Abstract
The role of carbapenem-resistant Acinetobacter baumannii (CRAb) in polymicrobial infection remains elusive. Having observed the ability of CRAb to shelter other susceptible bacteria from carbapenem killing, we sought to determine the factors contributing to this sheltering effect by transforming different recombinant plasmids into recipient A. baumannii cells. The sheltering effects of CRAb were reproduced in recipient A. baumannii cells that highly expressed carbapenem-hydrolyzing class D β-lactamases (CHDLs) through their associated strong promoter. With the use of Western blot analysis and a bioassay, the highly expressed CHDLs were found to be extracellularly released and led to hydrolysis of carbapenem. The level of extracellular CHDLs increased after challenge with a higher concentration of CHDL substrates, such as carbapenem and ticarcillin. This increased CHDL may, in part, be attributed to cell lysis, as indicated by the presence of extracellular gyrase. In the planktonic condition, the sheltering effect for the cocultured susceptible bacteria might represent an indirect and passive effect of the CRAb self-defense mechanism, because coculture with the susceptible pathogen did not augment the amount of the extracellular CHDLs. Polymicrobial infection caused by CRAb and a susceptible counterpart exerted higher pathogenicity than monomicrobial infection caused by either pathogen alone in mice receiving carbapenem therapy. This study demonstrated that CHDL-producing CRAb appears to provide a sheltering effect for carbapenem-susceptible pathogens via the extracellular release of CHDLs and, by this mechanism, can enhance the pathogenesis of polymicrobial infection in the presence of carbapenem therapy.
INTRODUCTION
Microorganisms that exist in the same ecological niche can interact in complex ways (1, 2). Different pathogens may act synergistically or in succession to mediate polymicrobial infections (2, 3). Clinical course, disease severity, and antimicrobial therapy outcomes are all affected by the presence of multiple pathogens (2, 4). Under some circumstances, polymicrobial infections have poorer outcomes than monomicrobial infections (5, 6). The contributions of individual pathogens toward the facilitated pathogenesis of polymicrobial infections have been delineated in several cases but remain elusive in many others (2, 3).
Acinetobacter baumannii is a leading pathogen of nosocomial infections worldwide. The rapid evolvement of A. baumannii strains resistant to multiple antimicrobial agents, including carbapenem, has severely limited therapeutic options (7, 8). Carbapenem resistance in A. baumannii has mainly been attributed to the production of carbapenemases, particularly carbapenem-hydrolyzing class D β-lactamases (CHDLs), which include the OXA-23, -40, -51, -58 (9), and -143 classes of β-lactamases (10). Polymicrobial infection has been found in 20 to 50% of A. baumannii infections (11–13). Although A. baumannii itself is considered to be a low-virulence pathogen, its role in polymicrobial infection has not been delineated.
We observed several patients who had breakthrough polymicrobial bacteremia due to combined infection by carbapenem-susceptible microorganisms and carbapenem-resistant A. baumannii (CRAb) during the course of carbapenem therapy (see Table S1 in the supplemental material). The unexpected in vivo isolation of carbapenem-susceptible microorganisms in the presence of carbapenem could be due to factors such as inadequate drug concentration at the site of infection, a compromised host immune system, phenotypic resistance such as biofilm formation of the susceptible pathogen (14), horizontal transfer of the carbapenemase gene from CRAb to the susceptible pathogen (15, 16), or a sheltering effect from the resistant counterpart (17). As described in this report, we observed that CRAb can provide a sheltering effect for susceptible pathogens, thereby protecting them from carbapenem therapy. In this study, we sought to determine the factors that contribute to this sheltering effect, by using recombinant plasmids transformed into A. baumannii cells and a mouse pneumonia model.
MATERIALS AND METHODS
Bacterial strains, plasmids, molecular techniques, chemicals, and antimicrobial susceptibility testing.
Bacterial strains and plasmids used in this study are listed in Table 1, and primers are shown in Table S2 in the supplemental material. A. baumannii was identified using a multiplex PCR method (18). Bacteria belonging to the family Enterobacteriaceae and Pseudomonas aeruginosa were identified using a Vitek II system (bioMérieux, Marcy l'Etoile, France). Genes encoding class A, B, and D carbapenemases and the upstream insertion sequence of CHDL genes were detected by a PCR method, as previously described (19, 20). Phenotypic tests were used to identify class B metallo-β-lactamases, as previously described (20). Imipenem, ticarcillin, and kanamycin were purchased from Sigma-Aldrich (St. Louis, MO). Restriction enzymes were purchased from New England BioLabs (Beverly, MA, USA). Antimicrobial susceptibility testing was determined by an agar dilution test (21) or Etest (AB Biodisk, Solna, Sweden). The breakpoint was defined as recommended by the Clinical and Laboratory Standards Institute (22).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description and/or usea |
|---|---|
| Strains | |
| Ec1003 | Clinical carbapenem-susceptible Escherichia coli isolate (IPM MIC, 0.125 mg/liter) recovered concomitantly with A. baumannii Ab1969. This isolate was used in most of the experiments to demonstrate the sheltering effect of carbapenem-resistant Acinetobacter baumannii (CRAb). Clinical characteristics of the patient from whom it was isolated are given in Table S1 in the supplemental material. |
