Abstract
Increased anxiety is co-morbid with human immunodeficiency virus (HIV) infection. Actions of the neurotoxic HIV-1 regulatory protein, Tat, may contribute to affective dysfunction. We hypothesized that Tat expression would increase anxiety-like behavior of female GT-tg bigenic mice that express HIV-1 Tat protein in the brain in a doxycycline-dependent manner. Furthermore, given reports that HIV-induced anxiety may occur at lower rates among women, and that the neurotoxic effects of Tat are ameliorated by sex steroids in vitro, we hypothesized that 17β-estradiol and/or progesterone would ameliorate Tat-induced anxiety-like effects. Among naturally-cycling proestrous and diestrous mice, Tat-induction via 7 days of doxycycline treatment significantly increased anxiety-like responding in an open field, elevated plus maze and a marble-burying task, compared to treatment with saline. Proestrous mice demonstrated less anxiety-like behavior than diestrous mice in the open field and elevated plus maze, but these effects did not significantly interact with Tat-induction. Among ovariectomized mice, doxycycline-induced Tat protein significantly increased anxiety-like behavior in an elevated plus maze and a marble burying task compared to saline-treated mice, but not an open field (where anxiety-like responding was already maximal). Co-administration of progesterone (4 mg/kg), but not 17β-estradiol (0.09 mg/kg), with doxycycline significantly ameliorated anxiety-like responding in the elevated plus maze and marble burying tasks. When administered together, 17β-estradiol partially antagonized the protective effects of progesterone on Tat-induced anxiety-like behavior. These findings support evidence of steroid-protection over HIV-1 proteins, and extend them by demonstrating the protective capacity of progesterone on Tat-induced anxiety-like behavior of ovariectomized female mice.
Keywords: 17β-estradiol, elevated plus maze, estrous cycle, GT-tg bigenic mice, marble burying, neuroAIDS, open field, ovariectomy, Tat1–86, transactivating transcriptor
Introduction
The incidence of mood disorders (e.g., anxiety, depression) is greater among human immunodeficiency virus (HIV)-positive individuals compared to the general population (Bing et al. 2001; Evans et al., 2005; Kagee and Martin, 2010; Orlando et al., 2002; Pence et al., 2007; Scharko, 2006), and greater than that reported for other terminal illnesses such as cancer (Massie, 2004). Antiretroviral therapies have proven successful at reducing HIV load, limiting viral transmission, prolonging lifespans and deferring progression of acquired immunodeficiency syndrome (AIDS; Cohen et al., 2011). However, a growing number of reports suggest these therapies cannot fully ameliorate the neurological dysfunction associated with HIV infection (Boissé et al., 2008; Kraft-Terry et al., 2010; Mollace et al., 2001; Pence et al., 2007), possibly due to the chronic activity of HIV proteins continually produced from persistent viral reservoirs within the CNS (Chen et al., 2010; McArthur, 2004).
One viral factor that may influence affective dysfunction is the HIV-1 regulatory protein, transactivating transcriptor (Tat). Tat drives efficient HIV transcription (Parada and Roeder, 1999) and replication (Jeang et al., 1991). Exposure to Tat protein produces neuronal dysfunction (Maragos et al., 2002; Wallace et al., 2006; Zhu et al., 2009a, 2011) and promotes cell death in vitro (Kruman et al., 1998; Magnuson et al., 1995; Nath et al., 1996; Zhu et al., 2009b) and in vivo (Starling et al., 1999; Royal et al., 2012), suggesting possible biological mechanisms by which HIV-1 Tat might influence neuropsychiatric status. Consistent with this, intracerebroventricular injection of Tat increases depressive-like behavior of male mice (Lawson et al., 2011) and we have recently demonstrated that inducing brain-limited expression of Tat in transgenic male mice elevates anxiety-like behavior in a manner consistent with Tat protein expression (Paris et al., 2013).
Although HIV is a leading cause of death among African-American women (Evans et al., 2002), clinical investigations of HIV-related mood disorders are rarely stratified by gender (Sordo del Castillo et al., 2010). Curiously, some clinical investigations have reported a lower vulnerability to HIV-associated anxiety and depression among infected women compared to men (Goggin et al., 1998; Lopes et al., 2012). The male gender may also be predictive of affective disorder in cases of HIV (Orlando et al., 2002), a reversal of the typically-observed sex difference (Kessler et al., 1993). Findings that the progression of HIV-related neurological dysfunction may be reduced or delayed among women (Cabrera-Muñoz et al., 2012a) suggest an effect perhaps influenced by sex steroids. Indeed, 17β-estradiol (E2) and/or progesterone (P4) may protect against HIV cellular infection (Cabrera-Muñoz et al., 2012b; Rodriguez-Garcia et al., 2013) and HIV progression (Cabrera-Muñoz et al., 2012b), and have been shown in vitro to reduce HIV-1 Tat-mediated neurotoxicity (Kendall et al., 2005; Wallace et al., 2006). However, it is not known whether sex-steroid hormones influence HIV-related anxiety.
To investigate this question, we utilized female GT-tg bigenic mice (Kim et al., 2003). These animals conditionally express HIV-1 Tat protein in the brain when treated with doxycycline (Carey et al., 2012; Paris et al., 2013. We hypothesized that central actions of HIV-1 Tat would increase anxiety-like behavior in female mice, and endogenous cycling or exogenous enhancement of E2 and/or P4 would ameliorate these effects.
Material and Methods
These methods were pre-approved by the Institutional Animal Care and Use Committee at the Torrey Pines Institute for Molecular Studies (Port Saint Lucie, FL) and were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85-23).
Subjects and housing
GT-tg bigenic mouse model
To assess the influence of central HIV-1 Tat protein on affective behavior, doxycycline-inducible GFAP/Tat bigenic (GT-tg) mice (originally derived by Dr. Johnny He, Indiana University School of Medicine, Indianapolis, IN) were utilized. Briefly, separate lines of founder mice (C3HeB x FeJ offspring; original strains obtained from The Jackson Laboratory, Bar Harbor, ME) were derived with a GFAP-localized doxycycline-inducible promoter (G-tg) or human Tat1–86 doxycycline-controlled (T-tg) transgene. Transgenic lines were crossed onto a C57BL/6J background to produce stabile transgene expression and mated to produce the GT-tg bigenic model (Kim et al., 2003). When transgene expression is induced via doxycycline administration, astrocyte-derived HIV-1 Tat1–86 protein is expressed selectively in the GT-tg mouse brain, but not in peripheral tissues, and the copy numbers of the Tat transgene correlate with Tat RNA expressed (Kim et al., 2003). The expression of Tat1–86 protein in the GT-tg brain is dependent on the dose- and duration of doxycycline treatment (Carey et al., 2012; Paris et al., 2013). Tat expression in this model recapitulates clinical findings of HIV characterized by central macrophage/monocyte infiltration, T-lymphocyte infiltration, neuronal cell death (Kim et al., 2003), as well as dose-dependent reductions in limbic gray matter density (Carey et al., 2013), reductions in cognitive performance (Carey et al., 2012), increased anxietylike responding among males (Paris et al., 2013), and enhancement of psychostimulant reward (Paris et al., 2014).
Housing
Female GT-tg mice (N = 210) were generated in our colony in the Torrey Pines Institute for Molecular Studies vivarium (Port Saint Lucie, FL). GT-tg breeders have been previously back-crossed 7 generations onto the C57BL/6J strain. Mice (approximately 70 days of age) were housed 4 – 5 / cage and were maintained in a temperature- and humidity-controlled room on a 12:12 h light / dark cycle (lights off at 19:00 h) with ad libitum access to food and water.
