Abstract
Iron-sulfur flavoproteins (Isf) are flavin mononucleotide (FMN)- and FeS cluster-containing proteins commonly encountered in anaerobic prokaryotes. However, with the exception of Isf from Methanosarcina thermophila, which participates in oxidative stress management by removing oxygen and hydrogen peroxide, none of these proteins has been characterized in terms of function. Trichomonas vaginalis, a sexually transmitted eukaryotic parasite of humans, was found to express several iron-sulfur flavoprotein (TvIsf) homologs in its hydrogenosomes. We show here that in addition to having oxygen-reducing activity, the recombinant TvIsf also functions as a detoxifying reductase of metronidazole and chloramphenicol, both of which are antibiotics effective against a variety of anaerobic microbes. TvIsf can utilize both NADH and reduced ferredoxin as electron donors. Given the prevalence of Isf in anaerobic prokaryotes, we propose that these proteins are central to a novel defense mechanism against xenobiotics.
INTRODUCTION
Iron-sulfur flavoproteins (Isf) constitute a relatively recently recognized family of proteins commonly present in diverse representatives of the Bacteria and Archaea domains, particularly in species inhabiting anaerobic environments (1). The only eukaryotic species found to possess an Isf homolog in its genome was the anaerobic intestinal pathogen Entamoeba histolytica (1). These proteins have subunits of approximately 20 kDa, harbor a noncovalently bound flavin mononucleotide (FMN) cofactor, and contain a conserved, compact, four-cysteine motif responsible for coordinating the low-redox-potential 4Fe-4S redox center (2, 3). Given the presence and properties of these redox cofactors, it has been suggested that Isf play a role in electron transport, possibly functioning as a one- to two-electron switch (2).
The prototype Isf from Methanosarcina thermophila (MtIsf), a strictly anaerobic methane-producing thermophile (1), has been characterized as a functional homodimer interacting with ferredoxin as a physiological electron donor (4, 5). The protein was eventually found to catalyze the reduction of dioxygen and hydrogen peroxide to water, and its role in combating oxidative stress in strictly anaerobic prokaryotes had been proposed (6). Despite the broad distribution of Isf proteins in bacteria (including human pathogens), to date, the purported physiological role of Isf proteins has been explored only in the case of MtIsf.
The anaerobic eukaryotic parasite Trichomonas vaginalis is a causative agent of human vaginitis and urethritis, a widespread infection affecting approximately 170 million people per year (7, 8). The disease is treated with derivatives of 5′-nitroimidazoles, such as metronidazole (MTZ) and tinidazole. These drugs are reductively activated within the susceptible cells and are highly effective against many anaerobic microorganisms, though the emergence of resistant isolates has been reported (9). Trichomonads are flagellated protists belonging to the supergroup Excavata. One of their distinctive features is the absence of cristate oxygen-respiring mitochondria. Instead, trichomonads possess hydrogenosomes, organelles of mitochondrial ancestry that produce molecular hydrogen and ATP in the reactions of extended glycolysis (10). The enzymes involved in hydrogen formation, pyruvate:ferredoxin oxidoreductase (PFO), which oxidatively decarboxylates pyruvate and forms acetyl coenzyme A (acetyl-CoA; subsequently utilized in the substrate-level synthesis of ATP), and Fe-Fe hydrogenase (the terminal oxidoreductase which forms hydrogen using pyruvate-derived electrons transferred by 2Fe-2S ferredoxin), are particularly oxygen-sensitive proteins (11). Nevertheless, T. vaginalis is exposed to oxygen concentrations of up to 60 μM in its natural environment (12) and must be able to effectively scavenge oxygen and reactive oxygen species (ROS) to protect its vital proteins from oxidative damage. Indeed, trichomonads are equipped with a number of oxygen and ROS detoxification systems, including oxygen-reducing NADH oxidase (13), superoxide-removing superoxide dismutase (12, 14), rubrerythrin (15) and the thioredoxin system (16) aimed at peroxides, and flavodiiron protein (FDP), which functions as a ferredoxin-dependent oxygen reductase and forms water (17). Annotation of the T. vaginalis genome (18) confirmed the presence of genes coding for the above-mentioned enzymes and identified several other candidate proteins possibly involved in ROS or xenobiotic detoxification. Among these were seven distinct paralogs of Isf; thus, T. vaginalis is the second eukaryote (in addition to Entamoeba histolytica) identified to possess otherwise strictly prokaryotic Isf proteins. The results of subsequent proteomic studies showed that three Isf paralogs are expressed in T. vaginalis hydrogenosomes under standard in vitro cultivation conditions (19, 20).
In this communication, we report the characterization and novel catalytic properties of Isf from T. vaginalis hydrogenosomes and suggest that this protein plays a general role in the defense against xenobiotics.
MATERIALS AND METHODS
Organism.
The T. vaginalis strain T1 (J.-H. Tai, Institute of Biomedical Sciences, Taipei, Taiwan) was grown in Diamond's TYM (Trypticase-yeast extract-maltose) medium without agar, as previously described (21). The T. vaginalis cell line used for immunolocalization of the Isf paralog (in this work named TvIsf3) by fluorescence microscopy was prepared by transforming T. vaginalis T1 with a TagVag vector (22) carrying the complete TvIsf3 gene (TVAG_154730) fused with a C-terminal 2× hemagglutinin (HA) tag.
T. vaginalis cell fractionation.
The T. vaginalis cell fractions used in the Western blot analysis were isolated from the cell homogenate obtained by sonication. The lysate was fractionated using differential centrifugation, followed by isopycnic centrifugation of the hydrogenosome-enriched fraction on a self-forming gradient of 45% Percoll (Sigma), as previously described (23).
Expression and purification of TvISf3.
The TvIsf3 gene was amplified and cloned into the pET-42b vector (Novagen).
Recombinant TvIsf3 with a 6× His tag at the C terminus and without an amino-terminal hydrogenosomal targeting presequence was expressed in Escherichia coli BL21(DE3) cells grown anaerobically in 1-liter screw-cap bottles in LB medium supplemented with 2 mM NaNO3 as an electron acceptor. The bacterial culture was induced with 0.25 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and the cells were grown for 12 h at 18°C in LB medium supplemented with 400 μM ammonium ferrous sulfate, 200 μM flavin mononucleotide (FMN), and 250 μM cysteine. The bacteria (1- to 3-liter cultures) were harvested by centrifugation, washed with buffer containing 20 mM imidazole, 50 mM NaH2PO4, 300 mM NaCl, and 10% glycerol (pH 8.0), and homogenized by passage through a French press at 18,000 lb/in2. The soluble fraction (approximately 25 ml) obtained after ultracentrifugation (250,000 × g, 45 min, and 4°C) of the cell lysate was applied onto a nickel-nitrilotriacetic acid (Ni-NTA) Superflow agarose column (Qiagen) and eluted with a stepwise gradient of 20 mM to 400 mM imidazole in buffer containing 50 mM NaH2PO4, 300 mM NaCl, and 10% glycerol (pH 8.0) at a flow rate of 1 ml min−1 using a BioLogic HR system (Bio-Rad). The TvIsf3 was specifically released from the column with 250 mM imidazole. The purity of the isolated TvIsf3 was verified by SDS-PAGE.
Characterization of TvISf3.
The flavin cofactor extracted from recombinant TvIsf3 was characterized by thin-layer chromatography (TLC) (HPTLC-Alufolien; Merck) as previously described (17), and the recombinant protein isolated from bacteria grown in LB medium without addition of FMN was used for the flavin determination. The quantity of flavin in TvIsf3 was determined after protein denaturation with 80% trichloroacetic acid using an extinction coefficient of 12,500 M−1c−1 at a λ of 450 nm (24). The iron content of TvIsf3 was determined using the 2,4,6-tripyridyl-1,3,5-triazine method (25). The native molecular mass of TvIsf3 was determined by gel filtration chromatography using a BioLogic HR system (Bio-Rad). The purified recombinant TvIsf3 was applied onto Superdex 75 10/300 GL and Superdex 200 10/300 GL columns (GE Healthcare) equilibrated with sodium phosphate buffer (50 mM NaH2PO4, 150 mM NaCl, 10% glycerol [pH 8.0]) using a flow rate of 0.5 ml min−1. The native molecular mass of TvIsf3 was calculated from the calibration curves determined by running appropriate protein standards under the same conditions.
