Abstract
Alcohol abuse changes behavior and can induce major mood disorders such as depression. Recent evidence in pre-clinical rodent models and humans now supports the conclusion that the innate immune system is an important physiological link between alcoholism and major depressive disorders. Deficiency of toll-like receptor 4 (TLR4), a protein that has been known to immunologists for 50 years, not only prevents lipopolysaccaride (LPS)-induced sickness behavior but recently has been demonstrated to induce resistance to chronic alcohol ingestion. Activation of the immune system by acute administration of LPS, a TLR4 agonist, as well as chronic infection with Bacille Calmette-Guérin, (BCG) causes development of depressive-like behaviors in pre-clinical rodent models. Induction of an enzyme expressed primarily in macrophages and microglia, 2,3 indoleamine dioxygenase, shunts tryptophan catabolism to form kynurenine metabolites. This enzyme is both necessary and sufficient for expression of inflammation-induced depressive-like behaviors in mice. New findings have extended these concepts to humans by showing that tryptophan catabolites of 2,3 indoleamine dioxygenase are elevated in the cerebrospinal fluid of cancer patients treated with the recombinant cytokine interferon-α. The remarkable conservation from mice to humans of the impact of inflammation on mood emphasizes the ever-expanding role for cross-talk among diverse physiological symptoms that are likely to be involved in the pathogenesis of alcohol abuse. These findings present new and challenging opportunities for scientists who are engaged in brain, behavior and immunity research.
Keywords: Alcoholism; Pro-inflammatory Cytokines; 2,3 Indoleamine 2,3 Dioxygenase; Kynurenine; Depressive-like Behavior; Sickness Behavior
Alcohol Abuse Causes Activation of Immune to Brain Inflammatory Signals
Alcohol intoxication is well known to affect the liver, but the brain is also a target. It is therefore not surprising that alcohol addiction shares a high rate of co-morbidity with numerous mood disorders (Kessler et al., 1994), with symptoms of depression being the most common (Swendsen et al., 1998). Even after years of research in humans and animals, using models of acute and chronic exposure to alcohol as well as withdrawal from alcohol, there is no real consensus as to whether alcoholism predisposes to development of depression or vice versa (reviewed in (Spanagel, 2009)). In reality, the line that connects alcoholism to depression probably has arrows that point both ways. During the past few years, a new player has emerged in the field of alcohol abuse and alcoholism. Surprisingly, that player is the immune system in the form of systemic and CNS inflammation (Irwin and Miller, 2007). Among other biochemical changes, recent evidence has established that the brains of alcoholics display an increase in monocyte chemoattractant protein-1 (CCL2) and in well accepted region-specific microglial markers such as ionized calcium binding adaptor protein-1 (Iba-1) (He and Crews, 2008). Systemic concentrations of cytokines are increased in alcoholic subjects (Achur et al., 2010; He and Crews, 2008; He et al., 2009). Similarly, a very conventional marker of systemic inflammation, high sensitivity C-reactive protein (hs-CRP), is elevated in men who consume excessive amounts (>280 g/day) of alcohol (Alho et al., 2004; Liukkonen et al., 2006). These same observations have been described in men, but not women, with clinical depression (Liukkonen et al., 2006). As might be expected, subjects in this Liukkonen et al. study who were heavy drinkers accounted for a significant amount of variability between hs-CRP and clinical depression. Finally, alcohol abuse not only induces inflammation in the body and brain, but it also causes significant changes in immunity (Irwin and Miller, 2007) and increases susceptibility to a variety of infections, particularly those of pulmonary origin (Zhang et al., 2008).
In many ways, these exciting discoveries about relationships between alcohol abuse, behavior and the immune system have followed a route that mirrors earlier developments in the field of brain, behavior and immunity. Initial findings in this area focused on the role of stress hormones on immune responses that affect host resistance to infectious, autoimmune and neoplastic diseases. In a colloquial sense, this was a top-down, neuroendocrine to immune system loop that involves stress hormones like glucocorticoids, norepinephrine, epinephrine, growth hormone and prolactin as well as expression of their receptors on leukocytes. Subsequently, scientists in this field discovered that products of an activated immune system, most generally characterized as cytokines produced by cells of the immune system, change the behavior of animals and humans. This was an example of a bottom-up, immune system to central nervous system loop. These very same issues have been investigated in the field of alcoholism research, with an emphasis on gene by environment interactions related mostly to acute and chronic stress (Spanagel, 2009). It is therefore amazing that very few scientists who are members of the Psychoneuroimmunology Research Society (PNIRS), the official scientific organization that sponsors this journal, have been actively involved in research aimed at alcohol abuse. This oversight has occurred even though two innovative scientists and their collaborators, Professor Anna Taylor and Professor Michael Irwin, were some of the earliest pioneers in the PNIRS community to study alcohol abuse. Professor Taylor used rodent models to describe the neuroimmune effects of fetal alcohol exposure (Nelson et al., 1986; Taylor et al., 1981), whereas Professor Irwin published data showing that alcoholism reduces natural killer cell activity and causes some symptoms of depression (e.g., sleep disturbances, reviewed in (Irwin and Rinetti, 2004)). Similarly, his group has recently reported that administration of the TNF antagonist, etanercept, causes large reductions in rapid eye movement sleep in abstinent, alcohol-dependent men (Irwin et al, 2009). This observation is important because increases in rapid eye movement sleep are often related to alcohol relapse.