Determination of estrous cycle phase
In Experiment 1 (Fig. 1, left panel), estrous cycle phase was determined as previously described (Paris et al., 2011). Briefly, epithelial tissue was collected via vaginal lavage and was assessed under a light microscope at 50 X and 200 X magnification. Proestrous (majority presence of nucleated epithelial cells), estrous (majority presence of cornified cells), metestrous (similarly proportioned cornified and leukocytic cells), and diestrous (majority presence of leukocytic cells) cycle phases were identified daily for 7 consecutive days prior to inclusion in the study. Transitions between cycle phase often occur overnight (Bronson and Vom Saal, 1979). As such, cycle phase was determined at the beginning of the light phase (∼09:00 h) of the light / dark cycle so that mice could be assessed and tested in the afternoon (∼13:00 h) on the day of diestrus (when concentrations of estrogens are beginning to rise and progestogens have fallen to nadir) or the day of proestrus (when concentrations of estrogens have peaked and progestogens are rising to peak; Michael, 1976; Scharfman and MacLusky, 2006; Schumacher et al., 2014; Wood et al., 2005). Testing at these times cannot parse the individual effects of E2 vs. P4, but is associated with a reduction of anxiety-like responding in the proestrous, compared to the diestrous, phases of the cycle that co-occur with elevated circulating E2 and progestogens (Koonce et al., 2012; Frye and Rhodes, 2006). Mice that did not demonstrate a 4 – 5 day estrous cycle were excluded (n = 8). To avoid confounds of carryover performance issues within the same assay, mice were behaviorally tested only once: either in the next proestrous or diestrous cycle phase that followed pharmacological manipulation.
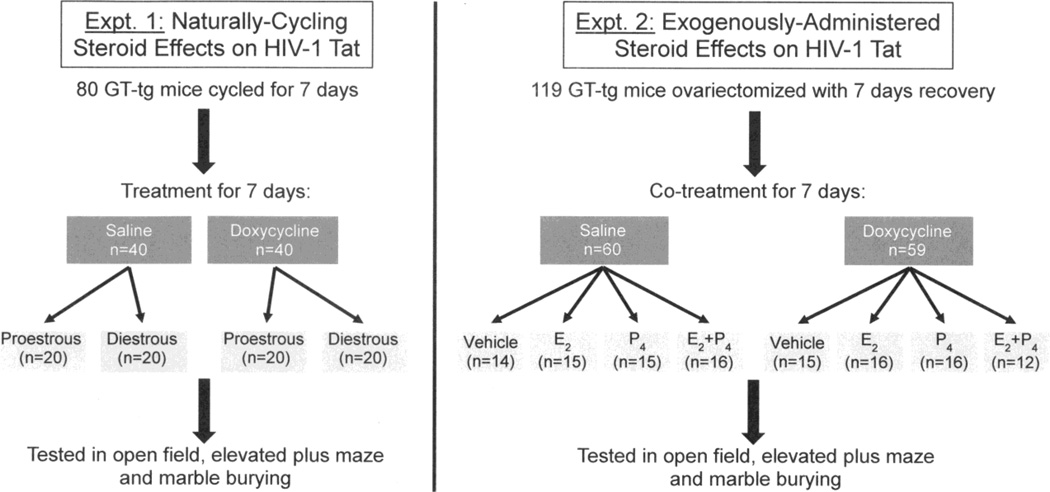
Figure 1.
Depicts the experimental design for female GT-tg mice that were intraperitoneally-administered saline (0.9 %) or doxycycline (100 mg/kg; to induce HIV-1 Tat) for 7 days while naturally-cycling (left panel) or ovariectomized and co-administered subcutaneous vehicle, 17β-estradiol (0.09 mg/kg), and/or progesterone (4 mg/kg; right panel). All mice were assessed for anxiety-like behavior in an open field, elevated plus maze, and marble burying task.
Surgical manipulation
In Experiment 2 (Fig. 1, right panel), mice were ovariectomized (OVX) via bilateral flank incisions while under isoflurane (4 %) anesthesia. Mice were allowed 7 days for surgical recovery and endogenous hormone washout. Following surgery, mice were monitored to ensure weight gain, muscle tone, and proper neurological response and general health (Crawley and Paylor, 1997). Three mice failed to recover and were excluded from the study. After recovery, mice underwent pharmacological manipulation (described below) for an additional 7 days prior to testing.
Chemicals
For both experiments, doxycycline hyclate, obtained from Sigma-Aldrich (St. Louis, MO), was dissolved in sterile 0.9 % saline, diluted to a concentration of 100 mg/kg (0.1 ml volume administered per 10 g body weight), and administered once daily for 7 consecutive days. Studies of doxycycline dose (0, 25, 50, or 125 mg/kg, i.p.) and treatment duration (0, 1, 3, 5, 7, or 14 days) have revealed this dosing regimen to optimally express central HIV-1 Tat protein in the GT-tg bigenic brain (Carey et al., 2012; Paris et al., 2013) commensurate with behavioral (Carey et al., 2012; Paris et al., 2013, 2014) and neurodegenerative (Carey et al., 2013) changes.
Steroid hormone replacement
Seven days following OVX, mice in Experiment 2 began treatment with saline (0.9 %, i.p. for 7 days) or doxycycline (100 mg/kg, i.p. for 7 days) and were co-administered dual vehicles subcutaneously (0.1 ml volume administered per 10 g body weight). Note that two vehicles were used; one injection of oil as the carrier for E2, and one injection of 10% EtOH in oil as the carrier for P4. Additional sets of mice instead received E2 (one injection of E2, 0.09 mg/kg in oil, and one injection of 10% EtOH in oil), P4 (one injection of oil and one injection of P4, 4 mg/kg in 10% EtOH in oil), or E2/P4 (one injection of E2, 0.09 mg/kg in oil, and one injection of P4, 4 mg/kg in 10% EtOH in oil) daily for 7 days (Fig. 1, right panel). Ovariectomy largely depletes circulating E2 and P4 concentrations (with trace remaining steroids primarily secreted from the adrenals), contributing to lower concentrations in the brain. The greatest elevations in rodent anxiety-like behavior are observed after ovariectomy/adrenalectomy > ovariectomy > diestrus cycle phase > proestrus cycle phase (with the last demonstrating the lowest anxiety-like responding; Frye and Paris, 2011). When administered acutely, this steroid hormone dosing approximates circulating and central E2 and P4 concentrations that are observed in the proestrous phase of the estrous cycle (Walf et al., 2006). Herein, we administered these steroids chronically in conjunction with saline or doxycycline to ascertain whether supra-physiological sex steroid concentrations could influence the effects of Tat.
Behavioral assays
For both experiments, testing was conducted in a battery of assays for anxiety-like behavior (open field, followed by elevated plus maze, followed by marble burying; Fig. 1). All mice were tested in the same order of the three tasks, given that the use of multiple anxiety assays increases confidence of the behavioral constructs assessed, and similar anxiety testing batteries have demonstrated minimal carry-over effects between assays (Crawley and Paylor, 1997; Lad et al., 2010; McIlwain et al., 2001). In between tasks, mice were returned to their housing cage for only as much time as it took to clean the previously-used apparatuses (approximately 2 min between each task to clean the open field and elevated plus maze with quatricide; 15 g/L). Each mouse was tested in their own marble burying apparatus, which were set up prior to testing each day, with enough apparatuses for all mice to be tested on that day. At the end of the testing day, used marbles were cleansed with quatricide and cages used for marble burying were cleaned.
Open field test
The open field test assesses anxiety-like behavior and ataxia (Hall and Ballachey, 1932; Paris et al., 2013, 2014). Briefly, mice were placed in the lower left corner of a square Plexiglas box (46 × 46 x 30 cm) and allowed to explore for 5 min. Movement was monitored and digitally encoded by a Noldus (Leesburg, VA) EthovisionPro3 image capture software package. A greater latency to, and lesser frequency of, entries into the brightly-lit center (∼24 cm square) were considered indices of greater anxiety-like behavior. The velocity (cm / s) of exploration was further utilized as an index of locomotor behavior.
Elevated plus maze test
The elevated plus maze assesses anxiety-like behavior (Lister, 1987) and consisted of black Plexiglas with two open arms (30 × 3.5 cm) and two enclosed arms of the same size (14 cm high walls). The four arms were connected by a central square (6 × 6 cm square) and were elevated approximately 74 cm from the ground. Briefly, mice were placed in the center square and allowed to explore for 5 min. A greater amount of raw time and a greater proportion of time (%) spent on the brightly-lit open arms of the elevated plus maze was considered an index of anxiety-like behavior. The percentage of open arm entries were also calculated but did not significantly differ across gonadally-intact or OVX groups. The total number of arm entries was utilized as an index of locomotor behavior.