The protein concentrations were determined by the Lowry assay (26) using bovine serum albumin as a standard.
Protein localization.
T. vaginalis cells overexpressing TvIsf3 fused with a 2× HA tag were used for the immunodetection of the protein within the cell. The cells were allowed to adhere to glass slides coated with 3-aminopropyltriethoxysilane (Sigma) and then fixed with methanol (5 min) and permeabilized with acetone (5 min) (both steps at −18°C). The slides were preincubated for 1 h in phosphate-buffered saline with 0.25% bovine serum albumin and 0.25% gelatin and then treated with antibodies as described previously (23). An anti-HA tag monoclonal antibody (Exbio) and hydrogenosomal malic enzyme polyclonal antiserum (Eurogentec) were used as the primary antibodies. Anti-mouse IgG labeled with Alexa Fluor 488 (Molecular Probes) and anti-rabbit IgG labeled with Alexa Fluor 594 (Molecular Probes) were used as the secondary antibodies for fluorescent immunolocalization. Anti-TvIsf3 rabbit polyclonal serum (Eurogentec) and anti-rabbit IgG conjugated with horseradish peroxidase were used for Western blotting to visualize TvIsf3 in T. vaginalis subcellular fractions obtained from untransformed wild-type trichomonads.
Activity assays.
The ability of various compounds to accept electrons from chemically reduced TvIsf3 was initially assayed by recording the visible spectra using a Shimadzu UV-1601 spectrophotometer and 1-ml stoppered cuvettes with a silicone septum in phosphate buffer (50 mM NaH2PO4, 150 mM NaCl, 10% glycerol [pH 8.0]) (assay buffer) at 25°C. The anaerobiosis of the system was achieved by degassing the buffer by evacuation, followed by extensive flushing with N2 and the addition of oxygen- and hydrogen peroxide-scavenging system consisting of glucose oxidase (28 U), catalase (104 U), and glucose (3 mM) to the reaction mixture (27). Prior to initiation of the reaction by the addition of potential electron acceptors, TvIsf3 (20 to 30 nmol) was reduced by the stepwise addition of Tris-buffered 30 mM sodium dithionite (pH 9.0); particular attention was paid during this step not to overtitrate the protein with an excess of dithionite. The enzymatic oxygen- and hydrogen peroxide-scavenging system was omitted from experiments aimed at identifying the reaction products of TvIsf3 with oxygen. Hydrogen peroxide was determined using the FOX assay as described previously (28). The assays with dipropylenetriamine NONOate (DPTA-NONOate; Alexis Biochemicals) used as a nitric oxide donor (12 μM to 1 mM final concentration) and hydrogen peroxide (35 μM final concentration) were prepared in the assay buffer at a pH of 5.5; this lower pH allowed for a faster evolution of NO from DPTA-NONOate and prevented the decomposition of hydrogen peroxide. The assay mixture with hydrogen peroxide as a substrate was supplemented with glucose oxidase (28 U) and glucose (3 mM) to eliminate the oxygen generated during the decomposition of hydrogen peroxide. Reoxidation of TvIsf3 with NaNO3, NaNO2, and hydroxylamine was tested with final concentrations of acceptors up to 1 mM. Assays with metronidazole and chloramphenicol were performed using acceptor concentrations of up to 200 μM. All the acceptors used in the assays were rendered anaerobic by flushing the stock solutions in stoppered vials with nitrogen.
Activity determinations under continuous, turnover conditions were performed with 220 μM NADH as the electron donor in spectrophotometric assays at 340 nm and 37°C. In addition to DPTA-NONOate, NO-saturated water (obtained by purging degassed water with pure NO to yield approximately 2 mM NO solution) was used as the NO source for assays at pH 8.0. Otherwise, the conditions were identical to those used to determine the activity of dithionite-reduced TvIsf3 as described above. Kinetic parameters were calculated using Lineweaver-Burk plots and UVProbe software (Shimadzu).
Enzymatic reduction of TvIsf3.
The experiment aimed at determining whether hydrogenosomal ferredoxin is capable of reducing TvIsf3 was performed using the anaerobic spectrophotometric assay system described above with 20 to 30 nmol of TvIsf3, 5 mM pyruvate, 0.25 mM coenzyme A (CoA), ∼40 μg of recombinant T. vaginalis ferredoxin 1 (29), and ∼10 μg of T. vaginalis PFO purified from hydrogenosomes (17) as the components of the reducing system.
Determination of metronidazole radical.
The electron paramagnetic resonance (EPR) spectra were recorded using an EMXplus-10/12 CW (continuous wave) spectrometer (Bruker) equipped with the Premium X-band microwave bridge. The experiment was performed under turnover conditions using 5 mM NADH and 3 mM metronidazole in 1 ml of anaerobic assay buffer. The reaction was started by the addition of ∼20 nmol of TvIsf3. The mixture was drawn into the quartz flat EPR cell (ER160FC-Q; Bruker), which was immediately closed and inserted into the standard rectangular cavity (ER 4102003ST; Bruker). Prior to insertion, the EPR cell was flushed with gaseous N2 to minimize the influence of oxygen in the air on the sample. The positive control giving rise to metronidazole nitroradical anion consisted of the hydrogenosomes (∼400 μg) supplemented with the components of the PFO reaction and metronidazole and/or of the ferredoxin-reducing NADH dehydrogenase module of complex 1 isolated from Trichomonas hydrogenosomes, NADH, T. vaginalis ferredoxin 1, and metronidazole as previously described (30).
The following parameters were used to record the EPR spectra: sweep width of 20 mT, power of 5 mW, modulation amplitude of 0.05 mT (or 0.4 mT), time constant of 10.24 ms, conversion time of 20 ms, and resolution of 0.01 mT. The experimental temperature was 25°C.
RESULTS
The TvIsf paralog herein named TvIsf3 was selected for our study because its presence in hydrogenosomes was confirmed by two independent proteomic studies (19, 20) and because our previous analysis revealed that the mRNA of this gene was produced at the highest level of all seven TvIsf paralogs (unpublished data).
The polypeptide derived from TvIsf3 (TVAG_154730) consists of 223 amino acids and has a calculated molecular mass of 25.4 kDa. The amino acid sequence contains the typical conserved motif CX2CX2CX10C (Cys56, Cys59, Cys62, and Cys73) of iron-sulfur flavoproteins shown to coordinate the 4Fe-4S cluster (2, 3); however, the number of amino acids between Cys62 and Cys73 is higher (10 residues) in TvIsf3 than in many prokaryotic homologs (usually 4 to 7 amino acid residues) (2).
TvIsf3 is colinear with and highly similar to its prokaryotic counterparts, displaying approximately 16% pairwise identity with the prototype MtIsf, 31% with C. acetobutylicum, and 22% with A. fulgidus homologs. However, the trichomonad protein contains a short extension, of 8 amino acids, at its amino terminus that is not present in the prokaryotic homologs (Fig. 1). Similar presequences identified in a number of hydrogenosomal proteins are implicated in protein targeting and in the translocation of proteins into the hydrogenosomal matrix; these presequences are cleaved (usually with the conserved arginine residue in the −2 position relative to the processing site) upon maturation of the cytosolically translated protein within the hydrogenosome (31, 32).
FIG 1.

Amino acid sequence alignment for comparison of Isf homologs from Trichomonas vaginalis (GenBank accession no. XP_001313900.1; TVAG_154730), Clostridium acetobutylicum (NP_349133.1), Methanosarcina thermophila (Q50562.2), and Archaeoglobus fulgidus (NP_070721.1). The cleavable hydrogenosomal targeting sequence of TvIsf3 is underlined. The conserved cysteines binding the iron-sulfur cluster are shaded. The amino acid residues implicated in FMN binding in M. thermophila and A. fulgidus based on the crystal structures (2) are highlighted with a black background.