| EcYT439 | Clinical carbapenem-susceptible E. coli isolate (IPM MIC, 0.25 mg/liter), used in the expt in Fig. 1D to demonstrate the sheltering of CRAb. |
| E. coli strain BL21 | Used in antigen production (Invitrogen, Carlsbad, CA). |
| KpTL425 | Clinical carbapenem-susceptible Klebsiella pneumoniae isolate (IPM MIC, 0.25 mg/liter), used in the expt in Fig. 1D to demonstrate the sheltering of CRAb. Clinical characteristics of the patient from whom it was isolated are given in Table S1. |
| EntCYT240 | Clinical carbapenem-susceptible Enterobacter cloacae isolate (IPM MIC, 0.5 mg/liter) recovered from a urine sample. It was used in the expt in Fig. 1D to demonstrate the sheltering of CRAb. |
| PaTL424 | Clinical carbapenem-susceptible Pseudomonas aeruginosa isolate (IPM MIC, 2 mg/liter), used in the expt in Fig. 1D to demonstrate the sheltering of CRAb. Clinical characteristics of the patient from whom it was isolated are given in Table S1. |
| Ab1969 | A. baumannii isolate overproducing OXA-58, isolated from a patient with polymicrobial bacteremia that concomitantly grew Ec1003. Clinical characteristics of the patient from whom it was isolated are given in Table S1. |
| Ab290 | Clinical A. baumannii isolate susceptible to multiple antimicrobials, including TIC, used as a recipient in transformation. |
| Plasmids | |
| pET-28a | Novagen commercial vector (Madison, WI) used in gene fragment cloning to generate polyclonal antibodies. |
| pYMAb-2 | A. baumannii-E. coli shuttle vector, in which a replicon of a plasmid from A. baumannii reference strain ATCC 19606T was inserted into pET-28a. No other component of the plasmid, including mob that encodes a protein assisting the mobilization of the plasmid, was included; KANr. |
| pOXA-58-2 | IS1008-ΔISAba3-blaOXA-58 amplified by primers IS1008 (XbaI)F and OXA-58 (XhoI)R and cloned into the XbaI and XhoI sites of pYMAb-2. |
| pOXA-58ΔP2-1 to -3 | Partial or total P2 promoter on IS1008–ΔISAba3-blaOXA-58 deleted by amplification using either primer IS1008-ΔP2-1(XbaI)F, IS1008-ΔP2-2 (XbaI)F, or IS1008-ΔP2-3 (XbaI)F and primer OXA-58 (XhoI)R, and then cloned into the XbaI and XhoI sites of pYMAb-2. |
| pOXA-23 | blaOXA-23 and its promoter in ISAba1 amplified by primers ISAba1(XbaI)F and OXA23-like (XhoI)R and cloned into the XbaI and XhoI sites of pYMAb-2. OXA-23 was His tagged. |
| pOXA-72 | blaOXA-72 and its promoter amplified by primers OXA-24(XbaI)F and OXA-24-like (XhoI)R and cloned into the XbaI and XhoI sites of pYMAb-2. OXA-72 was His tagged. |
| pOXA-83 | blaOXA-83 and its promoter in ISAba1 amplified by primers ISAba1(XbaI)F and OXA-51-like (XhoI)R and cloned into the XbaI and XhoI sites of pYMAb-2. OXA-83 was His tagged. |
IPM, imipenem; TIC, ticarcillin; KANr, kanamycin resistant.
Coculture of bacteria and determination of colony count.
Overnight cultures of different bacteria and A. baumannii strains were diluted 100-fold and cocultured in 5 ml of Luria-Bertani (LB; Difco, Detroit, MI) broth in a Pyrex culture tube (Corning Life Sciences, Manassas, VA) at 37°C for 2 h before antimicrobial agents were added. At given time points, 100-μl portions of 10-fold-diluted coculture were plated on an appropriate agar plate (see Fig. S1 in the supplemental material). A. baumannii and other bacterial species were discriminated by the colony characteristics of each species on the plates after overnight culture. Randomly selected colonies were subjected to species validation by the identification methods mentioned above.
Preparation of cellular fractions.
Overnight cultures of A. baumannii transformants, including strains Ab290(pOXA-58-2), Ab290(pOXA-58ΔP2-1), Ab290(pOXA-58ΔP2-2), and Ab290(pOXA-58ΔP2-3), were diluted 100-fold and cultured in 100 ml of LB broth. In some experiments, antimicrobials, with or without carbapenem-susceptible Ec1003 (100-fold-diluted overnight culture), were added to the culture of Ab290(pOXA-58-2). Cultures were grown to a logarithmic phase, and the cell densities (CFU/ml) were determined. The cultures were centrifuged at 10,000 × g for 15 min at 4°C. The supernatants, consisting of the extracellular fraction, were collected, filtered with a 0.22-μm Acrodisc filter (Sigma-Aldrich), and concentrated using Amicon Ultra columns (Millipore, Bedford, MA). Periplasmic and cytoplasmic fractions of the cells were fractionated as described previously (23). All cellular fractions were stored at −20°C prior to use.
Antibodies.
The internal fragment of blaOXA-58 or gyrA from A. baumannii was amplified with the primer pair OXA-58-21 (BamHI)F (F stands for forward) and OXA-58-21 (XhoI)R (R stands for reverse) or Gyrase-A(KpnI) P1 and Gyrase-A(PstI) P2, respectively (see Table S2 in the supplemental material). These fragments were subcloned into a pET-28a vector (Novagen, Madison, WI) to generate His-tagged proteins. Polyclonal antibodies against the OXA-58 and gyrase proteins of A. baumannii were generated from rabbits as previously described (24). The quality of purified polyclonal antibodies was examined using Western blot analysis with the preimmune serum as a negative control. The antibody for the detection of His-tagged protein was purchased from Anogen (Ontario, Canada).
Western blot analysis.
Protein samples of cellular fractions from ∼2 × 108 CFU bacteria were separated by polyacrylamide gel electrophoresis and immobilized on nitrocellulose membranes. The membranes were incubated with primary antibodies, followed by horseradish peroxidase-conjugated goat anti-rabbit (Sigma-Aldrich) or goat anti-mouse (Jackson ImmunoResearch Labs, West Grove, PA) secondary antibodies. An enhanced chemiluminescence (ECL) Western blot kit (PerkinElmer, Boston, MA) was used to detect chemiluminescence.