Marble burying test
The marble burying test utilizes spontaneous digging behavior, characteristic of rodents, to assess anxiety-like/compulsive behavior (Broekkamp et al. 1986; Paris et al., 2013; Poling et al. 1981). Briefly, mice were individually placed in a standard mouse housing cage (28 × 16 x 13 cm) with 20 marbles (1.5 cm diameter; evenly spaced in 5 rows of 4) located on a 5 cm layer of woodchip bedding. Mice were introduced to the cages and allowed to behave. After 30 min, the number of marbles that were completely buried were counted. A greater number of buried marbles was considered an index of greater anxiety/compulsive-like behavior.
Statistical analyses
Dependent measures on behavioral tasks were assessed via separate two-way analyses of variance with hormone status (Expt. 1: proestrous or diestrous; Expt. 2: vehicle, E2, P4, or E2/P4) and conditional Tat-induction status (Expts. 1 and 2: saline or doxycycline) as factors. Fisher’s Protected Least Significant Difference post-hoc tests determined group differences following main effects. Interactions were delineated via simple main effects and main effect contrasts with alpha controlled for multiple comparisons. Analyses were considered significant when p < 0.05. To indicate the proportion of variance each effect accounted for, η2 is reported following omnibus inferential test statistics. Cohen’s d is reported for each post-hoc comparison to estimate effect size.
Results
Expt. 1: Induction of HIV-1 Tat protein increases anxiety-like behavior across the estrous cycle
After one week of regular estrous cycling, GT-tg females were administered saline or doxycycline for 7 days (Fig. 2a). Neither saline nor doxycycline administration disrupted estrous cyclicity (1 – 2 days in the proestrous cycle phase were observed among all mice during the 7 days of saline or doxycycline treatment). In week 3, mice were assessed for anxiety-like behavior on the next day of proestrus (at a time when both E2 and P4 levels are elevated) or diestrus (at a time when both E2 and P4 levels are low). Mean time from the end of saline or doxycycline treatment to the time of testing was 2.8 ± 0.5 days across a range of 1–7 days.
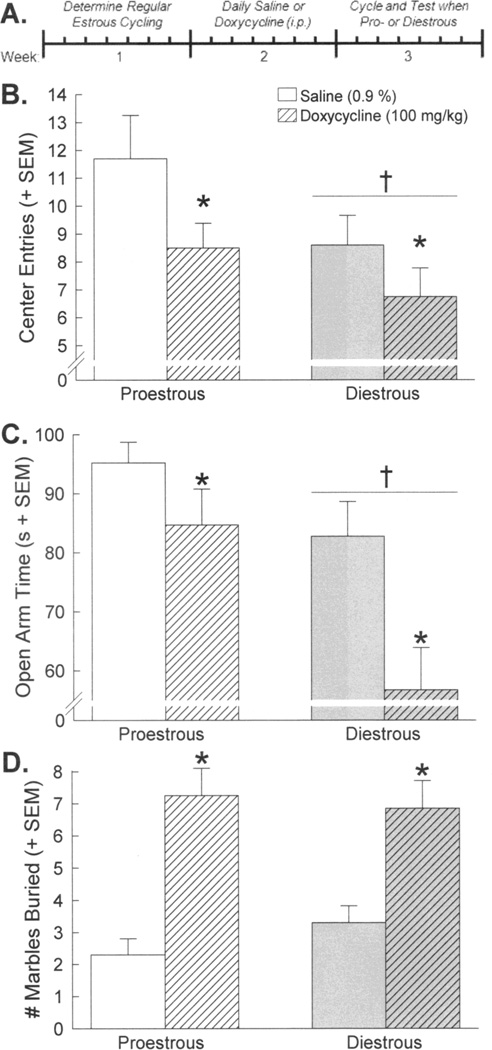
Figure 2.
(A) Regularly estrous cycling female GT-tg mice were treated with 7 days of saline (as a control; open bars) or doxycycline (to centrally induce HIV-1 Tat protein; striped bars) and tested in the next proestrous (left bars) or diestrous (right bars) phase of their cycle (n = 20 / group). Anxiety-like behavior was assessed in (B) an open field, (C) an elevated plus maze, and (D) a marble burying task. * indicates significant main effect for doxycycline-treated mice to differ from saline-treated mice. † indicates significant main effect for diestrous mice to differ from proestrous mice, p < 0.05.
Conditionally inducing HIV-1 Tat expression in the brain of female GT-tg mice increased anxiety-like behavior. Compared to uninduced controls, there were significant main effects for Tat-induced females irrespective of estrous cycle phase to display a significantly greater latency to enter the brightly-lit center of an open field [F(1,76) = 7.22, p < 0.05, η 2 = 0.083] (Table 1), to make fewer entries into the brightly-lit center of an open field [F(1,76) = 4.73, p < 0.05, η 2 = 0.055] (Fig. 2b), to spend less time [F(1,76) = 9.03, p < 0.05, η 2 = 0.092] (Fig. 2c) and a lesser proportion of time [F(1,76) = 8.87, p < 0.05, η 2 = 0.093] (Table 1) on the open arms of an elevated plus maze, and to bury more marbles in an anxiogenic marble burying task [F(1,76) = 36.19, p < 0.05, η2 = 0.319] (Fig. 2d). Estrous cycle differences in anxiety-like behavior were also observed with proestrous mice entering the center of an open field significantly sooner [F(1,76) = 3.99, p < 0.05, η 2 = 0.046] (Table 1), making significantly more central entries in an open field [F(1,76) = 4.37, p < 0.05, η 2 = 0.051] (Fig. 2b), accumulating significantly more open arm time [F(1,76) = 11.06, p < 0.05, η 2 = 0.113] (Fig. 2c) and spending a greater proportion of time on the open arms [F(1,76) = 9.48, p < 0.05, η 2 = 0.099] (Table 1) of an elevated plus as compared to their diestrous counterparts. No significant effects of doxycycline treatment or estrous cycle phase were detected on the percentage of arm choices in the elevated plus maze or on motor function (Table 1).
Table 1.
Proestrous and diestrous GT-tg mice treated i.p. for 7 days with saline as a control or doxycycline (100 mg/kg) to induce HIV-1 Tat protein were assessed for motor behavior (movement velocity, total arm entries) and anxiety-like behavior (latency to enter the brightly-lit center, % time spent on brightly-lit open arms) in an open field and elevated plus, respectively.
| Proestrous | Diestrous | ||||
|---|---|---|---|---|---|
| Saline (n=20) |
Doxycycline (n=20) |
Saline (n=20) |
Doxycycline (n=20) |
||
| Motor Measures | |||||
| Movement Velocity (cm / s ± SEM) |
7.2 ± 0.3 | 6.1 ± 0.2 | 6.5 ± 0.3 | 6.6 ± 0.4 | |
| Total Arm Entries (mean ± SEM) |
20 ± 1 | 16 ± 1 | 18 ± 1 | 21 ± 4 | |
| Anxiety-like Measures | |||||
| Latency to Central Entry (s ± SEM) |
30 ± 5 | 60 ± 16* | 50 ± 12† | 97 ± 20*† | |
| % Open Arm Time (% ± SEM) |
36 ± 2 | 32 ± 2* | 32 ± 2† | 22 ± 3*† | |
A main effect for doxycycline condition indicates groups significantly differ from saline treatment(*), irrespective of estrous cycle phase.
A main effect for estrous cycle phase indicates groups significantly differ from proestrous mice (†), irrespective of doxycycline treatment, p < 0.05.
Expt. 2: Progesterone administration is protective against the anxiety-like effects of central Tat induction
After one week of surgical recovery, OVX GT-tg females were administered saline or doxycycline while receiving subcutaneous injections of vehicle, E2, and/or P4 (Fig. 3a). Mice were assessed for anxiety-like behavior after 7 days of co-administration (14 days from the time of surgery).
