Key Points
MRD eradication is a desirable end point in chronic lymphocytic leukemia.
Early MRD eradication may prompt treatment discontinuation.
Abstract
The high complete remission rate with first-line combined fludarabine, cyclophosphamide, and rituximab (FCR) begs the question of the value of minimal residual disease (MRD)–negative status as a treatment end point. We report on 237 patients with chronic lymphocytic leukemia who received first-line FCR. MRD was prospectively assessed by 4-color flow cytometry in bone marrow after course 3 and at final response assessment. After course 3 and at final response assessment, 17% and 43% of patients were MRD negative in bone marrow, respectively. A mutated immunoglobulin heavy chain variable gene and trisomy 12 were independently associated with MRD-negative status both after 3 courses of FCR and at final response assessment in multivariable analyses (MVAs). MRD-negative status was independently associated with significantly longer progression-free survival (PFS) and overall survival (OS) in MVA (P = .03 and .02, respectively). This association was confirmed also on landmark MVA at the time of MRD assessment (P = .04 and .05, respectively). MRD-negative patients had comparable PFS and OS, independent of the number of courses received or interim staging. Early MRD eradication may be a desirable goal, prompting consideration of early discontinuation of treatment. This trial was registered at www.clinicaltrials.gov as #NCT00759798.
Introduction
Combined fludarabine, cyclophosphamide, and rituximab (FCR) was a significant advance in treatment of patients with chronic lymphocytic leukemia (CLL). First-line treatment with FCR results in a complete remission (CR) rate of 44% to 72% and an overall response rate of 90% to 95%; improved overall survival (OS) was demonstrated over fludarabine and cyclophosphamide.1,2
Similar to other types of leukemia, minimal residual disease (MRD) eradication can be a realistic desirable treatment goal for CLL.3-5 Available methods to study MRD in CLL include consensus and allele-specific oligonucleotide polymerase chain reaction for the clonal immunoglobulin heavy chain variable (IGHV) gene and multicolor flow cytometry.6,7 Allele-specific oligonucleotide polymerase chain reaction and 4-color flow cytometry can reach the .01% sensitivity recommended by International Workshop on CLL updating the National Cancer Institute-Working Group (IWCLL/NCI-WG) guidelines for clinical trials.8 The European Research Initiative on CLL established an international standardized approach for flow cytometric MRD evaluation, currently used at MD Anderson Cancer Center.9,10
Recently, several retrospective studies using different treatment regimens demonstrated the association between MRD-negative status (by different measures) and improved clinical outcomes.11-13 The German CLL study group CLL8 trial provided prospective evidence of the value of achieving MRD-negative status after treatment with fludarabine and cyclophosphamide or FCR.14 MRD was assessed by 4-color flow cytometry in blood before starting therapy, after 3 courses, 1 and 2 (final response assessment) months after the last course, and then every 3 months in follow-up. In cases of CR, MRD was also assessed in bone marrow at final response assessment. In multivariable analysis (MVA) when also considering patient pretreatment characteristics, MRD-negative status was independently associated with longer progression-free survival (PFS) and OS. However, blood is a less sensitive site than bone marrow to detect MRD, especially during and for several months after treatment with regimens containing monoclonal antibody.15 Rawstron highlighted the increased sensitivity of bone marrow over blood in evaluating for MRD after treatment with rituximab-containing regimens, such as with FCR in the CLL8 trial results of the German CLL study group (Andy C. Rawstron, St. James Institute of Oncology, oral communication, September 9, 2013). A remaining question is whether there is additional benefit with continued FCR treatment in patients who achieve early (prior to 6 courses) MRD-negative status.
We prospectively evaluated pretreatment patient characteristics and response, including bone marrow MRD evaluation, in 237 patients with CLL who received first-line FCR and followed patients for disease progression and survival.
Patients and methods
Patients
The study was approved by and conducted according to the Institutional Review Board of the University of Texas MD Anderson Cancer Center guidelines and was conducted in accordance with the principles of the Declaration of Helsinki. Between September 2008 and September 2012, 237 previously untreated patients with CLL requiring therapy according to IWCLL/NCI-WG indications8 provided informed consent, received up to 6 courses of standard FCR, and were evaluated for response (IWCLL/NCI-WG criteria,8 including 4-color flow cytometry) and follow-up on this prospective study.