Early Discoveries Relating Systemic Inflammation to Neuroinflammation
The field of brain, behavior and immunity is an integrative discipline that investigates reciprocal systems of communication between the brain and immune system. An important cornerstone in this discipline was published in the late 1980s. Those data established that the first recombinant cytokine, IL-1, given systemically not only induces fever but that it also activates the hypothalamic-pituitary-adrenal (HPA) axis (Berkenbosch et al., 1987; Sapolsky et al., 1987). At that time, the major focus of research in the field was still aimed at determining how hormones and neurotransmitters affect cells of the immune system (Kelley et al., 2007; Malarkey and Mills, 2007). The idea was that the endotoxin lipopolysaccharide (LPS), which is a component of the outer membrane of many types of gram-negative microorganisms, induces the synthesis and release of IL-1 from mononuclear myeloid cells into the bloodstream. This systemic IL-1 somehow communicates with the central nervous system to activate the HPA axis, thereby providing negative feedback to inhibit exuberant immune responses. Following these ground-breaking findings, the first discovery that IL-1 binding sites are expressed in the brain was published (Farrar et al., 1987), with the binding sites being found mostly in the hippocampus, dentate gyrus and choroid plexus. These data were quickly confirmed using more specific neuroanatomical approaches (Ban et al., 1991; Cunningham et al., 1992). Subsequently, expression of steady-state mRNA for both types of IL-1 receptors in the brain and pituitary was reported (Cunningham et al., 1992; French et al., 1999; Parnet et al., 1994; Parnet et al., 1993), and immunohistochemistry with specific antibodies for IL-1 receptors confirmed that the IL-1 receptor proteins were expressed at the protein level (French et al., 1999; French et al., 1996). Activation of IL-1 receptors in the periphery communicates with these IL-1 receptors in the brain and causes changes in motivated behaviors, such as reward-based food consumption and social interactions (Kent et al., 1992a; Kent et al., 1994). These changes in motivational behavior caused by acute systemic inflammation occur along with activation of the HPA axis and other indices of acute sickness, including fever, sleepiness and a general feeling of achiness (Watkins and Maier, 2000). Collectively, this constellation of physiological changes caused by acute activation of the immune system was coined as sickness behaviors (Dantzer and Kelley, 1989; Hart, 1988; Kelley, 1988; Kent et al., 1992b).
Inflammation Differs from Organ to Organ
Local inflammation was originally defined by the following clinical signs: heat (calor), redness (rubor), swelling (tumor/tugor), pain (dolor) and ultimately loss of function (functio laesa). As our understanding of inflammation has matured, the concept of inflammation has changed from its original Latin definition: the word inflammare means to set on fire. It is now recognized that there are several levels of inflammation. At a minimum, there is local inflammation, systemic inflammation, neuroinflammation and ultimately the systemic inflammatory response syndrome (SIRS) that can lead to ensuing sepsis and death (Kelley et al., 2011). Neuroinflammation is often considered to be the equivalent of inflammation in the periphery, but these two forms of inflammation are clearly different (Galea et al., 2007). For example, injection of LPS under the skin causes a rapid (2 h) influx of both polymorphonuclear cells and monocytes. Similarly, LPS injection into the ventricles of the brain causes a similar accumulation of inflammatory cells in the choroid plexus and meninges. However, this rapid myelomonocytic infiltration does not occur in the brain following parenchymal injection of LPS, although monocytes can accumulate at later times and in response to higher doses of intraparenchymal injection of LPS. Data such as these have led to the consensus that the role of IL-1 in the brain is different than it is in the periphery (Dantzer et al., 2008a). It should therefore be understood that neuroinflammation employs some of the same molecules and processes that occur in both local and systemic inflammation. However, the presence of IL-1 alone in brain tissue should not be considered as inflammation per se, particularly in the absence of functional CNS changes such as can be measured as outcomes in behavior.
Inflammation-Induced Changes in Mood in Humans
Type I interferon-α was the first human cytokine that was cloned (Nagata et al., 1980) and subsequently expressed in sufficient quantities in recombinant form to become available for clinical studies. This protein is an anti-viral agent that also inhibits tumor growth, and it was dubbed as the first most promising cancer treatment of the new biotechnology era of the 1980s. Interferon-α is currently approved by the US Food and Drug Association for treatment of multiple sclerosis, hepatitis B and C, malignant melanoma, basal cell carcinoma, actinic keratosis, papilloma and genital warts. Recognized side effects are neutropenia, flu-like symptoms, fever, headache, achiness, fatigue and clinical depression. Many of these symptoms have been described in sickness behavior. For example, Lucile Capuron described several features of depression caused by interferon-α and interleukin-2 in cancer patients (Capuron and Ravaud, 1999; Capuron et al., 2000). Andrew Miller and colleagues hypothesized that treatment with interferon-α of humans afflicted with malignant melanoma would cause depressive symptoms and that the selective serotonin reuptake inhibitor, paroxetine, would prevent this inflammation-induced depression (Musselman et al., 2001). During the first three months of treatment with interferon-α, 45% of the subjects developed symptoms of depression. Clinical symptoms were sufficiently severe in several of these subjects to contraindicate continued treatment with interferon-α, which was subsequently discontinued because of the risk of suicide in these patients. However, paroxetine treatment significantly reduced the proportion of subjects with depressive-like behavior to 11% and increased the number of subjects who were able to complete a full 12-week treatment with interferon-α. Subsequent psychiatric developments in clinical depression induced by interferon-α have been reviewed (Miller, 2009; Raison et al., 2006).