Bioassay for the detection of carbapenem inactivation.
Imipenem or kanamycin was diluted in 10 μl of phosphate-buffered saline (PBS) and preincubated with 10 μl of a concentrated extracellular fraction of A. baumannii transformants for 1 h at 37°C. Each 20-μl mixture was loaded onto a blank disk (Becton, Dickinson and Company, Franklin Lakes, NJ) that was placed in an agar plate containing a lawn of Ec1003, a carbapenem-susceptible Escherichia coli. Inhibitory zones were measured after incubating the plates overnight at 37°C. Antimicrobial inactivation was characterized by a decrease in the area of the inhibitory zone in the presence of the extracellular fraction from carbapenemase-producing transformants compared to that from the vector-alone transformants.
Murine model of polymicrobial infection.
Animal experiments were approved by the Ethical Committee for Animal Experiments of National Yang-Ming University. C57BL/6N mice (6 to 7 weeks old, 16 to 18 g) were used (National Laboratory Animal Center, Taiwan). Bacteria were grown to logarithmic phase at 37°C, washed, resuspended in 50 μl of sterile isotonic saline, and premixed with 50 μl of 10% porcine mucin (Sigma-Aldrich). Mice were anesthetized, and 100-μl amounts of bacterial solution were intratracheally introduced into their lungs. The 50% lethal dose (LD50) in mice was calculated using the SigmaPlot (version 7.0) program from SPSS Inc. (Chicago, IL).
In the treatment studies (10 mice/group), mice were intratracheally inoculated with E. coli Ec1003 (∼3 × 104 CFU) and/or A. baumannii transformants (∼3 × 106 CFU). Three hours after inoculation, the mice were intraperitoneally treated with either imipenem (40 mg/kg of body weight, every 8 h) or the equivalent volume of PBS for 2 days. Their survival was recorded. In separate experiments, mice were sacrificed at different time points after inoculation to determine the bacterial counts in the lung. These mice were anesthetized and sacrificed in a CO2 chamber. The whole lungs were removed, weighed, and homogenized in 1 ml of PBS. Homogenates were serially diluted and plated onto MacConkey agar to determine the A. baumannii and E. coli colony counts.
Statistical analysis.
Continuous variables were analyzed by one-way analysis of variance (ANOVA), followed by the Scheffe posthoc test or Student's t tests. Mouse mortality was analyzed with the Kaplan-Meier survival analysis (log rank test). Analyses were performed using SPSS (Statistical Package for the Social Sciences) version 18.0 (Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
RESULTS
Sheltering effect of CHDL-producing A. baumannii.
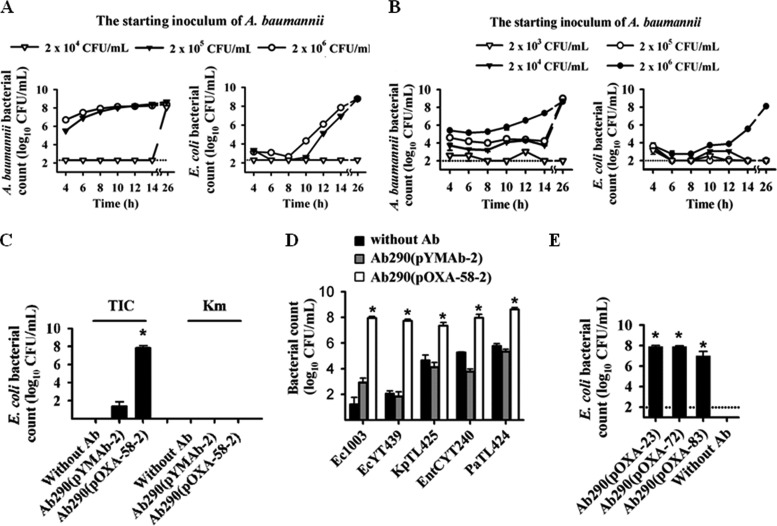
Isolates from the first patients (see Table S1 in the supplemental material), which included a carbapenem-resistant A. baumannii (CRAb) (Ab1969) and a carbapenem-susceptible E. coli (Ec1003), were used to demonstrate the carbapenem sheltering effect. Strain Ec1003 could survive only in carbapenem-containing broth (imipenem, 8 mg/liter) in the presence of strain Ab1969 (Fig. 1A). The Ab1969 strain carried a carbapenemase-encoding gene, blaOXA-58. Upstream of blaOXA-58 was ISAba3, which was inserted by IS1008 (IS1008–ΔISAba3-blaOXA-58). The ISAba3 sequence provides a promoter (P1) for blaOXA-58 expression (25). Together, these elements conferred resistance to ticarcillin (MIC, 256 mg/liter) but not to carbapenem (MIC, 1 mg/liter). Insertion of IS1008 in ISAba3 generates a stronger hybrid promoter (P2) for blaOXA-58 expression. The construct IS1008–ΔISAba3-blaOXA-58 has been shown to confer carbapenem resistance (MIC, 64 mg/liter) and a higher level of ticarcillin resistance (MIC > 1,024 mg/liter) than construct ΔISAba3-blaOXA-58 (with deletion of P2) confers (25). Strain Ab1969 also carries an intrinsic blaOXA-66 that lacks ISAba1 as the immediate upstream element (26). Thus, blaOXA-66 was not overexpressed in this strain and, therefore, was not responsible for the carbapenem resistance in this isolate (26). No other known class A or B carbapenemase gene was detected.
FIG 1.