All patients had pretreatment evaluation including medical history, physical examination, complete blood count, β-2 microglobulin (B2M) level, blood chemistry, and bone marrow aspiration and biopsy. The latter included fluorescent in situ hybridization (FISH) for common CLL chromosome abnormalities, analysis of the mutation status of the IGHV gene, CD38 expression by flow cytometry, and zeta-chain-associated protein kinase 70 (ZAP70) expression by immunohistochemistry. All patients had adequate baseline hepatic and renal function and received standard-dose FCR as previously described.1,16
MRD
Bone marrow MRD was quantified by flow cytometry in samples after course 3 and 2 months after the last course (response assessment). Four-color flow cytometry was performed according to the international standardized approach of European Research Initiative on CLL.9 Quantitative MRD results were categorized as positive (≥.01%) or negative (<.01%). Assessment was performed on patients achieving CR or partial remission (PR). Among patients who achieved bone marrow MRD-negative status after 3 courses of FCR, the median number of leukocytes evaluated in 4-color flow cytometry was 200 000 (range, 195 312-366 982); MRD evaluation was originally performed on 222 patients, but 28 MRD-negative cases were excluded because of not reaching adequate assay sensitivity (.01%). Among patients achieving MRD-negative status at the end of treatment, the median number of leukocytes evaluated in 4-color flow cytometry was 200 000 (range, 200 000-366 982); MRD evaluation was originally available for 220 patients, but 59 MRD-negative cases were excluded because of not reaching adequate assay sensitivity (.01%).
Statistical analysis
PFS was defined as time from start of treatment to progression; nonprogressers were censored at last follow-up. OS was defined as time from start of treatment to death or last follow-up. Survival curves were calculated using the method of Kaplan and Meier, and univariable comparisons were made using the log-rank test. MVA was performed using Cox regression with forward and backward stepwise selection. A landmark analysis at time of MRD final assessment was performed for PFS and OS. Categorical and continuous variables were compared using the χ2 or Fisher’s exact tests, and the Mann-Whitney U test, as appropriate. Logistic regression was used for MVA of categorical variable (IBM SPSS 19). All P values were 2-sided and considered significant if P ≤ .05.
Results
Baseline and treatment characteristics
Baseline patient characteristics are shown (Table 1); 75% received more than 3 courses of FCR, and 25% received 1-3 courses. CR was achieved in 65% of patients, CR with incomplete marrow recovery in 7%, nodular PR in 12%, and PR in 13%, for an overall response rate of 97%. Bone marrow MRD was negative in 17% of 194 patients evaluated after course 3. Bone marrow MRD was negative in 43% of 161 patients at final response assessment.
Table 1.
Patient characteristics
| Pretreatment characteristic (N = 237) | Number (%), median (range) |
|---|---|
| Males | 144 (61) |
| Age ≥65 years | 51 (21) |
| Age (years) | 59 (32-84) |
| Rai stage III-IV | 96 (40) |
| ALC (K/μL) | 75 (1-425) |
| ALC ≥75 K/μL | 119 (50) |
| Hb (g/dL) | 12 (6.9-16.5) |
| PLT (K/μL) | 142 (12-398) |
| B2M ≥4 mg/L (n = 231) | 95 (41) |
| B2M (mg/L) | 3.6 (1.3-14.1) |
| Unmutated IGHV gene (n = 208) | 126 (61) |
| CD38 ≥30% (n = 225) | 97 (43) |
| CD38 (%) | 16 (0.1-99.9) |
| ZAP70 IHC positive (n= 214) | 138 (64) |
| FISH (n = 222) | |
| del(13q) | 73 (33) |
| negative | 47 (21) |
| +12 | 39 (18) |
| del(11q) | 47 (21) |
| del(17p) | 16 (7) |
ALC, absolute lymphocyte count; Hb, hemoglobin; IHC, immunohistochemistry; PLT, platelet.
Factors associated with achieving MRD-negative status
At final response assessment, 70 (43%) patients were MRD negative, and 91 were MRD positive; MRD-negative status was achieved in 62 (63%) patients in CR, 3 (33%) patients in CR with incomplete marrow recovery, and 5 (17%) patients in PR. None of the patients in nodular PR achieved an MRD-negative status. Pretreatment characteristics were evaluated to determine factors independently associated with MRD-negative status at final response assessment. In univariable analyses (UVAs), factors associated with MRD-negative status were B2M <4 mg/L (P = .03), mutated IGHV gene (P = .02), trisomy 12 (+12; P = .004), and absence of deletion 17p [del(17p)] (P = .04). Factors not significantly associated (P > .05) with MRD-negative status in UVA included the following: age older than 65, Rai stage III-IV, absolute lymphocyte count (ALC) >75 K/μL; CD38 ≥30%; ZAP70 positivity; FISH: deletion (del)(13q), negative, and del(11q); and receiving more than 3 courses of FCR. In MVA, factors independently associated with MRD-negative status at final response assessment were mutated IGHV gene (odds ratio [OR], 2.5; 95% confidence interval [CI], 1.2-5.2; P = .01) and +12 (OR, 2.5; 95% CI, 1.5-4.2; P < .001) (Table 2).