It is important to note that inflammation-induced mood changes can be caused by much less radical treatments than systemic injection of cytokines. For example, a simple vaccination with an innocuous typhoid antigen promotes decrements in neurovegetative mood, and this negative mood is associated with a rise in IL-6 following vaccination (Harrison et al., 2009a; Harrison et al., 2009b; Wright et al., 2005). Immunization for typhoid also synergizes with psychological stress to promote greater decrements in mood (Brydon et al., 2009). Similarly, injection of a low-dose LPS that increased proinflammatory cytokines levels in a safe manner in volunteers also enhanced self-rated and observer-rated depressed mood and reduced ventral striatal responses to a monetary reward (Eisenberger et al., 2010).
Pre-clinical Rodent Models of Depressive-like Behavior
Cloning and expression of recombinant cytokines for humans, such as interferon-α, IL-1 and IL-2, permitted early clinical trials, mostly as potential treatments for a variety of cancers. Invariably, systemic treatment with these recombinant cytokines in cancer patients caused flu-like symptoms and neuropsychiatric side-effects (reviewed in (Dantzer and Kelley, 1989)). In 1996, Raz Yirmiya made a seminal observation by connecting the dots that linked an activated immune system to depressive-like behavior in rodents (Musselman et al., 2001; Yirmiya, 1996). There are a number of models of depressive-like behaviors in rodent systems that have predictive ability for development of anti-depressants as well as face validity (Nestler and Hyman, 2010). By using reward-based behaviors with face validity in male rats, such as preference for sucrose and arousing sexual stimuli with a receptive female, these experiments revealed that systemic injection of the cytokine inducer (LPS) reduced both types of behaviors four h later. Chronic, but not acute, injections of the tricyclic antidepressant imipramine reversed the LPS-induced decrease in sucrose consumption. However, sickness behaviors that were caused by systemic injection of LPS, including anorexia, loss of body weight, investigation of a novel juvenile, ambulation and rearing, remained significantly lower in imipramine-treated rats.
When using rodent models of depressive-like behavior, it is important to dissociate symptoms of sickness when measuring depressive-like behaviors. This is because the early stages of sickness behavior are accompanied by a reduction in appetite and motor activity that can interfere with performance in behavioral tests of depression (Dunn and Swiergiel, 2005). We therefore extended these early findings by measuring murine behaviors not only at a time point when locomotor behavior is maximally depressed (6 h) but at a point in time after these sickness behaviors had subsided (Frenois et al., 2007). As hypothesized, line crossings and rearings were decreased whereas duration of immobility in two predictive tests of clinical efficacy of antidepressant drugs, the tail suspension test and the forced swim test, were increased 6 h following an intraperitoneal injection of LPS. However, the changes in motor activity normalized 24 h after LPS even though the increased duration of immobility in both tests of depressive-like behavior remained. In the same manner, preference to consume sucrose rather than water remained lower at both 24 and 48 h following systemic injection of LPS even though food intake returned to normal. These data were subsequently confirmed and extended by showing that aged mice fail to recover from LPS-induced behavioral changes as fast as younger mice, as assessed by increased duration of immobility in the tail suspension and forced swim tests, compared to younger adult mice (Godbout et al., 2008). These findings with LPS, as well as following a chronic infection with Mycobacterium bovis (vide infra), are interpreted to indicate that inflammation-induced depressive-like behaviors develop on a background of initial sickness behavior and that the two behaviors can be dissociated in time (Figure 1).
Figure 1.
Time-dependent dissociation of sickness and depressive-like behaviors. Acute sickness is induced following induction of proinflammatory cytokines in the brain. IDO is activated by these proteins, which leads to the induction of depressive-like behavior at a later point in time.
The behavioral evidence in favor of dissociation between sickness and depressive-like behaviors was reinforced with immunochemistry experiments that mapped the brain distribution of Delta FosB in addition to c-Fos, the protein product of the early activation gene c-fos (Frenois et al., 2007). The former protein is a truncated splice variant of FosB that is characterized by a long half-life, which leads to its accumulation. As expected, there was a marked increase in c-Fos labeling in a number of brain structures at 6 h following LPS injection. Most of this c-Fos immunoreactivity had dissipated 24 h later. However, the LPS-induced depressive-like behavior described above was associated with delayed cellular activity, as characterized by FosB/Δ FosB immunostaining, particularly in the extended amygdala, hippocampus and hypothalamus. These data established that there is also a partial dissociation in the neural correlates of LPS-induced sickness behavior and depressive-like behavior.
Depression is well-known to be associated with more chronic, insidious infectious diseases that can last for days, months and years. We therefore sought to develop a more chronic model for of inflammation-induced depressive-like behavior, and for this we chose Bacillus Calmette-Guerin (BCG). This bacillus is an attenuated form of Mycobacterium bovis that is used in Western Europe as a vaccine for tuberculosis. Inoculation with BCG by the intraperitoneal route leads to infection of macrophages throughout the body, with the exception of the central nervous system. These experiments demonstrated that BCG initially causes a sickness response. However, this lassitude and fever disappear within a week, and these symptoms are followed by development of depressive-like behaviors (Moreau et al., 2008).