Production, purification, and biochemical characterization.
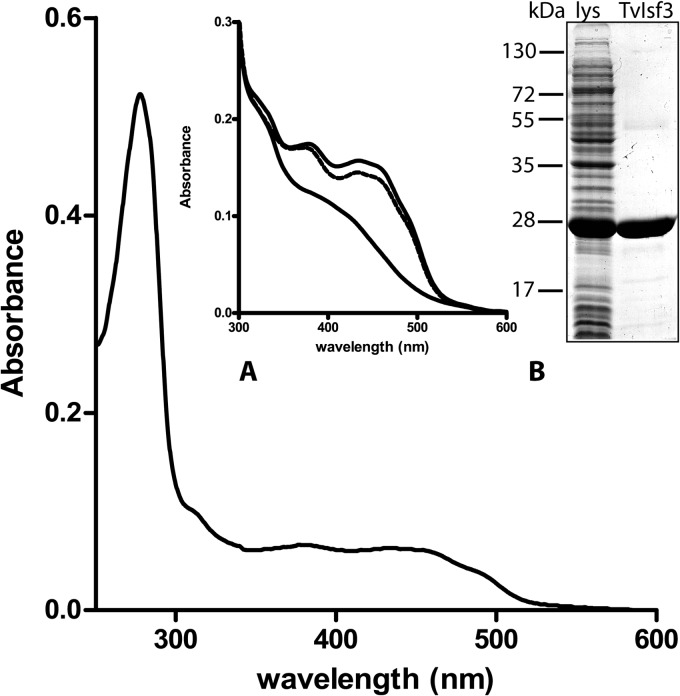
The TvIsf3 without the predicted hydrogenosomal targeting sequence (Fig. 1) was overproduced in E. coli. The protein with the 6× His tag at the C terminus was affinity purified close to homogeneity, as confirmed by SDS-PAGE in which the protein migrated as a polypeptide with an apparent molecular mass of approximately 26 kDa. Gel filtration chromatography used to determine the native molecular mass of TvIsf3 recovered the protein in a dominant peak corresponding to a size of approximately 51 kDa, indicating that TvIsf3 forms dimers; studies of Isf from Methanosarcina thermophila and three other prokaryotic species also showed the existence of a dimeric structure for these proteins (1, 2, 5). However, the major peak of the TvIsf3 dimer was preceded by a small shoulder with a retention time corresponding to a protein of approximately 90 kDa, indicating that a minor portion (estimated at less than 5%) of TvIsf3 was eluted from the column as a tetramer (data not shown). The UV-visible (UV-vis) spectrum of purified TvIsf3 displayed a complex pattern similar to that of other previously characterized Isf proteins (1, 5), with absorbance maxima at 490, 460, 435, 385, and 278 nm (Fig. 2 and 3). The mild acidification of the protein sample destroyed the iron-sulfur cluster and resulted in spectra typical of flavins, with maxima at 373 and 447 nm (Fig. 2, inset).
FIG 2.
UV-visible spectroscopic analysis of recombinant TvIsf3. The UV-visible spectrum of TvIsf3 exhibits the characteristic features of iron-sulfur flavoproteins. The lower trace represents the partial spectrum obtained after the reduction of TvIsf3 with sodium dithionite. The dashed line shows TvIsf3 reoxidized by air. The inset shows a typical flavin spectrum obtained after iron-sulfur cluster degradation by acidification of the protein sample.
FIG 3.
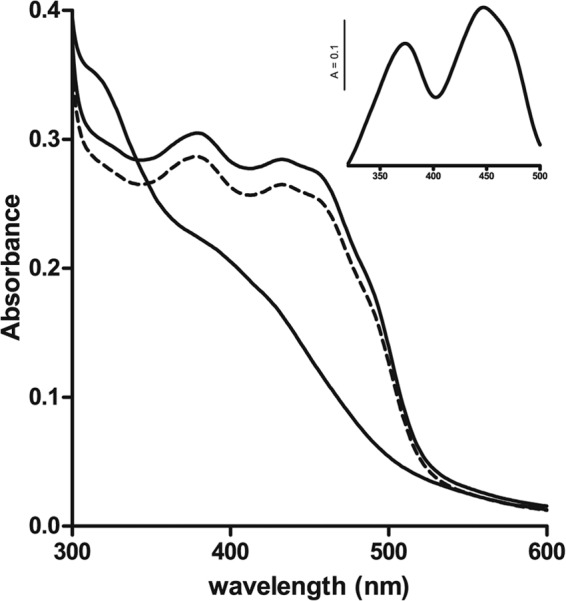
Complete UV-visible spectrum of affinity-purified TvIsf3. The partial representative spectrum in inset A shows the reoxidation (dashed line) of sodium dithionite-reduced (lower trace) TvIsf3 (approximately 25 nmol; upper trace) with several electron acceptors (H2O2, NO, metronidazole, and chloramphenicol). Details of the experimental setup are described in Materials and Methods. Inset B shows SDS-PAGE of the lysate of TvIsf3-expressing E. coli and the purified TvIsf3.
The TLC analysis of the flavin extracted from TvIsf3 purified from bacteria grown without flavin supplementation identified the cofactor as FMN (data not shown). The flavin quantification showed that there were approximately 0.57 mol of FMN per mol of Isf monomer, suggesting that 2 FMN molecules are bound to each homodimer.
The amount of nonheme iron in the different protein preparations ranged from 2.4 to 3.4 mol per mol of monomer, indicating the coordination of the 3Fe-4S or 4Fe-4S cluster in the active center of the protein. However, lower-than-stoichiometric levels of both FMN and iron indicated some cofactor loss, most likely during the purification procedure.
Subcellular localization of TvIsf3.
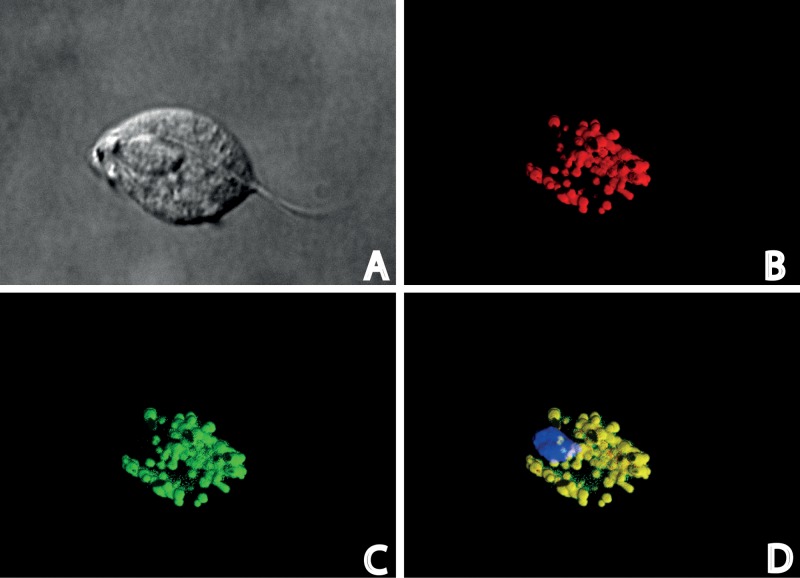
Subcellular fractions of wild-type trichomonads prepared by differential centrifugation were analyzed by Western blotting using polyclonal antibodies against TvIsf3. As expected, TvIsf3 was found in the hydrogenosomal fraction (Fig. 4). In addition, the hydrogenosomal localization of TvIsf3 was further confirmed by immunofluorescence microscopy using T. vaginalis cells transformed with a plasmid carrying the complete TvIsf3 gene with the N-terminal hydrogenosomal targeting presequence and the hemagglutinin tag at the C terminus. TvIsf3 was colocalized with the hydrogenosomal marker protein malic enzyme (Fig. 5).
FIG 4.