Table 2.
Multivariable model for MRD-negative status at end of treatment
| Characteristic | n | MRD-neg (n = 70) | MRD-pos (n = 91) | OR (95% CI) | P |
|---|---|---|---|---|---|
| Mutated IGHV gene | 50 | 28 (56%) | 22 (44%) | 2.5 (1.2-5.2) | .01 |
| +12 | 29 | 20 (69%) | 9 (31%) | 2.5 (1.5-4.2) | <.001 |
The ORs were adjusted from the multivariable logistic regression model. Variables not significant (P > .05) in MVA: B2M ≥4 mg/L; FISH positive for del(17p).
neg, negative; pos, positive.
After course 3 of FCR, MRD-negative status was achieved in 34/194 (17%) patients. Pretreatment characteristics were evaluated to determine factors associated with MRD-negative status after course 3 of therapy. In UVA, factors associated with MRD-negative status were mutated IGHV gene (P = .02), ZAP70 negative (P = .04), and +12 (P = .05). Factors not significantly associated (P > .05) with MRD-negative status in UVA were age older than 65, Rai stage III-IV; ALC ≥75 K/μL; B2M ≥4 mg/L; CD38 ≥30%; and FISH: del(13q), negative, del(11q), and del(17p). In MVA, factors independently associated with MRD-negative status after course 3 of FCR were mutated IGHV gene (OR, 2.7; 95% CI, 1.1-6.3; P = .02) and +12 (OR, 2.7; 95% CI, 1.1-7.2; P = .05) (Table 3).
Table 3.
Multivariable model for MRD-negative status after course 3 of FCR
| Characteristic | n | MRD-neg (n = 34) | MRD-pos (n = 160) | OR (95% CI) | P |
|---|---|---|---|---|---|
| Mutated IGHV gene | 62 | 16 (26%) | 46 (74%) | 2.7 (1.1-6.3) | .02 |
| +12 | 32 | 10 (31%) | 22 (69%) | 2.7 (1.1-7.2) | .05 |
The ORs were adjusted from the multivariable logistic regression model. Variables not significant (P > .05) in MVA: ZAP70 positive.
PFS and OS
After a median follow-up of 28 months (range, 4-53), the overall median PFS (30 patients progressed) and OS (18 deaths) cannot be estimated. Survival curves were compared according to pretreatment and treatment characteristics. Factors associated with a longer PFS in UVA were age younger than 65 years (P = .02), B2M <4 mg/L (P = .04), mutated IGHV gene (P = .006), ZAP70 negative (P = .03), absence of del(17p) (P < .001), achievement of CR (P < .001) or overall remission (P < .001), and MRD-negative status at final response assessment (P < .001) (Figure 1A). PFS was not significantly associated (P > .05) in UVA with the following patient characteristics: Rai stage III-IV; ALC ≥75 K/μL; CD38 ≥30%; and FISH: del(13q), negative, +12, and del(11q). The multivariable model for PFS included the following significant independent characteristics: MRD-negative status (hazard ratio [HR], 0.1; 95% CI, 0.01-0.8; P = .03), achievement of CR (HR, 0.2; 95% CI, 0.05-0.6; P = .007) or overall remission (HR, 0.1; 95% CI, 0.03-0.5; P = .003), and absence of del(17p) (HR, 0.08; 95% CI, 0.02-0.3; P < .001) (Table 4).