Neuroinflammation Induced by Alcohol Requires Toll-like Receptor-4
All of these preceding data would be of no importance to research on alcohol abuse if it were not for the demonstration that alcoholism is associated with chronic inflammation. A substantial body of data now indicates that chronic alcohol abuse causes an increase in both systemic and brain levels of cytokines (Crews et al., 2006; Crews and Nixon, 2009). In rats, several months of alcohol consumption increases iNOS, COX-2 and IL-1β; and all three major families of stress kinases, ERK 1/2, p38 and JNK, in astrocytes (Valles et al., 2004). A similar event occurs in macrophages treated in vitro with moderate levels (10 – 50 mM) of alcohol (Fernandez-Lizarbe et al., 2008). Similar to astrocytes, macrophages and microglia express abundant amounts of a protein known as toll-like receptor 4 (TLR4) (reviewed in (Mallard et al., 2009)). Data such as these reinforce the idea that the balance of NFκβ with other transcription factors such as cAMP responsive element-binding protein (CREB) is crucial to alcohol-induced brain damage (Crews et al., 2006; Crews and Nixon, 2009).
Around 50 years ago, the C3H/HeJ mouse strain was identified to have a spontaneous mutation at the LPS response locus, making C3H/HeJ mice very resistant to this endotoxin. Several years ago we demonstrated that these mice that are known to lack functional TLR4 are also resistant to development of LPS-induced sickness behavior (Johnson et al., 1997; Segreti et al., 1997). It has been recently established that alcohol treatment (10 mM) of astrocytes in vitro activates the major inflammatory pathways within minutes in wild-type C57BL/6 control mice (Alfonso-Loeches et al., 2010). Surprisingly, these effects do not occur in TLR4-/- mice. Similarly, chronic alcohol consumption causes nearly the same effects in the cortex of the control mice, as well as significantly increases concentrations of TNFα and IL-1β. As occurred in vitro, these in vivo alcohol-induced increases in cortical cytokines do not occur in TLR4-/- mice. The TLR4 deficient mice also fail to up-regulate caspase-3 in the cortex, as occurs in the wild-type mice. This latter effect, if it occurs in humans, is likely to be associated with CNS neurodegeneration that has been observed in the brains of some alcoholics.
Activation of the Tryptophan Degrading Enzyme Indoleamine 2,3-dioxygenase (IDO) is Required for Development of Depressive-Like Behavior in Pre-Clinical Models
If inflammation induces major depressive disorders, there must be a mechanism impacted by inflammatory mediators that ultimately results in mood changes. New theories of depression are always confronted with the big white elephant in the corner of the room, which is the monoamine deficiency hypothesis of depression that posits a deficiency in norepinephrine or serotonin transmission in the brain (Belmaker and Agam, 2008). This controversy has been discussed many times in scholarly journals, so the pros and cons of this hypothesis will not be described again. Unfortunately, the practical issue is that at least one-third of clinically-depressed patients do not find long term relief from currently available anti-depressant drugs (Mathew and Charney, 2009), and the side effects of many of these treatments are clinically significant. It has been known for years that persons with chronic infectious, autoimmune and neoplastic diseases often become clinically depressed. Unfortunately, a unifying mechanism other than “stress” has never been reliably substantiated.
It is seldom recognized that the original monoamine deficiency hypothesis noted the potential maladaptive effects of shunting tryptophan metabolism from serotonin to kynurenine and its metabolites (Lapin and Oxenkrug, 1969). In a search for other players involved in clinical depression, Smith was the first to hypothesize that macrophages, which are activated at some stage in many diseases, synthesize monokines that lead to depression (Maes et al., 1995; Smith, 1991). Maes extended this hypothesis to products of T lymphocytes, as well as monocytes and acute phase proteins, consistent with studies showing an increase in activity of the innate immune system in depressed patients (Maes et al., 1995). Because of earlier observations showing that cancer patients treated therapeutically with cytokines develop a statistically significant increase in depressive symptoms, Lucile Capuron and colleagues tested a potential mechanism in humans by analyzing blood samples from cytokine-treated cancer patients (Capuron et al., 2002). They found a reduction in serum concentrations of tryptophan, without a change in other large neutral amino acids (e.g., tyrosine, valine, leucine, isoleucine and phenylalanine,) after one month of therapy. The lower the circulating levels of tryptophan the higher the severity of depressive symptoms.
Since the precursor of serotonin is tryptophan, the search began for the mechanism that might reduce serum concentration of this essential amino acid. Nearly a dozen years earlier, Fuchs described a significant relationship between neuropsychiatric symptoms and reduced tryptophan concentration in the serum of HIV patients (Fuchs et al., 1990). Tryptophan is catabolized by two major enzymes, indoleamine 2, 3 dioxygenase (IDO) and tryptophan 2, 3 dioxygenase (TDO), to form kynurenine. The former enzyme is expressed mostly in macrophages and microglia and is activated by proinflammatory cytokines, particularly interferon-α (IFNα). The latter enzyme is found predominantly in hepatocytes, and its activity is increased by glucocorticoids. Using an intraperitoneal injection of LPS, a standard model of acute systemic inflammation, Lestage and colleagues reported that IDO enzymatic activity is increased in the brains of mice 24 h later (Lestage et al., 2002). Using a classic model of a microorganism that chronically infects macrophages and increases expression of IFNα, BCG, we subsequently reported that serum tryptophan is reduced 7 d after infection whereas serum kynurenine is significantly elevated 15 to 32 d later (Moreau et al., 2005). Similar to kynurenine, serum IFNα is elevated during this same time period. Although the activity of TDO in the liver is reduced at all times following infection, enzymatic activity of IDO in both the lung and brain is significantly increased by infection with BCG.
