby B, Eriksson B, Odkvist LM. Effects on the vestibulo- and opto-oculomotor system in rats by lesions of the commissural vestibular fibres. Acta Otolaryngol. 1989;108:372–377. doi: 10.3109/00016488909125542. [DOI] [PubMed] [Google Scholar]
- van der Meer MA, Knierim JJ, Yoganarasimha D, Wood ER, van Rossum MC. Anticipation in the rodent head direction system can be explained by an interaction of head movements and vestibular firing properties. J Neurophysiol. 2007;98:1883–1897. doi: 10.1152/jn.00233.2007. [DOI] [PubMed] [Google Scholar]
- Valerio S, Taube JS. Abstract Viewer/Itinerary Planner. Washington DC: Society for Neuroscience; 2012. Head direction cell activity is absent in mice without horizontal semicircular canals. Program No. 265.15. 2012 Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal PP, Corvisier J, Berthoz A. Eye and neck motor signals in periabducens reticular neurons of the alert cat. Exp Brain Res. 1983;53:16–28. doi: 10.1007/BF00239394. [DOI] [PubMed] [Google Scholar]
- Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. 1985;228:199–202. doi: 10.1126/science.3871968. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Sawinski J, Greenberg DS, Sawinski J, Rulla S, Notaro G, Kerr JND. Rats maintain an overhead binocular field at the expense of constant fusion. Nature. 2013;498:65–69. doi: 10.1038/nature12153. [DOI] [PubMed] [Google Scholar]
- Wearne S, Raphan T, Cohen B. Control of spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol. 1998;79:2690–2715. doi: 10.1152/jn.1998.79.5.2690. [DOI] [PubMed] [Google Scholar]
- Yakusheva TA, Shaikh AG, Green AM, Blazquez PM, Dickman JD, Angelaki DE. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron. 2007;54:973–985. doi: 10.1016/j.neuron.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Taube JS. Head direction cell activity in mice: robust directional signal depends on intact otoliths. J Neurosci. 2009;29:1061–1076. doi: 10.1523/JNEUROSCI.1679-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Brown JE, Lamia MV, Valerio S, Shinder ME, Taube JS. Both visual and idiothetic cues contribute to head direction cell stability during navigation along complex routes. J Neurophysiol. 2011;105:2989–3001. doi: 10.1152/jn.01041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Ma WJ, Angelaki DE. Supervised calibration relies on the multisensory percept. Neuron. 2013;80:1544–1557. doi: 10.1016/j.neuron.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaro MB, Tabuchi E, Fouquier C, Berthoz A, Wiener SI. Active locomotion increases peak firing rates of anterordorsal thalamic head direction cells. J Neurophysiol. 2001;86:692–702. doi: 10.1152/jn.2001.86.2.692. [DOI] [PubMed] [Google Scholar]