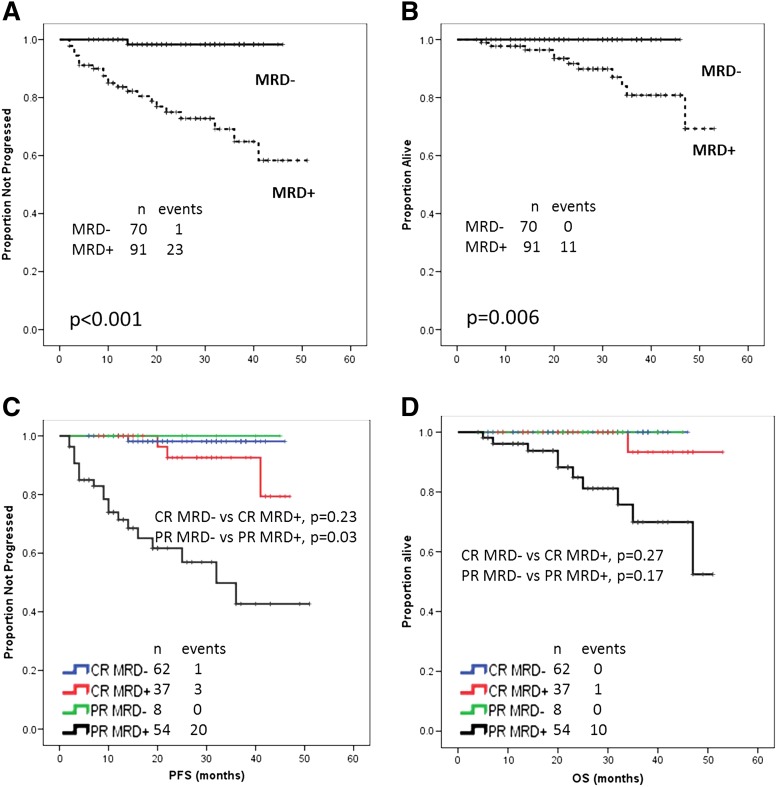
Figure 1.
Survival by MRD status. (A) Median PFS not reached in both groups; relapses among MRD-positive patients were 23/91; relapses among MRD-negative patients were 1/70 (P < .001). (B) Median OS not reached in both groups; deaths among MRD-positive patients were 11/91; there were no deaths among the 70 MRD-negative patients (P = .006). (C) PFS according to MRD status and IWCLL/NCI-WG. Relapses among MRD-positive patients in CR were 3/37; relapses among MRD-negative patients in CR were 1/62 (P = .23). Relapses among MRD-positive patients in PR were 20/54; there were no relapses among the 8 MRD-negative patients in PR (P = .03). (D) OS according to MRD status and IWCLL/NCI-WG response. Deaths among MRD-positive patients in CR were 1/37; there were no deaths among the 62 MRD-negative patients in CR (P = .27). Deaths among MRD-positive patients in PR were 10/54; there were no deaths among the 8 MRD-negative patients in PR (P = .17).
Table 4.
Multivariable models for PFS and OS by baseline and response characteristics
| Characteristic | Median PFS (mos) | HR (95% CI) | P | Median OS (mos) | HR (95% CI) | P |
|---|---|---|---|---|---|---|
| Absence of del(17p) | NR | 0.08 (0.02-0.3) | <.001 | NR | 0.2 (0.04-0.7) | .02 |
| CR | NR | 0.2 (0.05-0.6) | .007 | NR | 0.7 (0.5-0.9) | .05 |
| Overall remission | NR | 0.1 (0.03-0.5) | .003 | NR | NS | NS |
| MRD response-negative | NR | 0.1 (0.01-0.8) | .03 | NR | 0.6 (0.4-0.9) | .02 |
The HRs were adjusted from the multivariable Cox regression model. Variables not significant (P > .05) on MVA for PFS: age ≥65 years; B2M ≥4 mg/L; unmutated IGHV gene; ZAP70 positive. Variables not significant (P > .05) on MVA for OS: age ≥65 years; B2M ≥4 mg/L; overall remission.
mos, months NR, not reached; NS, not significant.
Factors associated with a longer OS in UVA were age younger than 65 (P = .02), B2M <4 mg/L (P = .03), absence of del(17p) (P = .001), achievement of CR (P < .001) or overall remission (P < .001), and MRD-negative status at final response assessment (P = .006) (Figure 1B). Variables not significantly associated (P > .05) with OS in UVA were Rai stage III-IV; ALC ≥75 K/μL; unmutated IGHV gene; ZAP70 positive; CD38 ≥30%; and FISH: del(13q), no FISH abnormality, +12, and del(11q).
OS was independently associated with the following characteristics in MVA: MRD-negative status (HR, 0.6; 95% CI, 0.4-0.9; P = .02), achievement of CR (HR, 0.7; 95% CI, 0.5-0.9; P = .05), and absence of del(17p) (HR, 0.2; 95% CI, 0.04-0.7; P = .02) (Table 4). MRD-negative status was not correlated with either PFS or OS when analyzing only patients who achieved CR with current follow-up (Figure 1C-D). Of interest, among MRD-positive patients in PR, median MRD positivity was 18.8% (range, 0.02% to 95%); whereas among MRD-positive patients who were in CR, median MRD positivity was 0.56% (range, 0.03% to 83.1%).