Carbapenem-resistant Acinetobacter baumannii producing carbapenem-hydrolyzing class D β-lactamases (CHDLs) confer shelter to carbapenem-susceptible bacteria. (A and B) A carbapenem-susceptible Escherichia coli strain (Ec1003) (104 CFU/ml) was cocultured with different amounts of a clinical A. baumannii isolate producing OXA-58 (Ab1969) (A) or an A. baumannii transformant producing OXA-58 [termed Ab290(pOXA-58-2)] (B) in the presence of imipenem. In panel B, although A. baumannii Ab290(pOXA-58-2) could survive under the culture condition at an inoculum of 104 CFU/ml or higher (left panel), E. coli Ec1003 was protected from carbapenem killing only at 106 CFU/ml of Ab290(pOXA-58-2) (right panel). (C) At 106 CFU/ml, Ab290(pOXA-58-2) sheltered Ec1003 against ticarcillin (TIC) but not kanamycin (Km) killing. The control Ab290(pYMAb-2) transformant, which carries an empty shuttle vector, failed to exert the sheltering effect. The experiment was also performed without A. baumannii (Without Ab). (D) Sheltering effect of A. baumannii Ab290(pOXA-58-2) for other Gram-negative bacteria against carbapenem-induced death. The first two or four letters of the strain designations indicate the species as follows: Ec, E. coli; Kp, Klebsiella pneumoniae; EntC, Enterobacter cloacae; Pa, Pseudomonas aeruginosa. (E) Ab290 transformants producing other CHDL proteins, including OXA-23, -72, and -83, sheltered Ec1003 against carbapenem-induced death. Each symbol in panels A and B is the mean of triplicate results. The dotted lines in panels A, B, and E indicate the detection limit. The bars and error bars in panels C to E indicate the means and standard deviations, respectively, of triplicate tests. Values that are significantly different (P < 0.05) are indicated by an asterisk.
Next, we sought to determine whether OXA-58, but not other factors from strain Ab1969, is responsible for sheltering strain Ec1003 from carbapenem killing. Ab290(pOXA-58-2) is a transformant carrying an A. baumannii-E. coli shuttle vector (pYMAb-2) harboring IS1008-ΔISAba3-blaOXA-58. When Ab290(pOXA-58-2) was cocultured with Ec1003 in the presence of imipenem, Ec1003 was sheltered from carbapenem killing in the presence of a high inoculum of Ab290(pOXA-58-2) (2 × 106 CFU/ml; Fig. 1B and Fig. S1 in the supplemental material). In contrast, the Ab290 transformant bearing the shuttle vector pYMAb-2 failed to exert a sheltering effect (Fig. 1D). Ec1003 cells recovered from the plate cocultured with either strain Ab1969 or Ab290(pOXA-58-2) retained their susceptibility to imipenem after subculture in the absence of carbapenem-resistant Acinetobacter strains. In addition, PCR also did not detect blaOXA-58 in these Ec1003 cells (data not shown), indicating that the growth of these Ec1003 cells in the presence of carbapenem was not due to spontaneous genetic changes or to the acquisition of resistant determinants, including blaOXA-58.
Ab290(pOXA-58-2) also resisted ticarcillin (conferred by blaOXA-58) and kanamycin (conferred by aph on the shuttle vector). However, while this variant exhibited a sheltering effect against ticarcillin, it did not protect Ec1003 against kanamycin-induced death (Fig. 1C). These results demonstrate the presence of a specific sheltering effect conferred by OXA-58 expression in the Ab290 variant. The carbapenem sheltering effect of Ab290(pOXA-58-2) could also be demonstrated using another carbapenem-susceptible E. coli strain (EcYT439), as well as Klebsiella pneumoniae (strain KpTL425), Enterobacter cloacae (strain EntC YT240), and P. aeruginosa (strain PaTL424) (Fig. 1D; see Fig. S1 in the supplemental material). Ab290 transformants producing other proteins in the CHDL family, including OXA-23, -72, and -83, were also shown to exert a similar carbapenem sheltering effect (Fig. 1E).
Detection of active OXA-58 in the extracellular fraction of A. baumannii overproducing OXA-58.
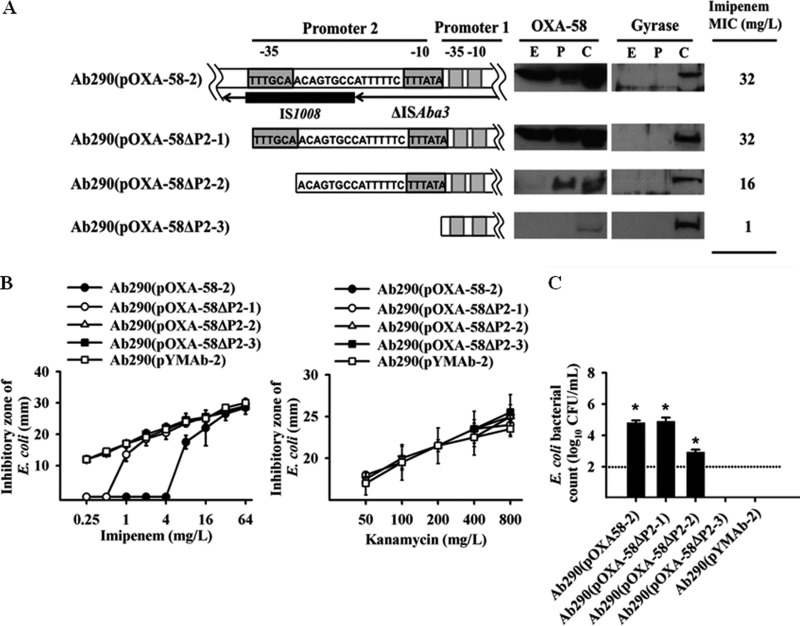
We have shown that the CRAb sheltering effect could be demonstrated in planktonic cultures. Therefore, we hypothesized that OXA-58 is released extracellularly by the CRAb strain to confer carbapenem shelter to susceptible bacteria. Western blot analysis confirmed the presence of extracellular OXA-58 in A. baumannii Ab290(pOXA-58-2) cultures (Fig. 2A), but not Ab290(pYMAb-2) cultures (see Fig. S2 in the supplemental material). Other CHDL proteins, including OXA-83, OXA-72, and OXA-23 could also be detected in the extracellular fraction of transformants producing these enzymes (Fig. S2). This extracellular OXA-58 likely resulted from cellular secretion or leakage, as opposed to cell lysis, as indicated by the absence of gyrase immunoreactivity that is typically observed in the bacterial cytoplasmic fraction (Fig. 2A).