These data are consistent with the notion that an increase in tryptophan catabolism occurs during systemic inflammation and is associated with symptoms of depression. However, a causal relationship had yet to be established. To test this possibility, we treated mice with an anti-inflammatory compound, minocycline, or a specific competitive inhibitor of IDO, 1-methyltryptophan (1-MT), prior to an intraperitoneal injection of LPS (O’Connor et al., 2009c). As expected, pretreatment with minocycline inhibited LPS-induced depressive-like behavior and CNS expression of TNFα, IL-1β and IFNα. More importantly, minocycline reversed the increase in the kynurenine/tryptophan ratio in both blood and brain and blocked expression of IDO steady-state transcripts in the brain. In contrast, pre-treatment with the IDO inhibitor, 1-MT, did not affect the expression of TNFα, IL-1β or IFNα in the brain. However, 1-MT did reverse the rise in the ratio of kynurenine/tryptophan in both blood and brain, blocked the expression of IDO and prevented the LPS-induced increase in depressive-like behavior. These data were interpreted to indicate that the LPS-induced proinflammatory cytokines (blocked by minocycline) cause the activation of IDO (blocked by 1-MT), which subsequently causes expression of depressive-like behaviors in this preclinical model. We reported similar results in the chronic, BCG-induced model of inflammation-induced depressive-like behavior by using both pharmacological (1-MT and the TNFα antagonist, etanercept) and genetic (IDO and IFNα receptor knockout mice) approaches (O’Connor et al., 2009a; O’Connor et al., 2009b).
We recently summarized all these data on inflammation-induced depressive-like behavior (Dantzer et al., 2008b; Dantzer et al., 2011; Luo et al., 2010). This summary concluded that in these models, depressive-like behavior develops on a background of inflammation-induced expression of proinflammatory cytokines in the brain, which are the cause of sickness behavior (Figure 1). These cytokines induce the downstream expression of IDO. It is the induction of IDO rather than a depletion of either tryptophan or serotonin that causes depressive-like behavior in rodents. This conclusion has recently been supported in hepatitis C patients given systemic treatment with interferon-α (Raison et al., 2009). In these subjects, treatment with interferon-α reduces plasma concentration of tryptophan but has no effect on the concentration of tryptophan in the cerebrospinal fluid. However, the increase in plasma kynurenine was mirrored by a similar statistically significant increase in kynurenine in the cerebrospinal fluid, and this was associated with a significant rise in both quinolinic acid and kynurenic acid in the cerebrospinal fluid. These findings are consistent with the idea that central IDO was activated in these patients. In this sense, IDO is a master regulator that is responsible for the switch from sickness to depressive-like behavior (Figure 1).
New findings using another well-accepted model of depressive-like behavior have demonstrated that chronic mild stress induces depressive-like behavior and impairs hippocampal neurogenesis in rodents (Goshen et al., 2008; Koo and Duman, 2008). In this model, chronic mild stress increases both IL-1 synthesis (Goshen et al., 2008) and IDO expression (Laugeray et al., 2010) in the brain. The resulting depressive-like behavior can be induced by peripheral injection of IL-1 and does not occur in IL-1 receptor knockout mice or in mice that overexpress the IL-1 receptor antagonist in the brain (Goshen et al., 2008). Pharmacological inhibition of NFκβ has also been recently demonstrated to inhibit stress-induced induction of hippocampal neurogenesis and expression of depressive-like behavior (Koo et al., 2010). These results are consistent with the recent findings of induction of IL-1β by peripheral nerve injury and its interaction of this type of pain with stress in the induction of depressive-like behavior in mice (Norman et al., 2010).
Does Indoleamine 2,3-dioxygenase Mediate Alcohol-associated Depression as well as Inflammation-induced Depression?
It is possible that depression associated with chronic alcohol abuse also involves tryptophan catabolism because LPS, which requires TLR4 for expression of its biological activity, induces IDO expression and enzymatic activity. Unfortunately, the mechanism by which moderate amounts of alcohol activate TLR4 is not certain. Current evidence favors the clustering of TLR4 with downstream signals such as MyD88 and CD14 into lipid rafts (Fernandez-Lizarbe et al., 2008). Another possibility is the well-known oxidation of polyunsaturated fatty acids by the liver caused by alcohol abuse, resulting in an increase in the concentration of circulating oxidized phospholipids (Yang et al., 2010). A third potential mechanism, particularly in the brain, is via an indirect route. Chronic alcohol ingestion may cause the release of damage-associated molecular patterns (DAMP) such as high-mobility group box 1 (HMGB1; (Garg et al., 2010)). For example, neurons that are exposed to alcohol insults are likely to release HMGB1, which is a member of the alarmin family of proteins. HMGB1 is known to bind and activate TLR4, as well as TLR-2 and RAGE (Huang et al., 2010). Regardless of the specific details of how alcohol leads to activation of TLR4, it is important for other research scientists to replicate the findings of Alfonso-Loeches et al (2010) that show a requirement for TLR4 for alcohol-induced neuroinflammation. Confirmation of this finding will lay a very firm foundation for a physiological role of the immune system as a critical link between the brain and behavior caused by alcohol consumption.