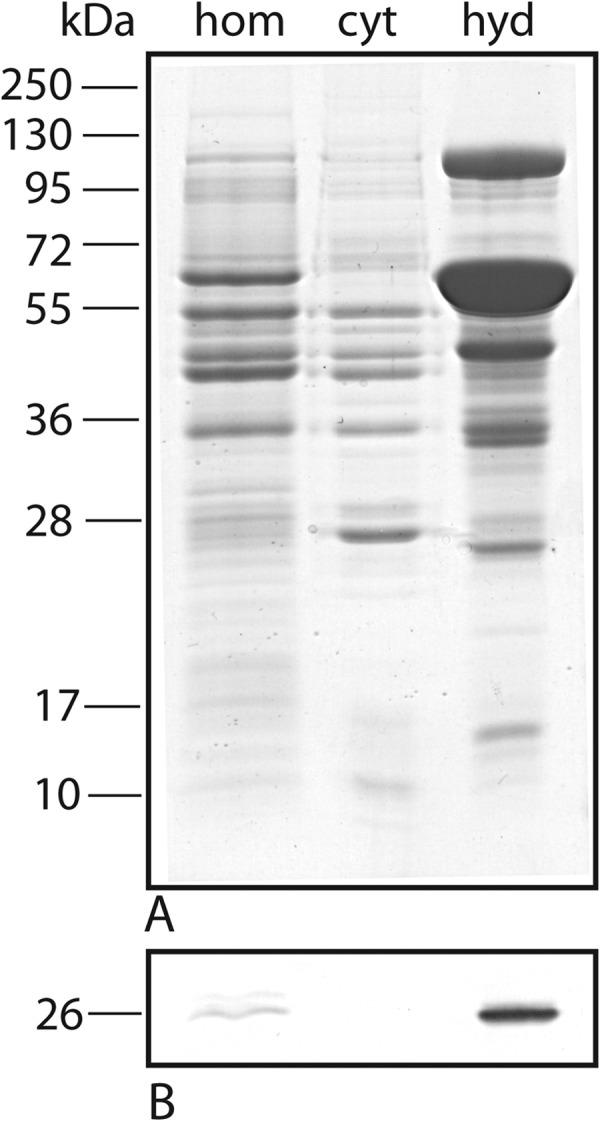
Subcellular localization of TvIsf3. (A) SDS-PAGE analysis of subcellular fractions of T. vaginalis. (B) Western blot probed with TvIsf3 polyclonal antiserum. The molecular mass standards are indicated in kilodaltons. hom, homogenate; cyt, cytosol; hyd, hydrogenosomes.
FIG 5.
Immunodetection of TvIsf3 in T. vaginalis cells. (A) Nomarski differential contrast; (B) visualization of malic enzyme, a hydrogenosomal marker; (C) TvIsf3 labeling; (D) merged image of color channels showing the localization of TvIsf3 within the hydrogenosome with DAPI (4′,6′-diamidino-2-phenylindole) staining for nuclei.
Function of TvIsf3.
The recombinant, affinity-purified, His-tagged version of TvIsf3 (Fig. 3) was used in the experiments aimed at determining the potential electron acceptors that could function as physiological substrates. Under anaerobic conditions, with caution taken not to overtitrate the protein with an excess of reductant, the protein in a sealable spectrophotometric cuvette was almost completely stepwise reduced with sodium dithionite. Following reduction, the ability of a variety of electron acceptors to reoxidize the TvIsf3 was assessed. Reoxidation of the protein was monitored as a regression of its UV-vis spectrum to the original oxidized state.
When the reduced protein was exposed to air by opening the cuvette, the spectrum immediately reassumed an oxidized pattern (Fig. 2). This observation shows that oxygen is reduced readily and is in agreement with the previous finding that Isf from Methanosarcina thermophila reduces oxygen (6).
Hydrogen peroxide, the other substrate shown to be reduced by MtIsf (6), was then tested. The reaction mixture (pH 5.5) was supplemented with an oxygen-scavenging and H2O2-regenerating system consisting of glucose oxidase and glucose to prevent oxygen from affecting the results. TvIsf3 was readily reoxidized by hydrogen peroxide (Fig. 3).
The ability of T. vaginalis lysates to reduce NO has been documented (33), but no enzyme responsible for this ability has been identified thus far. Therefore, we also tested the ability of NO to reoxidize the reduced TvIsf3. Approximately 25 nmol of dithionite-reduced TvIsf3 was exposed to 40 nmol of chemically generated NO, with the pH of the buffer (5.5) facilitating the rapid evolution of NO from DPTA-NONOate. The reduced protein was immediately reoxidized by NO (Fig. 3).
Another potential electron acceptor we tested was the antitrichomonad drug metronidazole. When 30 nmol (0.5 μl of 10 mg ml−1 of anaerobic MTZ solution) of metronidazole was added to approximately 20 nmol of chemically reduced protein, the spectrum of TvIsf3 immediately returned to its original oxidized state, indicating that MTZ can serve as an acceptor for TvIsf3 (Fig. 3).
Chloramphenicol, another antibiotic with a nitro group (NO2), was also able to serve as a reducible acceptor. Approximately 20 nmol of almost completely dithionite-reduced TvIsf3 was instantly reoxidized by equimolar amounts of an anaerobic solution of the drug (Fig. 3). However, other nitrogen compounds, such as hydroxylamine, NaNO2, and NaNO3, were unable to reoxidize the dithionite-reduced TvIsf3 protein.
Unexpectedly, and in contrast to the published data on MtIsf (6), TvIsf3 was found to utilize the pyridine nucleotide coenzyme NADH (but not NADPH) as an electron donor. This enabled the direct determination of kinetic parameters of TvIsf3 under turnover conditions with the electron acceptors identified in previous experiments with dithionite-reduced protein (Fig. 6). Due to higher concentrations of reactants, the turnover assays also allowed for a more reliable identification of reaction end products and, thus, the stoichiometry of electron transfer. This way, the oxygen reduction by TvIsf3 was first assayed. Upon consumption of most of the NADH, the cuvette content (an aerobic reaction mixture without O2-scavenging enzymes) was immediately probed for the presence of hydrogen peroxide using the FOX assay (28) to determine the final product of O2 reduction. In contrast to the results previously reported for MtIsf (6), the assay did indicate the presence of hydrogen peroxide. To corroborate this result, we determined the ratio of oxygen reduced per NADH oxidized using anaerobic conditions and known amounts of air-saturated water (the oxygen concentration in water at 37°C was taken as 199 μM) as a substrate. One mole of reduced oxygen resulted in 1 mol of oxidized NADH, confirming the two-electron transfer from NADH to oxygen and the formation of hydrogen peroxide (data not shown). The two nitro-antibiotics, metronidazole and chloramphenicol, were reduced by TvIsf3 with NADH as a reductant as well (Fig. 6). The kinetic data of TvIsf3 with oxygen, metronidazole, and chloramphenicol are summarized in Table 1. The observed values seem to fall within the physiologically relevant range.
FIG 6.
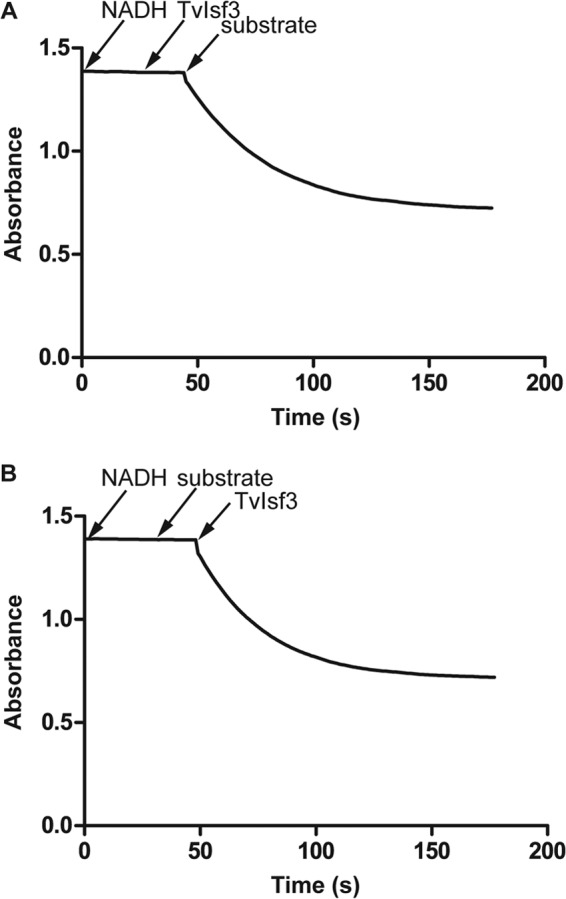
NADH-dependent reduction of acceptors by TvIsf3. Panels A and B demonstrate that both NADH and a substrate are required to observe the turnover activity of TvIsf3. The anaerobic spectrophotometric assays were performed in an assay buffer with air-saturated water, metronidazole, or chloramphenicol as a substrate and NADH as a reductant. The details are described in Materials and Methods.