FIG 2.
Detection of active CHDL in extracellular fractions from A. baumannii cells highly expressing OXA-58. (A) OXA-58 was detected in the extracellular fraction of A. baumannii Ab290(pOXA-58-2) by Western blotting. Constructs carrying a deletion at promoter 2 (P2) are demonstrated in the schematic map. Deletion of the −35 component of P2, or the entire P2, drastically diminished extracellular levels of OXA-58. The extracellular fraction (E), periplasmic fraction (P), and cytoplasmic fraction (C) of the transformants are indicated in the gels. (B) The imipenem (IPM) hydrolytic activity of several transformants was detected using a bioassay, in which failure of the transformants to hydrolyze kanamycin indicated specific IPM hydrolysis by OXA-58. (C) The sheltering effect of carbapenem-resistant A. baumannii (CRAb) was associated with a high level of OXA-58 expression. Colony counts were determined after 6-h cultures to determine the difference in the sheltering effect by transformants harboring different deleted promoters. Values that are significantly different (P < 0.05) are indicated by an asterisk. The dotted line indicates the detection limit. In panels B and C, data are presented as the means and standard deviations (error bars) of triplicate tests.
OXA-58 production by the hybrid promoter (P2) driving IS1008-ΔISAba3 was required for the presence of extracellular OXA-58 (Fig. 2A). In A. baumannii Ab290(pOXA-58ΔP2-3) cells, in which the P1 blaOXA-58 promoter was retained by deletion of the P2 promoter, the concentration of OXA-58 in the cytoplasmic fraction was decreased, and OXA-58 immunoreactivity was not detected in the periplasmic and extracellular fractions. The extracellular fraction of Ab290(pOXA-58-2) could hydrolyze imipenem (Fig. 2B), but not kanamycin (Fig. 2B), indicating the specificity of the hydrolytic activity of extracellular OXA-58. This hydrolytic activity was positively correlated with the amount of OXA-58 that was overexpressed (Fig. 2B). No imipenem hydrolysis was observed in the extracellular fraction of Ab290(pOXA-58ΔP2-3), in which the imipenem inhibitory zone was comparable to that of the Ab290(pYMAb-2) control. The coculture data also indicated a crucial role for OXA-58 production by hybrid promoter P2 in the CRAb sheltering effect (Fig. 2C).
Treatment with antimicrobial agents increases extracellular CHDL.
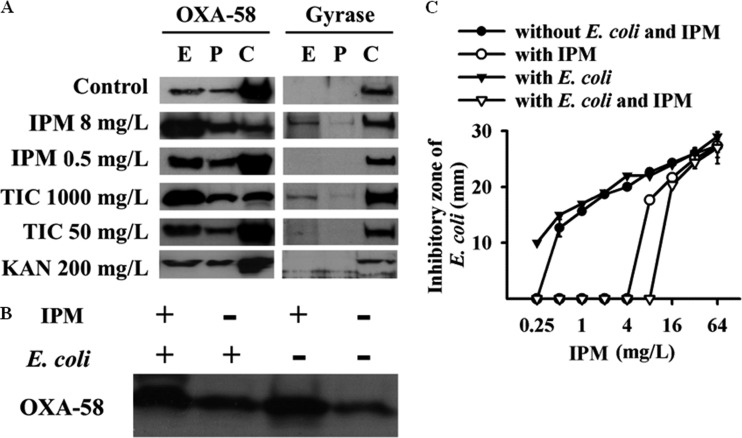
The amount of extracellular OXA-58 of A. baumannii Ab290(pOXA-58-2) was increased in the presence of higher concentrations of OXA-58 substrate, including imipenem (8 mg/liter) and ticarcillin (1,000 mg/liter), but not in the presence of higher concentrations of kanamycin (Fig. 3A). This increased OXA-58 may, in part, be attributed to cell lysis, as indicated by the presence of extracellular gyrase. No augmentation of the extracellular OXA-58 from Ab290(pOXA-58-2) was detected when this strain was cocultured in planktonic culture with E. coli, as indicated by the results of Western blot analysis (Fig. 3B) and the imipenem hydrolysis assay (Fig. 3C).
FIG 3.
Extracellular OXA-58 is increased in the presence of antimicrobials in CRAb cultures. (A) Extracellular OXA-58 in A. baumannii Ab290(pOXA-58-2) cultures was increased in the presence of higher concentrations of imipenem (IPM) and ticarcillin (TIC), but not kanamycin (KAN).The extracellular fraction (E), periplasmic fraction (P), and cytoplasmic fraction (C) of the transformants are indicated. (B and C) The presence of E. coli (Ec1003) did not augment the extracellular release of OXA-58, as demonstrated by Western blotting (B) and IPM hydrolysis assay (C). In panel C, values are means and standard deviations (error bars) of triplicate tests.
In vivo sheltering effect and indirect pathogenic role of CHDL-producing A. baumannii.
In the murine model, E. coli Ec1003 alone was more virulent than either A. baumannii Ab290(pYMAb-2) or Ab290(pOXA-58-2) alone, with LD50 values of 1.795 × 104, 1.563 × 106, and 7.096 × 107 CFU, respectively. However, only Ab290(pOXA-58-2) (with inoculums of ∼3 × 106 CFU), but not Ec1003 (∼3 × 104 CFU) or Ab290(pYMAb-2) (∼3 × 106 CFU), was able to survive in imipenem-treated mice for 48 h after inoculation (data not shown).