Several years ago, it was shown that chronic exposure (1 month) of rats to alcohol augments enzymatic activity of TDO in the liver and increases the amount of kynurenine excreted in urine (Branchey and Lieber, 1982). Using tryptophan loading, these workers subsequently established that urinary kynurenine is higher in human alcoholics following 3 days of abstinence compared to 1 month of abstinence (Buydens-Branchey et al., 1988). Since plasma cortisol mirrored these findings, it is likely that glucocorticoids were responsible for the induction of hepatic TDO. More recently, it has been established in humans that even a short, 2 h exposure to alcohol causes a significant reduction in plasma tryptophan and leads to an increase in kynurenine (Badawy et al., 2009). Data such as these in control human subjects establish that short term alcohol intoxication drives tryptophan metabolism toward the kynurenine pathway. Collectively, all these data point to the possibility that neuroinflammation caused by alcohol abuse activates IDO and leads to symptoms of depression. These concepts are summarized in Figure 2.
Figure 2.
An emerging view of alcohol abuse, the immune system and behavior. Chronic ingestion of alcohol leads to both systemic and neuroinflammation by activating TLR4. Systemically, this occurs on Kupffer cells in the liver sinusoids and in the brain it occurs by activation of TL4 on microglia and astrocytes. Chronic alcohol consumption is likely to prime Kupffer cells so that they synthesize and release enhanced amounts of IL-6, TNFα into the circulation. Activity of the enzyme 2, 3 tryptophan dioxygenase (TDO) is also increased. Similarly, hepatocytes synthesize greater amounts of C-reactive protein and there is an increase in oxidation of polyunsaturated fatty acids. In the brain, chronic alcohol consumption somehow leads to activation of TLR4, perhaps by causing the release of damage-associated molecular patterns such as high-mobility group box 1 (HMGB1) which subsequently binds TLR4. Downstream signaling pathways are activated, such as NFκβ, and microglia express chemotactic proteins such as CCL2 (MCP-1) that can attract monocytes into the CNS. Proinflammatory cytokines such as interferon-α and TNFα are synthesized, which may cause an increase in expression of indoleamine 2, 3 dioxygenase (IDO). The downstream tryptophan metabolites of IDO are likely to cause functional changes in neuronal circuits that culminate in inflammation-associated changes in mood, such as depression.
Summary
When this journal, Brain, Behavior, and Immunity, published its first issue in 1987, it was never imagined that the link between alcoholism and behavior involves the immune system. Much like the trajectory of scientific discoveries in the field of brain, behavior, and immunity that first studied the role of stress on immunity, scientists focused on investigating the effects of alcohol consumption on functioning of the immune system. The discovery that the immune system communicates with the brain via synthesis of proinflammatory cytokines is now mirrored by similar experiments showing that chronic alcohol consumption augments proinflammatory cytokine production in the brain. In contrast, moderate amounts of alcohol consumption have been shown to reduce risk of type 2 diabetes and coronary heart disease, perhaps by exerting anti-inflammatory properties (reviewed in (Hendriks and van Tol, 2005)). If so, the beneficial and detrimental properties of alcohol consumption would appear to follow the shape of a J- or a U-shape curve. A similar model has recently been proposed to explain the beneficial and detrimental effects of proinflammatory cytokines on learning and memory (Figure 3; Yirmiya and Goshen, 2011). These newly-discovered links between alcoholism, neuroinflammation and depression demand new approaches in pre-clinical and clinical experiments in the continuing search for new and effective treatments for alcohol abuse.
Figure 3.
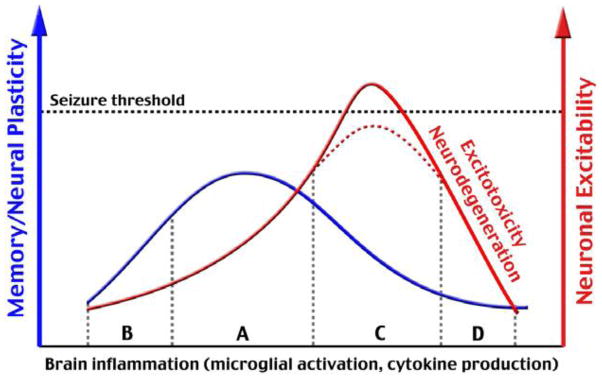
A conceptual model of the role for neuroinflammation in cognition (Section A on abscissa) as extended to mood changes caused by alcohol consumption. Low amounts of proinflammatory cytokines such as found in IL-1 receptor-deficient animals reduce learning and memory (section B on the abscissa). An optimal amount of cytokines is required for beneficial effects on cognition, and similar effects may occur with the effect of moderate alcohol consumption on mood. However, if proinflammatory cytokines increase to excessive levels in the CNS as caused by chronic alcohol consumption, learning and memory as well as depressive-like behavior can occur (part C on abscissa). Although enhanced excitability of glutamatergic signals seldom leads to epileptic-like seizures, a further elevation in brain inflammation can lead to neuronal death (section C). Figure from (Yirmiya and Goshen, 2011), with permission.
Acknowledgments
Supported by NIH grants to KWK (R01 AG 029573) and RD (R01 MH 079829).