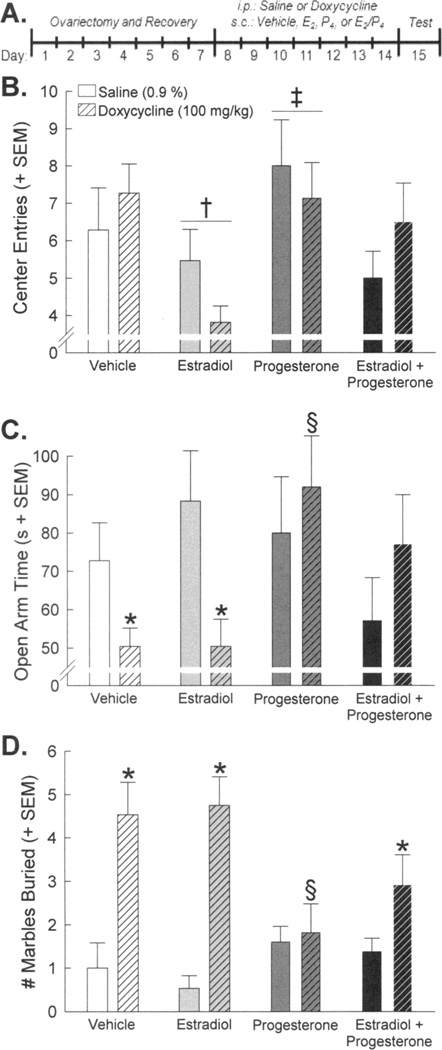
Figure 3.
(A) GT-tg female mice were ovariectomized. After one week of recovery, mice (n = 12 – 16 / group) were intraperitoneally treated with 7 days of saline (as a control; open bars) or doxycycline (to centrally induce HIV-1 Tat protein; striped bars) while subcutaneously co-administered vehicle, estradiol (0.09 mg/kg), and/or progesterone (4 mg/kg). Anxiety-like behavior was assessed in (B) an open field, (C) an elevated plus maze, and (D) a marble burying task. * indicates significant interaction wherein indicated doxycycline-treated group differs from respective saline-treated control group. † indicates significant main effect for estradiol-administered mice to differ from vehicle-treated mice. ‡ indicates significant main effect for progesterone-administered mice to differ from estradiol- or estradiol/progesterone-administered mice. § indicates significant interaction wherein doxycycline/progesterone-treated group differs from doxycycline/vehicle- or doxycycline-estradiol-treated groups, p < 0.05.
Doxycycline treatment did not significantly alter motor function (Table 2) or behavior in an open field, although anxiety-like responding among OVX GT-tg control mice already appeared maximal compared to performances of gonadally-intact mice (Fig. 2b). Steroid hormone administration did influence anxiety-like and exploratory behavior with P4 administration significantly reducing the latency to enter the brightly-lit center of an open field [F(3,111) = 3.46, p < 0.05, η2 = 0.083] (Table 2) compared to OVX mice administered vehicle (p = 0.008, d = 0.78) or E2 and P4 jointly (p = 0.004, d = 0.80). Estradiol and P4 had opposing effects on the velocity of open field exploration [F(3,111) = 3.07, p < 0.05, η2 = 0.123] (Table 2) and the frequency of central entry [F(3,111) = 4.06, p < 0.05, η2 = 0.097] (Fig. 2b). Compared to vehicle administration, E2 significantly reduced exploration speed, with (p = 0.02, d = 0.63) or without (p = 0.007, d = 0.66) co-administration of P4 (Table 2), and E2 administration significantly reduced the number of central entries (p = 0.02, d = 0.70) made in an open field (Fig. 3b). Conversely, progesterone did not influence exploration velocity (Table 2) and significantly enhanced the frequency of entries made into the center of an open field compared to administration of E2, with (p = 0.04, d = 0.53) or without (p = 0.001, d = 0.83) co-administration of P4, but did not differ from vehicle administration (Fig. 3b).
Table 2.
Ovariectomized GT-tg mice were treated i.p. for 7 days with saline as a control, or doxycycline (100 mg/kg) to induce HIV-1 Tat protein, concurrent with administration of s.c. vehicle, estradiol (0.09 mg/kg), and/or progesterone (4 mg/kg) and were assessed for motor behavior (movement velocity, total arm entries) and anxiety-like behavior (latency to enter the brightly-lit center, % time spent on brightly-lit open arms) in an open field and elevated plus, respectively.
| Vehicle | Estradiol | Progesterone | Estradiol/ Progesterone |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Saline (n=14) |
Doxycycline (n=15) |
Saline (n=15) |
Doxycyclin (n=16) |
Saline (n=15) |
Doxycycline (n=16) |
Saline (n=16) |
Doxycycline (n=12) |
||
|
Motor Measures |
|||||||||
| Movement Velocity (cm / s ±SEM) |
6.8 ±0.3 | 7.2 ± 0.3 | 6.4 ±0.3† | 6.0 ± 0.4† | 6.6 ±0.2 | 6.8 ± 0.3 | 6.3 ±0.3† | 6.3 ± 0.3† | |
| Total Arm Entries (mean±SEM) |
18 ±1 | 17 ± 1 | 19 ±1 | 14 ± 2 | 19 ±1 | 17 ± 1 | 15 ±1 | 16 ± 2 | |
|
Anxiety-like Measures |
|||||||||
| Latency to Central Entry (s±SEM) |
88 ±19 | 75 ± 21 | 55 ±16 | 73 ± 13 | 28 ±6†‡ | 42 ± 12†‡ | 104 ±24 | 62 ± 16 | |
| % Open Arm Time (% ± SEM) |
27 ±4 | 20 ± 2 | 33 ±5 | 19 ± 3^ | 30 ±5 | 33 ± 5§ | 22 ±4 | 29 ± 5 | |
A main effect of hormone treatment indicates groups significantly differ from vehicle-treated mice (†) or estradiol/progesterone-treated mice (‡), irrespective of doxycycline condition.
An interaction between hormone and doxycycline conditions indicates that estradiol/doxycycline-treated mice significantly differ from their estradiol/saline-treated counterparts (^) and progesterone/doxycycline-treated mice significantly differ from estradiol/doxycycline-treated mice or vehicle/doxycycline-treated controls (§), p < 0.05.
Central HIV-1 Tat induction and systemic steroid hormone administration significantly interacted to influence anxiety-like responding in an elevated plus maze [raw open arm time: F(3,111) = 2.97, p < 0.05, η2 = 0.071, (Fig. 3c); % time on open arms: F(3,111) = 2.84, p < 0.05, η2 = 0.068, (Table 2)] or a marble burying task [F(3,111) = 5.44, p < 0.05, η2 = 0.098] (Fig. 3d). Compared to uninduced controls, Tat-induced OVX mice demonstrated decreased open arm time (Fig. 3c), a decreased proportion of time on open vs. closed arms (Table 2), and increased marble burying behavior (Fig. 3d) when treated with systemic vehicle (praw open arm time = 0.047, d = 0.80; pmarbles buried = 0.001, d = 1.42) or E2 (praw open arm time = 0.01, d = 0.97; p% open arm time = 0.02, d = 0.90; pmarbles buried < 0.0001, d = 2.12). These effects of doxycycline treatment were ameliorated by administration of P4 which significantly increased raw open arm time (Fig. 3c), as well as the proportion of open arm time (Table 2), and decreased the number of marbles buried (Fig. 3d) compared to Tat-induced OVX females that were administered either vehicle (praw open arm time = 0.004, d = 1.06; p% open arm time = 0.009, d = 0.96; pmarbles buried = 0.006, d = 1.01) or E2 (praw arm time = 0.004, d = 1.01; p% open arm time = 0.006, d = 0.93; pmarbles buried = 0.003, d = 1.43). When E2 and P4 were co-administered, the protective effects of P4 were partially antagonized such that Tat-induced reduction in the raw seconds (Fig. 3c) and the proportion (Table 2) of open arm time on the elevated plus maze were still attenuated, but were no longer significantly enhanced beyond Tat-induced control levels. Similarly, co-administration of E2 and P4 appeared to reduce Tat-induced marble burying behavior by ∼40 % compared to treatment with vehicle or E2 alone, but did not significantly attenuate marble burying behavior when compared to uninduced E2/P4-treated controls (p = 0.04, d = 0.87; Fig. 3d).