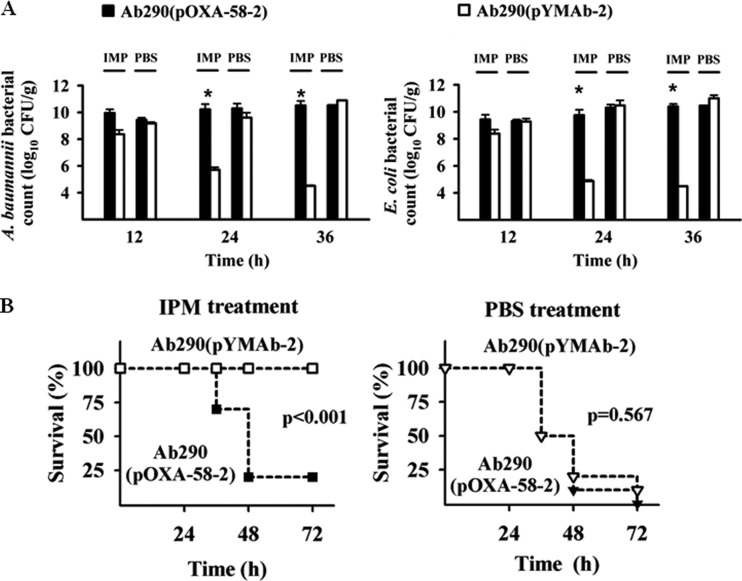
In the polymicrobial model mice, the amount of E. coli Ec1003 in mice coinfected with A. baumannii Ab290(pOXA-58-2) was significantly higher than in those coinfected with Ab290(pYMAb-2) at 24 and 36 h after inoculation in the imipenem-treated group (Fig. 4A). The amount of Ec1003 in mice coinfected with Ab290(pOXA-58-2) did not differ between those treated with imipenem or PBS (Fig. 4A). These results indicate that Ab290 expressing OXA-58 demonstrated an in vivo sheltering effect for Ec1003.
FIG 4.
A. baumannii producing OXA-58 exhibits an in vivo sheltering effect. Mice were coinfected with E. coli Ec1003 and A. baumannii Ab290(pOXA-58-2) or Ec1003 and Ab290(pYMAb-2), and treated with IPM or PBS 3 h later. The numbers of bacteria in the mouse lung and the mortality rates of mice were determined at different time points after inoculation. (A) A. baumannii Ab290(pOXA-58-2) shelters E. coli Ec1003 from IPM killing in vivo. (B) Together, these bacteria exhibited enhanced pathogenicity and resulted in higher mortality. The bars and error bars in panel A indicate the means and standard deviations of triplicate tests. *, P < 0.05.
In the imipenem-treated groups (Fig. 4B), the 72-h mortality was significantly higher in mice coinfected with E. coli Ec1003 and A. baumannii Ab290(pOXA-58-2) (80%) or Ab290(pOXA-72) (50% [see Fig. S3 in the supplemental material]) than in those coinfected with Ec1003 and Ab290(pYMAb-2) (0%) or in those that received monomicrobial Ab290(pOXA-58-2) (0% [data not shown]). Taken together, these results indicate that the in vivo imipenem sheltering effect of Ab290(pOXA-58-2) for Ec1003 was associated with enhanced pathogenicity in polymicrobial, compared to monomicrobial, CRAb infection during imipenem therapy.
DISCUSSION
In this study, we demonstrated a novel role for CRAb in the clinical setting in addition to its impact in severely ill patients (11). In polymicrobial infection, CRAb may shelter carbapenem-susceptible bacteria from carbapenem killing. In a murine pneumonia model, polymicrobial infection resulting from inoculation with CRAb and a carbapenem-susceptible, but more virulent, pathogen displayed higher pathogenicity than did a monomicrobial infection caused by either pathogen alone during carbapenem therapy. This result has emphasized the importance of early identification of CRAb in polymicrobial infection and highlighted the necessity of antibiotic regimens that can eradicate CRAb in polymicrobial infections.
In this study, the sheltering effect of CRAb could be attributed, at least in part, to extracellularly released carbapenem-hydrolyzing CHDLs. Bacteria can also provide antimicrobial resistance to susceptible recipients through horizontal gene transfer (15), formation of biofilm (27), the conveyance of antimicrobial inactivation enzymes through type VI secretion systems (28), membrane vesicles (29), or nanotube connections (30), and the production of volatile compounds (e.g., ammonia) to reduce the membrane permeability of susceptible recipients (31). The advantage of the mechanism we describe (i.e., sheltering conferred by extracellular CHDL) is that even relatively remote bacteria can be protected.
Extracellular β-lactamases, including those found in the biofilm matrix, were previously considered to be the result of cell lysis after exposure to antimicrobials (27). Our results indicate that the CHDLs were extracellularly released in the absence of antimicrobials. The extracellular CHDLs were not the results of cell lysis, as indicated by the absence of gyrase. This extracellular release of CHDLs was intimately linked to their high expression with a strong promoter. Although the exact mechanism is currently undetermined, the extracellular release of highly expressed periplasmic proteins has been demonstrated previously (32, 33). The maintenance of some extracellular CHDLs might act as a front-line protective strategy for A. baumannii to hydrolyze relevant antimicrobials, such as carbapenem or ticarcillin, before they reach the cells. We observed that an increase in the concentrations of these antimicrobials can trigger cell lysis and increase the extracellular CHDL concentration, which represents a more rapid and effective method to cope with large amounts of antimicrobial challenges.
We did not determine whether the carbapenem sheltering effect depended on additional mechanisms in the cocultured susceptible bacteria, such as changes in membrane permeability or increased expression of efflux pumps. In planktonic cultures, the sheltering effect for the cocultured susceptible bacteria might represent an indirect and passive effect of the CRAb self-defense mechanism, because the presence of E. coli did not significantly augment the amount of the extracellular CHDLs. Another explanation for this result is that the limited sensitivity of our detection methods may have failed to discriminate subtle differences in CHDL levels.