Landmark analysis at MRD final assessment for survival
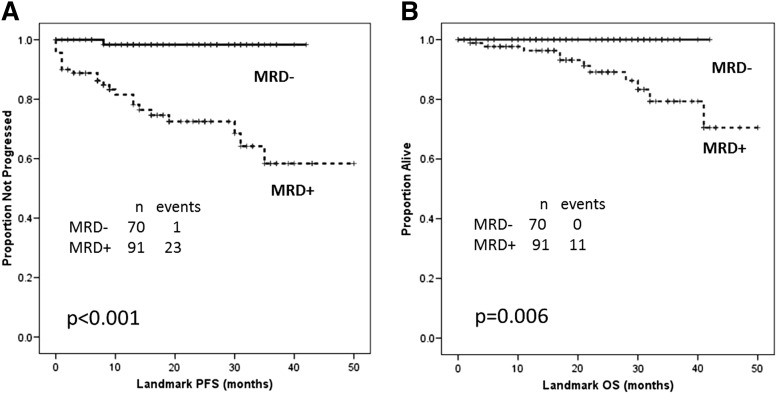
A landmark analysis for survival dated from when final MRD assessment was performed. After a median follow-up of 24 months (range, 1-50), overall median PFS and OS by landmark analysis cannot be estimated. Survival curves were compared according to baseline and treatment characteristics. Factors associated with a longer PFS in landmark UVAs were age younger than 65 (P = .02), B2M <4 mg/L (P = .05), mutated IGHV gene (P = .006), ZAP70 negative (P = .04), absence of del(17p) (P < .001), achievement of CR (P < .001) or overall remission (P < .001), and MRD-negative status at final response assessment (P < .001) (Figure 2A). Variables not significantly associated (P > .05) with PFS in landmark UVAs were Rai stage III-IV; ALC ≥75 K/μL; CD38 ≥30%; and FISH: del(13q), negative, +12, and del(11q). The landmark multivariable model included MRD-negative status (HR, 0.1, 95% CI, 0.05-0.9; P = .04), achievement of CR (HR, 0.2; 95% CI, 0.05-0.9; P = .04), and absence of del(17p) (HR, 0.05; 95% CI, 0.01-0.2; P < .001) independently associated with PFS (Table 5). Of interest, IGHV mutation status was not significant in the multivariable landmark model for PFS.
Figure 2.
Landmark analysis at MRD final assessment for survival by MRD status. (A) Median PFS not reached in both groups; relapses among MRD-positive patients were 23/91; relapses among MRD-negative patients were 1/70 (P < .001). (B) Median OS not reached in both groups; deaths among MRD-positive patients were 11/91; there were no deaths among MRD-negative patients (P = .006).
Table 5.
Landmark MVA for PFS and OS by baseline and response characteristics
| Characteristic | Median PFS (mos) | HR (95% CI) | P | Median OS (mos) | HR (95% CI) | P |
|---|---|---|---|---|---|---|
| Absence of del(17p) | NR | 0.05 (0.01-0.2) | <.001 | NR | 0.3 (0.09-0.9) | .05 |
| CR | NR | 0.2 (0.05-0.9) | .04 | NR | 0.3 (0.1-0.8) | .02 |
| MRD response-negative | NR | 0.1 (0.01-0.9) | .04 | NR | 0.7 (0.5-0.9) | .05 |
The HRs were adjusted from the multivariable Cox regression model. Variables not significant (P > .05) on MVA for PFS: age ≥65 years; B2M ≥4 mg/L; unmutated IGHV gene; ZAP70 positive; overall remission. Variables not significant (P > .05) on MVA for OS: age ≥65 years; B2M ≥4 mg/L; overall remission.
mos, months; NR, not reached.