Discussion
The present findings upheld the hypothesis that exposure to HIV-1 Tat would increase anxiety-like behavior of female GT-tg mice. Among naturally-cycling females (Expt. 1), treatment with doxycycline under conditions previously shown to be efficacious in male mice (Paris et al., 2013) significantly increased the latency to, and reduced the frequency of, central entries in an open field, reduced the amount and proportion of open arm time accumulated in an elevated plus maze, and increased anxiogenic marble burying (summarized in Table 3, left panel). Despite some apparent resilience to Tat’s effects among proestrous mice (which have elevated circulating and central E2 and P4 compared to diestrous mice), doxycycline treatment did not significantly interact with estrous cycle phase (summarized in Table 3, right panel). As such, endogenous, cyclical fluctuations in steroid hormones were not enough to overcome the anxiogenic effects of the present Tat-induction regimen. However, the hypothesis that exogenous steroid hormone administration would ameliorate the anxiety-like effects of Tat induction was upheld (Expt. 2). Tat-induction further increased anxiety-like behavior of OVX mice (in which circulating E2 and P4 are largely depleted and central concentrations are lower than those observed among gonadally-intact mice) in an elevated plus maze or a marble burying task (summarized in Table 3, left panel) and daily co-administration of P4 attenuated these effects (summarized in Table 3, right panel). Conversely, the present E2 regimen was not observed to protect against the anxiogenic effects of Tat induction and partially antagonized the protective effects of P4 when both steroids were co-administered.
Table 3.
Summary of major findings with anxiety-like effects of Tat-induction (left) among gonadally-intact and ovariectomized mice presented separately from anti-anxiety-like effects of steroid hormone milieu (right) among naturally-cycling and ovariectomized mice that were administered vehicle, estradiol (E2; 0.09 mg/kg, s.c.) and/or progesterone (P4; 4 mg/kg, s.c.).
| Anxiety-Enhancing Effects of Tat Induction (vs. Saline) |
Anxiety-Ameliorating Effects of Elevated Hormonal Milieu (vs. Reduced or Depleted Milieu) |
|||
|---|---|---|---|---|
| Expt. 1: Gonadally-Intact Mice |
Expt. 2: Ovariectomized Mice |
Expt. 1: Gonadally-Intact Mice |
Expt. 2: Ovariectomized Mice |
|
| Open Field |
•Tat induction ↑ anxiety |
(No effects) | •Proestrous ↓ anxiety |
•P4 ↓ anxiety |
| -↑ latency to center |
-↓ latency to center |
-↓ latency to center vs. vehicle or E2+P4 |
||
| -↓ central entries |
-↑ central entries |
-↑ central entries vs. E2 or E2+P4 |
||
| Elevated Plus Maze |
•Tat induction ↑ anxiety |
•Tat induction ↑ anxiety |
•Proestrous ↓ anxiety |
•Tat-induced P4 ↓ anxiety |
| -↓ open arm time |
-↓ open arm time |
-↑ open arm time |
-↑ open arm time vs. vehicle or E2 |
|
| Marble Burying |
•Tat induction ↑ anxiety |
•Tat induction ↑ anxiety |
(No effects) | • Tat-induced P4 ↓ anxiety |
| -↑ # marbles buried |
-↑ # marbles buried |
-↓ # marbles buried vs. vehicle or E2 |
||
These data confirm and extend previous findings of sex steroid interactions with HIV and HIV-1 Tat protein. Actions of E2 and P4 may influence the course of HIV infection (Cabrera-Muñoz et al., 2012ab). Apart from influences on HIV acquisition (wherein synthetic hormones that reduce endometrial thickness may facilitate infection; Clemetson et al., 1993; Mostad et al., 1997; Wang et al., 2004), sex steroids may ameliorate viral progression once infection has occurred. Indeed, Tat-mediated toxicity is notably associated with the upregulation of proinflammatory cytokines (Royal et al., 2012; Yadav and Collman, 2009) and/or NF-κB-regulated chemokines (El-Hage et al., 2005; Vergote et al., 2006) that can be inhibited by downstream actions of E2 or P4 (Härkönen and Väänänen, 2006; Su et al., 2009). To a greater extent than E2, P4 may also exert direct actions on HIV replication. In peripheral blood mononuclear cells, high-dose P4 consistently down-regulated HIV replication whereas low-dose E2 consistently up-regulated replication when cells came from a female donor (Ragupathy et al., 2013). Beneficial effects were not observed in cells from male donors and effects were variable amongst HIV subtypes (Ragupathy et al., 2013). Differences in gender and HIV clade may contribute to conflicting data as to whether the virological response (i.e., HIV copy numbers) is altered with endogenous changes that influence peripheral steroid production, such as age. In support, some studies find antiretroviral therapies to be less effective in geriatric populations, while others report no difference or a greater response (reviewed in Imai et al., 2013). Accordingly, we investigated changes in endogenous steroids by utilizing an in vivo animal model that expresses only one viral protein (Tat) that may contribute to HIV-relevant mood disorders. Notably, previous work with male GT-tg mice was shown to recapitulate neurological effects of HIV infection including deficits in cognition (Carey et al., 2012), affect (Paris et al., 2013), and vulnerability to psychostimulant reward (Paris et al., 2014), supporting the face validity of the present female mouse model.
Some of the behavioral sequelae associated with HIV-1 Tat are thought to occur partly via its effects to induce neuronal apoptosis in vitro (Nath et al., 1996; New et al., 1998) and in vivo (Starling et al., 1999; Royal et al., 2012). In particular, Tat exerts neurotoxic effects in vitro via NMDA receptor-mediated excitotoxicity (Magnuson et al. 1995; Haughey et al., 1999) and promotes loss of hippocampal synaptic density via actions downstream of NMDA receptors (Kim et al., 2008). These findings are germane to the present study, as we have previously observed that male GT-tg bigenic mice exposed to Tat demonstrate volumetric loss of gray matter in limbic brain regions associated with emotionality, including the amygdala, amygdalo-hippocampal area, peri-/entorhinal cortex, and hypothalamus (Carey et al., 2013). Anxiogenic effects of Tat on females may be associated with similar central pathology given that these regions are steroid-sensitive, expressing all of the necessary enzymes to metabolize the peripheral prohormones investigated (Melcangi et al., 1998). Importantly, in vitro findings indicate that some Tat-mediated microstructural changes in the hippocampus can be reversed (Kim et al., 2008), suggesting a potential therapeutic window during which centrally-acting steroids may be efficacious.
Steroids may play a potential ameliorating role in the neurodegeneration and dysfunction that is associated with HIV-1 Tat. In vitro findings support a protective role for both E2 and P4 on Tat-induced neurotoxicity. In human or rat tissue cultures, 17β-estradiol ameliorates the oxidative stress effects of combined Tat and the HIV glycoprotein, gp120, as well as apoptosis caused by combined Tat, gp120, and psychostimulant drugs (Adams et al., 2010, 2012; Turchan et al., 2001; Wallace et al., 2006). While we did not observe protective effects of the present E2 regimen over Tat-mediated anxiety-like behavior, it should be noted that this does not preclude cellular E2-mediated protection or alternate dosing schedules that may be efficacious. Similar to the present findings, others have reported high, chronic E2 to be anxiogenic among OVX mice (Morgan and Pfaff, 2001, 2002) whereas acute and lower dosing regimens have marked effects to reduce anxiety-like behavior of rodents in testing batteries such as the one presently used (Frye et al., 2008; Walf et al., 2009). Cholesterol or P4 also demonstrated smaller dose-dependent effects to attenuate neurotoxicity caused by Tat, gp120, and psychostimulants in vitro (Kendall et al., 2005). In concordance, we observed the present P4 dosing regimen to protect mice from the anxiogenic effects of Tat. The extent to which this indicates an amelioration of Tat-mediated brain pathology is unknown; yet, preclinical evidence of the neuroprotective effects of P4 for ischemic and traumatic brain injury are well-elucidated (Sayeed and Stein, 2009; Sayeed et al., 2006), leading to its clinical consideration in a NIH-sponsored Phase III clinical trial (Stein, 2013). Future investigations aiming to directly manipulate central steroid formation and their actions within implicated brain regions may provide insight into the important central sites of steroid and Tat interaction.