Interestingly, the kanamycin-resistant determinant did not display similar sheltering effects. One reason for this finding may be that the kanamycin-resistant determinants did not secrete into the extracellular space. Another explanation may be the high energy cost for aminoglycoside modification, which would inhibit the extensive inactivation of aminoglycoside (34).
Previous studies have not consistently demonstrated that β-lactamase-producing bacteria (BLPB) can shelter susceptible partners from β-lactam killing (17, 35–37). The BLPB sheltering effect may vary with the type of β-lactamase produced, as well as with the microorganisms that produce them (37). In the current study, we demonstrated that the inoculum of CRAb and the level of CHDL expression play deterministic roles in the sheltering effect.
In our model, the susceptible bacteria, including A. baumannii, are not eradicated in the mice after treatment with imipenem for 36 h. However, all the mice that survived 48 h of imipenem treatment had their carbapenem-susceptible pathogen eradicated (data not shown). The survival of carbapenem-susceptible A. baumannii in the lungs of mice treated with imipenem (120 mg/kg/day, divided into 3 doses) had also been demonstrated in a previous study which used the same pneumonia model (38). The mucin included in the inoculation might hinder the clearance of these susceptible pathogens.
In conclusion, we have demonstrated that CRAb plays a novel role in polymicrobial infection by sheltering carbapenem-susceptible pathogens, thereby exacerbating the pathogenesis of polymicrobial infection during carbapenem therapy. These results indicate a greater importance for the presence of CRAb in clinical settings and suggest a need for more-aggressive controls of CRAb in polymicrobial infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Chan-Jung Li for his technical support in the animal study.
Te-Li Chen is a medical advisor of TTY Biopharm. All other authors have no conflicts to report.
This work was supported by grants from the Taipei Veterans General Hospital (V101E4-003, V101A-017, and V101C-021), the National Science Council (101-2314-B-010-027-MY3), and the National Health Research Institute (IV-101-PP-12).
Footnotes
Published ahead of print 5 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02636-13.
REFERENCES
- 1.Singer M. 2010. Pathogen-pathogen interaction: a syndemic model of complex biosocial processes in disease. Virulence 1:10–18. 10.4161/viru.1.1.9933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters BM, Jabra-Rizk MA, O'May GA, Costerton JW, Shirtliff ME. 2012. Polymicrobial interactions: impact on pathogenesis and human disease. Clin. Microbiol. Rev. 25:193–213. 10.1128/CMR.00013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakaletz LO. 2004. Developing animal models for polymicrobial diseases. Nat. Rev. Microbiol. 2:552–568. 10.1038/nrmicro928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armbruster CE, Hong W, Pang B, Weimer KE, Juneau RA, Turner J, Swords WE. 2010. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio 1(3):e00102–10. 10.1128/mBio.00102-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittet D, Li N, Wenzel RP. 1993. Association of secondary and polymicrobial nosocomial bloodstream infections with higher mortality. Eur. J. Clin. Microbiol. Infect. Dis. 12:813–819. 10.1007/BF02000400 [DOI] [PubMed] [Google Scholar]
- 6.Zheng YL, Wan YF, Zhou LY, Ye ML, Liu S, Xu CQ, He YQ, Chen JH. 2013. Risk factors and mortality of patients with nosocomial carbapenem-resistant Acinetobacter baumannii pneumonia. Am. J. Infect. Control 41:e59–e63. 10.1016/j.ajic.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 7.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5:939–951. 10.1038/nrmicro1789 [DOI] [PubMed] [Google Scholar]
- 9.Poirel L, Nordmann P. 2006. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin. Microbiol. Infect. 12:826–836. 10.1111/j.1469-0691.2006.01456.x [DOI] [PubMed] [Google Scholar]
- 10.Higgins PG, Poirel L, Lehmann M, Nordmann P, Seifert H. 2009. OXA-143, a novel carbapenem-hydrolyzing class D beta-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:5035–5038. 10.1128/AAC.00856-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YT, Kuo SC, Yang SP, Lin YT, Tseng FC, Chen TL, Fung CP. 2012. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin. Infect. Dis. 55:209–215. 10.1093/cid/cis385 [DOI] [PubMed] [Google Scholar]
- 12.Lee YC, Huang YT, Tan CK, Kuo YW, Liao CH, Lee PI, Hsueh PR. 2011. Acinetobacter baumannii and Acinetobacter genospecies 13TU and 3 bacteraemia: comparison of clinical features, prognostic factors and outcomes. J. Antimicrob. Chemother. 66:1839–1846. 10.1093/jac/dkr200 [DOI] [PubMed] [Google Scholar]
- 13.Trottier V, Namias N, Pust DG, Nuwayhid Z, Manning R, Marttos AC, Jr, Dunham MB, Schulman CI, McKenney MG. 2007. Outcomes of Acinetobacter baumannii infection in critically ill surgical patients. Surg. Infect. (Larchmt.) 8:437–443. 10.1089/sur.2006.029 [DOI] [PubMed] [Google Scholar]
- 14.Levin BR, Rozen DE. 2006. Non-inherited antibiotic resistance. Nat. Rev. Microbiol. 4:556–562. 10.1038/nrmicro1445 [DOI] [PubMed] [Google Scholar]