TABLE 1.
Apparent kinetic parameters of recombinant, affinity-purified TvIsf3 with 220 μM NADH as an electron donora
| Substrate | kcat (s−1) | Km (μM) | kcat/Km |
|---|---|---|---|
| Chloramphenicol | 130 ± 13 | 77.63 ± 3.55 | 1.67 |
| Metronidazole | 56 ± 7 | 27.28 ± 2.83 | 2.05 |
| Oxygen | 10 ± 1 | 5.64 ± 0.69 | 1.77 |
The means were calculated from at least three separate determinations.
Using EPR spectroscopy and high concentrations of NADH and metronidazole in a turnover assay, we attempted to determine whether the product of MTZ reduction by TvIsf3 was a nitroradical anion, the species most suspected of being responsible for the toxic effect of MTZ on susceptible organisms (34, 35). We were unable to detect the typical signal of MTZ radical anion, indicating that MTZ is likely reduced by an even number of electrons. The control consisting of hydrogenosomes, metronidazole, and reactants of the PFO reaction as well as the single-enzyme system consisting of a purified, ferredoxin-reducing NADH dehydrogenase module of complex 1 and T. vaginalis ferredoxin 1 (30) produced an easily detectable signal (data not shown).
Virtually no activity under turnover conditions was observed with hydrogen peroxide and nitric oxide, although both substrates were able to immediately reoxidize the dithionite-reduced protein. To exclude the negative effect of low pH in the reaction of TvIsf3 with DPTA-NONOate, the assay was repeated in pH 8.0 assay buffer with NO-saturated water, with the same result.
Ferredoxin-mediated reduction.
Isf protein from Methanosarcina thermophila was proposed to interact with ferredoxin as a physiological electron donor (4). To determine whether ferredoxin can also function as a reductant of TvIsf3, we reconstructed in vitro the anaerobic reaction system consisting of PFO purified from T. vaginalis hydrogenosomes (17), recombinant hydrogenosomal ferredoxin 1 (29), recombinant TvIsf3, and pyruvate and CoA as substrates. Electrons released from pyruvate were transferred to ferredoxin 1 by the activity of PFO and subsequently to TvIsf3, as documented by the continuous regression of the TvIsf3 UV-vis spectrum to the reduced state (Fig. 7). In contrast, the control reaction without ferredoxin did not show any appreciable reduction of TvIsf3 over a period of 20 min. When air was introduced into the cuvette, the TvIsf3 spectrum immediately returned to its oxidized pattern; when the cuvette was sealed again, the reduction of TvIsf3 resumed, confirming the turnover and thus the functionality of the components of the enzymatic system. This multicomponent setup nevertheless could not be used to determine the parameters of the electron transfer from ferredoxin to TvIsf3 because reduced ferredoxin rapidly oxidized in the presence of all electron acceptors tested that at the same time were capable of reoxidation of TvIsf3.
FIG 7.
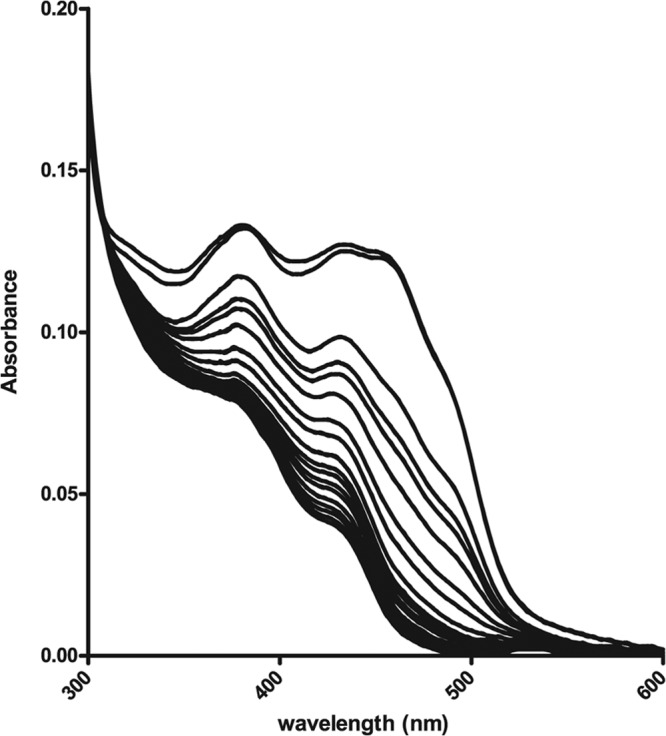
Ferredoxin-mediated reduction of TvIsf3. The UV-visible spectra demonstrate the continuous reduction of TvIsf3 (approximately 20 μM) by recombinant T. vaginalis ferredoxin 1 (∼40 μg) in an anaerobic system consisting of 0.25 mM CoA, 5 mM pyruvate, and T. vaginalis PFO (∼10 μg) in an assay buffer supplemented with oxygen-scavenging enzymes. The recording time for one spectrum was 38 s.
DISCUSSION
In this study, we characterized the iron-sulfur flavoprotein (TvIsf3) of T. vaginalis, the only eukaryote in addition to members of the Entamoeba genus in which such proteins could be detected using searches of sequence databases. In contrast, Isf homologs are common in the genomes of diverse anaerobic Bacteria and Archaea. Nonetheless, despite the broad distribution among prokaryotes, the Isf from the methanoarchaeon Methanosarcina thermophila has been the only Isf studied in terms of function to date. Because of its activity as an oxygen and hydrogen peroxide reductase, the general function of MtIsf and its homologs in oxidative stress protection has been proposed (6).
The phylogenetic relationships of T. vaginalis Isf were not studied in detail, but the protein, most likely acquired through horizontal gene transfer, is a close homolog of its counterparts from both the Archaea and Bacteria domains. In T. vaginalis, the protein is localized in the hydrogenosomes, and this localization is consistent with the presence of an amino-terminal hydrogenosomal targeting sequence that is absent in otherwise highly similar prokaryotic homologs.
TvIsf3 binds one molecule of FMN per subunit, and the complex pattern of the UV-visible spectrum (which is sensitive to acidification), the conserved four-cysteine motif, and the ∼3.5 mol of nonheme iron per subunit of the affinity-purified protein are indicative of the presence of the 4Fe-4S redox cluster. The same flavin and FeS cluster were identified in MtIsf (4). Gel filtration chromatography showed that the overwhelming majority of the enzymatically active TvIsf3 protein had a dimeric composition, in agreement with an original report on several bacterial Isf proteins (1). However, in the present work, a small portion of TvIsf3 was also recovered from the column in the form of a tetramer, an observation that may have been overlooked if not for a more recent structural study of MtIsf and Isf from Archaeoglobus fulgidus showing that these proteins formed tetramers (2). Thus, it may be possible that a relatively dilute protein tends to disintegrate into stable dimers under the dynamic conditions of column chromatography and potentially suboptimal buffer composition, whereas the native quaternary structure is tetrameric under physiological conditions.
The activity of TvIsf3 was first assessed by the ability of various substrates to reoxidize the dithionite-reduced protein. Using this approach, oxygen, hydrogen peroxide, nitric oxide, and the broad-spectrum nitro-antibiotics metronidazole and chloramphenicol were identified as electron acceptors of TvIsf3. Subsequently and rather surprisingly, we found that TvIsf3 can accept electrons from the coenzyme NADH. This observation was unexpected because the sequences of Isf proteins, including T. vaginalis paralogs, do not contain a recognizable Rossmann fold with a glycine-rich, NAD(P)-binding motif, and the prototype MtIsf was reported to utilize ferredoxin rather than pyridine nucleotide coenzyme as an electron donor (6). The activity of TvIsf3 with NADH enabled the continuous spectrophotometric assays and the determination of the kinetic parameters of the enzyme.