Factors associated with a longer OS in landmark UVAs were age younger than 65 (P = .03), B2M <4 mg/L (P = .03), absence of del(17p) (P = .001), achievement of CR (P = .001) or overall remission (P < .001), and MRD-negative status at final response assessment (P < .006) (Figure 2B). Variables not significant (P > .05) in landmark UVAs for OS were Rai stage III-IV; ALC ≥75 K/μL; unmutated IGHV gene; ZAP70 positive; CD38 ≥30%; and FISH: del(13q), negative, +12, and del(11q). The multivariable landmark model included MRD-negative status (HR, 0.7; 95% CI, 0.5-0.9; P = .05), achievement of CR (HR, 0.3; 95% CI, 0.1-0.8; P = .02), and absence of del(17p) (HR, 0.3; 95% CI, 0.5-0.9; P = .05) as independently associated with OS (Table 5).
Achieving early MRD-negative status
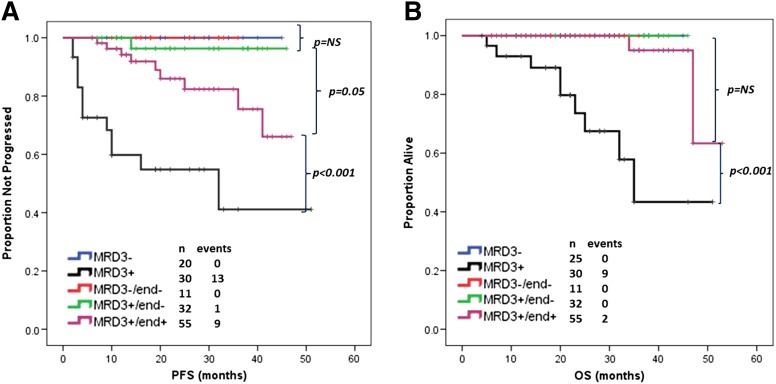
In order to potentially evaluate the significance of achieving early MRD-negative status, patients included in this study were divided according to the final number of FCR courses received and MRD status after course 3 and at final response assessment. Five patient groups were defined: patients who received 3 or fewer total courses of FCR and were MRD negative (n = 20) or positive (n = 30), patients who received more than 3 courses and were MRD negative both after course 3 and at final response assessment (n = 11), those who were MRD positive at both time points (n = 55), and those who were MRD positive after course 3 but negative at final response assessment (n = 32). Thirteen patients who had more than 3 courses of FCR but who had not undergone MRD evaluation after course 3 were not included in the analysis. PFS and OS were compared among the 5 groups. No significant differences in baseline characteristics were observed among the 3 MRD-negative groups (see supplemental Table, available on the Blood Web site).
There was no significant difference in PFS with current follow-up among patients achieving MRD negativity, regardless of number of courses and/or interim MRD status. The shortest PFS was observed for patients who received a total of 3 or fewer FCR courses and were MRD positive (P = .05), followed next by patients who received more than 3 total courses of FCR and were MRD positive at final response assessment (Figure 3A). For patients who were MRD negative after course 3 and who continued on treatment, there did not appear to be improved PFS for patients who stopped treatment vs those who achieved MRD-negative status upon continued treatment. A significantly shorter OS was observed only for patients who were MRD positive and received no more than 3 total courses of FCR (P < .001) (Figure 3B).
Figure 3.
PFS and OS according to MRD/therapy groups. (A) Patients achieving MRD-negative status after 3 or more courses of FCR, irrespective of MRD status at intermediate staging, had similar PFS (P = NS). Patients with MRD-negative status had a longer PFS than patients with MRD-positive status receiving 3 (P < .001) or more (P = .05) courses of FCR. (B) Patients achieving MRD-negative status after 3 or more courses of FCR, irrespective of MRD status at intermediate staging, or MRD-positive patients receiving more than 3 courses of FCR, had similar OS (P = NS). Patients with MRD-negative status or MRD-positive status but receiving more than 3 courses of FCR had a longer OS than patients with MRD-positive status receiving only 3 courses of FCR (P < .001). NS, not significant.
Discussion
Achieving an MRD-negative remission has been validated as an important treatment end point for some leukemias.3-5 The improved outcomes in CLL achieved with chemoimmunotherapy1,2 raise the question about the value of achieving MRD-negative remission as a therapeutic end point in CLL. Four-color flow cytometry is feasible in blood or bone marrow and can achieve the .01% sensitivity threshold requested by IWCLL/NCI-WG criteria for clinical trials.8
Evaluation for MRD in blood vs bone marrow remains an issue for study. Historically, with chemotherapy- and chemoimmunotherapy-based treatment, bone marrow was the last site to clear disease with response to treatment. High concordance between blood and bone marrow MRD status has been reported for regimens not including rituximab or alemtuzumab.9 Most data supporting the prognostic significance of blood MRD with rituximab-containing regimens do not provide direct comparison with matched, paired bone marrow samples.14,17 Rawstron analyzed 236 patients who received first-line FCR with paired bone marrow and blood MRD samples (Andy C. Rawstron, St. James Institute of Oncology, oral communication, September 9, 2013), reporting a 75% and a 85% concordance at 3 and 6 months after the completion of therapy, respectively, with marrow showing residual disease in discordant cases where blood was negative. Comparison with very small sample size (12 patients) showed comparable median PFS for patients with disconcordant (positive bone marrow, negative peripheral blood) samples.18 Our study evaluates bone marrow MRD and does not provide comparative MRD data for bone marrow vs blood. We feel that in the absence of definitive data, bone marrow should be the site for evaluation of MRD, especially in cases where blood is MRD negative.