It should be noted that nontraditional actions of progestogens overlap with targets of HIV-1 Tat, and may partly underlie observed protective effects. Progestogens dose-dependently negatively modulate NMDA receptor activity associated with Tat-induced excitotoxicity (Monnet et al., 1995; Smith, 1991), likely through an allosteric mechanism (Johansson et al., 2005, Malayev et al., 2002). In particular, the P4 metabolite, allopregnanolone, binds NMDA receptor-2B subunits (Johansson et al., 2005) and reduces NMDA receptor excitation (Maurice et al., 2006) in cortical cells. Unlike P4, allopregnanolone acts at extracellular membrane-bound neurotransmitter targets to exert rapid effects on behavior (reviewed in Schumacher et al., 2014). Such nontraditional actions of progestogens are important for regulating anxiety-like behavior of rodents (Bali and Jaggi, 2013; Frye and Paris, 2011) and their utility in treating HIV-mediated neurotoxicity has been recently proposed (Perumal and Dhanasekaran, 2012). Indeed, the rate-limiting enzyme necessary to catalyze P4’s conversion to allopregnanolone is upregulated by E2 (Micevych and Sinchak, 2011), indicating this nontraditional progestogen as a common product between these two prohormones that may partly underlie their overlapping effects for neuroprotection and behavior. Consistent with these observations, P4’s effects on chemokines in peripheral blood mononuclear cells are independent of actions at progestin receptors (Cabrera-Muñoz et al., 2012ab) suggesting the involvement of nontraditional sites of action. These data support the present findings of P4-mediated protection over Tat-induced anxiety-like behavior of mice and extend them to suggest a novel therapeutic target for P4 effects on HIV-1-related pathology.
Given HIV’s transition to a chronic-care disease with the advent of antiretroviral therapies, the incidence of associated mood disorders is expected to rise (McArthur et al., 2003; Sacktor et al., 2002). For the first time, HIV-afflicted women are able to live to post-menopause, characterized by erratic fluctuations in circulatory E2, reduced gonadal P4, and an increased incidence in affective disorder (Prior and Hitchcock, 2011; Soares, 2014). In 2011, 45–49 year olds were the largest group of women estimated to be living with HIV in the United States (Imai et al., 2013). The effects of infection on the climacteric are under-investigated; however, recent studies suggest that HIV-afflicted women transition to post-menopause sooner and experience greater symptomology than non-afflicted controls (Boonyanurak et al., 2012; Fan et al., 2008; Kanapathipillai et al. 2013; Rubin et al., 2014). Moreover, antiretroviral therapies may contribute to the worsening of some post-menopausal symptoms (Kanapathipillai et al. 2013). Moreover, HIV-infected premenopausal women have been found to have lower circulating E2 compared to uninfected controls (Karim et al., 2013). These findings are consistent with the notion that infection-related reductions in circulating steroids may result in a concordant loss of neuroprotection and a possible exacerbation of neuropsychiatric sequelae later in life. It is difficult to predict whether post-menopausal women afflicted with HIV will benefit from the available hormone replacement therapies currently used. Evidence indicates that non-metabolizing synthetic progestins may actually worsen the progression of HIV (Baeten et al., 2005; Stringer et al., 2007, 2009), whereas natural P4 may be beneficial (Vassiliadou et al., 1999). In the present study, we observed chronic P4 co-administration with Tat-induction to be beneficial for anxiety-like behavior among OVX mice, adding to its therapeutic potential which is already recognized for its intravenous efficacy in treating brain injury (Stein, 2014). Admittedly, the menopausal milieu is quite different from surgical ovariectomy and often presents with greater E2 levels in circulation (Prior and Hitchcock, 2011). Given the number of survivors now entering the climacteric, there is a critical need to identify hormone replacement therapies that are beneficial for aging HIV-afflicted individuals.
The current findings are among the first to demonstrate a role for HIV-1 Tat on affective behavior and further indicate a role for exogenous P4 protection, but must be considered with some caveats. Assessing the effects of chronic manipulations can be challenging in a naturally-cycling animal model. As such, in Expt. 1 it was necessary to accept a range of days from treatment within which animals could be assessed in either the proestrous or diestrous cycle phases. However, no animal was tested more than 7 days from the end of Tat-induction and we have demonstrated Tat-mediated changes in several behavioral measures to be stable for at least 14 days (Carey et al., 2012; Paris et al., 2013, 2014). In Expt. 2, OVX depletes the primary source of circulating steroid, enhancing anxiety-like behavior. As such, anxiety-like responding in the open field was increased among OVX controls compared to gonadally-intact proestrous or diestrous mice and Tat-induction was not observed to further increase anxiety-like behavior. However, additional anxiety tasks were more resilient to the apparent “ceiling” effects observed in the open field allowing the efficacy of exogenous steroid hormones to be assessed. Future investigations might further discount these concerns by utilizing measures that can be directly correlated across tasks, such as the latency to central entry in the open field and the latency to open arm entry in the elevated plus maze. Furthermore, a variety of hormone-replacement regimens should be examined. Herein, steroids were chronically-administered to OVX mice, potentially accumulating in supraphysiological concentrations. Accordingly, we cannot delineate the extent to which pharmacological (rather than physiological) effects of steroids at their receptor targets contributed to the observed outcomes, nor whether longer treatment durations (commensurate to clinical use) would yield the same effects on anxiety. Rodent models of natural estropause do express a divergent central steroid milieu (Paris et al., 2011) compared to OVX rodent models, and may be a more responsive model better suited for hormone replacement studies (Walf et al., 2009). Finally, the identity of the active metabolites and molecular targets that underlie these actions remain uncertain, requiring future investigation.
Conclusion
The present data are the first to demonstrate P4’s protective capacity over Tat-induced anxiety-like behavior in a whole animal model. Induction of central HIV-1 Tat increased anxiety-like behavior of female mice, irrespective of estrous cycle phase. Among OVX mice, administration of P4, but not E2, throughout Tat-induction attenuated anxiogenic effects.
Highlights.
Induction of central HIV-1 Tat increased anxiety-like behavior in female mice
Anxiety-like effects of Tat were not prevented by endogenous hormone fluctuations
Co-administration of systemic progesterone ameliorated Tat-induced anxiety
Estradiol was without effect on Tat-induced anxiety
Estradiol partly attenuated the protective effects of progesterone against Tat
Acknowledgments
We thank Johnny He for the gift of the GT-tg transgenic breeder mice. This work was supported by funding from the National Institute of Mental Health (MH085607 to JPM) and funds from the State of Florida, Executive Office of the Governor’s Department of Economic Opportunity. The sources of funding had no involvement in the planning, execution, or presentation of these data in any manner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jason J. Paris, Email: jparis@tpims.org.
Jason Fenwick, Email: jfenwick@tpims.org.
Jay P. McLaughlin, Email: jmclaughlin@tpims.org.