With its capacity to reduce oxygen, TvIsf3 is similar to MtIsf (6). However, in contrast to MtIsf, which was reported to reduce oxygen with four electrons and form water (6), the trichomonad protein reduced oxygen to hydrogen peroxide using two electrons. The kinetic parameters of the reaction seem physiologically relevant; thus, the activity of TvIsf3 may account for the formation of toxic hydrogen peroxide within hydrogenosomes under aerobic conditions. Nevertheless, the organelles are equipped with defense mechanisms to cope with peroxidative stress (15, 16).
Hydrogen peroxide, the other substrate shown to interact with MtIsf, was reduced by TvIsf3 only at a very low rate in an NADH-dependent continuous assay, although it immediately reoxidized the dithionite-reduced protein. The same observation was made also for nitric oxide. While NO rapidly reoxidized the chemically reduced TvIsf, it could not serve as a substrate that would effectively support the continuous oxidation of NADH under turnover conditions. The easiest explanation of this discrepancy is that hydrogen peroxide and nitric oxide, both strong oxidants, in a thermodynamically favorable reaction simply reoxidize the reduced FMN in the TvIsf3. However, the structurally related flavodiiron proteins also use oxygen and nitric oxide as electron acceptors; some representatives reduce both substrates, while others display strong substrate specificity (24, 36, 37). For example, the flavodiiron protein from T. vaginalis hydrogenosomes is strictly specific for oxygen, and nitric oxide could not reoxidize its FMN cofactor (17). Exactly which structural features of the flavodiiron proteins affect the substrate specificity is currently unclear. Therefore, the possibility exists that the reactivity of reduced TvIsf3 with hydrogen peroxide and NO actually reflects the genuine protein specificity. It cannot be excluded that while NADH does not serve as an electron donor for the reduction of hydrogen peroxide and NO, ferredoxin could. If this is indeed the case, then an intriguing question arises as to what is the physiological electron donor to TvIsf3. It would seem logical that if the protein utilizes NADH, this would be its natural redox partner. Trichomonad hydrogenosomes contain several NAD-dependent enzymes, and the most abundant one, the malic enzyme, provides NADH during oxidative decarboxylation of malate, one of the catabolic substrates of hydrogenosomes (10). On the other hand, ferredoxin and not the pyridine nucleotide coenzyme was identified as an electron donor to Isf from Methanosarcina for the reduction of oxygen and hydrogen peroxide (6). T. vaginalis encodes seven paralogs of ferredoxin (18), and most of them, if not all, are expressed in the hydrogenosomes (19, 20), where they serve as one-electron carriers that link the crucial reactions of hydrogenosomal metabolism (10). Ferredoxin is an electron donor to the oxygen-reducing and water-forming flavodiiron protein (17); in this work, we show that upon in vitro reduction by hydrogenosomal PFO, ferredoxin can also transfer electrons to TvIsf3. A comparison of the kinetic parameters of TvIsf3 with NADH versus ferredoxin would help to assess which of the donors is the more likely redox partner; however, this seemingly straightforward experiment was not possible because trichomonad ferredoxin 1 rapidly oxidized in the presence of all active acceptors used in this study.
If under in vivo conditions TvIsf3 is indeed capable of NO reduction, this activity would be physiologically relevant. NO is a microbicidal molecule that is released by macrophages during inflammation (38), and its toxic effect on T. vaginalis under microaerobic conditions has been described previously (39). Nevertheless, trichomonads infesting the vaginal epithelium cause chronic, non-self-limiting infections (40), and the parasite must therefore be able to effectively resist the immune response of the host. It has been shown that T. vaginalis can degrade NO (33), although it remained unclear which protein was responsible for this capability. As mentioned above, the proteins of the flavodiiron superfamily, which are similarly widespread in anaerobic bacteria as Isf and are present in certain anaerobic eukaryotic parasites, including T. vaginalis (17), function as oxygen and NO reductases with variable affinities for these acceptors (24, 36, 37). It was originally suggested that the flavodiiron protein could be responsible for the NO reduction observed in T. vaginalis lysates (33); however, subsequent experiments showed that eukaryotic FDP homologs, including the one from T. vaginalis hydrogenosomes, lack NO reductase activity (17, 41, 42). Other proteins thus should be present that afford the protection against nitrosative stress. In addition to TvIsf3 and two other Isf paralogs present in the hydrogenosomes (19, 20), among those to be considered are three other putative flavodiiron protein homologs, all lacking hydrogenosomal targeting signals (18). However, no experimental data on these proteins are currently available.
The results of the experiment showing the possible activity of TvIsf3 with NO prompted us to test the ability of other nitrogen compounds to function as potential electron acceptors, including metronidazole, a 5′-nitroimidazole drug used to treat anaerobic infections, including trichomoniasis. Indeed, dithionite-reduced TvIsf3 was immediately reoxidized upon the addition of an anaerobic metronidazole solution, showing that metronidazole is reduced by TvIsf3. This observation was confirmed in a continuous assay with NADH as the electron donor. The nitroimidazole radical anion arising from the ferredoxin-mediated, one-electron reduction of the nitro group is formed in the cell lysates of trichomonads as well as in isolated hydrogenosomes and is considered to be the most toxic form of the drug (30, 43). The characteristic complex signal of the metronidazole radical anion is generally detectable by EPR spectroscopy (30), and upon completing the reaction, we probed the mixture originally containing TvIsf3, NADH, and metronidazole for the presence of such a radical anion. No such signal was observed, indicating that metronidazole was reduced by an even number of electrons, thus obviating the formation of the radical and giving rise to a more reduced, less toxic product. Reductive inactivation of metronidazole to a nontoxic amine derivative by nitroimidazole reductases (Nim proteins) has been associated with 5′-nitroimidazole resistance in certain bacterial strains (44, 45). It is noteworthy that bacterial Nim protein homologs are encoded by T. vaginalis, along with several nitroreductases (18) that, on the other hand, are responsible for reductive activation of nitromidazole drugs in microaerophilic bacteria such as Helicobacter pylori (46). However, the activities of these proteins in T. vaginalis remain to be studied, and no relationship between the transcription levels of corresponding mRNAs and metronidazole sensitivity was found in either T. vaginalis or E. histolytica that also possesses such genes (47).
We also tested chloramphenicol, a broad-spectrum antibiotic with a nitro group effective against both Gram-positive and Gram-negative bacteria (including anaerobes) (48), for its ability to function as an electron acceptor. We found that chloramphenicol was able to accept electrons from TvIsf3 in the same rapid manner as the other active acceptors we assayed, and similar to oxygen and metronidazole, it supported the oxidation of NADH under turnover conditions. It is noteworthy that several chloramphenicol reduction products that have been tested for antibiotic activity do not appear to have appreciable microbicidal effects (49). Moreover, the reductive inactivation of chloramphenicol has been shown to occur in actively growing cultures of Clostridium acetobutylicum, an anaerobic bacterium that possesses Isf homologs, and the ferredoxin-dependent, pyruvate-stimulated reduction of chloramphenicol and other aryl-nitro compounds by cell extracts of Clostridium has been described (50). It is possible that the mechanism described here, that is, the reduction of various acceptors by Isf using the electrons released from pyruvate by PFO and transferred by ferredoxin, is responsible for this decades-old observation.