In this study, we prospectively evaluated bone marrow MRD status during treatment and at final response assessment in patients receiving first-line FCR treatment. MRD-negative status correlated with longer PFS and OS. Mutated IGHV gene and +12 were independently associated with achieving MRD-negative status both after 3 courses of FCR and at final response assessment. Inferior outcome with first-line FCR was previously reported for patients with unmutated IGHV gene.2,19 A higher level of CD20 expression associated with +12 CLL20 could favor a greater sensitivity to rituximab and improve outcome. Keeping in mind MRD eradication as a desirable goal, these findings support the use of first-line FCR for this genetic subgroup.
Similar to the CLL8 trial, our study showed that MRD independently correlated with PFS and OS. This was also confirmed by landmark analysis at final MRD assessment. Of interest, the independent prognostic role played by MRD-negative status was stronger in patients achieving PR than patients in CR. This is most likely related to the current relatively limited follow-up period. Moreover, the low level of MRD positivity observed among patients in CR may be associated with the better outcome. PR in patients who achieved MRD-negative status was related to persistent lymphadenopathy (defined by physical examination, confirmed by computed tomography scan in a few patients). Given the favorable outcome among this group, the persistent lymphadenopathy may be scar tissue or fibrosis and not disease related in these patients. Biopsy would need to be performed for confirmation; this observation illustrates the weakness that bone marrow MRD evaluation is only evaluating for residual disease at a single potential disease site, when the disease can reside in other sites not being assessed, such as lymph nodes.
In our analysis, 20 patients achieved MRD-negative status after course 3 and stopped treatment, mostly owing to patients’ performance status, comorbidities, or myelosuppression. Their PFS outcome was comparable with patients who were MRD negative and continued to receive additional FCR courses or patients who achieved MRD-negative status after receiving more than 3 courses of FCR, despite comparable baseline characteristics. Moreover, on MVA, receiving more than 3 courses of FCR did not correlate with a higher probability of achieving MRD-negative status. This raises the prospect of stopping treatment upon achieving MRD-negative status, rather than requiring administration of a defined number of courses. Such a strategy could reduce exposure to cytotoxic chemotherapy, thereby potentially reducing associated secondary complications like infection, myelosuppression, myelodysplastic syndrome and acute myeloid leukemia, and other malignancies. Although feasible, the FCR regimen can be associated with complications, particularly in elderly patients.21 Moreover, in young patients with a longer life expectancy, there is concern about second malignancies, including myelodysplastic syndrome and acute myeloid leukemia.22,23 This underscores the potential value of minimizing the total number of courses administered for maximum clinical benefit. Moreover, consolidation or maintenance treatment with less aggressive regimens may be considered for patients who remain MRD positive after the completion of 6 courses of FCR. Bone marrow evaluation after 3 cycles of treatment is not currently recommended by IWCLL guidelines and may cause discomfort.8 Moreover, this study has a limited follow-up and number of events. These data will need to be confirmed in a large prospective randomized trial.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
Presented in part at the 18th annual meeting of the European Hematology Association, Stockholm, Sweden, June 13-16, 2013, and at the 15th biannual meeting of the International Workshop on Chronic Lymphocytic Leukemia, Cologne, Germany, September 9-11, 2013.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: W.G.W. designed, performed, and analyzed the trial, provided clinical care to patients, and wrote the manuscript; P.S. analyzed data, performed statistical analysis, and wrote the manuscript; M.J.K., S.M.O., J.B., A.F., N.J., F.P.T., Z.E., and S.H.F. provided clinical care to patients and coauthored the manuscript; and J.J. and P.C. analyzed laboratory data and coauthored the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William G. Wierda, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: wwierda@mdanderson.org.