- 15.Zarrilli R, Vitale D, Di Popolo A, Bagattini M, Daoud Z, Khan AU, Afif C, Triassi M. 2008. A plasmid-borne blaOXA-58 gene confers imipenem resistance to Acinetobacter baumannii isolates from a Lebanese hospital. Antimicrob. Agents Chemother. 52:4115–4120. 10.1128/AAC.00366-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rumbo C, Fernandez-Moreira E, Merino M, Poza M, Mendez JA, Soares NC, Mosquera A, Chaves F, Bou G. 2011. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55:3084–3090. 10.1128/AAC.00929-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brook I. 2009. The role of beta-lactamase-producing-bacteria in mixed infections. BMC Infect. Dis. 9:202. 10.1186/1471-2334-9-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen TL, Siu LK, Wu RC, Shaio MF, Huang LY, Fung CP, Lee CM, Cho WL. 2007. Comparison of one-tube multiplex PCR, automated ribotyping and intergenic spacer (ITS) sequencing for rapid identification of Acinetobacter baumannii. Clin. Microbiol. Infect. 13:801–806. 10.1111/j.1469-0691.2007.01744.x [DOI] [PubMed] [Google Scholar]
- 19.Chen TL, Chang WC, Kuo SC, Lee YT, Chen CP, Siu LK, Cho WL, Fung CP. 2010. Contribution of a plasmid-borne blaOXA-58 gene with its hybrid promoter provided by IS1006 and an ISAba3-like element to beta-lactam resistance in Acinetobacter genomic species 13TU. Antimicrob. Agents Chemother. 54:3107–3112. 10.1128/AAC.00128-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YT, Huang LY, Chiang DH, Chen CP, Chen TL, Wang FD, Fung CP, Siu LK, Cho WL. 2009. Differences in phenotypic and genotypic characteristics among imipenem-non-susceptible Acinetobacter isolates belonging to different genomic species in Taiwan. Int. J. Antimicrob. Agents 34:580–584. 10.1016/j.ijantimicag.2009.06.027 [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—9th ed. M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing: 24th informational supplement M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 23.Valenzuela JK, Thomas L, Partridge SR, van der Reijden T, Dijkshoorn L, Iredell J. 2007. Horizontal gene transfer in a polyclonal outbreak of carbapenem-resistant Acinetobacter baumannii. J. Clin. Microbiol. 45:453–460. 10.1128/JCM.01971-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen-Chih Wu R, Shaio MF, Cho WL. 2007. A p38 MAP kinase regulates the expression of the Aedes aegypti defensin gene in mosquito cells. Insect Mol. Biol. 16:389–399. 10.1111/j.1365-2583.2007.00734.x [DOI] [PubMed] [Google Scholar]
- 25.Chen TL, Wu RC, Shaio MF, Fung CP, Cho WL. 2008. Acquisition of a plasmid-borne blaOXA-58 gene with an upstream IS1008 insertion conferring a high level of carbapenem resistance to Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:2573–2580. 10.1128/AAC.00393-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, Pitt TL. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 258:72–77. 10.1111/j.1574-6968.2006.00195.x [DOI] [PubMed] [Google Scholar]
- 27.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35:322–332. 10.1016/j.ijantimicag.2009.12.011 [DOI] [PubMed] [Google Scholar]
- 28.Tashiro Y, Yawata Y, Toyofuku M, Uchiyama H, Nomura N. 2013. Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes Environ. 28:13–24. 10.1264/jsme2.ME12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaar V, Nordstrom T, Morgelin M, Riesbeck K. 2011. Moraxella catarrhalis outer membrane vesicles carry beta-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob. Agents Chemother. 55:3845–3853. 10.1128/AAC.01772-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubey GP, Ben-Yehuda S. 2011. Intercellular nanotubes mediate bacterial communication. Cell 144:590–600. 10.1016/j.cell.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 31.Bernier SP, Letoffe S, Delepierre M, Ghigo JM. 2011. Biogenic ammonia modifies antibiotic resistance at a distance in physically separated bacteria. Mol. Microbiol. 81:705–716. 10.1111/j.1365-2958.2011.07724.x [DOI] [PubMed] [Google Scholar]
- 32.Pages JM, Anba J, Lazdunski C. 1987. Conditions leading to secretion of a normally periplasmic protein in Escherichia coli. J. Bacteriol. 169:1386–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazzaroni JC, Atlan D, Portalier RC. 1985. Excretion of alkaline phosphatase by Escherichia coli K-12 pho constitutive mutants transformed with plasmids carrying the alkaline phosphatase structural gene. J. Bacteriol. 164:1376–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connell HA, Kottkamp GS, Eppelbaum JL, Stubblefield BA, Gilbert SE, Gilbert ES. 2006. Influences of biofilm structure and antibiotic resistance mechanisms on indirect pathogenicity in a model polymicrobial biofilm. Appl. Environ. Microbiol. 72:5013–5019. 10.1128/AEM.02474-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renneberg J, Walder M. 1989. The role of beta-lactamase in mixed infections in mice in relation to treatment with ampicillin. J. Infect. Dis. 160:337–341. 10.1093/infdis/160.2.337 [DOI] [PubMed] [Google Scholar]
- 36.Kataoka D, Fujiwara H, Kawakami T, Tanaka Y, Tanimoto A, Ikawa S. 2003. The indirect pathogenicity of Stenotrophomonas maltophilia. Int. J. Antimicrob. Agents 22:601–606. 10.1016/S0924-8579(03)00244-9 [DOI] [PubMed] [Google Scholar]
- 37.Westman E, Lundin S, Hermansson A, Melhus A. 2004. Beta-lactamase-producing nontypeable Haemophilus influenzae fails to protect Streptococcus pneumoniae from amoxicillin during experimental acute otitis media. Antimicrob. Agents Chemother. 48:3536–3542. 10.1128/AAC.48.9.3536-3542.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Hernandez MJ, Pachon J, Pichardo C, Cuberos L, Ibanez-Martinez J, Garcia-Curiel A, Caballero FJ, Moreno I, Jimenez-Mejias ME. 2000. Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J. Antimicrob. Chemother. 45:493–501. 10.1093/jac/45.4.493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.