Footnotes
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alho H, Sillanaukee P, Kalela A, Jaakkola O, Laine S, Nikkari ST. Alcohol misuse increases serum antibodies to oxidized LDL and C-reactive protein. Alcohol and alcoholism (Oxford, Oxfordshire) 2004;39:312–315. doi: 10.1093/alcalc/agh059. [DOI] [PubMed] [Google Scholar]
- Badawy AA, Doughrty DM, Marsh-Richard DM, Steptoe A. Activation of liver tryptophan pyrrolase mediates the decrease in tryptophan availability to the brain after acute alcohol consumption by normal subjects. Alcohol and alcoholism (Oxford, Oxfordshire) 2009;44:267–271. doi: 10.1093/alcalc/agp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban E, Milon G, Prudhomme N, Fillion G, Haour F. Receptors for interleukin-1 (alpha and beta) in mouse brain: mapping and neuronal localization in hippocampus. Neuroscience. 1991;43:21–30. doi: 10.1016/0306-4522(91)90412-h. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. The New England journal of medicine. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Branchey L, Lieber CS. Activation of tryptophan pyrrolase after chronic alcohol administration. Substance and alcohol actions/misuse. 1982;3:225–229. [PubMed] [Google Scholar]
- Brydon L, Walker C, Wawrzyniak A, Whitehead D, Okamura H, Yajima J, Tsuda A, Steptoe A. Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain, behavior, and immunity. 2009;23:217–224. doi: 10.1016/j.bbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Worner TM, Zucker D, Aramsombatdee E, Lieber CS. Increase in tryptophan oxygenase activity in alcoholic patients. Alcoholism, clinical and experimental research. 1988;12:163–167. doi: 10.1111/j.1530-0277.1988.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient’s initial affective state. The New England journal of medicine. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Molecular psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcoholism, clinical and experimental research. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and alcoholism (Oxford, Oxfordshire) 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Wada E, Carter DB, Tracey DE, Battey JF, De Souza EB. In situ histochemical localization of type I interleukin-1 receptor messenger RNA in the central nervous system, pituitary, and adrenal gland of the mouse. J Neurosci. 1992;12:1101–1114. doi: 10.1523/JNEUROSCI.12-03-01101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, Perry VH, Rousey S, Yirmiya R. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008a;33:18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life Sci. 1989;44:1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008b;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-Associated Depression: From Serotonin to Kynurenine. Psychoneuroendocrinology. 2011. http://dx.doi.org/10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed]
- Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacology, biochemistry, and behavior. 2005;81:688–693. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987;139:459–463. [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Gascon MS, Blanco A, Guerri C. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Molecular immunology. 2008;45:2007–2016. doi: 10.1016/j.molimm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- French RA, VanHoy RW, Chizzonite R, Zachary JF, Dantzer R, Parnet P, Bluthe RM, Kelley KW. Expression and localization of p80 and p68 interleukin-1 receptor proteins in the brain of adult mice. Journal of neuroimmunology. 1999;93:194–202. doi: 10.1016/s0165-5728(98)00224-0. [DOI] [PubMed] [Google Scholar]
- French RA, Zachary JF, Dantzer R, Frawley LS, Chizzonite R, Parnet P, Kelley KW. Dual expression of p80 type I and p68 type II interleukin-I receptors on anterior pituitary cells synthesizing growth hormone. Endocrinology. 1996;137:4027–4036. doi: 10.1210/endo.137.9.8756580. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor JC, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs D, Moller AA, Reibnegger G, Stockle E, Werner ER, Wachter H. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. Journal of acquired immune deficiency syndromes (1999) 1990;3:873–876. [PubMed] [Google Scholar]
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends in immunology. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, JOC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Molecular psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological psychiatry. 2009a;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biological psychiatry. 2009b;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience and Biobehavioral Reviews. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental neurology. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Overstreet DH, Crews FT. Abstinence from moderate alcohol self-administration alters progenitor cell proliferation and differentiation in multiple brain regions of male and female P rats. Alcoholism, clinical and experimental research. 2009;33:129–138. doi: 10.1111/j.1530-0277.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- Hendriks HF, van Tol A. Alcohol. Handbook of experimental pharmacology. 2005:339–361. doi: 10.1007/3-540-27661-0_12. [DOI] [PubMed] [Google Scholar]
- Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry. 2009;66:191–195. doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MF, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behavior, and Immunity. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Rinetti G. Disordered sleep, nocturnal cytokines, and immunity: interactions between alcohol dependence and African-American ethnicity. Alcohol (Fayetteville, NY) 2004;32:53–61. doi: 10.1016/j.alcohol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Gheusi G, Segreti S, Dantzer R, Kelley KW. C3H/HeJ mice are refractory to lipopolysaccharide in the brain. Brain research. 1997;752:219–226. doi: 10.1016/s0006-8993(96)01454-0. [DOI] [PubMed] [Google Scholar]
- Kelley KW. Cross-talk between the immune and endocrine systems. Journal of animal science. 1988;66:2095–2108. doi: 10.2527/jas1988.6682095x. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Aubert A, Dantzer R. Inflammation and behavior. In: Demas GE, Nelson RJ, editors. Eco-Immunology and Behavior. Oxford University Press; Oxford, New York: 2011. [Google Scholar]