References
- 1.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Fischer K, Fingerle-Rowson G, et al. International Group of Investigators; German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 3.Grimwade D, Vyas P, Freeman S. Assessment of minimal residual disease in acute myeloid leukemia. Curr Opin Oncol. 2010;22(6):656–663. doi: 10.1097/CCO.0b013e32833ed831. [DOI] [PubMed] [Google Scholar]
- 4.Campana D. Should minimal residual disease monitoring in acute lymphoblastic leukemia be standard of care? Curr Hematol Malig Rep. 2012;7(2):170–177. doi: 10.1007/s11899-012-0115-4. [DOI] [PubMed] [Google Scholar]
- 5.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2012 update on diagnosis, monitoring, and management. Am J Hematol. 2012;87(11):1037–1045. doi: 10.1002/ajh.23282. [DOI] [PubMed] [Google Scholar]
- 6.Moreno C, Ritgen M, Rawstron A. Is MRD eradication a desirable goal in CLL? Best Pract Res Clin Haematol. 2010;23(1):97–107. doi: 10.1016/j.beha.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Van Den Neste E, Letestu R, Aurran-Schleinitz T, et al. Post-remission intervention with alemtuzumab or rituximab to eradicate minimal residual disease in chronic lymphocytic leukemia: where do we stand? Leuk Lymphoma. 2012;53(3):362–370. doi: 10.3109/10428194.2011.608450. [DOI] [PubMed] [Google Scholar]
- 8.Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21(5):956–964. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]
- 10.Rawstron AC, Böttcher S, Letestu R, et al. European Research Initiative in CLL. Improving efficiency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia. 2013;27(1):142–149. doi: 10.1038/leu.2012.216. [DOI] [PubMed] [Google Scholar]
- 11.Wierda W, O’Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 12.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25(35):5616–5623. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 13.Moreton P, Kennedy B, Lucas G, et al. Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol. 2005;23(13):2971–2979. doi: 10.1200/JCO.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Böttcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30(9):980–988. doi: 10.1200/JCO.2011.36.9348. [DOI] [PubMed] [Google Scholar]
- 15.Böttcher S, Stilgenbauer S, Busch R, et al. Standardized MRD flow and ASO IGH RQ-PCR for MRD quantification in CLL patients after rituximab-containing immunochemotherapy: a comparative analysis. Leukemia. 2009;23(11):2007–2017. doi: 10.1038/leu.2009.140. [DOI] [PubMed] [Google Scholar]
- 16.Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117(11):3016–3024. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouvet E, Borel C, Obéric L, et al. Impact of dose intensity on outcome of fludarabine, cyclophosphamide, and rituximab regimen given in the first-line therapy for chronic lymphocytic leukemia. Haematologica. 2013;98(1):65–70. doi: 10.3324/haematol.2012.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrisqueta P, Villamor N, Terol MJ, et al. Rituximab maintenance after first-line therapy with rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) for chronic lymphocytic leukemia. Blood. 2013;122(24):3951–3959. doi: 10.1182/blood-2013-05-502773. [DOI] [PubMed] [Google Scholar]
- 19.Lin KI, Tam CS, Keating MJ, et al. Relevance of the immunoglobulin VH somatic mutation status in patients with chronic lymphocytic leukemia treated with fludarabine, cyclophosphamide, and rituximab (FCR) or related chemoimmunotherapy regimens. Blood. 2009;113(14):3168–3171. doi: 10.1182/blood-2008-10-184853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matutes E, Oscier D, Garcia-Marco J, et al. Trisomy 12 defines a group of CLL with atypical morphology: correlation between cytogenetic, clinical and laboratory features in 544 patients. Br J Haematol. 1996;92(2):382–388. doi: 10.1046/j.1365-2141.1996.d01-1478.x. [DOI] [PubMed] [Google Scholar]
- 21.Lamanna N. Treatment of older patients with chronic lymphocytic leukemia. Curr Hematol Malig Rep. 2012;7(1):21–25. doi: 10.1007/s11899-011-0111-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Tang G, Medeiros LJ, et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol. 2012;25(2):237–245. doi: 10.1038/modpathol.2011.158. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki S, Nakamura F, Nannya Y, Nakagawa M, Ichikawa M, Kurokawa M. Early-onset therapy-related myelodysplastic syndrome originating from prolonged myelosuppression after fludarabine-based therapy. Intern Med. 2012;51(24):3427–3430. doi: 10.2169/internalmedicine.51.8310. [DOI] [PubMed] [Google Scholar]