References
- Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. ER-β mediates 17β-estradiol attenuation of HIV-1 Tat-induced apoptotic signaling. Synapse. 2010;64:829–838. doi: 10.1002/syn.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Soy isoflavones genistein and daidzein exert anti-apoptotic actions via a selective ER-mediated mechanism in neurons following HIV-1 Tat(1–86) exposure. PLoS One. 2012;7:e37540. doi: 10.1371/journal.pone.0037540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SC, Kim GY, Kim JH, Baik SW, Han MK, Lee HJ, Moon DO, Lee CM, Kang JH, Kim BH, Oh YH, Park YM. Epigallocatechin-3-gallate, constituent of green tea, suppresses the LPS-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and NF-kappaB. Biochem. Biophys. Res. Commun. 2004;313:148–155. doi: 10.1016/j.bbrc.2003.11.108. [DOI] [PubMed] [Google Scholar]
- Baeten JM, Lavreys L, Sagar M, Kreiss JK, Richardson BA, Chohan B, Panteleeff D, Mandaliya K, Ndinya-Achola JO, Overbaugh J, Farley T, Mwachari C, Cohen C, Chipato T, Jaisamrarn U, Kiriwat O, Duerr A. Effect of contraceptive methods on natural history of HIV: studies from the Mombasa cohort. J. Acquir. Immune Defic. Syndr. 2005;38:S18–S21. doi: 10.1097/01.qai.0000167030.18278.0e. [DOI] [PubMed] [Google Scholar]
- Bali A, Jaggi AS. Multifunctional aspects of allopregnanolone in stress and related disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;48C:64–78. doi: 10.1016/j.pnpbp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch. Gen. Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Boissé L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol. Clin. 2008;26:799–819. doi: 10.1016/j.ncl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Boonyanurak P, Bunupuradah T, Wilawan K, Lueanyod A, Thongpaeng P, Chatvong D, Sophonphan J, Saeloo S, Ananworanich J, Chaithongwongwatthana S. Age at menopause and menopause-related symptoms in human immunodeficiency virus-infected Thai women. Menopause. 2012;19:820–824. doi: 10.1097/gme.0b013e31824cfc0f. [DOI] [PubMed] [Google Scholar]
- Broekkamp CL, Rijk HW, Joly Gelouin D, Lloyd KL. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim induced grooming in mice. Eur. J. Pharmacol. 1986;126:223–229. doi: 10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology. 1979;104:1247–1255. doi: 10.1210/endo-104-5-1247. [DOI] [PubMed] [Google Scholar]
- Cabrera-Muñoz E, Hernández-Hernández OT, Camacho-Arroyo I. Role of estradiol and progesterone in HIV susceptibility and disease progression. Mini. Rev. Med. Chem. 2012a;12:1049–1054. doi: 10.2174/138955712802762185. [DOI] [PubMed] [Google Scholar]
- Cabrera-Muñoz E, Fuentes-Romero LL, Zamora-Chávez J, Camacho-Arroyo I, Soto-Ramírez LE. Effects of progesterone on the content of CCR5 and CXCR4 coreceptors in PBMCs of seropositive and exposed but uninfected Mexican women to HIV-1. J. Steroid Biochem. Mol. Biol. 2012b;132:66–72. doi: 10.1016/j.jsbmb.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Carey AN, Liu X, Mintzopoulos D, Paris JJ, Muschamp JW, McLaughlin JP, Kaufman MJ. Conditional Tat protein expression in the GT-tg bigenic mouse brain induces gray matter density reductions. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;43:49–54. doi: 10.1016/j.pnpbp.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav. Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MF, Soldan S, Kolson DL. HIV neuroinvasion: early events, late manifestations. In: Meucci O, editor. Chemokine Receptors and NeuroAIDS: Beyond Co-Receptor Function and Links to Other Neuropathologies. Springer, New York: pp. 5–32. [Google Scholar]
- Clemetson DB, Moss GB, Willerford DM, Hensel M, Emonyi W, Holmes KK, Plummer F, Ndinya-Achola J, Roberts PL, Hillier S, Kreiss JK. Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA. 1993;269:2860–2864. [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm. Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr., Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol. Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Evans DL, Mason K, Bauer R, Leserman J, Pettito J. Neuropsychiatric manifestations of HIV-1 infection and AIDS. In: Charney D, Coyle J, Davis K, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. New York: Raven Press; 2002. pp. 1281–1399. [Google Scholar]
- Fan MD, Maslow BS, Santoro N, Schoenbaum E. HIV and the menopause. Menopause Int. 2008;14:163–168. doi: 10.1258/mi.2008.008027. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ. Effects of neurosteroid actions at N-methyl-D-aspartate and GABA A receptors in the midbrain ventral tegmental area for anxiety-like and mating behavior of female rats. Psychopharmacology (Berl) 2011;213:93–103. doi: 10.1007/s00213-010-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Estrogen is necessary for 5α-pregnan-3α-ol-20-one (3α,5α-THP) infusion to the ventral tegmental area to facilitate social and sexual, but neither exploratory nor affective behavior of ovariectomized rats. Pharmacol. Biochem. Behav. 2008;91:261–270. doi: 10.1016/j.pbb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006;83:336–347. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggin K, Engelson ES, Rabkin JG, Kotler DP. The relationship of mood, endocrine, and sexual disorders in human immunodeficiency virus positive (HIV+) women: an exploratory study. Psychosom. Med. 1998;60:11–16. doi: 10.1097/00006842-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Hall C, Ballachey EL. A study of the rat’s behavior in a field: a contribution to method in comparative psychology. Univ. Calif. Publ. Psychol. 1932;6:1–12. [Google Scholar]
- Härkönen PL, Väänänen HK. Monocyte-macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann. N. Y. Acad. Sci. 2006;1089:218–227. doi: 10.1196/annals.1386.045. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein tat. J. Neurochem. 1999;73:1363–1374. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- Imai K, Sutton MY, Mdodo R, Del Rio C. HIV and menopause: a systematic review of the effects of HIV infection on age at menopause and the effects of menopause on response to antiretroviral therapy. Obstet. Gynecol. Int. 2013;2013:340309. doi: 10.1155/2013/340309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang KT, Chang Y, Berkhout B, Hammarskjöld ML, Rekosh D. Regulation of HIV expression: mechanisms of action of Tat and Rev. AIDS. 1991;5:S3–S14. [PubMed] [Google Scholar]
- Johansson T, Le Grevès P. The effect of dehydroepiandrosterone sulfate and allopregnanolone sulfate on the binding of [3H]ifenprodil to the N-methyl-D-aspartate receptor in rat frontal cortex membrane. J. Steroid Biochem. Mol. Biol. 2005;94:263–266. doi: 10.1016/j.jsbmb.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Kagee A, Martin L. Symptoms of depression and anxiety among a sample of South African patients living with HIV. AIDS Care. 2010;22:159–165. doi: 10.1080/09540120903111445. [DOI] [PubMed] [Google Scholar]
- Kanapathipillai R, Hickey M, Giles M. Human immunodeficiency virus and menopause. Menopause. 2013;20:983–990. doi: 10.1097/GME.0b013e318282aa57. [DOI] [PubMed] [Google Scholar]
- Karim R, Mack WJ, Kono N, Tien PC, Anastos K, Lazar J, Young M, Cohen M, Golub E, Greenblatt RM, Kaplan RC, Hodis HN. Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the women′s interagency HIV study (WIHS) J. Clin. Endocrinol. Metab. 2013;98:E610–E618. doi: 10.1210/jc.2012-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall SL, Anderson CF, Nath A, Turchan-Cholewo J, Land CL, Mactutus CF, Booze RM. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: testosterone and ICI 182,780 sensitive mechanism. BMC Neurosci. 2005;6:40. doi: 10.1186/1471-2202-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J. Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Martemyanov KA, Thayer SA. Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J. Neurosci. 2008;28:12604–12613. doi: 10.1523/JNEUROSCI.2958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce CJ, Walf AA, Frye CA. Type 1 5α-reductase may be required for estrous cycle changes in affective behaviors of female mice. Behav. Brain Res. 2012;226:376–380. doi: 10.1016/j.bbr.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol. Dis. 2010;37:542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp. Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lad HV, Liu L, Paya-Cano JL, Parsons MJ, Kember R, Fernandes C, Schalkwyk LC. Behavioural battery testing: evaluation and behavioural outcomes in 8 inbred mouse strains. Physiol. Behav. 2010;99:301–316. doi: 10.1016/j.physbeh.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Kelley KW, Dantzer R. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain Behav. Immun. 2011;25:1569–1575. doi: 10.1016/j.bbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lopes M, Olfson M, Rabkin J, Hasin DS, Alegría AA, Lin KH, Grant BF, Blanco C. Gender, HIV status, and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry. 2012;73:384–391. doi: 10.4088/JCP.10m06304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann. Neurol. 1995;37:373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- Malayev A, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br. J. Pharmacol. 2002;135:901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J. Neurochem. 2002;83:955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- Massie MJ. Prevalence of depression in patients with cancer. J. Natl. Cancer Inst. Monogr. 2004;32:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- Maurice T, Grégoire C, Espallergues J. Neuro(active)steroids actions at the neuromodulatory sigma1 (sigma1) receptor: biochemical and physiological evidences, consequences in neuroprotection. Pharmacol. Biochem. Behav. 