- Kelley KW, Weigent DA, Kooijman R. Protein hormones and immunity. Brain, behavior, and immunity. 2007;21:384–392. doi: 10.1016/j.bbi.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proceedings of the National Academy of Sciences of the United States of America. 1992a;89:9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992b;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Kent S, Rodriguez F, Kelley KW, Dantzer R. Reduction in food and water intake induced by microinjection of interleukin-1 beta in the ventromedial hypothalamus of the rat. Physiology & behavior. 1994;56:1031–1036. doi: 10.1016/0031-9384(94)90339-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of general psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapin IP, Oxenkrug GF. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet. 1969;293:132–136. doi: 10.1016/s0140-6736(69)91140-4. [DOI] [PubMed] [Google Scholar]
- Laugeray A, Launay JM, Callebert J, Surget A, Belzung C, Barone PR. Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression. Behavioural brain research. 2010;210:84–91. doi: 10.1016/j.bbr.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain, behavior, and immunity. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Liukkonen T, Silvennoinen-Kassinen S, Jokelainen J, Rasanen P, Leinonen M, Meyer-Rochow VB, Timonen M. The association between C-reactive protein levels and depression: Results from the northern Finland 1966 birth cohort study. Biological psychiatry. 2006;60:825–830. doi: 10.1016/j.biopsych.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Luo L, Rodriguez E, Jerbi K, Lachaux JP, Martinerie J, Corbetta M, Shulman GL, Piomelli D, Turrigiano GG, Nelson SB, Joels M, de Kloet ER, Holsboer F, Amodio DM, Frith CD, Block ML, Zecca L, Hong JS, Dantzer R, Kelley KW, Bud Craig AD. Ten years of Nature Reviews Neuroscience: insights from the highly cited. Nature reviews. 2010;11:718–726. doi: 10.1038/nrn2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–116. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- Malarkey WB, Mills PJ. Endocrinology: the active partner in PNI research. Brain, behavior, and immunity. 2007;21:161–168. doi: 10.1016/j.bbi.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard C, Wang X, Hagberg H. The role of Toll-like receptors in perinatal brain injury. Clinics in perinatology. 2009;36:763–772. v–vi. doi: 10.1016/j.clp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Charney DS. Publication bias and the efficacy of antidepressants. The American journal of psychiatry. 2009;166:140–145. doi: 10.1176/appi.ajp.2008.08071102. [DOI] [PubMed] [Google Scholar]
- Miller AH. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23:149–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Andre C, O’Connor JC, Dumich SA, Woods JA, Kelley KW, Dantzer R, Lestage J, Castanon N. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun. 2008;22:1087–1095. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, Castanon N. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. The Journal of infectious diseases. 2005;192:537–544. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. The New England journal of medicine. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Nagata S, Taira H, Hall A, Johnsrud L, Streuli M, Ecsodi J, Boll W, Cantell K, Weissmann C. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature. 1980;284:316–320. doi: 10.1038/284316a0. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Taylor AN, Lewis JW, Poland RE, Redei E, Branch BJ. Pituitary-adrenal responses to morphine and footshock stress are enhanced following prenatal alcohol exposure. Alcoholism, clinical and experimental research. 1986;10:397–402. doi: 10.1111/j.1530-0277.1986.tb05112.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nature neuroscience. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, Devries AC. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Molecular psychiatry. 2010;15:404–414. doi: 10.1038/mp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009a;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009b;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular psychiatry. 2009c;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnet P, Amindari S, Wu C, Brunke-Reese D, Goujon E, Weyhenmeyer JA, Dantzer R, Kelley KW. Expression of type I and type II interleukin-1 receptors in mouse brain. Brain research. 1994;27:63–70. doi: 10.1016/0169-328x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Parnet P, Brunke DL, Goujon E, Mainard JD, Biragyn A, Arkins S, Dantzer R, Kelley KW. Molecular identification of two types of interleukin-1 receptors in the murine pituitary gland. Journal of neuroendocrinology. 1993;5:213–219. doi: 10.1111/j.1365-2826.1993.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Molecular psychiatry. 2009 doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science (New York, NY) 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Segreti J, Gheusi G, Dantzer R, Kelley KW, Johnson RW. Defect in interleukin-1beta secretion prevents sickness behavior in C3H/HeJ mice. Physiology & behavior. 1997;61:873–878. doi: 10.1016/s0031-9384(96)00611-7. [DOI] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Medical hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiological reviews. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR, Canino GJ, Kessler RC, Rubio-Stipec M, Angst J. The comorbidity of alcoholism with anxiety and depressive disorders in four geographic communities. Comprehensive psychiatry. 1998;39:176–184. doi: 10.1016/s0010-440x(98)90058-x. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Kokka N. Neuroendocrine effects of fetal alcohol exposure. Progress in biochemical pharmacology. 1981;18:99–110. [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain pathology (Zurich, Switzerland) 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain, behavior, and immunity. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yang L, Latchoumycandane C, McMullen MR, Pratt BT, Zhang R, Papouchado BG, Nagy LE, Feldstein AE, McIntyre TM. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. The Journal of biological chemistry. 2010;285:22211–22220. doi: 10.1074/jbc.M110.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain research. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, behavior, and immunity. 2011 doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Happel KI, Raasch CE, Nelson S. Alcohol abuse, immunosuppression, and pulmonary infection. Current drug abuse reviews. 2008;1:56–67. doi: 10.2174/1874473710801010056. [DOI] [PubMed] [Google Scholar]