In summary, we found that the bacterial-type Isf protein present in T. vaginalis hydrogenosomes could reduce a variety of substrates, all of them being molecules toxic for anaerobic microbes. While the chloramphenicol-reducing activity of TvIsf3 is probably an inherited trait of a bacterial protein with no adaptive function in trichomonads, the relevance of reductive inactivation of metronidazole by trichomonad Isf paralogs for the drug resistance occurring in the field and induced in in vitro cultures remains to be explored. With the exception of oxygen and hydrogen peroxide reduction, the Isf activities described in this report have not been observed in prokaryotic Isf proteins; regardless, the ability of bacterial Isf homologs to fulfill these other roles seems likely and should be verified experimentally. After all, it has been noted that many anaerobic bacteria possess multiple Isf paralogs, and it was suggested that these proteins perform for anaerobes universally important and possibly diverse functions (1). The same applies also to T. vaginalis, which encodes seven distinct Isf paralogs and expresses at least three of them in its hydrogenosomes. Both metronidazole and chloramphenicol (and numerous other nitro compounds) are naturally occurring antibiotics, and reactive oxygen and nitrogen species belong to the repertoire of antimicrobial response of the immune system. The activity of Isf described here may, therefore, represent a novel mechanism of defense against xenobiotics that is unique in trichomonads and amoebae but likely common among prokaryotic anaerobes.
ACKNOWLEDGMENTS
This work was supported by grant GC13-09208J of the Czech Science Foundation to J. Tachezy, by Charles University grant GAUK 407911 to K.P., and by Charles University grant UNCE 204017 to T.S. The kind support of the Institute of Organic Chemistry and Biochemistry (RVO: 61388963) is acknowledged by J. Tarábek.
Footnotes
Published ahead of print 24 March 2014
REFERENCES
- 1.Zhao T, Cruz F, Ferry JG. 2001. Iron-sulfur flavoprotein (Isf) from Methanosarcina thermophila is the prototype of a widely distributed family. J. Bacteriol. 183:6225–6233. 10.1128/JB.183.21.6225-6233.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade SLA, Cruz F, Drennan CL, Ramakrishnan V, Rees DC, Ferry JG, Einsle O. 2005. Structures of the iron-sulfur flavoproteins from Methanosarcina thermophila and Archaeoglobus fulgidus. J. Bacteriol. 187:3848–3854. 10.1128/JB.187.11.3848-3854.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leartsakulpanich U, Antonkine ML, Ferry JG. 2000. Site-specific mutational analysis of a novel cysteine motif proposed to ligate the 4Fe-4S cluster in the iron-sulfur flavoprotein of the thermophilic methanoarchaeon Methanosarcina thermophila. J. Bacteriol. 182:5309–5316. 10.1128/JB.182.19.5309-5316.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker DF, Leartsakulpanich U, Surerus KK, Ferry JG, Ragsdale SW. 1998. Electrochemical and spectroscopic properties of the iron-sulfur flavoprotein from Methanosarcina thermophila. J. Biol. Chem. 273:26462–26469. 10.1074/jbc.273.41.26462 [DOI] [PubMed] [Google Scholar]
- 5.Latimer MT, Painter MH, Ferry JG. 1996. Characterization of an iron-sulfur flavoprotein from Methanosarcina thermophila. J. Biol. Chem. 271:24023–24028. 10.1074/jbc.271.39.24023 [DOI] [PubMed] [Google Scholar]
- 6.Cruz F, Ferry JG. 2006. Interaction of iron-sulfur flavoprotein with oxygen and hydrogen peroxide. Biochim. Biophys. Acta 1760:858–864. 10.1016/j.bbagen.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 7.Johnston VJ, Mabey DC. 2008. Global epidemiology and control of Trichomonas vaginalis. Curr. Opin. Infect. Dis. 21:56–64. 10.1097/QCO.0b013e3282f3d999 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. 2001. Global prevalence and incidence of selected curable sexually transmitted infections. World Health Organization, Geneva, Switzerland [Google Scholar]
- 9.Petrin D, Delgaty K, Bhatt R, Garber G. 1998. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 11:300–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hrdý I, Tachezy J, Muller M. 2008. Metabolism of trichomonad hydrogenosomes. Microbiol. Monogr. 9:113–145 [Google Scholar]
- 11.Lloyd D, Kristensen B. 1985. Metronidazole inhibition of hydrogen production in vivo in drug-sensitive and resistant strains of Trichomonas vaginalis. J. Gen. Microbiol. 131:849–853. 10.1099/00221287-131-4-849 [DOI] [PubMed] [Google Scholar]
- 12.Ellis JE, Yarlett N, Cole D, Humphreys MJ, Lloyd D. 1994. Antioxidant defences in the microaerophilic protozoan Trichomonas vaginalis: comparison of metronidazole-resistant and sensitive strains. Microbiology 140(Part 9):2489–2494 [DOI] [PubMed] [Google Scholar]
- 13.Linstead DJ, Bradley S. 1988. The purification and properties of two soluble reduced nicotinamide: acceptor oxidoreductases from Trichomonas vaginalis. Mol. Biochem. Parasitol. 27:125–133. 10.1016/0166-6851(88)90032-1 [DOI] [PubMed] [Google Scholar]
- 14.Lindmark DG, Muller M. 1974. Superoxide dismutase in the anaerobic flagellates, Tritrichomonas foetus and Monocercomonas sp. J. Biol. Chem. 249:4634–4637 [PubMed] [Google Scholar]
- 15.Pütz S, Gelius-Dietrich G, Piotrowski M, Henze K. 2005. Rubrerythrin and peroxiredoxin: two novel putative peroxidases in the hydrogenosomes of the microaerophilic protozoon Trichomonas vaginalis. Mol. Biochem. Parasitol. 142:212–223. 10.1016/j.molbiopara.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 16.Mentel M, Zimorski V, Haferkamp P, Martin W, Henze K. 2008. Protein import into hydrogenosomes of Trichomonas vaginalis involves both N-terminal and internal targeting signals: a case study of thioredoxin reductases. Eukaryot. Cell 7:1750–1757. 10.1128/EC.00206-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smutná T, Goncalves VL, Saraiva LM, Tachezy J, Teixeira M, Hrdy I. 2009. Flavodiiron protein from Trichomonas vaginalis hydrogenosomes: the terminal oxygen reductase. Eukaryot. Cell 8:47–55. 10.1128/EC.00276-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UCM, Besteiro S, Sicheritz-Ponten T, Noel CJ, Dacks JB, Foster PG, Simillion C, Van de Peer Y, Miranda-Saavedra D, Barton GJ, Westrop GD, Muller S, Dessi D, Fiori PL, Ren QH, Paulsen I, Zhang HB, Bastida-Corcuera FD, Simoes-Barbosa A, Brown MT, Hayes RD, Mukherjee M, Okumura CY, Schneider R, Smith AJ, Vanacova S, Villalvazo M, Haas BJ, Pertea M, Feldblyum TV, Utterback TR, Shu CL, Osoegawa K, de Jong PJ, Hrdy I, Horvathova L, Zubacova Z, Dolezal P, Malik SB, Logsdon JM, Henze K, Gupta A, Wang CC, Dunne RL, Upcroft JA, Upcroft P, White O, Salzberg SL, Tang P, Chiu CH, Lee YS, Embley TM, Coombs GH, Mottram JC, Tachezy J, Fraser-Liggett CM, Johnson PJ. 2007. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315:207–212. 10.1126/science.1132894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider RE, Brown MT, Shiflett AM, Dyall SD, Hayes RD, Xie YM, Loo JA, Johnson PJ. 2011. The Trichomonas vaginalis hydrogenosome proteome is highly reduced relative to mitochondria, yet complex compared with mitosomes. Int. J. Parasitol. 41:1421–1434. 10.1016/j.ijpara.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beltrán NC, Horvathova L, Jedelsky PL, Sedinova M, Rada P, Marcincikova M, Hrdy I, Tachezy J. 2013. Iron-induced changes in the proteome of Trichomonas vaginalis hydrogenosomes. PLoS One 8:e65148. 10.1371/journal.pone.0065148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertens E, Muller M. 1990. Glucokinase and fructokinase of Trichomonas vaginalis and Tritrichomonas foetus. J. Protozool. 37:384–388. 10.1111/j.1550-7408.1990.tb01161.x [DOI] [PubMed] [Google Scholar]