2006;84:581–597. doi: 10.1016/j.pbb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L. The 5α-reductase in the central nervous system: expression and modes of control. J. Steroid Biochem. Mol. Biol. 1998;65:295–299. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- Micevych P, Sinchak K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front. Endocrinol. (Lausanne) 2011;2:90. doi: 10.3389/fendo.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael SD. Plasma prolactin and progesterone during the estrous cycle in the mouse. Proc Soc. Exp. Biol. Med. 1976;153:254–257. doi: 10.3181/00379727-153-39522. [DOI] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J. Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol. Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;24:411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Mahé V, Robel P, Baulieu EE. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm. Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behav. Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- Mostad SB, Overbaugh J, DeVange DM, Welch MJ, Chohan B, Mandaliya K, Nyange P, Martin HL, Jr., Ndinya-Achola J, Bwayo JJ, Kreiss JK. Hormonal contraception, vitamin A deficiency, and other risk factors for shedding of HIV-1 infected cells from the cervix and vagina. Lancet. 1997;350:922–927. doi: 10.1016/S0140-6736(97)04240-2. [DOI] [PubMed] [Google Scholar]
- Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J. Virol. 1996;70:1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New DR, Maggirwar SB, Epstein LG, Dewhurst S, Gelbard HA. HIV-1 Tat induces neuronal death via tumor necrosis factor-alpha and activation of non-N-methyl-D-aspartate receptors by a NFkappaB-independent mechanism. J. Biol. Chem. 1998;273:17852–17858. doi: 10.1074/jbc.273.28.17852. [DOI] [PubMed] [Google Scholar]
- Orlando M, Burnam MA, Beckman R, Morton SC, London AS, Bing EG, Fleishman JA. Re-estimating the prevalence of psychiatric disorders in a nationally representative sample of persons receiving care for HIV: results from the HIV Cost and Services Utilization Study. Int. J. Methods Psychiatr. Res. 2002;11:75–82. doi: 10.1002/mpr.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Roeder RG. A novel RNA polymerase II-containing complex potentiates Tat-enhanced HIV-1 transcription. EMBO J. 1999;18:3688–3701. doi: 10.1093/emboj/18.13.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, McLaughlin JP. Effects of Conditional Central Expression of HIV-1 Tat Protein to Potentiate Cocaine-Mediated Psychostimulation and Reward Among Male Mice. Neuropsychopharmacology. 2014;39:380–388. doi: 10.1038/npp.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3385-1. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Walf AA, Frye CA. II. Cognitive performance of middle-aged female rats is influenced by capacity to metabolize progesterone in the prefrontal cortex and hippocampus. Brain Res. 2011;1379:149–163. doi: 10.1016/j.brainres.2010.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Miller WC, Gaynes BN, Eron JJ., Jr. Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2007;44:159–166. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- Perumal MB, Dhanasekaran S. HIV associated dementia: role for neurosteroids. Med. Hypotheses. 2012;78:672–674. doi: 10.1016/j.mehy.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Poling A, Cleary J, Monaghan M. Burying by rats in response to aversive and nonaversive stimuli. J. Exp. Anal. Behav. 1981;35:31–44. doi: 10.1901/jeab.1981.35-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior JC, Hitchcock CL. The endocrinology of perimenopause: need for a paradigm shift. Front Biosci (Schol Ed) 2011;3:474–486. doi: 10.2741/s166. [DOI] [PubMed] [Google Scholar]
- Ragupathy V, Devadas K, Tang S, Wood O, Lee S, Dastyer A, Wang X, Dayton A, Hewlett I. Effect of sex steroid hormones on replication and transmission of major HIV subtypes. J. Steroid Biochem. Mol. Biol. 2013;138C:63–71. doi: 10.1016/j.jsbmb.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Garcia M, Biswas N, Patel MV, Barr FD, Crist SG, Ochsenbauer C, Fahey JV, Wira CR. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PLoS One. 2013;8:e62069. doi: 10.1371/journal.pone.0062069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W, 3rd, Zhang Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J. Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Sundermann EE, Cook JA, Martin EM, Golub ET, Weber KM, Cohen MH, Crystal H, Cederbaum JA, Anastos K, Young M, Greenblatt RM, Maki PM. Investigation of menopausal stage and symptoms on cognition in human immunodeficiency virus-infected women. Menopause. 2014 doi: 10.1097/GME.0000000000000203. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann. Emerg. Med. 2006;47:381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Stein DG. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog. Brain Res. 2009;175:219–237. doi: 10.1016/S0079-6123(09)17515-5. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharko AM. DSM psychiatric disorders in the context of pediatric HIV/AIDS. AIDS care. 2006;18:441–445. doi: 10.1080/09540120500213487. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Mattern C, Ghoumari A, Oudinet JP, Liere P, Labombarda F, Sitruk-Ware R, De Nicola AF, Guennoun R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog. Neurobiol. 2014;113:6–39. doi: 10.1016/j.pneurobio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Smith SS. Progesterone administration attenuates excitatory amino acid responses of cerebellar Purkinje cells. Neuroscience. 1991;42:309–320. doi: 10.1016/0306-4522(91)90377-z. [DOI] [PubMed] [Google Scholar]
- Soares CN. Mood disorders in midlife women: understanding the critical window and its clinical implications. Menopause. 2014;21:198–206. doi: 10.1097/GME.0000000000000193. [DOI] [PubMed] [Google Scholar]
- Sordo del Castillo L, Ruiz-Pérez I, Olry de Labry Lima A. Biological, psychosocial, therapeutic and quality of life inequalities between HIV-positive men and women - a review from a gender perspective. AIDS Rev. 2010;12:113–120. [PubMed] [Google Scholar]
- Starling I, Wright A, Arbuthnott G, Harkiss G. Acute in vivo neurotoxicity of peptides from Maedi Visna virus transactivating protein Tat. Brain Res. 1999;830:285–291. doi: 10.1016/s0006-8993(99)01407-9. [DOI] [PubMed] [Google Scholar]
- Stein DG. A clinical/translational perspective: can a developmental hormone play a role in the treatment of traumatic brain injury? Horm. Behav. 2013;63:291–300. doi: 10.1016/j.yhbeh.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Stringer EM, Kaseba C, Levy J, Sinkala M, Goldenberg RL, Chi BH, Matongo I, Vermund SH, Mwanahamuntu M, Stringer JS. A randomized trial of the intrauterine contraceptive device vs hormonal contraception in women who are infected with the human immunodeficiency virus. Am. J. Obstet. Gynecol. 2007;197:144. doi: 10.1016/j.ajog.2007.03.031. e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer EM, Levy J, Sinkala M, Chi BH, Matongo I, Chintu N, Stringer JS. HIV disease progression by hormonal contraceptive method: secondary analysis of a randomized trial. AIDS. 2009;23:1377–1382. doi: 10.1097/QAD.0b013e32832cbca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Sun Y, Ma F, Lü P, Huang H, Zhou J. Progesterone inhibits Toll-like receptor 4-mediated innate immune response in macrophages by suppressing NF-kappaB activation and enhancing SOCS1 expression. Immunol. Lett. 2009;125:151–155. doi: 10.1016/j.imlet.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman I, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliadou N, Tucker L, Anderson DJ. Progesterone-induced inhibition of chemokine receptor expression on peripheral blood mononuclear cells correlates with reduced HIV-1 infectability in vitro. J. Immunol. 1999;162:7510–7518. [PubMed] [Google Scholar]
- Vergote D, Butler GS, Ooms M, Cox JH, Silva C, Hollenberg MD, Jhamandas JH, Overall CM, Power C. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19182–19187. doi: 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909–916. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol. Learn. Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wang CC, McClelland RS, Overbaugh J, Reilly M, Panteleeff DD, Mandaliya K, Chohan B, Lavreys L, Ndinya-Achola J, Kreiss JK. The effect of hormonal contraception on genital tract shedding of HIV-1. AIDS. 2004;18:205–209. doi: 10.1097/00002030-200401230-00009. [DOI] [PubMed] [Google Scholar]
- Wood PA, Bove K, You S, Chambers A, Hrushesky WJ. Cancer growth and spread are saltatory and phase-locked to the reproductive cycle through mediators of angiogenesis. Mol. Cancer Ther. 2005;4:1065–1075. doi: 10.1158/1535-7163.MCT-05-0028. [DOI] [PubMed] [Google Scholar]
- Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J. Neuroimmune Pharmacol. 2009;4:430–447. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM. Recombinant human immunodeficiency virus-1 transactivator of transcription 1-86 allosterically modulates dopamine transporter activity. Synapse. 2011;65:1251–1254. doi: 10.1002/syn.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J. Pharmacol. Exp. Ther. 2009a;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Yao H, Peng F, Callen S, Buch S. PDGF-mediated protection of SH-SY5Y cells against Tat toxin involves regulation of extracellular glutamate and intracellular calcium. Toxicol. Appl. Pharmacol. 2009b;240:286–291. doi: 10.1016/j.taap.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]