- 22.Delgadillo MG, Liston DR, Niazi K, Johnson PJ. 1997. Transient and selectable transformation of the parasitic protist Trichomonas vaginalis. Proc. Natl. Acad. Sci. U. S. A. 94:4716–4720. 10.1073/pnas.94.9.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutak R, Dolezal P, Fiumera HL, Hrdy I, Dancis A, Delgadillo-Correa M, Johnson PJ, Muller M, Tachezy J. 2004. Mitochondrial-type assembly of FeS centers in the hydrogenosomes of the amitochondriate eukaryote Trichomonas vaginalis. Proc. Natl. Acad. Sci. U. S. A. 101:10368–10373. 10.1073/pnas.0401319101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes CM, Giuffre A, Forte E, Vicente JB, Saraiva LM, Brunori M, Teixeira M. 2002. A novel type of nitric-oxide reductase. Escherichia coli flavorubredoxin. J. Biol. Chem. 277:25273–25276. 10.1074/jbc.M203886200 [DOI] [PubMed] [Google Scholar]
- 25.Fischer DS, Price DC. 1964. A simple serum iron method using the new sensitive chromogen tripyridyl-s-triazine. Clin. Chem. 10:21–31. 10.1016/0009-8981(64)90210-4 [DOI] [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 27.Vicente JB, Justino MC, Goncalves VL, Saraiva LM, Teixeira M. 2008. Biochemical, spectroscopic, and thermodynamic properties of flavodiiron proteins. Methods Enzymol. 437:21–45. 10.1016/S0076-6879(07)37002-X [DOI] [PubMed] [Google Scholar]
- 28.Wolff SP. 1994. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 233:182–189. 10.1016/S0076-6879(94)33021-2 [DOI] [Google Scholar]
- 29.Vidakovic MS, Fraczkiewicz G, Germanas JP. 1996. Expression and spectroscopic characterization of the hydrogenosomal [2Fe-2S] ferredoxin from the protozoan Trichomonas vaginalis. J. Biol. Chem. 271:14734–14739. 10.1074/jbc.271.25.14734 [DOI] [PubMed] [Google Scholar]
- 30.Hrdý I, Cammack R, Stopka P, Kulda J, Tachezy J. 2005. Alternative pathway of metronidazole activation in Trichomonas vaginalis hydrogenosomes. Antimicrob. Agents Chemother. 49:5033–5036. 10.1128/AAC.49.12.5033-5036.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley PJ, Lahti CJ, Plumper E, Johnson PJ. 1997. Targeting and translocation of proteins into the hydrogenosome of the protist Trichomonas: similarities with mitochondrial protein import. EMBO J. 16:3484–3493. 10.1093/emboj/16.12.3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown MT, Goldstone HM, Bastida-Corcuera F, Delgadillo-Correa MG, McArthur AG, Johnson PJ. 2007. A functionally divergent hydrogenosomal peptidase with protomitochondrial ancestry. Mol. Microbiol. 64:1154–1163. 10.1111/j.1365-2958.2007.05719.x [DOI] [PubMed] [Google Scholar]
- 33.Sarti P, Fiori PL, Forte E, Rappelli P, Teixeira M, Mastronicola D, Sanciu G, Giuffre A, Brunori M. 2004. Trichomonas vaginalis degrades nitric oxide and expresses a flavorubredoxin-like protein: a new pathogenic mechanism? Cell. Mol. Life Sci. 61:618–623. 10.1007/s00018-003-3413-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman A, Cammack R, Linstead D, Lloyd D. 1985. The generation of metronidazole radicals in hydrogenosomes isolated from Trichomonas vaginalis. J. Gen. Microbiol. 131:2141–2144 [DOI] [PubMed] [Google Scholar]
- 35.Yarlett N, Gorrell TE, Marczak R, Muller M. 1985. Reduction of nitroimidazole derivatives by hydrogenosomal extracts of Trichomonas vaginalis. Mol. Biochem. Parasitol. 14:29–40. 10.1016/0166-6851(85)90103-3 [DOI] [PubMed] [Google Scholar]
- 36.Gomes CM, Silva G, Oliveira S, LeGall J, Liu MY, Xavier AV, Rodrigues-Pousada C, Teixeira M. 1997. Studies on the redox centers of the terminal oxidase from Desulfovibrio gigas and evidence for its interaction with rubredoxin. J. Biol. Chem. 272:22502–22508. 10.1074/jbc.272.36.22502 [DOI] [PubMed] [Google Scholar]
- 37.Seedorf H, Dreisbach A, Hedderich R, Shima S, Thauer RK. 2004. F420H2 oxidase (FprA) from Methanobrevibacter arboriphilus, a coenzyme F420-dependent enzyme involved in O2 detoxification. Arch. Microbiol. 182:126–137. 10.1007/s00203-004-0675-3 [DOI] [PubMed] [Google Scholar]
- 38.Bogdan C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907–916. 10.1038/ni1001-907 [DOI] [PubMed] [Google Scholar]
- 39.Park GC, Ryu JS, Min DY. 1997. The role of nitric oxide as an effector of macrophage-mediated cytotoxicity against Trichomonas vaginalis. Korean J. Parasitol. 35:189–195. 10.3347/kjp.1997.35.3.189 [DOI] [PubMed] [Google Scholar]
- 40.Schwebke JR, Burgess D. 2004. Trichomoniasis. Clin. Microbiol. Rev. 17:794–803. 10.1128/CMR.17.4.794-803.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Matteo A, Scandurra FM, Testa F, Forte E, Sarti P, Brunori M, Giuffre A. 2008. The O2-scavenging flavodiiron protein in the human parasite Giardia intestinalis. J. Biol. Chem. 283:4061–4068. 10.1074/jbc.M705605200 [DOI] [PubMed] [Google Scholar]
- 42.Vicente JB, Tran V, Pinto L, Teixeira M, Singh U. 2012. A detoxifying oxygen reductase in the anaerobic protozoan Entamoeba histolytica. Eukaryot. Cell 11:1112–1118. 10.1128/EC.00149-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreno SN, Mason RP, Docampo R. 1984. Distinct reduction of nitrofurans and metronidazole to free radical metabolites by Tritrichomonas foetus hydrogenosomal and cytosolic enzymes. J. Biol. Chem. 259:8252–8259 [PubMed] [Google Scholar]
- 44.Carlier JP, Sellier N, Rager MN, Reysset G. 1997. Metabolism of a 5-nitroimidazole in susceptible and resistant isogenic strains of Bacteroides fragilis. Antimicrob. Agents Chemother. 41:1495–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leiros HK, Kozielski-Stuhrmann S, Kapp U, Terradot L, Leonard GA, McSweeney SM. 2004. Structural basis of 5-nitroimidazole antibiotic resistance: the crystal structure of NimA from Deinococcus radiodurans. J. Biol. Chem. 279:55840–55849. 10.1074/jbc.M408044200 [DOI] [PubMed] [Google Scholar]
- 46.Mendz GL, Megraud F. 2002. Is the molecular basis of metronidazole resistance in microaerophilic organisms understood? Trends Microbiol. 10:370–375. 10.1016/S0966-842X(02)02405-8 [DOI] [PubMed] [Google Scholar]
- 47.Pal D, Banerjee S, Cui J, Schwartz A, Ghosh SK, Samuelson J. 2009. Giardia, Entamoeba, and Trichomonas enzymes activate metronidazole (nitroreductases) and inactivate metronidazole (nitroimidazole reductases). Antimicrob. Agents Chemother. 53:458–464. 10.1128/AAC.00909-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vazquez D. 1966. Mode of action of chloramphenicol and related antibiotics p 169–191 In Newton PA, Reynolds PE. (ed), Symposium of the Society for General Microbiology, vol 16 Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 49.Corbett MD, Chipko BR. 1978. Synthesis and antibiotic properties of chloramphenicol reduction products. Antimicrob. Agents Chemother. 13:193–198. 10.1128/AAC.13.2.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Brien RW, Morris JG. 1971. The ferredoxin-dependent reduction of chloramphenicol by Clostridium acetobutylicum. J. Gen. Microbiol. 67:265–271. 10.1099/00221287-67-3-265 [DOI] [PubMed] [Google Scholar]